Introduction
The advantages of total intravenous anesthesia
(TIVA) over inhalational anesthesia are well recognized (1). However, the use of TIVA during daily
clinical practice is limited in certain instances. The application
of TIVA may be considered as unpractical when compared with
inhalational anesthesia. Preparation of the drugs for infusion may
be time-consuming and in addition, TIVA requires infusion pumps,
infusion sets and connecting tubes that increase the cost. The
management of infusion rates during anesthesia maintenance is not
straightforward when converting ‘µg/kg/min’ or ‘mg/kg/h’ to
‘ml/h’.
Remifentanil and propofol are commonly used for TIVA
due to their characteristics of ease of titration, as well as rapid
onset and offset of action. It may be more appropriate to use
remifentanil and propofol with target-controlled infusion (or
‘smart’) pumps and monitoring using processed
electroencephalographic signals, but access to such devices is
limited. In general practice, remifentanil and propofol infusions
are usually adjusted according to clinical signs of depth of
anesthesia with the guidance of manual infusion schemes that have
been proposed to maintain a constant blood concentration during
anesthesia, depending on the pharmacokinetics (2).
As a practical implementation for TIVA, using
remifentanil and propofol as a mixture at proper concentrations
allows managing one infusion instead of two and has been described
in the literature (1,3-18)
and certain textbooks (19-22)
and has been suggested by various institutes (23-25).
Studies on the stability and compatibility of drugs in the
remifentanil-propofol mixture (MIXTIVA) are conflicting (26,27).
However, in clinical practice, there may be other possible
drawbacks of this technique: When infusing drugs as a mixture, when
it is intended to administer one drug at a certain infusion rate,
the other drug may be overdosed or its blood concentration may not
reach the therapeutic level. The aim of the present study was to
investigate the applicability and possible disadvantages of MIXTIVA
infusion for the maintenance of TIVA and the changes in clinical
outcomes when compared with the standard technique using propofol
and remifentanil infusions separately.
Materials and methods
Patients
The present prospective randomized controlled trial
was performed at Bezmialem Vakıf University Medical Faculty
(Istanbul, Turkey) between January 2013 and April 2014. After
approval by the Bezmialem Vakif University Ethics Committee
(reference no. 71306642/050-01-04), patients aged 18-65 years
scheduled for elective thyroidectomy and with an American Society
of Anesthesiologists (ASA) physical status of I or II were included
in the present study. The exclusion criteria were as follows: ASA
physical status of III or above; a body mass index >35
kg/m2; pregnant, breast-feeding or menstruating females;
patients who were not euthyroid; uncontrolled hypertension;
hepatic, renal or cardiac insufficiency; alcohol, opioid or drug
abuse; allergy or contraindication to any of the study drugs.
Investigators MB and UT recruited the patients and written informed
consent was obtained from each patient prior to randomization.
Drug preparation
Remifentanil (Ultiva; GlaxoSmithKline) was diluted
with sterile water and the concentration of the reconstituted
solution was 1 mg/ml. The remifentanil-propofol mixture MIXTIVA was
prepared in a 50-ml bottle of propofol 1% (Fresenius Kabi) by
adding either 1 or 1.5 mg of remifentanil to achieve a remifentanil
concentration of 20 or 30 µg/ml and a remifentanil/propofol
proportion of 2/1,000 or 3/1,000, respectively. Mixtures were
always prepared immediately prior to administration, checked for
visual stability to verify that they exhibited no evidence of
precipitation or separation and used within 2 h of preparation. All
infusions were administered with an Alaris GW Volumetric Pump
(Cardinal Health) using an Alaris infusion set.
Randomization and blinding
Patients were randomly allocated to 3 groups to
either receive anesthesia maintenance with remifentanil and
propofol infusions separately (control group, Group I) or with
MIXTIVA infusion with a remifentanil/propofol proportion of 2/1,000
or 3/1,000 (Group II or Group III, respectively). Simple
randomization was performed using 96 opaque sealed envelopes, 32
for each group, indicating group assignments. Prior to anesthesia
induction, an anesthesiologist (TU) opened the next envelope and
prepared the study medications. This anesthesiologist was not
involved in preoperative and postoperative data collection or
anesthesia management of the patients. Resident anesthesiologists
(EYG and HU) who were blinded to the study groups (Group II and
III) performed all procedures.
