Introduction
Rheumatoid arthritis (RA) is a debilitating,
systemic and chronic autoimmune disease (1). The characteristics of RA include
synovial hyperplasia, joint destruction and extra-articular
manifestations, which finally leads to tissue destruction (2). A combination of gene, environment and
immunology factors has been well-documented to be responsible for
the onset and development of RA (3). Furthermore, multiple inflammatory
mediators (small factors that are secreted by cells and then induce
an inflammatory response), immune cells and non-immune cells are
collectively involved in the inflammatory processes in RA (4). Interleukin (IL)-1β, IL-6, IL-17,
IL-22, IL-23 and tumor necrosis factor (TNF)-α are inflammatory
mediators that serve an important role in RA mainly at the synovial
level, evoking significant pathological changes (5); among these changes, the infiltration
of self-reactive T and B cells, including Th17 cells (immune
cells), initiates a complex autoimmune response and produces
certain cytokines and proliferation factors that drive fibroblasts
(non-immune cells) differentiate into synoviocyte-like cells,
called synovial fibroblasts (SFs) (6). Furthermore, loss of balance in SF
proliferation and apoptosis leads to hypertrophied synovium or
pannus formation, which is a hallmark of RA (7).
At present, clinically used drugs for RA have been
advanced, including nonsteroidal anti-inflammatory drugs,
immunosuppressive agents, glucocorticoids and biological agents
(8). However, only 50-60% of
patients with RA respond to these drugs owning to their inherent
limitations in the clinical treatment of RA (9). Over the past few years, certain
studies have suggested that genetic approach may offer novel
therapeutic methods for RA (6,10).
Furthermore, microRNAs (miRNAs) have been revealed to demonstrate
diverse crucial roles in the pathological process of RA (11). miRNAs are short, non-coding RNA
sequences that repress gene expression generally by binding to the
3'-untranslated region (3'-UTR) of target messenger RNAs (mRNAs).
Accumulating data have revealed miRNAs as multifunctional
regulators in numerous physiological processes, including
proliferation, apoptosis and differentiation (12). Pathologically, miRNAs have been
extensively studied and applied as biomarkers and targets for
cancers. Furthermore, recent studies have indicated that miRNAs are
gaining increasing recognition for their involvement in autoimmune
diseases (13-15),
including RA. Altered expression of miRNAs is reported to be
associated with the occurrence and development of RA (16,17).
Several studies have evaluated miRNAs expression in
RA patient samples, including plasma, synovial tissue and SFs
(18-20).
Among these miRNAs, miR-27a-3p is downregulated in patients with RA
and serves a crucial role in RASF migration and invasion, which are
partly answerable for the spread of arthritic destruction to
distant joints (18,21). Furthermore, expression of miR-27a-3p
is associated with the patient's responsiveness to therapy
(13,18). However, its role in RA has not been
completely elucidated. Based on the existing data stating the
dysregulation of miR-27a-3p in synovial tissues and SFs from
patients with RA (13,18,21),
and the present bioinformatics data from DianaTools (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index)
predicting the association between miR-27a-3p and TLRs; therefore,
miR-27a-3p and TLR5 received attention to be further investigated
regarding their role in RA pathogenesis and progression in
RASFs.
The present study isolated human synovial tissues
and RASFs from patients with RA and further investigated the
expression of miR-27a-3p. The functional roles of miR-27a-3p and
TLR5 in RASF proliferation, apoptosis and inflammation were
determined, as well as the association between miR-27a-3p and
TLR5.
Materials and methods
Collection of tissue samples
The present study was performed according to the
recommendations of the Declaration of Helsinki. A total of 27
patients with RA and 27 non-RA control subjects between January
2013 and December 2016 were recruited from General Hospital of
Central Theater Command (Wuhan, China). The present study was
performed with the approval of the Research Ethics Committee of
General Hospital of Central Theater Command. The patients with RA
were diagnosed with the 2010 American College of
Rheumatology/European League against Rheumatism classification
criteria (2010 ACR/EULAR) for RA (22). The control subjects were patients
with osteoarthritis and joint trauma but free of autoimmune disease
and infectious disease. Patients with RA who achieved a score of at
least 6 out of 10 points according to the 2010 ACR/EULAR were
included in the present study. The exclusion criteria for the
patients were as follows: i) Presence of malignant diseases such as
cancer; ii) presence of central or peripheral nervous system
diseases such as trigeminal neuralgia; iii) presence of cardiac
diseases such as coronary heart disease, and iv) presence of
endocrine disorders (especially diabetes mellitus). The demographic
characteristic of patients with RA are presented in Table I. The synovial tissue samples from
patients were obtained while undergoing joint surgery at the
hospital. Tissue samples were obtained following the written
informed consents being obtained from all patients. All tissue
samples were immediately frozen in liquid nitrogen and then stored
at -80˚C prior to use.
 | Table IDemographic characteristics and
miR-27a-3p expression in synovial tissue samples from patients with
RA. |
Table I
Demographic characteristics and
miR-27a-3p expression in synovial tissue samples from patients with
RA.
| Features | Low miR-27a-3p
expression (n=15) | High miR-27a-3p
expression (n=12) | P-value |
|---|
| Age, mean ± SD | 52.43±8.59 | 51.35±10.25 | 0.698 |
| Male/female, n | 7/8 | 5/7 | 0.325 |
| CRP, mg/l | 35.57±15.33 | 20.35±10.25 | 0.037a |
| DAS28 | 13.59±3.58 | 5.21±1.02 | 0.003a |
| ESR, mm/h | 22.33±3.83 | 14.08±2.65 | 0.046a |
| RF positive, % | 56.25 | 51.39 | 1.029 |
| Anti-CCP | 198.25±56.32 | 193.57±42.85 | 1.058 |
| Swollen joint
count | 2.56±0.58 | 0.53±0.22 | 0.024a |
Isolation and culture of RASFs
As previously described (23), RASFs and control synovial
fibroblasts (CSFs) were isolated from eight indicated patients,
respectively. In short, synovial tissues were washed with sterile
phosphate buffer solution (PBS), followed by being minced and
digested using 1 mg/ml collagenase type II (Invitrogen; Thermo
Fisher Scientific, Inc.) for 4 h at 37˚C. The cells were collected
and incubated in RPMI-1640 (HyClone; GE Healthcare Life Sciences),
containing 10% fetal bovine serum (HyClone; GE Healthcare Life
Sciences) and 4 mM glutamine at 37˚C in 5% CO2 for 1
month with a change of culture medium every 3 days. All experiments
were conducted using cultured cells between passages 3-6.
