Introduction
According to the World Health Organization, the
failure to achieve a clinical pregnancy after 1 year or more of
regular unprotected sexual intercourse is defined as infertility
(1). So far, ~10-15% of couples
experience infertility worldwide (2). Among all these infertility cases, the
male infertility factor accounts for ~50% (3). It is well known that traditional
treatments of male infertility include in vitro
fertilization and intrauterine insemination (3). However, intrauterine insemination only
achieves modest outcomes (3) and
in vitro fertilization with intracytoplasmic sperm injection
is invasive and expensive. In addition, both of the traditional
treatments are inefficient in treating specific causes of
infertility (3). Therefore,
additional research studies have to be conducted on novel treatment
strategies specifically targeting the causes of male
infertility.
Leydig cells are a group of cells found in clusters
that are located in the interstitial space of the testes between
seminiferous tubules (4). Leydig
cells produce testosterone and their dysfunction causes
seminiferous tubule dysfunction and fall in testosterone levels,
which are common features of infertile men (4). The association between Leydig cell
insufficiency and hypospermatogenesis in humans has already been
confirmed (4). In addition,
specific toxins that affect the function and morphology of Leydig
cells may result in male infertility (5). Therefore, protecting Leydig cells from
toxin-induced dysfunction may aid the development of treatment
strategies for male infertility.
Mycotoxins and pesticides are typical
endocrine-disrupting chemicals, which can cause poor sperm quality
(5). Zearalenone (ZEN) is a
non-steroidal estrogenic mycotoxin, which is a frequent contaminant
of cereal crops worldwide (6). The
toxic effects of ZEN on the reproductive system and its associated
reproductive disorders have been previously shown (7).
MicroRNAs (miRNAs/miRs) are a family of short
non-coding RNA molecules that are ~18-23 nucleotides in length
(8). miRNAs regulate gene
expression at the post-transcriptional level by binding to the
3'-untranslated region (3'-UTR) of their target mRNAs and inducing
translational repression. As a class of small non-coding RNAs,
miRNA play an essential role in the process of spermatogenesis
(8,9). Several miRNAs, including miR-96-5p,
miR-19a-3p and miR-210-5p, were found to be associated with
ZEN-induced toxicity noted in Leydig cells (10). Among these miRNAs, miR-96-5p
exhibited significantly increased expression levels (10). Moreover, miR-96-5p expression was
associated with cell proliferation effectors, such as Foxo1, AKT2
and PTEN (10). Therefore, the
present study aimed to further investigate the role of miR-96-5p on
protecting Leydig cells against ZEN-induced toxicity.
Materials and methods
Cell culture and reagents
TM3 (mouse Leydig cells) were obtained from the
American Type Culture Collection and cultured in DMEM/F12 media
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin in a humidified incubator
containing 5% CO2 at 37˚C. TM3 cells were cultured
overnight to allow adherence prior to treatment with ZEN (50 µM;
Sigma-Aldrich; Merck KGaA) for 48 h at 37˚C. 3-Methyladenine (3MA)
was purchased from Sigma-Aldrich; Merck KGaA and used at a
concentration of 5 mM.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay kit (Dojindo Molecular Technologies,
Inc.) was used to evaluate cell viability. TM3 cells were seeded
into 96-well plates at a density of 5x104 cells/well
overnight. Following treatment with certain concentrations of ZEN
(0, 10, 25, 50 and 75 µM) at 37˚C for 48 h, the cells were
incubated with 10 µl CCK-8 solution for an additional 2 h at 37˚C
prior to measuring the absorbance at 450 nm with a microplate
reader (Bio-Rad Laboratories, Inc.). ZEN (50 µM) was selected in
the subsequent experiments to induce a moderate decrease in cell
viability. For the combined treatment, TM3 cells were transfected
with the miR-96-5p inhibitor (10 nM) for 6 h using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.)
followed by incubation with ZEN (50 µM) for an additional 48 h, as
described previously (11).
Subsequently, the CCK-8 assay was conducted following the
aforementioned protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total cellular RNA was isolated from TM3 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The extracted total RNA was reverse transcribed into cDNA
using a PrimeScript RT-PCR kit (Takara Bio, Inc.) according to the
manufacturer's instructions. RT-qPCR was performed to determine the
levels of miR-96-5p using SYBR premix Ex Taq (Takara Bio, Inc.)
following the manufacturer's instructions. The primer sequences
used were as follows: miR-96-5p forward,
5'-TGGCACTAGCACATTTTTGC-3'; miR-96-5p reverse,
5'-CTCAACTGGTGTCGTGGAGTC-3'; U6 forward, 5'-CTCGCTTCGGCAGCACAT-3';
and U6 reverse, 5'-AACGCTTCACGAATTTGCGT-3'. The relative expression
levels of miR-96-5p were calculated using the comparative
2-ΔΔCq method (12). U6
was used as the internal control. The temperature conditions for
amplification were as follows: Pre-incubation at 98˚C for 3 min,
followed by 40 cycles at 95˚C for 30 sec, 56˚C for 40 sec and 72˚C
for 40 sec.
Cell transfection
miR-96-5p mimics (5'-UUUGGCACUAGCACAUUUUUGCU-3'),
inhibitors (5'-AGCAAAAAUGUGCUAGUGCCAAA-3'), and negative control
(mimic NC, 5'-UUGUACUACACAAAAGUACUG-3'; inhibitor NC,
5'-CAGUACUUUUGUGUAGUACAA-3') sequences were obtained from
GenePharm, Inc. miR-96-5p mimics could mimic endogenous miR-96-5p,
while miR-96-5p inhibitor (antisense single-stranded
oligonucleotides for miRNA inhibition) could inhibit endogenous
miR-96-5p. TM3 cells were seeded into 6-well cell culture plates
(1.5x104 cells/well) and cultured overnight prior to
transfection. Cells were transfected with 10 nM miR-96-5p mimics,
miR-96-5p inhibitor by using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) as previously
described (13). The efficiency of
transfection was determined using RT-qPCR following 48 h of cell
incubation.