Surgical preparation of patients
Patients received all of their regular medications
on the morning of surgery. On arrival at the operating room, ECG,
noninvasive blood pressure (at the ankle), pulse oximetry,
temperature and bispectral index (BIS; Aspect Medical Systems)
monitoring were applied. After premedication with intravenous
(i.v.) midazolam (0.03 mg/kg), baseline heart rate and mean
arterial blood pressure (MAP) values, were determined as the
average of three consecutive measurements. Noninvasive blood
pressure (NIBP) was assessed with intervals at least 3 min during
anesthesia and BIS scores at the time of NIBP measurements were
automatically recorded. An intravenous crystalloid solution
(Isolyte-S; Koçak Farma İlaç ve Kimya Sanayi A.Ş) was administered
as a 5-ml/kg bolus prior to induction and infusion at 5 ml/kg/h was
started.
Anesthesia
Induction of anesthesia was as follows: In Group I,
remifentanil (30 µg/ml) and propofol 1% were prepared for infusion
separately and both started at 0.5 ml/kg/h. In Group II and III,
MIXTIVA infusion at 0.5 ml/kg/h was started. All patients received
lidocaine 1 mg/kg, propofol 1.5 mg/kg and vecuronium 0.1 mg/kg. At
the 4th min of infusions, intubation was performed.
For anesthesia maintenance, in Group I, remifentanil
infusion was adjusted to maintain the MAP within ±20% of the
baseline value (preferably under the baseline value) and the
propofol infusion rate was adjusted to maintain the BIS between 30
and 50 (target: 40). In Group II and III, the primary goal of
anesthesia management was to maintain the MAP within ±20% of the
baseline value (preferably under the baseline value) by adjusting
MIXTIVA infusion between 0.3-1.2 ml/kg/h (dose chart for MIXTIVA
infusion is provided in Table I).
When the MAP was above the baseline value, MIXTIVA infusion was
increased with a 0.5-1 ml bolus administration. When the MAP was
within ±20% of the baseline value, the secondary goal was to
maintain BIS values between 30 and 50 (target: 40). Intravenous
crystalloid infusion was also adjusted to maintain the MAP within
the desired levels.
 | Table IDose chart for remifentanil-propofol
mixture (MIXTIVA). |
Table I
Dose chart for remifentanil-propofol
mixture (MIXTIVA).
| Infusion rate,
ml/kg/h | Infusion
ratea, ml/h | Propofolb dose, µg/kg/min (mg/kg/h) |
Remifentanilc dose, µg/kg/min |
Remifentanild dose, µg/kg/min |
|---|
| 1.2 | 84 | 200(12) | 0.4 | 0.6 |
| 1 | 70 | 166(10) | 0.33 | 0.5 |
| 0.8 | 56 | 133(8) | 0.26 | 0.4 |
| 0.6 | 42 | 100(6) | 0.2 | 0.3 |
| 0.5 | 35 | 83(5) | 0.17 | 0.25 |
| 0.4 | 28 | 66(4) | 0.13 | 0.2 |
| 0.3 | 21 | 50(3) | 0.1 | 0.15 |
In case of hypotension (defined as MAP <60 mmHg),
anesthesia infusions were decreased or stopped for 1-2 min, i.v.
bolus crystalloid infusion 3-5 ml/kg was administered and if
hypotension persisted in two consecutive measurements,
norepinephrine 5-10 µg was administered. When hypotension was
accompanied with BIS values >60, ketamine 20-30 mg was
administered. In case of hypertension (defined as systolic arterial
pressure ≥150 mmHg persisting in two consecutive measurements
despite maximum infusion rates), a bolus dose of 0.1-0.2 mg
nitroglycerine was administered i.v. Bradycardia (heart rate <45
bpm) was treated with atropine 0.5-1 mg i.v.
The lungs were mechanically ventilated with a
mixture of oxygen and air (fraction of inspired O2, 50%;
tidal volume, 6-8 ml/kg; respiratory rate, 10-14/min) to obtain an
end-tidal CO2 value between 30 and 35 mmHg.
