Introduction
Cerebral aneurysm (CA) is a cystic dilation of
cerebral vascular wall caused by congenital or postnatal factors
(1,2). CA is characterized by the destruction
of the integrity of arterial wall, endothelial dysfunction and
extracellular matrix disorder (3).
There are reports that 20-50% of patients with CA will experience
the rupture of CA, leading to subarachnoid hemorrhage with high
disability rate, high mortality rate and poor prognosis (4-7).
Unfortunately, most patients are painless and asymptomatic when the
aneurysm is not ruptured, which is a challenge for early treatment
(8). At present, the occurrence and
rupture of CA is closely associated with inflammation (9), and macrophages are the key effector
cells (10). Monocyte
chemoattractant protein-1 (MCP-1) induces macrophages to infiltrate
CA walls and stimulates macrophages to differentiate into
inflammatory phenotypes, thus affecting the formation and rupture
of CA (11). In addition, the low
wall shear stress of the aneurysm wall induces the continuous
expression of MCP-1 in vascular smooth muscle cells (12,13).
However, the pathogenesis of aneurysm remains to be explored.
Therefore, it is urgent to identify useful biomarkers for the
diagnosis and prognosis of CA.
MicroRNAs (miRNAs) are short strands of noncoding
RNAs composed of 20-25 nucleotides (14,15).
miRNAs can inhibit the expression of target genes by binding with
the 3'-untranslated region (UTR) region of target mRNA to inhibit
the translation of mRNA or promote its degradation, thus regulating
most biological processes such as cellular proliferation,
migration, invasion and apoptosis (16-19).
The dysregulation of miRNAs is frequently found in the pathogenesis
of inflammatory diseases (20). For
example, Kawano and Nakamachi (21)
found that miR-124 can regulate the process of rheumatoid
arthritis, and researchers also confirmed that the inhibition of
miR-124 can activate the inflammatory response of retina and
central neurons (22,23). The formation and rupture of CA are
attributed to the inflammatory dysfunction of endothelial cells,
and some researchers have confirmed that miRNAs are associated with
the formation of CA (24,25). Therefore, it is speculated that
miRNAs are involved in the inflammatory process of CA. It has been
found that miR-124-5p can regulate macrophage phagocytosis and
inhibit inflammatory pathways (26,27).
In addition, miR-124-5p has been shown to regulate angiogenesis
(27). However, the role and
molecular mechanism of miR-124-5p in CA has not been confirmed. The
present study aims to explore the effect and the molecular
mechanism of miR-124-5p on the occurrence and development of
CA.
Materials and methods
Clinical samples
Peripheral blood samples from 30 patients with CA
and 30 healthy individuals were collected from the Handan Central
Hospital between August 2018 and January 2020. Before surgery, all
patients did not receive treatment. Written informed consent was
obtained from all participants and their families. The
institutional ethics committee of Handan Central Hospital has
approved this study on August 20, 2018.
In vitro CA model construction
Human umbilical vein endothelial cells (HUVECs) were
chosen as the model cells in the present study, since it is a
common cell in vascular system in vitro research, and are
easier to obtain compared with other endothelial cells (28). In addition, the cellular function of
HUVECs inhibited by inflammatory smooth muscle cells that are
associated with CA (28). HUVECs
purchased from Thermo Fisher Scientific, Inc. (cat. no. C0155C),
and the cells were cultured in 37˚C with 5% CO2 for 2
days in DMEM complete medium (Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.). HUVECs were plated in 24-well plates (3x105
cells/well) and treated with 10 ng/ml IL-1b (Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 day at 37˚C to establish the
inflammatory model of CA.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA according to
the manufacturer's instructions. The cDNA was synthesized using the
First Strand cDNA Synthesis kit at 55˚C for 30 min (Thermo Fisher
Scientific, Inc.). Then, the mixture system including SYBR Green
qPCR Master mix, cDNA templates and primers were applied for qPCR
according to standard methods (95˚C for 3 min to carry on
pre-denaturation; 95˚C for 30 sec to carry out denaturation; 55˚C
for 30 sec to subject to annealing; and 72˚C for 60 sec to subject
to elongation) to detect the expression of miR-124-5p or FoxO1.
Finally, 1.5% agarose gel was used to electrophorese and visualize
the products of PCR. The relative expression was calculated by the
2-ΔΔCq method (29). U6
small nuclear RNA and GAPDH were used as the internal controls of
miR-124-5p and FoxO1, respectively. The primer sequences were:
miR-124-5p forward, 5'-CGCGTGTTCACAGCGGAC-3'; miR-124-5p reverse,
5'-AGTGCAGGGTCCGAGGTATT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3'; U6 reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; FoxO1 forward,
5'-GGCTGAGGGTTAGTGAGCAG-3'; FoxO1 reverse,
5'-AAAGGGAGTTGGTGAAAGACA-3' (30);
GAPDH forward, 5'-TCGACAGTCAGCCGCATCTTCTTT-3'; and GAPDH reverse,
5'-GCCCAATACGACCAAATCCGTTGA-3' (31).
Cell transfection
miR-124-5p-inhibitor, miR-124-5p-mimics, FoxO1-small
interfering (si)RNA (si-FoxO1) and negative controls (inhibitor-NC,
mimic-NC and si-NC) were designed and construct by Shanghai Gene
Pharmaceutical Technology Comsenz Inc. In addition, the
Lipofectamine™ 3000 Transfection kit was purchased from Thermo
Fisher Scientific, Inc. Three different siRNAs were used to
knockdown FoxO1 and si-FoxO1-1 was screened out as the most
specific for subsequent experiments (Fig. S1). The cells were cultured with
trypsin for 1 h, and then the cells were transfected according to
the instructions of the Lipofectamine™ 3000 Transfection kit.
Subsequently, the cells were cultured in an incubator with 5%
CO2 at 37˚C for 48 h. Meanwhile, the transfection
efficiency of miR-124-5p-mimic/inhibitor was also detected by PCR
(Fig. S2).
