Introduction
18β-Glycyrrhetinic acid (18β-GA) and 18α-GA are the
active components of Glycyrrhiza glabra (1). As the natural availability of the
18α-GA isomer is low and that of 18β-GA is higher, there is a
greater research focus on 18β-GA (2). Previous studies have demonstrated the
diverse favourable effects of 18β-GA, including its
hepatoprotective, renoprotective, antioxidant and inflammation
relief effects (3-5).
Zhou et al (6) indicated
that 18β-GA suppressed autoimmune encephalomyelitis by inhibiting
the activation of microglia and facilitating remyelination via
inhibition of the MAPK signalling pathway. Oztanir et al
(7) suggested that treatment with
18β-GA for 10 days after cerebral ischaemia/reperfusion (I/R)
altered the neurodegenerative effect of I/R on brain tissue due to
its powerful antioxidant effect and capability to scavenge
radicals. Kao et al (8)
indicated that 18β-GA protected PC12 cells from
6-hydroxydopamine-induced harm through PI3K/Akt signalling and the
Bcl-2 family. However, the protective mechanism of 18β-GA in
Schwann cells has remained elusive. As Schwann cells have a vital
impact on nerve repair (9), the
present study explored the protective capacity of 18β-GA against
H2O2-induced injury in Schwann cells.
Peripheral nerve injury is a common problem and may
result in severe disability and an economic burden (10). Despite the fact that peripheral
nerve cells have a specific regenerative ability, functional
prognosis is not optimal (11).
Schwann cells are proliferative glial cells in the peripheral
nervous system and they are essential for nerve repair after
injuries to maintain normal nerve function (12). Increasing evidence indicates that
Schwann cell death is the major cellular event in the pathogenesis
of peripheral nerve injuries, which tend to manifest in the form of
apoptosis (13). In addition,
oxidative stress after injury has a considerable role in neuronal
death. The most important genes associated with apoptosis are
proteins of the Bcl family and caspases (14,15).
Su et al (3) indicated that
18β-GA lessened the severity of radiation-induced skin injury and
decreased inflammatory infiltration and the levels of TNF-α, IL-1β
and IL-6 in dermal tissues by decreasing NADPH oxidase activity and
reactive oxygen species (ROS) production and suppressing the
activation of p38 MAPK and NF-κB signalling. 18β-GA demonstrated
considerable functions similar to those of Pyr3 or 2-aminoethyl
diphenylborinate inhibitors and suppressed high glucose-induced
effects, including the blockade of transient receptor potential
(TRP) cation channel subfamily C member 3 (TRPC3) and TRPC6 protein
expression and decreases in ROS and inducible nitric oxide synthase
expression (16). Based on the
determinant role of 18β-GA in inhibiting ROS generation and
inflammatory infiltration, it was hypothesized that 18β-GA protects
against nerve injury caused by hydrogen peroxide
(H2O2). However, the mechanism of the
neuroprotective effect of 18β-GA has so far remained elusive.
Network pharmacology has illustrated the synergistic
effects and underlying mechanisms of herbs via network analysis,
which is an appropriate way to measure the effectiveness and
demonstrate the functional mechanisms of novel bioactive compounds.
In the present study, network pharmacology was applied to identify
related targets of 18β-GA for the treatment of neuronal injury and
these targets were then experimentally verified. This experimental
verification suggested that 18β-GA protected Schwann cells from
H2O2-induced injury by inhibiting the
phosphorylation of p38 MAPK.
Methods
Pathway prediction based on network
pharmacology
The SMILES annotation for the drug 18β-GA was found
in PubChem. After searching Swiss Target Prediction, the Similarity
Ensemble Approach (SEA) Search Server and TargetNet (http://www.swisstargetprediction.ch, http://sea.bkslab.org and http://targetnet.scbdd.com/home/index, respectively),
SMILES for monomer drugs was selected and the species was limited
to Homo sapiens. The obtained protein targets and
corresponding UniProt IDs were imported into Excel. By entering the
keywords ‘injury’, ‘damage’ and ‘harm’ into the ‘GeneCards
database’ (https://www.genecards.org), reported
injury-related genes were acquired. The common targets of injury
and 18β-GA were screened by Wayne diagram analysis. These targets
were imported into the WebGestalt database (http://www.webgestalt.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; https://www.genome.jp/dbget-bin/www_bget?pathway:map04750)
pathway analysis was performed with P<0.05 and gene count
≥5.
Reagents and cell line
18β-GA (cat. no. G109796; 98.0%) was acquired from
Shanghai Aladdin Biochemical Technology Co., Ltd. PBS, Triton X,
TBS and neutral balsam were purchased from Shuyan Biological
Technology Co., Ltd. Trypsin was acquired from Guangzhou Saiguo
Biological Co., Ltd. PageRµLer Prestained Protein Ladder and Marker
(cat. no. P12083) were obtained from Shanghai Bioscience Technology
Co. Ltd. SB203580 (a p38 MAPK inhibitor) was purchased from Selleck
Chemicals. Polyvinylidene difluoride (PVDF) membranes were obtained
from MilliporeSigma. Hoechst 33258 stain (cat. no. RYS580),
phosphatase inhibitor cocktail 1 (cat. no. RBG2012) and a BCA
protein content kit (cat. no. P0010S) were acquired from Guangzhou
Junji Biotechnology Co., Ltd. RIPA buffer (cat. no. WB-0071) was
purchased from Beijing Dingguo Biological Co., Ltd. Rabbit
antibodies against TRP vanilloid 1 (TRPV1), TRP ankyrin 1 (TRPA1),
β-actin, cleaved-caspase-3, cleaved-caspase-7, Bcl-xL, p38 MAPK,
phosphorylated (p)-p38 MAPK, Bcl-2, Bax and Bad (cat. nos. ab6166,
NB110-40763, 9662S, 9664S, 8438S, 2764S, 8690S, 4511S, ab117115,
2772 and 9292, respectively) were acquired from Guangzhou Juyan
Biological Co., Ltd. Goat anti-rabbit secondary antibody (cat. no.
CW0103) was acquired from Guangzhou Juyan Biological Co., Ltd.
Meilunbio® ECL reagent (cat. no. MA0186-100ML) was
acquired by Guangzhou Jiayan Biological Co., Ltd. A Cell Counting
Kit-8 (CCK-8; cat. no. 96992) was acquired from Sigma-Aldrich
(Merck KGaA). The Annexin V FITC Apoptosis Detection Kit I (cat.
no. 556547) was obtained from Guangzhou Juyan Biological Co., Ltd.
Diluted primary antibody, diluted secondary antibody, western blot
transfer solution and western blot electrophoresis solution were
obtained from Servicebio. RNAiso Plus was acquired from Takara
Biotechnology, Co., Ltd. SYBR®-Green Premix qPCR, an Evo
M-MLV RT-PCR kit and RNase-free water (cat. nos. AG11701, AG11602
and AG11012) were obtained from Accurate Biotechnology Co., Ltd.
The Schwann cell line RSC96 was acquired from Shanghai Institute of
Cell (cat. no. GNR6). The cell line used in the experiments was
between passages 8 and 13.
