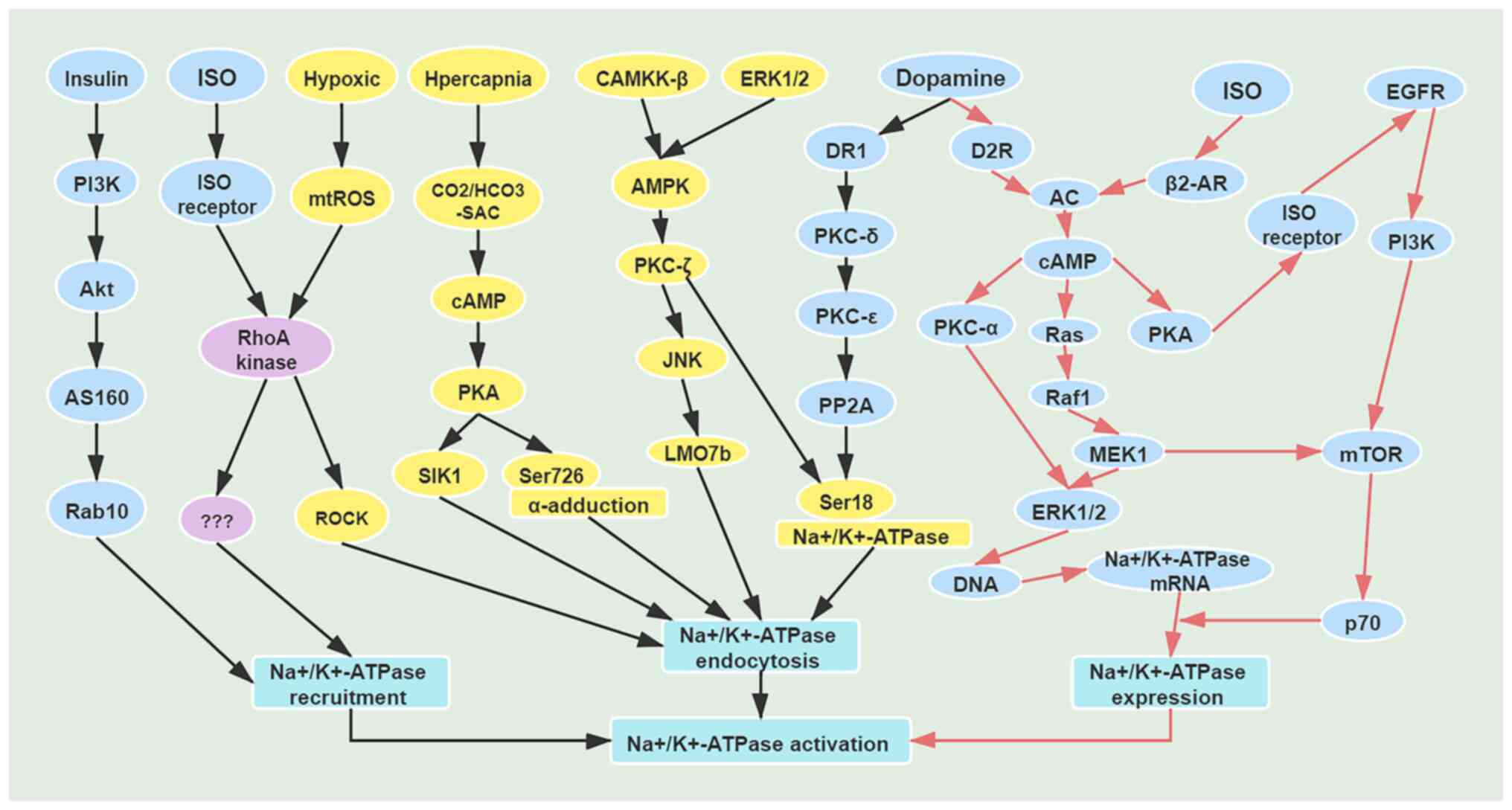
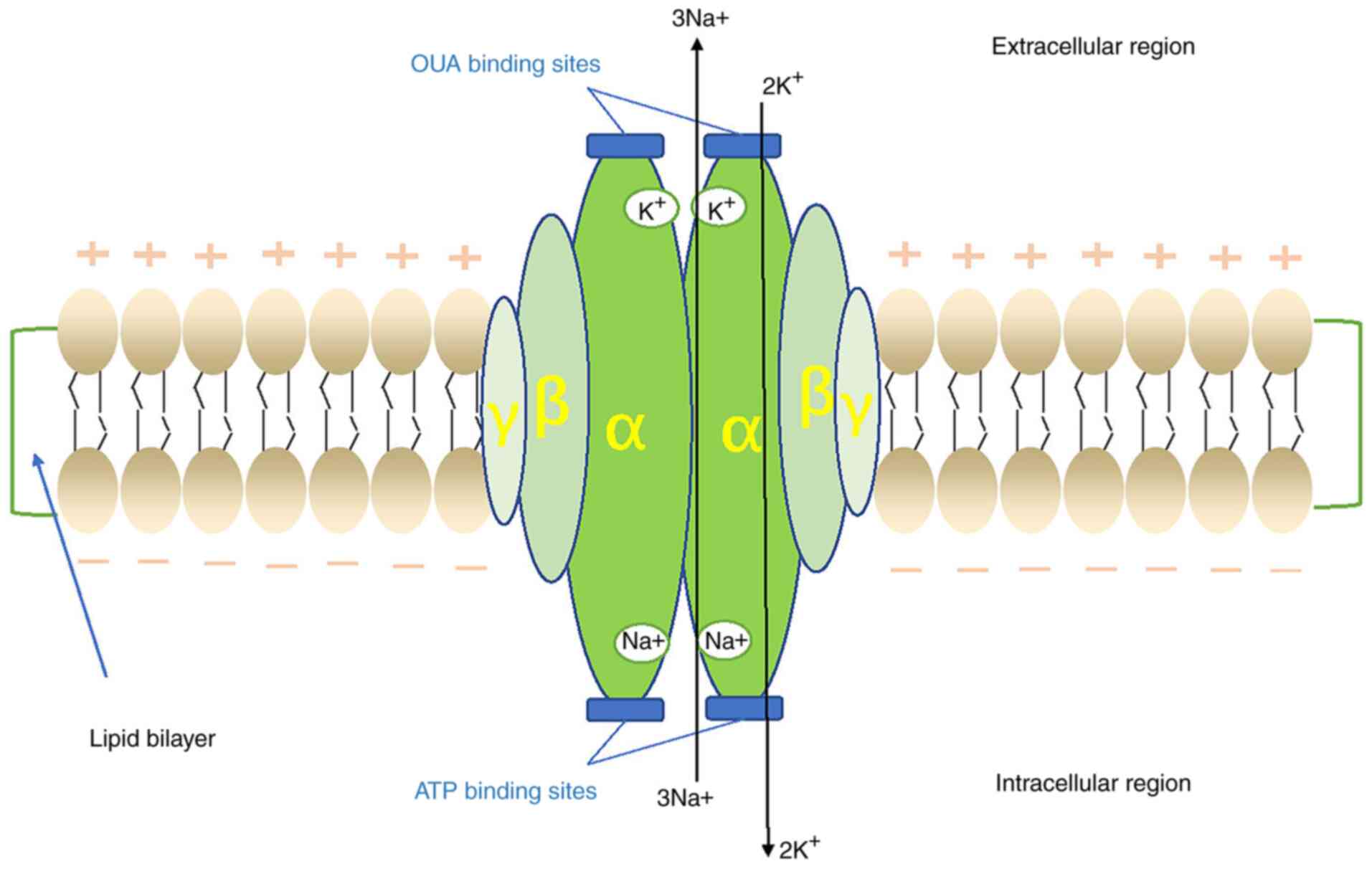