Enzyme-linked immunosorbent assay
(ELISA)
Human IL-6 ELISA kit (cat. no. ab178013; Abcam),
Human TNF alpha ELISA kit (cat. no. ab181421; Abcam) and Human IL-8
ELISA kit (cat. no. ab214030; Abcam) were used to detect the levels
of inflammatory cytokines including interleukin-6 (IL-6),
interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α). According
to the instructions of the kits, 50 µl samples or standards were
added to the appropriate wells of 96-well plates that were
incubated with antibodies cocktail for 1 h at 37˚C. Then, each well
was washed by 350 µl 1X Wash Buffer PT for 3 times. Next, 100 µl
tetramethylbenzidine development solution were added into each well
and incubated for 10 min in the dark. Finally, the results were
detected by a Microplate Reader at 450 nm after the color reaction
stopped. Each sample was assayed using three replicates.
Western blotting
The expression of nuclear factor (NF)-κB,
intercellular adhesion molecule (ICAM)-1 and FoxO1 were detected by
western blotting. The HUVECs were lysed by RIPA total protein
lysate (150 mM NaCl, 0.5% sodium deoxycholate, 50 mM Tris-HCl, pH
7.4, 1% NP-40) to obtain protein samples. The BCA protein
quantitative detection kit was used to determine the protein
concentration, according to the instructions. Firstly, the protein
standard solution was prepared and added to the 96-well plates
according to the gradient concentration, and the appropriate
concentration of sample protein was added. Next, BCA solution
(A:B=50:1) was added to the protein solution and incubated at 37˚C
for 30 min. Finally, the absorbance was measured and the standard
curve was drawn to determine the concentration of sample protein.
Next, 40 µg protein of each group was loaded into 10% SDS-PAGE gel,
and GAPDH was used as the internal control to carry out
concentrated gel electrophoresis and separation gel
electrophoresis. Then, the transmembrane electrophoresis was
implemented by using the polyvinylidene fluoride (PVDF) membrane.
The PVDF membrane was blocked with 5% bovine serum albumin (BSA)
for 1 h at 37˚C. The membrane was then incubated with primary
antibodies purchased from Abcam, including anti-NF-NF-κB (cat. no.
ab16502; 0.5 µg/ml), anti-ICAM-1 (cat. no. ab109361; 1:10,000),
anti-FoxO1 (cat. no. ab70382; 1:10,000) and anti-GADPH (cat. no.
ab9485; 1/12,500) antibodies, for 14 h at 4˚C. Subsequently, the
secondary antibody Goat Anti-Rabbit IgG H&L (HRP) (cat. no.
ab205718; Abcam; 1:50,000) was added to the PVDF membranes and
incubated for 90 min at 37˚C. Eventually, the PVDF membrane was put
into the gel imager with ECL luminescent reagent for exposure for
30 sec. The proteins were quantified using ImageJ software (v1.8.0;
National Institutes of Health).
Dual luciferase reporter assay
The binding site of FoxO1 in miR-124-5p was
predicted using the TargetScan website (http://www.targetscan.org/mmu_72/), and the double
luciferase reporter gene analysis was applied to identify the
targeting association between miR-124-5p and FoxO1. The FoxO1
3'-UTR containing the miR-124-5p binding sites was synthesized and
inserted into the pGL3 control vector between the BamH1 site
and Xhal site to construct the FoxO1 wild-type (FoxO1-WT)
reporter vector. The FoxO1-WT reporter vector (100 ng) and
miR-124-5p mimics (50 nM) or mimic-NC (100 nM) were co-transfected
into the cells at 37˚C for 8 h using Lipofectamine™ 3000
Transfection kit. The aforementioned process was also applied to
the corresponding FoxO1 mutant (FoxO1 MUT) reporter vector. The
activity of luciferase was determined by the Dual-Luciferase
Reporter Assay System kit (cat. no. E1910; Shanghai Haoran
Biotechnology Co., Ltd.) after transfection for 48 h.
Transwell migration/invasion
assay
The migration and invasion abilities of the HUVECs
were detected by using Transwell chambers. After digestion and
washing, the cells at the logarithmic growth stage were resuspended
in serum-free medium at a cell concentration of 2x105
cells/ml. Next, 650 µl medium containing 10% FBS was added to the
lower chamber, 150 µl cell suspension was added to the upper
chamber, and the chamber was incubated for 24 h at 37˚C. Then, the
cells on the upper surface of the membrane were removed slowly
using a cotton swab. Subsequently, the cells on the lower surface
of the membrane were fixed with 4% methanol for 30 min at room
temperature after the membrane was dried, and the cells were
stained by Giemsa for 25 min at room temperature. Ultimately images
were captured with a Leica DC 300F light microscope (magnification,
x400) and the cells were counted using the ImageJ software (v1.8.0;
National Institutes of Health) to assess the ability of cell
migration. The invasion assay was performed as aforementioned for
the migration assay, except that Matrigel gel diluted by serum-free
DMEM medium was added to the upper chamber.