Cell viability and cytotoxicity
assays
The viability of Schwann cells was determined by the
CCK-8 assay. First, Schwann cells were seeded into 96-well plates
at a density of 6x103 cells/well for 24 h. To assess
H2O2-induced injury, cells were incubated
with H2O2 at various concentrations (0, 20,
50, 100, 150, 200, 250 and 300 µM) for 4 h and then subjected to
the CCK-8 assay. For the 18β-GA-mediated protection assay, Schwann
cells were pre-treated with 18β-GA (0, 2.5, 5, 7.5, 10, 15, 20 and
30 µM) 24 h prior to being exposed to H2O2
(200 µM) for 4 h. After the incubation, the medium was discarded
and the cells were then incubated with CCK-8 solution at 37˚C for 1
h. The absorbance (450 nm) was them measured by using a microplate
reader (Bio-Tek Instruments, Inc.).
Experimental grouping
The experimental groups were as follows: Control,
model (200 µM H2O2), 10 µM SB203580 (200 µM
H2O2 + 10 µM SB203580), 5 µM GA
(H2O2 200 µM + 5 µM 18β-GA; GA5) and 10 µM GA
(200 µM H2O2 + 10 µM 18β-GA; GA10) groups. In
brief, Schwann cells (1.2x105 cells/well) were
cultivated in 6-well plates. The medium was then discarded and the
cells were washed with PBS. The cells were then incubated with
18β-GA at 5 or 10 µM concentrations or SB203580 for 24 h. After the
medium was discarded, the cells were washed with PBS and they were
incubated with 200 µM H2O2 (dissolved in PBS)
for 4 h at 37˚C.
Intracellular ROS measurement
An intracellular ROS measurement assay was performed
as previously described (17).
After being cultivated for 24 h, Schwann cells were incubated at
37˚C for 20 min in PBS containing 20 µM
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA). Subsequently,
the cells were treated with 18β-GA at two fixed concentrations or
SB203580 and then incubated with 200 µM H2O2
in PBS. After the PBS had been removed, intracellular ROS
production was measured on an inverted fluorescence microscope.
Photomicrographs of three fields were taken for each well. The
amount of intracellular ROS was determined based on the
fluorescence intensity via Image-Pro Plus 6.0 (Media Cybernetics,
Inc.).
Hoechst 33258 staining
After pre-treatment and incubation with 200 µM
H2O2 in PBS at 37˚C for 4 h, cells were
incubated with Hoechst 33258 (5 µl in 1.0 ml of PBS) in each well
for 20 min. After washing twice with PBS, fluorescence images were
acquired using an inverted fluorescence microscope. Three
photomicrographs were captured per well, and Image-Pro Plus 6.0 was
used for analysis.
Cell apoptosis detection via flow
cytometry
Annexin V FITC and propidium iodide (PI) were used
to evaluate the apoptotic rates of Schwann cells in different
groups. After pre-treatment, cells were incubated with 200 µM
H2O2 in PBS at 37˚C for 4 h. Cells were
collected with trypsin and washed with PBS. Subsequently,
1x106 cells were placed in binding buffer and
double-stained with Annexin V FITC and PI in the dark for 15 min at
4˚C. The proportion of early + late apoptotic cells was then
analysed on a flow cytometer (CytExpert 2.3; Beckman Coulter, Inc.)
to determine the apoptotic rate.
Reverse-transcription quantitative
(RT-qPCR)
According to the manufacturer's protocol, total RNA
was isolated using RNAiso Plus. Subsequently, cDNA was synthesized
based on the instructions of the RT-PCR kit. Then, a Bio-Rad CFX96
Real-Time PCR System (Bio-Rad Laboratories, Inc.) was used to
perform qPCR. The amplification parameters were 95˚C for 30 sec,
followed by 40 cycles of 95˚C for 5 sec and 60˚C for 34 sec, 95˚C
