1. Introduction
Alpha-lipoic acid (ALA) is an organosulfur compound
(chemical formula:
C8H14O2S2), with a
molecular weight of 206.32 Da, which is synthesized by plants,
animals, as well as humans (1,2).
Endogenous ALA is synthesized in the mitochondria
from octanoic acid. This natural molecule, which is essential for a
number of metabolic processes, is inconsistently synthesized by the
human body in insufficient quantities. Consequently, ALA must be
obtained from exogenous sources, such as food and dietary
supplements, with red meat (e.g., liver, heart and kidney)
representing a major supply source. Significant quantities of this
molecule are also found in vegetables, such as tomatoes, spinach,
Brussels sprouts, peas, potatoes, broccoli and brown rice (2). Lipoic acid plays a key role in energy
and amino acid metabolism through forming covalent bonds with
specific proteins, serving as part of the essential mitochondrial
multi-enzyme complexes (2). There
is currently a growing scientific and medical interest in the
therapeutic use of lipoic acid, and it was lately introduced in
pregnancies at risk of miscarriage/premature delivery, or in cases
of intrauterine growth restriction (3).
Once absorbed, the oxidized form of the ALA molecule
is transformed by a specific enzymatic mechanism (dihydrolipoamide
dehydrogenase, thioredoxin reductase, or glutathione reductase)
into its reduced form, dihydrolipoic acid (DHLA) (4). Both forms may be identified in living
organisms, exercising a biological function under different
circumstances. ALA and DHLA comprise a solid redox couple. Their
physical activity is interchangeable and depends on the
microenvironmental conditions (the cytoplasm is a reducing medium;
thus, ALA is reduced to DHLA after entering the cell) (5).
Pharmacokinetic studies have shown that different
doses (50-600 mg) of orally administered ALA are completely
absorbed within 30-60 min, with a plasma half-life of 30 min
(6). Rapidly metabolized ALA has a
bioavailability after the first passage of ~30% (range, 20-38%).
ALA is mainly stored in the heart, kidneys and liver (7,8).
Spontaneous abortion in the first 20 weeks of
pregnancy, particularly in the first trimester, is a common problem
during pregnancy, with the most common causes being genetic
abnormalities (5,9), inflammatory processes (10) and immune dysfunction (11). The primary manifestation is vaginal
bleeding, which is frequently accompanied by lumbar-abdominal pain
and a feeling of pressure in the pelvis. A total of 18% of vaginal
bleeding cases in the first trimester develop a subchorionic
hematoma. The risk of miscarriage in early pregnancy is 20%, while
half of these cases have a subsequent increased risk of premature
delivery (12).
The aim of the present review was to discuss the
positive effect of ALA administration in high-risk pregnancies.
Miscarriage and premature birth share a common pathophysiological
pathway, manifested by an imbalance between pro- and
anti-inflammatory cytokines. As the ALA molecule has well-known
antioxidant, anti-inflammatory and immunomodulatory properties, it
may be of value in this setting, and is already being utilized on a
limited scale.
2. ALA/DHLA action
Most studies describe that ALA/DHLA exert direct
antioxidant, anti-inflammatory and immunomodulatory effects,
whereas they may also exert indirect antioxidant effects by
contributing to the regeneration of other essential antioxidant
molecules, such as coenzyme Q10, vitamin C and vitamin E. ALA/DHLA
may also chelate a number of heavy metals implicated in oxidative
processes, such as iron, lead, cadmium, mercury, copper and arsenic
(5-7,13,14).
Antioxidant activity
Increased generation of oxygen free radicals has
been reported to be involved in the pathophysiology of
first-trimester miscarriage (15).
Serum prolidase activity, sulfhydryl levels and total antioxidant
capacity (markers of oxidative stress) have shown statistically
significant changes in such cases (16). Patients at risk of abortion exhibit
changes in the regulation of oxidase activity of peripheral blood
granulocytes (17). Peroxiredoxins
are antioxidant proteins expressed by cytotrophoblastic cells, and
their downregulation is often associated with miscarriage (15).
The ALA/DHLA redox couple is involved in the repair
of biological molecules, such as proteins, lipids and DNA, which
are damaged by oxidation. At the protein level, oxidation occurs at
the level of amino acids such as methionine, cysteine, histidine,
tyrosine and tryptophan. The oxidation of methionine may cause
protein inactivation and the alteration or inhibition of their
enzymatic, hormonal and/or chemotactic functions. Lipoamide, the
neutral amide of ALA, is involved in the repairing process of
oxidized methionine (18).
Thus, ALA/DHLA exert antioxidant effects in
vitro through four different mechanisms (19): i) Elimination of oxidants; ii)
regeneration of endogenous antioxidants; iii) chelation of
transition metals; and iv) reparative action of oxidative
damage.
Some researchers dispute the in vivo
antioxidant activity of ALA/DHLA, mainly due to the inability to
reach a sufficient serum concentration by oral administration to
achieve elimination of free radicals (20).
