Introduction
Diabetic patients are susceptible to the development
of chronic wounds, especially on the feet (1). Diabetic foot ulcer (DFU)-derived
fibroblasts contribute to defective matrices and chronic wound
pathogenesis (1). Chronic
hyperglycemia contributes to the formation of advanced glycation
end products (AGEs), which are harmful compounds formed by
glycation reactions and are considered to serve a causative role in
the development and worsening of diabetic wound healing (2,3).
Diabetes is a chronic condition associated with
elevated oxidative stress and inflammation, involving a continuum
of tissue and cellular insults that leads to the development of
DFUs (4-6).
Previous studies have demonstrated that the diabetic environment is
characterized by elevated oxidative stress indices [reactive oxygen
species (ROS), malondialdehyde (MDA) and 8-hydroxydeoxyguanosine
(8-OHdG) (4,5)] and increased proinflammatory
cytokines, such as TNF-α, IL-6 and IL-1β (6,7).
However, the human body possesses several defense mechanisms
against oxidative stress and inflammatory insults. For example,
heme oxygenase-1 (HO-1) is an antioxidative, anti-inflammatory and
cytoprotective enzyme that is increased as a protective response to
stress. It has been revealed that HO-1 is upregulated in response
to oxidative stress that leads to impaired wound healing (8). It has been indicated that HO-1 exerted
potent antidiabetic and insulin-sensitizing effects, in addition to
suppressing immune/inflammatory insults (9). In a previous study, HO-1 improved the
biological behavior of fibroblasts and reduced oxidative damage
caused by a high-glucose (HG) environment (10). The present study examined HO-1
expression in rat dermal fibroblasts in experimental groups before
and after the hemin induction of HO-1 expression to elucidate the
antioxidative and anti-inflammatory effects of HO-1 and its
cytoprotective role in high AGE environments.
Materials and methods
Cell culture and groupings
Rat dermal fibroblasts (cat. no. CRL-1213; American
Type Culture Collection) were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) without any supplementary antibiotics in an
incubator (37˚C; 5% CO2). Fibroblasts were cultured in
DMEM containing 10% FBS under twelve different conditions
(according to the groupings) for 72 h after 24 h of serum
deprivation (0.5% FBS). All manipulations were performed in dim
lighting, and the plates were wrapped in aluminum foil to protect
them from the light. Cells were sub-cultured after they had grown
to confluence. All experiments were performed in triplicate.
The cells were divided into the following groups: i)
normal glucose (NG) (NG 1.0 g/l; Gibco; Thermo Fisher Scientific,
Inc.); ii) NG + Hemin (hemin 5 µM; MilliporeSigma); iii) NG +
chromium mesoporphyrin (CrMP) (CrMP 20 µM; MilliporeSigma); iv) NG
+ Hemin + CrMP; v) AGEs + NG (AGEs 100 µg/ml; Gibco; Thermo Fisher
Scientific, Inc.); vi) AGEs + NG + Hemin; vii) AGEs + NG + CrMP;
viii) AGEs + NG + Hemin + CrMP; ix) AGEs + HG (HG 4.5 g/l); x) AGEs
+ HG + Hemin; xi) AGEs + HG + CrMP; and xii) AGEs + HG + Hemin +
CrMP group (9-11).
Determination of HO-1 mRNA
expression
Rat fibroblasts were cultured in 6-well plates at a
density of 1.0-2.0x105 cells/well and harvested after 72
h of treatment. HO-1 mRNA expression was determined via reverse
transcription-quantitative PCR. RNA extraction was performed using
Trizol reagent (cat. no R0016; Beyotime Institute of Biotechnology)
according to the manufacturer's guidelines. Then the M-MLV Reverse
Transcriptase Kit (cat. no. MLV100K; ProFoldin) was used for the
reverse transcription of RNA samples according to the
manufacturer's guidelines. The primers were designed by Invitrogen
(Thermo Fisher Scientific, Inc.), according to the sequences of rat
HO-1 and EF2 in GenBank (http://www.ncbi.nlm.nih.gov/), as follows: HO-1
(forward, 5'-AGAGTCCCTCACAGACAGAGTTT-3' and reverse,
5'-CCTGCAGAGAGAAGGCTACATGA-3'); EF2 (forward,
5'-GACATCACCAAGGGTGTGCAG-3' and reverse,
5'-TCAGCACACTGGCATAGAGGC-3').
Amplification was performed according to the
instructions of the SYBR PrimeScript RT-PCR Kit (cat. no. RR055A;
Takara Bio, Inc.). Briefly, 10 µl SYBR Premix Ex Taq™ (2X), 0.4 µl
PCR forward primer (10 µM), 0.4 µl PCR reverse frimer (10 µM), 0.4
µl ROX reference dye (50X) and 2.0 µl cDNA template were added into
a microfuge tube along with distilled water to make a total volume
of 20.0 µl. The PCR reactions were performed in a LightCycler 480
real-time-PCR system (Roche Diagnostics), with denaturation at 95˚
for 30 sec, followed by 40 cycles of 95˚ for 5 sec and 60˚ for 20
sec. A melting curve analysis was performed to ensure the
specificity of the amplification, and the products were quantified
using the 2-ΔΔCq method (12). The difference between the Ct value
of HO-1 and the corresponding EF2 value in each sample was used to
determine the relative HO-1 value (10,11).
Determination of HO-1 protein
expression
HO-1 protein expression was measured using western
blot analysis. Proteins were extracted from fibroblasts with 100 µl
RIPA and 1 µl PMSF (Beijing Solarbio Science & Technology Co.,
Ltd.). The lysate centrifuged at 11,000 x g for 20 min at 4˚, and
the supernatant was stored at -80˚. Protein concentrations were
determined using a BCA kit (Beijing Solarbio Science &
Technology Co., Ltd.). A total of 40 µg protein were
electrophoresed in 10% denaturing SDS-PAGE. The proteins were
transferred to PDVF membranes and incubated in blocking buffer [5%
nonfat milk in PBS containing 0.1% Tween 20 (PBS-T)] for 1.5 h at
room temperature, followed by an overnight incubation at 4˚C in
1:1,000 dilution of monoclonal antibody to HO-1 (cat. no.
SAB5700731; Sigma-Aldrich; Merck KGaA). The membrane was PBS washed
three times and incubated with a 1:1,000 dilution of horseradish
peroxidase-linked goat anti-rabbit IgG (cat. no. SR134; Beijing
Solarbio Science & Technology Co., Ltd.) in PBS-T containing 1%
nonfat milk for 1.5 h at room temperature. Membranes were washed
again and developed with a chemiluminescent agent (cat. no. SW2010;
Beijing Solarbio Science & Technology Co., Ltd.). The band
densities were measured using TINA image software (Elysia-raytest
GmbH) (10).
Determination of HO-1 activity
HO-1 activity in fibroblast microsomes was measured
using bilirubin generation based on the instructions of the
bilirubin kit (cat. no. BS-E11055R2; Jiangsu Boshen Biological
Technology Co., Ltd.). The level of extracted bilirubin was
calculated based on the difference in absorption at 510 nm
(extinction coefficient 40/mmol/cm for bilirubin). HO-1 activity
was expressed as pmol bilirubin/mg protein/h, as previously
reported (10).
Analysis of oxidative stress and
inflammatory insult markers
Rat fibroblasts were cultured in 6-well plates at a
density of 1.0-2.0x105 cells/well and the supernatants
were collected after 72 h treatment. The levels of 8-OHdG (cat. no.