Other medications
All patients received intravenous dexamethasone 8
mg, metoclopramide 10 mg, ranitidine 50 mg and dexketoprophen
trometamol 50 mg after anesthesia induction; tramadol 100 mg and
paracetamol 1 g, 15 min before the end of surgery. In addition,
skin infiltration with lidocaine 2% was achieved prior to surgical
incision. Additional vecuronium was preferably not used after the
induction dose to retain the option of recurrent laryngeal nerve
monitoring. All infusions were terminated during skin closure and
residual neuromuscular blockade was antagonized with neostigmine
0.05 mg/kg and atropine 0.02 mg/kg.
Design of the study
To determine the proportions of the drugs for the
mixtures, a pilot study was performed with 10 patients who
underwent thyroidectomy with TIVA using remifentanil and propofol
infusions separately (the same anesthesia protocol in the control
group). The mean infusion rates for remifentanil and propofol were
0.26±0.09 and 81±23 µg/kg/min, respectively. The proportion of
total drug consumptions for remifentanil and propofol was 3.2/1,000
in the pilot study, which was supposed to be compared with the
routine management at our institution using a drug proportion of
2/1,000.
Statistical analysis
The primary outcome measure was the extubation time
after all infusions were stopped. The sample size requirement was
based on the preliminary data from the pilot study in which the
extubation time was 8±2.4 min. Thus, at an alpha risk of 0.05, 32
patients per group would provide 80% statistical power and detect a
50% difference in extubation time. Secondary outcome measures were
the incidence of undesirable events (e.g. hypotension,
hypertension, bradycardia, intraoperative movement of the
patient).
One-way ANOVA for parametric variables and the
Kruskal-Wallis test for non-parametric variables were used. The
chi-squared test was used for comparison of adverse effects. The
