Introduction
Systemic lupus erythematosus (SLE) is a serious
chronic autoimmune disease characterized by loss of tolerance to
autoantigens, a variety of immune abnormalities and high titer of
autoantibodies against nuclear components (1). It is a highly heterogeneous disease
and different patients may have distinct symptoms and clinical
characteristics (2). The
pathogenesis of SLE is complex and it is thought that genetic
susceptibilities and environmental factors have a key role in the
development of SLE (3). Lupus
nephritis (LN), one of the most serious manifestations of SLE, is
the major cause of a large number of SLE-related deaths (4,5). In
the internationally recognized SLE classification standard,
complement component 3 (C3) and C4 are among the immunological
diagnostic items for SLE (6,7).
Although plasma levels of C3 and C4 are of certain diagnostic value
for SLE, they are not specific diagnostic indicators (8). Therefore, it is critical to identify
more diagnostic indicators and therapeutic targets for SLE.
The importance of non-coding RNAs (ncRNAs), such as
microRNAs (miRNAs), has been emphasized in numerous biological and
pathological processes (9). Several
studies have demonstrated the feasibility of using miRNAs in bodily
fluids as a biomarker for the diagnosis of SLE (10,11).
Although miRNAs have an important role in SLE, they account for
only a small fraction of the non-coding region of the mammalian
genome. Unlike miRNAs, long non-coding RNAs (lncRNAs) are highly
expressed, including large intergenic ncRNAs (lincRNAs) (12). LincRNAs have been indicated to have
an important role in the course of autoimmune diseases and studies
on rheumatoid arthritis (RA) (13)
and autoimmune thyroid diseases (14) have provided evidence to confirm this
hypothesis. The relationship between SLE and lincRNAs has been a
research hotspot and has been preliminarily explored in several
reports. Wu et al (15)
reported that the expression levels of linc0597 and linc0949 in
peripheral blood mononuclear cells of patients with SLE were
significantly downregulated compared with those of healthy
controls, suggesting that linc0597 and linc0949 may be biomarkers
for the diagnosis of SLE. Zheng et al (16) determined that soluble TNF receptor 1
(sTNF-R1 and linc0597 may serve as biomarkers for the diagnosis of
LN and were associated with disease activity in SLE. In another
study of 24 patients with SLE, the lncRNA growth arrest-specific 5
was identified as a potential biomarker for SLE (17). Li et al (18) measured the expression of lincRNAs by
reverse transcription-quantitative PCR (RT-qPCR) after 8 h of
treatment of THP-1 macrophages with Pam3-Cys-Ser-Lys4 (Pam3CSK4).
Among them, there was a clear decrease in the expression levels of
linc7190 and linc0597, while the expression of linc8986 increased
significantly. In addition, linc7190 was indicated to have a
regulatory effect on the secretion of interleukin-6 (IL-6) and
TNF-α, both of which have been indicated to be involved in the
pathogenesis of SLE (19).
There is evidence that lincRNAs are stable in human
plasma. In the present study, the expression levels of three
lincRNAs, namely linc7190, linc0597 and linc8986, we measured in
the plasma of patients with SLE and their relationship with the
clinical characteristics and organ damage status of the patients
was analyzed. The present study provided a basis for the use of new
potential biomarkers for the diagnosis and treatment of SLE.
Materials and methods
Patients and healthy controls
A total of 54 patients with SLE, 24 patients with RA
and 24 patients with Sjögren's syndrome (SS) were enrolled at the
Department of Rheumatology and Immunology of the First Affiliated
Hospital of Harbin Medical University (Harbin, China), as well as
22 healthy individuals matched to the patients with SLE for sex and
age from the Physical Examination Center at the First Affiliated
Hospital of Harbin Medical University (Harbin, China) between April
2018 and October 2018. Demographic characteristics, disease
activity and laboratory parameters were collected from the
patients' medical records. All patients with SLE were diagnosed
according to the American College of Rheumatology (ACR) 1997
revised criteria (20). Renal
damage in SLE was defined by the ACR standard and includes any one
of the following: i) Persistent proteinuria ≥0.5 g/day; ii)
presence of active tubular cells; iii) biopsy evidence of LN. RA
was diagnosed according to the ACR/European League Against
Rheumatism 2010 classification criteria (21). SS was diagnosed in accordance with
the revised 2002 American-European criteria (22). The exclusion criteria for all
patients were as follows: i) Patients with malignant tumors; ii)
patients with acute infection within one month prior to admission;
and iii) patients with other autoimmune diseases.
The present study was approved by the Research
Ethics Committee of the First Affiliated Hospital of Harbin Medical
University (Harbin, China). All participants included in the study
provided written informed consent.
Obtainment of peripheral blood samples
and RNA processing
Blood samples from each donor were collected in
EDTA-anticoagulant tubes. The plasma was separated by
centrifugation at 1,500 x g for 10 min at room temperature,
followed by high-speed centrifugation at 12,000 x g for 10 min at
room temperature to completely remove cell debris. The supernatant
plasma was recovered and stored at -80˚C until further analysis.
Total RNA was extracted from 400 µl plasma by TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The total RNA concentration was
measured using a spectrophotometer (Thermo Fisher Scientific, Inc.)
and 200-600 ng of total RNA was obtained from 400 µl plasma.