Cell transfection
RASFs were seeded into 6-well plates (Corning
Incorporate) for 24 h prior to transfection. Plasmids (3 µg) and
miRNA/siRNA (100 nM) were transfected into RASFs using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 4-6 h, according to the
manufacturer's protocol. Transfected miR-27a-3p mimic (miR-27a-3p;
5'-UUCACAGUGGCUAAGUUCCGC-3'), miR-27a-3p inhibitor (in-miR-27a-3p;
5'-GCGGAACUUAGCCACUGUGAA-3') and siRNA targeting toll like receptor
5 (si-TLR5; 5'-CUGUGA UGAGAUUCCUAUAGU-3' and 5'UAUAGGAAUCUCAUC
ACAGUG-3') were purchased from Guangzhou Ribobio Co., Ltd., as well
as the negative controls miR-NC (5'-ACGUGACAC GUUCGGAGAATT-3'),
in-miR-NC (UUCUCCGAACGUGUC ACGUTT) and si-NC
(5'UUCUCCGAACGUGUCACGUTT-3' and 5'ACGUGACACGUUCGGAGAATT-3'). The
full-length human TLR5 coding domain sequence was cloned into
pcDNA4.1 (Invitrogen; Thermo Fisher Scientific, Inc.). In rescue
experiments, co-transfection was launched with 1.5 µg plasmid and
60 nM miR-27a-3p or miR-NC. Cells were subsequently cultured for
another 30 h prior to further study.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and
RASFs using Qiagen miRNeasy Mini kit (Qiagen GmbH), according to
the manufacturer's protocol. The cDNAs were synthesized depending
on total RNA and a reverse transcription kit (Abcam, Canada). The
reverse transcription protocol was as follows: 25˚C for 5 min, 55˚C
for 15 min and 85˚C for 5 min. The amplification of cDNAs was
performed using a SYBR®-Green Master mix kit (Qiagen
GmbH) on an Applied Biosystems 7500 Real-Time PCR system (Thermo
Fisher Scientific, Inc.). The primers were as follows: miR-27a-3p
forward, 5'-ACACTCCAGCTGGGTTCACAGTG GCTAAG-3' and reverse,
5'-AGGGCTTAGCTGCTTGTGA GCA-3'; TLR5 forward,
5'-TGCCACTGTTGAGTGCAAGTC-3' and reverse,
5'-ACCTGGAGAAGCCGAAGGTAA-3'; GAPDH forward,
5'-GACAGTCAGCCGCATCTTCT-3' and reverse, 5'-GCGCCCAATACGACCAAA-3';
U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'. The thermocycling conditions used
were 95˚C for 10 min, followed by 40 cycles of 95˚C for 15 sec,
60˚C for 30 sec and 60˚C for 15 sec. The reaction was held at 74˚C
for continuous for melting curve analysis. The relative expression
levels of miR-27a-3p and TLR5 mRNA were calculated by
2-ΔΔCT methods (24)
with normalization to U6 small nuclear RNA (U6) and GAPDH,
respectively. All PCR reactions were performed in triplicate.
Protein extraction and western
blotting
Total protein was extracted from cultivated RASFs
using RIPA lysis buffer (Beyotime Institute of Biotechnology), and
protein concentration was determined using the BCA method. TLR5
protein expression was measured using western blotting, which was
performed according to standard procedures (25). A total of 20 µg was protein per lane
lane and separated using 8-10% SDS-PAGE. PVDF membranes (EMD
Millipore) were blocked with 3% BSA (Sigma-Aldrich; Merck KGaA) at
25˚C for 1 h. β-actin on the same membrane was used as the loading
control. The antibodies were purchased from Abcam and were as
follows: anti-TLR5 (1:2,000; cat. no. ab168382), anti-β-actin
(1:10,000; cat. no. ab227387;), horse-radish peroxidase
(HRP)-labelled anti-Rabbit IgG (1:50,000; cat. no. ab205718).
Primary antibody incubation was at 4˚C overnight and secondary
antibody incubation was performed at 25˚C for 1 h. The proteins
were visualized using ECL procedure (EMD Millipore), and ImageJ
v1.8.0 software (National Institutes of Health, Bethesda) was used
to analyze the gray intensity of the bands.
MTT assay
Transfected RASFs (5,000 cells) were seeded onto
96-well plates (Corning Incorporated) for 0, 24, 48 and 72 h. The
cell proliferation was determined by MTT staining. 5 mg/ml MTT (20
µl; Sigma-Aldrich; Merck KGaA) was added for another 4
h-incubation; the formazan was dissolved in 150 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA). The absorbance at 450 nm was
measured on a microplate reader (Molecular devices, LLC). All
experiments were performed in triplicate.
Apoptosis assay
The apoptotic rate of transfected RASFs was analyzed
using a Annexin V-FITC/propidium iodide (PI) kit (Beyotime
Institute of Biotechnology) using flow cytometry. Following
transfection for 30 h, apoptotic cells were dual-labelled with
Annexin V-FITC and PI for 30 min in the dark, and fluorescence was
analyzed on a cytoFLEX LX flow cytometer (Beckman Coulter, Inc.),
supplemented with CytExpert v.2.0 software (Beckman Coulter, Inc.).
Quadrants were positioned on Annexin V/PI plots to distinguish
apoptotic cells (Annexin V+/PI-, Annexin
V+/PI+). Apoptosis (%) = apoptotic
cells/total cells x100%.
ELISA
ELISA was conducted to measure the concentrations of
IL-1β, IL-6 and TNF-α released in the culture medium of RASFs.
Following transfection for 30 h, the culture supernatant of RASFs
was collected and subjected to the ELISA kits purchased from Abcam:
Human IL-1β ELISA kit (cat. no. ab100562), human IL-6 ELISA kit
(cat. no. ab46027) and human TNF-α ELISA kit (cat. no. ab46087).
All operations were performed according to the manufacturer's
protocols, and the reactions were set in triplicate for each
sample.
Bioinformatics analysis
According to in silico data on DIANA TOOLS
v3.0 (DianaTools; http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index),
potential binding sites of TLR5 (ENSG00000187554) and
hsa-miR-27a-3p were investigated.
Luciferase reporter assay
The fragment sequence of TLR5 3'-UTR containing the
potential binding sites of miR-27a-3p was cloned by PCR methods
into pGL4 vector (Invitrogen; Thermo Fisher Scientific, Inc.) to
construct pGL4-TLR5 3'-UTR-WT vectors; similarly, pGL4-TLR5
3'-UTR-MUT vectors were constructed to carry the TLR5 3'-UTR
fragment containing the mutations of miR-27a-3p responsive
elements. RASFs were co-transfected with pGL4-TLR5 3'-UTR-WT/MUT
vectors and miRNA mimics or inhibitors for 48 h using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and cell lysate was collected to measure Firefly
and Renilla luciferase activities using the dual-luciferase
reporter assay system (Promega Corporation). At least three
independent transfections were set in each group and the relative
luciferase activity was presented as fold-change normalized to the
negative control group.
Biotin-coupled miRNA pull-down
assay
Biotin-labelled miR-27a-3p mimic (Bio-miR-27a-3p;
biotin-UUCACAGUGGCUAAGUUCC) and biotin-labelled miR-27a-3p-MUT
mimic (Bio-miR-27a-3p MUT; bio-AAGUGUCUGGCUAAGUUCC), as well as
biotin-labelled miR-NC mimic (Bio-miR-NC;
biotin-UUCUCCGAACGUGUCACGU) were synthesized by Guangzhou Ribobio
Co., Ltd. RASFs were transfected with 50 µM of the aforementioned
biotinylated miRNAs for 30 h. Next, cell lysates of transfected
RASFs were obtained by lysing cells in lysis buffer and sonicate.
Subsequently, cell lysates were incubated with 50 µl
streptavidin-coated magnetic beads (Sigma-Aldrich; Merck KGaA) at
room temperature for 2 h, and biotin-coupled miRNA capture was
subjected to Qiagen miRNeasy Mini kit (Qiagen GmbH) to recycle the
precipitated RNAs. Expression of TLR5 mRNA in the aforementioned
precipitated RNAs was analyzed using RT-qPCR.
