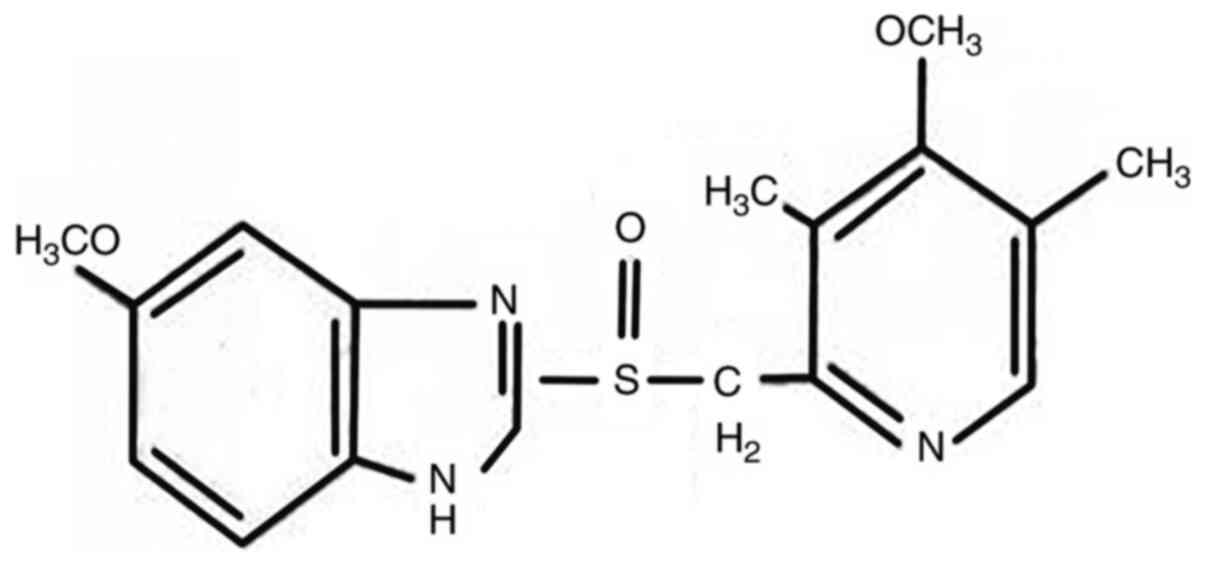
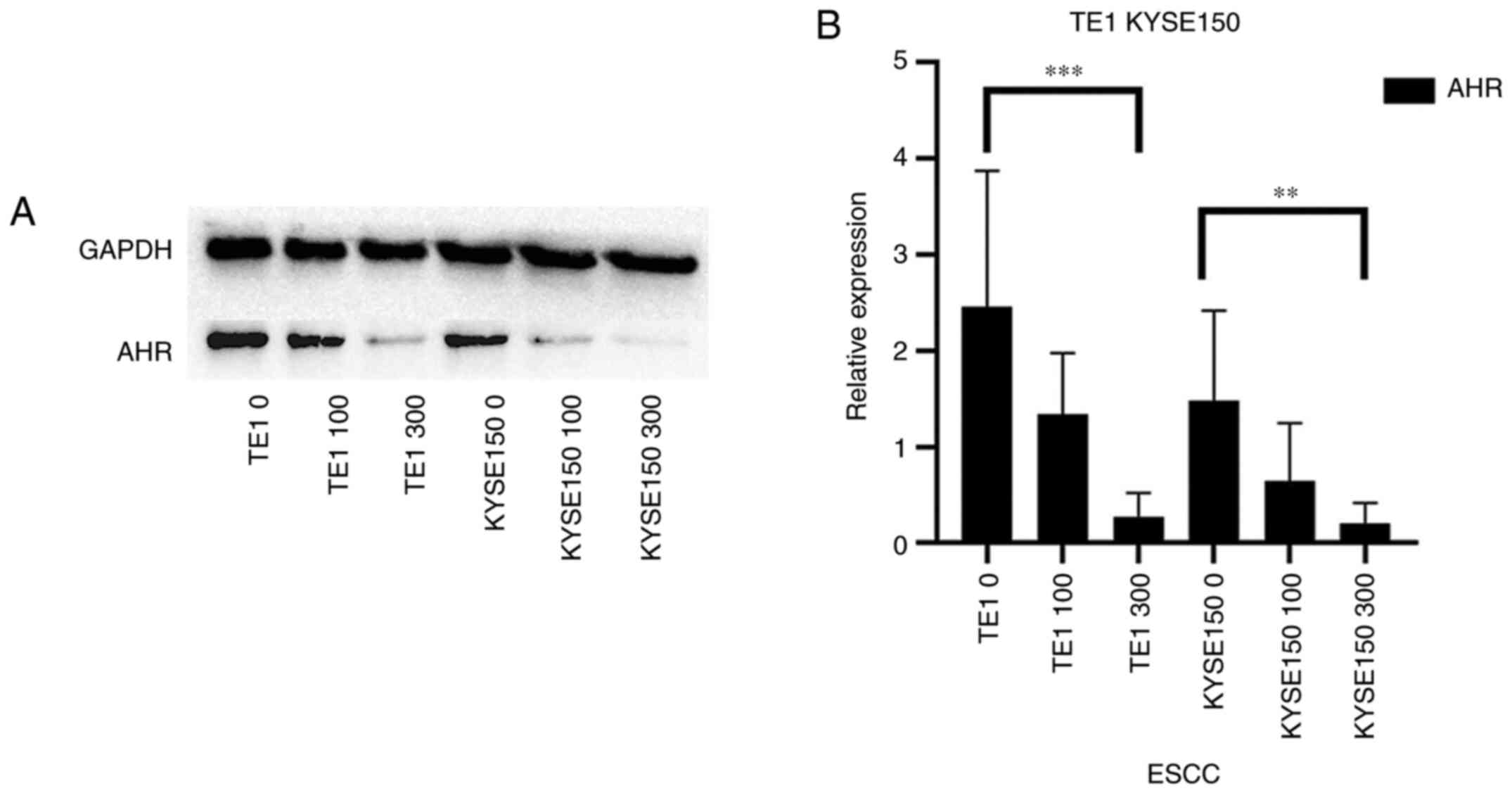
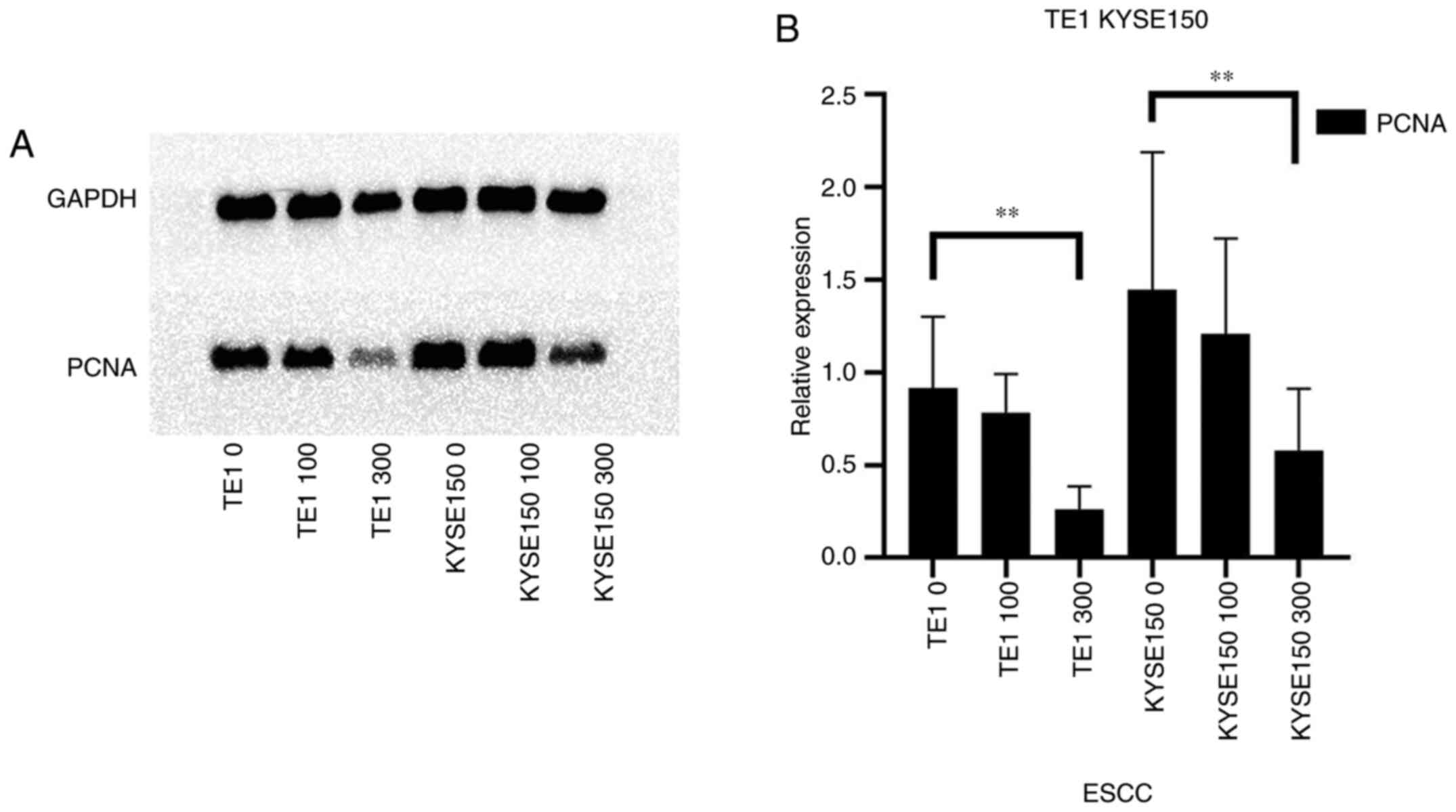