RT-qPCR
The PrimeScript™ RT Kit (Takara Bio Inc.) was used
to eliminate genomic DNA from the RNA samples and to
reverse-transcribe RNA into cDNA. To quantify the expression of the
three lincRNAs (linc0597, lin8986 and linc7190), cDNA was amplified
by PCR using a SYBR Green kit (SYBR® PremixEx Taq™ II;
Takara Bio, Inc.) in 20-µl reactions containing 10 µl SYBR Green,
0.4 µl ROX Reference Dye II, 0.2 µM forward primer, 0.2 µM reverse
primer, 6 µl sterile deionized water and 2 µl cDNA. The relative
expression level of each lincRNA was normalized to GAPDH (23). The following thermocycling
conditions were used for PCR: 95˚C for 1 min, followed by 40 cycles
of 95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 1 min. After the
reaction, the quantification cycle (Cq) value was determined using
a fixed threshold setting. The relative expression of the lincRNAs
was calculated using the 2-ΔΔCq method (24). The PCR primer sequences were as
follows: Linc0597 forward, TTGGATTCATCCCGTTCACCTCCA and reverse,
AAAGAAGCAGGACTACCCACT; linc8986 forward, ATCTTGGCCCACAGAGGAGGAAAT
and reverse, ATTCCCAGTGACTGCACTGAAGGT; linc7190 forward,
CTGCTTTGGAGCAGTTGGGAACTT and reverse, AGACAGGTTTGCTGACGAAGGTCT; and
GAPDH forward, CCAACATGCTGACTCACCCTTCC and reverse,
ATGGAGTCTCGCTCTGTCACCCA.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software version 25.0 (IBM Corporation). Values are
expressed as the mean ± standard deviation. The t-test was used to
compare age and the Fisher's exact test was used to compare sex
between patients with SLE and healthy controls. The nonparametric
Mann-Whitney U-test was used to compare differences in the
expression of each lincRNA within two groups. The nonparametric
Kruskal-Wallis test was used to compare data among three groups.
Spearman's rank correlation analysis was used to analyze the
correlation between lincRNAs and clinical characteristics and to
calculate the correlation coefficient. Receiver operating
characteristic (ROC) curve analysis was performed by MedCalc
version 18.9 (MedCalc Software bvba). P<0.05 was considered to
indicate statistical significance.
Results
Identification of candidate lincRNAs
in SLE
The expression levels of three unique lincRNAs,
namely linc0597, linc8986 and linc7190, in human plasma were
analyzed in 54 patients with SLE (50 females and 4 males; mean age,
39.4±6.3 years) and 22 healthy donors (19 females and 3 males; mean
age, 35.9±5.5 years) using RT-qPCR. The mean age and sex
distribution did not differ significantly between the patients with
SLE and the healthy donors (Table
I). As presented in Fig. 1A and
B, patients with SLE had
significantly higher levels of linc0597 and linc8986 than the
healthy controls (both P<0.001). However, the difference in
linc7190 levels between patients with SLE and the healthy donors
was not significant (P=0.052; Fig.
1C). In addition, in order to determine whether there is an
association between lincRNA levels and LN, the patients with SLE
were divided into an LN group (n=24) and a non-LN group (n=30). The
results indicated that the expression level of linc0597 in the LN
and the non-LN groups were significantly higher compared with the
healthy controls (P<0.001 and P=0.006) and the linc0597 in the
LN group was significantly higher than that in the non-LN group
(P=0.044; Fig. 1D). In addition,
the levels of linc8986 in the LN and the non-LN groups were
significantly higher compared with those in the healthy controls
(P<0.001), but there was not significantly different between the
LN and non-LN groups (P=0.523; Fig.
1E). The levels of linc7190 in the LN and the non-LN groups
were not significantly different compared with the healthy controls
(P>0.05), and there was also no significant difference between
the LN and non-LN groups (P=0.122; Fig.
1F). To further evaluate the diagnostic capacity of linc0597
and linc8986, 24 patients with RA and 24 patients with SS were
selected for analysis and comparison with the healthy control
group. As presented in Fig. 1G and
H, no significant difference in the
plasma levels of linc0597 and linc8986 was observed between the
patients with RA, the patients with SS and the healthy controls
(P=0.468 and P=0.674, respectively). Overall, the results suggested
that increased levels of linc0597 and linc8986 in plasma may be
associated with SLE.