Anti-inflammatory and immunomodulatory
action
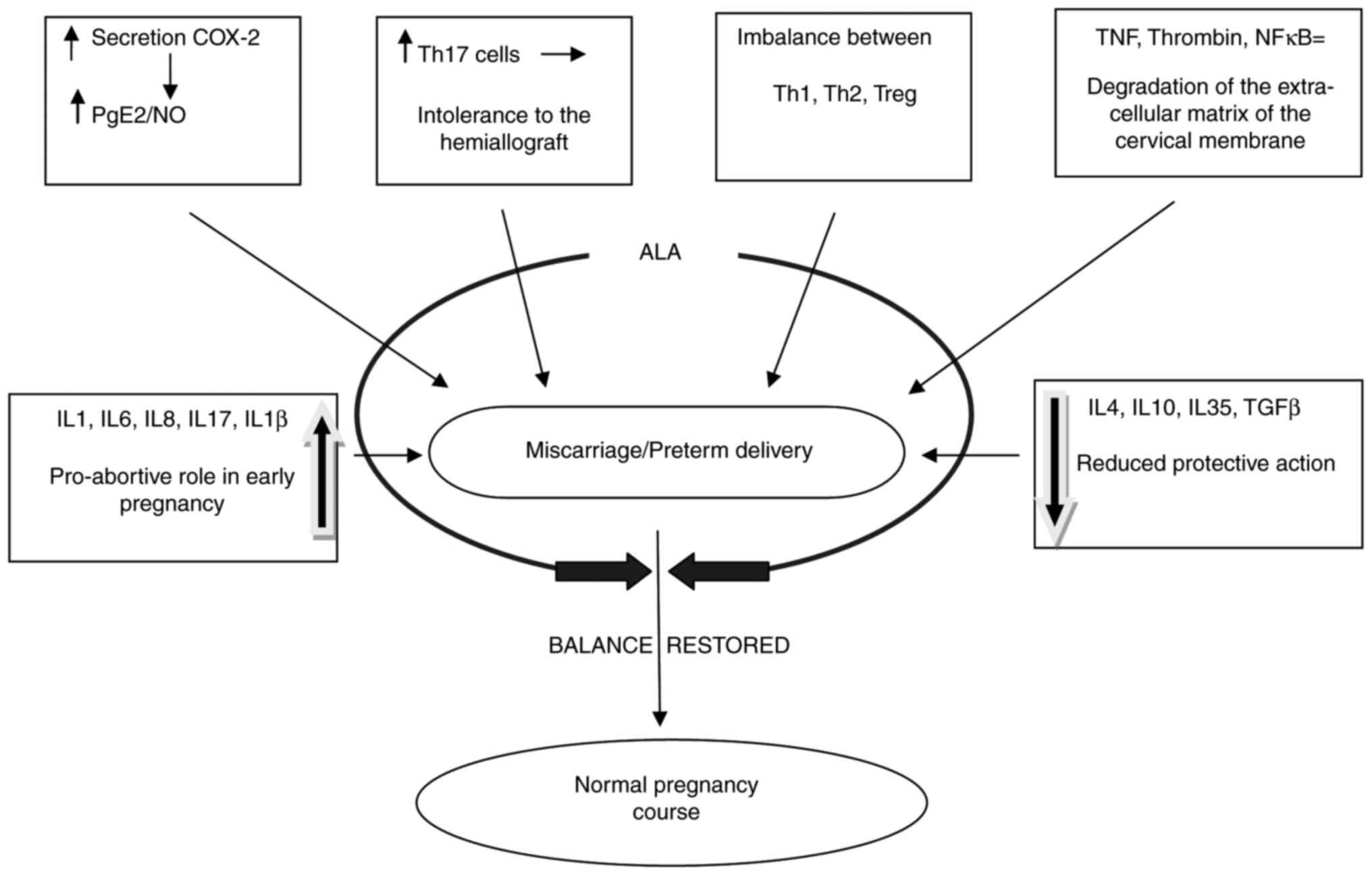
Recent research has shown that high titers of
pro-inflammatory cytokines (IL-1 and IL-6) (14,15,18)
and/or low titers of anti-inflammatory cytokines (IL-4 and IL-10)
(8) increase the risk of
miscarriage and preterm delivery (Fig.
1).
Current studies have described the role of specific
cytokines, growth factors, chemokines and helper T cells in the
etiopathogenesis of miscarriage and preterm delivery. There are two
subsets of CD4+ lymphocytes, namely T helper 1 (Th1) and
T helper 2 (Th2) cells, classified according to the type of
secreted cytokines (5,21).
Th1 cells predominantly secrete TNF-β, IFN-γ, IL-2,
TNF-α and TGF-β. TNF-α protects the fetoplacental unit (22,23),
but it is also involved in the immunopathology of various pregnancy
complications. TNF-α increases the level of the trophoblast-derived
plasminogen activator inhibitor-1 and decreases its invasive
capacity (24,25). Th1 cells also secrete IL-10, albeit
in small amounts, which is a cytokine with a critical role in
controlling inflammation and self-regulating Th1 cell activation
(21).
On the other hand, Th2 lymphocytes predominantly
secrete IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13 and TGF-β, and
small amounts of TNF-α and IL-2. All these molecules are involved
to different extents in the implantation process and the evolution
of normal/abnormal placentation (26). In particular, IL-4, which has an
anti-inflammatory role, and IL-6, which has a pro-inflammatory
role, are involved in modulating the risk of miscarriage (27).
Prins et al (28) demonstrated a significant increase in
serum IL-6 concentrations in women who had suffered habitual
abortion, supporting the pro-inflammatory and pro-abortive role of
this cytokine in early pregnancy.
