Introduction
Late-onset hypogonadism (LOH) syndrome is an
endocrine disorder that is characterized by low levels of androgen
and a subsequent decline in various physical functions (1). LOH syndrome has a well-established
concept of disorder in men, but what differentiates it from the
so-called ‘menopausal disorders’ is that testosterone levels
included into the diagnostic criteria (1).
In males, serum testosterone levels remain stable
until ~40 years of age, after which the circulating total
testosterone and biologically active free testosterone (FT) levels
decrease by 1-2 and 2-3% annually, respectively (2,3).
Testosterone is strongly bound to sex hormone-binding globulin
(SHBG) and relatively loosely bound to albumin. The subsequent
addition of FT to these hormones gives the total testosterone (TT).
Furthermore, since albumin-bound testosterone and FT are
biologically meaningful, they are referred to as bioavailable
testosterone.
The diagnosis of LOH syndrome is based on serum
testosterone levels, taking clinical symptoms and signs into
account. The European Endocrine Society and the International
Society for the Study of the Ageing Male recommend measuring TT
(4). In Japan, serum FT levels are
used as the diagnostic criterion for LOH syndrome (5), as Okamura et al (6) and Iwamoto et al (7) reported that TT does not change with
age. Furthermore, it has also been reported that LOH-associated
symptoms are not significantly associated with serum levels of TT,
analog ligand FT, estradiol, luteinizing hormone (LH),
follicle-stimulating hormone (FSH), dehydroepiandrosterone sulfate
or growth hormone (8).
Regarding the treatment of LOH syndrome, the use of
several agents, primarily testosterone replacement therapy (TRT),
has been reported (9). TRT includes
administration of oral, injectable and skin-absorbing agents, but
in Japan, only testosterone enanthate (T enanthate) injection is
covered by insurance, and 125-250 mg is usually injected
intramuscularly every 2 weeks. In Europe and the USA, an increasing
number of patients prefer gels (9).
The effects of TRT include improvements in muscle
mass, strength, bone density, serum fat profile, insulin
sensitivity, libido and overall health (10). For erectile dysfunction, TRT was
determined to increase the effect of phosphodiesterase 5 inhibitors
(PDE5i) (11). In a meta-analysis
of clinical trials on TRT, the frequency of adverse events in which
prostate cancer occurs in association with TRT did not change
between the TRT treatment group and the placebo group (12). However, certain patients with low
testosterone levels had high-grade malignancies despite relatively
low prostate-specific antigen (PSA) levels (13). Therefore, in accordance with
Japanese guidelines, PSA is measured; if it is 2.0 ng/ml or higher,
the patient should be referred to a urologist to rule out prostate
cancer (5). LOH syndrome may lead
to a decline in numerous biological functions linked to activities
of daily living (ADL) and quality of life (QOL), including
cognitive function, muscle strength, bone density, sexual function
and metabolism (14-17).
Approximately 20% of males over 60 years of age may have LOH
syndrome. These patients may improve their ADL and prevent
lifestyle-related diseases (LRDs) such as metabolic syndrome via
TRT (16). In a double-blind
clinical trial, 1,677 local residents aged 65 years or above were
screened, of which 274 patients with a weak constitution, weight
loss, low exercise, muscle weakness, fatigue, slow walking and
serum testosterone levels <345 ng/dl, who had no prostate
disease or dysuria, were randomly assigned to the testosterone gel
(50 mg/day) treatment group and the placebo group (18). The results indicated a significant
increase in knee joint muscle strength, increased body mass index
(BMI), decreased fat mass, improved physical function, and improved
QOL index (physical symptoms, sexual function symptoms) within six
months in the TRT treatment group (18).
In addition to TRT, certain therapies for LOH
syndrome commonly include counseling, antidepressants, PDE5i and
herbal medicine (Kampo). Various herbal medicines have been used to
treat LOH syndrome (19). Tsujimura
et al (20) recently
reported on herbal medicine as a possible treatment option.
Oxidative stress is cited as a cause of various
diseases. Oxidative stress, and occasionally anticancer activity
(21), are useful biomarkers for
predicting severe erectile dysfunction (ED) and LOH syndrome in
middle-aged males. Therefore, PDE5i may improve LOH syndrome
symptoms, as PDE5i has been demonstrated to increase testosterone
through its antioxidant activity (22,23).
Amano et al (24) reported
that testosterone ointment (Glowmin; DAITO Pharmaceutical Co.)
applied to patients with LOH syndrome for three months
significantly improved their functional capacity and their
psychological and mental health.
In the present study, the effectiveness of initial
treatment by combination therapy using TRT, herbal medicine and
PDE5i in male patients with LOH were determined. The patients'
clinical background, changes in symptom scores and clinical
parameters were analyzed to verify the effectiveness of the
treatment intervention.
Patients and methods
Patients
Between April 2017 and September 2018, 21 patients
with LOH-associated symptoms, including chief complaints of
decreased libido, ED, depression and general fatigue, visited the
menopausal outpatient clinic at Kyoei-kai Okubo Hospital (Ibaraki,
Japan). Those who had undergone previous treatments were included
in the present study (Table I). No
patients with any serious condition, such as malignancy, sleep
apnea syndrome (SAS), suicidal depression or high PSA levels
(>2.0 ng/ml), or who refused treatment, were included according
to Japanese guidelines (5). All
patients provided written informed consent to participate in the
present study.
 | Table IClinical features of the patients
with late-onset hypogonadism syndrome (n=21). |
Table I
Clinical features of the patients
with late-onset hypogonadism syndrome (n=21).
| Characteristic | Value |
|---|
| Age (years) | 50 (33-65) |
| BMI
(kg/m2) | 23.1
(17.0-35.2) |
| FT (pg/ml) | 8.5 (4.9-15.2) |
| LH (mIU/ml) | 4.0 (1.4-11.8) |
| FSH (mIU/ml) | 2.5 (1.1-7.3) |
| PRL (ng/ml) | 3.0 (3.5-64.9) |
| Hb (g/dl) | 15.4
(12.9-16.8) |
| T-Cho (mg/dl) | 196 (152-319) |
| AMS score | 51 (36-68) |
| IIEF5 score | 12 (5-22) |
| IPSS score | 6 (0-28) |
| PD
(with/without) | 4/17 |
| LRD
(with/without) | 9/12 |
| Treatment | |
|
TRT
monotherapy | 14 |
|
T
enanthate 250 mg | 13 |
|
Testosterone
ointment | 1 |
|
TRT
combination (T enanthate 250 mg + PDE5i) | 3 |
|
Herbal
medicines | 4 |
|
Hochuekkito | 2 |
|
Kamishoyosan | 1 |
|
Shakuyakukanzoto | 1 |
Examination
At their first visit, the patients were evaluated by
reviewing their medical history, a physical examination and
detection of clinical parameters, and their general LOH symptoms
were judged according to Heinemann's Ageing Males' Symptoms (AMS)
scale (25). Voiding function was
evaluated by the International Prostate Symptom Score (IPSS)
(26) and sexual function was
evaluated by the International Index of Erectile Function-5
(IIEF-5) score. Blood screening, including hemoglobin (Hb),
aspartate transaminase, alanine transaminase, creatinine, total
cholesterol (T-Cho), triglyceride, blood sugar and PSA, and
endocrinological variables [including FT (RIA method), LH and FSH]
were determined until 11 a.m. prior to monitoring endocrinological
variables for any treatment of LOH. The patients in the present
study had not received any medications for LOH, such as TRT,
previously.
The questionnaire to determine the AMS score as a
disease-specific measure was administered at baseline and after 12
weeks. The AMS score comprises 17 items scored on a five-point
scale (25). The 17 items are
distributed over three subdomains: A total of five questions
address psychological factors, seven address physical factors and
five address factors of sexual function. The total AMS score
defines symptom severity as ‘no/little’ (17-26 points), ‘mild’
(27-36 points), ‘moderate’ (37-49 points) or ‘severe’ (≥50 points)
(25).
Furthermore, the IPSS questionnaire for lower
urinary tract symptoms and IIEF-5 questionnaire for sexual function
were also administered. The IPSS contains seven symptom questions
that assess weak urine stream, intermittency and straining (voiding
symptoms), frequency, urgency and nocturia (storage symptoms), and
a feeling of incomplete bladder emptying (post-voiding symptom).
The response options range from ‘not at all’ (0 points) to ‘almost
always’ (5 points), with a maximum total of 35 points. The total
IPSS score defines symptom severity as ‘no/little’ (0-7 points),
‘moderate’ (8-19 points) or ‘severe’ (≥20 points) (26). The IIEF-5 score comprises only five
questions and each item is scored on a five-point ordinal scale,
wherein lower values indicate poor sexual function. The possible
IIEF-5 scores range from 1 to 25 points (one question may receive a
score from 1 to 5; all others may receive scores from 0 to 5); a
score of >21 points is considered to indicate normal erectile
function, whereas scores at or below this cut-off indicate ED
(27).
Metabolic syndrome (Mets) and the BMI have been
reported to be related, so as an indicator of the potential Mets of
the body type of individual patients (28), the BMI was calculated initially for
each patient. According to the World Health Organization
guidelines, a BMI <25 kg/m2 represents a normal body
weight and a BMI ≥25 kg/m2 represents overweight status
(29). Thus, the patients were then
grouped by their BMI-defined body type using a threshold value of
25 kg/m2 (29). In
addition, to determine the impact of diseases, such as LRDs or
psychiatric disorders (PDs), that are closely linked to LOH
syndrome, the patients were further divided into groups with or
without LRDs or PDs (30).