for 15 sec, 60˚C for 60 sec and 95˚C for 15 sec. The relative
expression of mRNA was calculated by the 2-ΔΔCq method
(18) after normalization to
β-actin. For this procedure, SYBR®-Green Premix qPCR and
primers (Table I) were used.
 | Table IPrimer sequences used for PCR. |
Table I
Primer sequences used for PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| TRPA1 |
AAATGCCACAGTTCTCAA |
TCTTCGTGTTGCCCTTAT |
| TRPV1 |
TTCAAGGGTTCCACGAGA |
AGTGCCGACACCTATCCA |
| TNF-α |
GCGTGTTCATCCGTTCTCTACC |
TACTTCAGCGTCTCGTGTGTTTCT |
| IL-1β |
AGGAGAGACAAGCAACGACA |
CTTTTCCATCTTCTTCTTTGGGTAT |
| β-actin |
GAGAGGGAAATCGTGCGT |
GGAGGAAGAGGATGCGG |
Western blot analysis
Changes in the expression of Bcl-xl, Bcl-2, Bad,
Bax, cleaved-caspase 3 and cleaved-caspase 7, which are related to
apoptosis pathways, were assessed by western blot analysis. The
levels of TRPA1 and TRPV1, which are closely related to injury,
were also measured. RIPA buffer was used to lyse the cells and
obtain the proteins from the supernatant. The protein concentration
was determined via a BCA assay, and samples (30 µg) were separated
via 4-10% SDS-PAGE followed by transfer to PVDF membranes and
blocking with 5% skim milk at 37˚C for 1 h. PVDF membranes were
incubated with primary antibody (1:1,000 dilution) overnight at 4˚C
for 24 h and then with secondary antibody (1:5,000 dilution) for 45
min at 37˚C. Finally, chemiluminescence was used to visualize the
bands for assessment of the images via Image-Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analyses
Values are expressed as the mean ± standard
deviation. Experiments were repeated three times. GraphPad Prism 8
(GraphPad Software, Inc.) and SPSS 13.0 (SPSS, Inc.) software were
used to perform statistical analysis. The data were analyzed by
one-way ANOVA. Bonferroni's test was the post hoc test after ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
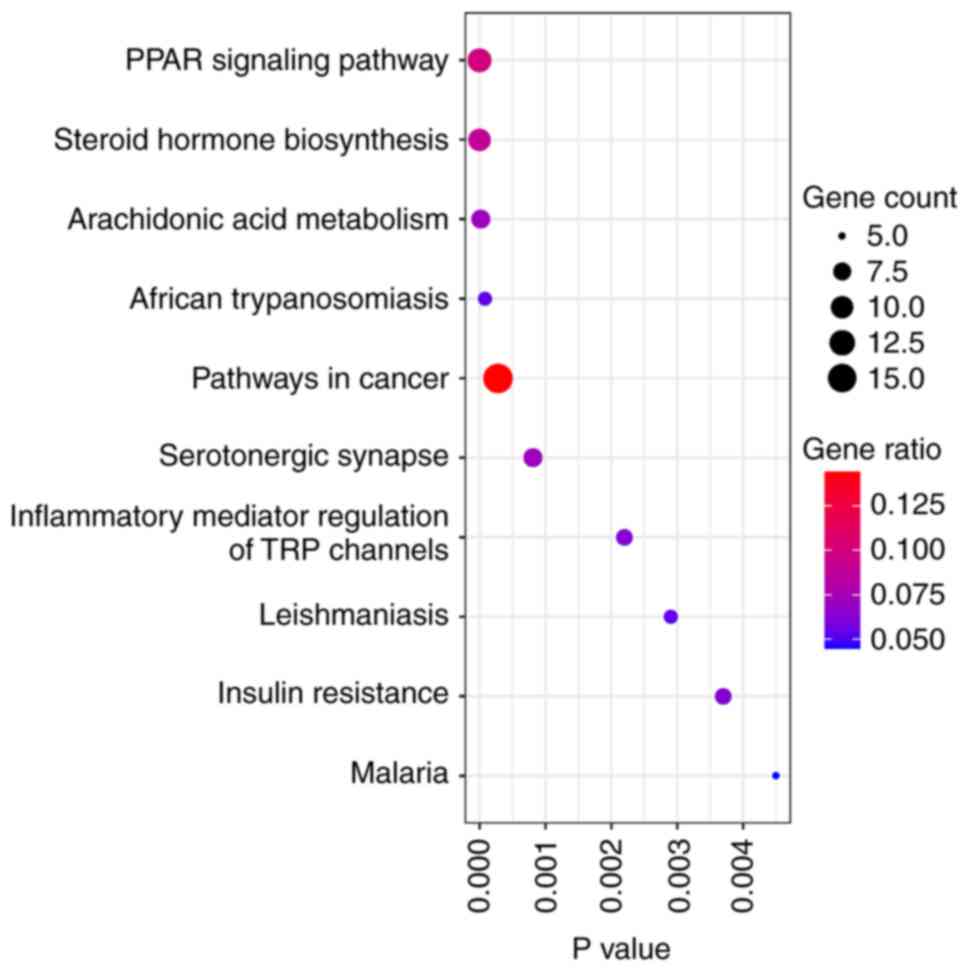
Target of 18β-GA activity
Through PubChem, the SMILES annotation for 18β-GA
was obtained, which is
‘CC1(C2CCC3(C(C2(CCC1O)C)C(=O)C=C4C3(CCC5(C4CC
(CC5)(C)C(=O)O)C)C)C)C’. This sequence was inputted into Swiss
Target Prediction, the SEA Search Server and TargetNet, which
identified 126 active targets. In addition, 15,327 targets
associated with injury were identified. A total of 111 potential
targets associated with 18β-GA for injury management were further
identified. Targets of 18β-GA with potential for injury treatment
were inputted into the STRING database along with the species. The
top 10 pathways were ranked and are presented in Fig. 1. In a previous experiment, it was
indicated that model rats with chronic constriction injury of the
sciatic nerve exhibited increased inflammation (19). Therefore, ‘Inflammatory mediator
regulation of TRP channels’, was chosen for experimental
verification.
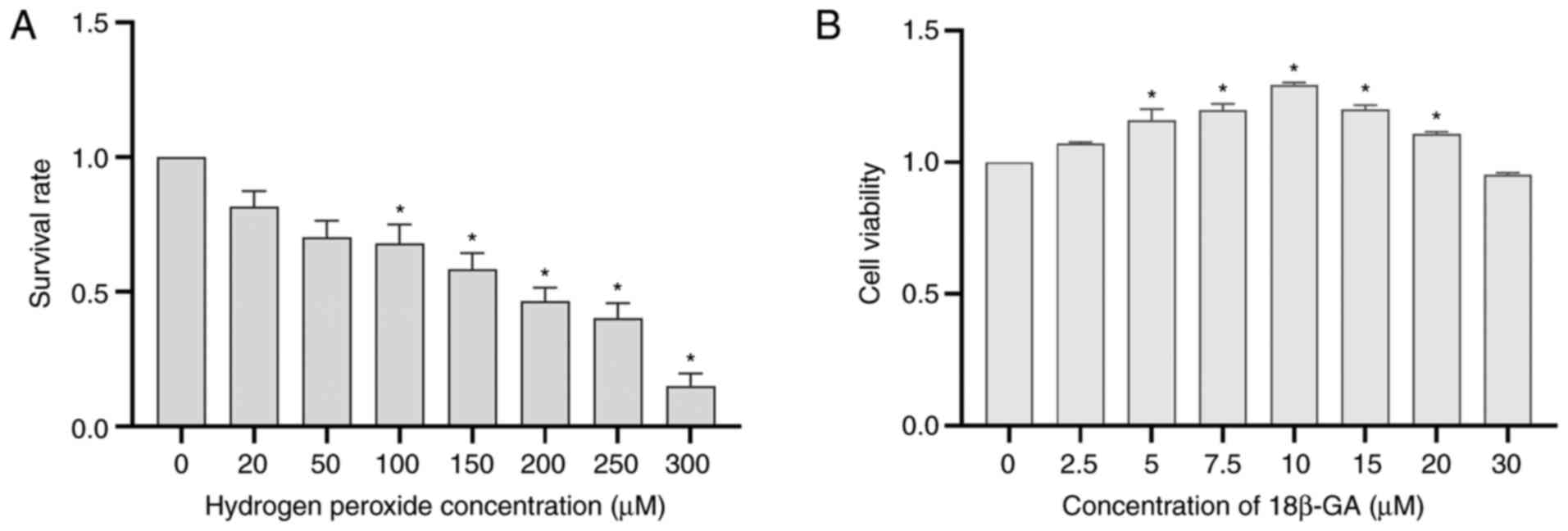
Effects of H2O2
and 18β-GA on Schwann cell viability
H2O2 decreased cell viability
in a concentration-dependent manner. A moderate response (~50%) was
induced by 200 µM H2O2 (Fig. 2A). To examine the cytotoxicity of
18β-GA, Schwann cells were incubated with various doses of 18β-GA
at 37˚C for 24 h. Cytotoxicity was determined based on the results
of the CCK-8 assays. Treatment with 10 µM 18β-GA significantly
increased cell viability (P<0.05; Fig. 2B), confirming that a suitable
concentration had been used. The concentrations of 10 and 5 µM were
subsequently applied to achieve a dose-effect relationship and it
was more suitable to choose 5 µM than 7.5 µM 18β-GA.