Immunofluorescence assay
TM3 cells were transfected with miR-96-5p inhibitor
(10 nM) for 6 h followed by incubation with ZEN (50 µM) at 37˚C for
an additional 48 h. Following treatment, the cells were fixed in 4%
paraformaldehyde for 30 min at 4˚C, followed by permeabilization
with 0.1% Triton X-100 (Sigma-Aldrich; Merck KGaA) at 4˚C and
blocking with 5% bovine serum album (BSA; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Subsequently, the cells were
incubated with the primary antibodies overnight at 4˚C. The primary
antibodies used were as follows: Ki67 (1:200; cat. no. ab15580;
Abcam) and LC3 (1:200; cat. no. ab192890; Abcam). The following
morning, the cells were washed with PBS and incubated with goat
anti-rabbit secondary antibodies (1:500; cat. no. ab7090; Abcam)
for 1 h at room temperature. Subsequently, the cells were
counterstained with 10 mg/ml DAPI fluorescence for 2 min to detect
the nuclei. The stained cells were visualized and mounted using a
LSM710 confocal fluorescence microscope (Carl Zeiss AG;
magnification, x200).
Monodansylcadaverine (MDC)
staining
After the indicated treatment conditions, the cells
were plated into a 6-well plate (3x106 cells/well) and
cultured overnight, followed by staining with 0.05 mM MDC
(Sigma-Aldrich; Merck KGaA) at 37˚C for 30 min. Following washing
with PBS for three times, the cells were fixed with 4%
paraformaldehyde at room temperature for 10 min and immediately
observed under a fluorescence microscope (magnification, x200).
Dual-luciferase reporter assay
miRDB (http://mirdb.org/) and TargetScan (www.targetscan.org/vert_71) were used to predict
the target of miR-96-5p. The dual luciferase reporter assay was
performed to verify the targeting association between miR-96-5p and
ATG9A. Wild-type (WT) and mutant (MT) sequences of autophagy
related 9A (ATG9A) were synthesized by Shanghai GeneChem Co., Ltd.
The MT and WT 3'-UTR of ATG9A was cloned into a pMIR-REPORT plasmid
(H306; Obio Technology) following the manufacturer's instructions.
Subsequently, the cells were co-transfected with 0.1 µg WT-ATG9A or
MT-ATG9A and miR-96-5p mimics or vector control (50 nM final
concentration) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 48 h of transfection, the Dual-Glo
Luciferase Assay system (Promega Corporation) was used to measure
luciferase activity according to the manufacturer's protocol. The
firefly luciferase activity was normalized to the Renilla
luciferase activity and presented as relative luciferase
activity.
Western blot analysis
Total proteins were obtained from lysates of
cultured cells using RIPA buffer (Shanghai GenePharma Co., Ltd.).
The concentration levels of the proteins were measured with a BCA
protein assay kit (Beyotime Institute of Biotechnology).
Subsequently, total protein (30 µg/lane) was loaded onto SDS gels
(10%) and separated by electrophoresis. The gels were transferred
to PVDF membranes. Following blocking with 5% skimmed milk for 1 h
at room temperature, the membrane was probed with antibodies
against ATG9A (1:1,000; cat. no. ab108338; Abcam), cleaved
caspase-3 (1:1,000; cat. no. ab32042; Abcam), Bcl-2 (1:1,000; cat.
no. ab32124; Abcam) and beclin 1 (1:1,000; cat. no. ab210498;
Abcam) overnight at 4˚C. Subsequently, the membrane was incubated
with appropriate horseradish peroxidase-conjugated anti-rabbit
secondary antibody (1:3,000; cat. no. ab7090; Abcam). The
visualization was performed using an ECL chemiluminescent kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The integrated density of each band
was normalized to that of the corresponding β-actin (1:1,000; cat.
no. ab8226; Abcam) band. ImageJ software (version 2.0; National
Institutes of Health) was used to quantify the intensity of the
bands.
Apoptosis assay
Following the indicated treatment, cells were
harvested and resuspended at a density of 5x105
cells/ml. Subsequently, the cells were centrifuged (300 x g) at
room temperature for 5 min, followed by washing with PBS 3 times. A
total of 1x105 cells were collected in each tube. The
Annexin V-FITC Apoptosis Detection kit (Thermo Fisher Scientific,
Inc.) was used to quantify the percentage of apoptotic cells. The
cell pellets were resuspended in 1 ml Annexin V binding buffer,
followed by staining with 5 µl Annexin V and 10 µl propidium iodide
(PI) for 15 min at room temperature. Flow cytometry analysis (BD
FACS Aria; BD Biosciences) was conducted within 1 h to detect the
orange-red fluorescence of Annexin V/PI. The data was quantified by
FlowJo (v7.6.5; FlowJo LLC).
Statistical analysis
All experiments were performed in triplicate. All
data are presented as mean ± SD. GraphPad Prism 7 (GraphPad
Software, Inc.) was used for statistical analysis. The differences
among multiple groups were analyzed using one-way ANOVA followed by
Tukey's multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Downregulation of miR-96-5p expression
reverses ZEN-induced cytotoxicity in TM3 cells
TM3 cells were cultured with various concentrations
of ZEN (0, 10, 25, 50 and 75 µM) for 48 h and the effect of this
compound on cell viability was evaluated by the CCK-8 assay. The
results indicated that ZEN inhibited the proliferation of TM3 cells
in a dose-dependent manner (Fig.
1A). Treatment of the cells with ZEN (50 µM) resulted in
moderate cell growth inhibition of ~50% (Fig. 1A). Therefore, ZEN was used in the
following experiments at a concentration of 50 µM. At the
concentration of 75 µM, ZEN induced severe toxicity. It has been
previously reported that miR-96-5p is closely associated with the
toxicity of ZEN in Leydig cells (10). Therefore, the expression levels of
miR-96-5p in ZEN-treated TM3 cells were detected by RT-qPCR. ZEN
notably upregulated the expression levels of miR-96-5p in a
dose-dependent manner (Fig. 1B). In
addition, ZEN (50 µM) markedly inhibited the viability of TM3 cells
in a time-dependent manner (Fig.