Treatment
After confirming the examination and blood test
results, TRT or an oral treatment, such as herbal medicines or
PDE5i, was provided for each symptom. For the TRT, T enanthate (250
mg; Enarmon Depot®; ASKA Pharmaceutical Co., Ltd.) was
administered via intramuscular injection every four weeks for a
total of 12 weeks or testosterone ointment was applied to a 2.5-cm
area once a day. The most appropriate herbal medicine was
administered to the patients according to their pathogenic
alterations. A total of three traditional Japanese herbal
medicines, namely Kamishoyosan (TJ-24), Hochuekkito (TJ-41) and
Shakuyakukanzoto (TJ-68) (Tsumura & Co.), were candidates for
LOH treatment in the present study. The composition of each of
these three herbal medicines is described in Table II. The basic selection criteria are
presented in Table III. All
herbal drugs were given orally 3 times a day (before meals) to a
total dose of 7.5 g/day.
 | Table IIComposition of the three herbal
medicines for the treatment of late-onset hypogonadism
syndrome. |
Table II
Composition of the three herbal
medicines for the treatment of late-onset hypogonadism
syndrome.
| Name | Preparation | Herbal
components |
|---|
| Kamishoyosan
(TJ-24) | 7.5 g of extract
granules contain 4.0 g of a dried extract of the mixed crude
herbs. | Bupleurum root, 3.0
g |
| | | Peony root, 3.0
g |
| | | Atractylodes lancea
rhizome, 3.0 g |
| | | Angelica root, 3.0
g |
| | | Poria sclerotium,
3.0 g |
| | | Gardenia fruit, 2.0
g |
| | | Moutan bark, 2.0
g |
| | | Glycyrrhiza, 1.5
g |
| | | Ginger, 1.0 g |
| | | Mentha herb, 1.0
g |
| Hochuekkito
(TJ-41) | 7.5 g of extract
granules contain 5.0 g of a dried extract of the mixed crude
herbs. | Astragalus root,
4.0 g |
| | | Atractylodes lancea
rhizome, 4.0 g |
| | | Ginseng, 4.0 g |
| | | Angelica root, 3.0
g |
| | | Bupleurum root, 2.0
g |
| | | Jujube, 2.0 g |
| | | Citrus unshiu peel,
6.0 g |
| | | Glycyrrhiza,
1.5g |
| | | Cimicifuga rhizome,
1.0 g |
| | | Ginger, 0.5 g |
| Shakuyakukanzoto
(TJ-68) | 7.5 g of extract
granules contain 2.5 g of a dried extract of the mixed crude
herbs. | Peony root, 6.0
g |
| | | Glycyrrhiza, 6.0
g |
 | Table IIIIndex for the selection of herbal
medicine corresponding to the conditions of patients with
late-onset hypogonadism syndrome. |
Table III
Index for the selection of herbal
medicine corresponding to the conditions of patients with
late-onset hypogonadism syndrome.
| Formulation | Conditions |
|---|
| Kamishoyosan
(TJ-24) | Shoulder stiffness,
fatigue, anxiety |
| Hochuekkito
(TJ-41) | Severe fatigue,
pollakisuria, erectile dysfunction |
| Shakuyakukanzoto
(TJ-68) | Relief of pain,
myalgia, arthralgia, gastric pain, abdominal pain |
All treatments were repeated over 12 weeks from the
start of treatment. Laboratory and endocrinological values and
LOH-related symptoms determined prior to and after treatment were
compared to evaluate the efficacy and safety of the combination
therapy for LOH.
If patients wished to be further treated, their
current treatment was continued or switched to another treatment,
continued for another 12 weeks and then evaluated again via blood
sample analyses. Those results were not included in the present
study. There were no adverse events that required treatment
interruption in any treatment group during the treatment
period.
Statistical analysis
All statistical analyses were performed using
StatView (version 5.0; SAS Institute, Inc.). Continuous variables
were presented as the mean ± SD or the median (range). Based on the
AMS scores, the patients were categorized into three groups: None
or mild (17-26 points and 27-36 points), moderate (37-49 points)
and severe (50 points or more). The FT was also categorized into
three groups: Low (<8.5 pg/ml), borderline zone (≥8.5 and
<11.8 pg/ml) and normal (≥11.8 pg/ml). In addition, the IPSS
scores were categorized into three groups: Mild (0-7 points),
moderate (8-19 points) and severe (20 points or more). A
χ2 test was used to compare the age, FT and the presence
of PD and LRD among groups stratified by their AMS score. An
unpaired t-test was used to compare clinical features, including
the AMS score, age, FT, BMI, IPSS score, IIEF-5 score, and with LRD
groups. Correlations among various clinical parameters were
determined using the Spearman's rank correlation test (Figs. 5 and 7). P<0.05 was considered to indicate
statistical significance.
Results
Clinical features and patient
treatment
The clinical characteristics of the 21 patients with
LOH syndrome are presented in Table
I. The median patient age was 50 years (range, 33-65 years).
The median BMI was 23.1 kg/m2 (range, 17.0-35.2
kg/m2) and the median AMS score was 51 (range, 36-68).
Only 9 of the 21 patients had an LRD.
The treatment details are as follows: TRT
monotherapy in 14 cases (T enanthate 250 mg in 13 cases,
testosterone ointment in 1 case), TRT (T enanthate 250 mg + PDE5i)
in 3 cases and herbal medicines in 4 cases (Hochuekkito in 2 cases,
Kamishoyosan in 1 case and Shakuyakukanzoto in 1 case) (Table I).
Comparison of AMS score and age, FT,
PDs and LRDs
As presented in Table
IV, the patients were stratified based on the AMS scores into
mild, moderate and severe groups, and it was examined whether there
was any bias in any of the groups in terms of age (cut-off, 50, 55
years), FT (low, borderline zone and normal), and the presence or
absence of PDs and LRDs by using the χ2 test. There was
no significant difference among any of the groups.
 | Table IVComparison of AMS score between
patients stratified by age, FT, psychiatric disorders and
LRDsa before
treatment. |
Table IV
Comparison of AMS score between
patients stratified by age, FT, psychiatric disorders and
LRDsa before
treatment.
| Factor | <37 | 37-50 | >50 | Total |
|---|
| Age (years) | | | | |
|
≥55 | 0 | 2 | 5 | 7 |
|
50-54 | 0 | 1 | 4 | 5 |
|
<50 | 1 | 3 | 5 | 9 |
| FT (pg/ml) | | | | |
|
>11.8 | 0 | 0 | 3 | 3 |
|
8.5-11.8 | 1 | 3 | 4 | 8 |
|
<8.5 | 0 | 3 | 7 | 10 |
| PD | | | | |
|
Yes | 0 | 3 | 11 | 14 |
|
No | 1 | 3 | 3 | 7 |
| LRD | | | | |
|
Yes | 0 | 3 | 11 | 14 |
|
No | 1 | 3 | 3 | 7 |
| Total (n=21) | 1 | 6 | 14 | 21 |
Comparison of FT and BMI and IPSS
score
As presented in Table
V, the FT values were divided into low, borderline and normal
groups, and it was examined whether there was any bias among the
groups regarding the BMI (cut-off, 25 kg/m2) and IPSS
score (no/little, moderate and severe). There was no significant
bias among the groups.
 | Table VComparison of FT between patients
stratified by BMI and IPSS scoresa before treatment. |
Table V
Comparison of FT between patients
stratified by BMI and IPSS scoresa before treatment.