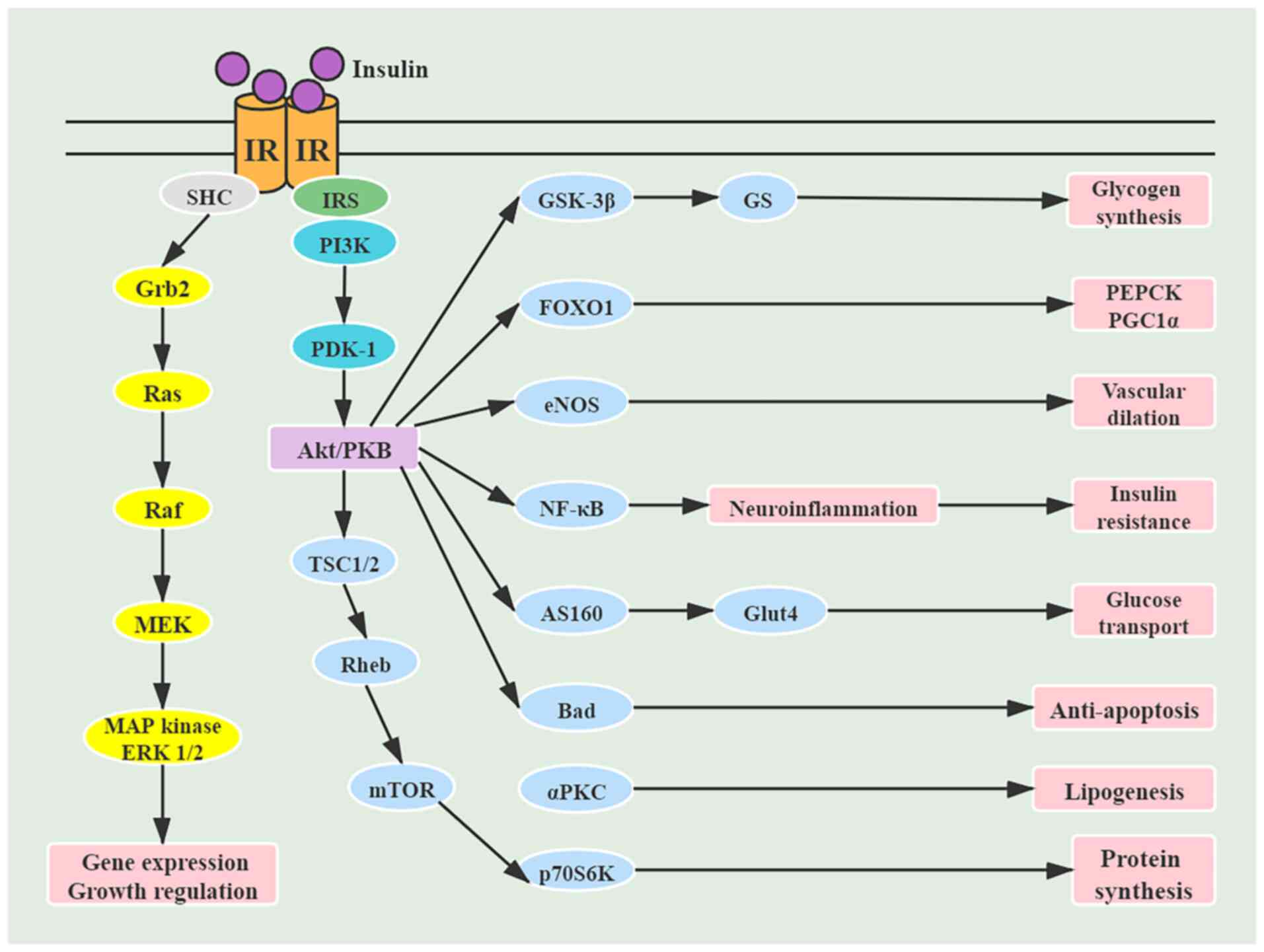