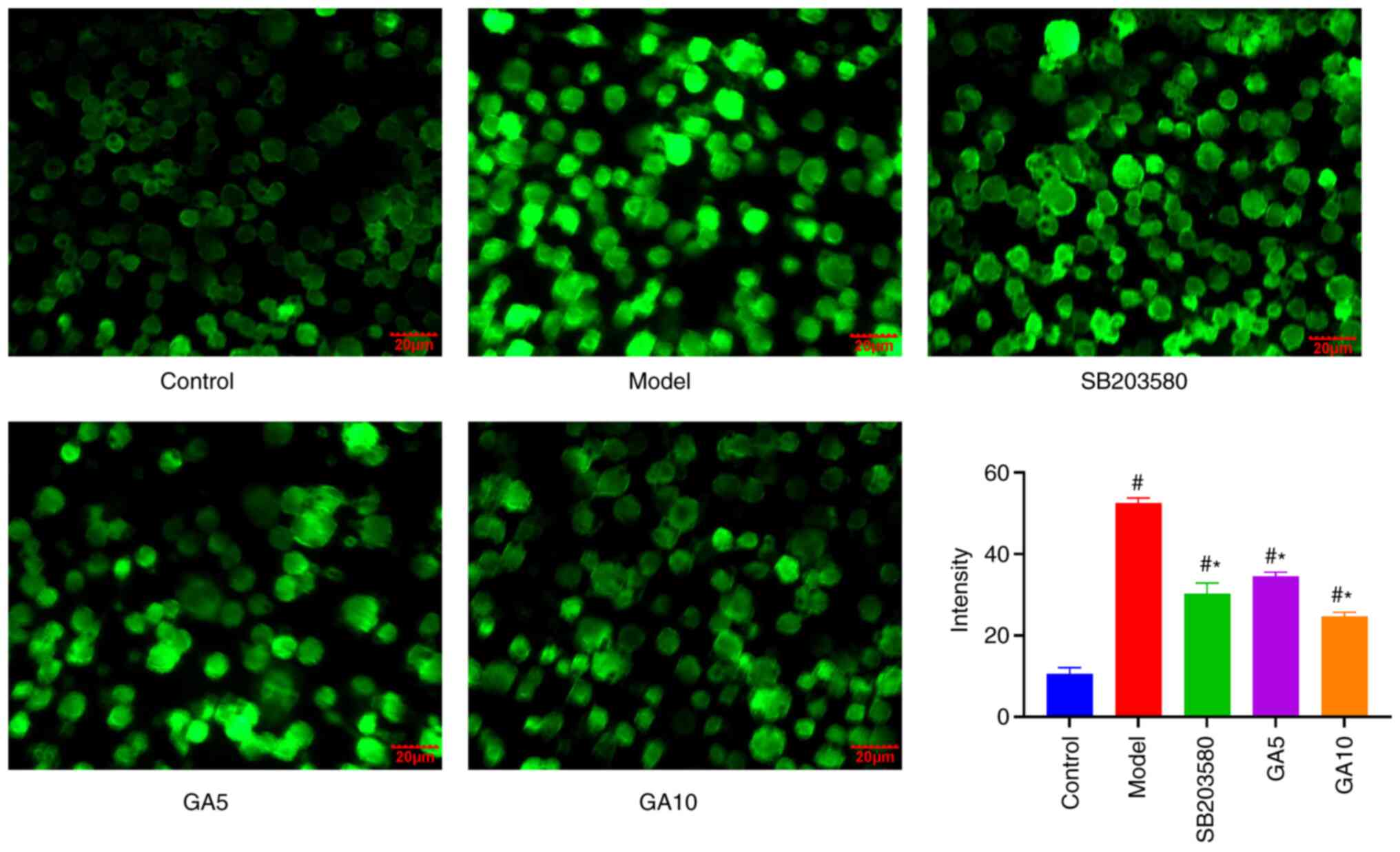
18β-GA inhibits
H2O2-induced ROS production in Schwann
cells
To determine whether the cytoprotective effects of
18β-GA are an intracellular effect, it was investigated whether
18β-GA is able to be transported into Schwann cells to inhibit
H2O2-induced intracellular radical
production. In brief, cells were first stressed with 18β-GA; 20 µM
DCFH-DA was then added before intracellular ROS levels were
evaluated. In this way, as 18β-GA and H2O2
did not come into contact in the extracellular space, any reduction
in ROS levels was attributed to an intracellular effect. Treatment
of Schwann cells with H2O2 increased
intracellular ROS levels compared with those of untreated cells.
However, 18β-GA and SB203580 attenuated ROS accumulation. The
difference in ROS production under treatment with 200 µM
H2O2 with and without 18β-GA was significant
(Fig. 3). These results indicated
that 18β-GA and SB203850 are able to decrease
H2O2-induced ROS production in Schwann
cells.
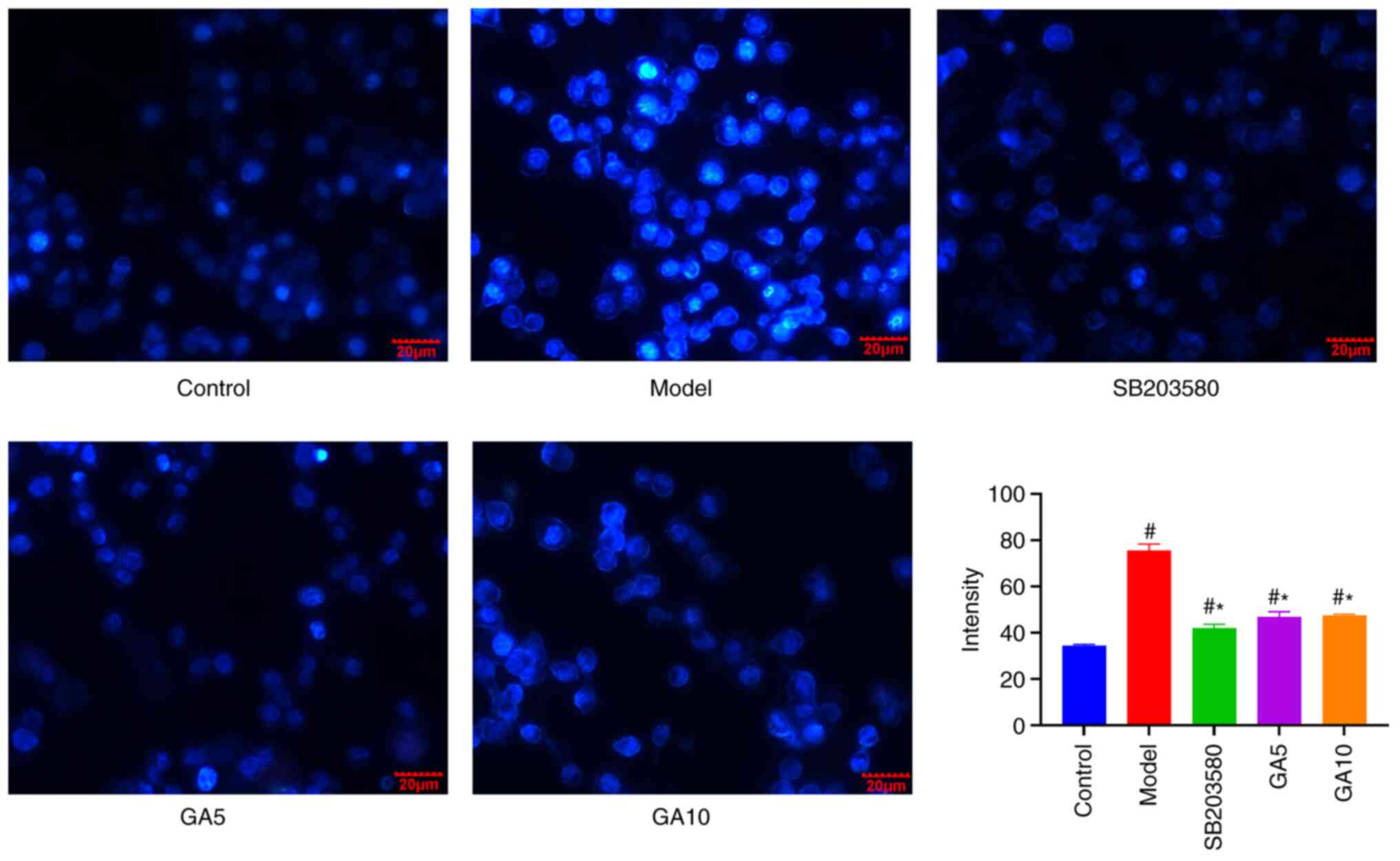
Hoechst 33258 staining
To determine whether 18β-GA protects the nucleus
from damage, nuclei were subjected to Hoechst 33258 staining. After
H2O2 treatment, Schwann cells exhibited
apoptotic nuclei, but pre-treatment with 18β-GA and SB203580
markedly abrogated these effects. 18β-GA and SB203580 inhibited the
formation of apoptotic nuclei induced by H2O2
treatment (Fig. 4).