Statistical analysis
Statistics were analyzed by SPSS 21.0 (IBM Corp.)
and data are presented as the mean ± standard deviation (Table SI). The independent samples t-test
method was utilized for comparison between two groups, and one-way
analysis of variance (ANOVA) was for comparison among multiple
groups. Tukey's post hoc test was applied following ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
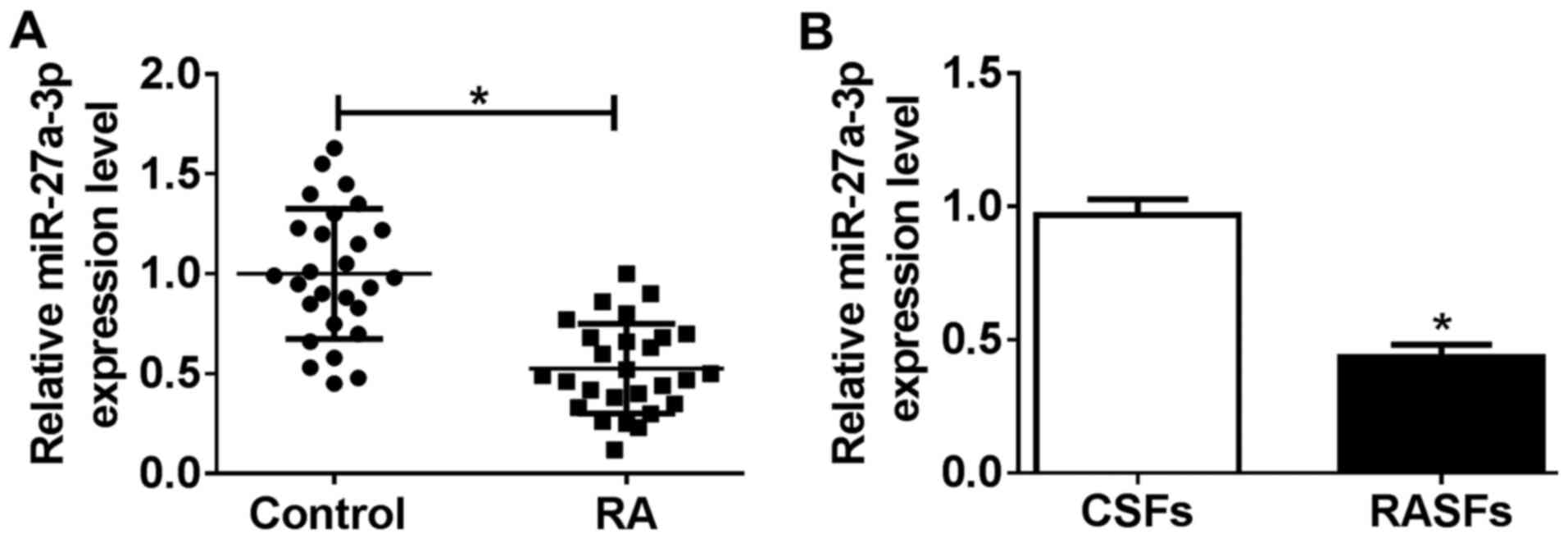
Expression of miR-27a-3p is
downregulated in human RA synovial tissues and RASFs
To investigate the role of miR-27a-3p in
pathological and physiological changes in patients with RA, the
present study investigated miR-27a-3p expression in RA specimens.
According to RT-qPCR analysis, miR-27a-3p levels were significantly
downregulated (0.51-fold; P<0.05) in RA synovial tissues,
compared with that from non-RA controls (Fig. 1A). Furthermore, RASFs and CSFs were
isolated from eight patients with RA and eight non-RA controls,
respectively. As shown in Fig. 1B,
the level of miR-27a-3p was decreased (0.45-fold; P<0.05) in
RASFs, compared with that in CSFs. Furthermore, low expression of
miR-27a-3p was correlated with several RA features, including a
disease activity score of 28-joint (DAS28), swollen joint count,
C-reactive protein (CRP) level and erythrocyte sedimentation rate
(ESR; Table I). Therefore, we
hypothesized that dysregulation of miR-27a-3p may serve a potential
role in RASF dysfunction, and that miR-27a-3p may be a potential
clinical diagnostic marker in RA.
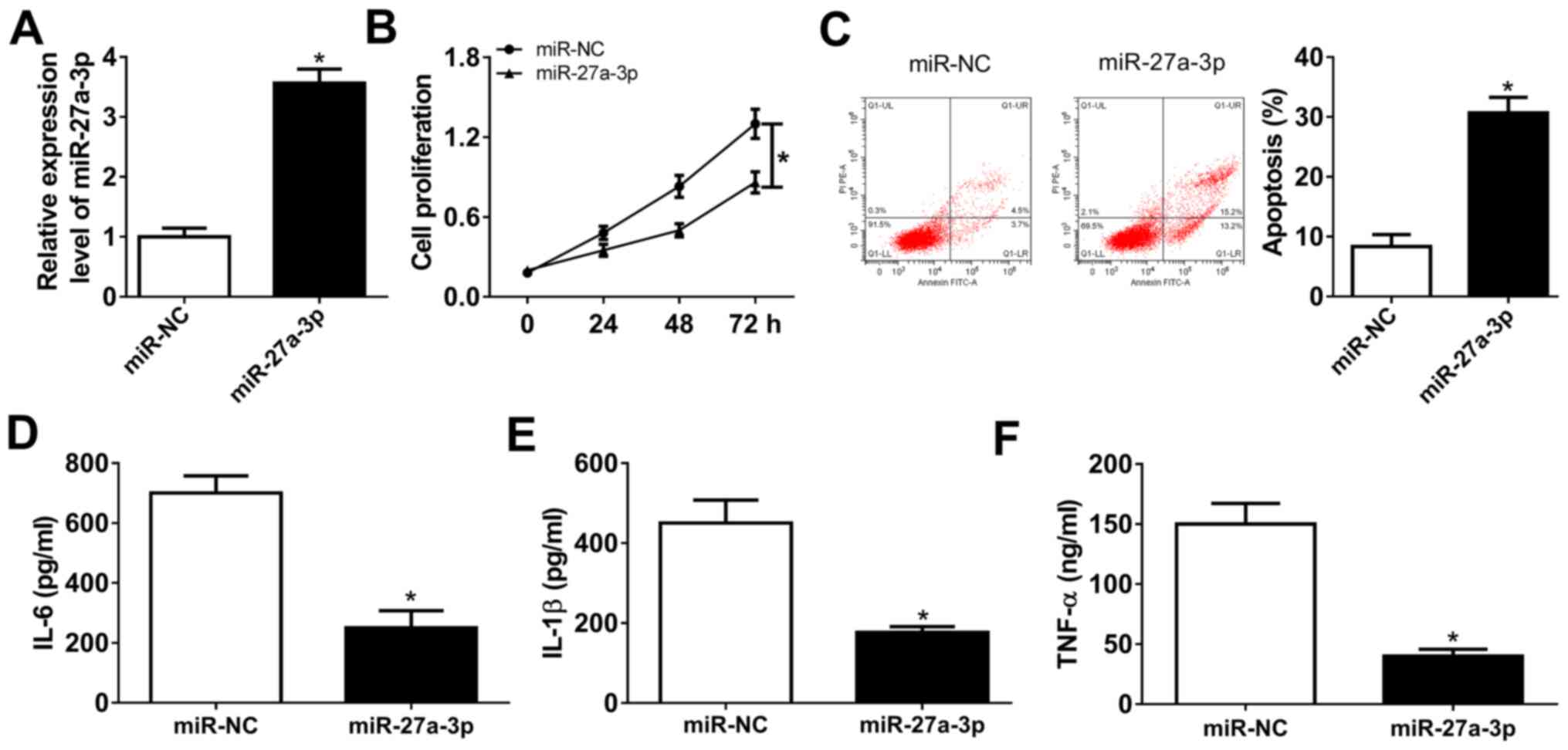
Upregulation of miR-27a-3p suppresses
proliferation and inflammatory response of human RASFs
Isolated RASFs were cultured and miR-27a-3p was
highly expressed ex-vivo. Gain-of-function experiments were
performed in RASFs following mimic transfection. To begin with,
transfection efficiency was validated by RT-qPCR, and miR-27a-3p
was significantly overexpressed (3.7-fold; P<0.05) in RASFs in
the presence of exogenous miR-27a-3p, compared with miR-NC
(Fig. 2A). The MTT assay
demonstrated that the cell proliferation of RASFs was attenuated by
miR-27a-3p mimic transfection (Fig.