1C). After 24 h of incubation, ZEN (50 µM) resulted in moderate
cell growth inhibition of ~20%; after 48 h of incubation, ZEN (50
µM) resulted in moderate cell growth inhibition of ~50% (Fig. 1C). Therefore, TM3 cells that were
treated with ZEN (50 µM) for 48 h were utilized in the following
experiments.
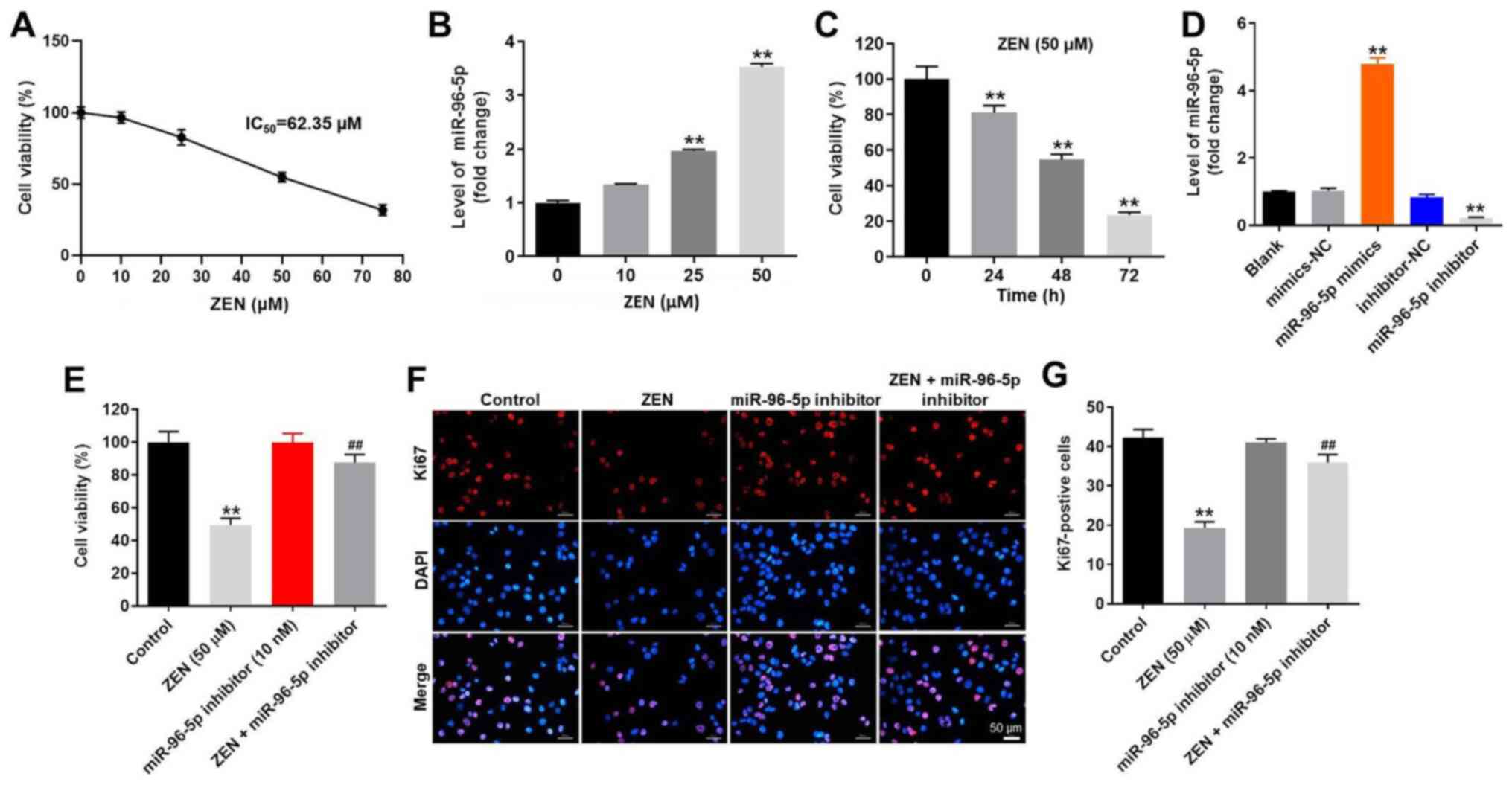 | Figure 1Downregulation of miR-96-5p reversed
ZEN-induced cell viability decline in TM3 cells. (A) TM3 cells were
treated with different concentrations of ZEN (0, 10, 25, 50 and 75
µM) for 48 h. CCK-8 assay was used to evaluate cell viability. (B)
TM3 cells were treated with different concentrations of ZEN (10,
25, 50 and 75 µM) for 48 h. TM3 cells treated with PBS was used as
control. The level of miR-96-5p was determined by RT-qPCR. (C) TM3
cells were treated with 50 µM ZEN for 0, 24, 48 and 72 h. CCK-8
assay was used to evaluate cell viability. (D) TM3 cells were
transfected with NC, miR-96-5p mimics and miR-96-5p inhibitor,
respectively. The level of miR-96-5p was determined by RT-qPCR. (E)
The cells were treated with ZEN (50 µM), miR-96-5p inhibitor (10
nM) and ZEN + miR-96-5p inhibitor (10 nM), respectively. (F) Ki67
staining indicated cell proliferation. Magnification, x200. (G)
Ki67-positive cells were counted. **P<0.01 vs.