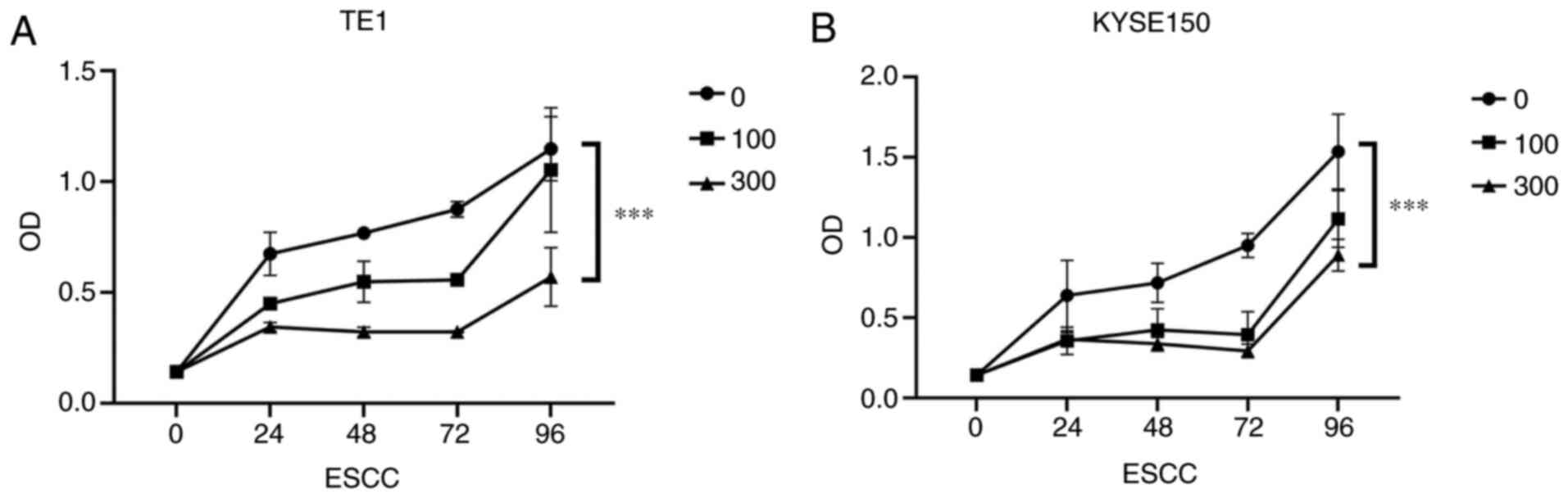
|
1
|
Ilic M, Kocic S, Radovanovic D, Macuzic IZ
and Ilic I: Trend in esophageal cancer mortality in Serbia,
1991-2015 (a population-based study): An age-period-cohort analysis
and a joinpoint regression analysis. J BUON. 24:1233–1239.