E4442-100; BioVision, Inc.), ROS (cat. no. MS-21264R2; Shanghai
Maisha Biological Technology Co., Ltd.), MDA (cat. no. KTE100650;
Abbkine Scientific Co., Ltd.), TNF-α (cat. no. PT516; Beyotime
Institute of Biotechnology), IL-6 (cat. no. ab100772; Abcam) and
IL-1β (cat. no. ab100767; Abcam) were measured using ELISA
according to the manufacturers' instructions and a plate reader
(SpectraMax 340PC; Molecular Devices, LLC) (4,7,10).
Collagen (hydroxyproline) secretion
and viability assay
Rat fibroblasts were cultured in 6-well plates at a
density of 1.0-2.0x105 cells/well. After treatment for
72 h as aforementioned, collagen (hydroxyproline) secretion was
measured via ELISA. Hydroxyproline was measured according to the
instructions of the rat hydroxyproline ELISA kit (cat. no.
RTEB1742; Assay Genie). Cell viability was assessed using a CCK-8
kit (cat. no. ER0808; Wuhan Fine Biotech Co., Ltd.). The cells were
inoculated into 96-well plates at a density of
2.0-3.0x103 cells/well. Premixed CCK-8 reagent and
complete cell culture medium (10 µl:100 µl) was added into the
96-well plates, and the cells were incubated for 0.5-1 h at 37˚C,
and A450 values were obtained subsequently with a 3550 automatic
detector (Beckman Coulter, Inc.) (10,13).
Proliferation and apoptosis assay
Rat fibroblasts were cultured in 6-well plates at a
density of 1.0-2.0x105 cells/well and were harvested
after 72 h of treatment as aforementioned. Cells were centrifuged
at 11,000 x g for 6 min at 4˚C, and the supernatant was discarded.
Cells were resuspended with 1.5 ml PBS. Subsequently, 300 µl of the
cell suspension was added into 800 µl ice-cold ethanol, and fixed
overnight at 4˚C in dark. The next day, the cell suspension was
centrifuged at 11,000 x g for 10 min at 4˚C, and the supernatant
was discarded. The cells were resuspended again with 500 µl PBS
containing RNase A (100 U/ml), and incubated for 30 min at 37˚C.
Then, ethidium bromide (2 mg/ml) was added to a final concentration
of 50 µg/ml and incubated for 30 min in dark at 4˚C. Cell cycle was
detected by standard procedures of flow cytometry (FACSCalibur; BD
Biosciences), and the S-phase cell ratio and proliferation index
were calculated at the same time. S-phase cell ratio was calculated
as S/(G0/G1 + S + G2/M), and
proliferation index was calculated as (S + G2/M)/
(G0/G1 + S + G2/M) (10).
Cell apoptosis was assessed with Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection Kit (Beyotime Institute of Biotechnology) according to
the manufacturer's guidelines. Briefly, cells were collected and
then resuspended as aforementioned. Subsequently, 5 µl
AnnexinV-FITC and 5 µl PI were added and maintained for 15 min in
the dark at room temperature. Lastly, cell apoptosis was examined
with a FACScan flow cytometry analyzer (BD Biosciences). The
results were expressed as the mean ± SD of three determinations per
sample for each experiment, as previously reported (10).
Migration assay
Horizontal migration was assessed using the scratch
test. Rat fibroblasts were cultured in 6-well plates at a density
of 1.0-2.0x105 cells/well. After 72 h of treatment as
aforementioned, the bottom of 6-well plates were scratched with a
10-µl tip. Subsequently, the cells were cultured for 24 h without
serum. Five different horizontal areas were selected and the width
of the wound at 0 and 24 h after scratching was measured. The mean
distance was the horizontal migration rate, which was calculated as
follows: (Width at 0 h-width at 24 h)/width at 0 h x100% (10).
Statistical analysis
Data are presented as the mean ± SD. Comparisons
between two groups were performed using unpaired Student's t-test.
Pairwise comparisons among three groups were performed using
one-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
Pairwise comparisons among >3 groups were performed using
one-way ANOVA followed by Tukey's post hoc test. Data were analyzed
with Microsoft Excel 2003 (Microsoft Corporation) and SPSS 13.0
(SPSS, Inc.) for Windows. Statistical analyses were performed using
the average results of three experimental repeats under identical
conditions. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of the fibroblast
functional disorder model
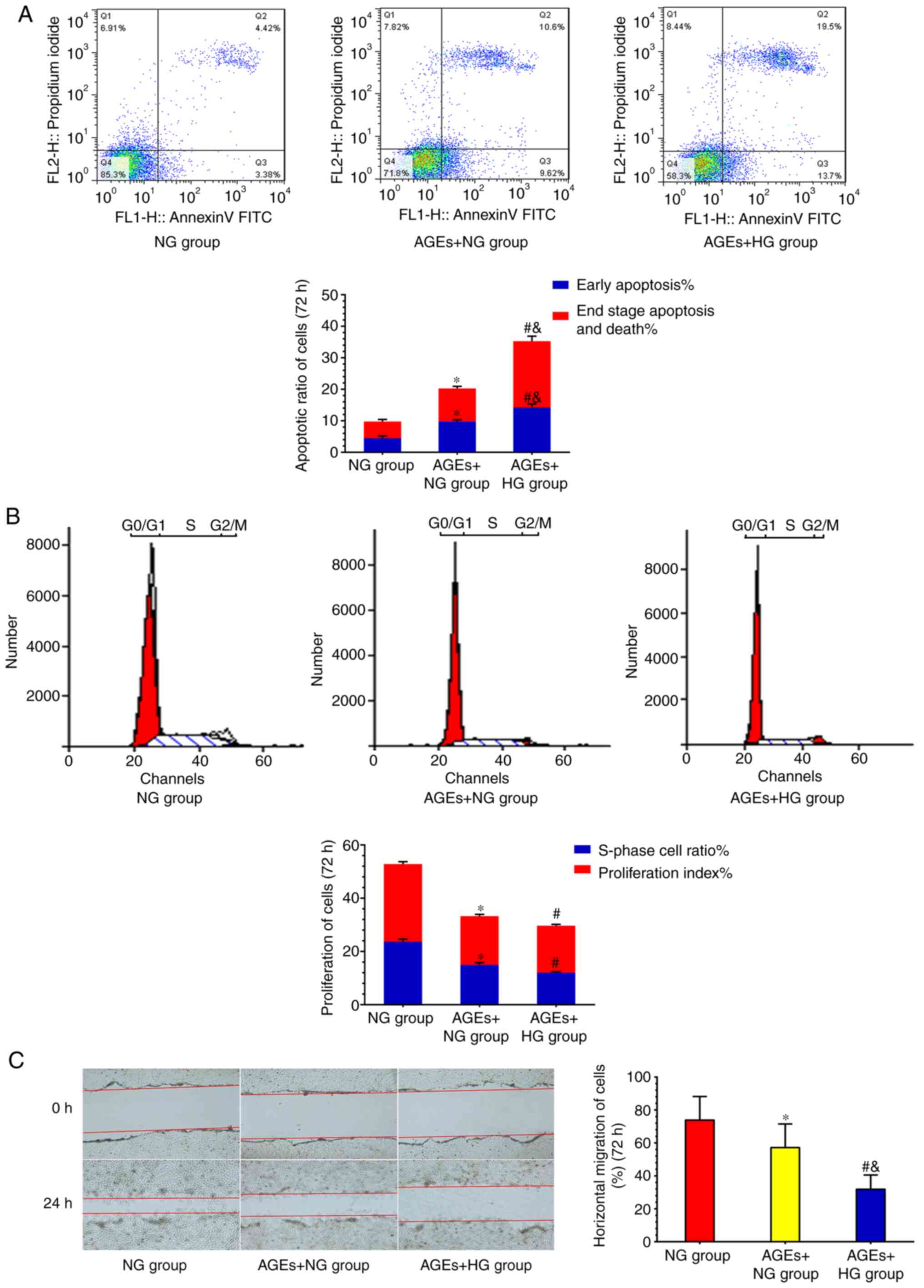
Compared with normal glucose conditions, AGEs
induced oxidative stress, inflammation and biological behavioral
disorders in fibroblasts, and more severe cell damage was caused by
the combination of AGEs and HG conditions.