Statistical analysis
All experiments were repeated three times, and all
data are presented as means ± SD. The data were evaluated using
statistical approach, including one-way ANOVA followed by Sidak's
multiple comparisons test or Student's t-test, using the GraphPad
Prism 7.0 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
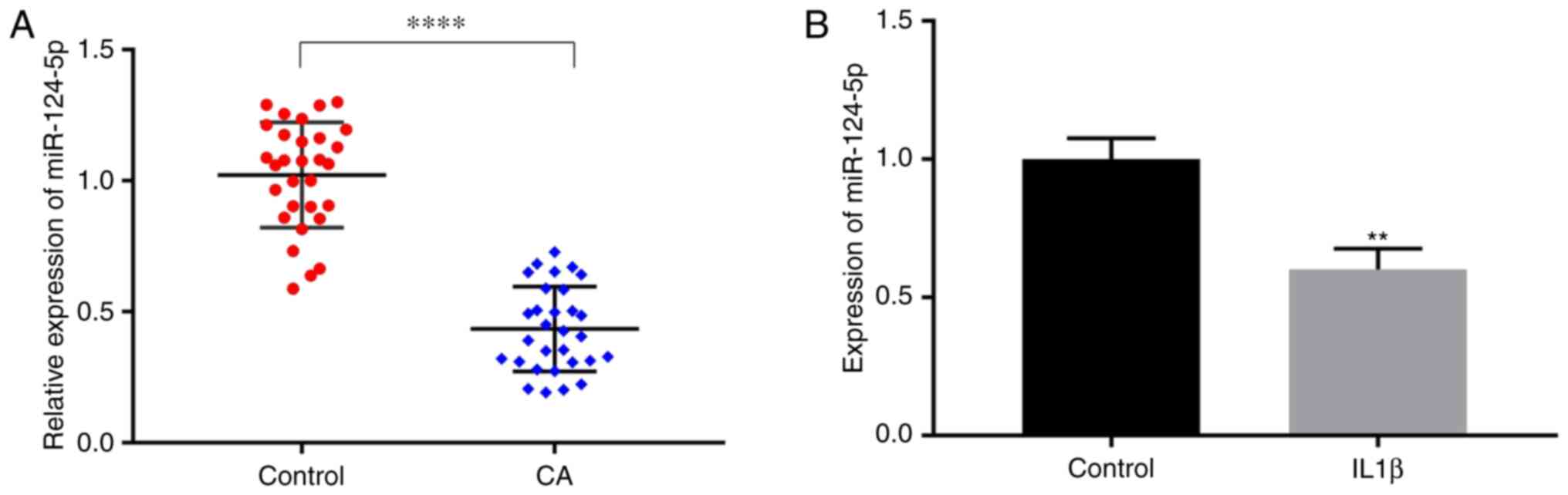
Downregulation of miR-124-5p in CA in
vivo and in vitro
The difference in the expression of miR-124-5p in
the peripheral blood of 30 patients with CA and 30 healthy
individuals was analyzed by using RT-qPCR. The results showed that
the expression of miR-124-5p was lower in patients with CA compared
with that of healthy individuals (Fig.
1A). In addition, according to previous studies, It is known
that IL-1β can activate vascular endothelial cells and induce their
secretion of adhesion factors such as ICAM-1, in order to recruit
inflammatory cells and trigger the inflammatory reaction of
arterial walls, thus forming aneurysm (32). In the present study, HUVECs were
used as an in vitro model system, and the IL-1β were used to
stimulate HUVECs to simulate the inflammatory state of CA. Then,
the expression of miR-124-5p was detected in HUVECs that were
simulated by IL-1β and the non-stimulated HUVECs using RT-qPCR. The
results showed that the expression of miR-124-5p in the IL-1β group
was significantly lower compared with that of the control group
(Fig. 1B).
Inhibition of miR-124-5p can enhance
the cell function of HUVECs and promote the release of inflammatory
factors
The changes in the cell function of HUVECs and the
associated inflammatory factors were detected following the
downregulation of miR-124-5p by the miR-124-5p-inhibitor. The
results of RT-qPCR showed that IL-1β could inhibit the expression
of miR-124-5p, and there was no significant difference between the
IL-1β group and IL-1β + miR-124-5p-inhibitor-negative control (NC)
group (Fig. 2A). Furthermore, the
application of miR-124-5p-inhibitor could enhance the inhibitory
effect of miR-124-5p compared with the IL-1β +
miR-124-5p-inhibitor-NC group (Fig.
2A). The results of the cell invasion assay indicated that the
IL-1β group had higher invasion ability compared with the control
group, and the invasion ability was further enhanced by
miR-124-5p-inhibitor compared with the miR-124-5p-inhibitor-NC
(Fig. 2B). Compared with the