Flow cytometry results
Flow cytometry was performed to investigate whether
18β-GA protects Schwann cells against
H2O2-induced apoptosis (Fig. 5). The proportion of apoptotic cells
was obviously lower in the control group (4.34±0.27%) than in the
model group (12.63±1.21%). In addition, the percentage of apoptotic
cells was markedly lower in the group pre-treated with SB203580 or
5 or 10 µM 18β-GA (7.02±0.30, 4.83±0.55 and 4.88±0.50%,
respectively) than in the model group (P<0.05). 18β-GA enabled
the recovery of cell viability to its normal level. These results
suggested that 18β-GA and SB203580 markedly inhibited
H2O2-induced apoptosis.
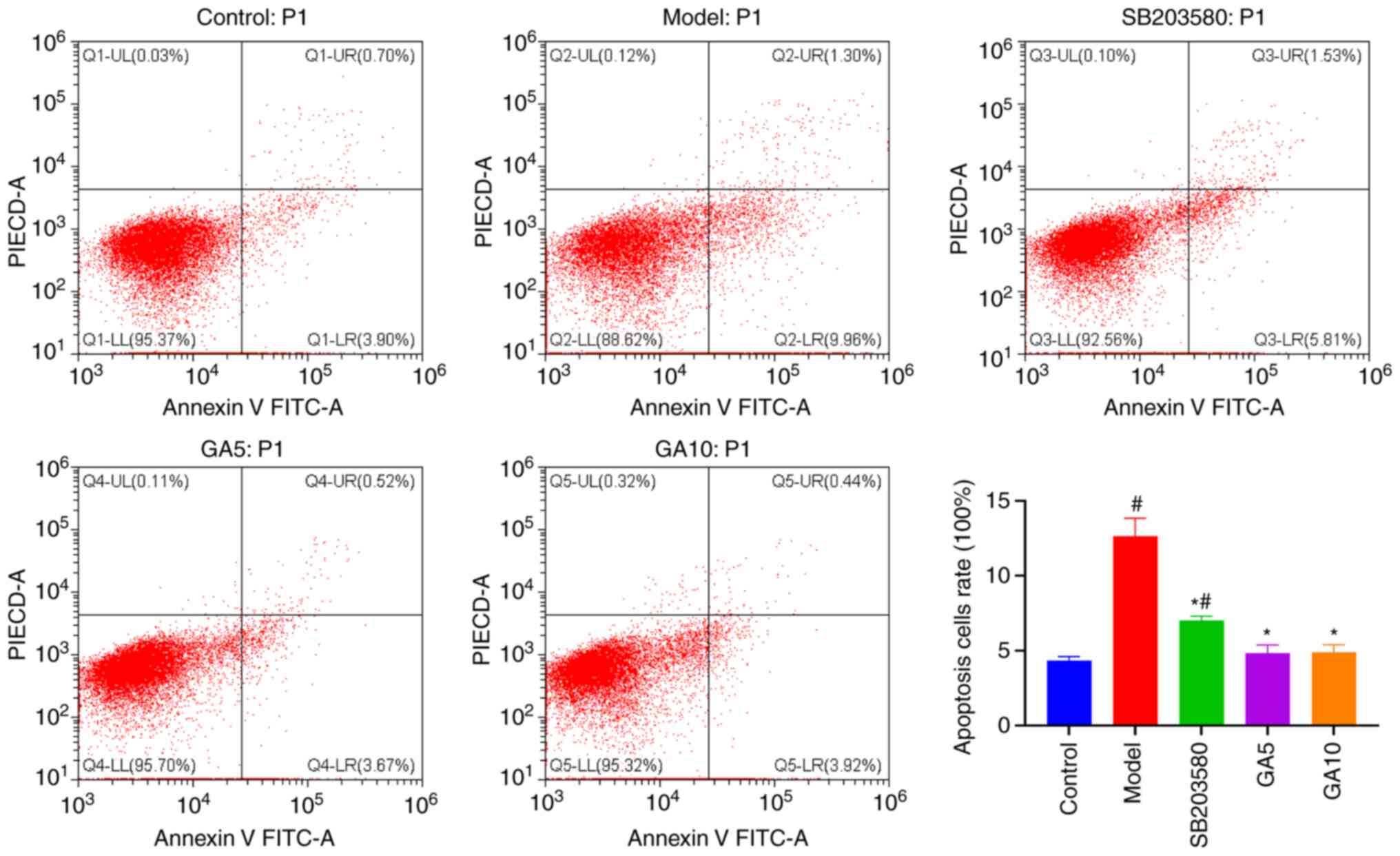 | Figure 5Protective effect of 18β-GA against
H2O2-induced apoptosis. Flow cytometry
revealed that H2O2, 18β-GA and SB203580
affected the levels of cell apoptosis. #P<0.05 vs.