2019.PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Malhotra G, Yanala U, Ravipati A, Follet
M, Vijayakumar M and Are C: Global trends in esophageal cancer. J
Surg Oncol. 115:564–579. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smyth E, Lagergren J, Fitzgerald R,
Lordick F, Shah M, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis primers. 3(17048)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arnal MJ, Arenas ÁA and Arbeloa ÁL:
Esophageal cancer: Risk factors, screening and endoscopic treatment
in Western and Eastern countries. World J Gastroenterol.
21:7933–7943. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schulte K, Green E, Wilz A, Platten M and
Daumke O: Structural basis for aryl hydrocarbon receptor-mediated
gene activation. Structure. 25:1025–1033. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang F, Gao J, Mimura J, Kobayashi A,
Sogawa K and Fujii-Kuriyama Y: Structure and expression of the
mouse AhR nuclear translocator (mArnt) gene. J Biol Chem.
273:24867–24873. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nebert D: Aryl hydrocarbon receptor (AHR):
‘Pioneer member’ of the basic-helix/loop/helix per-Arnt-sim
(bHLH/PAS) family of ‘sensors’ of foreign and endogenous signals.
Prog Lipid Res. 67:38–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shiizaki K, Ohsako S, Kawanishi M and Yagi
T: Omeprazole alleviates benzo[a]pyrene cytotoxicity by inhibition
of CYP1A1 activity in human and mouse hepatoma cells. Basic Clin
Pharmacol Toxicol. 103:468–475. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao W, Li HY, Wang LX, Hao LJ, Gao JL,
Zheng RJ, Cai CJ and Si YL: Protective effect of omeprazole on
gastric mucosal of cirrhotic portal hypertension rats. Asian Pac J
Trop Med. 7:402–406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weil J, Bell GD, Powell K, Morden A,
Harrison G, Gant PW, Jones PH and Trowell JE: Omeprazole and
helicobacter pylori: Temporary suppression rather than true
eradication. Aliment Pharmacol Ther. 5:309–313. 1991.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blum R: Lansoprazole and omeprazole in the
treatment of acid peptic disorders. Am J Health Syst Pharm.
53:1401–1415. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jin UH, Lee SO and Safe S: Aryl
hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR
modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol
Exp Ther. 343:333–341. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nguyen LP and Bradfield CA: The search for
endogenous activators of the aryl hydrocarbon receptor. Chem Res
Toxicol. 21:102–116. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin UH, Kim SB and Safe S: Omeprazole
inhibits pancreatic cancer cell invasion through a nongenomic aryl
hydrocarbon receptor pathway. Chem Res Toxicol. 28:907–918.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Murray IA, Patterson AD and Perdew GH:
Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat
Rev Cancer. 14:801–814. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Patrizi B and Siciliani de Cumis M: TCDD
toxicity mediated by epigenetic mechanisms. Int J Mol Sci.