ELISA was used to measure markers of oxidative
stress (ROS, MDA and 8-OHdG) and inflammatory insult (TNF-α, IL-6
and IL-1β). ROS, MDA and 8-OHdG levels in the AGEs + NG group were
4.2-, 2.6- and 2.8-fold higher, respectively, compared with those
in the NG group at 72 h (all P<0.05). ROS, MDA and 8-OHdG levels
in the AGEs + HG group were 5.5-, 3.6- and 3.7-fold higher,
respectively, compared with those in the NG group (all P<0.05),
and were 1.3-, 1.4- and 1.3-fold higher, respectively, in the AGEs
+ HG group compared with the AGEs + NG group (all P<0.05)
(Table I). TNF-α, IL-6 and IL-1β
levels in the AGEs + NG group were 3.6-, 3.8- and 3.2-fold higher,
respectively, compared with those in the NG group at 72 h (all
P<0.05). In the AGEs + HG group, TNF-α, IL-6 and IL-1β levels
were 4.7-, 4.6- and 3.9-fold higher, respectively, compared with
those in the NG group (all P<0.05), and were 1.3-, 1.2 and
1.2-fold higher, respectively, in the AGEs + HG group compared with
the AGEs + NG group (all P<0.05) (Table I). These results indicated that AGEs
caused oxidative stress and inflammatory insult in fibroblasts, and
severe damage was caused by the combination of AGEs and HG
conditions.
 | Table IOxidative stress and inflammatory
injury index of fibroblasts under different conditions. |
Table I
Oxidative stress and inflammatory
injury index of fibroblasts under different conditions.
| Group | ROS, fluorescence
intensity | MDA, nmol/ml | 8-OHdG, ng/ml | TNF-α, pg/ml | IL-6, pg/ml | IL-1β, pg/ml |
|---|
| NG | 214.03±24.91 | 2.42±0.51 | 2.99±0.62 | 8.51±1.84 | 10.56±2.13 | 15.25±3.48 |
| NG + Hemin |
135.73±12.42a |
1.59±0.23a |
1.41±0.20a |
3.48±0.82a |
4.71±0.82a |
6.59±1.97a |
| NG + CrMP | 238.46±27.31 | 2.68±0.65 | 3.55±0.71 | 10.72±1.98 | 12.92±2.99 | 18.65±4.09 |
| NG + Hemin +
CrMP | 227.52±23.74 | 2.51±0.42 | 3.02±0.63 | 9.13±1.79 | 11.37±2.11 | 16.99±4.31 |
| AGEs + NG |
892.48±50.27a |
6.27±0.78a |
8.39±0.85a |
30.81±4.63a |
39.88±5.97a |
48.85±6.27a |
| AGEs + NG +
Hemin |
485.32±38.64b |
3.98±0.71b |
3.22±0.72b |
11.56±2.01b |
21.96±3.63b |
23.26±5.24b |
| AGEs + NG +
CrMP | 917.60±62.84 | 6.94±0.81 | 9.78±0.96 | 38.55±5.09 | 42.42±6.56 | 55.43±7.38 |
| AGEs + NG + Hemin +
CrMP | 901.25±60.35 | 6.68±0.79 | 8.52±0.84 | 32.79±5.11 | 40.71±6.71 | 52.83±7.50 |
| AGEs + HG |
1,183.73+79.37a,b |
8.83±0.86a,b |
10.97±1.23a,b |
39.63±5.94a,b |
48.68±7.02a,b |
60.13±7.62a,b |
| AGEs + HG +
Hemin |
582.16±41.38c |
4.62±0.76c |
5.63±0.88c |
15.38±2.32c |
22.53±4.39c |
32.86±5.89c |
| AGEs + HG +
CrMP | 1,294.31±83.65 | 9.41±0.92 | 12.85±1.40 | 45.83±6.84 | 52.50±7.58 | 65.73±7.97 |
| AGEs + HG + Hemin +
CrMP | 1,206.39±80.15 | 8.96±0.83 | 11.09±1.14 | 42.09±6.58 | 50.15±6.91 | 62.92±7.25 |
Subsequently, ELISA was used to measure fibroblast
collagen secretion and viability. Cell proliferation and apoptosis
were measured by flow cytometry, and the scratch test was used to
evaluate cell migration. Fibroblast collagen secretion
(595.37±61.26 pg/ml), viability (1.46±0.18), the S-phase cell ratio
(14.93±1.97%), the proliferation index (18.10±1.93%) and the
horizontal migration rate (0.47±0.12%) were inhibited in the AGEs +
NG group compared with the NG group (943.61±92.17 pg/ml, 1.87±0.21,
23.67±2.67%, 29.14±2.55% and 0.73±0.19%, respectively; all
P<0.05). Early apoptosis (9.87±1.99%) and end-stage apoptosis
and death (10.53±2.02%) were increased in the AGEs + NG group
compared with the NG group (4.36±1.42 and 5.37±1.63%, respectively;
both P<0.05). Collagen secretion (354.21±49.34 pg/ml), cell
viability (1.09±0.17), the S-phase cell ratio (12.00±1.67%), the
proliferation index (17.90±2.54%) and the horizontal migration rate
(0.28±0.11%) were inhibited in the AGEs + HG group compared with
the NG group (all P<0.05). Early apoptosis (14.12±2.38%) and
end-stage apoptosis and death (20.72%±4.18) were increased in the
AGEs + HG group compared with the NG group (both P<0.05).
Collagen secretion, cell viability and migration rate were
inhibited in the AGEs + HG group compared with the AGEs + NG group
(P<0.05). Cell apoptosis rate was increased in the AGEs + HG
group compared with the AGEs + NG group (both P<0.05) (Table II) (Fig. 1). In the presence of AGEs,
fibroblast collagen secretion, viability, proliferation and
migration were decreased, and apoptosis was increased. Severe
fibroblast biological behavioral disorders were caused by the
combination of AGEs and HG conditions.
 | Table IIAlterations of fibroblast biological
behavior under different conditions. |
Table II
Alterations of fibroblast biological
behavior under different conditions.