control group, the migration ability of HUVECs was strengthened by
IL-1β, and miR-124-5p-inhibitor further promoted the cell migration
compared with the IL-1β + miR-124-5p-inhibitor-NC group (Fig. 2C). Meanwhile, western blotting and
ELISA were used to detect the expression of inflammatory factors.
Western blotting showed that the expression of NF-κB and ICAM-1 was
increased in the IL-1β group compared with the control group, and
the transfection of miR-124-5p-inhibitor enhanced the expression of
inflammatory factors compared with the IL-1β +
miR-124-5p-inhibitor-NC group (Fig.
2D). The results of ELISA indicated that IL-1β upregulated the
expression levels of IL-6, IL-8 and TNF-α compared with the control
group, and the miR-124-5p-inhibitor further promoted the increase
in the expression of inflammatory factors (Fig. 2E-G). The aforementioned experimental
results demonstrate that the inhibition of miR-124-5p could enhance
the cellular function of HUVECs and promote the release of
inflammatory factors.
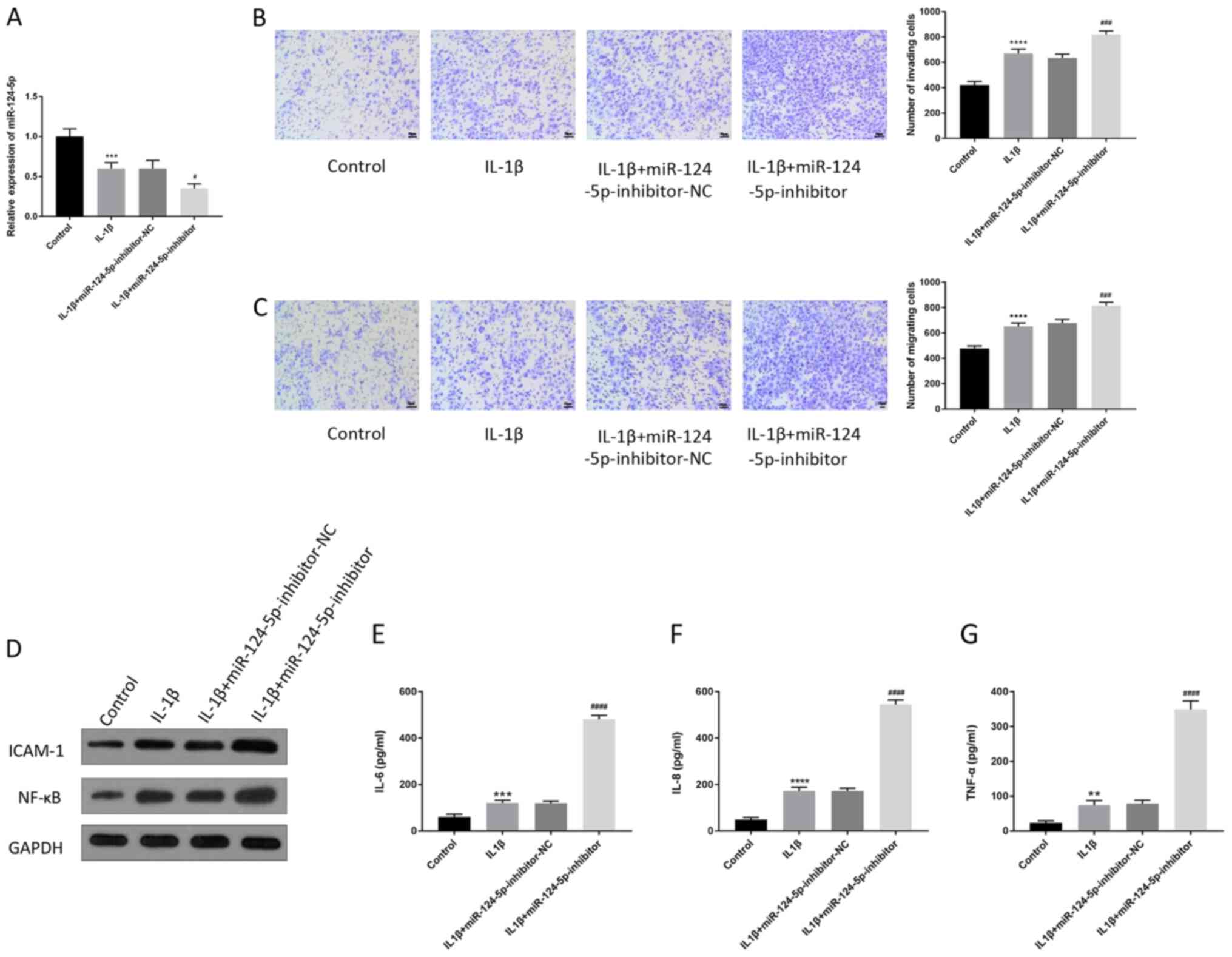 | Figure 2Inhibition of miR-124-5p can enhance
the cell function of HUVECs and promote the release of inflammatory
factors. In this group of experiment, HUVECs were treated
differently and divided into four groups: Control group; IL-1β
group; IL-1β + miR-124-5p-inhibitor-NC; IL-1β +
miR-124-5p-inhibitor. The transfection of miR-124-5p-inhibitor
decreased the expression of miR-124-5p, and miR-124-5p-inhibitor-NC
was the negative control. (A) The changes in miR-124-5p expression
in HUVECs were detected by reverse transcription-quantitative PCR.
The (B) invasion and (C) migration abilities of HUVECs were
detected by Transwell assay. Scale bar, 50 µm. (D) The changes in
the expression of ICAM-1 and NF-κB in HUVECs were detected by
western blotting. GAPDH was used as the internal reference. The
expression of (E) IL-6, (F) IL-8 and (G) TNF-α in HUVECs was
detected by enzyme-linked immunosorbent assay.
**P<0.01, ***P<0.001,
****P<0.0001 vs. control; #P<0.05,
###P<0.001, ####P<0.0001, vs. IL-1β +
miR-124-5p-inhibitor-NC. miR, microRNA; NC, negative control;
IL-1β/6/8, interleukin-1β/6/8; TNF-α, tumor necrosis factor-α;
ICAM1, intercellular adhesion molecule; NF-κB, nuclear
factor-κB. |
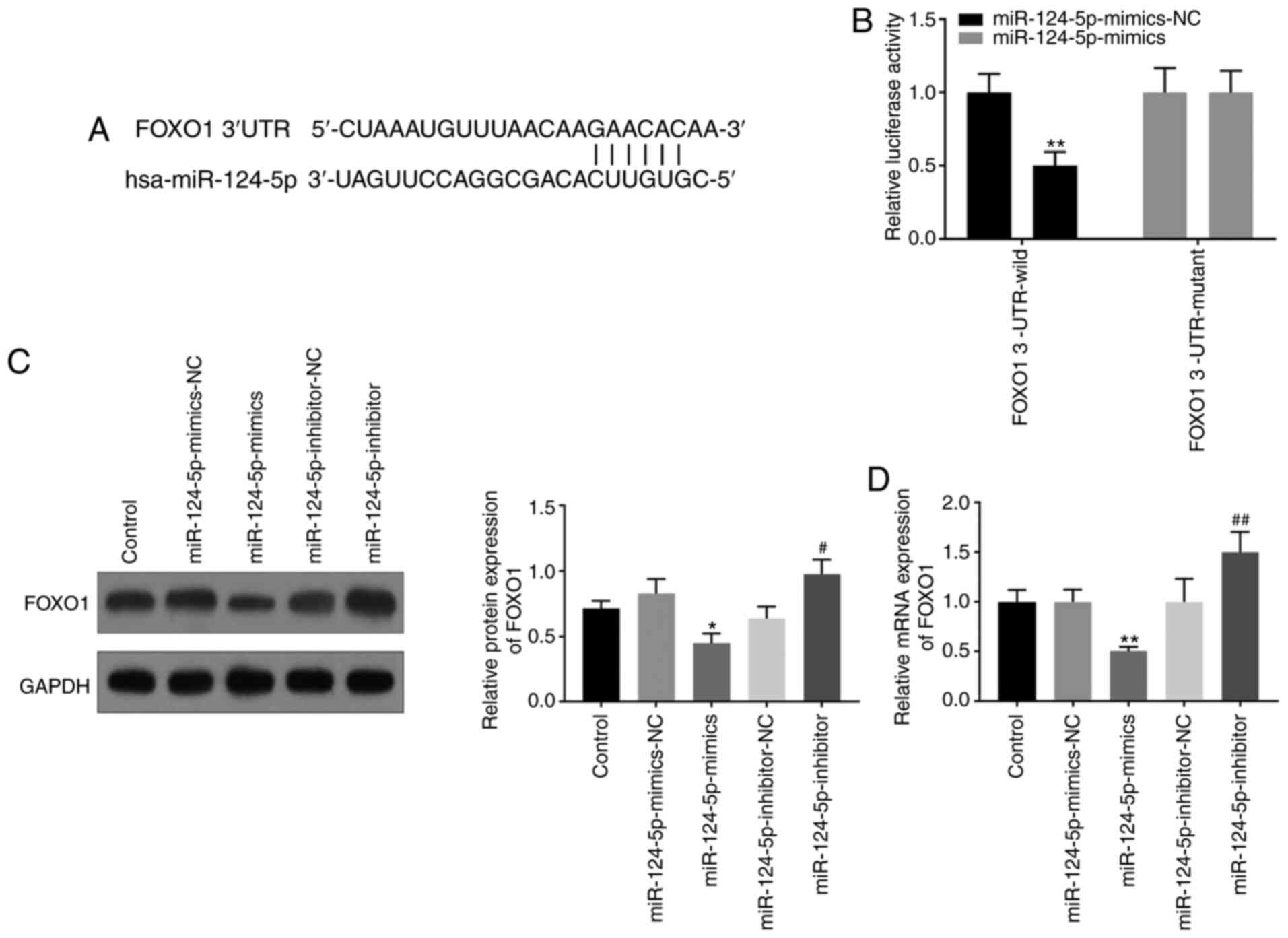
FoxO1 is the target gene of
miR-124-5p
It was predicted that FoxO1 is the target gene of