control group; ##P<0.01, vs. ZEN (50 µM) treated
group. Control group, vehicle control. miR, microRNA; ZEN,
zearalenone; CCK-8, Cell Counting Kit-8; NC, negative control;
RT-qPCR, reverse transcription-quantitative PCR. |
In order to assess the function of miR-96-5p
further, TM3 cells were transfected with miR-96-5p mimics,
miR-96-5p inhibitor or NC sequences. The efficiency of transfection
was evaluated using RT-qPCR and the data indicated that TM3 cells
were successfully transfected with miR-96-5p mimics or inhibitor
(Fig. 1D). In addition, the results
of the CCK-8 assay indicated that ZEN (50 µM) resulted in a
significant reduction of cell viability (Fig. 1E), while the miR-96-5p inhibitor (10
nM) exhibited no significant effect on cell viability (Fig. 1D). In addition, the decrease in cell
viability induced by ZEN was remarkably attenuated by the miR-96-5p
inhibitor (Fig. 1E). Furthermore,
the results of Ki67 staining indicated that the miR-96-5p inhibitor
efficiently reversed the decrease in cell proliferation induced by
ZEN in TM3 cells (Fig. 1F and
G). Taken together, the results
indicated that downregulation of miR-96-5p expression significantly
attenuated ZEN-induced cytotoxicity in TM3 cells.
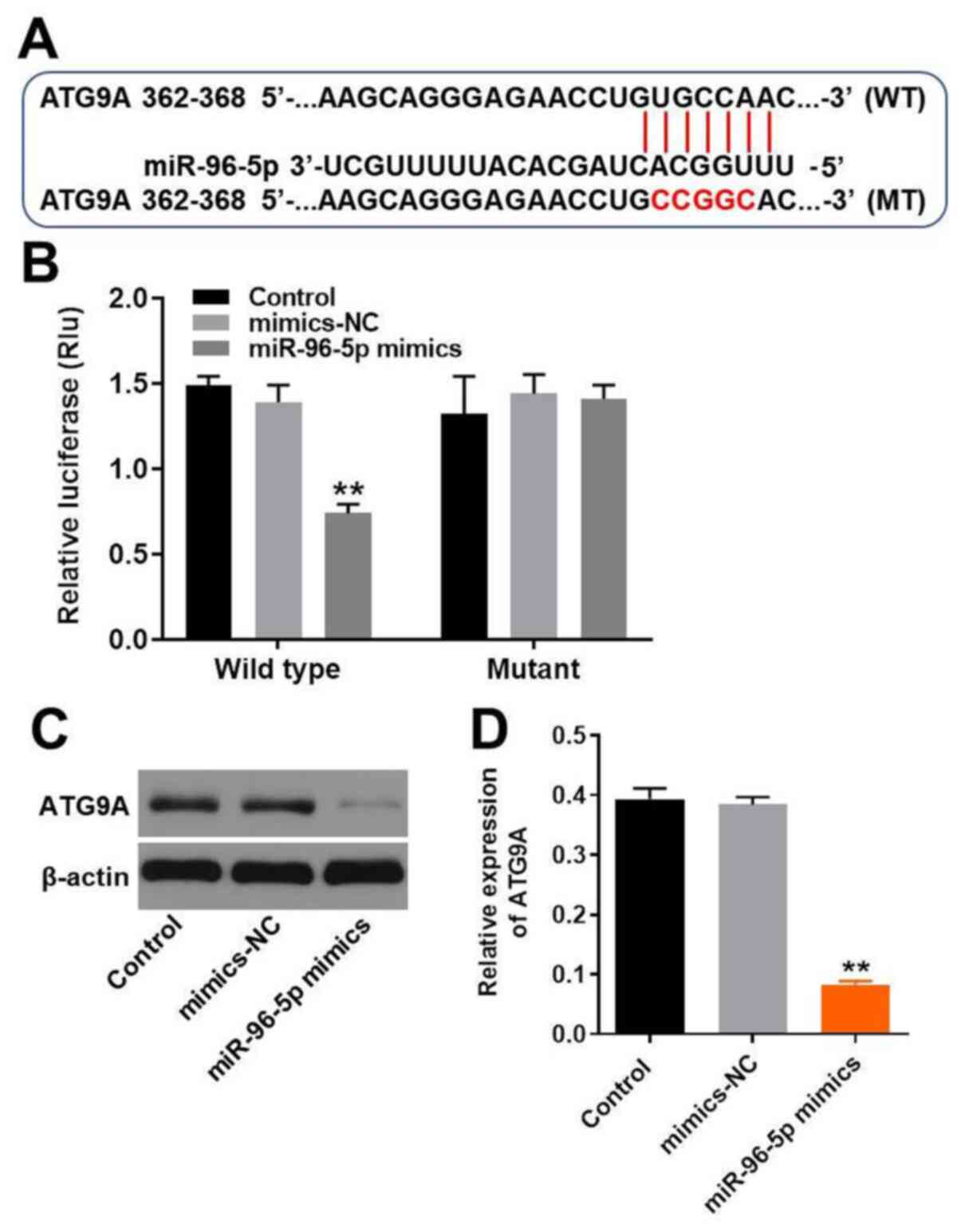
ATG9A is the target of miR-96-5p
The target of miR-96-5p was predicted using the
online databases miRDB (http://www.mirdb.org/) and TargetScan (http://www.targetscan.org/vert_72/). Accordingly
to previous studies, miR-96-5p was reported to have a closed
association with autophagy (11,13,14).
Thus, the present study focused on analyzing autophagy-associated
genes and found that ATG9A was the putative target of miR-96-5p
(Fig. 2A). Subsequently, the
association between miR-96-5p and ATG9A was verified by
dual-luciferase reporter assays. The relative luciferase activity
of the cells co-transfected with wild type ATG9A and miR-96-5p
mimics was markedly decreased (Fig.
2B). This result demonstrated that miR-96-5p was able to bind
directly to the WT 3'-UTR of ATG9A. Therefore, ATG9A was confirmed
as a direct target of miR-96-5p. In addition, western blot analysis
was used to validate the association between miR-96-5p and ATG9A.
The expression levels of ATG9A were downregulated by miR-96-5p
mimics, confirming that ATG9A was a direct target of miR-96-5p
(Fig. 2C and D). Since ATG9A is a transmembrane protein
and plays an essential role in autophagy (15), the induction of autophagy in TM3
cells was investigated in the subsequent experiments.