| | Collagen | Cell viability | Cell
proliferation | | Cell apoptosis | | Cell migration |
|---|
| Group | Hydroxyproline,
pg/ml | CCK-8 OD value | S-phase cell ratio,
% | Proliferation
index, % | Early apoptosis,
% | End stage apoptosis
and death, % | Horizontal
migration of cells, % |
|---|
| NG | 943.61±92.17 | 1.87±0.21 | 23.67±2.67 | 29.14±2.55 | 4.36±1.42 | 5.37±1.63 | 0.73±0.19 |
| NG + Hemin |
1,823.34±118.28a |
2.27±0.34a |
32.25±3.38a |
41.33±4.90a |
2.18±0.87a |
3.03±0.84a |
0.95±0.21a |
| NG + CrMP | 892.32±93.39 | 1.69±0.19 | 16.35±2.30 | 19.90±3.01 | 5.58±1.67 | 6.02±1.13 | 0.61±0.16 |
| NG + Hemin +
CrMP | 912.58±98.16 | 1.72±0.23 | 22.53±2.03 | 30.93±2.83 | 4.67±0.99 | 7.12±1.98 | 0.70±0.17 |
| AGEs + NG |
595.37±61.26a |
1.46±0.18a |
14.93±1.97a |
18.10±1.93a |
9.87±1.99a |
10.53±2.02a |
0.47±0.12a |
| AGEs + NG +
Hemin |
1,096.15±89.25b |
1.89±0.25b |
27.05±4.80b |
35.69±5.47b |
6.44±1.24b |
6.94±1.65b |
0.71±0.18b |
| AGEs + NG +
CrMP | 559.21±68.59 | 1.31±0.16 | 12.42±1.92 | 17.71±1.85 | 9.05±2.09 | 11.31±2.13 | 0.30±0.13 |
| AGEs + NG + Hemin +
CrMP | 574.47±65.13 | 1.39±0.20 | 12.14±1.75 | 15.92±2.71 | 9.07±1.92 | 10.77±2.26 | 0.38±0.15 |
| AGEs + HG |
354.21±49.34a,b |
1.09±0.17a,b |
12.00±1.67a |
17.90±2.54a |
14.12±2.38a,b |
20.72±4.18a,b |
0.28±0.11a,b |
| AGEs + HG +
Hemin |
984.35±88.47c |
1.57±0.19c | 17.55±2.24 | 20.20±3.28 | 10.97±1.87 |
17.07±2.99c |
0.59±0.14c |
| AGEs + HG +
CrMP | 308.25±46.72 | 0.92±0.12 | 8.61±1.94 | 12.53±2.93 | 14.83±2.52 | 21.07±3.97 | 0.17±0.09 |
| AGEs + HG + Hemin +
CrMP | 333.75±46.92 | 1.01±0.13 | 11.17±0.89 | 16.05±1.68 | 13.78±2.54 | 19.91±3.78 | 0.21±0.10 |
HO-1 expression in AGE-treated
models
HO-1 expression exhibited time-dependent alterations
and was the lowest at 72 h in the AGEs + NG and AGEs + HG group
compared with the NG group (Fig.
2A). HO-1 mRNA levels, protein levels and protease activity
decreased by 52.1, 61.6 and 44.7%, respectively, at 72 h in the
AGEs + NG group compared with the NG group (Fig. 2A, E,
F and G; all P<0.05), and were decreased by
67.5, 80.2 and 58.7%, respectively, in the AGEs + HG group compared
with the NG group (Fig. 2A,
E, F and G;
all P<0.05). HO-1 protein expression and protease activity
decreased by 46.3 and 28.7%, respectively, in the AGEs + HG group
compared with the AGEs + NG group (both P<0.05) (Fig. 2E-G). The results also demonstrated
that hemin induced high HO-1 expression at least within 72 h of
treatment (Fig. 2B-D). HO-1 mRNA
expression, protein expression and protease activity in the NG +
Hemin group were 9.9-, 9.7- and 10.1-fold higher, respectively,
than those in the NG group at 72 h (Fig. 2B, E,
F and H; all P<0.05), were 9.0-, 10.5- and
9.2-fold higher, respectively, in the AGEs + NG + Hemin group
compared with the AGEs + NG group (Fig.
2C, E, F and I;
all P<0.05), and were 8.6-, 9.7- and 10.2-fold higher,
respectively, in the AGEs + HG + Hemin group than in the AGEs + HG
group (Fig. 2D, E, F and
J; all P<0.05). CrMP exerted
selective effects against HO-1 activity. HO-1 protease activity
decreased by 25.2 and 10.7% in the NG + CrMP group and NG + Hemin +
CrMP group, respectively, compared with the NG group at 72 h
(Fig. 2H; both P<0.05),
decreased by 28.7 and 8.1% in the AGEs + NG + CrMP group and AGEs +
NG + Hemin + CrMP group, respectively, compared with the AGEs + NG
group (Fig. 2I; both P<0.05),
and decreased by 24.3 and 10.3% in the AGEs + HG + CrMP group and
AGEs + HG + Hemin + CrMP group, respectively, compared with the
AGEs + HG group (Fig. 2J; both
P<0.05). These results suggested that hemin treatment induced
HO-1 expression, and CrMP abolished the effects of hemin.
Therefore, a fibroblast model of high HO-1 expression was
successfully established.
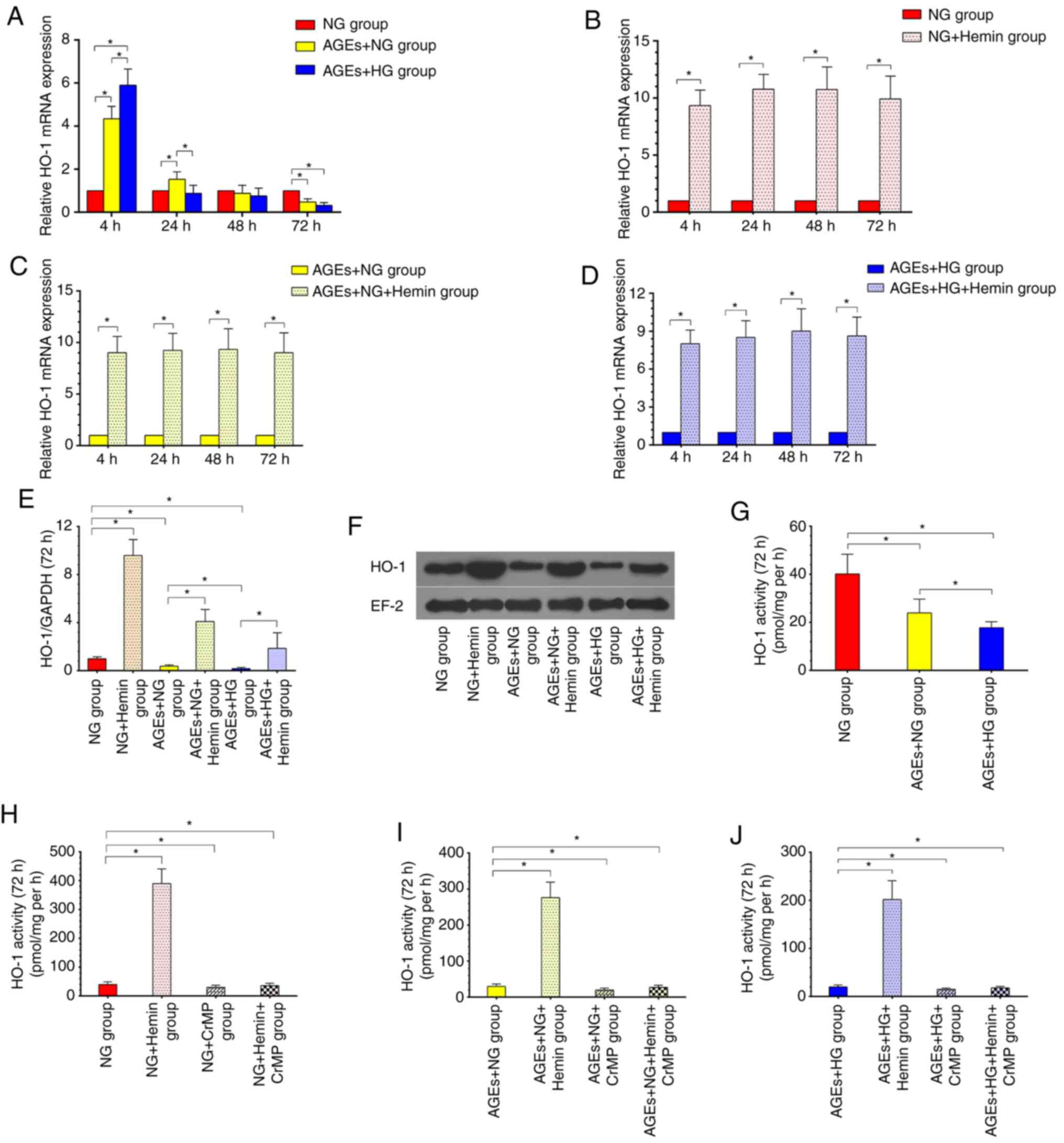 | Figure 2Establishment of the high HO-1
expression model. (A) HO-1 expression exhibited time-dependent
alterations. Within 4 h, HO-1 expression was increased, after which
it gradually declined and reached the lowest level at 72 h. (B)
Hemin induced HO-1 mRNA expression in NG + Hemin group. (C) Hemin
induced HO-1 mRNA expression in AGEs + NG + Hemin group. (D) Hemin
induced HO-1 mRNA expression in AGEs + HG + Hemin group. (E and F)
Hemin induced HO-1 protein expression in the hemin groups, as
indicated by western blotting. (G) In the AGES + NG and AGEs + HG
groups, the HO-1 activity was lower than that of the NG group. (H)
Hemin increased HO-1 activity, and CrMP abolished the effects of
hemin in the NG group. (I) Hemin increased HO-1 activity, and CrMP
abolished the effects of hemin in the AGEs + NG group. (J) Hemin
increased HO-1 activity, and CrMP abolished the effects of hemin in
the AGEs + HG group. All experiments were performed in triplicate,
and the data are presented as the means ± SD.