Tukey-Kramer test was used to compare groups individually. All
statistical analyses were performed using the commercially
available SPSS v.16.0 software package (SPSS Inc.). P<0.05 was
considered to indicate statistical significance.
Results
Enrollment
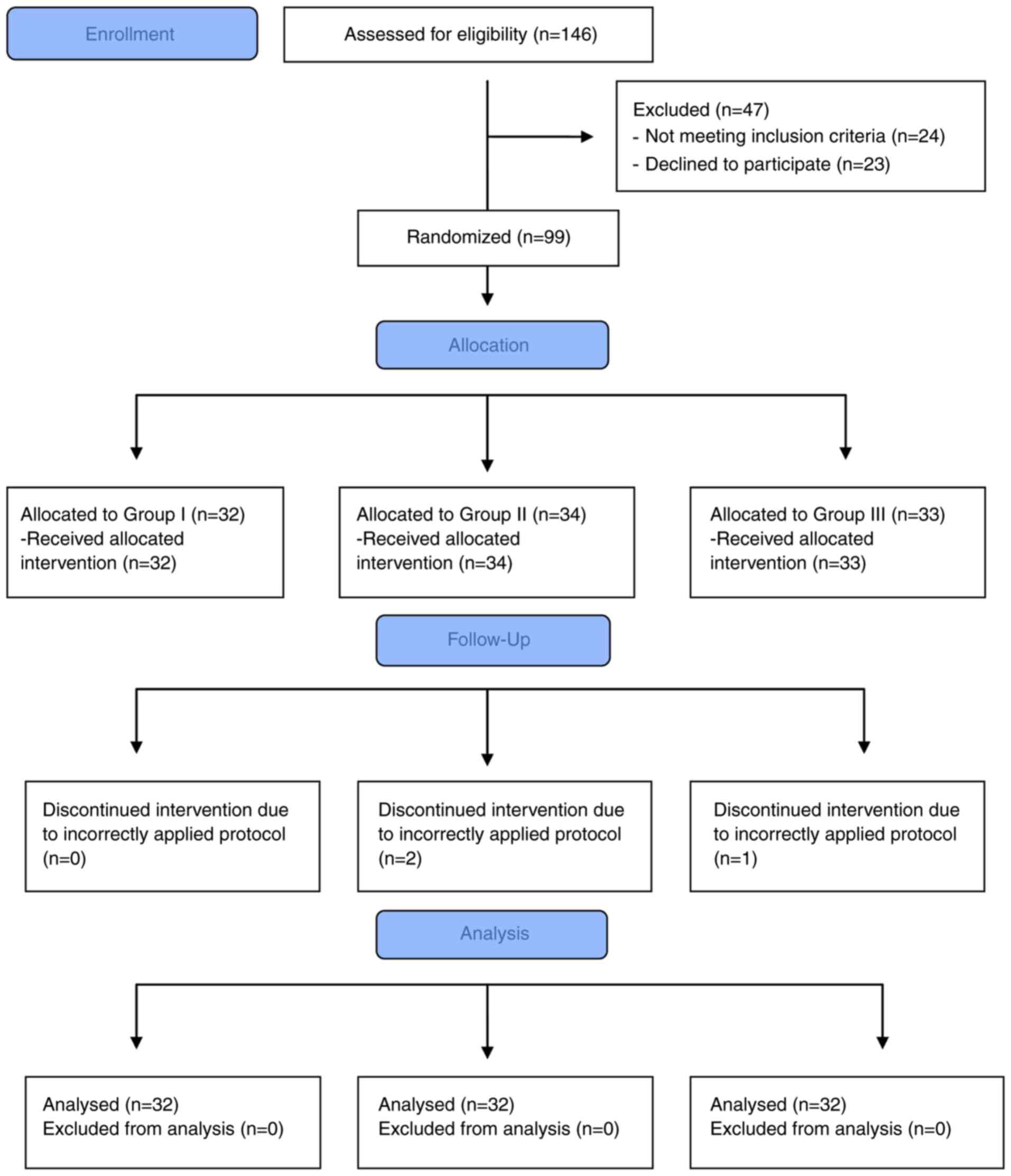
Of the 146 patients approached, 24 did not meet the
criteria for inclusion, 23 refused to participate in the study and
the protocol was incorrectly applied in 3, leaving 96 patients
suitable to be enrolled in the present trial (Fig. 1).
Demographic characteristics
Data of demographic characteristics of patients are
presented in Table II. The age and
sex distribution of the patients and other patient characteristics
were not significantly different between the groups.
 | Table IIDemographic characteristics of
patients. |
Table II
Demographic characteristics of
patients.
| Item | Group I (n=32) | Group II
(n=32) | Group III
(n=32) | P-value |
|---|
| Sex
(male/female) | 6/26 | 7/25 | 4/28 | NS |
| Age (years) | 44.6±11.4
(40.5-48.7) | 46.1±10.8
(42.2-50) | 42.4±11.6
(38.3-46.6) | NS |
| Body weight
(kg) | 76.3±16
(70.5-82.1) | 74.4±15.6
(68.8-80.1) | 71.7±14
(66.6-76.7) | NS |
| Body height
(m) | 1.65±0.09
(1.62-1.68) | 1.65±0.08
(1.62-1.68) | 1.65±0.07
(1.63-1.68) | NS |
| BMI
(kg/cm2) | 28±4.8
(26.2-29.7) | 27.3±4.6
(25.6-29) | 26.2±4.2
(24.7-27.7) | NS |
| ASA I/II | 25/7 | 26/6 | 29/3 | NS |
Anesthesia characteristics
While the duration of anesthesia and surgery and the
mean propofol infusion rate (total propofol consumption/body
weight/infusion time) were comparable between the groups, the mean
remifentanil infusion rate (total remifentanil consumption/body
weight/infusion time) in Group III was significantly higher when
compared with that in the other groups (P<0.05). The primary
outcome of the study (the extubation time) was comparable among the
groups (Table III).
 | Table IIIAnesthesia characteristics. |
Table III
Anesthesia characteristics.