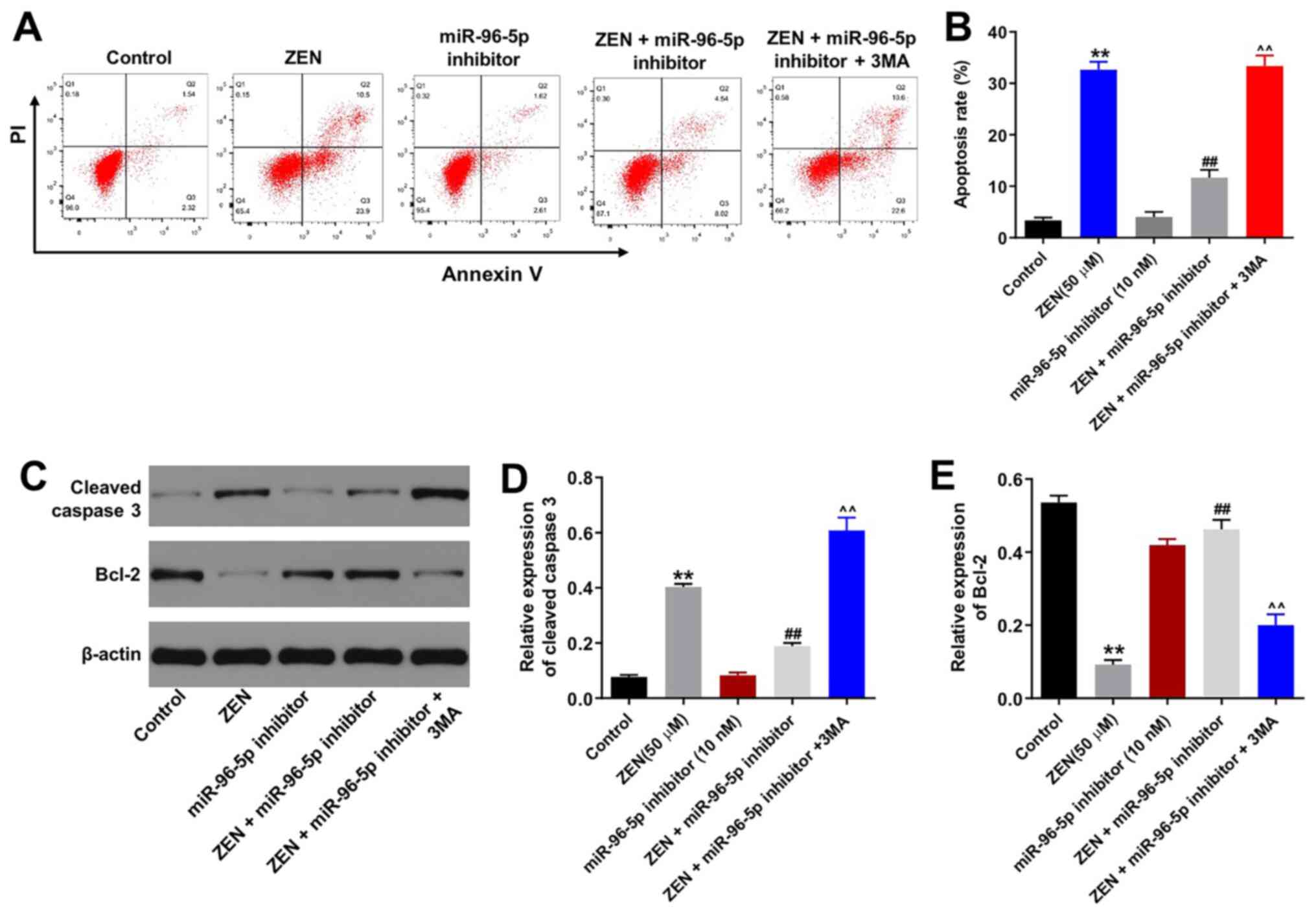
Downregulation of miR-96-5p expression
protects TM3 cells from ZEN-induced apoptosis
Subsequently, the induction of TM3 cell apoptosis
was detected by Annexin V/PI staining. ZEN (50 µM) induced TM3 cell
apoptosis (Fig. 3A and B), whereas the miR-96-5p inhibitor had no
effect on cell apoptosis (Fig. 3A
and B). In addition, downregulation
of miR-96-5p expression markedly decreased ZEN-induced cell
apoptosis. It is interesting to note that the protective effect of
the miR-96-5p inhibitor against ZEN-induced apoptosis was abolished
by 3MA, which is an autophagy inhibitor. Moreover, the expression
levels of the apoptosis-associated proteins (cleaved caspase-3 and
Bcl-2) were evaluated by western blot analysis. The results
indicated that ZEN-induced upregulation of cleaved caspase-3
expression was reversed by miR-96-5p inhibitor transfection in the
cells (Fig. 3C-E). Similarly, the
ZEN-induced decrease in Bcl-2 expression levels was reversed by
miR-96-5p inhibitor transfection in the cells (Fig. 3C-E). Moreover, the effects of the
miR-96-5p inhibitor on cleaved caspase-3 and Bcl-2 expression
levels were eliminated in the presence of 3MA, which was consistent
with the aforementioned findings. Taken together, the results
indicated that downregulation of miR-96-5p expression protected TM3
cells against ZEN-induced apoptosis by regulating autophagy.
Downregulation of miR-96-5p expression
protects TM3 cells against ZEN cytotoxicity by promoting
autophagy
In order to confirm the association between the
miR-96-5p inhibitor and the induction of cell autophagy, MDC
staining was performed. ZEN (50 µM) decreased the number of
autophagosomes in TM3 cells, whereas this effect was reversed by
transfection of the cells with the miR-96-5p inhibitor (Fig. 4A and B). As expected, the autophagy-promoting
effect of the miR-96-5p inhibitor was neutralized by 3MA (Fig. 4A and B). Furthermore, the expression levels of
the autophagy-associated proteins (ATG9A and beclin 1) were
assessed (Fig. 4C-E) (16). ZEN (50 µM) significantly decreased
the expression levels of ATG9A and Beclin 1 (Fig. 4C-E), while the miR-96-5p inhibitor
(10 nM) triggered the upregulation of ATG9A expression compared
with the control group (Fig. 4C).