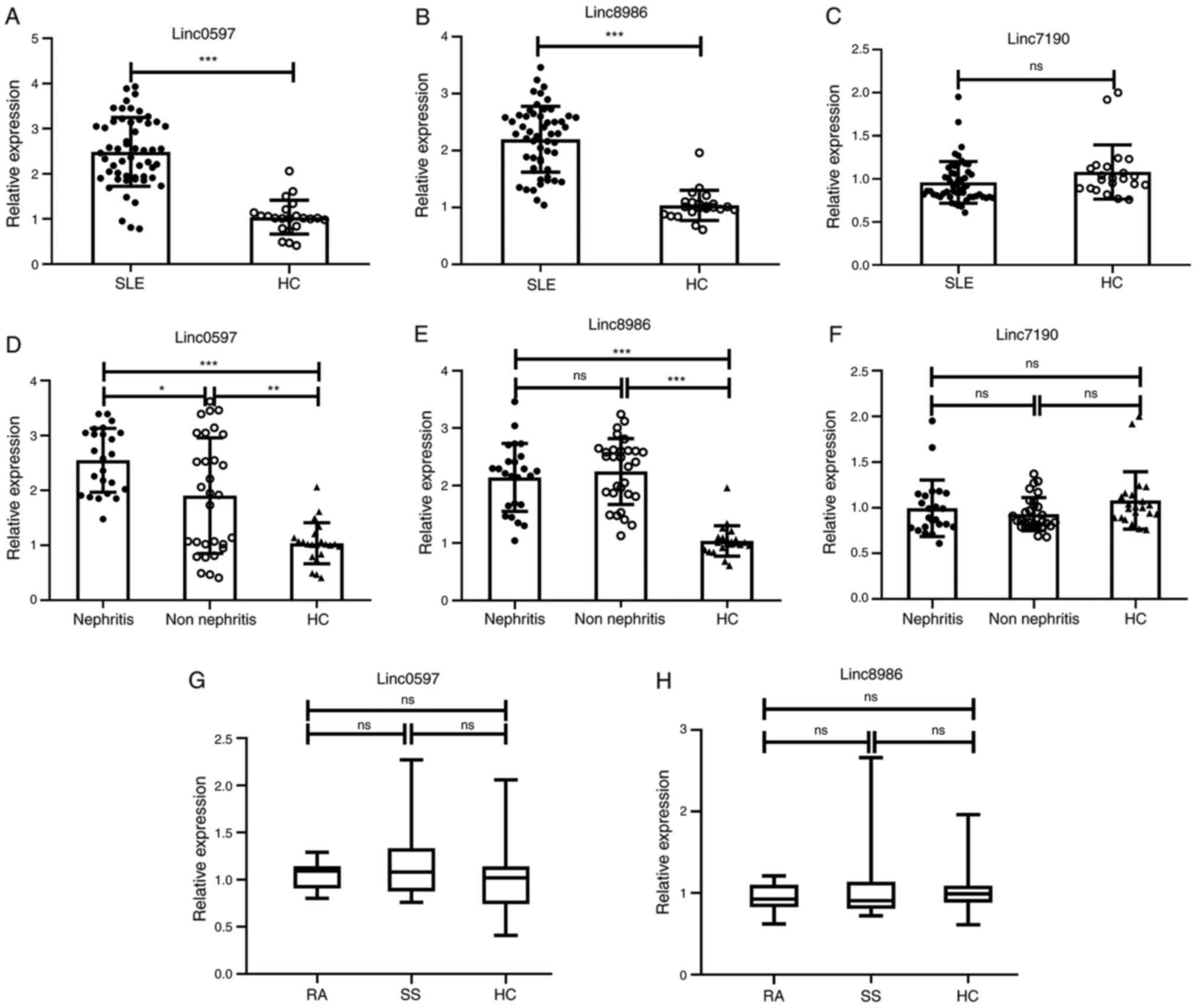 | Figure 1Validation of candidate lncRNAs
(linc0597, linc8986 and linc7190) identified in plasma of patients
with SLE and HCs. The expression levels of the three candidate
lncRNAs in 54 patients with SLE and 24 HCs were analyzed by reverse
transcription-quantitative PCR and normalized to GAPDH. (A)
Increased expression of linc0597 in total patients with SLE vs.
HCs. (B) Increased expression of linc8986 in total patients with
SLE vs. HCs. (C) Expression of linc7190 in total patients with SLE
vs. HCs. (D) Increased expression of linc0597 in patients with SLE
with and without nephritis vs. HCs. (E) Increased expression of
linc8986 in patients with SLE with and without nephritis vs. HCs.
(F) Expression of linc7190 in patients with SLE with and without
nephritis vs. HCs. Each symbol represents an individual subject;
horizontal lines indicate median values. (G) Expression of linc0597
in patients with RA vs. patients with SS vs. HCs. (H) Expression of
linc8986 in patients with RA vs. patients with SS vs. HCs. The
three horizontal lines in the figure indicate the upper quartile,
the median and the lower quartile values, respectively.
*P<0.05, **P<0.01,
***P<0.001. ns, no significance; HC, healthy control;
SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS,
Sjögren's syndrome; lncRNA, long non-coding RNA; linc, large
intergenic non-coding RNA. |
 | Table IClinical features of patients with
systemic lupus erythematosus. |
Table I
Clinical features of patients with
systemic lupus erythematosus.
| Characteristic | HC (n=22) | Patients
(n=54) | P-value |
|---|
| Age, years | 35.9±5.5 | 39.4±6.3 | 0.245 |
| Sex
(male/female) | 3/19 | 4/50 | 0.406 |
| Anti-dsDNA
(P/N)a | - | 27/22 | |
| Disease duration,
years | - | 2.5±1.5 | |
| LN (P/N) | - | 24/30 | |
| ESR
(P/N)a | - | 37/14 | |
| C3 level,
mg/dla | | | |
|
<80 | - | 38 | |
|
≥80 | - | 13 | |
| Methylprednisolone
dosea, mg/day | | | |
|
≥30 | - | 33 | |
|
<30 | - | 19 | |
Correlation between the candidate
lincRNAs and clinical features of SLE
To determine whether the experssion levels of
lincRNA was related to clinical features, autoantibody profiles and
hormone therapy, we analyzed the correlation of the expression of
plasma linc0597 and linc8986 with clinical parameters of patients
with SLE (Table II). The
expression levels of linc0597 and linc8986 were both negatively
correlated with C3 levels (P=0.004 and P<0.001, respectively;
Table II) and the expression
levels of linc0597 was positively correlated with erythrocyte
sedimentation rate (ESR) (P=0.039; Table II).
 | Table IICorrelation of the expression of
plasma linc0597 and linc8986 with clinical parameters of patients
with systemic lupus erythematosus. |
Table II
Correlation of the expression of
plasma linc0597 and linc8986 with clinical parameters of patients
with systemic lupus erythematosus.