| Item | Group I (n=32) | Group II
(n=32) | Group III
(n=32) | P-value |
|---|
| Duration of
anesthesia (min) | 103 (100-131) | 114 (101-122) | 107 (96-114) | NS |
| Duration of surgery
(min) | 90 (88-119) | 103 (88-108) | 92 (82-100) | NS |
| Mean propofol
infusion rate (µg/kg/min) | 88±25 (79-98) | 106±25
(97-115) | 100±41
(85-115) | NS |
| Mean remifentanil
infusion rate (µg/kg/min) | 0.25±0.09
(0.22-0.29) | 0.21±0.05
(0.19-0.23) |
0.31±0.11a (0.27-0.35) | <0.0001 |
| Mean BIS value | 41±5 (39-42) | 39±6 (37-42) | 42±5 (40-44) | NS |
| Extubation time
(min) | 8 (7.2-10.2) | 8.5 (7.7-10.2) | 9.5 (8.4-10.8) | NS |
| Orientation time
(min) | 9 (8.6-11.8) | 10 (9-11.8) | 11 (9.9-12.8) | NS |
| Intraoperative
bradycardia | 1(3) | 1(3) | 0 (0) | NS |
| Intraoperative
hypertension | 5(16) | 6(19) | 2(6) | NS |
| Intraoperative
hypotension | 7(22) | 6(19) | 5(16) | NS |
| Sympathomimetic
use | 3(9) | 1(3) | 2(6) | NS |
| Intraoperative
movement | 3(9) | 2(6) | 0 (0) | NS |
| Additional
vecuronium | 4(13) | 3(9) | 2(6) | NS |
| Recorded BIS
>60 | 7(22) | 6(19) | 9(28) | NS |
| Ketamine use | 0 (0) | 0 (0) | 1(3) | NS |
| PONV | 3(9) | 2(6) | 3(9) | NS |
| Postoperative
shivering | 2(6) | 2(6) | 3(9) | NS |
Hemodynamics
The numbers of patients in whom intraoperative
hypotension, hypertension or bradycardia episodes were encountered
during anesthesia were comparable among the groups (Table III). Hypotension episodes were
mostly encountered after induction or prior to delayed surgery.
Hypertension episodes mostly occurred after endotracheal intubation
or at the beginning of delayed surgery.
BIS scores and recovery
characteristics
The mean BIS scores (the average of values recorded
during anesthesia) and recovery characteristics (extubation and
orientation time) of the patients were comparable among groups
(Table III). The numbers of
patients who had a BIS >60 recorded during anesthesia were also
comparable (in most of them, BIS was between 60 and 65). A high BIS
was mostly encountered after endotracheal intubation or at the
beginning of delayed surgery. Hypotension accompanied with a high
BIS (up to 70) was encountered in one patient in Group III who was
treated with norepinephrine and ketamine (Table III). No intraoperative awareness
was noted in any of the groups.
Other adverse events
In addition, no serious respiratory adverse event
leading to desaturation (peripheral oxygen saturation <90%
lasting >1 min) was noted in any of the groups. The incidence of
postoperative nausea and vomiting, which required ondansetron
administration, and shivering, which required meperidine
administration, were also comparable between groups (Table III).
Discussion
In the present study, TIVA with MIXTIVA
(remifentanil/propofol proportion of 2/1,000 or 3/1,000) using a
single-infusion technique was not associated with any statistically
significant difference in clinical outcomes (recovery
characteristics, incidence of hemodynamic fluctuations and other
undesirable events) when compared with the standard TIVA technique
using separate drug infusions.
Recently, Bagshaw et al (3) evaluated 873 pediatric patients who
underwent mostly gastroenterology and ear, nose and throat
procedures using MIXTIVA infusion. The incidence of serious adverse
events such as desaturation, apnea, abdominal/chest rigidity, cough
requiring paralysis, ventilatory problems and hypotension was 1.7%
and they had occurred mostly at induction and were not attributed
directly to the use of the mixture itself.
Although it is mostly not recommended, mixing
intravenous agents appears to be popular in clinical practice.
Propofol is frequently mixed with lidocaine to minimize the
injection pain. Mixtures of alfentanil and propofol were used for
TIVA before remifentanil became more popular (28,29).
Mixtures of ketamine and propofol, ‘ketofol’, was recommended,
particularly for sedation and analgesia for short and painful
procedures (30-32).
Mixtures of propofol with other intravenous drugs such as
thiopental (33), ephedrine
(34), methohexital (35) and metoclopramide (36), and mixtures of remifentanil with
tramadol (37) have been reported,
but most of these implementations may be considered as
‘experimental’.
In the literature, remifentanil-propofol mixture has
been used mostly for patient-controlled sedation (9,10,12,17) or
deep sedation with spontaneous ventilation (5-8,11,13,14,16,18,19).