2B). FCM revealed a distinctive augmentation of apoptosis rate
(from 8.3 to 30.7%; P<0.05) in RASFs transfected with miR-27a-3p
with normalization to control transfection (Fig. 2C). Furthermore, the secretions of
inflammatory cytokines were detected by ELISA kits, and products of
IL-6, IL-1β and TNF-α were markedly declined in RASFs with
miR-27a-3p-overexpression via transfection (Fig. 2D-F). These results demonstrated that
miR-27a-3p upregulation may promote apoptosis and suppress
proliferation and inflammation of RASFs.
TLR5, serving as a downstream target
for miR-27a-3p, is upregulated in patients with RA
Bioinformatics analysis indicated that TLR5 was a
potential one (Fig. 3A). There were
potential responsive elements of miR-27a-3p ‘seed sequence’
(UCACAG) in TLR5 3'-UTR: position 157-169 (conserved). To confirm
this prediction, dual-luciferase reporter and biotin-coupled miRNA
pull-down assays were conducted. The relative luciferase activity
of pGL4-TLR5 3'-UTR-WT vectors was significantly decreased
(0.35-fold; P<0.05) in RASFs with miR-27a-3p-overexpression via
mimic transfection, and was increased (2.50-fold; P<0.05) with
miR-27a-3p-silencing via inhibitor transfection (Fig. 3B and C). However, there was no difference in
pGL4-TLR5 3'-UTR-MUT vector-transfected RASFs when miR-27a-3p was
overexpressed or downregulated. Furthermore, TLR5 expression was
significantly enriched (4.43-fold; P<0.05) by Bio-miR-27a-3p
instead of Bio-miR-27a-3p-MUT, compared with the control group
(Fig. 3D). Additionally, the
expression of TLR5 in RA was also investigated. As shown in
Fig. 3E and F, expression levels of TLR5 were
significantly upregulated (2.14-fold and 2.27-fold; P<0.05) in
synovial tissues and RASFs from patients with RA. Western blotting
revealed that TLR5 protein expression was decreased (0.32-fold;
P<0.05) in miR-27a-3p-overexpressed RASFs via mimic
transfection, and was increased (2.20-fold; P<0.05) in
miR-27a-3p-silenced RASFs (Fig.
3G). These data suggested that TLR5 was a downstream target for
miR-27a-3p, and its expression was upregulated in patients with
RA.
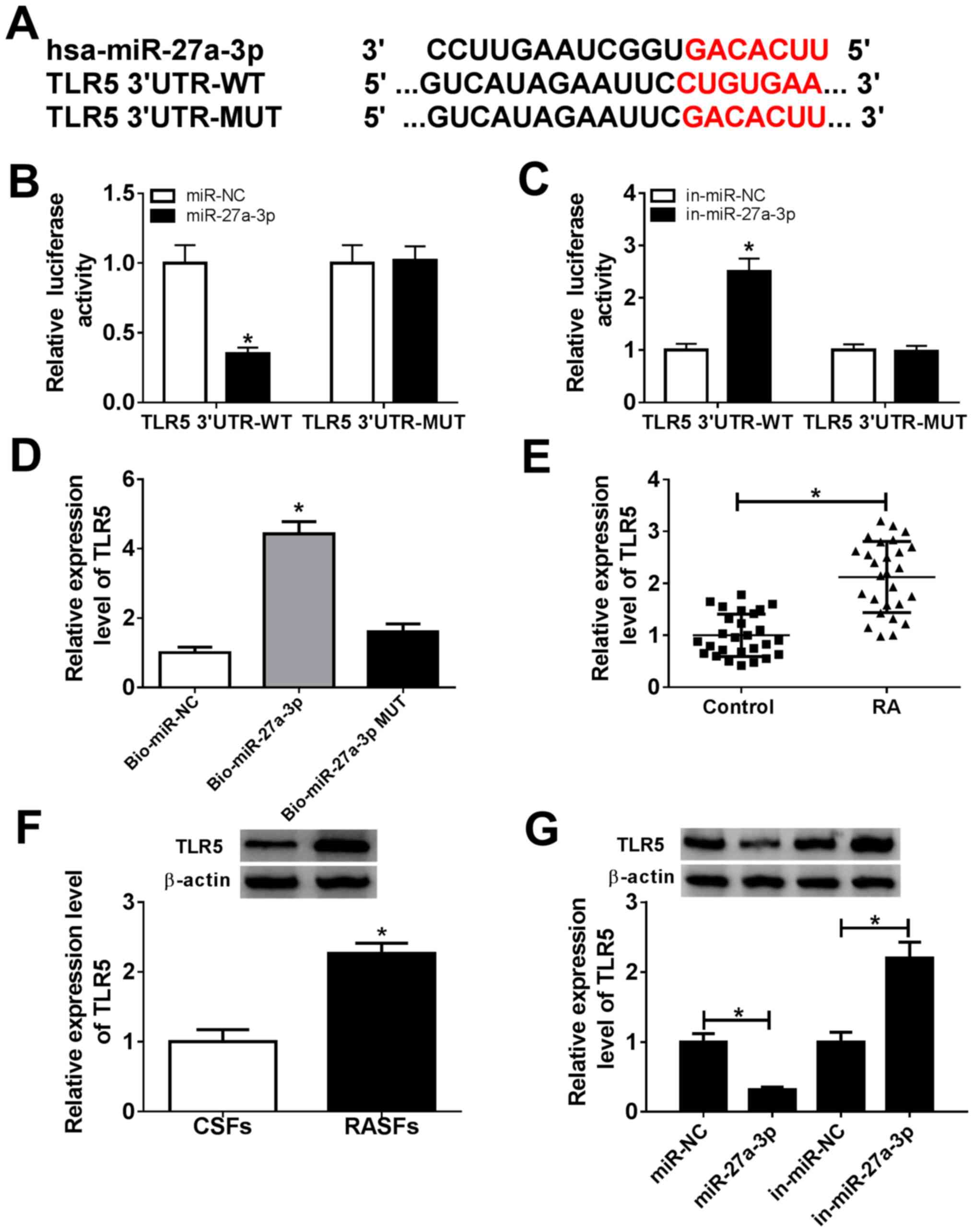 | Figure 3miR-27a-3p negatively regulates TLR5
expression by target binding. (A) Schematic diagram demonstrating
the potential miR-27a-3p responsive elements in wild-type and
mutant of TLR5 3'-UTR (TLR5 3'-UTR-WT and TLR5 3'-UTR-MUT). (B and
C) Dual-luciferase reporter assay detected the luciferase of
pGL4-TLR5 3'-UTR-WT/MUT vectors in RASFs co-transfected with
miR-27a-3p/NC or miR-27a-3p/NC inhibitors (in-miR-27a-3p/NC).
*P<0.05, compared with the miR-NC group or in-miR-NC
group. (D) RNA pull-down assay measured the TLR5 level in RASFs
transfected with biotin-labelled miR-27a-3p (Bio-miR-27a-3p) or its
mutant (Bio-miR-27a-3p MUT). *P<0.05, compared with
biotin-labelled miR-NC (Bio-miR-NC). (E) Reverse
transcription-quantitative polymerase chain reaction detected the
TLR5 mRNA level in RA synovial tissue (n=27) and Control synovial
tissue (n=27). *P<0.05, compared with the Control.