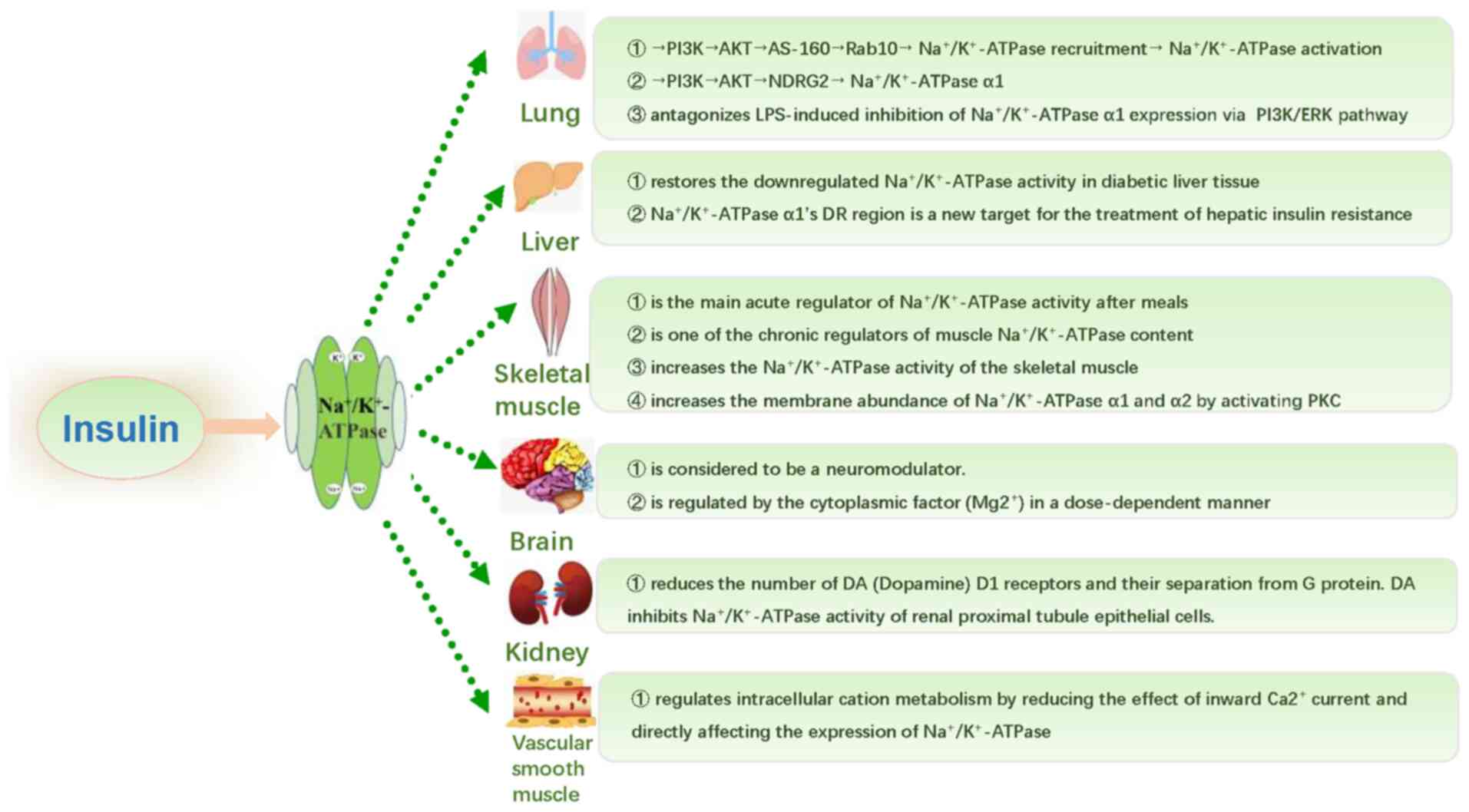
|
1
|
Liu J, Lilly MN and Shapiro JI: Targeting
Na/K-ATPase signaling: A new approach to control oxidative stress.