Research on the use of MIXTIVA for maintenance of general
anesthesia is limited (1,3,15,20,22).
While certain recommendations have been given for MIXTIVA usage in
general anesthesia (23-25),
the remifentanil/propofol proportion varies (0.5/1,000 to 5/1,000)
among centers and the possible drawbacks of this technique and
optimum proportion of drugs remain to be investigated.
Stewart et al (26) demonstrated that remifentanil and
propofol may be mixed in polypropylene syringes and used for up to
36 h when the remifentanil/propofol proportion was 5/1,000.
However, when polyvinylchloride bags and lower concentrations of
remifentanil were used (0.5/1,000), the duration of stability was
decreased to 1 h. O'Connor et al (27) reported that when remifentanil
solution and propofol emulsion were mixed in the same syringe,
separation and layering of the drugs may result in significant
differences of drug concentrations at the top and bottom of the
syringe (remifentanil having a greater concentration at the top and
propofol having a greater concentration at the bottom of the
syringe). The lack of control groups (drugs also had to be
evaluated separately without mixing) may be the major limitation of
that study. In addition, they measured drug concentrations at the
top and bottom of vertically mounted syringes, while in most of the
syringe infusion pumps in clinical use, syringes are mounted
horizontally and the drug mixture exits the syringe from the
midpoint of the mixture instead of the bottom. In the present
study, volumetric pumps were used and the mixtures were prepared
immediately prior to infusion in 50-ml glass bottles of propofol
instead of polypropylene syringes and mixtures were used within
<2 h.
The use of MIXTIVA infusion may be recommended to
clinicians after gaining experience with the standard TIVA
technique using separate drug infusions and administrating propofol
infusion with the guidance of BIS monitoring. In clinical practice,
BIS monitoring is not common and in order to decrease the risk of
awareness and recall during TIVA, premedication with midazolam,
adding ketamine 0.3-0.5 mg/kg to the anesthesia protocol and (as
the movement of the patient under general anesthesia constitutes a
warning of rising consciousness or pain) minimizing the use of
neuromuscular blocking agents (NMBAs) during TIVA are recommended.
General anesthesia for short procedures using a laryngeal mask
airway without NMBAs appears to be best suited for MIXTIVA
technique. Adjusting the remifentanil/propofol proportion of
MIXTIVA according to the type of surgery (increasing the proportion
for more painful procedures or hypotensive anesthesia) or to the
condition of the patient (decreasing the proportion in elderly
patients) may be favorable. According to the pharmacokinetics of
propofol, for a constant blood or effect-site concentration, a
gradual decrease in the initial propofol infusion rate is required
(2). For long procedures (>2 h),
it may be preferred to gradually increase the proportion, starting
with 2/1,000 in the first 50 ml (with or without ketamine
administration) and continuing with 3-4/1,000 in the following.
The primary outcome of the recent study was the
extubation time and sample size estimation was performed according
to this outcome. As a consequence, the study may be underpowered to
obtain any differences in other clinical outcomes. In addition, a
‘BIS-blinded’ study would be more appropriate to determine the risk
of awareness.
In conclusion, compared with the standard TIVA
technique using separate drug infusions, when TIVA, using a
single-infusion technique with MIXTIVA (remifentanil/propofol
proportion, 2/1,000 or 3/1,000) was applied for thyroidectomies, no
statistically significant difference in recovery and clinical
outcome was obtained. This technique may be considered as a
practical implementation for busy ambulatory surgery centers
performing general anesthesia. Adjustment of the
remifentanil-propofol proportion for different types of surgery and
patient groups must be considered and further studies with larger
patient populations are warranted.
Acknowledgements
The results of the present study were presented on a
poster at the 47th National Congress of the Turkish Society of
Anesthesiology and Reanimation (20-24 October 2013; Abstract no.