| | FT (pg/ml) | |
|---|
| Parameter | <8.5 | 8.5-11.8 | >11.8 | Total |
|---|
| BMI
(kg/m2) | | | | |
|
<25 | 7 | 5 | 2 | 14 |
|
≥25 | 3 | 3 | 1 | 7 |
| IPSS | | | | |
|
>20 | 3 | 1 | 0 | 4 |
|
8-20 | 3 | 2 | 0 | 5 |
|
<8 | 4 | 5 | 3 | 12 |
| Total (n=21) | 10 | 8 | 3 | 21 |
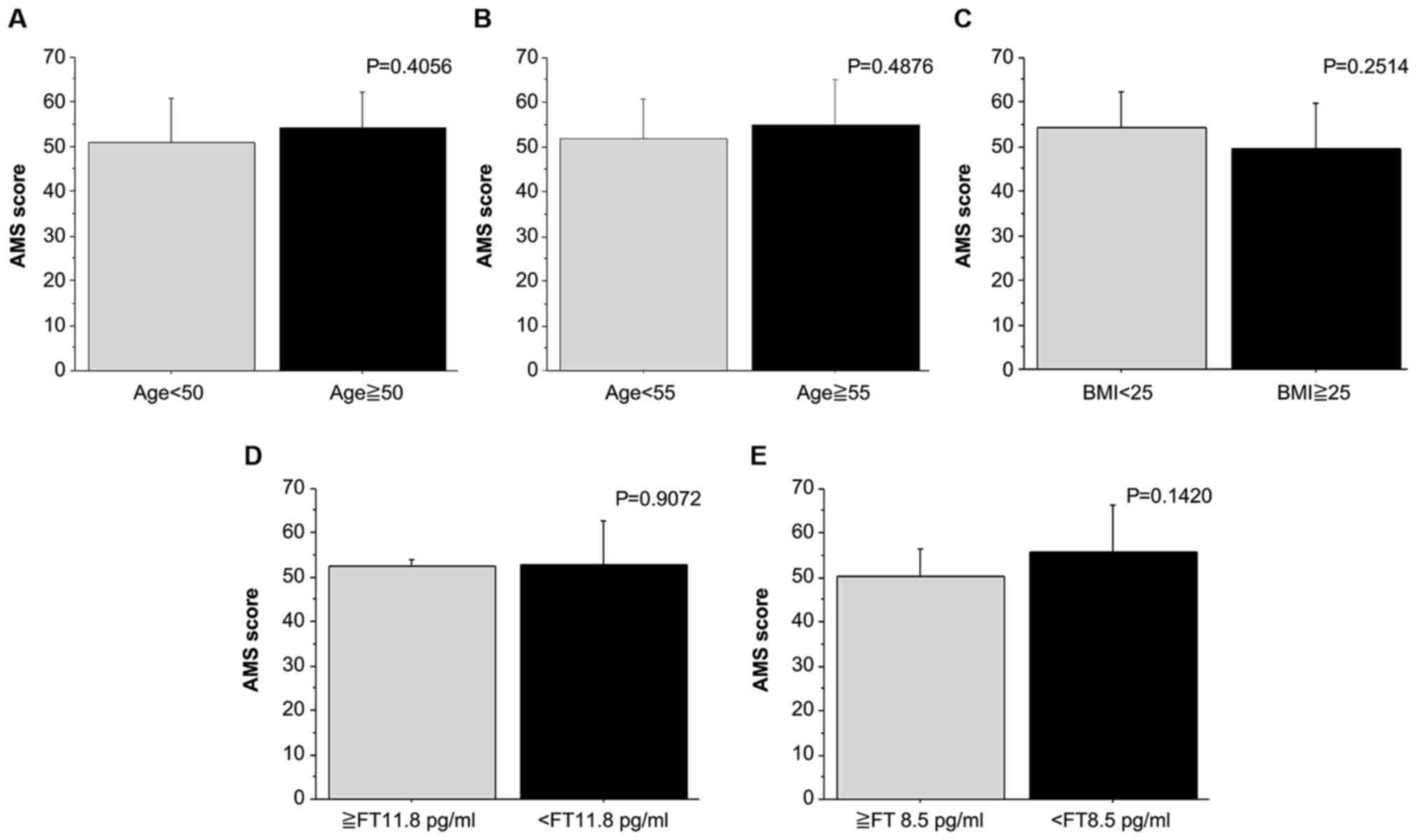
Associations between AMS score and
various clinical parameters
Differences in AMS scores between groups stratified
based on various parameters were examined using Student's t-test
(Fig. 1). There was no significant
difference between the average AMS value between age groups
stratified with a cutoff at 50 and 55 years. There was also no
significant difference in the average AMS value between the
normal-BMI (<25 kg/m2) and high-BMI (≥25
kg/m2) groups. There was also no significant difference
in the average AMS value between patients with different FT values
stratified with a cutoff at 8.5 and 11.8 pg/ml.
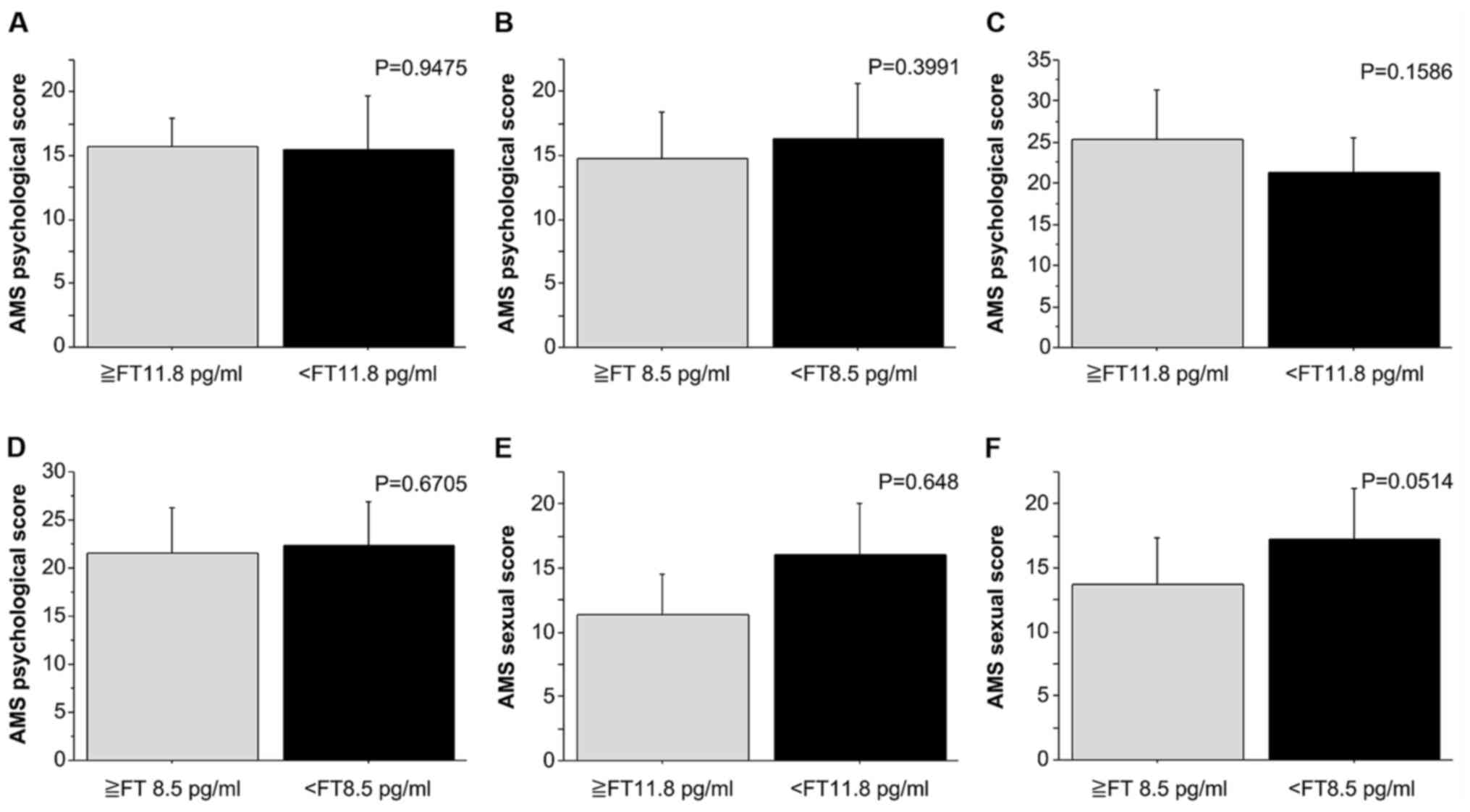
Association between the FT value and
the AMS score (psychological, physiological and sexual
function)
The possible association of the FT value with each
category of the AMS score (psychological, physiological and sexual
function) was examined using Student's t-test (Fig. 2). There was no significant
difference in the average AMS score in each category
(psychological, bodily and sexual function) between patients with
different FT values stratified with a cutoff at 8.5 and 11.8
pg/ml.
Association of various clinical
parameters with the FT value
The association between the FT value and various
parameters was examined using Student's t-test (Fig. 3). There was no significant
difference in the average FT value between age groups stratified
with a cutoff at 50 and 55 years. There was also no significant
difference in the average FT value between the normal-BMI (<25
kg/m2) and high-BMI (≥25 kg/m2) groups.
Furthermore, there was no significant difference in the average FT
value between different groups of patients stratified based on the
IIEF-5 scores (cutoff was at 9, 16 and 21). There was also no
significant difference in the average FT value between subgroups by
IPSS score (no/little, moderate and severe; cutoff was at 8 and 16,
respectively).
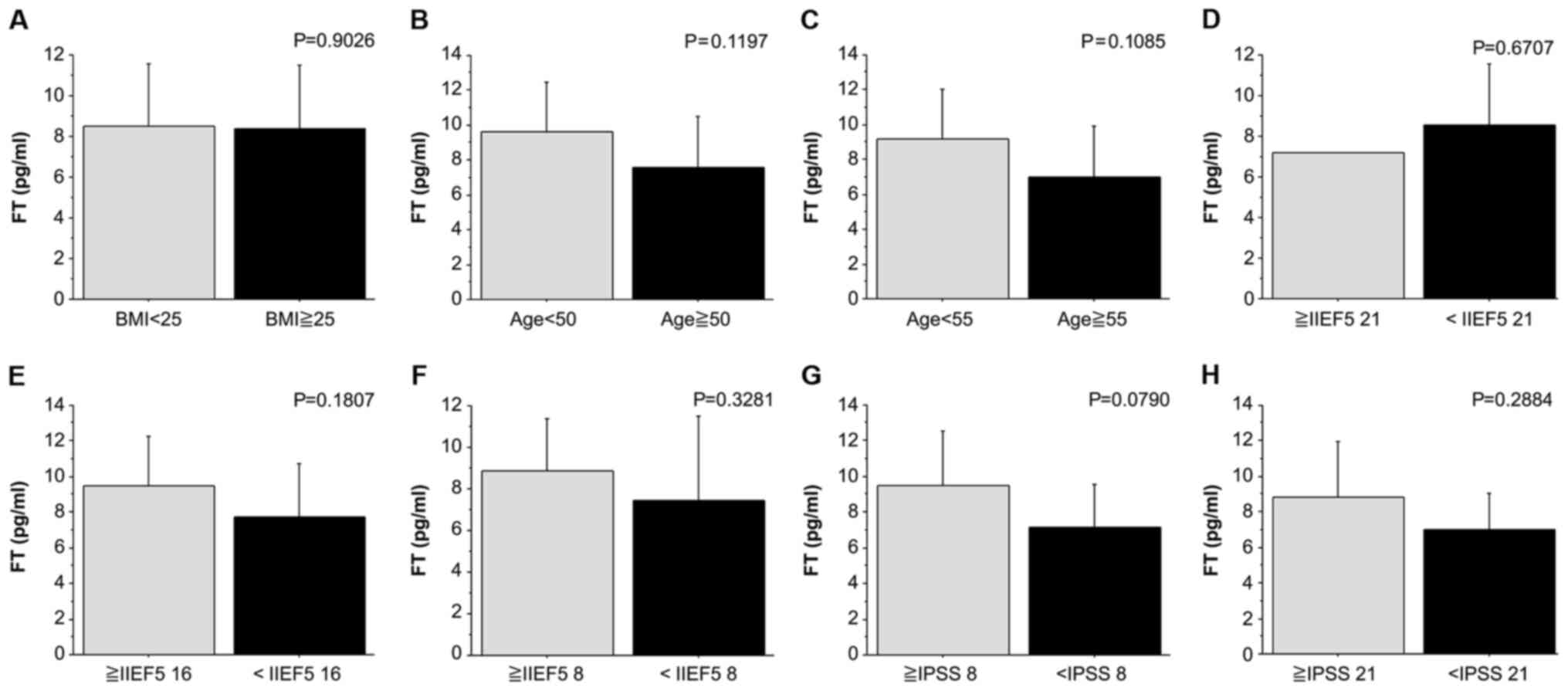 | Figure 3Comparisons of FT values in patients
with late-onset hypogonadism syndrome stratified based on various
clinical parameters. (A) Comparison by BMI (cut-off, 25
kg/m2). (B) Comparison by age (cut-off, 50
kg/m2). (C) Comparison by age (cut-off, 55 years). (D)
Comparison by IIEF5 score (cut-off, 21). (E) Comparison by IIEF5
score (cut-off, 16). (F) Comparison by IIEF5 score (cut-off, 8).