Curr Pharm Des. 24:359–364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaplan JH: Biochemistry of Na,K-ATPase.
Annu Rev Biochem. 71:511–535. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suhail M: Na, K-ATPase: Ubiquitous
multifunctional transmembrane protein and its relevance to various
pathophysiological conditions. J Clin Med Res. 2:1–17.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shrivastava AN, Triller A and Melki R:
Cell biology and dynamics of Neuronal
Na+/K+-ATPase in health and diseases.
Neuropharmacology. 169(107461)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang P, Cartwright C, Efuet E, Hamilton
SR, Wistuba II, Menter D, Addington C, Shureiqi I and Newman RA:
Cellular location and expression of Na+, K+
-ATPase α subunits affect the anti-proliferative activity of
oleandrin. Mol Carcinog. 53:253–263. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clausen MV, Hilbers F and Poulsen H: The
structure and function of the Na,K-ATPase isoforms in health and
disease. Front Physiol. 8(371)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tidow H, Aperia A and Nissen P: How are
ion pumps and agrin signaling integrated? Trends Biochem Sci.
35:653–659. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Z and Langhans SA: Transcriptional
regulators of Na,K-ATPase subunits. Front Cell Dev Biol.
3(66)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brazee PL, Soni PN, Tokhtaeva E, Magnani
N, Yemelyanov A, Perlman HR, Ridge KM, Sznajder JI, Vagin O and
Dada LA: FXYD5 Is an essential mediator of the inflammatory
response during lung injury. Front Immunol. 8(623)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marck PV and Pierre SV: Na/K-ATPase
signaling and cardiac pre/postconditioning with cardiotonic
steroids. Int J Mol Sci. 19(2336)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lingrel JB: The physiological significance
of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase.
Annu Rev Physiol. 72:395–412. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Johnson MD, Widdicombe JH, Allen L, Barbry
P and Dobbs LG: Alveolar epithelial type I cells contain transport
proteins and transport sodium, supporting an active role for type I
cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci
USA. 99:1966–1971. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blanco G: Na,K-ATPase subunit
heterogeneity as a mechanism for tissue-specific ion regulation.
Semin Nephrol. 25:292–303. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang HY and O'doherty GA: Modulators of
Na/K-ATPase: A patent review. Expert Opin Ther Pat. 22:587–605.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geering K: Functional roles of Na,K-ATPase
subunits. Curr Opin Nephrol Hypertens. 17:526–532. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Toyoshima C, Kanai R and Cornelius F:
First crystal structures of Na+,K+-ATPase:
New light on the oldest ion pump. Structure. 19:1732–1738.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mijatovic T, Dufrasne F and Kiss R:
Na+/K+-ATPase and cancer. Pharm Pat Anal.
1:91–106. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Herold S, Gabrielli NM and Vadász I: Novel
concepts of acute lung injury and alveolar-capillary barrier
dysfunction. Am J Physiol Lung Cell Mol Physiol. 305:L665–L681.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Laffey JG and Matthay MA: Fifty years of
research in ARDS. Cell-based therapy for acute respiratory distress
syndrome. Biology and potential therapeutic value. Am J Respir Crit
Care Med. 196:266–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vadász I, Raviv S and Sznajder JI:
Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive
Care Med. 33:1243–1251. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mutlu GM and Sznajder JI: Mechanisms of
pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol.