By contrast, IL-4, similar to IL-10, is an
anti-inflammatory cytokine with a preventive role in miscarriage.
Chatterjee et al (29)
reported that a decrease in serum IL-4 below average values exerted
pro-inflammatory effects and increased the risk of abortion.
Saito et al (27) and Kaminski et al (30) investigated the role of regulatory T
cells (Tregs) and Th17 cells, responsible for the release of IL-17,
and their dual action in pregnancy. Th17 cells serve an immune
protective role against the maternal microbiome in the uterus
(5). Average IL-17 levels determine
the normal degree of inflammation that accompanies the oocyte
fertilization process in pregnancy (31), whereas elevated IL-17 levels explain
the excessive pro-inflammatory mechanism underlying spontaneous
abortion (32-34).
Tregs and Th1 cells can be converted to Th17 cells
(35,36), thus ensuring the fine
immunomodulation and tolerance to the fetal hemiallograft. The
cells release the anti-inflammatory cytokines TGF-β, IL-10 and
IL-35, which directly or indirectly block the secretion and
activity of pro-inflammatory cytokines. There is an interdependence
between the Treg, Th1 and Th2 cell types and the cytokines released
by them in normal pregnancies.
Therefore, a balance between Th1 and Th2-mediated
immunity (predominantly Th2) is required to maintain a normal
pregnancy (5), without exacerbated
secretion of certain cytokines to compensate for the deficiency of
others (Fig. 1).
3. ALA impact on high-risk pregnancies
Medication for women at risk of abortion and
premature birth must modulate pro and anti-inflammatory cytokines
to restore the delicate balance. DHLA, the reduced form of ALA, has
demonstrated marked efficacy as a free radical scavenger and
modifier of oxidative stress and inflammation-related pathways
(37).
Numerous scientific studies have shown that ALA
administration may be beneficial in high-risk pregnancies (Table I).
 | Table IALA proven effects on
miscarriage/preterm delivery |
Table I
ALA proven effects on
miscarriage/preterm delivery
| Study, year | Design | Patient no. and
duration | Pregnancy
condition | Treatment | Effects | (Refs.) |
|---|
| Parente et
al, 2014 | Randomized
double-blind; clinical trial; controlled | 300 patients; 14-34
weeks of pregnancy; duration: Until delivery | Preterm uterine
contractions | ALA 100 mg/day and
magnesium 225 mg/day; 50 patients on placebo | Reduction in the
inci dence of premature uterine contractions and hospitalization
rate | (42) |
| Porcaro et
al, 2015 | Randomized;
clinical trial; preliminary Results | 16 patients; 6-13
weeks of pregnancy; duration: Up to the full mending of the
clinical picture | Pregnancy with
pelvic pain, vaginal bleeding and subcho rionic hematomas | ALA orally 600
mg/day + progesterone 400 mg/day vs. progesterone 400 mg/day
(CG) | Decrease in
specific symptoms; faster improvement in the clinical picture and
in the remission/reduction of the subchorionic hematoma | (38) |
| Costantino et
al, 2016 | Randomized;
clinical trial; controlled | 62 patients; 7-12
weeks of pregnancy; duration: 60 days | Imminent abortion
in the first trimester | ALA (vaginal
capsule) 10 mg/day vs. progesterone (vaginal soft gel) 400 mg/day;
CG without treatment (on request) | Faster resorption
of subchori onic hematoma; decreased number of miscarriages in the
ALA group | (39) |
| Grandi et
al, 2017 | Randomized;
clinical trial; controlled | 40 patients; 24-30
weeks of pregnancy; duration: 30 days | Women hospitalized
for a first preterm labor episode (cervical change diagnosed by TV
US) | ALA (vaginal
capsule) 400 mg/day | Vaginal ALA
inhibited the cervical effacement after a preterm labor event | (49) |
| Vitrano et
al, 2018 | Prospective;
observational; controlled | 60 patients; 24-33
weeks of pregnancy; duration: 30 days orally, 10 days
vaginally | Cervical shortening
diag nosed by TV US and/or pelvic pain | ALA orally 2x300
mg/day and vaginally 10 mg/day | ALA alleviated the
symptoms | (50) |
Beneficial effects of ALA on
threatened abortion
In patients at risk of spontaneous abortion, ALA
administration starting from the first trimester has shown efficacy
by accelerating the resorption of subchorionic hematoma, with a
significant decrease in the accompanying abdominal pain (38-40).
ALA is implicated in fine immunomodulation and interdependence
among Tregs, Th1 and Th2 cells.
ALA reduces the levels of pro-inflammatory cytokines
(IL-1β, IL-6, IL-8 and IL-17), while it increases the secretion of
anti-inflammatory cytokines (IL-10) (5,41). It
also inhibits cyclooxygenase 2, which causes a decrease in
prostaglandin E2 and nitric oxide (NO) secretion, thus reducing the
risk of miscarriage in the first trimester of pregnancy (5).
Recent studies have reported the benefits of oral or
intravaginal ALA administration in pregnant women at risk of
miscarriage in the first trimester (2,5,42).