P-27: Antalya, Turkey).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB designed the study. MB and TU were involved in
performing the study procedures, data analysis and writing of the
manuscript. UT contributed to data analysis and writing of the
manuscript. EYG and HU were involved in performing the study
procedures and data analysis. EO contributed to design of the study
and was involved in writing the manuscript. All authors confirm the
authenticity of the raw data, and read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Bezmialem Vakif University (Istanbul, Turkey;
reference no. 71306642/050-01-04). Informed consent was obtained
from all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson BJ and Bagshaw O: Practicalities
of total intravenous anesthesia and target-controlled infusion in
children. Anesthesiology. 131:164–185. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roberts FL, Dixon J, Lewis GT, Tackley RM
and Prys-Roberts C: Induction and maintenance of propofol
anaesthesia. A manual infusion scheme. Anaesthesia. 43
(Suppl):S14–S17. 1988.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bagshaw O, McCormack J, Brooks P, Marriott
D and Baxter A: The safety profile and effectiveness of
propofol-remifentanil mixtures for total intravenous anesthesia in
children. Paediatr Anaesth. 30:1331–1339. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bagshaw O: Pediatric anesthesia
editorial-propofol and remifentanil: To mix or not to mix. Paediatr
Anaesth. 26:677–679. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goudra BG, Singh PM and Sinha A: Providing
anesthesia in a remote location for radiation oncology in an
adult-Problems and solutions. J Anaesthesiol Clin Pharmacol.
30:114–116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pedersen NA, Jensen AG, Kilmose L and
Olsen KS: Propofol-remifentanil or sevoflurane for children
undergoing magnetic resonance imaging? A randomized study. Acta
Anaesthesiol Scand. 57:988–995. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lan C, Shen X, Cui H, Liu H, Li P, Wan X,
Lan L and Chen D: Comparison of nitrous oxide to no sedation and
deep sedation for diagnostic upper gastrointestinal endoscopy. J
Gastrointest Surg. 17:1066–1072. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kramer KJ, Ganzberg S, Prior S and Rashid
RG: Comparison of propofol-remifentanil versus propofol-ketamine
deep sedation for third molar surgery. Anesth Prog. 59:107–117.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Joo JD, In JH, Kim DW, Jung HS, Kang JH,
Yeom JH, Yeom JH and Choi JW: The comparison of sedation quality,
side effect and recovery profiles on different dosage of
remifentanil patient-controlled sedation during breast biopsy
surgery. Korean J Anesthesiol. 63:431–435. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mazanikov M, Udd M, Kylänpää L, Mustonen
H, Lindström O, Halttunen J, Färkkilä M and Pöyhiä R:
Patient-controlled sedation for ERCP: A randomized double-blind
comparison of alfentanil and remifentanil. Endoscopy. 44:487–492.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ryu JH, Kim M, Bahk JH, Do SH, Cheong IY
and Kim YC: A comparison of retrobulbar block, sub-Tenon block, and
topical anesthesia during cataract surgery. Eur J Ophthalmol.
19:240–246. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mandel JE, Tanner JW, Lichtenstein GR,
Metz DC, Katzka DA, Ginsberg GG and Kochman ML: A randomized,
controlled, double-blind trial of patient-controlled sedation with
propofol/remifentanil versus midazolam/fentanyl for colonoscopy.
Anesth Analg. 106:434–439. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Melville D, Hartsilver EL and Hart A:
Bolus dosing of remifentanil with propofol for gynaecological day
case surgery. J One Day Surg. 18:88–91. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsui BC, Wagner A, Usher AG, Cave DA and
Tang C: Combined propofol and remifentanil intravenous anesthesia
for pediatric patients undergoing magnetic resonance imaging.
Paediatr Anaesth. 15:397–401. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brady WJ, Meenan DR, Shankar TR, Balon JA
and Mennett DR: Use of a remifentanil and propofol combination in
outpatients to facilitate rapid discharge home. AANA J. 73:207–210.
2005.PubMed/NCBI
|
|
16
|
Berkenbosch JW, Graff GR, Stark JM, Ner Z
and Tobias JD: Use of a remifentanil-propofol mixture for pediatric
flexible fiberoptic bronchoscopy sedation. Paediatr Anaesth.
14:941–946. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Joo HS, Perks WJ, Kataoka MT, Errett L,
Pace K and Honey RJ: A comparison of patient-controlled sedation
using either remifentanil or remifentanil-propofol for shock wave
lithotripsy. Anesth Analg. 93:1227–1232. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fish WH, Hobbs AJ and Daniels MV:
Comparison of sevoflurane and total intravenous anaesthesia for
daycase urological surgery. Anaesthesia. 54:999–1006.