289:L685–L695. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lecuona E, Trejo HE and Sznajder JI:
Regulation of Na,K-ATPase during acute lung injury. J Bioenerg
Biomembr. 39:391–395. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hunter T: The age of crosstalk:
Phosphorylation, ubiquitination, and beyond. Mol Cell. 28:730–738.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calistri A, Munegato D, Carli I, Parolin C
and Palù G: The ubiquitin-conjugating system: Multiple roles in
viral replication and infection. Cells. 3:386–417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heaton SM, Borg NA and Dixit VM: Ubiquitin
in the activation and attenuation of innate antiviral immunity. J
Exp Med. 213:1–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Coppi MV and Guidotti G: Ubiquitination of
Na,K-ATPase alpha1 and alpha2 subunits. FEBS Lett. 405:281–284.
1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dada LA, Welch LC, Zhou G, Ben-Saadon R,
Ciechanover A and Sznajder JI: Phosphorylation and ubiquitination
are necessary for Na,K-ATPase endocytosis during hypoxia. Cell
Signal. 19:1893–1898. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Comellas AP, Dada LA, Lecuona E, Pesce LM,
Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A and
Sznajder JI: Hypoxia-mediated degradation of Na,K-ATPase via
mitochondrial reactive oxygen species and the ubiquitin-conjugating
system. Circ Res. 98:1314–1322. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Helenius IT, Dada LA and Sznajder JI: Role
of ubiquitination in Na,K-ATPase regulation during lung injury.
Proc Am Thorac Soc. 7:65–70. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoxhaj G, Najafov A, Toth R, Campbell DG,
Prescott AR and Mackintosh C: ZNRF2 is released from membranes by
growth factors and, together with ZNRF1, regulates the
Na+/K+ATPase. J Cell Sci. 125:4662–4675.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ryter SW, Bhatia D and Choi ME: Autophagy:
A lysosome-dependent process with implications in cellular redox
homeostasis and human disease. Antioxid Redox Signal. 30:138–159.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu M, Cao L, Xiong S, Sun H, Wu Z and
Bian JS: Na+/K+-ATPase-dependent autophagy
protects brain against ischemic injury. Signal Transduct Target
Ther. 5(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Felippe Gonçalves-de-Albuquerque C,
Ribeiro Silva A, Ignácio da Silva C, Caire Castro-Faria-Neto H and
Burth P: Na/K pump and beyond: Na/K-ATPase as a modulator of
apoptosis and autophagy. Molecules. 22(578)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu Y, Shoji-Kawata S, Sumpter RM Jr, Wei
Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, et al:
Autosis is a Na+,K+-ATPase-regulated form of
cell death triggered by autophagy-inducing peptides, starvation,
and hypoxia-ischemia. Proc Natl Acad Sci USA. 110:20364–20371.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21(29)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin X, Li S, Zhao Y, Ma X, Zhang K, He X
and Wang Z: Interaction domains of p62: A bridge between p62 and
selective autophagy. DNA Cell Biol. 32:220–227. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wurzer B, Zaffagnini G, Fracchiolla D,
Turco E, Abert C, Romanov J and Martens S: Oligomerization of p62
allows for selection of ubiquitinated cargo and isolation membrane
during selective autophagy. Elife. 4(e08941)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hancock ML, Meyer RC, Mistry M, Khetani
RS, Wagschal A, Shin T, Ho Sui SJ, Naar AM and Flanagan JG: Insulin
receptor associates with promoters genome-wide and regulates gene
expression. Cell. 177:722–736.e22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tokarz VL, MacDonald PE and Klip A: The
cell biology of systemic insulin function. J Cell Biol.
217:2273–2289. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Haeusler RA, McGraw TE and Accili D:
Biochemical and cellular properties of insulin receptor signalling.
Nat Rev Mol Cell Biol. 19:31–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Borge PD, Moibi J, Greene SR, Trucco M,
Young RA, Gao Z and Wolf BA: Insulin receptor signaling and
sarco/endoplasmic reticulum calcium ATPase in beta-cells. Diabetes.