A survey reflecting the positive effects of ALA was
conducted by Porcaro et al (38) on two groups of pregnant women. The
study sample consisted of pregnant women in the first 13 weeks of
pregnancy diagnosed with threatened or imminent abortion,
presenting with subchorionic hematoma occupying between 20% and
>50% of the gestational sac surface, vaginal bleeding,
lumbar-abdominal pain, or uterine contractions. In the first group,
pregnant women received 200 mg vaginal progesterone twice/day,
whereas pregnant women in the second group were administered 200 mg
vaginal progesterone twice/day combined with 300 mg oral ALA
twice/day. The results demonstrated a decrease in symptoms specific
to this clinical entity (having a synergistic effect with the
vaginal progesterone treatment). A faster improvement in the
clinical symptoms was demonstrated in pregnant women who received
oral ALA at 600 mg/day combined with progesterone, compared with
pregnant women who received vaginal progesterone alone as follows:
89 vs. 71% remission of vaginal bleeding, 78 vs. 43% improvement in
pelvic pain after 1 week and 100% remission of symptoms for
ALA-treated patients at 3 weeks. Combined ALA/vaginal progesterone
treatment was also associated with a faster remission/reduction of
the subchorionic hematoma, with a 50% resorption in the first week,
and 90% resorption after 15 days (38).
Constantino et al (39) reported results from a pilot study at
the Women's Health Center (Ferrara, Italy). The study group
comprised pregnant women with imminent abortion in the first
trimester (7-12 weeks of gestation), with or without subchorionic
hematoma. The study evaluated the vaginal administration of a
preparation containing ALA (10 mg/day). The data were compared with
those obtained from pregnant women who received progesterone (400
mg/day) and from the control group (patients who did not receive
any medication). The results demonstrated that vaginal
administration of ALA resulted in faster resorption of subchorionic
hematoma, while avoiding insufficient intestinal absorption (80%
resorption of the hematoma in ALA-treated patients at 20 days).
Regarding symptomatology (low back pain, pelvic pain and vaginal
bleeding), 2 women from the progesterone alone and 3 from the
control group still suffered from pelvic pain at the 20-day
check-up, whereas ALA-treated patients reported full remission of
pelvic pain and vaginal bleeding in the first 20 days of treatment.
No significant differences were observed between the study groups
receiving treatment. Final assessments were performed on pregnant
women who reached 20 weeks of gestation.
Progesterone serves an immunosuppressive role in
pregnancy, whereas ALA exerts fine immunomodulatory effects among
different cytokines. Therefore, ALA may represent a new therapeutic
option in this setting.
Regular uterine contractions between 26 and 37 weeks
of gestation are responsible for premature delivery, which
represents a public health concern. To date, certain strategies
have been developed for early diagnosis and suppression of
premature uterine contractions through appropriate treatment.
Premature contractions have a multifactorial etiology explained by
multiple pathophysiological mechanisms. The treatment regimens must
be combined and individualized for each patient. The obstetricians
must carefully select the tocolytic agent to ensure maximum
efficiency with minimal side effects. The most frequently
recommended treatment includes administering calcium antagonists,
oxytocin antagonists, prostaglandin synthesis inhibitors, NO
donors, betamimetics and magnesium sulfate (43,44).
Although several tocolytic compounds are available,
consensus of the optimal first-line tocolytic agent is still
lacking. Recent research on innovative, effective and safe
therapies describes preparations containing ALA as the missing link
(42). This molecule acts as a
neutralizer of oxygen free radicals, exhibiting prominent
anti-inflammatory and immunomodulatory properties. ALA works
synergistically with magnesium in ameliorating/amending premature
uterine contractions.
Parente et al (42) conducted a study involving 300
pregnant women at 14-34 weeks of gestation, with premature uterine
contractions but no vaginal infections, among whom 50 women had a
history of miscarriage and premature delivery. The subjects
received 100 mg ALA/day and 225 mg magnesium/day. They were
followed up on an outpatient basis every 4 weeks, recording events
such as sporadic and persistent episodes of premature contractions
requiring combined tocolytic therapy and possible hospitalization.
The findings demonstrated that the administration of magnesium and
ALA starting from the 14th gestational week led to a reduction in
the incidence of premature uterine contractions and hospitalization
rate. A total of 52% reported the absence of premature uterine
contractions throughout the pregnancy, while 28% reported sporadic
episodes of uterine contractions that did not require other
tocolytic medication.
Experimental studies have shown that the
pathophysiological mechanisms involved in the onset and persistence
of premature uterine contractions are distinct and they are most
commonly associated with the degradation of the extracellular
matrix of the cervical membrane (45,46).
Myometrial activation may occur with or without the premature
rupture of membranes (46).
Premature degradation and rupture of amniotic membranes may result
from collagen alteration at this level, caused by the TNF-α and
thrombin, mediated by certain metalloproteases. Recent in
vitro studies have shown that ALA inhibits the action of TNF-α
and thrombin on the amniotic membrane, thus inhibiting their
degradation and fragility (47,48).
The mechanism through which ALA inhibits amniotic
membrane degradation remains elusive. It has been shown that ALA
inhibits NF-κB, a protein complex that controls the transcription
of DNA that is activated by cytokines and thrombin (47).
Therefore, ALA has antioxidant and NF-κB-inhibitory
properties, and its association with magnesium counteracts
premature uterine contractions and prevents premature rupture of
amniotic membranes, lowering the hospitalization rate in these
women. However, the precise effects of this combination must be
verified in a significantly larger number of subjects.