miR-124-5p by using the TargetScan website (Fig. 3A); this was verified using dual
luciferase reporter and western blotting assays. The results of the
dual luciferase assay showed decreased luciferase activity in the
FoxO1 3'-UTR wild-type group, following transfection with
miR-124-5p-mimics, compared with the miR-124-5p-mimics-NC group,
but there was no significant change in the FoxO1 3'-UTR mutant
group (Fig. 3B). Results of western
blotting displayed that the miR-124-5p-inhibitor increased the
expression of FoxO1, while miR-124-5p-mimics decreased the
expression of FoxO1 (Fig. 3C). The
results of RT-qPCR also showed that the miR-124-5p-mimics group had
lower expression of FoxO1 compared with the control group, and the
miR-124-5p-inhibitor group had the opposite results (Fig. 3D). All the experimental results
indicated that FoxO1 was the target gene of miR-124-5p, and
miR-124-5p could negatively regulate FoxO1.
miR-124-5p regulates the cell
migration and invasion, as well as the release of inflammatory
factors, through mediating FoxO1 expression
A series of experiments were conducted to verify the
interaction of FoxO1 with miR-124-5p in HUVECs. The results of
western blotting showed that the upregulated expression of FoxO1
induced by miR-124-5p-inhibitor was weakened by the si-FoxO1
(Fig. 4A). The results of cell
invasion and migration experiments indicated that inhibition of
miR-124-5p promoted the invasion and migration of HUVECs, which was
weakened after the cells were co-transfected with
miR-124-5p-inhibitor and si-FoxO1 (Fig.
4B and C). At the same time,
the increase in NF-κB, ICAM-1, IL-6, IL-8 and TNF-α that was
induced by miR-124-5p-inhibitor were downregulated by FoxO1
knockdown (Fig. 4A, D-F). All the experimental results showed
that inhibition of miR-124-5p promoted the cell function and the
release of inflammatory factors of HUVECs, which could be reversed
by FoxO1 knockdown. Hence, miR-124-5p regulated the cell function
and the release of inflammatory factors of HUVECs through mediating
FoxO1 expression.
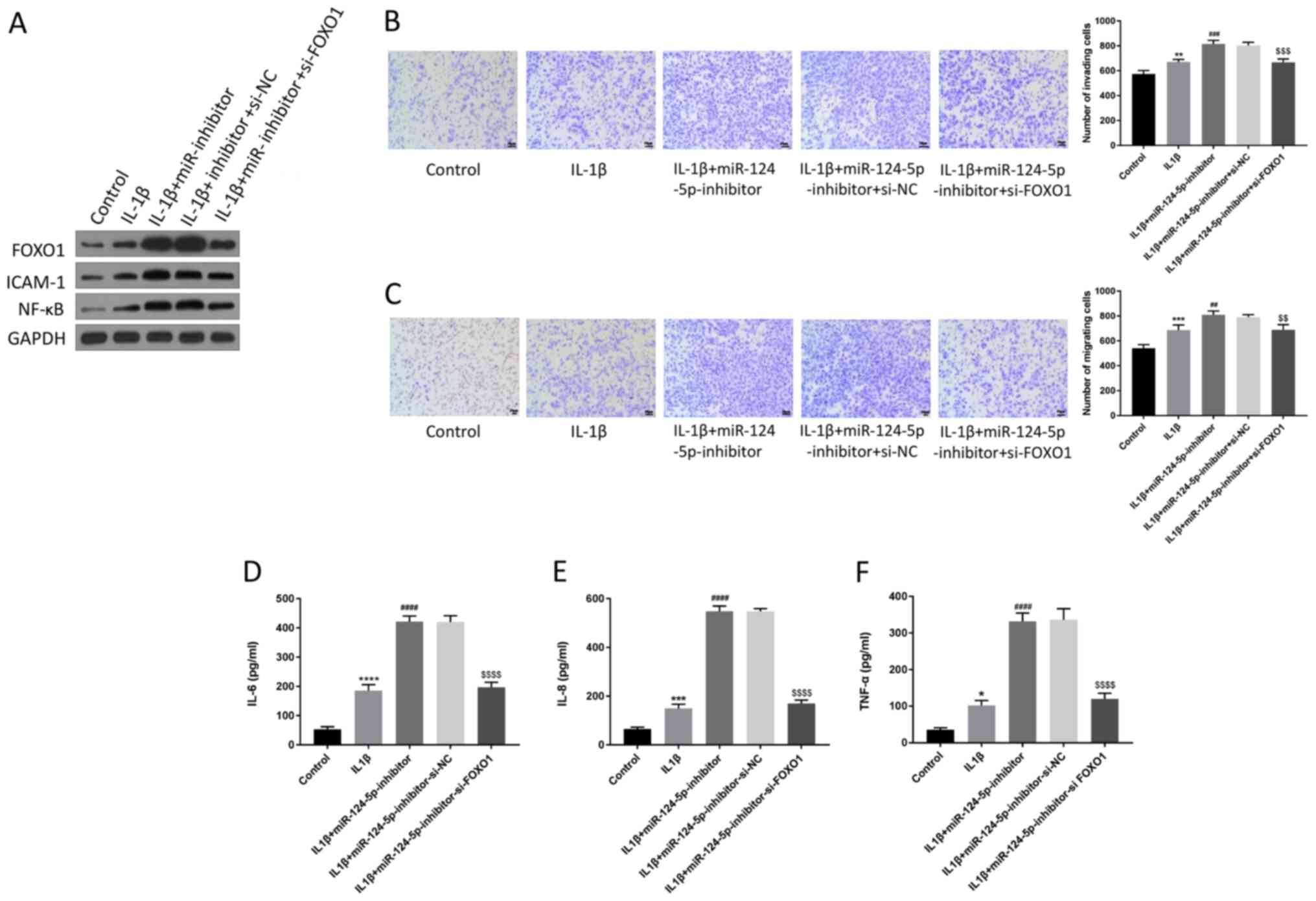 | Figure 4miR-124-5p regulates cell migration
and invasion, as well as the release of inflammatory factors,
through mediating FoxO1 expression. (A) The changes in the
expression levels of FoxO1, ICAM-1 and NF-κB in HUVECs after
transfecting with siRNA-FoxO1 was detected by western blotting.