*P<0.05. NG, normal glucose; HG, high glucose; AGEs,
advanced glycation end products; HO-1, heme oxygenase-1; CrMP,
chromium mesoporphyrin. |
HO-1 alleviates fibroblast oxidative
stress
Hemin treatment for 72 h inhibited ROS, MDA and
8-OHdG production in the NG + Hemin group by 36.6, 34.3 and 52.8%,
respectively, compared with the NG group (all P<0.05). In
addition, 45.6, 36.5 and 61.6% inhibition was observed in the AGEs
+ NG + Hemin group compared with the AGEs + NG group (all
P<0.05), and 50.8, 47.7 and 48.7% inhibition was observed in the
AGEs + HG + Hemin group compared with the AGEs + HG group (all
P<0.05). CrMP abolished the effects of hemin. ROS, MDA and
8-OHdG levels in NG + Hemin + CrMP group were 1.06-, 1.03- and
1.01-fold higher, respectively, than those in the NG group at 72 h,
were 1.00-, 1.06- and 1.02-fold higher, respectively, in the AGEs +
NG + Hemin + CrMP group than those in the AGEs + NG group, and were
1.02-, 1.01- and 1.01-fold higher, respectively, in the AGEs + HG +
Hemin + CrMP group than those in the AGEs + HG group. These results
suggested that the hemin-induced HO-1 expression may alleviate
oxidative stress in fibroblasts, but CrMP abolished the effects of
hemin (Table I).
HO-1 alleviates the fibroblast
inflammatory response
Hemin treatment for 72 h inhibited TNF-α, IL-6 and
IL-1β production in the NG + Hemin group by 59.1, 55.4 and 56.8%,
respectively, compared with the NG group (all P<0.05). In
addition, 62.5, 44.9 and 52.4% inhibition was observed in the AGEs
+ NG + Hemin group compared with the AGEs + NG group (all
P<0.05), and 61.2, 53.7 and 45.4% inhibition was observed in the
AGEs + HG + Hemin group compared with the AGEs + HG group (all
P<0.05). However, TNF-α, IL-6 and IL-1β levels in the NG + Hemin
+ CrMP group were 1.07-, 1.08- and 1.11-fold higher, respectively,
than those in the NG group at 72 h, were 1.06-, 1.02- and 1.08-fold
higher, respectively, in the AGEs + NG + Hemin + CrMP group
compared with those in the AGEs + NG group, and were 1.06-, 1.03-
and 1.05-fold higher, respectively, in the AGEs + HG + Hemin + CrMP
group compared with those in the AGEs + HG group. These results
suggested that the hemin-induced HO-1 expression may alleviate the
inflammatory insult in fibroblasts, but CrMP abolished the effects
of hemin (Table I).
HO-1 improves fibroblast biological
behaviors
Hemin treatment for 72 h increased fibroblast
collagen secretion (1823.34±118.28 pg/ml), cell viability
(2.27±0.34), the S-phase cell ratio (32.25±3.38%), the
proliferation index (41.33±4.90%) and the horizontal migration rate
(0.95±0.21%) in the NG + Hemin group compared with the NG group
(943.61±92.17 pg/ml, 1.87±0.21, 23.67±2.67%, 29.14±2.55% and
0.73±0.19%, respectively; all P<0.05). Early apoptosis
(2.18±0.87%) and end-stage apoptosis and death (3.03±0.84%) were
decreased in the NG + Hemin group compared with the NG group
(4.36±1.42% and 5.37±1.63%, respectively; both P<0.05).
Fibroblast collagen secretion (1096.15±89.25 pg/ml), cell viability
(1.89±0.25), the S-phase cell ratio (27.05±4.80%), the
proliferation index (35.69±5.47%) and the horizontal migration rate
(0.71±0.18%) were increased in the AGEs + NG + Hemin group compared
with the AGEs + NG group (595.37±61.26 pg/ml, 1.46±0.18,
14.93±1.97%, 18.10±1.93% and 0.47±0.12%, respectively; all
P<0.05). Early apoptosis (6.44±1.24%) and end-stage apoptosis
and death (6.94±1.65%) were decreased in the AGEs + NG + Hemin
group compared with the AGEs + NG group (9.87±1.99% and
10.53±2.02%, respectively; both P<0.05). Fibroblast collagen
secretion (984.35±88.47 pg/ml), cell viability (1.57±0.19), and the
horizontal migration rate (0.59±0.14%) were increased in the AGEs +
HG + Hemin group compared with the AGEs + HG group (354.21±49.34
pg/ml, 1.09±0.17, 12.00±1.67%, 17.90±2.54% and 0.28±0.11%,
respectively) (P<0.05), and the S-phase cell ratio (17.55%±2.24)
and proliferation index (20.20±3.28%) were also increased.
End-stage apoptosis and death (17.07±2.99%; P<0.05) was
decreased in the AGEs + HG + Hemin group compared with the AGEs +
HG group (20.72±4.18%) (Table
II).
CrMP abolished the effects of hemin. Fibroblast
collagen secretion (912.58±98.16 pg/ml), cell viability
(1.72±0.23), the S-phase cell ratio (22.53±2.03%), and the
horizontal migration rate (0.70±0.17%) were inhibited in the NG +
Hemin + CrMP group compared with the NG group. Early apoptosis
(4.67±0.99%) and end-stage apoptosis and death (7.12±1.98%) were
increased in the NG + Hemin + CrMP group compared with the NG
group. Fibroblast collagen secretion (574.47±65.13 pg/ml), cell
viability (1.39±0.20), the S-phase cell ratio (12.14±1.75%), the
proliferation index (15.92±2.71%) and the horizontal migration rate
(0.38±0.15%) were inhibited in the AGEs + NG + Hemin + CrMP group
compared with the AGEs + NG group. End-stage apoptosis and death
(10.77±2.26%) was increased in the AGEs + NG + Hemin + CrMP group
compared with the AGEs + NG group. Fibroblast collagen secretion
(333.75±46.92 pg/ml), cell viability (1.01±0.13), the S-phase cell
ratio (11.17±0.89%), the proliferation index (16.05±1.68%) and the
horizontal migration rate (0.21±0.10%) were inhibited in the AGEs +
HG + Hemin + CrMP group compared with the AGEs + HG group. Early
apoptosis (13.78±2.54%) and end-stage apoptosis and death
(19.91±3.78%) were decreased in the AGEs + HG + Hemin + CrMP group
compared with the AGEs + HG group (Table II; Fig. S1). Collagen secretion, viability,
proliferation, apoptosis and migration are important biological
behaviors of fibroblasts (1). The
results of the present study suggested that the hemin-induced HO-1
expression may improve the biological behaviors of fibroblasts, but
CrMP abolished the effects of hemin.