(F) Western blotting investigated the TLR5 protein level in RASFs
and CSFs. *P<0.05, compared with CSFs. (G) Western
blotting investigated the TLR5 protein level in RASFs transfected
with miR-27a-3p/NC or in-miR-27a-3p/NC. *P<0.05,
compared with miR-NC or in-miR-NC. miR, microRNA; TLR5, toll-like
receptor 5; UTR, untranslated region; WT, wild-type; MUT, mutant;
RASFs, RA synovial fibroblasts; NC, negative control; in,
inhibitor; RA, rheumatoid arthritis; CSFs, control synovial
fibroblasts. |
Silencing of TLR5 depresses
proliferation and inflammatory response in human RASFs
Loss-of-function experiments were performed in RASFs
transfected with si-TLR5 or si-NC. The transfection efficiency was
first validated by western blotting, and TLR5 protein expression
was distinctively downregulated (0.31-fold; P<0.05) in the
presence of si-TLR5, compared with si-NC (Fig. 4A). Cell proliferation was inhibited
and the apoptosis rate was promoted in RASFs with si-TLR5
transfection, as evidenced by MTT assay and FCM method (Fig. 4B and C). Furthermore, ELISA kits revealed that
the secretions of IL-6, IL-1β and TNF-α were markedly decreased in
RASFs with TLR5-knockdown via transfection (Fig. 4D-F). These results demonstrated that
TLR5 downregulation may promote apoptosis and suppress
proliferation and inflammation in RASFs, which was similar to role
of miR-27a-3p upregulation (Fig.
2A-F).
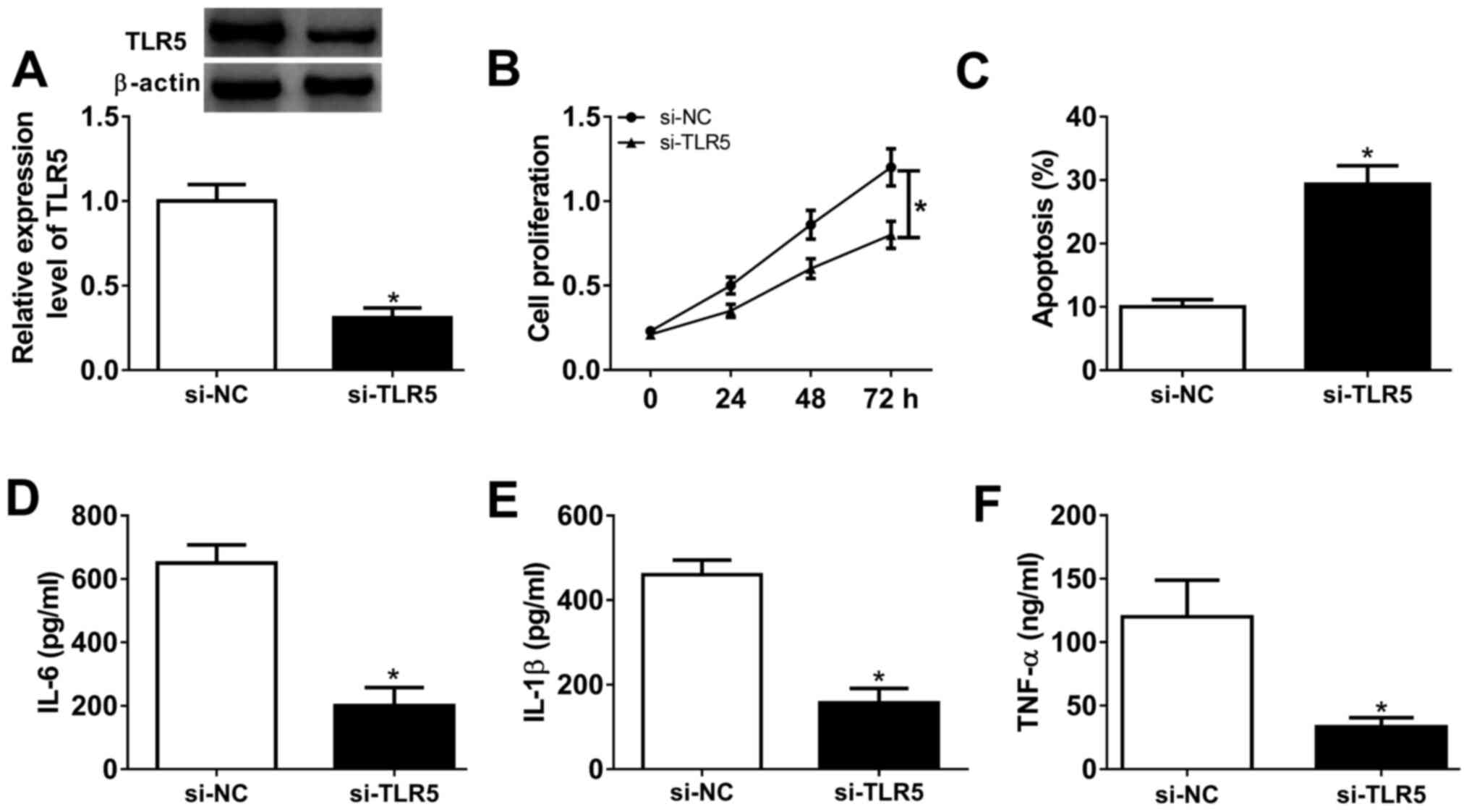 | Figure 4Effect of TLR5 on the proliferation,
apoptosis and inflammation in RASFs. TLR5 was exogenously silenced
in RASFs by transfecting siRNA targeting TLR5 (si-TLR5). (A)
Western blotting detected the TLR5 level. (B and C) Cell
proliferation and apoptosis were investigated using MTT assay and
flow cytometry. (D-F) Products of IL-6, IL-1β and TNF-α were