GAPDH was used as the internal reference. The changes in (B)
invasion and (C) migration abilities of HUVECs were detected by
using Transwell assays after FoxO1 knockdown. Scale bar, 50 µm. The
changes in (D) IL-6, (E) IL-8 and (F) TNF-α of HUVECs were detected
by enzyme-linked immunosorbent assay after FoxO1 knockdown.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. control;
##P<0.01, ###P<0.001,
####P<0.0001 vs. IL-β; $$P<0.01,
$$$P<0.001, $$$$P<0.0001 vs. IL-1β +
miR-124-5p-Inhibitor-si-NC. miR, microRNA; NC, negative control;
TNF-α, tumor necrosis factor-α; ICAM1, intercellular adhesion
molecule; NF-κB, nuclear factor-κB; siRNA/si-, small interfering
RNA; IL-6/8, interleukin-6/8. |
Discussion
The present study demonstrated that the expression
of miR-124-5p in patients with CA and in HUVECs that were
stimulated by IL-1β was lower compared with that in healthy
individuals or cells. The lower expression of miR-124-5p could
promote the ability of cell invasion, migration and the
inflammatory response of HUVECs. Then, FoxO1 was predicted as a
target gene of miR-124-5p. The inhibition of miR-124-5p promoted
the cell migration, cell invasion and the release of inflammatory
factors, which could be reversed by FoxO1 knockdown. Hence, it was
speculated that miR-124-5p could delay the progression of CA by
inhibiting the expression of FoxO1.
The environmental and genetic factors of the
formation of CA have been reported, but the pathological factors of
its occurrence and development have not been fully acquainted
(33). Furthermore, the most
recognized factors that promote the development of CA are
inflammation and hemodynamics (34). NF-κB is one of the key components to
regulate the expression of proinflammatory genes and it induces the
expression of proinflammatory cytokines, chemokines and adhesion
molecules (35). It has been
reported that the expression of NF-κB in CA is significantly higher
compare with normal tissues and the activation of NF-κB is a risk
factor in the occurrence and development of CA (36-38).
In inflammatory reaction, endotoxin and some chemokines can promote
the expression of ICAM-1 in endothelial cells and leukocytes in the
wall of the porch (39).
Furthermore, the activated leukocytes adhere to the vascular wall
by ICAM-1, and then pass through the vessel wall and enter the
focus of infection (39). TNF-α
plays a role in vascular inflammation when the vascular
inflammation is closely associate with the progression of aneurysm
(40-42).
Some studies have confirmed that hemodynamic stress increases the
expression of TNF-α, and TNF-α inhibitors can inhibit the formation
of aneurysm and delay the disease process of aneurysm (42,43).
Studies have shown that cytokines such as IL-6 and IL-8 can
downregulate procollagen biosynthesis at the transcription level,
while the decrease in arterial wall collagen is a significant
pathological feature of CA (44).
Since the aforementioned inflammatory factors are closely
associated with the occurrence and process of CA, the present study
examined the changes in these inflammatory factors under various
experimental treatments.
Previous studies have shown that miRNAs can affect
multiple signaling pathways to regulate the cell function, such as
the ability of migration and invasion of cancer cells (45-48).
However, according to the research of miR-124-5p in diseases, the
effect of miR-124-5p on the ability of migration and invasion for
glioblastoma multiforme cells is not obvious (27). Because the influence of miR-124-5p
on the cell function of CA cells has not been studied, the present
study attempted to study about CA by using HUVECs. The results
indicated that the expression level of miR-124-5p in patients with
CA or in cells was lower compared with that in normal individuals
or cells. It was also found that the miR-124-5p inhibitor increased
the ability of cell migration and invasion of HUVECs stimulated by
IL-1β. It has been reported that miRNAs can regulate the expression
of a variety of inflammatory factors; for example, Wu et al
(49) found that miR-124-5p can
relieve the cerebral injury that is caused by inflammation by
downregulating the expression of IL-6 and TNF-α. The present study
further indicated that the decrease in miR-124-5p could increase
the release of inflammatory factors such as IL-6, IL-8, TNF-α,
ICAM-1 and NF-κB.
In order to further explore the molecular mechanism
of miR-124-5p in CA, target gene FoxO1 of miR-124-5p was identified
by using the TargetScan website. Previous studies have shown that
FoxO1 knockdown can inhibit inflammatory response; for example,
Malik et al (50) found that
FoxO1 knockdown can decrease the expression of IL-1β, thus,
relieving the symptoms of diabetic retinopathy, and Wu et al
(51) found that the allergic
inflammation in asthmatic patients can be lowered by the decrease
in IL-9 that is regulated by knocking down FoxO1. The present
results indicated that inhibition of miR-124-5p enhanced the
release of inflammatory factors (IL-6, IL-8, TNF-α, NF-κB and
ICAM-1), which was reversed by FoxO1 knockdown. Thus, the present
study showed the inhibition on the inflammation caused by FoxO1
knockdown is in agreement with the previous reports. Furthermore,
the promotion of migration and invasion of HUVECs induced by
miR-124-5p inhibitors was weakened by the introduction of FoxO1
siRNA. Therefore, miR-124-5p regulated the migration and invasion
abilities and the release of inflammatory factors of HUVECs through
mediating FoxO1 expression.