control group; *P<0.05 vs. model group. 18β-GA,
18β-glycyrrhetinic acid; PI, propidium iodide; Q, quadrant; UL,
upper left; UR, upper right; LL, lower left; LR, lower right; GA5,
5 µM 18β-GA; GA10, 10 µM 18β-GA. |
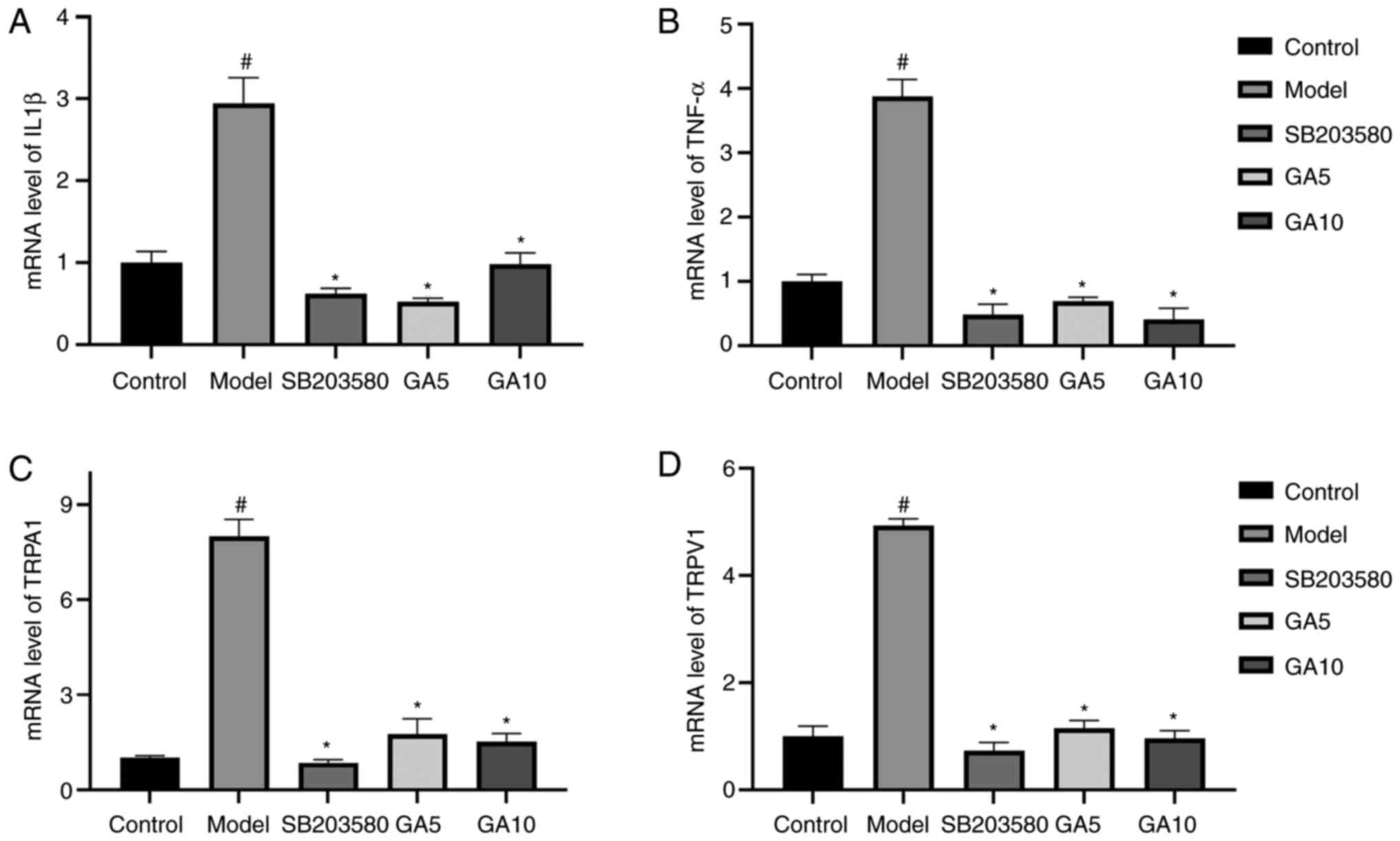
mRNA levels of TRPA1, TRPV1, IL1β and
TNF-α
To explore the mRNA expression of TRPV1, TRPA1,
IL-1β and TNF-α, RT-qPCR analysis was applied (Fig. 6). The mRNA expression of TRPV1,
TRPA1, IL-1β and TNF-α in the control group was evidently enhanced
after treatment with H2O2 (P<0.05). By
contrast, 18β-GA decreased the expression of these genes
(P<0.05). The mRNA levels of the four genes returned to normal
in the groups treated with SB203580 or 5 or 10 µM 18β-GA. Thus,
18β-GA had an anti-inflammatory effect.
Expression of TRP, apoptotic,
antiapoptotic and p38 MAPK proteins
Western blot analysis was used to determine the
expression of various proteins (Fig.
7). H2O2 decreased the expression of
Bcl-2 and Bcl-xl (P<0.05), which are antiapoptotic proteins,
while pre-treatment with 18β-GA abrogated the effect (P<0.05).
Furthermore, H2O2 was indicated to increase
the expression of Bax, Bad, cleaved-caspase-3 and cleaved-caspase-7
(P<0.05), which are proapoptotic proteins. Of note, in
comparison to the H2O2-treated model group,
SB203580 and 18β-GA enhanced the levels of Bcl-2 and Bcl-xl, and
decreased the Bax, Bad, cleaved-caspase-3 and cleaved-caspase-7
levels (P<0.05), which were nearly normal.
The levels of TRPV1, TRPA1 and p-p38 MAPK in the
model group were clearly increased after treatment with
H2O2 (P<0.05). However, the p38 MAPK
levels did not vary among the groups. 18β-GA and SB203580 reduced
the expression of the three proteins compared with those in the
model group (P<0.05). Furthermore, 18β-GA decreased TRPA1 to
near-normal levels. However, SB203580 only slightly decreased the
levels of TRPA1 and they remained significantly higher than those
in the control group (P<0.05). Overall, the inhibitory effects
of 18β-GA and SB203580 at different concentrations were similar,
suggesting that 18β-GA was able to suppress the activation of p38
MAPK and the protein levels of TRPA1 and TRPV1.
Discussion
For network pharmacology research, the top 10 KEGG
pathways are typically selected. In the present study, the pathway
‘Inflammatory mediator regulation of TRP channels’ was selected,
which contains IL-1β, phospholipase Cγ1 (PLCG1), protein kinase Cα
(PRKCA), PRKCH, prostaglandin E receptor 2 (PTGER2), PTGER4 and
TRPA1, for experimental verification. The experiments of the
present study demonstrated that H2O2 induced
oxidative stress and increased the proportion of apoptotic nuclei
and the apoptotic rate in Schwann cells. H2O2
increased intracellular ROS and the levels of Bax, Bad,
cleaved-caspase-3 and cleaved-caspase-7, while decreasing the
levels of Bcl-2, Bcl-xl, TRPA1 and TRPV1 and p-p38 MAPK. In
addition, H2O2 exposure increased the mRNA
levels of TRPA1, TRPV1, IL-1β and TNF-α. However, pre-treatment
with 18β-GA and SB203580 notably decreased the level of ROS and
particularly prevented nuclear and Schwann cell apoptosis. In
addition, pre-treatment with 18β-GA and SB203580 clearly reduced
the protein levels of Bax, Bad, cleaved-caspase-3,
cleaved-caspase-7, TRPA1, TRPV1 and p-p38 MAPK and the mRNA levels
of TRPA1, TRPV1, IL-1β and TNF-α, and enhanced the levels of Bcl-xl
and Bcl-2 in comparison with the model group.