HO-1 partially reverses fibroblast
functional disorders
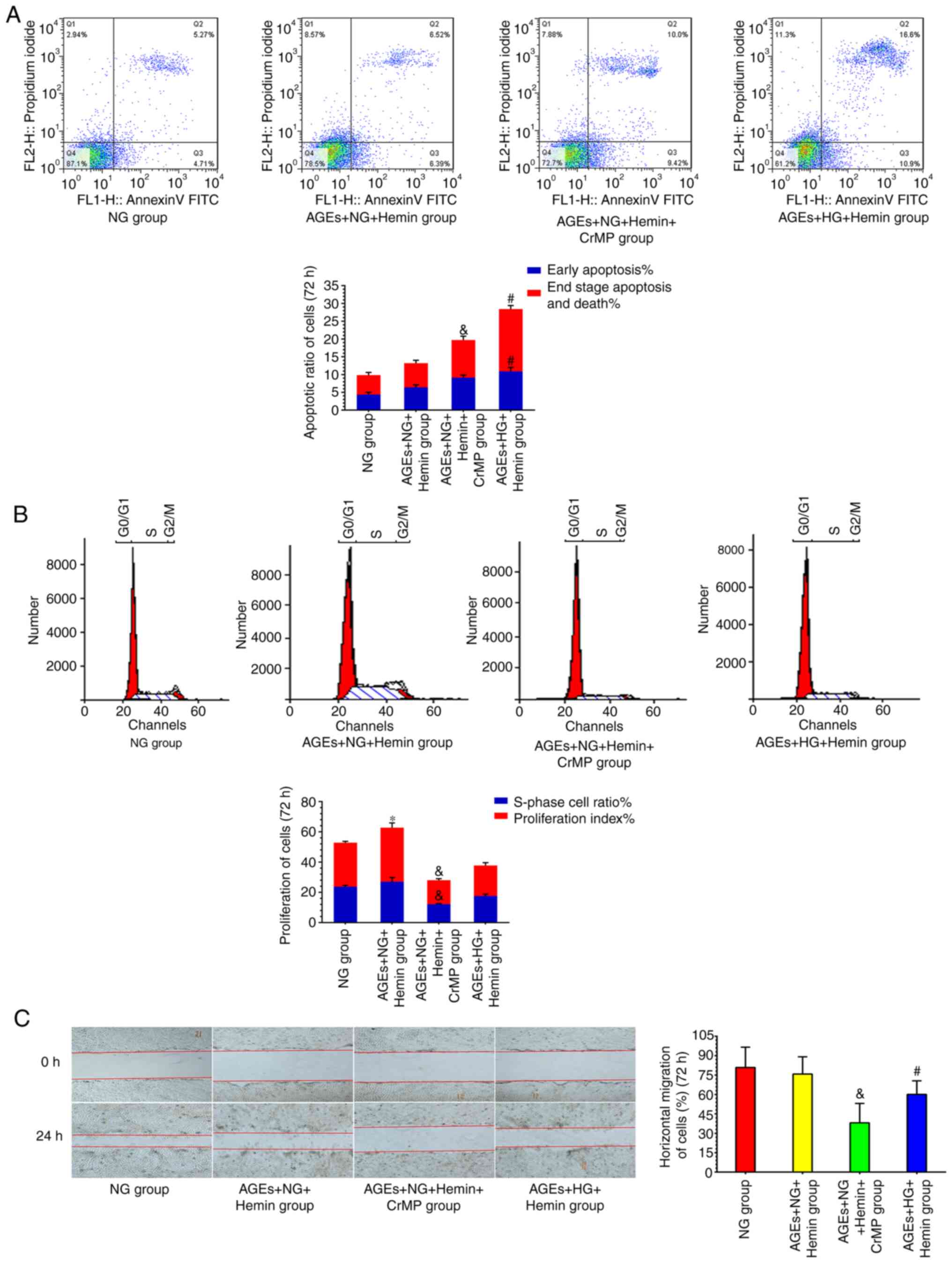
In the AGEs + NG + Hemin and AGEs + HG + Hemin
groups, markers of oxidative stress (ROS, MDA and 8-OHdG) and
inflammatory insult (TNF-α, IL-6 and IL-1β) were higher than those
in the NG group (Table I). Compared
with the NG group, fibroblast collagen secretion, viability and
proliferation were increased, the horizontal migration rate was
decreased, and the apoptosis rate was increased in the AGEs + NG +
Hemin group (Table II) (Fig. 3). In the AGEs + HG + Hemin group,
fibroblast collagen secretion was increased, cell viability,
proliferation and the horizontal migration rate were decreased, and
the cell apoptosis rate was increased compared with those in the NG
group (Table II) (Fig. 3).
The results suggested that in the AGEs + NG + Hemin
group, the hemin-induced HO-1 expression partially restored
cellular functions to levels similar to those of the NG group.
However, in the AGEs + HG + Hemin group, HO-1 expression hardly
restored cellular functions to levels similar to those of the NG
group.
Discussion
DFUs are nonhealing chronic wounds that are a
serious complication of diabetes (1,2). AGEs,
which are harmful compounds associated with diabetes, induce cell
apoptosis, oxidative and inflammatory insults, and previous studies
have demonstrated that AGEs result in delayed or impaired wound
repair in DFUs (14-16).
Various hyperglycemia-induced metabolic and hemodynamic
derangements, such as increased AGE deposition, enhance the
production of ROS, and stimulate protein kinase C, which may
contribute to DFUs (15). AGEs act
via their receptor for advanced glycosylation end product and have
been implicated in chronic diabetic wounds and inflammation
(16). AGEs have been implicated in
diabetes-related complications, including diabetic neuropathy,
diabetic nephropathy and DFU-related delayed wound healing
(17,18).
Diabetes is characterized by increases in oxidative
and inflammatory insults (19,20). A
previous study indicated that in a HG environment, rat dermal
fibroblast biological behavior was disrupted, and oxidative stress
indices (ROS and 8-OHdG) were increased (10). In the present study, alterations in
fibroblast biological behaviors, oxidative stress and inflammatory
insult indices were observed in the presence of AGEs. Oxidative
stress (ROS, MDA and 8-OHdG) and inflammatory insult indices
(TNF-α, IL-6 and IL-1β) were significantly increased in the present
of AGEs, and the biological behaviors of fibroblasts were impaired.
Specific alterations were observed, including increased cell
apoptosis and decreased collagen synthesis, viability, cell
proliferation and migration. AGEs caused oxidative stress,
inflammatory insult and fibroblast biological behavioral disorders,
and severe fibroblast functional damage was induced by the
combination of AGEs and high glucose. High glucose toxicity and AGE
deposits are associated with cellular dysfunction, and when
combined these factors can cause further damage to the cells
(10,14).