ALA may protect against premature
cervical shortening
Thus far, when addressing preterm birth, we can only
intervene on uterine contractility with tocolytic drugs; however,
not all the pathophysiological elements can be assessed and
resolved. The imbalance between pro-inflammatory and
anti-inflammatory cytokines may serve as a foundation for premature
cervical shortening. Recent studies analyzed the inflammatory
mediators synthesized by fetal tissues and the maternal genital
tract and their preterm delivery role (49,50).
Compiled information suggests that infection and inflammation are
latent causes determining preterm birth (51). The subtle and defining changes
observed in these women are associated with
modifications/alterations in the cervicovaginal fluid composition
and qualities. Increased local levels of pro-inflammatory
cytokines, such as IL-8, prostaglandins and MMPs (mainly MMP-9) are
involved in the process of cervical ripening by inducing changes in
the extracellular matrix (50).
The efficiency of ALA in reducing the expression of
MMP-9 and inhibiting the TNF-α- and thrombin-induced reduction in
the strength of human fetal membranes has been previously reported
(47,48,52).
Grandi et al (49) conducted a pilot study and concluded
that ALA administered vaginally maintained the length of the cervix
and kept it closed after an episode of preterm labor. The survey
included 40 pregnant women at 24-30 weeks of pregnancy, 20 of whom
received 400 mg ALA administered vaginally for 30 days. The main
finding of the study was that vaginal ALA administration exerted a
distinct anti-inflammatory effect at the cervical level compared
with placebo. In the treatment group, the monthly cervical
shortening was arrested at the normal expected values of 3 mm,
whereas double this rate was observed in women receiving placebo
(48). ALA treatment is strongly
associated with the stabilization of the cervix, which typically
undergoes shortening. As regards tolerability, transient vaginal
discomfort was reported by 2 patients from the vaginal ALA
group.
In a prospective observational study, Vitrano et
al (50) tested the effects of
ALA on women at risk of preterm birth, with cervical shortening
diagnosed by transvaginal ultrasound and/or pelvic pain. The survey
included 60 pregnant women at 24-33 weeks pregnancy in two groups:
50 patients received combined oral ALA at 2x300 mg/day for 30 days
and vaginal ALA at 10 mg/day for 10 days, and 10 patients refused
the treatment. A total of 44 patients had symptoms, 40 of whom
received ALA and 37 reported amelioration, while 3 patients did not
display any improvement. In the 4 untreated patients, the clinical
symptoms persisted. These results support ALA supplementation for
reducing the risk of preterm labor onset and symptomatology and for
delaying cervical shortening in women at risk of preterm delivery.
ALA administered at therapeutic doses was safe and was not
associated with major side effects.
As the surveys conducted to date included small
sample populations, the success of ALA in preventing threatened
preterm delivery requires further validation in extended groups of
patients to ratify therapy effectiveness.
Future prospects
Pregnancy represents a metabolic challenge
characterized by increased metabolic rate and increased
mitochondrial activity, with the placenta itself being a source of
oxidative stress. Stress is triggered by the imbalance between free
radicals (oxidants) and antioxidants (52,53),
testing the ability of the body to eliminate the amount of reactive
oxygen species. The ability of placental antioxidants to attenuate
potentially harmful free radicals is crucial for normal placental
function and optimal fetal growth and development (54).
Preeclampsia is a dysfunction caused by abnormal
placentation and an excessive maternal inflammatory vascular
response. The mechanisms that determine the evolution towards
preeclampsia are numerous, some of which remain incompletely
understood.
Abnormal cytotrophoblast invasion in spiral
arterioles leads to inappropriate vascular remodeling, endothelial
cell dysfunction and placental insufficiency. Preeclampsia leads to
extreme hypoxia in the placental bed and an exaggerated oxidative
reaction with the release of oxygen free radicals (55,56),
the source of which are the placental mitochondria (57). Exaggerated lipid peroxidation
reactions occur, along with increased xanthine oxidase levels in
the blood, placental tissue and umbilical cord. At the placental
endoplasmic reticulum level, faulty protein synthesis occurs, which
leads to cell apoptosis. This pathway is considered to contribute
to intrauterine growth restriction and the development of cytokine
and prostaglandin-induced preeclampsia (58).
The association between fetoplacental hypoxia,
oxidative stress and fetal brain impairment during the perinatal
period represents information validated by extensive research with
specific topics (59,60). When there is a variation in the
placental blood flow or placental insufficiency, a series of
mechanisms help the fetus cope with the acute or chronic reduction
in oxygen availability. Tissue hypoxia caused by hypoxemia,
hypoperfusion, and ischemia/reperfusion generates an excess of
oxygen free radicals and causes a decrease in the antioxidant
defense capacity. Under these conditions of increased oxidative
stress, the fetal tissues, particularly the fetal brain, are
exposed to the deleterious effects of free radicals (60).
Traditionally, it has been accepted that this
imbalance leads to cell damage by the accumulation of reactive
oxygen species in tissues, oxidation of lipids and intracellular
proteins, resulting in cell death by apoptosis or necrosis
(59-61).
Overproduction of free radicals triggers a systemic inflammatory
response in the fetal body, given the already compromised oxygen
availability.
As oxidative stress plays a key role in developing
preeclampsia and intrauterine growth restriction, antioxidant
supplementation may prove essential for disease prevention.