Moreover, the inhibition of miR-96-5p reversed ZEN-induced decrease
in ATG9A and Beclin 1 expression levels (Fig. 4C-E). Similarly, the effects of the
miR-96-5p inhibitor were neutralized by 3MA (Fig. 4C-E). In addition to MDC staining,
LC3 staining was used to confirm the induction of autophagy in
ZEN-treated TM3 cells. ZEN-induced decline in autophagy was
ameliorated by treatment of the cells with the miR-96-5p inhibitor,
while the enhancement of autophagy by the miR-96-5p inhibitor was
abrogated following 3MA treatment (Fig.
5A and B). Taken together, the
data demonstrated that downregulation of miR-96-5p expression
protected TM3 cells against ZEN cytotoxicity by promoting
autophagy.
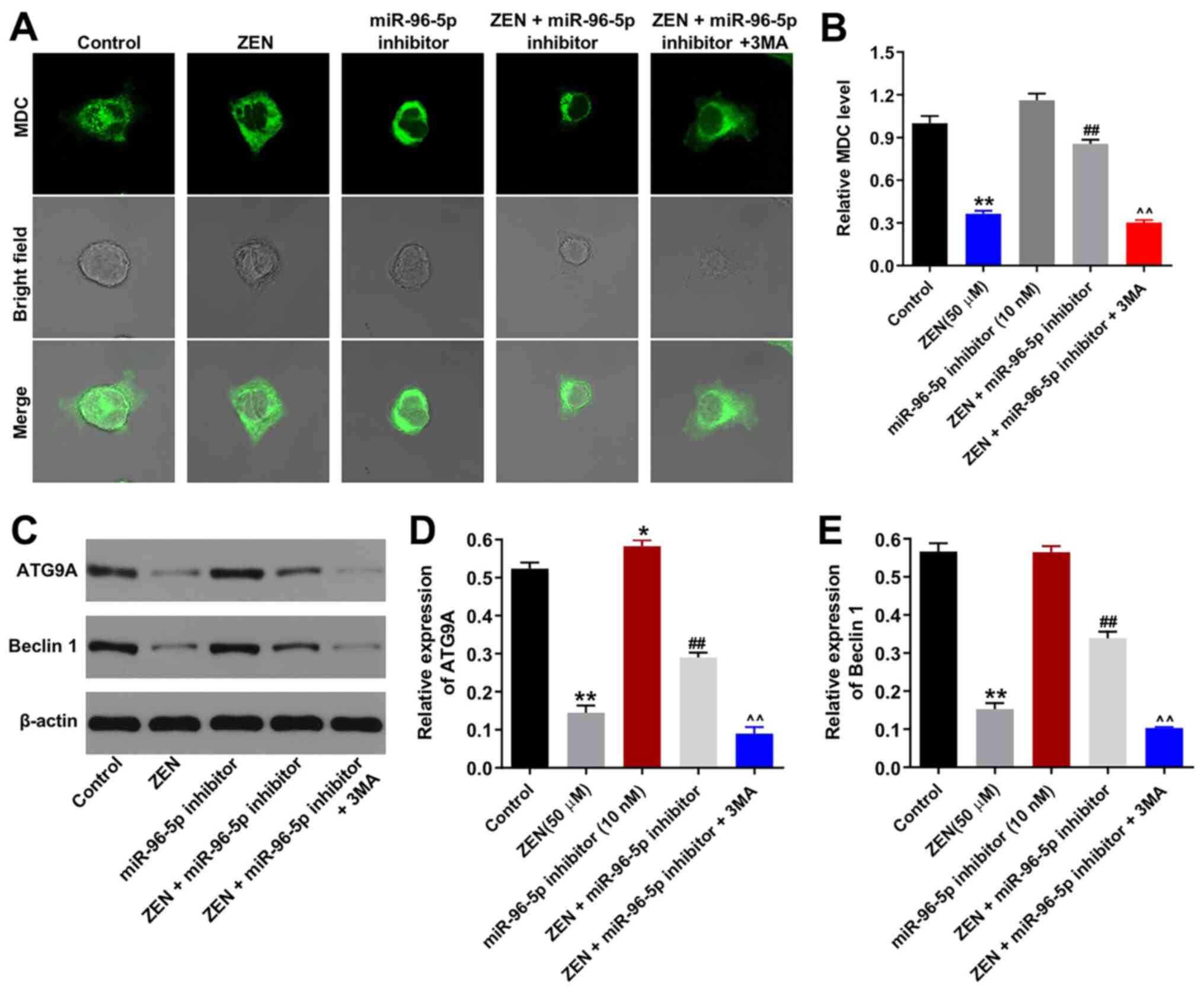 | Figure 4Downregulation of miR-96-5p protects
TM3 cells against ZEN cytotoxicity via promoting autophagy. TM3
cells were transfected with miR-96-5p inhibitor (10 nM) for 6 h,
then cells were treated with 50 µM ZEN and/or 5 mM 3MA for 48 h.
(A) MDC-stained autophagosome in TM3 cells from each group.
Magnification, x400. (B) MDC-positive autophagosomes were counted.
(C) The expression levels of ATG9A and beclin 1 were detected using
western blotting and (D and E) quantified. ##P<0.01,
vs. ZEN (50 µM) group. ^^P<0.01, vs. ZEN + miR-96
inhibitor group. *P<0.05, **P<0.01, vs.