(G) Comparison by IPSS score (cut-off, 8). (H) Comparison by IPSS
score (cut-off, 21). Continuous variables were presented as the
mean ± standard deviation. FT, free testosterone; BMI, body mass
index; IEF5, International Index of Erectile Function-5; IPSS,
International Prostate Scale Score. |
Association of LRDs and various
parameters
The association of the presence of LRDs with various
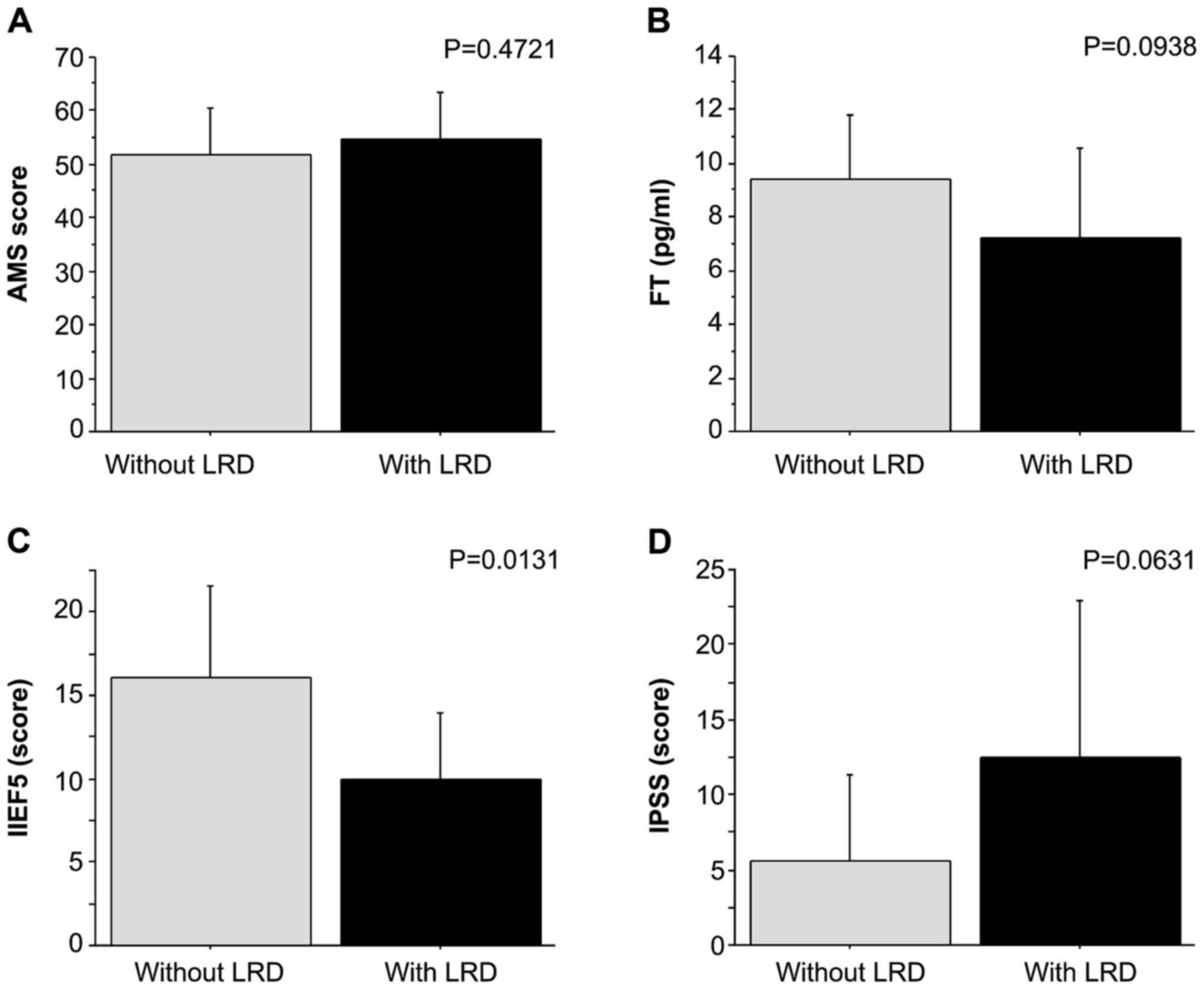
parameters was examined using Student's t-test (Fig. 4). There was no significant
difference in the mean values of the AMS, FT and IPSS scores
between patients with and without LRDs. Of note, the mean IIEF-5
score in patients with an LRD was significantly higher than in
those without an LRD, indicating that patients with an LRD may have
a significantly lower sexual function (P=0.0131; Fig. 4C).
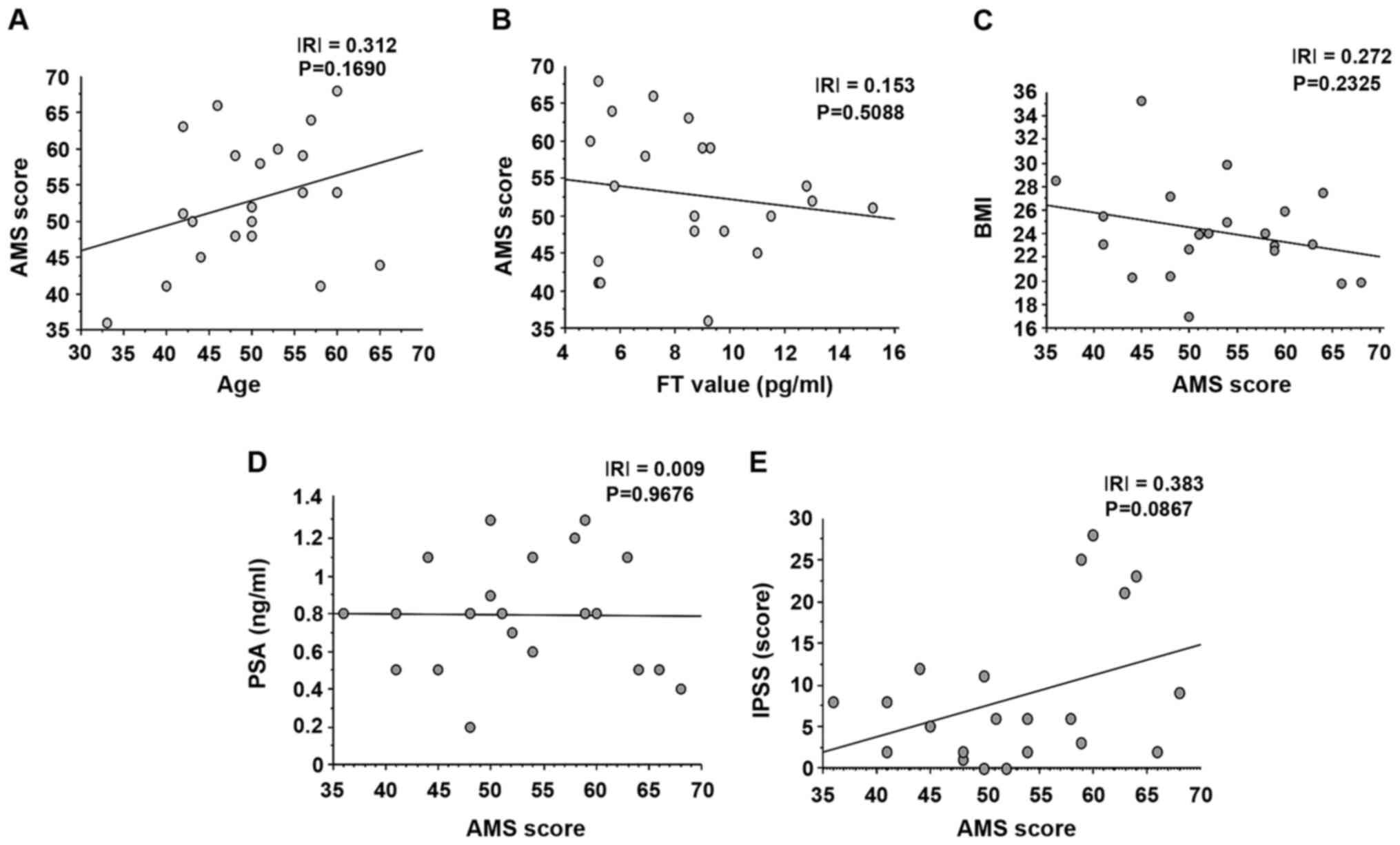
Correlation between AMS score and
various clinical parameters
The correlation between the AMS score and various
clinicopathological parameters, including age, FT, BMI, PSA and
IPSS score, in patients with LOH syndrome was examined using
Spearman's correlation test (Fig.