Regarding the inflammatory pathway by which TRP
channels are regulated, IL-1β, PLCG1, PRKCA, PRKCH, PTGER2 and
PTGER4 are the upstream targets of TRPA1, as indicated in a
signalling pathway on the official KEGG website (pathway map:
map04750). Through this pathway of inflammation-mediated regulation
of TRP channels, the expression of TRPA1 may be regulated by the
p38 signalling pathway. Indeed, TRPA1 and TRPV1 are generally
co-expressed in cells (20).
Therefore, TRPA1, TRPV1 and p38 MAPK were selected as proteins from
the inflammation-mediated regulation of the TRP channel pathway for
analysis in the present study. According to differences in the
expression levels determined by PCR and western blot analysis, a
p38 inhibitor (SB203580) attenuated the
H2O2-induced increase in TRPA1, TRPV1 and
p-p38 MAPK at the protein level. These results suggested that TRPA1
and TRPV1 are related to injury and apoptosis. Furthermore, TRPA1
and TRPV1 were indicated to mediate cigarette smoke extract-induced
damage by regulating oxidative stress, inflammatory infiltration
and mitochondrial injury in bronchial epithelial cells (21).
Oxidative stress is involved in injury and
apoptosis. The accumulation of ROS may result in various forms of
oxidative protein, lipid and DNA modifications, leading to cellular
damage. 18β-GA pre-treatment strongly regulated these oxidative
conditions, and 18β-GA and SB203580 attenuated ROS accumulation.
Previous studies demonstrated that Bcl-xl and Bcl-2 were associated
with apoptosis induced by ROS accumulation (22); furthermore, ROS activate
caspase-3(22). Su et al
(3) determined that 18β-GA
treatment decreased the accumulation of ROS in RAW264.7 cells after
exposure to X-ray radiation. SB203580 decreased the expression of
cleaved-caspase 3 reduced the level of cleaved-caspase 7. SB203580
increased the expression of Bcl-xl and Bcl-2 after
H2O2 treatment. In addition, the proportion
of apoptotic cells was obviously lower in the group pre-treated
with SB203580 than in the group pre-treated with
H2O2 alone. Similarly, SB203580 reversed the
increase in condensed chromatin and apoptotic nuclei after
treatment with H2O2. Initial reports have
suggested that p38 MAPK regulates mitochondria in drug-induced
cancer cell apoptosis (23). These
results are in agreement with the generally accepted knowledge that
the inhibition of p38 MAPK phosphorylation prevents apoptosis
(24). The p38 MAPK inhibitor
SB202190 reduced TNF-α-induced TRPA1 expression.
Coskun et al (25) indicated that TNF-α induced tissue
damage mediated by neutrophils. After treatment with
H2O2, the mRNA levels of IL-1β and TNF-α
increased sharply. Pre-treatment with 18β-GA reversed this trend.
Previously, 18β-GA was reported to significantly inhibit
lipopolysaccharide-induced TNF-α production (26). Ishida et al (27) indicated that the binding of
hydroxypropyl-γ-cyclodextrin and 18β-GA had a negative effect on
IL-6, IL-1β, TNF-α and mRNA expression and enhanced intestinal
injury induced by indomethacin. Furthermore, Su et al
(3) reported that 18β-GA inhibited
radiation-induced inflammation by decreasing the accumulation of
inflammatory cytokines, including IL-6 and IL-1β, caused by
radiation. The results in the present study are similar to those of
the aforementioned studies. Therefore, 18β-GA is able to attenuate
the mRNA expression of TNF-α and IL1β, which may reduce the degree
of cell apoptosis.
18β-GA increased the expression of Bcl-xl and Bcl-2
after H2O2 treatment and decreased the
expression of Bax, Bad, cleaved-caspase-3 and cleaved-caspase-7. In
addition, the proportion of apoptotic cells was notably higher in
the group treated with H2O2 alone than in the
group pre-treated with 18β-GA. 18β-GA similarly inhibited the
protein levels of p-p38 MAPK. Su et al (3) indicated that 18β-GA exhibited
anti-inflammatory activity against radiation-induced skin damage by
inhibiting ROS accumulation and restricting the activation of the
NF-κB and p38 MAPK pathways. The present results suggested that
18β-GA prevented Schwann cell injury and apoptosis induced by
H2O2 via the p38 MAPK pathway.
There was no significant difference between 5 and 10
µM 18β-GA in terms of their anti-apoptosis effect and regulation of
various signaling factors. These two concentrations were selected
with the aim of obtaining a dose-effect relationship, but this was
not achieved. A CCK-8 assay was used to detect cell viability.
However, whilst this experiment indicates the toxicity of 18β-GA to
cells, it may not reveal anti-damage effects of a compound; this
may be the reason why the two concentrations of 18β-GA had the same
effect.
The typical manifestations of peripheral nerve
injury are increased oxidative stress (28,29),
cell damage (28) and inflammatory
infiltration (30). It was
indicated that 18β-GA was able to alleviate these effects by
decreasing inflammatory factors, decreasing the elevated expression
of TRP proteins and inhibiting p38 MAPK phosphorylation.
However, there were limitations to the present in
vitro experiments. Cell experiments are able to demonstrate
that a drug has therapeutic potential for peripheral nerve injury
prior to animal experiments. However, the main disadvantage of
in vitro experimental research is that it is challenging to
extrapolate the results to the biology of intact organisms, as the
body is not a single-celled organism.
In conclusion, H2O2 increased
intracellular ROS and the levels of Bax, Bad, cleaved-caspase-3 and
cleaved-caspase-7 and decreased the levels of Bcl-xl and Bcl-2.
Furthermore, H2O2 exposure increased the
protein levels of TRPA1 and TRPV1 and the phosphorylation of p38
MAPK. 18β-GA and SB203580 attenuated ROS accumulation, inhibited
the phosphorylation of p38 MAPK, enhanced the expression of Bcl-xl
and Bcl-2 and decreased the levels of cleaved-caspase-7,
cleaved-caspase-3, Bax and Bad, which indicated that 18β-GA may be
a candidate drug to prevent peripheral nerve injury.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81874404).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ, JS, SC and GZ made considerable contributions to
the experimental design, statistical data analysis and experimental
procedures. XL, HS, BJ, YZ, YL, GQ and YP assisted with the English
writing, and made substantial contributions towards the
experimental design and statistical analysis. DZ and JS checked and
confirmed the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pastorino G, Cornara L, Soares S,
Rodrigues F and Oliveira MBPP: Liquorice (Glycyrrhiza
glabra): A phytochemical and pharmacological review. Phytother
Res. 32:2323–2339. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Abd El-Twab SM, Hozayen WG, Hussein OE and
Mahmoud AM: 18β-glycyrrhetinic acid protects against
methotrexate-induced kidney injury by up-regulating the
Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren Fail.