determined by ELISA kits. *P<0.05, compared with
scrambled siRNA (si-NC). TLR5, toll-like receptor 5; RASFs, RA
synovial fibroblasts; NC, negative control; IL, interleukin; TNF,
tumor necrosis factor. TLR5, toll-like receptor 5; RASFs, RA
synovial fibroblasts; IL, interleukin; TNF, tumor necrosis factor;
NC, negative control. |
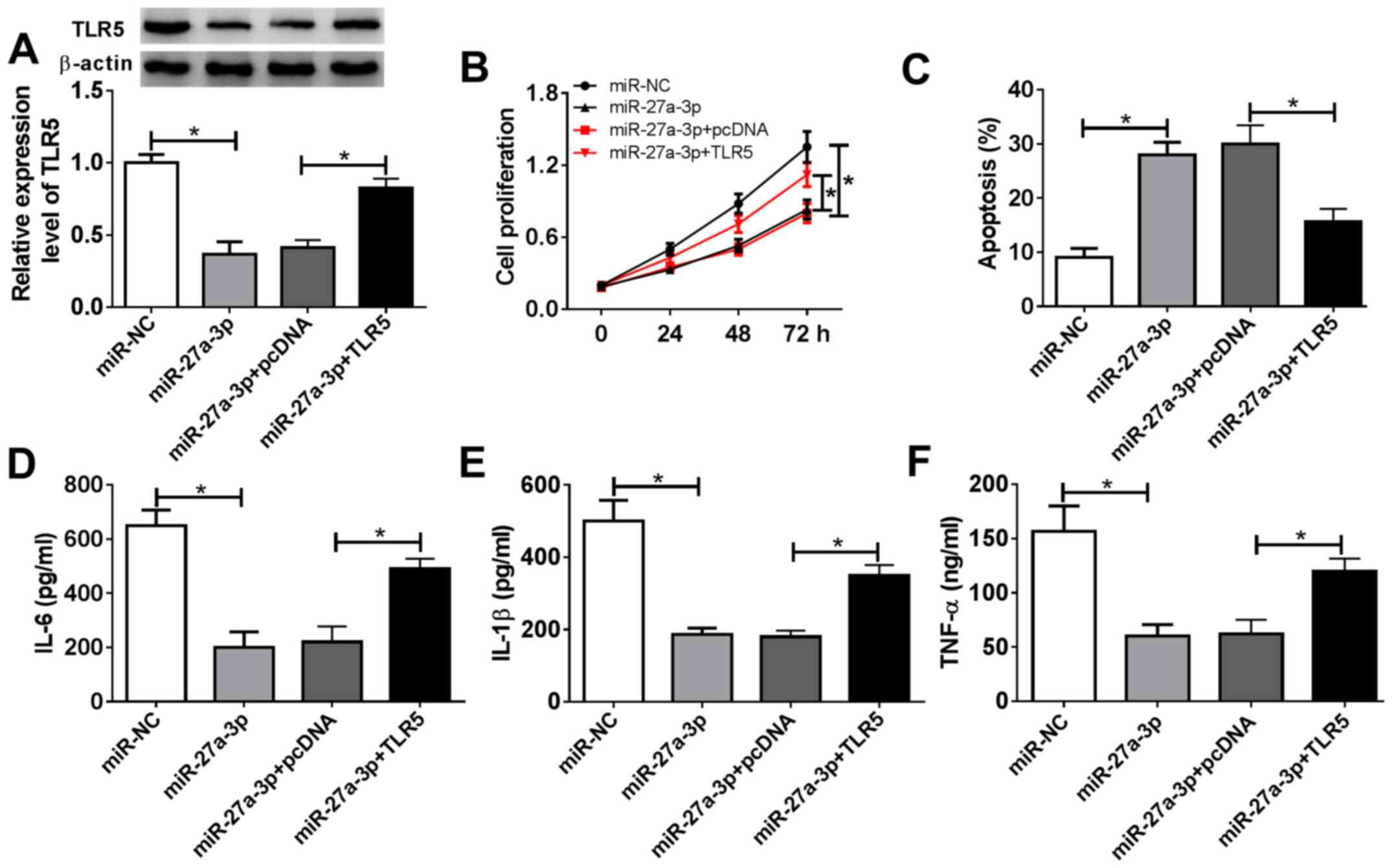
Restoration of TLR5 counteracts the
suppressive effect of miR-27a-3p-overexpression on human RASF
proliferation and inflammatory response
The present study aimed to investigate whether
miR-27a-3p exerted a suppressive effect on RASF proliferation and
inflammation through inhibiting its target TLR5. Therefore, rescue
experiments were performed. RASFs were co-transfected with
miR-27a-3p and a pcDNA-TLR5 (TLR5) vector or pcDNA vector.
Expression of TLR5 was measured by western blotting following
transfection; the decreased TLR5 protein levels mediated by
miR-27a-3p overexpression were improved (from 0.37-fold to
0.83-fold; P<0.05) in the co-transfection of miR-27a-3p mimic
and TLR5 vector (Fig. 5A). Cell
proliferation of RASFs was inhibited by miR-27a-3p-overexpression,
and then was rescued when miR-27a-3p and TLR5 were concurrently
overexpressed (Fig. 5B). miR-27a-3p
mimic-induced a higher apoptosis rate in RASFs, which was decreased
(from 28.0 to 15.6%; P<0.05) by also transfecting the TLR5
vector (Fig. 5C). Furthermore, TLR5
upregulation via vector transfection reversed the inhibitory effect
of miR-27a-3p mimic on the secretions of IL-6, IL-1β and TNF-α in
RASFs (Fig. 5D-F). These results
demonstrated that restoration of TLR5 counteracted the biological
effects of miR-27a-3p-overexpression in RASFs, suggesting a
miR-27a-3p/TLR5 axis in proliferation, apoptosis and inflammation
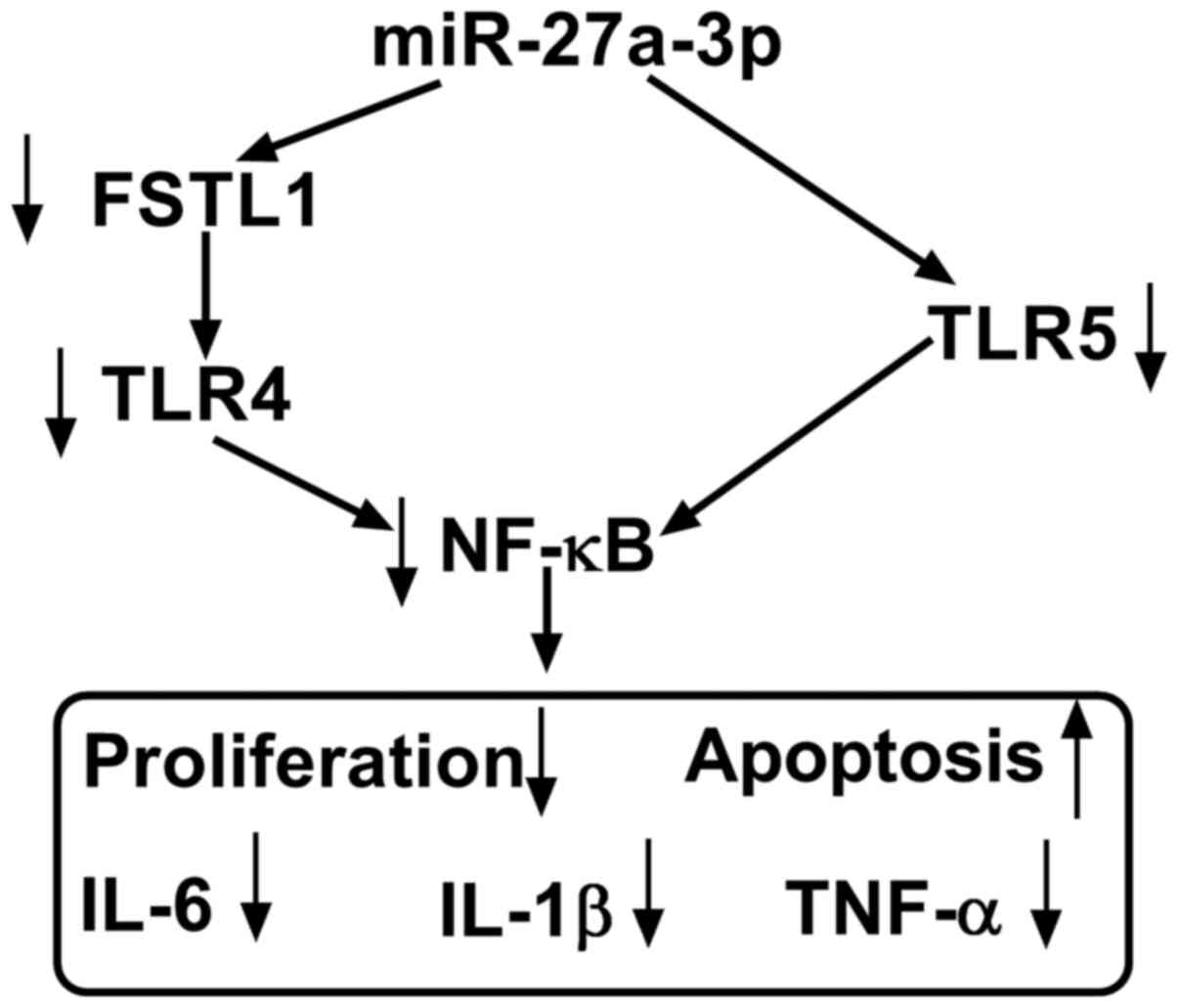
of RASFs (Fig. 6).