The present study focused on the regulation of
miR-124-5p and FoxO1 on the cellular function and the release of
inflammatory factors by HUVECs in vitro, however, whether
miR-124-5p/FoxO1 pathway is also associated with inflammatory
process or cellular function in CA animal models is the focus of
the follow-up study. In addition, whether factors (such as matrix
metalloproteinases) associated with CA in vivo or in
vitro are regulated by miR-124-5p/FoxO1 pathway will also be
studied in the future.
In conclusion, miR-124-5p was able to inhibit the
cellular function and the release of inflammatory factors of HUVECs
by downregulating the expression of FoxO1, which might prevent the
occurrence of CA or delay the development of CA. Therefore, the
present results provide the potential biomarkers and signaling
pathway for the treatment of CA, and supply another way to explore
the molecular mechanism of the occurrence and development of
CA.
Supplementary Material
Screening of specific siRNA and
detection of transfection efficiency. (A) Western blotting and (B)
reverse transcription-quantitative PCR assays were used to detect
the specific siRNA for FoxO1, and (A) the quantification of
proteins was performed by GraphPad Prism. **P<0.01,
***P<0.001, ****P<0.0001 vs. control;
##P<0.01, ###P<0.001,
####P<0.0001 vs. si-NC. siRNA/si-, small interfering
RNA; NC, negative control.
miR-124-5p-mimics/inhibitors were
successfully transfected into the cells. Reverse
transcription-quantitative assay was used to detect the
transfection efficiency of (A) miR-124-5p-mimics and (B)
miR-124-5p-inhibitors. **P<0.01,
****P<0.0001 vs. control; ##P<0.01,
####P<0.0001 vs. mimic-NC/inhibitor-NC. miR,
microRNA; NC, negative control.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RKW and GYY designed experiments; YYS, GYL and HTY
collected data; XJL, KFL and XZ analyzed experimental results. RKW
wrote the manuscript; GYY approved the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The institutional ethics committee of Handan Central
Hospital has approved this study. Written informed consent was
obtained from all participants and their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cebral JR and Raschi M: Suggested
connections between risk factors of intracranial aneurysms: A
review. Ann Biomed Eng. 41:1366–1383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sadasivan C, Fiorella DJ, Woo HH and
Lieber BB: Physical factors effecting cerebral aneurysm
pathophysiology. Ann Biomed Eng. 41:1347–1365. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kataoka H: Molecular mechanisms of the
formation and progression of intracranial aneurysms. Neurol Med
Chir (Tokyo). 55:214–229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alg VS, Sofat R, Houlden H and Werring DJ:
Genetic risk factors for intracranial aneurysms: A meta-analysis in
more than 116,000 individuals. Neurology. 80:2154–2165.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Juvela S: Prevalence of and risk factors
for intracranial aneurysms. Lancet Neurol. 10:595–597.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chalouhi N, Hoh BL and Hasan D: Review of
cerebral aneurysm formation, growth, and rupture. Stroke.
44:3613–3622. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wei L, Wang Q, Zhang Y, Yang C, Guan H,
Chen Y and Sun Z: Identification of key genes, transcription
factors and microRNAs involved in intracranial aneurysm. Mol Med
Rep. 17:891–897. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang WH, Wang YH, Zheng LL, Li XW, Hao F
and Guo D: MicroRNA-29a: A potential biomarker in the development
of intracranial aneurysm. J Neurol Sci. 364:84–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hosaka K and Hoh BL: Inflammation and
cerebral aneurysms. Transl Stroke Res. 5:190–198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kanematsu Y, Kanematsu M, Kurihara C, Tada
Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y
and Hashimoto T: Critical roles of macrophages in the formation of
intracranial aneurysm. Stroke. 42:173–178. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aoki T, Kataoka H, Ishibashi R, Nozaki K,
Egashira K and Hashimoto N: Impact of monocyte chemoattractant
protein-1 deficiency on cerebral aneurysm formation. Stroke.
40:942–951. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aoki T, Kataoka H, Nishimura M, Ishibashi
R, Morishita R and Miyamoto S: Ets-1 promotes the progression of
cerebral aneurysm by inducing the expression of MCP-1 in vascular
smooth muscle cells. Gene Ther. 17:1117–1123. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aoki T, Yamamoto K, Fukuda M, Shimogonya
Y, Fukuda S and Narumiya S: Sustained expression of MCP-1 by low
wall shear stress loading concomitant with turbulent flow on
endothelial cells of intracranial aneurysm. Acta Neuropathol
Commun. 4(48)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Zhao Y, Wang F, Bei Y, Xiao J and
Yang C: MicroRNAs in liver regeneration. Cell Physiol Biochem.
37:615–628. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Pal AS and Kasinski AL: Animal models to
study microRNA function. Adv Cancer Res. 135:53–118.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Henninger N and Mayasi Y: Nucleic acid
therapies for ischemic stroke. Neurotherapeutics. 16:299–313.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Martinez B and Peplow PV: Blood microRNAs
as potential diagnostic and prognostic markers in cerebral ischemic
injury. Neural Regen Res. 11:1375–1378. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chu-Tan JA, Rutar M, Saxena K, Aggio-Bruce
R, Essex RW, Valter K, Jiao H, Fernando N, Wooff Y, Madigan MC, et
al: MicroRNA-124 dysregulation is associated with retinal
inflammation and photoreceptor death in the degenerating retina.