Taking into consideration all these aspects, the
administration of an antioxidant with a fetal neuroprotective role
may represent an innovative therapeutic strategy. Recent theories
have reinforced the concept that antioxidants, such as ALA, can
protect the fetus against oxidative stress, particularly towards
the end of pregnancy. ALA has a low molecular weight and an
increased ability to cross biological membranes, including the
blood-brain barrier. Its efficiency depends on the dose,
administration route, and the time elapsed between treatment
administration and the onset of hypoxic cerebral injury. A study in
rats and mice conducted by Wolz and Krieglstein (62) reported that ALA acted as a
neuroprotector when administered subcutaneously, and was most
effective when administered 2 h prior to occlusion of the middle
cerebral artery. In 2017, Mei and Yang (63) demonstrated the antioxidant and
anti-inflammatory role of ALA in mice with cerebral hypoxia and
evolution to hypoxic-ischemic encephalopathy. Administering ALA 7
days prior to injury was associated with a decrease in the volume
of the cerebral infarct, the degree of cerebral edema, and the
levels of several inflammatory markers involved in oxidative
stress, such as TNF-α, NF-p65, IL-1β and IL-6. In addition, a
secondary increase in the expression of certain antioxidant enzymes
(superoxide dismutase, catalase and glutathione peroxidase) was
also observed.
4. Conclusions
ALA/DHLA acts as potent immunomodulatory redox
couple, with a specific role in preventing miscarriage and
premature delivery. The administration of ALA may represent a
promising therapeutic strategy in several obstetric pathologies,
such as complicated pregnancies with abnormal
placentation/exaggerated maternal vascular inflammatory response
(15), or hypoxia/perinatal
ischemia caused by various factors (64). Validation of this therapeutic
indication requires further studies. The onset of hypoxic or
ischemic injury should be diagnosed accurately to determine the
appropriate timing and dose for ALA administration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AP, MB and MEZ designed the review. NM, IGC, AB,
MCD, RCP and FS performed the literature research and drafted the
manuscript. AP, MB, NM and MEZ substantially contributed to the
conception of the study, revised and edited the final manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reed LJ: A trail of research from lipoic
acid to alpha-keto acid dehydrogenase complexes. J Biol Chem.
276:38329–38336. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parente E, Colannino G, Picconi O and
Monastra G: Safety of oral alpha-lipoic acid treatment in pregnant
women: A retrospective observational study. Eur Rev Med Pharmacol
Sci. 18:4219–4227. 2017.PubMed/NCBI
|
|
3
|
Smith AR, Shenv SV, Widlansky M, Suh JH
and Hagen TM: Lipoic acid as a potential therapy for chronic
diseases associated with oxidative stress. Curr Med Chem.
11:1135–1146. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mayr JA, Feichtinger RG, Tort F, Ribes A
and Sperl W: Lipoic acid biosynthesis defects. J Inherit Metab Dis.
37:553–563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Monastra G, De Gazia S, Cilaker Micili S,
Goker A and Unfer V: Immunomodulatory activities of alpha lipoic
acid with a special focus on its efficacy in preventing
miscarriage. Expert Opin Drug Deliv. 13:1695–1708. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gorąca A, Huk-Kolega H, Piechota A,
Keniewska P, Ciejka E and Skibska B: Lipoic acid-biological
activity and therapeutic potential. Pharmacol Rep. 63:849–858.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Biewenga GP, Haenen GR and Bast A: The
pharmacology of the antioxidant lipoic acid. Gen Pharmacol.
29:315–331. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hermann R, Niebch G, Borbe HO,
Fieger-Büschges H, Ruus P, Nowak H, Riethmüller-Winzen H, Peukert M
and Blume H: Enantioselective pharmacokinetics and bioavailability
of different racemic α-lipoic acid formulations in healthy
volunteers. Eur J Pharm Sci. 4:167–174. 1996.
|
|
9
|
Kliman HJ and Milano KM: The majority of
miscarriages are caused by genetic abnormalities. Fertil Steril.
100(S306)2013.
|
|
10
|
Kwak-Kim J, Yang KM and Gilman-Sachs A:
Recurrent pregnancy loss: A disease of inflammation and
coagulation. J Obstet Gynaecol Res. 35:609–622. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Christiansen OB, Nielsen HS and Kolte AM:
Inflammation and miscarriage. Semin Fetal Neonatal Med. 11:302–308.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yassaee F, Shekarriz-Foumani R, Afsari S
and Fallahian M: The effect of progesterone suppositories on
threatened abortion: A randomized clinical trial. J Reprod
Infertil. 15:147–151. 2014.PubMed/NCBI
|
|
13
|
Wada H, Shintani D and Ohlrogge J: Why do
mitochondria synthesize fatty acids? Evidence for involvement in
lipoic acid production. Proc Natl Acad Sci USA. 94:1591–1596.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Teichert J and Preiss R: HPLC-methods for
determination of lipoic acid and its reduced form in human plasma.
Int J Clin Pharmacol Ther Toxicol. 30:511–512. 1992.PubMed/NCBI
|
|
15
|
Duhig K, Chappell LC and Shennan AH:
Oxidative stress in pregnancy and reproduction. Obstet Med.
9:113–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Toy H, Camuzcuoglu H, Camuzcuoglu A, Celik
H and Aksoy N: Decreased serum prolidase activity and increased
oxidative stress in early pregnancy loss. Gynecol Obstet Invest.