Discussion
The prevalence of RA varied from 0.3-1.0% globally,
and it was more common in developed countries than others (26). Although current therapies offer
diverse choices for RA drugs, only 50-60% of patients with RA
responded to them (9). Aberrant
expression of miRNAs was associated with the patients'
responsiveness to therapy in RA. For example, Dudics et al
(13) observed that 8 special
miRNAs had the potential to be key regulators of arthritis
pathogenesis; among these, 6 miRNAs may serve as biomarkers of
therapeutic response of celastrol in patients with RA. Furthermore,
miR-27a-3p was one of the 8 upregulated miRNAs following RA
development, but was downregulated in response to celastrol
treatment. In a placebo-controlled clinical trial, Sode et
al (18) confirmed that the
high miR-27a-3p pretreatment level and the decrease in the
miR-27a-3p level with treatment for 3 months was associated with
ACR/EULAR Boolean remission at 12 months. Furthermore, miR-16-5p
and miR-22-3p pretreatment levels and their level changes over
3-months treatment served as predictive target of MTX response
after 3 and 12 months, respectively. These results proposed that
certain miRNAs, including miR-27a-3p, may serve as potent
biomarkers to monitor disease activity and therapeutic response in
RA.
By contrast to the other fibroblasts, RASFs had a
high-level of invasive ability (25), and shared certain similar features
with cancer cells (27), including
migration, invasion and resistance to apoptosis. Synoviocyte
migration may be essential to the pathology of RA, and contributed
toward the spread of arthritic destruction to distant joints
(28). On one hand, abnormal miRNA
expression may cause cell migration abnormality and inflammation in
RASFs. For instance, miR-27a-5p downregulation underlies the
molecular mechanism of lncRNA ZFAS1 promoting human RASF migration
and invasion (29). By contrast,
downregulation of miR-221 decreased RASF migration and invasion via
inhibiting expression of vascular endothelial growth factor, matrix
metalloproteinase (MMP)-3 and MMP-9(30).
However, loss of balance in RASF proliferation and
apoptosis also served an essential role in RA pathogenesis.
Furthermore, the activity of RASFs conduced to inflammatory
response, thereby driving the progression of RA into late stages
(31,32). For example, miR-140-5p prevented the
proliferation and secretion of IL-6 and IL-8 in RASFs by targeting
TLR4(33). Fu et al
(34) demonstrated the suppressive
role of miR-3926 in RASF proliferation and pro-inflammatory factors
(IL-1β, IL-6 and TNF-α) secretion through targeting TLR5. The
present study focused on the contribution of miR-27a-3p to RASF
proliferation, apoptosis and inflammation, instead of the migration
and invasion (21). As a result,
re-expressing miR-27a-3p may increase the apoptotic rate and
attenuate the proliferation ability and secretions of IL-1β, IL-6
and TNF-α in human RASFs ex-vivo (Fig. 6). Furthermore, inhibiting TLR5
displayed a suppressive role in RASF proliferation and
inflammation, and restoring TLR5 mitigated the anti-proliferation
and anti-inflammation role of miR-27a-3p-overexpression in RASFs.
Notably, TLR5 was identified as a novel target gene for miR-27a-3p.
Furthermore, Shi et al (21)
demonstrated that miR-27a-3p inhibited cell migration and invasion
of RASFs by targeting FSTL1. Additionally, TLR4/NF-κB and
TLR5/NF-κB signaling pathways had been discovered in fibroblasts
(21,35). Therefore, we hypothesized a possible
miR-27a-3p/TLRs/NF-κB regulatory mechanism underlying RASFs
proliferation, apoptosis, inflammation, migration and invasion
(Fig. 6). According to
aforementioned results, miRNA expression alteration, including
restoring miR-27a-3p, was considered to be a potential therapeutic
strategy for RA treatment.
TLR5 is a crucial member of the TLR family, which
was evolutionarily conserved innate immune receptors (36). TLRs serves a fundamental role in the
first-line defense against foreign molecules, and TLRs were
released following injury or pathogen infection. The functions of
TLR1/2/4/5 and 7 had been extensively studied in RA (37,38).
However, function of TLR5 in RA remain unknown. TLR5 was known to
specifically sense and recognize flagellin, the major structural
protein of bacterial flagella (39). For example, TLR5 was implicated in
the pathogenesis of autoimmune diseases, including systemic lupus
erythematosus and RA (37,40). Chamberlain et al (41) demonstrated that TLR5 expression in
myeloid was positively associated with TNF level and RA disease
activity (DAS28). Kim et al (42) reported that ligation of TLR5 may
promote myeloid cell infiltration and differentiation into mature
osteoclasts in RA and experimental arthritis. Furthermore, Fu et
al (34) demonstrated that the
miR-3926/TLR5 pathway may represent a novel target for the
prevention and treatment of RA. The present study observed a high
expression of TLR5 in human RA synovial tissues and RASFs, and this
data supported the results of a previous study (34). Additionally, low expression of TLR5
not only suppressed proliferation and secretions of TNF-α, IL-1β
and IL-6, but also promoted the apoptotic rate of RASFs
ex-vivo, which were in consistent with the results of a
previous study (34). Furthermore,
it was identified that TLR5 was a downstream target of
miR-27a-3p.
In conclusion, the present study highlighted that
overexpressing miR-27a-3p and/or silencing TLR5 may interfere with
RA progression by inhibiting RASF proliferation and inflammatory
response and facilitating apoptosis (Fig. 6). The results of the present study
may provide a potential therapeutic target for RA. Meanwhile, a
novel miR-27a-3p/TLR5 axis was discovered underlying RASF
dysfunction; however, the precise signaling pathway of
miR-27a-3p/TLR5, including NF-κB, should be better investigated in
the future.
Supplementary Material
Raw data obtained from the present
study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and QL conceived the study, designed the
methodology and performed the final analysis. QL, JC, RF and CY
performed the experiments and acquired the data. QL and JC
confirmed the authenticity of all the raw data and performed
statistical analysis. LC and QL wrote the original draft. LC
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Research Ethics
Committee of General Hospital of Central Theater Command (Wuhan,
China). Written informed consent was obtained from each
patient.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coras R, Narasimhan R and Guma M: Liquid
biopsies to guide therapeutic decisions in rheumatoid arthritis.
Transl Res. 201:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Araki Y and Mimura T: The mechanisms
underlying chronic inflammation in rheumatoid arthritis from the
perspective of the epigenetic landscape. J Immunol Res.
2016(6290682)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Croia C, Bursi R, Sutera D, Petrelli F,
Alunno A and Puxeddu I: One year in review 2019: Pathogenesis of
rheumatoid arthritis. Clin Exp Rheumatol. 37:347–357.
2019.PubMed/NCBI
|
|
4
|
Fang Q, Zhou C and Nandakumar KS:
Molecular and cellular pathways contributing to joint damage in
rheumatoid arthritis. Mediators Inflamm.
2020(3830212)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alunno A, Carubbi F, Giacomelli R and
Gerli R: Cytokines in the pathogenesis of rheumatoid arthritis: New
players and therapeutic targets. BMC Rheumatol. 1(3)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Falconer J, Murphy AN, Young SP, Clark AR,
Tiziani S, Guma M and Buckley CD: Review: Synovial cell metabolism
and chronic inflammation in rheumatoid arthritis. Arthritis
Rheumatol. 70:984–999. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi J, Ermann J, Weissman BN, Smith SE and
Mandell JC: Thinking beyond pannus: A review of retro-odontoid
pseudotumor due to rheumatoid and non-rheumatoid etiologies.
Skeletal Radiol. 48:1511–1523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Laev SS and Salakhutdinov NF:
Anti-arthritic agents: Progress and potential. Bioorg Med Chem.
23:3059–3080. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yuasa S, Yamaguchi H, Nakanishi Y,
Kawaminami S, Tabata R, Shimizu N, Kohno M, Shimizu T, Miyata J,
Nakayama M, et al: Treatment responses and their predictors in
patients with rheumatoid arthritis treated with biological agents.