5). There was no significant correlation between the AMS score
and age, FT, BMI, PSA or IPSS score in patients with LOH
syndrome.
Efficiency of combination therapy
using TRT, PDE5i and herbal medicine for patients with LOH
syndrome
The AMS scores (overall, psychological,
physiological and sexual function) prior to and after each
treatment were compared (Table
VI). Significant improvements in the overall AMS scores were
observed after treatment in the TRT group, T enanthate monotherapy
treatment group and T enanthate and PDE5i treatment group
(P<0.001; Table VIA). In the
herbal medicine group, the overall AMS scores were also slightly
improved after treatment, but there was no statistical significance
(P=0.144).
 | Table VIAMS scores at baseline and 12 weeks
after each treatment. |
Table VI
AMS scores at baseline and 12 weeks
after each treatment.
| A, Overall AMS
score |
|---|
| Method of
treatment | AMS score prior to
treatment (median) | AMS score after
treatment (median) | P-value |
|---|
| All (n=21) | 52.9±8.8 | 37.5±11.5 | <0.001 |
| TRT (n=14) | 54.6±8.2 | 38.2±10.6 | <0.001 |
| T enanthate
monotherapy | 53.4±7.6 | 37.0±9.9 | <0.001 |
| T enanthate and
PDE5i | 55.3±8.1 | 39.2±10.6 | <0.001 |
| Herbal
medicine | 45.5±8.4 | 34.8±16.4 | 0.144 |
| B, AMS sub-score
(physiological factors) |
| Method of
treatment | AMS score prior to
treatment (median) | AMS score after
treatment (median) | P-value |
| All (n=21) | 22.0±4.4 | 16.0±5.5 | <0.001 |
| TRT (n=14) | 22.0±3.7 | 15.9±4.4 | <0.001 |
| T enanthate
monotherapy | 21.3±3.8 | 15.1±3.8 | <0.001 |
| T enanthate and
PDE5i | 22.0±3.8 | 16.2±4.4 | <0.001 |
| Herbal
medicine | 22.0±7.7 | 16.8±9.7 | 0.017 |
| C, AMS sub-score
(psychological factors) |
| Method of
treatment | AMS score prior to
treatment (median) | AMS score after
treatment (median) | P-value |
| All (n=21) | 15.5±3.9 | 10.3±4.0 | <0.001 |
| TRT (n=14) | 16.5±3.1 | 10.6±4.0 | <0.001 |
| T enanthate
monotherapy | 16.6±2.8 | 10.3±4.3 | <0.001 |
| T enanthate and
PDE5i | 16.9±2.6 | 10.9±4.0 | <0.001 |
| Herbal
medicine | 11.3±4.6 | 9.3±4.0 | 0.415 |
| D, AMS sub-score
(sexual function) |
| Method of
treatment | AMS score prior to
treatment (median) | AMS score after
treatment (median) | P-value |
| All (n=21) | 15.4±4.1 | 11.1±4.1 | <0.001 |
| TRT (n=14) | 16.1±4.0 | 11.7±4.2 | 0.001 |
| T enanthate
monotherapy | 15.4±3.8 | 11.6±4.3 | 0.015 |
| T enanthate and
PDE5i | 16.3±4.0 | 12.1±4.1 | 0.003 |
| Herbal
medicine | 12.3±3.6 | 8.8±3.0 | 0.322 |
There was a significant improvement in the
physiological factors of the AMS after treatment in all treatment
groups (P<0.001; Table VIB). In
the TRT group, including the T enanthate monotherapy group
(P<0.001) and the T enanthate + PDE5i treatment group
(P<0.001), a significant improvement in physiological factors of
AMS was achieved after treatment. In the herbal medicine group, a
significant improvement in the psychological factors of AMS was
also observed after treatment (P=0.017).
There was a significant improvement in the
psychological factors of AMS after treatment in various treatment
groups (P<0.001; Table VIC).
There was a significant improvement in the psychological factors of
AMS after treatment in the TRT groups, including the T enanthate
monotherapy treatment group and the T enanthate + PDE5i treatment
group, but there was no significant difference in the herbal
treatment group (P=0.415).
There was a significant improvement in the sexual
functional factors of AMS after treatment in several treatment
groups (P<0.001; Table VID). In
the TRT groups, including the T enanthate monotherapy group and T
enanthate + PDE5i treatment group, the sexual function factors of
AMS were significantly improved after treatment (P<0.001). In
the herbal medicine group, the AMS sexual functional factors were
also slightly improved after treatment, but there was no
significant difference (P=0.322).
Associations among AMS score
improvement and FT value prior to treatment or AMS score prior to
treatment
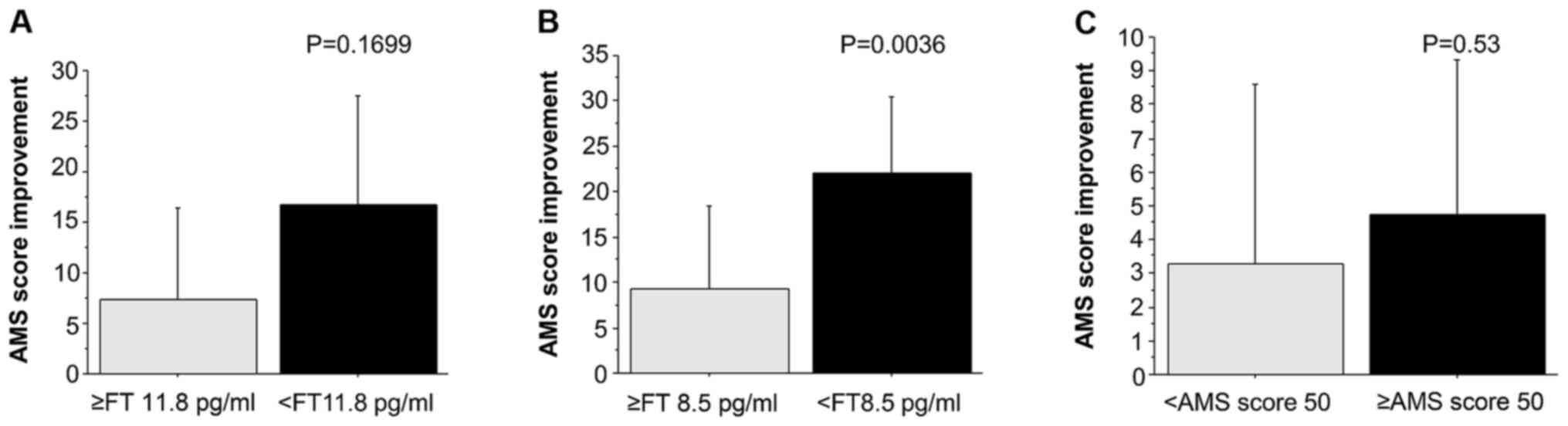
The association of the AMS score improvement with
the FT value prior to treatment or the AMS score prior to treatment
was examined using Student's t-test (Fig. 6). The average difference in the AMS
score prior to vs. after treatment, defined as the AMS score
improvement via treatment, was compared between patients with
different pre-treatment FT values stratified with cutoff values of
8.5 and 11.8 pg/ml. The results indicated that a lower
pre-treatment FT value was associated with a greater improvement in
AMS. Of note, the improvement in AMS was significantly higher in
the group with an FT value of <8.5 pg/ml prior to treatment as
compared with that in the group with FT ≥8.5 pg/ml (P=0.0036;
Fig. 6B).
The cohort was then divided based on the AMS score
prior to treatment with a cutoff at 50 points, and the AMS score
improvements via treatment were compared between these two
subgroups. A higher pre-treatment AMS score was associated with a
greater improvement in AMS score after treatment; however, this
difference was not significant. It may be concluded that patients
with a lower FT value and a higher AMS score prior to treatment may
exhibit a greater AMS score improvement following treatment. In
particular, the patient group with a pre-treatment FT value of
<8.5 pg/ml presented with a significantly higher therapeutic
effect.