Control group, vehicle + inhibitor-NC. miR, microRNA; NC, negative
control; ZEN, zearalenone; 3MA, 3-methyladenine; MDC,
monodansylcadaverine; ATG9A, autophagy related 9A. |
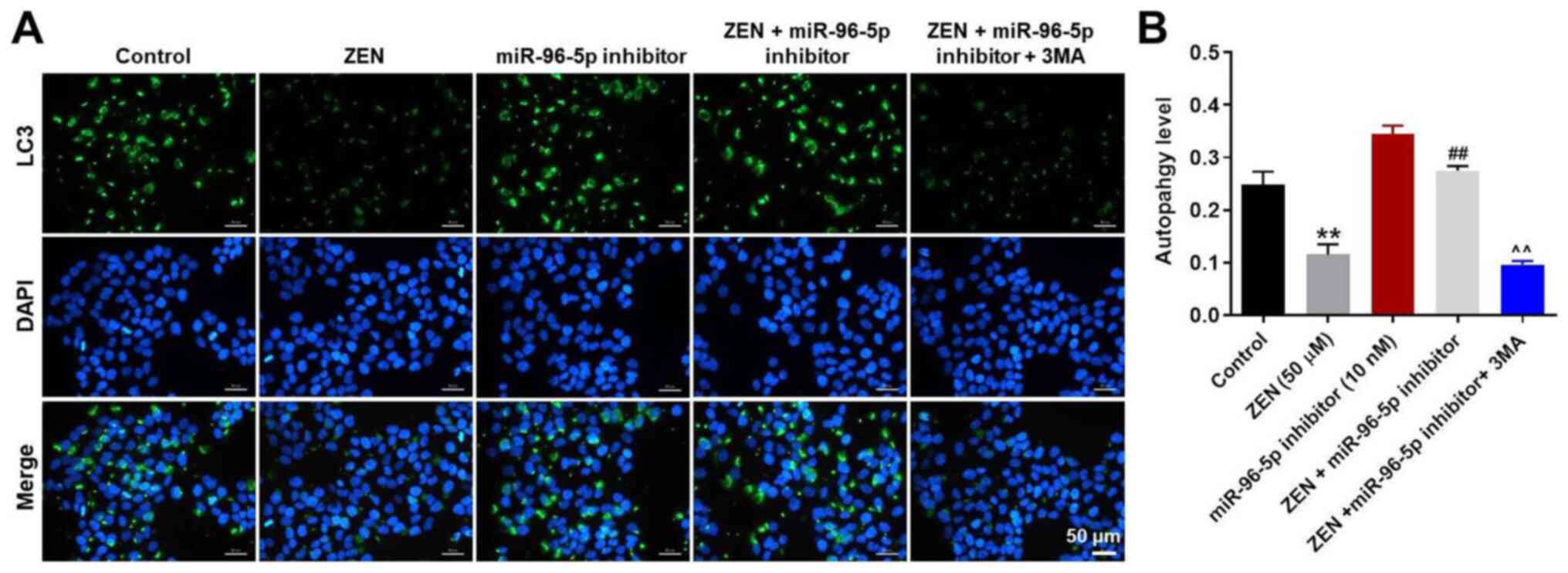 | Figure 5miR-96-5p inhibitor-induced autophagy
in TM3 cells is inhibited by 3MA treatment. TM3 cells were
transfected with miR-96-5p inhibitor (10 nM) for 6 h, then cells
were treated with 50 µM ZEN and/or 5 mM 3MA for 48 h. (A)
LC3-stained autophagosome in TM3 cells was detected with
fluorescence microscope. Magnification, x200. (B) LC3-positive
autophagosomes were counted. **P<0.01, vs. Control.
##P<0.01, vs. ZEN (50 µM) group.
^^P<0.01, vs. ZEN + miR-96 inhibitor group. Control
group, vehicle + inhibitor-NC. miR, microRNA; NC, negative control;
ZEN, zearalenone; 3MA, 3-methyladenine. |
Discussion
The insufficiency and dysfunction of Leydig cells is
associated with male infertility (4). Increasing sperm production or motility
by optimizing testosterone production from Leydig cells is the
typical medical therapy (17). In
the present study, downregulation of miR-96-5p expression levels
protected TM3 Leydig cells against ZEN-induced toxicity, providing
a potential new biomarker for male infertility.
In the present study, the data demonstrated that
downregulation of miR-96-5p expression protected TM3 cells against
ZEN-induced toxicity via promoting autophagy. A previous study
conducted by Yu et al (11)
demonstrated that inhibition of miR-96-5p expression promoted
autophagy in the human hepatic stellate cell line LX-2 via the
upregulation of ATG7. Moreover, Shi et al (14) demonstrated that the overexpression
of miR-96-5p was able to inhibit autophagy in the human breast
cancer cell lines MCF-7 and MDA-MB-231 through downregulation of
FOXO1. In addition, Ma et al (18) indicated that miR-96 could induce the
autophagy in prostate cancer cells via inhibition of mTOR. The
aforementioned studies indicated that miR-96-5p could regulate
autophagy via the regulation of several autophagy-associated genes
(such as ATG7 and mTOR) or autophagy-associated signaling molecules
(such as FOXO1). The present study demonstrated that ATG9A was a
direct binding target of miR-96-5p. ATG9A is a membrane protein,
that is essential for autophagy (19). Downregulation of miR-96-5p
significantly induced the autophagy of ZEN-treated TM3 cells via
the upregulation of ATG9A. However, one miRNA can regulate several
mRNAs, thus further studies are needed to investigate whether
miR-96-5p could regulate the progression of male infertility via
targeting other gene targets.
Furthermore, the present study data found that
miR-96-5p inhibitor could suppress the apoptosis of ZEN-treated TM3
cells. However, the inhibitory effects of miR-96-5p inhibitor on
apoptosis in ZEN-treated TM3 cells were reversed by the treatment
with 3MA. The results suggested that downregulation of miR-96-5p
could inhibit apoptosis in ZEN-treated TM3 cells via inducing
autophagy. In addition, the data demonstrated that the miR-96-5p
inhibitor alone exhibited no influence on the induction of cell
apoptosis; thus, miR-96-5p inhibitor did not regulate cell
apoptosis directly. In addition, rescue experiments demonstrated
that the miR-96-5p inhibitor protected TM3 cells against
ZEN-induced cytotoxicity by promoting autophagy. The enhanced
induction of autophagy by the miR-96-5p inhibitor may aid the
maintenance of cell homeostasis and enable the cells to survive
under adverse conditions, such as in the case of ZEN-induced
toxicity (20).