| | Linc0597 | Linc8986 |
|---|
| Clinical
feature | N | rs | P-value | rs | P-value |
|---|
| C3 | 51 | -0.380 | 0.004 | -0.565 | <0.001 |
| Anti-dsDNA | 51 | 0.127 | 0.116 | 0.114 | 0.545 |
| Prednisone | 52 | 0.076 | 0.339 | 0.174 | 0.194 |
| ESR | 51 | 0.291 | 0.039 | 0.143 | 0.051 |
| Age | 54 | 0.02 | 0.783 | 0.077 | 0.294 |
| Duration of
disease | 54 | 0.128 | 0.081 | 0.075 | 0.308 |
Association between the expression
levels of linc0597 and clinical characteristics in patients with
SLE
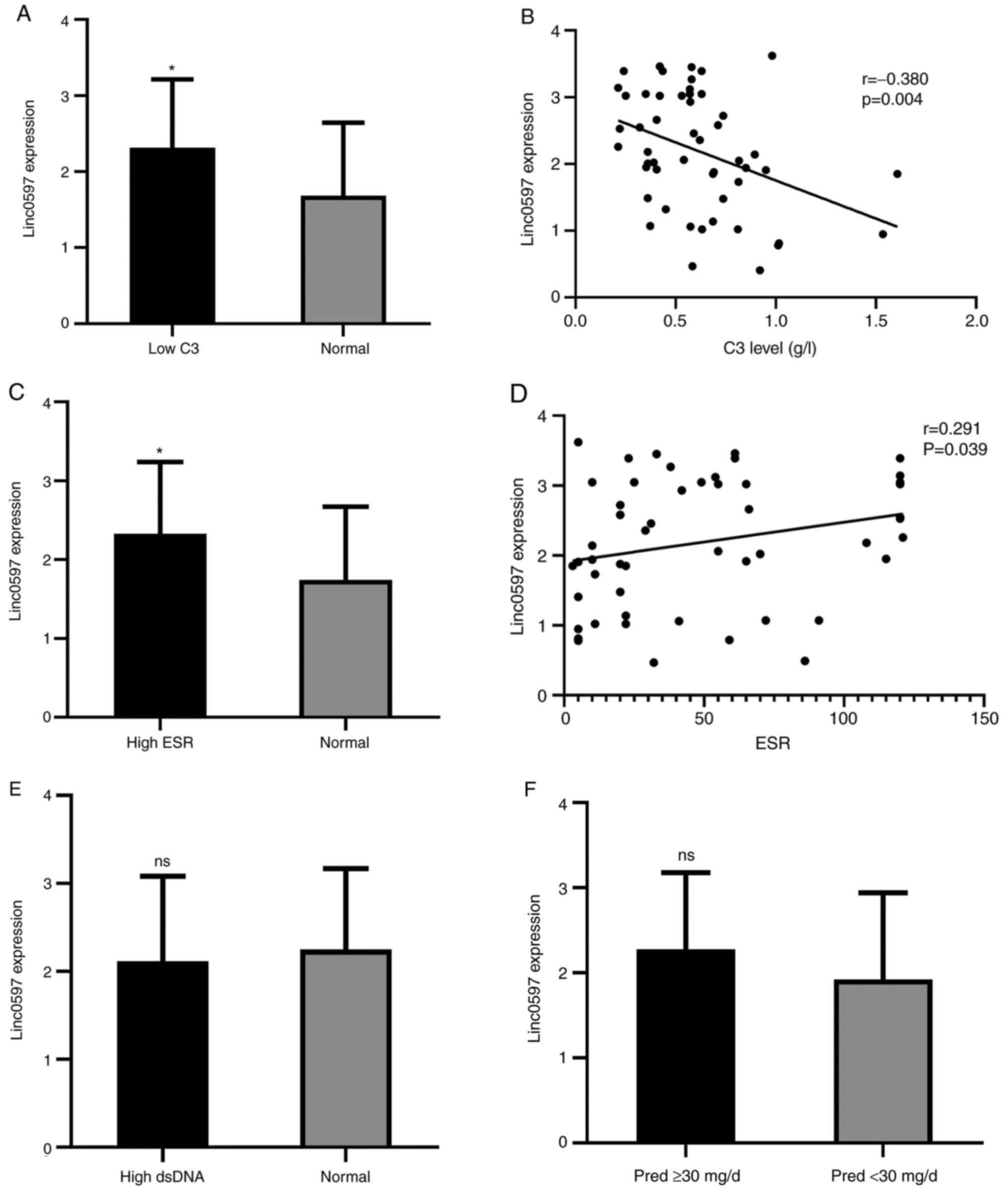
According to the expression levels of C3 and ESR in
patients with SLE, the group was divided into a low C3 group and
normal C3 group, as well as a high ESR group and a normal ESR
group. It was revealed that the level of linc0597 was significantly
higher in the low C3 group than in the normal C3 group (P=0.03;
Fig. 2A) and correlation analysis
between C3 and linc0597 indicated that the level of linc0597 in
patients with SLE was negatively correlated with C3 levels
(r=0.380, P=0.004; Fig. 2B). In
addition, the level of linc0597 in the high ESR group was
significantly higher than that in the normal ESR group (P=0.032;
Fig. 2C) and there was a weak
positive correlation between linc0597 levels and ESR (r=0.291,
P=0.039; Fig. 2D).
The association between lincRNA levels and
autoantibody profiles and medical treatments were also analyzed and
no significant difference in the level of linc0597 between the high
anti-double-stranded DNA (anti-dsDNA) antibodies group and the
normal anti-dsDNA antibody level group was obtained (P=0.609;
Fig. 2E). Furthermore, patients
with SLE receiving drug therapy were divided into a middle-to-high
dose of prednisone group (≥30 mg/day) and a low dose of prednisone
group (<30 mg/day). There was no significant difference in the
expression level of linc0597 between the two groups (P=0.247;
Fig. 2F).