Correlation between improvement in AMS
score (overall, psychological, physiological and sexual
functioning) and FT value prior to treatment
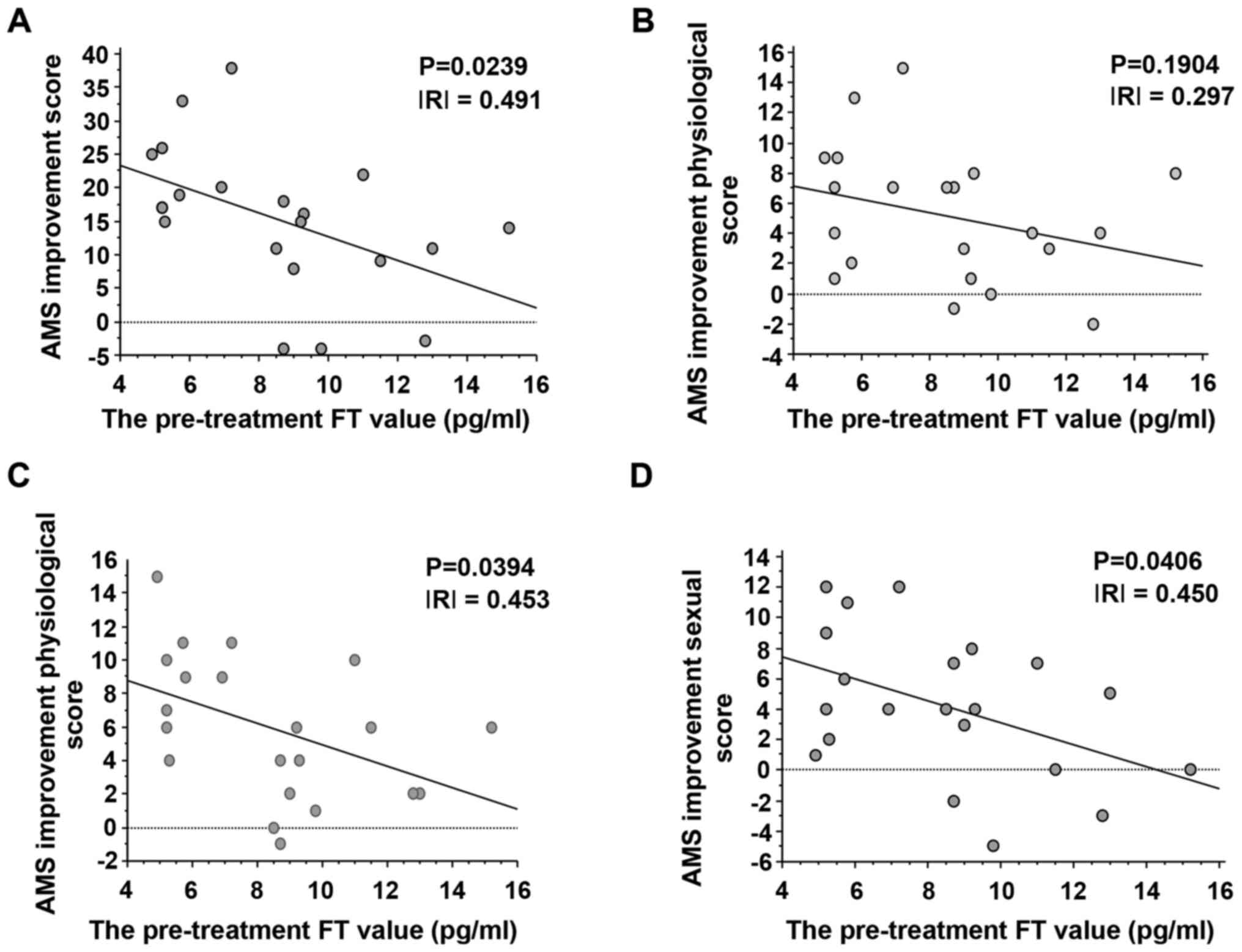
Upon examination using Spearman's correlation test,
the correlation between the improvement in the AMS score and the
pre-treatment FT value exhibited a significant negative correlation
(P=0.0239; Fig. 7A). In other
words, patients with a lower FT value prior to treatment had a
greater improvement in AMS score after the treatment.
Examination of the correlation between the
improvement in the psychological AMS score with the FT value prior
to treatment revealed no significant correlation (P=0.1904;
Fig. 7B). However, the improvement
of the AMS score of physiological and sexual functioning and the FT
value prior to treatment indicated a significant negative
correlation (P=0.0394 and P=0.0406; Fig. 7C and D, respectively). In conclusion, patients
with a lower FT value prior to treatment had significantly improved
overall AMS scores, as well as AMS scored of physical and sexual
function.
Examination of changes in each
clinical factor after vs. prior to treatment
Combining all treatments, a significant decrease in
serum LH after treatment was observed (P=0.007; Table VII). Furthermore, slight but
insignificant increases in FT levels, as well as an insignificant
decrease in the serum T-Cho were detected after treatment. A
significant increase in the IIEF-5 score was observed after
treatment (P=0.01; Table VII),
demonstrating that combination therapy using TRT, PDE5i and herbal
medicine may contribute to the treatment of sexual dysfunction.
Furthermore, there was a decrease in the IPSS score after
treatment, but it was not significant.
 | Table VIIClinical screening factors were not
significantly changed after all treatments compared with the
baseline value. |
Table VII
Clinical screening factors were not
significantly changed after all treatments compared with the
baseline value.
| Parameter | Prior to treatment
(median) | After treatment
(median) | P-value |
|---|
| LH | 2.8±1.5 | 1.6±1.8 | 0.007 |
| T-cho | 212.0±47.0 | 207.1±33.2 | 0.439 |
| Hb | 15.3±1.1 | 15.4±1.1 | 0.668 |
| FT | 8.5±3.0 | 9.6±4.0 | 0.368 |
| IIEF5 score | 13.4±5.7 | 16.0±5.1 | 0.010 |
| IPSS score | 8.6±8.6 | 6.4±5.9 | 0.072 |
Discussion
In the present study, the treatments were selected
based on the clinical background and initial therapeutic effects in
21 males treated for LOH syndrome as outpatients were evaluated and
good results regarding improvement of the symptoms based on the AMS
score were obtained in each treatment group.
The association between sex hormones and urological
diseases has been reported previously (31). In recent years, it has become widely
recognized that menopausal-like symptoms may occur in middle-aged
and elderly males, as well as in females, due to decreased androgen
production. The term LOH syndrome is now being used to describe
this condition and it is defined as a ‘syndrome by androgen
decrease according to age that may result in multi-organ
dysfunction and decrease in QOL’ (1). Testosterone and associated hormonal
changes due to ageing are more clearly reduced by ageing for FT
compared to TT. SHBG increases the decrease in FT and albumin-bound
testosterone. Adrenal androgens are also on the decline with
increasing age (2,3). Liu et al (32) reported that genetic variation among
individuals exists in the number of CAG repeats in the androgen
receptor and affects the therapeutic effect of genetic
polymorphisms, and that the number of repeats and androgenic
activity are inversely related. They also indicated that patients
with serum TT levels of 340 ng/dl or higher tend to develop LOH
symptoms if they have CAG repeat lengths of ≥25.
In the present study, patients with FT of <8.5
pg/ml at the first visit exhibited a significantly greater
improvement in their AMS score after treatment as compared with
those with an FT of ≥8.5 pg/ml. In addition, there was a negative
correlation between AMS score improvement following treatment and
FT levels at the first visit. This suggests that, in patients with
low FT, the symptoms of LOH syndrome may be improved by aggressive
intervention using combination therapy with TRT, herbal medicine
and PDE5i. Although various physical, psychological or sexual
dysfunction symptoms may occur in LOH syndrome, TRT is considered
the first treatment, as androgen deficiency is the essence of the
condition (5). However, it has also
been indicated that an increase in the blood testosterone
concentration due to testosterone administration is caused by aging
(33).
In the present study, a significant increase in the
IIEF-5 score after treatment was observed, suggesting that
combination therapy using TRT, herbal medicine and PDE5i may be
effective as a single treatment for sexual dysfunction. Sexual
decline is an important clinical manifestation of LOH syndrome. In
particular, when the three items of low sexual desire, i.e.,
decreased nocturnal and morning erections and ED, appear together,
LOH syndrome is more strongly indicated (34). As for the association between
testosterone and erectile ability, a Japanese study has reported
that IIEF-5 increases when testosterone increases (35); this trend was also indicated in a
study from the US (36). In the
present study, lower pre-treatment FT levels were associated with a
greater increase in total AMS score for physical and sexual
function following treatment. Various factors affecting the sexual
function of testosterone have been reported and the basic research
results expected to also be observed in clinical practice are as
follows: i) Nitric oxide synthase activity stimulation as a
physiological effect; ii) maintenance of penile structure and
function as a developmental effect; iii) development, maintenance,
function and plasticity of erectile nerve and pelvic plexus as
neurological effects; and iv) improvement in resistance of PDE5i
for ED as an anti-ageing effect (2,3).
Symptoms resulting from changes in testosterone with
age are defined as LOH syndrome. However, a decrease in
testosterone from various causes may be associated with the
development of symptoms as well as ageing. A study reported that
the rate of testosterone changes is a factor predicting the onset
of symptoms of LOH syndrome (37).
In addition, the threshold of symptom manifestation due to the
decrease in testosterone varies depending on the organ it affects
(38).
Decreased serum testosterone may be associated with
specific symptoms and metabolic risks in males (10). In the present study, no significant
correlation between PSA and testosterone was observed. PSA, a tumor
marker for prostate cancer, is used as an indicator for prostate
cancer diagnosis and treatment (39). Rastrelli et al (40) reported that PSA was a marker of the
testosterone concentration and that it may represent a novel
indicator for confirming hypogonadism. They also stated that
determining PSA levels may provide insight not only regarding the
circulating levels of TT but also its active fractions.
In the present study, the efficiency of a
combination treatment using several drugs that have been reported
to be efficacious in patients with LOH syndrome, i.e., T enanthate,
testosterone ointment, PDE5i and herbal medicines, was evaluated.
The effect of each treatment on LOH syndrome has been previously
reported, primarily that of T enanthate. Snyder et al
(41) reported on a randomized
controlled trial (RCT) that examined the effectiveness of TRT for
the most common complaints among the middle-aged and elderly. Their
results indicated that the effects on sexual function factors,
including sexual activity, libido and erectile ability, physical
function factors, including walking distance and vitality, and
psychological function, including depression symptoms, improved in
the TRT group. The present report had a patient cohort recruited
based on a tight selection criteria (n=790), testosterone was
controlled within the physiological range after TRT (41).
In the present study, there were no adverse events
requiring treatment interruption in any group during the drug
administration period. To date, no RCT with sufficient power to
adequately assess the impact of TRT on cardiovascular events and
death has been performed. A previous retrospective study including
almost 4,000 cases suggested that TRT does not increase the risk of
cardiovascular events (42).