19(4101)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Opitz C, Litzenburger U, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18(3249)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jankowski JAZ, de Caestecker J, Love SB,
Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks
C, et al: Esomeprazole and aspirin in Barrett's oesophagus
(AspECT): A randomised factorial trial. Lancet. 392:400–408.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shiizaki K, Kido K and Mizuta Y: Insight
into the relationship between aryl-hydrocarbon receptor and
β-catenin in human colon cancer cells. PLoS One.
14(e0224613)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tomblin JK, Arthur S, Primerano DA,
Chaudhry AR, Fan J, Denvir J and Salisbury TB: Aryl hydrocarbon
receptor (AHR) regulation of L-type amino acid transporter 1
(LAT-1) expression in MCF-7 and MDA-MB-231 breast cancer cells.
Biochem Pharmacol. 106:94–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Donovan M, Selmin O and Romagnolo D: Aryl
hydrocarbon receptor diet and breast cancer risk. Yale J Biol Med.
91:105–127. 2018.PubMed/NCBI
|
|
26
|
Terashima J, Jimma Y, Jimma K, Hakata S,
Yachi M, Habano W and Ozawa S: The regulation mechanism of AhR
activated by benzo[a]pyrene for CYP expression are different
between 2D and 3D culture of human lung cancer cells. Drug Metab
Pharmacokinet. 33:211–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gao H, Ye G, Lin Y, Chi Y and Dong S:
Benzo[a]pyrene at human blood equivalent level induces human lung
epithelial cell invasion and migration via aryl hydrocarbon
receptor signaling. J Appl Toxicol. 40:1087–1098. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Dong S, Zhu P and Zhang S: Expression of
collagen type 1 alpha 1 indicates lymph node metastasis and poor
outcomes in squamous cell carcinomas of the lung. PeerJ.
8(e10089)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoshinari K, Ueda R, Kusano K, Yoshimura
T, Nagata K and Yamazoe Y: Omeprazole transactivates human CYP1A1
and CYP1A2 expression through the common regulatory region
containing multiple xenobiotic-responsive elements. Biochem
Pharmacol. 76:139–145. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vogeley C, Esser C, Tüting T, Krutmann J
and Haarmann-Stemmann T: Role of the aryl hydrocarbon receptor in
environmentally induced skin aging and skin carcinogenesis. Int J
Mol Sci. 20(6005)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen Z, Cai A, Zheng H, Huang H, Sun R,
Cui X, Ye W, Yao Q, Chen R and Kou L: Carbidopa suppresses prostate
cancer via aryl hydrocarbon receptor-mediated ubiquitination and
degradation of androgen receptor. Oncogenesis. 9(49)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang L, Tang W, Yang S, He P, Wang J,
Gaedcke J, Ströbel P, Azizian A, Ried T, Gaida MM, et al: NO
/RUNX3/kynurenine metabolic signaling enhances disease
aggressiveness in pancreatic cancer. Int J Cancer. 146:3160–3169.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Masoudi S, Nemati AH, Fazli HR, Beygi S,
Moradzadeh M, Pourshams A and Mohamadkhani A: An increased level of
aryl hydrocarbon receptor in patients with pancreatic cancer.
Middle East J Dig Dis. 11:38–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu P, Yu H, Zhou K, Bai Y, Qi R and Zhang
S: 3,3'-Diindolylmethane modulates aryl hydrocarbon receptor of
esophageal squamous cell carcinoma to reverse
epithelial-mesenchymal transition through repressing
RhoA/ROCK1-mediated COX2/PGE2 pathway. J Exp Clin Cancer Res.
39(113)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu P, Zhou K, Lu S, Bai Y, Qi R and Zhang
S: Modulation of aryl hydrocarbon receptor inhibits esophageal
squamous cell carcinoma progression by repressing COX2/PGE2/STAT3
axis. J Cell Commun Signal. 14:175–192. 2020.PubMed/NCBI View Article : Google Scholar
|