Relationship between the expression
level of linc8986 and clinical characteristics of patients with
SLE
Compared to patients with normal C3 levels, the
expression level of linc8986 was significantly higher in patients
with SLE with low C3 levels (P<0.001; Fig. 2G). Further analysis revealed a
negative correlation between the level of linc8986 and C3 levels
(r=0.565, P<0.001; Fig. 2H). In
contrast to linc0597, there was no significant difference in the
expression level of linc8986 between the high ESR group and the
normal ESR group (P=0.135; Fig.
2I). However, the expression level of linc8986 in the high
anti-dsDNA group was significantly higher than that in the normal
anti-dsDNA group (P=0.009; Fig.
2J), but further analysis indicated no correlation between
linc8986 and anti-dsDNA (r=0.114, P=0.545; Fig. 2K). In addition, there was no
significant difference in the expression level of linc8986
(P=0.055; Fig. 2L) between the
middle-to-high dose prednisone group and the low-dose prednisone
group.
Identification of linc0597 and
linc8986 as potential biomarkers of SLE
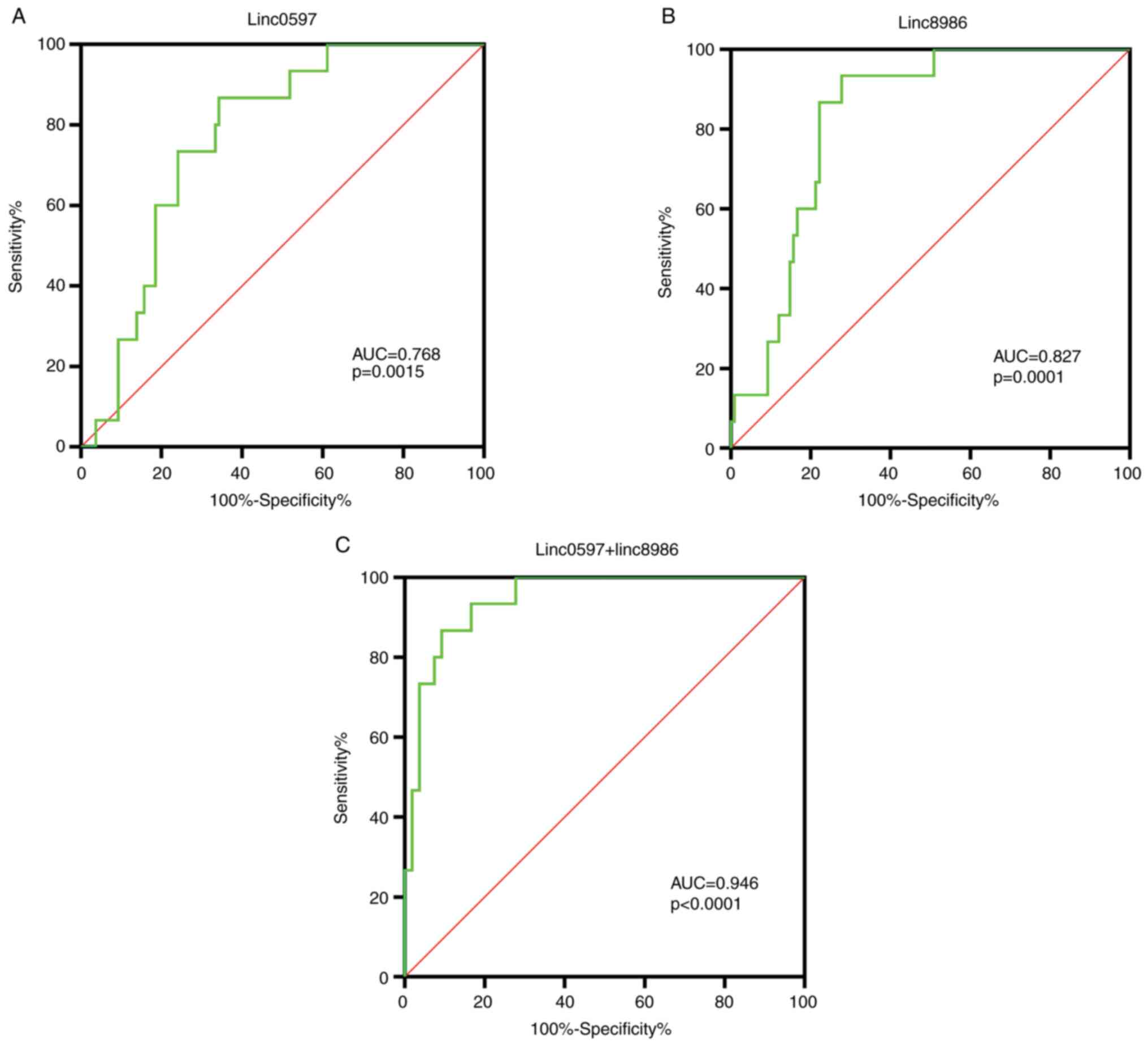
In order to determine whether linc0597 and linc8986
may be used as potential biomarkers of SLE, ROC curve analysis was
used to assess linc0597 and linc8986 separately and in combination,
as presented in Fig. 3.
The area under the ROC curve (AUC) of linc0597 was
0.768 (95%CI: 0.652-0.883; P=0.0015), the cut-off value was 0.515,
the sensitivity and the specificity was 64.8 and 78.7%. The AUC of
linc8986 was 0.827 (95%CI: 0.728-0.925; P=0.0001), the cut-off
value was 0.655, the sensitivity and the specificity was 72.2 and
84.6%. (Fig. 3A and B). The results revealed that both linc8986
and linc0597 may be associated with the pathogenesis of SLE, but
that linc8986 has higher sensitivity and may be a potential
biomarker for the diagnosis of SLE. Furthermore, the results
suggested that the combined application of linc8986 and linc0597
had an AUC of 0.946 (95% CI: 0.893-0.998, P<0.0001; Fig. 3C) and may thus provide higher
diagnostic accuracy. The cut-off value was 0.774, the sensitivity
and specificity of the combined application of linc8986 and
linc0597 were 90.7 and 86.7%, respectively.