1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Anderson BJ and Houghton J: Total
intravenous anesthesia and target-controlled infusion. In: A
Practice of Anesthesia for infants and children. 6th edition. Cote
CJ, Lerman J and Anderson BJ (eds). Elsevier, pp177-198, 2019.
|
|
20
|
Bennett JD and Butterfield K: Anesthetic
concepts and techniques. Vol. 1. In: Oral and Maxillofacial
Surgery. 3rd edition. Fonseca RJ (ed). Elsevier, St. Louis, MO,
pp211-224, 2018.
|
|
21
|
Robert RC, Liu S, Patel C and Gonzalez ML:
Advancements in office-based anesthesia in oral and maxillofacial
surgery. Atlas Oral Maxillofac Surg Clin North Am. 21:139–165.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Urman RD and Shapiro FE: Choosing
anesthetic agents. Which one? In: Manual of Office-Based Anesthesia
Procedures. Shapiro FE (ed). Lippincott Williams & Wilkins,
Philadelphia, PA, pp58-74, 2007.
|
|
23
|
https://www.rch.org.au/uploadedFiles/Main/Content/anaes/TIVA%20for%20tonsillectomy%202014.pdf.
Assessed October, 2020.
|
|
24
|
http://www.anesthesiologynews.com/ViewArticle.aspx?d_id=1&a_id=14586&ses=ogst.
Assessed October, 2020.
|
|
25
|
http://ether.stanford.edu/library/pediatric_anesthesia/anesthetic%20techniques/guideline-tiva.pdf.
Assessed October, 2020.
|
|
26
|
Stewart JT, Warren FW, Maddox FC,
Viswanathan K and Fox JL: The stability of remifentanil
hydrochloride and propofol mixtures in polypropylene syringes and
polyvinylchloride bags at 22 degrees-24 degrees C. Anesth Analg.
90:1450–1451. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
O'Connor S, Zhang YL, Christians U,
Morrison JE Jr and Friesen RH: Remifentanil and propofol undergo
separation and layering when mixed in the same syringe for total
intravenous anesthesia. Paediatr Anaesth. 26:703–709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wilson RJ and Ridley SA: The use of
propofol and alfentanil by infusion in military anaesthesia.
Anaesthesia. 47:231–233. 1992.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Collins SJ, Robinson AL and Holland HF: A
comparison between total intravenous anaesthesia using a
propofol/alfentanil mixture and an inhalational technique for
laparoscopic gynaecological sterilization. Eur J Anaesthesiol.
13:33–37. 1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Weatherall A and Venclovas R: Experience
with a propofol-ketamine mixture for sedation during pediatric
orthopedic surgery. Paediatr Anaesth. 20:1009–1016. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lamperti M: Adult procedural sedation: An
update. Curr Opin Anaesthesiol. 28:662–667. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ghojazadeh M, Sanaie S, Paknezhad SP,
Faghih SS and Soleimanpour H: Using ketamine and propofol for
procedural sedation of adults in the emergency department: A
systematic review and meta-analysis. Adv Pharm Bull. 9:5–11.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fahringer DL, Goodwin SR, Warde MK, Ye G,
Blackwelder B, Ajala AM and Gurgis FS: The effect of a 3: 1 volume
mixture of propofol 1% and thiopental 2.5% in reducing the pain on
injection of propofol in children. Paediatr Anaesth. 20:545–552.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Austin JD and Parke TJ: Admixture of
ephedrine to offset side effects of propofol: A randomized,
controlled trial. J Clin Anesth. 21:44–49. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thompson N and Robertson GS: Comparison of
propofol and a propofol-methohexitone mixture for induction of
day-case anaesthesia. Br J Anaesth. 77:213–216. 1996.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mecklem DW: Propofol injection pain:
Comparing the addition of lignocaine or metoclopramide. Anaesth
Intens Care. 22:568–570. 1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Unlugenc H, Tetiker S and Isik G: Addition
of remifentanil to patient-controlled tramadol for postoperative
analgesia: A double-blind, controlled, randomized trial after major
abdominal surgery. Eur J Anaesthesiol. 25:968–975. 2008.PubMed/NCBI View Article : Google Scholar
|