Previous studies have demonstrated that oxidative
stress and chronic inflammation are involved in the damage that
occurs in diabetes (19,20). HO-1 is an antioxidative,
anti-inflammatory and cytoprotective enzyme that is expressed as a
protective response to stress (6).
The HO system can suppress these injuries by generating carbon
monoxide, bilirubin/biliverdin and free divalent iron to oppose
apoptosis, inflammation and oxidative stress (6,10).
Basal HO activity is maintained by HO-2, while HO-1 is stimulated
by a wide variety of physical, chemical and pathophysiological
stimuli, including oxidative and inflammatory insults, as well as
metabolic and hemodynamic factors, such as high glucose, elevated
blood pressure and increased lipid levels (9,10).
Therefore, the expression of HO-1 is an important protective
response to a wide variety of stress types (8,9). It
has been revealed that HO-1 expression is differentially regulated
in organs under different disease states (6-10).
Our previous study indicated that high glucose could induce HO-1
expression, but this effect was short-lived (10). The present study revealed that in
the presence of AGEs, HO-1 expression exhibited a time-dependent
alteration.
HO-1 is an inducible isoform and is activated by a
variety of stimuli, such as hemin, lipopolysaccharide and
H2O2 (9). In
the present study, hemin was used to induce HO-1 expression, and
CrMP, which exhibits selective effects against HO activity
(11), was used to abolish the
effects of hemin. The results demonstrated that hemin markedly
induced HO-1 expression. On the other hand, the coadministration of
the HO blocker CrMP and HO inducer hemin abolished the effects of
hemin, whereas in the presence of CrMP alone, HO-1 exhibited
reduced expression. These results suggested that a high HO-1
expression model was successfully established.
Inducible HO-1 functions in a wide range of
processes that may be important in the resolution phase of wound
healing, such as the amelioration of oxidative injury and
inflammation and protection against cell apoptosis (8,10).
Oxidative stress serves an important role in the development of
diabetic foot, and the disruption of the redox balance contributes
to poor healing (5,19). The present study demonstrated that
AGE treatment increased the cellular ROS, MDA and 8-OHdG levels.
ROS are common oxidative damage indicators that are highly
expressed in diabetic conditions (4,8). MDA
is a marker of oxidative stress that is a product of lipid
peroxidation, and is involved in the oxidative conversion of
polyunsaturated fatty acids. This reaction is the most studied
biologically relevant free radical reaction (4). 8-OHdG is a sensitive indicator of
oxidative damage to DNA and is increased in diseases, such as
diabetes and obesity (5,21). Oxidative stress is one of the causes
of cell and tissue damage in diabetes (19,21).
It has been indicated that HO-1 exerts protective effects against
diabetes-induced oxidative stress, and could decrease MDA and ROS
levels (22). The induction of HO-1
with hemin can suppress oxidative stress in diabetic rats (9,23). In
the present study, the ROS, MDA and 8-OHdG levels were increased in
the presence of AGEs, but hemin treatment notably reduced ROS, MDA
and 8-OHdG levels. On the other hand, the coadministration of CrMP
and hemin abolished the effects of hemin, whereas treatment with
CrMP alone increased the levels of ROS, MDA and 8-OHdG. Therefore,
the results indicated that HO-1 alleviated AGE-induced oxidative
injury in fibroblasts.
Inflammatory insults serve an important role in the
development of diabetic foot, and several proinflammatory cytokines
have been indicated to be significantly elevated in diabetes
(19,24). It has been demonstrated that hemin
induces the HO-1-mediated suppression of proinflammatory cytokines
(TNF-α, IL-6 and IL-1β), which in turn activates the JNK and NF-κB
pathways, leading to a vicious circle that exacerbates diabetic
complications (7). The effects of
concomitantly activating the HO system with hemin treatment on
these cytokines were investigated. In the present study, the levels
of TNF-α, IL-6 and IL-1β were markedly elevated in the presence of
AGEs. Interestingly, hemin significantly abated the increases in
the levels of TNF-α, IL-6 and IL-1β. On the other hand, the
coadministration of CrMP and hemin abolished the effects of hemin,
whereas treatment with CrMP alone increased the levels of TNF-α,
IL-6 and IL-1β. Therefore, it was revealed that HO-1 alleviated
fibroblast inflammatory insults caused by AGEs.
Fibroblasts act as major repair cells in skin wounds
(25). Fibroblasts isolated from
DFUs are likely to be senescent and exhibit slow, declining
proliferative responses (1,25). The present study demonstrated that
fibroblast biological functions were impaired in the presence of
AGEs. The specific effects included increased apoptosis and
decreased collagen synthesis, viability, proliferation and
migration, with severe functional damage being caused by the
combination of AGEs and HG conditions. In normal skin, type I and
III collagen coexist in a ratio of ~3.5:1 (1,26). In
certain pathological conditions, such as diabetic wound healing,
AGE deposition, excessive inflammatory reactions and enhanced
oxidative stress damage, the proportion of type III collagen is
increased, and excessive type III collagen results in scar
hyperplasia or fibrosis (1,26). In the present study, fibroblast
collagen secretion decreased in the presence of AGEs, while HO-1
increased fibroblast collagen secretion and decreased the
inflammatory response and oxidative stress injury. HO-1 may reduce
fibroblast functional disorders induced by AGEs and accelerate the
healing of diabetic wounds by improving fibroblast biological
behaviors and reducing oxidative stress and inflammatory insults.
Further animal experiments on skin collagen components and
elucidation of the associated mechanisms are required.
Delayed diabetic wound healing is associated with
impaired fibroblast biological behaviors to a certain extent
(1,25). Keyse and Tyrrell (27) first suggested the cytoprotective
role of HO-1. Impaired wound healing in diabetic mice may be
associated with delayed HO-1 upregulation, and HO-1 gene transfer
has been indicated to improve wound healing (8,28). It
was observed that the biological behaviors of fibroblasts were
impaired in the presence of AGEs for 72 h. However, the HO-1
inducer hemin increased fibroblast collagen synthesis and cell
viability, improved proliferation and migration and decreased cell
apoptosis. On the other hand, the coadministration of CrMP and
hemin abolished the effects of hemin, whereas treatment with CrMP
alone exacerbated the disordered fibroblast biological behaviors.
It was hypothesized that HO-1 alleviated the disordered fibroblast
biological behavior caused by AGEs. HO-1 is a protective enzyme
that is highly expressed in response to stress (6-8).
In the present study, the antioxidative, anti-inflammatory and
cytoprotective effects of HO-1 were investigated. The results
suggested that HO-1 alleviated the fibroblast functional disorders
induced by AGEs, but it was difficult to reverse the functional
disorders and restore cellular functions to normal levels.
In conclusion, the results of the present study
indicated that compared with normal glucose conditions, AGEs
induced fibroblast oxidative stress, inflammatory insult and
biological behavioral disorders, and severe cell damage was caused
by the combination of AGEs and HG conditions. Hemin treatment
induced HO-1 expression, reduced oxidative stress (ROS, MDA and
8-OHdG) and inflammatory insult indicators (TNF-α, IL-6 and IL-1β)
and improved cell biological behaviors, including increased
cellular collagen synthesis, viability, proliferation and migration
and decreased cell apoptosis. These findings suggested that HO-1
may reduce fibroblast functional disorders and accelerate the
healing of diabetic wounds by improving fibroblast biological
behaviors and reducing oxidative stress and inflammatory responses.
Increasing HO-1 expression may represent a feasible strategy for
improving diabetic wound healing.