Discussion
Increasing evidence has indicated that lincRNAs have
important roles in vast biological processes, including stem cell
biology, cell differentiation, embryonic development and
tissue-specific expression (25).
LincRNAs, which were initially studied in the context of genomic
imprinting and cell differentiation, have been determined to
function as key regulators of various processes, particularly in
the molecular mechanisms of immune cells and autoimmunity (26).
In the present study, the expression levels of three
different lincRNAs (linc0597, linc8986, and linc7190) were analyzed
in plasma samples and investigated the association between their
expression and specific clinical features of SLE was determined.
The results indicated that linc0597 and linc8986 may be suitable
biomarkers for the diagnosis of SLE, while linc7190 is not. In
addition, the plasma level of linc0597 was correlated with the
levels of C3 and the ESR, while the plasma level of linc8986 was
correlated with the levels of C3 and anti-dsDNA antibodies. This
further raised the question of how complements are affected by
these lincRNAs. Song et al (27) indicated that elevated levels of the
lncRNA RPAIN regulate invasion and apoptosis of trophoblast cells
through complement C1q. Furthermore, it was indicated that
overexpression of RPAIN inhibits the expression of C1q and that C1q
was the functional target of RPAIN (27). It was also previously reported that
C3 and C4 have diagnostic value for SLE (6,7). In
the present study, elevated levels of linc8986 and linc0597 in the
plasma of patients with SLE were indicated to be negatively
correlated with C3, suggesting that high expression of linc8986 and
linc0597 may inhibit the expression of C3. Whether C3 is also the
target of these two lincRNAs requires further investigation.
Compared with the previously reported expression
levels of linc0597 in monocytes of patients with SLE, the plasma
level of linc0597 in patients with SLE is relatively high (15,28).
This discrepancy may be due to a number of reasons. One explanation
for the increased levels of linc0597 in the plasma of patients with
SLE may be the increase of cellular exocytosis resulting in a
decrease in intracellular content (29). In addition, the sequence length of
lincRNAs is high and it is unlikely to exist in its complete form
in bodily fluids, i.e., the lincRNAs in plasma may exist in a
fragmented form (30). Another
reason is that the expression of lincRNAs may be tissue-specific
(31).
To the best of our knowledge, the present study was
the first to identify a relationship between the plasma levels of
linc8986 and SLE. The results suggested that the expression level
of linc8986 in patients with SLE was higher than that in the
healthy controls. Further analysis revealed that the plasma levels
of linc8986 were not significantly different between patients with
RA, patients with SS and healthy controls, indicating that the
level of linc8986 in plasma was specific for the diagnosis of SLE.
Li et al (18) used Pam3CSK4
to stimulate THP-1 macrophages and determined that the expression
level of linc8986 increased significantly. However, only a small
number of studies have examined linc8986 and the next step is to
identify the molecular mechanisms in detail. Regarding linc7190, Li
et al (18) indicated that
the level of linc7190 increased significantly after 8 h of
treatment of THP-1 macrophages with Pam3CSK4 and linc7190 regulates
the secretion of IL-6 and TNF-α. Of note, both IL-6 and TNF-α are
involved in the pathogenesis of SLE. Previous studies suggested
that Toll-like receptors may trigger the regulatory factors NF-κB
and IFN, which regulate the expression of hundreds of genes
involved in the immune response, including the pro-inflammatory
cytokines TNF-α, IL-1 and IL-6 (32,33).
Therefore, Li et al (18)
revealed that under the stimulation of Pam3CSK4, the regulation of
linc7190 may have a priming process and linc7190 further regulates
the secretion of IL-6 and TNF-α and participates in the
pathogenesis of SLE.
LN is one of the most common complications of SLE.
Depending on the population, the incidence of LN may be as high as
50% and the incidence of end-stage nephropathy is 20% (5). Although significant progress has been
made in the diagnosis and treatment of LN in recent years, the
therapeutic effect remains unsatisfactory and the incidence of the
disease is on the rise (34). While
renal pathological biopsy is still the gold standard for diagnosing
patients suspected to have LN, it has a number of disadvantages,
such as increased trauma and pain associated with the procedure
(35). Wu et al (17) reported that plasma lncRNA dendritic
cells may be used as a biomarker for the diagnosis of SLE and to
distinguish between patients with and without LN in SLE. Zheng
et al (16) reported that
serum sTNF-R1 and linc0597 were upregulated in patients with SLE
and LN, suggesting that serum sTNF-R1 and linc0597 may be
biomarkers for SLE and LN. Therefore, in the present study, the
relationship between linc8986, linc0597 and linc7190 was also
analyzed in patients with LN. The present results indicated that
the expression level of linc0597 in patients with SLE with LN was
higher than that in patients with SLE without LN, but the
expression levels of linc8986 and linc7190 were not significantly
different between the two groups. This suggests that linc0597 and
linc8986 may be utilized for the diagnosis of SLE and that high
levels of linc0597 may be helpful for the differential diagnosis of
LN in patients with SLE.
Over the past few decades, much effort has been
invested into the study of SLE biomarkers and numerous researchers
have proposed potential biomarkers for SLE. For instance,
IFN-induced genes and chemokines may be used to determine the
activity and severity of SLE in patients (36,37).