It is unlikely that prostate cancer is caused by
TRT. In a meta-analysis of clinical trials on the use of TRT, the
frequency prostate cancer as an adverse event detected in the
placebo group and hormone replacement group was not significantly
different, but rather in patients with lower testosterone levels,
the rate of aggressive prostate cancer was relatively high
(43).
Although androgen is a steroid hormone that is
thought to affect not only male reproductive organs, but also
numerous other organs, tissues, biological functions and even
behaviors, the mechanisms of action of androgens have remained to
be fully elucidated. Conventional side effects that should be
considered when administering TRT include cardiovascular disease,
abnormal lipid metabolism, polycythemia, fluid retention, prostatic
hypertrophy, prostate cancer, hepatotoxicity, SAS, gynecomastia,
acne, testis atrophy, infertility, and behavioral and mood changes
(44,45). In the present study, the significant
decrease in the plasma levels of LH following testosterone
treatment may have been due to suppression of LH, the upstream
hormone of testosterone (46). This
is important for clinicians to note, since it may lead to
testicular atrophy and requires monitoring, including blood tests
during the course of treatment for LOH syndrome.
The present study covered initial treatment for LOH,
over three months from the start of treatment. In patients who
received medical intervention for LOH syndrome, the physical and
sexual function, as well as total AMS scores, improved after
treatment for three months. There was also a significant
improvement in the IIEF-5 score for sexual function factors
following treatment. Tsujimura et al (47) administered TRT for patients with LOH
for 7.3 months on average. Their analyses prior to treatment, after
treatment and three months after treatment indicated that, at three
months after treatment, testosterone had returned to pre-treatment
levels, but the total AMS and physical and psychological factor
scores were significantly improved by the treatment and were
maintained at three months after the end of treatment. Lifelong TRT
is not always required for patients with improved symptoms
(47). From this point of view, it
appears to be important to improve symptoms via initial treatment.
Although the present study was limited to an evaluation of the
initial three months of treatment, it is worthwhile due the
importance of the initial treatment.
There is no universally agreed threshold for
‘normal’ serum testosterone levels. In addition, there are no
generally accepted lower limits of normal TT. LOH syndrome
comprises non-specific, indefinite complaints and the symptoms are
particularly similar to those of peripheral depression. The target
serum testosterone levels for TRT are not absolutely diagnostic.
Careful differentiation of whether a symptom is due to LOH syndrome
is important for a more accurate diagnosis. To increase the
accuracy of LOH syndrome diagnosis, decreased vigor, decreased
energy and motivation, fatigue, depressive mood and sleep
disturbances may also be associated with LOH syndrome, and physical
symptoms may appear as obesity and increased blood levels of
cholesterol. The association between symptoms and testosterone
levels is not clear in numerous cases and inter-individual
differences in the appearance of these symptoms must be taken into
account. Furthermore, the diagnosis should be made by
comprehensively considering the decrease in serum testosterone
levels, the aforementioned symptoms and the results of a physical
examination (48).
According to Japanese guidelines, hormone
replacement therapy is not performed in patients with FT of ≥11.8
pg/ml and treatments should be considered depending on the symptoms
(5). In the present study, herbal
medicine treatment was also effective. The herbal medicines were
not as potent as the TRT treatments and no significant change was
achieved for numerous parameters. However, the herbal medicines
were instead given to the patients with less severe LOH at baseline
and with higher FT, so that the magnitude of the changes required
to reach a near-normal state were not as high. In particular, there
was a significant improvement in the scores related to physical
symptoms of AMS. In herbal medicine, Hochuekkito has been reported
to increase testosterone levels; Saikouka Ryubon-Oyakuto has no
effect on testosterone levels but has been reported to improve the
symptoms of LOH via cytokines (19,49).
In relation to Mets, including LRDs and urological
diseases, dysuria and urolithiasis are well known, but other
associations of Mets and LOH syndrome have also been reported
(50,51). Decreased testosterone levels are a
significant risk factor for type 2 diabetes and Mets (52,53).
RCTs have gradually demonstrated the effects of TRT on metabolic
factors. A recent meta-analysis of the effects TRT on metabolic
factors in a middle-aged group of patients in their 50s, based on
multiple RCTs, indicated that it should improve insulin sensitivity
and hyperlipidemia in addition to decreasing abdominal
circumference (54).
The limitations of the present study include the
small number of cases, the retrospective study design and the short
observation period. It is difficult to draw an affirmative
conclusion based on this number of patients. Therefore, the results
of the present study only suggest certain trends. Future tasks are
to acquire cases and follow their long-term progress, as well as to
determine the effectiveness of each treatment method, side effects
and drug combination effects. In addition, drug treatment and
examination of the effects of nutritional therapy on LOH syndrome
are necessary.
In conclusion, among the outpatients with LOH
syndrome who presented at our hospital, improved AMS scores were
observed in numerous cases following treatment intervention using
combination or single therapy with TRT, herbal medicine and PDE5i,
and the outcomes were favorable.
From these results, it was indicated that lower
serum FT levels prior to treatment were associated with a
significantly greater improvement in AMS score after treatment. In
particular, the physical and sexual function factors and AMS scores
after treatment were significantly more improved in the group with
FT <8.5 pg/ml at the first visit. These results suggest that
patients with LOH syndrome with low FT are likely to experience
symptom improvement by treatment intervention. There was also a
significant increase in the IIEF-5 score after treatment. This
suggests that combination therapy using TRT, herbal medicine and
PDE5i may be effective as a single treatment for sexual
dysfunction.
In addition to TRT, numerous treatments have been
reported for LOH syndrome, and these treatments achieved good
results in the present study. Thus, it is required to evaluate the
symptoms of each patient with LOH syndrome to judge whether they
require treatment intervention and determine the appropriate
treatment method.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT designed the study, contributed to analysis and
interpretation of data. HT and HO were involved in the treatment of
actual patients at the hospital, made a contribution to the
acquisition of data for this study and confirmed the authenticity
of all the raw data. Both authors approved the final version of the
manuscript, and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All procedures of the present study were approved by
the Ethical Committee of Kyoeikai Okubo Hospital (Ibaraki, Japan).
All patients provided written informed consent to participate in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nieschlag E, Swerdloff R, Behre HM, Gooren
LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang
C, et al: Investigation, treatment, and monitoring of late-onset
hypogonadism in males: ISA, ISSAM, and EAU recommendations. J
Androl. 27:135–137. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feldman HA, Longcope C, Derby CA, Johannes
CB, Araujo AB, Coviello AD, Bremner WJ and McKinlay JB: Age trends
in the level of serum testosterone and other hormones in
middle-aged men: Longitudinal results from the Massachusetts male
aging study. J Clin Endocrinol Metab. 87:589–598. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu FC, Tajar A, Pye SR, Silman AJ, Finn
JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, et
al: Hypothalamic-pituitary-testicular axis disruptions in older men
are differentially linked to age and modi able risk factors: The
European Male Aging Study. J Clin Endocrinol Metab. 93:2737–2745.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang C, Nieschlag E, Swerdloff R, Behre
HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B,
Morales A, et al: Investigation, treatment and monitoring of
late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA
recommendations. Eur J Endocrinol. 159:507–514. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Japanese Urological Association/Japanese
Men's Health Medicine Association: LOH symptom group - Aging Male
Hypogonadism Syndrome Medical Guidance. Jihou, Tokyo, 4-7,
2007.
|
|
6
|
Okamura K, Ando F and Shimokata H: Serum
total and free testosterone level of Japanese men: A
population-based study. Int J Urol. 12:810–814. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwamoto T, Yanase T, Horie H, Namiki M and
Okuyama A: Late-onset hypogonadism (LOH) and androgens: Validity of
the measurement of free testosterone levels in the diagnostic
criteria in Japan. Int J Urol. 16:168–174. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miwa Y, Kaneda T and Yokoyama O:
Correlation between the Aging Males' symptoms Scale and sex
steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth
hormone levels in ambulatory men. J Sex Med. 3:723–726.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nieschlag E: Testosterone treatment comes
of age: New options for hypogonadal men. Clin Endocrinol (Oxf).
65:275–281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang C, Swerdloff RS, Iranmanesh A, Dobs
A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T and Berman N:
Testosterone Gel Study Group. Transdermal testosteron gel improves
sexual function, mood, muscle strength, body composition parameters
in hypogonadal men. J Clin Endocrinol Metab. 85:2839–2853.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tenover JS: Effects of testosterone
supplementation in the aging male. J Clin Endocrinol Metab.
75:1092–1098. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buvat J, Maggi M, Gooren L, Guay AT,
Kaufman J, Morgentaler A, Schulman C, Tan HM, Torres LO, Yassin A
and Zitzmann M: Endocrine aspects of male sexual dysfunctions. J
Sex Med. 7:1627–1656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ide H, Yasuda M, Nishio K, Saito K,
Isotani S, Kamiyama Y, Muto S and Horie S: Development of a
nomogram for predicting high-grade prostate cancer on biopsy: The
significance of serum testosterone levels. Anticancer Res.
28C:2487–2492. 2008.PubMed/NCBI
|
|
14
|
Bagatell CJ and Bremner WJ: Androgens in
men: Uses and abuses. N Engl J Med. 334:707–714. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Coates JM and Herbert J: Endogenous
steroids and financial risk taking on a London trading floor. Proc
Natl Acad Sci USA. 105:6167–6172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Muller M, Grobbee DE, den Tonkelaar I,
Lamberts SW and van der Schouw YT: Endogenous sex hormones and
metabolic syndrome in aging men. J Clin Endocrinol Metab.