Supplementary Material
Supplementary material of fibroblast
apoptosis, proliferation and horizontal migration images. (A) Cell
apoptosis, (B) cell proliferation and (C) cell horizontal migration
of the remaining six groups is demonstrated. NG, normal glucose;
HG, high glucose; AGEs, advanced glycation end products; CrMP,
chromium mesoporphyrin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from National
Natural Science Foundation of China (grant no. 81801757), Natural
Science Foundation of Guangdong Province (grant no.
2018A030310322), Guangdong Basic and Applied Basic Research
Foundation (grant no. 2019A1515012051) and Guangdong Medical
Research Foundation (grant no. A2018106).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QLL and RMG confirm the authenticity of all the raw
data. QLL, SYL, RMG, QWL, LSS and YZ conceived and designed the
study, acquired, analyzed and interpreted data. QLL and SYL drafted
and revised the manuscript for important intellectual content. QWL
and LSS performed literature search. YZ and RMG edited the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maione AG, Smith A, Kashpur O, Yanez V,
Knight E, Mooney DJ, Veves A, Tomic-Canic M and Garlick JA: Altered
ECM deposition by diabetic foot ulcer-derived fibroblasts
implicates fibronectin in chronic wound repair. Wound Repair Regen.
24:630–643. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guo Y, Lin C, Xu P, Wu S, Fu X, Xia W and
Yao M: AGEs induced autophagy impairs cutaneous wound healing via
stimulating macrophage polarization to M1 in diabetes. Sci Rep.
6(36416)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rajaobelina K, Helmer C,
Vélayoudom-Céphise FL, Nov S, Farges B, Pupier E, Blanco L, Hugo M,
Gin H and Rigalleau V: Progression of skin autofluorescence of AGEs
over 4 years in patients with type 1 diabetes. Diabetes Metab Res
Rev. 33(e2917)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen Y, Wu Y, Gan X, Liu K, Lv X, Shen H,
Dai G and Xu H: Iridoid glycoside from Cornus officinalis
ameliorated diabetes mellitus-induced testicular damage in male
rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK
signaling pathway. J Ethnopharmacol. 194:850–860. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Aubaidy HA and Jelinek HF: Oxidative
DNA damage and obesity in type 2 diabetes mellitus. Eur J
Endocrinol. 164:899–904. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ndisang JF: Role of heme oxygenase in
inflammation, insulin-signalling, diabetes and obesity. Mediators
Inflamm. 2010(359732)2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ndisang JF and Jadhav A: Hemin therapy
improves kidney function in male streptozotocin-induced diabetic
rats: Role of the heme oxygenase/atrial natriuretic
peptide/adiponectin axis. Endocrinology. 155:215–229.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen QY, Wang GG, Li W, Jiang YX, Lu XH
and Zhou PP: Heme oxygenase-1 promotes delayed wound healing in
diabetic rats. J Diabetes Res. 2016(9726503)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ndisang JF and Jadhav A: Up-regulating the
hemoexygenase system enhances insulin sensitivity and improves
glycose metabolism in insulin-resistant diabetes in Goto-Kakizaki
rats. Endocrinology. 150:2627–2636. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li QL, Guo RM, Zhao K, Lin DZ, Ye XM and
Chen LH: Effects of heme oxygenase-1 expression on oxidative injury
and biological behaviours of rat dermal fibroblasts. J Wound Care.
27:780–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reis WL, Biancardi VC, Son S,
Antunes-Rodrigues J and Stern JE: Enhanced expression of heme
oxygenase-1 and carbon monoxide excitatory effects in oxytocin and
vasopressin neurones during water deprivation. J Neuroendocrinol.
24:653–663. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xue SN, Lei J, Yang C, Lin DZ and Yan L:
The biological behaviors of rat dermal fibroblasts can be inhibited
by high levels of MMP9. Exp Diabetes Res.
2012(494579)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Okano Y, Masaki H and Sakurai H:
Dysfunction of dermal fibroblasts induced by advanced glycation
end-products (AGEs) and the contribution of a nonspecific
interaction with cell membrane and AGEs. J Dermatol Sci.
29:171–180. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamagishi S, Maeda S, Matsui T, Ueda S,
Fukami K and Okuda S: Role of advanced glycation end products
(AGEs) and oxidative stress in vascular complications in diabetes.
Biochim Biophys Acta. 1820:663–671. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dong MW, Li M, Chen J, Fu TT, Lin KZ, Ye
GH, Han JG, Feng XP, Li XB, Yu LS and Fan YY: Activation of α7nAChR
Promotes Diabetic Wound Healing by Suppressing AGE-Induced TNF-α
Production. Inflammation. 39:687–699. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu H, Jiang H, Ren H, Hu X, Wang X and Han
C: AGEs and chronic subclinical inflammation in diabetes: disorders
of immune system. Diabetes Metab Res Rev. 31:127–137.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tesch G, Sourris KC, Summers SA, McCarthy
D, Ward MS, Borg DJ, Gallo LA, Fotheringham AK, Pettit AR, Yap FY,
et al: Deletion of bone-marrow-derived receptor for AGEs (RAGE)
improves renal function in an experimental mouse model of diabetes.
Diabetologia. 57:1977–1985. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vairamon SJ, Babu M and Viswanathan V:
Oxidative stress markers regulating the healing of foot ulcers in
patients with type 2 diabetes. Wounds. 21:273–279. 2009.PubMed/NCBI
|
|
20
|
Ingram JR, Cawley S, Coulman E, Gregory C,
Thomas-Jones E, Pickles T, Cannings-John R, Francis NA, Harding K,
Hood K and Piguet V: Levels of wound calprotectin and other
inflammatory biomarkers aid in deciding which patients with a
diabetic foot ulcer need antibiotic therapy (INDUCE study). Diabet
Med. 35:255–261. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zheng F, Lu W, Jia C, Li H, Wang Z and Jia
W: Relationships between glucose excursion and the activation of
oxidative stress in patients with newly diagnosed type 2 diabetes
or impaired glucose regulation. Endocrine. 37:201–208.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song Y, Huang L and Yu J: Effects of
blueberry anthocyanins on retinal oxidative stress and inflammation
in diabetes through Nrf2/HO-1 signaling. J Neuroimmunol. 301:1–6.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ndisang JF and Jadhav A: Heme oxygenase
system enhances insulin sensitivity and glucose metabolism in
streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab.
296:E829–E841. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Van Asten SA, Nichols A, La Fontaine J,
Bhavan K, Peters EJ and Lavery LA: The value of inflammatory
markers to diagnose and monitor diabetic foot osteomyelitis. Int
Wound J. 14:40–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Berlanga-Acosta J, Mendoza-Mari Y,
Martínez MD, Valdés-Perez C, Ojalvo AG and Armstrong DG: Expression
of cell proliferation cycle negative regulators in fibroblasts of
an ischemic diabetic foot ulcer. A clinical case report. Int Wound
J. 10:232–236. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Davison-Kotler E, Marshall WS and
García-Gareta E: Sources of collagen for biomaterials in skin wound
healing. Bioengineering (Basel). 6(56)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Keyse SM and Tyrrell RM: Heme oxygenase is
the major 32-kDa stress protein induced in human skin fibroblasts
by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl
Acad Sci USA. 86:99–103. 1989.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grochot-Przeczek A, Lach R, Mis J,
Skrzypek K, Gozdecka M, Sroczynska P, Dubiel M, Rutkowski A,
Kozakowska M, Zagorska A, et al: Heme oxygenase-1 accelerates
cutaneous wound healing in mice. PLoS One. 4(e5803)2009.PubMed/NCBI View Article : Google Scholar
|