However, the limitations of these biomarkers have gradually
emerged. Previous studies have reported that overexpression of the
IFN I type pathway has been confirmed in patients with RA, SS,
myositis and scleroderma. Thus, IFN is not sufficiently specific
for the diagnosis of SLE (38,39).
This highlights the urgent requirement for a specific biomarker to
diagnose SLE. As biomarkers, lincRNAs have a number of
characteristics that make them well-suited for use as biomarkers,
including being stable in plasma and their ease and low cost of
detection (40), making them ideal
biomarkers to evaluate disease activity and judge the severity of
SLE in patients.
In the present study, it was demonstrated that the
expression levels of linc0597 are specific to patients with SLE and
that the levels of linc0597 may be helpful for evaluating disease
activity and distinguishing between patients with LN and without
LN. However, further validation of linc0597 in large-scale
multicenter trials is necessary. In addition, the present study
also suggested that linc8986 in plasma may be used as a promising
diagnostic marker for SLE. To our knowledge, the present study was
the first to report the association of linc8986 with SLE.
Furthermore, in the ROC curve analysis, the AUC value of linc0597
and linc8986 was 0.768 and 0.827, respectively and combination of
linc8986 and linc0597 improved the diagnostic accuracy. Therefore,
the use of lincRNAs as novel biomarkers may become a valuable tool
for the clinical diagnosis of SLE.
However, there are still certain shortcomings to the
present study. The functions of the lincRNAs and their potential
mechanisms in SLE were not investigated. In addition, the level of
lincRNAs released from local target tissues was not evaluated. In
future studies, it will be investigated how these lincRNAs affect
IFN, IL-6, TNF and TGF signals and their relationship with LN
within a larger cohort and the influence of these molecules on the
STAT signaling pathway and the regulation of lincRNAs on the
post-transcriptional process will be further explored.
In conclusion, linc8986 and linc0597 may be used to
specifically identify patients with SLE and linc0597 has an
important role in the detection of nephritis in patients with SLE.
Combined application of linc0597 and linc8986 may improve the
diagnostic accuracy of SLE.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 81772261).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CR proposed the research, performed the data
analysis and wrote the manuscript. HX helped with the formal
analysis and data collection. CY and FW helped with the data
acquisition. HZ was involved in funding acquisition, and analysis
and interpretation of data for the study. XG was involved in
supervision and conception and design of the study. XG and HZ
confirmed the authenticity of the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of the First Affiliated Hospital of Harbin Medical
University (Harbin, China; approval no. IRB-AF/SC-04/01.0). All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herrada AA, Escobedo N, Iruretagoyena M,
Valenzuela RA, Burgos PI, Cuitino L and Llanos C: Innate immune
cells' contribution to systemic lupus erythematosus. Front Immunol.
10(772)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Luo Q, Li X, Xu C, Zeng L, Ye J, Guo Y,
Huang Z and Li J: Integrative analysis of long non-coding RNAs and
messenger RNA expression profiles in systemic lupus erythematosus.
Mol Med Rep. 17:3489–3496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang HT, Chen JM, Guo J, Lan Y and Wei
YS: The association of interleukin-31 polymorphisms with
interleukin-31 serum levels and risk of systemic lupus
erythematosus. Rheumatol Int. 36:799–805. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wenderfer SE and Eldin KW: Lupus
nephritis. Pediatr Clin North Am. 66:87–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thomas G, Mancini J, Jourde-Chiche N,
Sarlon G, Amoura Z, Harlé JR, Jougla E and Chiche L: Mortality
associated with systemic lupus erythematosus in france assessed by
multiple-cause-of-death analysis. Arthritis Rheumatol.
66:2503–2511. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Troldborg A, Jensen L, Deleuran B,
Stengaard-Pedersen K, Thiel S and Jensenius JC: The C3dg fragment
of complement is superior to conventional C3 as a diagnostic
biomarker in systemic lupus erythematosus. Front Immunol.
9(581)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mulvihill E, Ardoin S, Thompson SD, Zhou
B, Yu GR, King E, Singer N, Levy DM, Brunner H, Wu YL, et al:
Elevated serum complement levels and higher gene copy number of
complement C4B are associated with hypertension and effective
response to statin therapy in childhood-onset systemic lupus
erythematosus (SLE). Lupus Sci Med. 6(e000333)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qu C, Zhang J, Zhang X, Du J, Su B and Li
H: Value of combined detection of anti-nuclear antibody,
anti-double-stranded DNA antibody and C3, C4 complements in the
clinical diagnosis of systemic lupus erythematosus. Exp Ther Med.
17:1390–1394. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lekka E and Hall J: Noncoding RNAs in
disease. FEBS Lett. 592:2884–2900. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tian J, An X and Niu L: Role of microRNAs
in cardiac development and disease. Exp Ther Med. 13:3–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou Q, Yu Q, Gong Y, Liu Z, Xu H, Wang Y
and Shi Y: Construction of a lncRNA-miRNA-mRNA network to determine
the regulatory roles of lncRNAs in psoriasis. Exp Ther Med.
18:4011–4021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Messemaker TC, Frank-Bertoncelj M, Marques
RB, Adriaans A, Bakker AM, Daha N, Gay S, Huizinga TW, Toes REM,
Mikkers HMM and Kurreeman F: A novel long non-coding RNA in the
rheumatoid arthritis risk locus TRAF1-C5 influences C5 mRNA levels.