It is interesting to note that the changes in the
expression levels of cleaved caspase-3 and Bcl-2 were inconsistent
(Fig. 3D and E). It was deduced that cleaved caspase-3
may be activated by another pathway in addition to the typical
mitochondrial pathway of apoptosis. However, the specific pathway
by which cleaved caspase-3 was activated remained unclear.
In the present study, the data demonstrated that
downregulation of miR-96-5p expression reversed ZEN-induced
cytotoxicity in TM3 cells by targeting ATG9A. When the expression
of miR-96-5p was downregulated in TM3 cells, ATG9A-associated
autophagy was increased. The findings suggested that miR-96-5p may
serve as a potential biomarker for male infertility.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Natural Science Foundation
of Guangdong Province (grant no. 2016A030310075).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX conceived and supervised the study. LX, XYX and
XHX designed the study. XHX and YZ performed the experiments and
analyzed the data. LX and XYX confirmed the authenticity of all the
raw data. All authors reviewed the results and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zegers-Hochschild F, Adamson GD, de Mouzon
J, Ishihara O, Mansour R, Nygren K, Sullivan E and van der Poel S:
International Committee for Monitoring Assisted Reproductive
Technology; World Health Organization. The International Committee
For Monitoring Assisted Reproductive Technology (ICMART) and the
World Health Organization (WHO) revised glossary on ART
terminology, 2009. Hum Reprod. 24:2683–2687. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Okutman O, Rhouma MB, Benkhalifa M, Muller
J and Viville S: Genetic evaluation of patients with non-syndromic
male infertility. J Assist Reprod Genet. 35:1939–1951.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stojanov M, Baud D, Greub G and Vulliemoz
N: Male infertility: The intracellular bacterial hypothesis. New
Microbes New Infect. 26:37–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Winters SJ, Moore JP Jr and Clark BJ:
Leydig cell insufficiency in hypospermatogenesis: A paracrine
effect of activin-inhibin signaling? Andrology. 6:262–271.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eze UA, Huntriss J, Routledge MN and Gong
YY: Toxicological effects of regulated mycotoxins and persistent
organochloride pesticides: In vitro cytotoxic assessment of single
and defined mixtures on MA-10 murine Leydig cell line. Toxicol In
vitro. 48:93–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boeira SP, Filho CB, Del'Fabbro L, Royes
LF, Jessé CR, Oliveira MS and Furian AF: Possible role for
glutathione-S-transferase in the oligozoospermia elicited by acute
zearalenone administration in Swiss albino mice. Toxicon.
60:358–366. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou M, Yang L, Shao M, Wang Y, Yang W,
Huang L, Zhou X, Jiang S and Yang Z: Effects of zearalenone
exposure on the TGF-β1/Smad3 signaling pathway and the expression
of proliferation or apoptosis related genes of post-weaning gilts.
Toxins (Basel). 10(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao H, Wen H, Cao C, Dong D, Yang C, Xie
S, Zhang J, Huang X, Huang X, Yuan S and Dong W: Overexpression of
MicroRNA-10a in germ cells causes male infertility by targeting
Rad51 in mouse and human. Front Physiol. 10(765)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Wang Z and Gemeinhart RA:
Progress in microRNA delivery. J Control Release. 172:962–974.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang M, Wu W, Li L, He J, Huang S, Chen S,
Chen J, Long M, Yang S and Li P: Analysis of the miRNA expression
profiles in the zearalenone-exposed TM3 leydig cell line. Int J Mol
Sci. 20(635)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu K, Li N, Cheng Q, Zheng J, Zhu M, Bao
S, Chen M and Shi G: miR-96-5p prevents hepatic stellate cell
activation by inhibiting autophagy via ATG7. J Mol Med (Berl).
96:65–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mao Z, Yao M, Li Y, Fu Z, Li S, Zhang L,
Zhou Z, Tang Q, Han X and Xia Y: miR-96-5p and miR-101-3p as
potential intervention targets to rescue TiO2 NP-induced
autophagy and migration impairment of human trophoblastic cells.
Biomater Sci. 6:3273–3283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shi Y, Zhao Y, Shao N, Ye R, Lin Y, Zhang
N, Li W, Zhang Y and Wang S: Overexpression of microRNA-96-5p
inhibits autophagy and apoptosis and enhances the proliferation,
migration and invasiveness of human breast cancer cells. Oncol
Lett. 13:4402–4412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Judith D and Tooze SA: ATG9A supplies
PtdIns4P to the autophagosome initiation site. Autophagy.
15:1660–1661. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nishikawa M, Miyake H, Liu B and Fujisawa
M: Expression pattern of autophagy-related markers in
non-metastatic clear cell renal cell carcinoma: Association with
disease recurrence following radical nephrectomy. J Cancer Res Clin
Oncol. 141:1585–1591. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dabaja AA and Schlegel PN: Medical
treatment of male infertility. Transl Androl Urol. 3:9–16.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y,
Kong XM and Huang YR: Biphasic regulation of autophagy by miR-96 in
prostate cancer cells under hypoxia. Oncotarget. 5:9169–9182.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kong Y, Huang T, Zhang H, Zhang Q, Ren J,
Guo X, Fan H and Liu L: The lncRNA NEAT1/miR-29b/Atg9a axis
regulates IGFBPrP1-induced autophagy and activation of mouse
hepatic stellate cells. Life Sci. 237(116902)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kasprowska-Liśkiewicz D: The cell on the
edge of life and death: Crosstalk between autophagy and apoptosis.
Postepy Hig Med Dosw (Online). 71:825–841. 2017.PubMed/NCBI View Article : Google Scholar
|