Invest Ophthalmol Vis Sci. 59:4094–4105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawano S and Nakamachi Y: miR-124a as a
key regulator of proliferation and MCP-1 secretion in synoviocytes
from patients with rheumatoid arthritis. Ann Rheum Dis. 70 (Suppl
1):i88–i91. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun Y, Luo ZM, Guo XM, Su DF and Liu X: An
updated role of microRNA-124 in central nervous system disorders: A
review. Front Cell Neurosci. 9(193)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dong N, Xu B, Shi H and Lu Y: miR-124
regulates amadori-glycated albumin-induced retinal microglial
activation and inflammation by targeting Rac1. Invest Ophthalmol
Vis Sci. 57:2522–2532. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun L, Zhao M, Zhang J, Lv M, Li Y, Yang
X, Liu A and Wu Z: miR-29b downregulation induces phenotypic
modulation of vascular smooth muscle cells: Implication for
intracranial aneurysm formation and progression to rupture. Cell
Physiol Biochem. 41:510–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Signorelli F, Sela S, Gesualdo L, Chevrel
S, Tollet F, Pailler-Mattei C, Tacconi L, Turjman F, Vacca A and
Schul DB: Hemodynamic stress, inflammation, and intracranial
aneurysm development and rupture: A systematic review. World
Neurosurg. 115:234–244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Huang C, Chintagari NR, Xi D, Weng
T and Liu L: miR-124 regulates fetal pulmonary epithelial cell
maturation. Am J Physiol Lung Cell Mol Physiol. 309:L400–L413.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Q, Lu G, Cai Y, Li Y, Xu R, Ke Y and
Zhang S: miR-124-5p inhibits the growth of high-grade gliomas
through posttranscriptional regulation of LAMB1. Neuro Oncol.
16:637–651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu P, Shi Y, Fan Z, Zhou Y, Song Y, Liu
Y, Yu G, An Q and Zhu W: Inflammatory smooth muscle cells induce
endothelial cell alterations to influence cerebral aneurysm
progression via regulation of integrin and VEGF expression. Cell
Transplant. 28:713–722. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liang Y, Zhu D, Zhu L, Hou Y, Hou L, Huang
X, Li L, Wang Y, Li L, Zou H, et al: Dichloroacetate overcomes
oxaliplatin chemoresistance in colorectal cancer through the
miR-543/PTEN/Akt/mTOR pathway. J Cancer. 10:6037–6047.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tang Z, Gong Z and Sun X: LncRNA DANCR
involved osteolysis after total hip arthroplasty by regulating
FOXO1 expression to inhibit osteoblast differentiation. J Biomed
Sci. 25(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Skaria T, Bachli E and Schoedon G: RSPO3
impairs barrier function of human vascular endothelial monolayers
and synergizes with pro-inflammatory IL-1. Mol Med.
24(45)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chalouhi N, Ali MS, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ and Dumont AS:
Biology of intracranial aneurysms: Role of inflammation. J Cereb
Blood Flow Metab. 32:1659–1676. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tulamo R, Frösen J, Hernesniemi J and
Niemelä M: Inflammatory changes in the aneurysm wall: A review. J
Neurointerv Surg. 2:120–130. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Catrysse L and van Loo G: Inflammation and
the metabolic syndrome: The tissue-specific functions of NF-κB.
Trends Cell Biol. 27:417–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng WT and Wang N: Correlation between
MMP-2 and NF-κ B expression of intracranial aneurysm. Asian Pac J
Trop Med. 6:570–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aoki T, Kataoka H, Nishimura M, Ishibashi
R, Morishita R and Miyamoto S: Regression of intracranial aneurysms
by simultaneous inhibition of nuclear factor-κB and Ets with
chimeric decoy oligodeoxynucleotide treatment. Neurosurgery.
70:1534–1543. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sun W, Watanabe Y and Wang ZQ: Expression
and significance of ICAM-1 and its counter receptors LFA-1 and
Mac-1 in experimental acute pancreatitis of rats. World J
Gastroenterol. 12:5005–5009. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yagi K, Tada Y, Kitazato KT, Tamura T,
Satomi J and Nagahiro S: Ibudilast inhibits cerebral aneurysms by
down-regulating inflammation-related molecules in the vascular wall
of rats. Neurosurgery. 66:551–559. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ali MS, Starke RM, Jabbour PM, Tjoumakaris
SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH and
Dumont AS: TNF-α induces phenotypic modulation in cerebral vascular
smooth muscle cells: Implications for cerebral aneurysm pathology.
J Cereb Blood Flow Metab. 33:1564–1573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Starke RM, Chalouhi N, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K,
Hasan DM, Greig NH, et al: Critical role of TNF-α in cerebral
aneurysm formation and progression to rupture. J Neuroinflammation.
11(77)2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yokoi T, Isono T, Saitoh M, Yoshimura Y
and Nozaki K: Suppression of cerebral aneurysm formation in rats by
a tumor necrosis factor-α inhibitor. J Neurosurg. 120:1193–1200.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Aoki T, Kataoka H, Ishibashi R, Nozaki K,
Morishita R and Hashimoto N: Reduced collagen biosynthesis is the
hallmark of cerebral aneurysm: Contribution of interleukin-1beta
and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol.
29:1080–1086. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Garofalo M and Croce CM: MicroRNAs: Master
regulators as potential therapeutics in cancer. Annu Rev Pharmacol
Toxicol. 51:25–43. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Guo X, Guo L, Ji J, Zhang J, Zhang J, Chen
X, Cai Q, Li J, Gu Q, Liu B, et al: miRNA-331-3p directly targets
E2F1 and induces growth arrest in human gastric cancer. Biochem
Biophys Res Commun. 398:1–6. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Martello G, Rosato A, Ferrari F, Manfrin
A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T,
et al: A microRNA targeting dicer for metastasis control. Cell.
141:1195–1207. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Guo X, Jing C, Li L, Zhang L, Shi Y, Wang
J, Liu J and Li C: Down-regulation of VEZT gene expression in human
gastric cancer involves promoter methylation and miR-43c. Biochem
Biophys Res Commun. 404:622–627. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu Y, Yao J and Feng K: miR-124-5p/NOX2
axis modulates the ROS production and the inflammatory
microenvironment to protect against the cerebral I/R injury.
Neurochem Res. 45:404–417. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Malik S, Sadhu S, Elesela S, Pandey RP,
Chawla AS, Sharma D, Panda L, Rathore D, Ghosh B, Ahuja V and
Awasthi A: Transcription factor Foxo1 is essential for IL-9
induction in T helper cells. Nat Commun. 8(815)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu L, Guo F, Wu Y, Wang Q, Ma X, Zhao Y
and Qin G: The role of FoxO1 in interleukin-1β-induced
autostimulation in retina endothelial cells and retinas of diabetic
rats. Microvasc Res. 112:93–100. 2017.PubMed/NCBI View Article : Google Scholar
|