Genes Immun. 17:85–92. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yi R, Yang L, Zeng S and Su Y: Different
expression profile of mRNA and long noncoding RNA in autoimmune
thyroid diseases patients. J Cell Biochem. 120:19442–19456.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Y, Zhang F, Ma J, Zhang X, Wu L, Qu B,
Xia S, Chen S, Tang Y and Shen N: Association of large intergenic
noncoding RNA expression with disease activity and organ damage in
systemic lupus erythematosus. Arthritis Res Ther.
17(131)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng CZ, Yan WW, Luo YL, Wang TL and Shu
YB: Value of sTNF-R1 and linc0597 as indicators for disease
activity and diagnosis of lupus nephritis. Eur Rev Med Pharmacol
Sci. 24:5582–5591. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu GC, Li J, Leng RX, Li XP, Li XM, Wang
DG, Pan HF and Ye DQ: Identification of long non-coding RNAs GAS5,
linc0597 and lnc-DC in plasma as novel biomarkers for systemic
lupus erythematosus. Oncotarget. 8:23650–23663. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Z, Chao TC, Chang KY, Lin N, Patil VS,
Shimizu C, Head SR, Burns JC and Rana TM: The long noncoding RNA
THRIL regulates TNF expression through its interaction with hnRNPL.
Proc Natl Acad Sci USA. 111:1002–1007. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan S, Tang C, Chen D, Li F, Huang M, Ye
J, He Z, Li W, Chen Y, Lin X, et al: miR-98 modulates cytokine
production from human PBMCs in systemic lupus erythematosus by
targeting IL-6 mRNA. J Immunol Res. 2019(9827574)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hochberg MC: Updating the American college
of rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40(1725)1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American college of Rheumatology/European league against
rheumatism collaborative initiative. Arthritis Rheum. 69:1580–1588.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vitali C, Bombardieri S, Jonsson R,
Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox
RI, Kassan SS, et al: Classification criteria for sjögren's
syndrome: A revised version of the European criteria proposed by
the American-European consensus group. Ann Rheum Dis. 61:554–558.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tong YS, Wang XW, Zhou XL, Liu ZH, Yang
TX, Shi WH, Xie HW, Lv J, Wu QQ and Cao XF: Identification of the
long non-coding RNA POU3F3 in plasma as a novel biomarker for
diagnosis of esophageal squamous cell carcinoma. Mol Cancer.
14(3)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao CN, Mao YM, Liu LN, Li XM, Wang DG
and Pan HF: Emerging role of lncRNAs in systemic lupus
erythematosus. Biomed Pharmacother. 106:584–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu GC, Hu Y, Guan SY, Ye DQ and Pan HF:
Differential plasma expression profiles of long non-Coding RNAs
reveal potential biomarkers for systemic lupus erythematosus.
Biomolecules. 9(206)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Song X, Rui C, Meng L, Zhang R, Shen R,
Ding H, Li J, Li J and Long W: Long non-coding RNA RPAIN regulates
the invasion and apoptosis of trophoblast cell lines via complement
protein C1q. Oncotarget. 8:7637–7646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Q, Liang Y, Yuan H, Li S, Wang JB,
Li XM, Tao JH, Pan HF and Ye DQ: Integrated analysis of lncRNA,
miRNA and mRNA expression profiling in patients with systemic lupus
erythematosus. Arch Med Sci. 15:872–879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Müller N, Döring F, Klapper M, Neumann K,
Schulte DM, Türk K, Schröder JO, Zeuner RA, Freitag-Wolf S,
Schreiber S and Laudes M: Interleukin-6 and tumour necrosis
factor-α differentially regulate lincRNA transcripts in cells of
the innate immune system in vivo in human subjects with rheumatoid
arthritis. Cytokine. 68:65–68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J,
Wei M, Xu C, Wu C, Zhang Z, et al: Long non-coding RNA metastasis
associated in lung adenocarcinoma transcript 1 derived miniRNA as a
novel plasma-based biomarker for diagnosing prostate cancer. Eur J
Cancer. 49:2949–2959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
toll-like receptors. Nat Immunol. 11:373–384. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cansu DÜ, Temiz G, Açıkalın MF and Korkmaz
C: Pauci-immune lupus nephritis: Possibility or co-incidence? Eur J
Rheumatol. 4:73–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Haładyj E and Cervera R: Do we still need
renal biopsy in lupus nephritis? Reumatologia. 54:61–66.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chaichian Y, Wallace DJ and Weisman MH: A
promising approach to targeting type 1 IFN in systemic lupus
erythematosus. J Clin Invest. 129:958–961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Murayama G, Furusawa N, Chiba A, Yamaji K,
Tamura N and Miyake S: Enhanced IFN-α production is associated with
increased TLR7 retention in the lysosomes of palasmacytoid
dendritic cells in systemic lupus erythematosus. Arthritis Res
Ther. 19(234)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Higgs BW, Liu Z, White B, Zhu W, White WI,
Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, et al:
Patients with systemic lupus erythematosus, myositis, rheumatoid
arthritis and scleroderma share activation of a common type I
interferon pathway. Ann Rheum Dis. 70:2029–2036. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bodewes ILA, Huijser E, van
Helden-Meeuwsen CG, Tas L, Huizinga R, Dalm V, van Hagen PM, Groot
N, Kamphuis S, van Daele PLA and Versnel MA: TBK1: A key regulator
and potential treatment target for interferon positive sjögren's
syndrome, systemic lupus erythematosus and systemic sclerosis. J
Autoimmun. 91:97–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Luo Q, Xu C, Li X, Zeng L, Ye J, Guo Y,
Huang Z and Li J: Comprehensive analysis of long non-coding RNA and
mRNA expression profiles in rheumatoid arthritis. Exp Ther Med.
14:5965–5973. 2017.PubMed/NCBI View Article : Google Scholar
|