69:122–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Safronova VG, Matveeva NK, Avkhacheva NV,
Sidel'nikova VM, Van'ko LV and Sukhikh GT: Changes in regulation of
oxidase activity of peripheral blood granulocytes in women with
habitual abortions. Bull Exp Biol Med. 136:257–260. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spector A, Huang RR, Yan GZ and Wang RR:
Thioredoxin fragment 31-36 is reduced by dihydrolipoamide and
reduces oxidized protein. Biochem Biophys Res Commun. 150:156–162.
1988.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Salehi B, Berkay Yılmaz Y, Antika G,
Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, Akram M, Riaz
M, Capanoglu E, Sharopov F, et al: Insights on the Use of α-lipoic
acid for therapeutic purposes. Biomolecules. 9:9–356.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shay KP, Moreau RF, Smith EJ and Hagen TM:
Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and
therapeutic potential. Biochim Biophys Acta. 1790:1149–1160.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luckheeram RV, Zhou R, Verma AD and Xia B:
CD4+ T cells: Differentiation and functions. Clin Dev
Immunol. 2012(925135)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Torchinsky A, Shepshelovich J, Orenstein
H, Zaslavsky Z, Savion S, Carp H, Fein A and Toder V: TNF-alpha
protects embryos exposed to developmental toxicants. Am J Reproduct
Immunol. 49:159–168. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang W, Sung N, Gilman-Sachs A and
Kwak-Kim J: T Helper (Th) cell profiles in pregnancy and recurrent
pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol.
11(2025)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bauer S, Pollheimer J, Hartmann J,
Husslein P, Aplin JD and Knöfler M: Tumor necrosis factor-alpha
inhibits trophoblast migration through elevation of plasminogen
activator inhibitor-1 in first-trimester villous explant cultures.
J Clin Endocrinol Metabol. 89:812–822. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Renaud SJ, Postovit LM,
Macdonald-Goodfellow SK, McDonald GT, Caldwell JD and Graham CH:
Activated macrophages inhibit human cytotrophoblast invasiveness in
vitro. Biol Reprod. 73:237–243. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vitoratos N, Papadias C, Economou E,
Makrakis E, Panoulis C and Creatsas G: Elevated circulating
IL-1beta and TNF-alpha, and unaltered IL-6 in first-trimester
pregnancies complicated by threatened abortion with an adverse
outcome. Mediators Inflamm. 2006(30485)2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Prins JR, Gomez-Lopez N and Robertson SA:
Interleukin-6 in pregnancy and gestational disorders. J Reprod
Immunol. 95:1–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chatterjee P, Chiasson VL, Bounds KR and
Mitchell BM: Regulation of the anti-inflammatory cytokines
Interleukin-4 and Interleukin-10 during pregnancy. Front Immunol.
5(253)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kaminski VD, Ellwanger JH, Matte MCC,
Savaris RF, Vianna P and Chies JAB: IL-17 blood levels increase in
healthy pregnancy but not in spontaneous abortion. Mol Biol Rep.
45:1565–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lombardelli L, Logiodice F, Aguerre-Girr
M, Kullolli O, Haller H, Casart Y, Berrebi A, L'Faqihi-Olive FE,
Duplan V, Romagnani S, et al: Interleukin-17-producing decidual
CD4+ T cells are not deleterious for human pregnancy when they also
produce interleukin-4. Clin Mol Allergy. 14(1)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nakashima A, Ito M, Shima T, Bac ND,
Hidaka T and Saito S: Accumulation of IL-17-positive cells in
decidua of inevitable abortion cases. Am J Reprod Immunol. 64:4–11.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang WJ, Hao CF, Yi-Lin Yin GJ, Bao SH,
Qiu LH and Lin QD: Increased prevalence of T helper 17 (Th17) cells
in peripheral blood and decidua in unexplained recurrent
spontaneous abortion patients. J Reprod Immunol. 84:164–170.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou
GX, Luo LH and Luan HB: Study on the relationship between Th17
cells and unexplained recurrent spontaneous abortion. Am J Reprod
Immunol. 65:503–511. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Osorio F, Leibund Gut-Landmann S, Lochner
M, Lahl K, Sparwasser T, Eberl G and Reis e Sousa C: DC activated
via dectin-1 convert Treg into IL-17 producers. Eur J Immunol.
38:3274–3281. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu HP, Cao AT, Feng T, Li Q, Zhang W, Yao
S, Dann SM, Elson CO and Cong Y: TGF-β converts Th1 cells into Th17
cells through stimulation of Runx1 expression. Eur J Immunol.
45:1010–1018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Attia M, Essa EA, Zaki RM and Elkordy AA:
An overview of the antioxidant effects of ascorbic acid and alpha
lipoic acid (in liposomal forms) as adjuvant in cancer treatment.
Antioxidants (Basel). 9(359)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Porcaro G, Brillo E, Giardina I and Di
Iorio R: Alpha Lipoic Acid (ALA) effects on subchorionic hematoma:
Preliminary clinical results. Eur Rev Med Pharmacol Sci.
19:3426–3432. 2015.PubMed/NCBI
|
|
39
|
Costantino M, Guaraldi C and Costantino D:
Resolution of subchorionic hematoma and symptoms of threatened
miscarriage using vaginal alpha lipoic acid or progesterone:
Clinical evidences. Eur Rev Med Pharmacol Sci. 20:1656–1663.