90:2618–2623. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bhasin S, Cunningham GR, Hayes FJ,
Matsumoto AM, Snyder PJ, Swerdloff RS and Montori VM: Testosterone
therapy in adult men with androgen deficiency syndromes: An
endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 91:1995–2010. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Srinivas-Shankar U, Roberts SA, Connolly
MJ, O'Connell MD, Adams JE, Oldham JA and Wu FC: Effects of
testosterone on muscle strength, physical function, body
composition, and quality of life in intermediate-frail and frail
elderly men: A randomized, double-blind, placebo-controlled study.
J Clin Endocrinol Metab. 95:639–650. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Amano T, Imao T and Takemae K: Clinical
efficacy of Japanese traditional herbal medicine (Kampo) in
patients with late-onset hypogonadism. Aging Male. 13:166–173.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsujimura A, Takada S, Matsuoka Y,
Nakayama J, Takao T, Miyagawa Y, Nonomura N and Okuyama A: Clinical
trial of treatment with saikokaryukotsuboreito for eugonadal
patients with late-inset hypogonadism-related symptoms. Aging Male.
11:95–99. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takeuchi H, Taoka R, Mmeje CO, Jinesh GG,
Safe S and Kamat AM: CDODA-Me decreases specificity protein
transcription factors and induces apoptosis in bladder cancer cells
through induction of reactive oxygen species. Urol Oncol.
34:337.e11–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yasuda M, Ide H and Horie S, Yoshii T,
Nishio K, Saito K, Isotani S, Kamiyama Y, Muto S and Horie S:
Salivary 8-OHdG: A useful biomarker for predicting severe ED and
hypogonadism. J Sex Med. 5:1482–1491. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Podlasek CA, Mulhall J, Davies K, Wingard
CJ, Hannan JL, Bivalacqua TJ, Musicki B, Khera M, González-Cadavid
NF and Burnett AL II: Translational perspective on the role of
testosterone in sexual function and dysfunction. J Sex Med.
13:1183–1198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amano T, Iwamoto T, Sato Y, Imao T and
Earle C: The efficacy and safety of short-acting testosterone
ointment (Glowmin) for late-onset hypogonadism in accordance with
testosterone circadian rhythm. Aging Male. 21:170–175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heinemann LA, Zimmermann T, Vermeulen A,
Thiel C and Hummel W: A new ‘aging males’ symptoms' (AMS) rating
scale. Aging Male. 2:105–114. 1999.
|
|
26
|
Barry MJ, Fowler FJ Jr, O'Leary MP,
Bruskewitz RC, Holtgrewe HL, Mebust WK and Cockett AT: The American
urological association symptom index for benign prostatic
hyperplasia. The measurement committee of the American urological
association. J Urol. 148:1549–1557; discussion 1564.
1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosen RC, Cappelleri JC, Smith MD, Lipsky
J and Peña BM: Development and evaluation of an abridged, 5-item
version of the International index of erectile function (IIEF-5) as
a diagnostic tool for erectile dysfunction. Int J Impot Res.
11:319–326. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Frankenfield DC, Rowe WA, Cooney RN, Smith
JS and Becker D: Limits of body mass index to detect obesity and
predict body composition. Nutrition. 17:26–30. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
WHO Expert Consultation. Appropriate
body-mass index for Asian populations and its implications for
policy and intervention strategies. Lancet. 363:157–163.
2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sato Y, Tanda H, Kato S, Onishi S,
Nakajima H, Nanbu A, Nitta T, Koroku M, Akagashi K and Hanzawa T:
Prevalence of major depressive disorder in self-referred patients
in a late onset hypogonadism clinic. Int J Impot Res. 19:407–410.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeuchi H, Mmeje CO, Jinesh GG, Taoka R
and Kamat AM: Sequential gemcitabine and tamoxifen treatment
enhances apoptosis and blocks transformation in bladder cancer
cells. Oncol Rep. 34:2738–2744. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu CC, Lee YC, Wang CJ, Yeh HC, Li WM, Wu
WJ, Huang CN, Bao BY, Huang CH and Huang SP: The impact of androgen
receptor CAG repeat polymorphism on andropausal symptoms in
different serum testosterone levels. J Sex Med. 9:2429–2437.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coviello AD, Lakshman K, Mazer NA and
Bhasin S: Differences in the apparent metabolic clearance rate of
testosterone in young and older men with gonadotropin suppression
receiving graded doses of testosterone. J Clin Endocrinol Metab.
91:4669–4675. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu FC, Tajar A, Beynon JM, Pye SR, Silman
AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva FF, Forti G, et al:
Identification of late-onset hypogonadism in middle-aged and
elderly men. N Engl J Med. 363:123–135. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsujimura A, Matsumiya K, Matsuoka Y,
Takahashi T, Koga M, Iwasa A, Takeyama M and Okuyama A:
Bioavailable testosterone with age and erectile dysfunction. J
Urol. 170:2345–2347. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cunningham GR, Stephens-Shields AJ, Rosen
RC, Wang C, Ellenberg SS, Matsumoto AM, Bhasin S, Molitch ME,
Farrar JT, Cella D, et al: Association of sex hormones with sexual
function, vitality, and physical function of symptomatic older men
with low testosterone levels at baseline in the testosterone
trials. J Clin Endocrinol Metab. 100:1146–1155. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Holm AC, Fredrikson MG, Theodorsson E,
Palmefors LG, Karlsson PS, Joborn C and Hammar ML: Change in
testosterone concentrations over time is a better predictor than
the actual concentrations for symptoms of late onset hypogonadism.
Aging Male. 14:249–256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zitzmann M, Faber S and Nieschlag E:
Association of specific symptoms and metabolic risks with serum
testosterone in older men. J Clin Endocrinol Metab. 91:4335–4343.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takeuchi H, Ohori M and Tachibana M:
Clinical significance of the prostate-specific antigen doubling
time prior to and following radical prostatectomy to predict the
outcome of prostate cancer. Mol Clin Oncol. 6:249–254.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rastrelli G, Corona G, Vignozzi L,
Maseroli E, Silverii A, Monami M, Mannucci E, Forti G and Maggi M:
Serum PSA as a predictor of testosterone deficiency. J Sex Med.
10:2518–2528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Snyder PJ, Bhasin S, Cunningham GR,
Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM,
Barrett-Connor E, Swerdloff RS, Wang C, et al: Effects of
testosterone treatment in older men. N Engl J Med. 374:611–624.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Anderson JL, May HT, Lappé DL, Bair T, Le
V, Carlquist JF and Muhlestein JB: Impact of testosterone
replacement therapy on myocardial infarction, stroke, and death in
men with low testosterone concentrations in an integrated health
care system. Am J Cardiol. 117:794–799. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Feneley MR and Carruthers M: Is
testosterone treatment good for the prostate? Study of safety
during long-term treatment. J Sex Med. 9:2138–2149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Morales A and Lunenfeld B: International
Society for the Study of the Aging Male. Investigation, treatment
and monitoring of late-onset hypogonadism in males. Official
recommendations of ISSAM. International Society for the Study of
the Aging Male. Aging Male. 5:74–86. 2002.PubMed/NCBI
|
|
45
|
Rhoden EL and Morgentaler A: Risks of
testosterone-replacement therapy and recommendations for
monitoring. N Engl J Med. 350:482–492. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gregory SJ and Kaiser UB: Regulation of
gonadotropins by inhibin and activin. Semin Reprod Med. 22:253–267.
2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tsujimura A, Takada S, Matsuoka Y, Hirai
T, Takao T, Miyagawa Y, Nonomura N and Okuyama A: Is
discontinuation of hormone replacement therapy possible for
patients with late-onset hypogonadism? Int J Urol. 15:625–629.
2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Khera M, Adaikan G, Buvat J, Carrier S,
El-Meliegy A, Hatzimouratidis K, McCullough A, Morgentaler A,
Torres LO and Salonia A: Diagnosis and treatment of testosterone
deficiency: Recommendations from the Fourth International
consultation for sexual medicine (ICSM 2015). J Sex Med.
13:1787–1804. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tsujimura A, Miyagawa Y, Okuda H, Yamamoto
K, Fukuhara S, Nakayama J, Takao T, Nonomura N and Okuyama A:
Change in cytokine levels after administration of
saikokaryuukotsuboreito or testosterone in patients with symptoms
of late-onset hypogonadism. Aging Male. 14:76–81. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kupelian V, McVary KT, Kaplan SA, Hall SA,
Link CL, Aiyer LP, Mollon P, Tamimi N, Rosen RC and McKinlay JB:
Association of lower urinary tract symptoms and the metabolic
syndrome: Results from the Boston Area Community Health Survey. J
Urol. 182:616–625. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Takeuchi H and Aoyagi T: Clinical
characteristics in urolithiasis formation according to body mass
index. Biomed Rep. 11:38–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Brand JS, van der Tweel I, Grobbee DE,
Emmelot-Vonk MH and van der Schouw YT: Testosterone, sex
hormone-binding globulin and the metabolic syndrome: A systematic
review and meta-analysis of observational studies. Int J Epidemiol.
40:189–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tsujimura A, Miyagawa Y, Takezawa K, Okuda
H, Fukuhara S, Kiuchi H, Takao T, Yamamoto R, Nishida M,
Yamauchi-Takihara K, et al: Is low testosterone concentration a
risk factor for metabolic syndrome in healthy middle-aged men?
Urology. 82:814–819. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Corona G, Monami M, Rastrelli G, Aversa A,
Tishova Y, Saad F, Lenzi A, Forti G, Mannucci E and Maggi M:
Testosterone and metabolic syndrome: A meta-analysis study. J Sex
Med. 8:272–283. 2011.PubMed/NCBI View Article : Google Scholar
|