38:1516–1527. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Su L, Wang Z, Huang F, Lan R, Chen X, Han
D, Zhang L, Zhang W and Hong J: 18β-glycyrrhetinic acid mitigates
radiation-induced skin damage via NADPH oxidase/ROS/p38MAPK and
NF-κB pathways. Environ Toxicol Pharmacol. 60:82–90.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahmoud AM, Hussein OE, Hozayen WG and Abd
El-Twab SM: Methotrexate hepatotoxicity is associated with
oxidative stress, and down-regulation of PPARγ and Nrf2: Protective
effect of 18β-Glycyrrhetinic acid. Chem Biol Interact. 270:59–72.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheng X, Qiu L and Wang F:
18α-glycyrrhetinic acid (GA) ameliorates fructose-induced
nephropathy in mice by suppressing oxidative stress, dyslipidemia
and inflammation. Biomed Pharmacother. 125(109702)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou J, Cai W, Jin M, Xu J, Wang Y, Xiao
Y, Hao L, Wang B, Zhang Y, Han J and Huang R: 18β-glycyrrhetinic
acid suppresses experimental autoimmune encephalomyelitis through
inhibition of microglia activation and promotion of remyelination.
Sci Rep. 5(13713)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oztanir MN, Ciftci O, Cetin A, Durak MA,
Basak N and Akyuva Y: The beneficial effects of 18β-glycyrrhetinic
acid following oxidative and neuronal damage in brain tissue caused
by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model.
Neurol Sci. 35:1221–1228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kao TC, Shyu MH and Yen GC: Glycyrrhizic
acid and 18beta-glycyrrhetinic acid inhibit inflammation via
PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation.
J Agric Food Chem. 58:8623–8629. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gao L, Feng A, Yue P, Liu Y, Zhou Q, Zang
Q and Teng J: LncRNA BC083743 promotes the proliferation of Schwann
cells and axon regeneration through miR-103-3p/BDNF after sciatic
nerve crush. J Neuropathol Exp Neurol. 79:1100–1114.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chi GF, Kim DW, Jiang MH, Yoon KJ and Son
Y: Schwann-like cells from human melanocytes and their fate in
sciatic nerve injury. Neuroreport. 22:603–608. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Sanford MT, Xin Z, Lin G and Lue
TF: Role of Schwann cells in the regeneration of penile and
peripheral nerves. Asian J Androl. 17:776–782. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gonçalves NP, Mohseni S, El Soury M,
Ulrichsen M, Richner M, Xiao J, Wood RJ, Andersen OM, Coulson EJ,
Raimondo S, et al: Peripheral nerve regeneration is independent
from schwann cell p75NTR expression. Front Cell
Neurosci. 13(235)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gomez-Sanchez JA, Pilch KS, van der Lans
M, Fazal SV, Benito C, Wagstaff LJ, Mirsky R and Jessen KR: After
nerve injury, lineage tracing shows that myelin and remak schwann
cells elongate extensively and branch to form repair Schwann cells,
which shorten radically on remyelination. J Neurosci. 37:9086–9099.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Julien O and Wells JA: Caspases and their
substrates. Cell Death Differ. 24:1380–1389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kalkavan H and Green DR: MOMP, cell
suicide as a BCL-2 family business. Cell Death Differ. 25:46–55.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li ZY, Tung YT, Chen SY and Yen GC: Novel
findings of 18β-glycyrrhetinic acid on sRAGE secretion through
inhibition of transient receptor potential canonical channels in
high-glucose environment. Biofactors. 45:607–615. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wankun X, Wenzhen Y, Min Z, Weiyan Z, Huan
C, Wei D, Lvzhen H, Xu Y and Xiaoxin L: Protective effect of
paeoniflorin against oxidative stress in human retinal pigment
epithelium in vitro. Mol Vis. 17:3512–3522. 2011.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang D, Sun J, Yang B, Ma S, Zhang C and
Zhao G: Therapeutic effect of tetrapanax papyriferus and
hederagenin on chronic neuropathic pain of chronic constriction
injury of sciatic nerve rats based on KEGG pathway prediction and
experimental verification. Evid Based Complement Alternat Med.
2020(2545806)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gouin O, L'Herondelle K, Lebonvallet N, Le
Gall-Ianotto C, Sakka M, Buhé V, Plée-Gautier E, Carré JL, Lefeuvre
L, Misery L and Le Garrec R: TRPV1 and TRPA1 in cutaneous
neurogenic and chronic inflammation: Pro-inflammatory response
induced by their activation and their sensitization. Protein Cell.
8:644–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang M, Zhang Y, Xu M, Zhang H, Chen Y,
Chung KF, Adcock IM and Li F: Roles of TRPA1 and TRPV1 in cigarette
smoke-induced airway epithelial cell injury model. Free Radic Biol
Med. 134:229–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma G, Luo W, Lu J, Ma DL, Leung CH, Wang Y
and Chen X: Cucurbitacin E induces caspase-dependent apoptosis and
protective autophagy mediated by ROS in lung cancer cells. Chem
Biol Interact. 253:1–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kang N, Wang MM, Wang YH, Zhang ZN, Cao
HR, Lv YH, Yang Y, Fan PH, Qiu F and Gao XM: Tetrahydrocurcumin
induces G2/M cell cycle arrest and apoptosis involving p38 MAPK
activation in human breast cancer cells. Food Chem Toxicol.
67:193–200. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Coskun AK, Yigiter M, Oral A, Odabasoglu
F, Halici Z, Mentes O, Cadirci E, Atalay F and Suleyman H: The
effects of montelukast on antioxidant enzymes and proinflammatory
cytokines on the heart, liver, lungs, and kidneys in a rat model of
cecal ligation and puncture-induced sepsis. ScientificWorldJournal.
11:1341–1356. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ishida T, Mizushina Y, Yagi S, Irino Y,
Nishiumi S, Miki I, Kondo Y, Mizuno S, Yoshida H, Azuma T and
Yoshida M: Inhibitory effects of glycyrrhetinic acid on DNA
polymerase and inflammatory activities. Evid Based Complement
Alternat Med. 2012(650514)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ishida T, Miki I, Tanahashi T, Yagi S,
Kondo Y, Inoue J, Kawauchi S, Nishiumi S, Yoshida M, Maeda H, et
al: Effect of 18β-glycyrrhetinic acid and hydroxypropyl
γcyclodextrin complex on indomethacin-induced small intestinal
injury in mice. Eur J Pharmacol. 714:125–131. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Khezri MK, Turkkan A, Koc C, Salman B,
Levent P, Cakir A, Kafa IM, Cansev M and Bekar A: Anti-apoptotic
and anti-oxidant effects of systemic uridine treatment in an
experimental model of sciatic nerve injury. Turk Neurosurg.
31:373–378. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang D, Yang B, Chang SQ, Ma SS, Sun JX,
Yi L, Li X, Shi HM, Jing B, Zheng YC, et al: Protective effect of
paeoniflorin on H2O2 induced Schwann cells
injury based on network pharmacology and experimental validation.
Chin J Nat Med. 19:90–99. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kalinski AL, Yoon C, Huffman LD, Duncker
PC, Kohen R, Passino R, Hafner H, Johnson C, Kawaguchi R, Carbajal
KS, et al: Analysis of the immune response to sciatic nerve injury
identifies efferocytosis as a key mechanism of nerve debridement.
Elife. 9(e60223)2020.PubMed/NCBI View Article : Google Scholar
|