2016.PubMed/NCBI
|
|
40
|
Di Tucci C, Di Feliciantonio M, Vena F,
Capone C, Schiavi MC, Pietrangeli D, Muzii L and Benedetti Panici
P: Alpha lipoic acid in obstetrics and gynecology. Gynecol
Endocrinol. 34:729–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pashaj A, Xia M and Moreau R: α-Lipoic
acid as a triglyceride-lowering nutraceutical. Can J Physiol
Pharmacol. 93:1029–1041. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Parente E, Colannino G and Ferrara P:
Efficacy of magnesium and alpha lipoic acid supplementation in
reducing premature uterine contractions. Open J Obstet Gynecol.
4:578–583. 2014.
|
|
43
|
Carson RJ: Detection and prevention of
premature labour. Neuro Endocrinol Lett. 25 (Suppl 1):S35–S41.
2004.PubMed/NCBI
|
|
44
|
Hubinont C and Debieve F: Prevention of
preterm labour: 2011 update on tocolysis. J Pregnancy.
2011(941057)2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Connon CJ, Nakamura T, Hopkinson A,
Quantock A, Yagi N, Doutch J and Meek KM: The biomechanics of
amnion rupture: An X-ray diffraction study. PLoS One.
2(e1147)2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Snegovskikh V, Park JS and Norwitz ER:
Endocrinology of parturition. Endocrin Metabol Clin North Am.
35:173–191. 2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Moore RM, Novak JB, Kumar D, Mansour JM,
Mercer BM and Moore JJ: Alpha-Lipoic Acid inhibits tumor necrosis
factor-induced remodeling and weakening of human fetal membranes.
Biol Reprod. 80:781–787. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Moore RM, Schatz F, Kumar D, Mercer BM,
Abdelrahim A, Rangaswamy N, Bartel C, Mansour JM, Lockwood CJ and
Moore JJ: Alpha-lipoic acid inhibits thrombin-induced fetal
membrane weakening in vitro. Placenta. 31:886–892. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grandi G, Pignatti L, Ferrari F, Dante G,
Neri I and Facchinetti F: Vaginal alpha-lipoic acid shows an
anti-inflammatory effect on the cervix, preventing its shortening
after primary tocolysis. A pilot, randomized, placebo-controlled
study. J Matern Fetal Neonatal Med. 30:2243–2249. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vitrano G, Mocera G, Guardino M,
Giallombardo V and Venezia R: Oral plus vaginal alpha-lipoic acid
in women at risk for preterm delivery. IJMDAT. 1(e104)2018.
|
|
51
|
Wei SQ, Fraser W and Luo ZC: Inflammatory
cytokines and spontaneous preterm birth in asymptomatic women: A
systematic review. Obstet Gynecol. 116:393–401. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK,
Park JY, Lee KU, Kim JG and Lee IK: Alpha-lipoic acid inhibits
matrix metalloproteinase-9 expression by inhibiting NF-kappaB
transcriptional activity. Exp Mol Med. 39:106–113. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Myatt L and Cui X: Oxidative stress in the
placenta. Histochem Cell Biol. 122:369–382. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Walsh SW and Wang Y: Secretion of lipid
peroxides by the human placenta. Am J Obstet Gynecol.
169:1462–1466. 1993.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Redman CW and Sargent IL: Placental
debris, oxidative stress and preeclampsia. Placenta. 21:597–602.
2000.PubMed/NCBI View Article : Google Scholar
|
|
56
|
McKinney ET, Shouri R, Hunt RS, Ahokas RA
and Sibai BM: Plasma, urinary, and salivary 8-epi-prostaglandin
f2alpha levels in normotensive and preeclamptic pregnancies. Am J
Obstet Gynecol. 183:874–877. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Aouache R, Biquard L, Vaiman D and
Miralles F: Oxidative stress in preeclampsia and placental
diseases. Int J Mol Sci. 19(1496)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Buonocore G, Perrone S and Tataranno ML:
Oxygen toxicity: Chemistry and biology of reactive oxygen species.
Semin Fetal Neonatal Med. 15:186–190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Poston L, Igosheva N, Mistry HD, Seed PT,
Shennan AH, Rana S, Karumanchi SA and Chappell LC: Role of
oxidative stress and antioxidant supplementation in pregnancy
disorders. Am J Clin Nutr. 94 (6 Suppl):1980S–1985S.
2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lewen A, Matz P and Chan PH: Free radical
pathways in CNS injury. J Neurotrauma. 17:871–890. 2000.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gug C, Rațiu A, Navolan D, Drăgan I, Groza
IM, Păpurică M, Vaida MA, Mozoș I and Jurcă MC: Incidence and
spectrum of chromosome abnormalities in miscarriage samples: A
retrospective study of 330 cases. Cytogenet Genome Res.
158:171–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wolz P and Krieglstein J: Neuroprotective
effects of alpha-lipoic acid and its enantiomers demonstrated in
rodent models of focal cerebral ischemia. Neuropharmacology.
35:369–375. 1996.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mei XH and Yang YW: Neuroprotective
effects of α-lipoic acid against hypoxic-ischemic brain injury in
neonatal rats. Trop J Pharm Res. 16:1051–1058. 2017.
|
|
64
|
Miller SL, Wallace EM and Walker DW:
Antioxidant therapies: A potential role in perinatal medicine.
Neuroendocrinology. 96:13–23. 2012.PubMed/NCBI View Article : Google Scholar
|