J Med Invest. 60:77–90. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zamanpoor M: The genetic pathogenesis,
diagnosis and therapeutic insight of rheumatoid arthritis. Clin
Genet. 95:547–557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moran-Moguel MC, Petarra-Del Rio S,
Mayorquin-Galvan EE and Zavala-Cerna MG: Rheumatoid arthritis and
mirnas: A critical review through a functional view. J Immunol Res.
2018(2474529)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dong H, Lei J, Ding L, Wen Y, Ju H and
Zhang X: MicroRNA: Function, detection, and bioanalysis. Chem Rev.
113:6207–6233. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dudics S, Venkatesha SH and Moudgil KD:
The micro-RNA expression profiles of autoimmune arthritis reveal
novel biomarkers of the disease and therapeutic response. Int J Mol
Sci. 19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Huang X, Ye L, Guo G, Li X, Chen
C, Sun L, Li B, Chen N and Xue X: B cell-related circulating
MicroRNAs with the potential value of biomarkers in the
differential diagnosis, and distinguishment between the disease
activity and lupus nephritis for systemic lupus erythematosus.
Front Immunol. 9(1473)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Regev K, Paul A, Healy B, von Glenn F,
Diaz-Cruz C, Gholipour T, Mazzola MA, Raheja R, Nejad P, Glanz BI,
et al: Comprehensive evaluation of serum microRNAs as biomarkers in
multiple sclerosis. Neurol Neuroimmunol Neuroinflamm.
3(e267)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Evangelatos G, Fragoulis GE, Koulouri V
and Lambrou GI: MicroRNAs in rheumatoid arthritis: From
pathogenesis to clinical impact. Autoimmun Rev.
18(102391)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharma AR, Sharma G, Lee SS and
Chakraborty C: miRNA-regulated key components of cytokine signaling
pathways and inflammation in rheumatoid arthritis. Med Res Rev.
36:425–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sode J, Krintel SB, Carlsen AL, Hetland
ML, Johansen JS, Hørslev-Petersen K, Stengaard-Pedersen K,
Ellingsen T, Burton M, Junker P, et al: Plasma MicroRNA profiles in
patients with early rheumatoid arthritis responding to adalimumab
plus methotrexate vs, methotrexate alone: A Placebo-controlled
Clinical Trial. J Rheumatol. 45:53–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takamura Y, Aoki W, Satomura A, Shibasaki
S and Ueda M: Small RNAs detected in exosomes derived from the MH7A
synovial fibroblast cell line with TNF-α stimulation. PLoS One.
13(e0201851)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maeda Y, Farina NH, Matzelle MM, Fanning
PJ, Lian JB and Gravallese EM: Synovium-derived MicroRNAs regulate
bone pathways in rheumatoid arthritis. J Bone Miner Res.
32:461–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi DL, Shi GR, Xie J, Du XZ and Yang H:
MicroRNA-27a inhibits cell migration and invasion of
fibroblast-like synoviocytes by targeting follistatin-like protein
1 in rheumatoid arthritis. Mol Cells. 39:611–618. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American College of Rheumatology/European League Against
Rheumatism collaborative initiative. Ann Rheum Dis. 69:1580–1588.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li XJ, Xu M, Zhao XQ, Zhao JN, Chen FF, Yu
W, Gao DY and Luo B: Proteomic analysis of synovial fibroblast-like
synoviocytes from rheumatoid arthritis. Clin Exp Rheumatol.
31:552–558. 2013.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zou Y, Xu S, Xiao Y, Qiu Q, Shi M, Wang J,
Liang L, Zhan Z, Yang X, Olsen N, et al: Long noncoding RNA LERFS
negatively regulates rheumatoid synovial aggression and
proliferation. J Clin Invest. 128:4510–4524. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Alamanos Y and Drosos AA: Epidemiology of
adult rheumatoid arthritis. Autoimmun Rev. 4:130–136.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Pan YF, Xue YQ, Fang LK, Guo XH,
Guo X, Liu M, Mo BY, Yang MR, Liu F, et al: uPAR promotes
tumor-like biologic behaviors of fibroblast-like synoviocytes
through PI3K/Akt signaling pathway in patients with rheumatoid
arthritis. Cell Mol Immunol. 15:171–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai P, Jiang T, Li B, Qin X, Lu Z, Le Y,
Shen C, Yang Y, Zheng L and Zhao J: Comparison of rheumatoid
arthritis (RA) and osteoarthritis (OA) based on microarray profiles
of human joint fibroblast-like synoviocytes. Cell Biochem Funct.
37:31–41. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Ye Y, Gao X and Yang N: LncRNA ZFAS1
promotes cell migration and invasion of fibroblast-like
synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum
Cell. 31:14–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang S and Yang Y: Downregulation of
microRNA 221 decreases migration and invasion in fibroblast like
synoviocytes in rheumatoid arthritis. Mol Med Rep. 12:2395–2401.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Neumann E, Lefèvre S, Zimmermann B, Gay S
and Müller-Ladner U: Rheumatoid arthritis progression mediated by
activated synovial fibroblasts. Trends Mol Med. 16:458–468.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Karouzakis E, Gay RE, Gay S and Neidhart
M: Epigenetic control in rheumatoid arthritis synovial fibroblasts.
Nat Rev Rheumatol. 5:266–272. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li H, Guan SB, Lu Y and Wang F: MiR-140-5p
inhibits synovial fibroblasts proliferation and inflammatory
cytokines secretion through targeting TLR4. Biomed Pharmacother.
96:208–214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fu D, Xiao C, Xie Y, Gao J and Ye S:
MiR-3926 inhibits synovial fibroblasts proliferation and
inflammatory cytokines secretion through targeting toll like
receptor 5. Gene. 687:200–206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thunyakitpisal P, Ruangpornvisuti V,
Kengkwasing P, Chokboribal J and Sangvanich P: Acemannan increases
NF-κB/DNA binding and IL-6/-8 expression by selectively binding
Toll-like receptor-5 in human gingival fibroblasts. Carbohydr
Polym. 161:149–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sabroe I, Read RC, Whyte MK, Dockrell DH,
Vogel SN and Dower SK: Toll-like receptors in health and disease:
Complex questions remain. J Immunol. 171:1630–1635. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Elshabrawy HA, Essani AE, Szekanecz Z, Fox
DA and Shahrara S: TLRs, future potential therapeutic targets for
RA. Autoimmun Rev. 16:103–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Thwaites RS, Unterberger S, Chamberlain G,
Walker-Bone K, Davies KA and Sacre S: TLR1/2 and 5 induce elevated
cytokine levels from rheumatoid arthritis monocytes independent of
ACPA or RF autoantibody status. Rheumatology (Oxford).
59:3533–3539. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Smith KD, Andersen-Nissen E, Hayashi F,
Strobe K, Bergman MA, Barrett SL, Cookson BT and Aderem A:
Toll-like receptor 5 recognizes a conserved site on flagellin
required for protofilament formation and bacterial motility. Nat
Immunol. 4:1247–1253. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Wu YW, Tang W and Zuo JP: Toll-like
receptors: Potential targets for lupus treatment. Acta Pharmacol
Sin. 36:1395–1407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chamberlain ND, Vila OM, Volin MV, Volin
MV, Volkov S, Pope RM, Swedler W, Mandelin AM 2nd, Shahrara S, et
al: TLR5, a novel and unidentified inflammatory mediator in
rheumatoid arthritis that correlates with disease activity score
and joint TNF-α levels. J Immunol. 189:475–483. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kim SJ, Chen Z, Chamberlain ND, Essani AB,
Volin MV, Amin MA, Volkov S, Gravallese EM, Arami S, Swedler W, et
al: Ligation of TLR5 promotes myeloid cell infiltration and
differentiation into mature osteoclasts in rheumatoid arthritis and
experimental arthritis. J Immunol. 193:3902–3913. 2014.PubMed/NCBI View Article : Google Scholar
|