51 (Suppl 3):S427–S433. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Herchuelz A and Pachera N: The
Na+/Ca2+ exchanger and the Plasma Membrane
Ca2+-ATPase in β-cell function and diabetes. Neurosci
Lett. 663:72–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Herchuelz A, Nguidjoe E, Jiang L and
Pachera N: Na(+)/Ca (2+) exchange and the plasma membrane
Ca(2+)-ATPase in β-cell function and diabetes. Adv Exp Med Biol.
961:385–394. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Futai M, Sun-Wada GH, Wada Y, Matsumoto N
and Nakanishi-Matsui M: Vacuolar-type ATPase: A proton pump to
lysosomal trafficking. Proc Jpn Acad Ser B Phys Biol Sci.
95:261–277. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lichtstein D, Ilani A, Rosen H, Horesh N,
Singh SV, Buzaglo N and Hodes A: Na+,
K+-ATPase signaling and bipolar disorder. Int J Mol Sci.
19(2314)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Therien AG and Blostein R: Mechanisms of
sodium pump regulation. Am J Physiol Cell Physiol. 279:C541–C566.
2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shahidullah M, Mandal A and Delamere NA:
Src family kinase links insulin signaling to short term regulation
of Na,K-ATPase in nonpigmented ciliary epithelium. J Cell Physiol.
232:1489–1500. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen HI, Yeh DY, Liou HL and Kao SJ:
Insulin attenuates endotoxin-induced acute lung injury in conscious
rats. Crit Care Med. 34:758–764. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Brunkhorst FM, Engel C, Bloos F,
Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M,
Oppert M, Grond S, et al: Intensive insulin therapy and pentastarch
resuscitation in severe sepsis. N Engl J Med. 358:125–139.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Comellas AP, Kelly AM, Trejo HE, Briva A,
Lee J, Sznajder JI and Dada LA: Insulin regulates alveolar
epithelial function by inducing Na+/K+-ATPase
translocation to the plasma membrane in a process mediated by the
action of Akt. J Cell Sci. 123:1343–1351. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bruss MD, Arias EB, Lienhard GE and Cartee
GD: Increased phosphorylation of Akt substrate of 160 kDa (AS160)
in rat skeletal muscle in response to insulin or contractile
activity. Diabetes. 54:41–50. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ishikura S, Bilan PJ and Klip A: Rabs 8A
and 14 are targets of the insulin-regulated Rab-GAP AS160
regulating GLUT4 traffic in muscle cells. Biochem Biophys Res
Commun. 353:1074–1079. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
He J, Qi D, Wang DX, Deng W, Ye Y, Feng
LH, Zhu T, Zhao Y and Zhang CR: Insulin upregulates the expression
of epithelial sodium channel in vitro and in a mouse model of acute
lung injury: Role of mTORC2/SGK1 pathway. Exp Cell Res.
331:164–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Deng W, Li CY, Tong J, He J, Zhao Y and
Wang DX: Insulin ameliorates pulmonary edema through the
upregulation of epithelial sodium channel via the PI3K/SGK1 pathway
in mice with lipopolysaccharide-induced lung injury. Mol Med Rep.
19:1665–1677. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bashir SO: Concomitant administration of
resveratrol and insulin protects against diabetes mellitus
type-1-induced renal damage and impaired function via an
antioxidant-mediated mechanism and up-regulation of
Na+/K+-ATPase. Arch Physiol Biochem.
125:104–113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu H, Chen Z, Wu D, Huang X and Wan Q:
Insulin attenuates the inhibition effect of lipopolysaccharide on
Na+ -K+ -ATPase α1 via PI3 K/AKT and PI3
K/ERK pathway. Chin J Clin Pharm Therap. 24:896–902. 2019.
|
|
57
|
Obradovic M, Bjelogrlic P, Rizzo M,
Katsiki N, Haidara M, Stewart AJ, Jovanovic A and Isenovic ER:
Effects of obesity and estradiol on
Na+/K+-ATPase and their relevance to
cardiovascular diseases. J Endocrinol. 218:R13–R23. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Siddiqui MR, Moorthy K, Taha A, Hussain ME
and Baquer NZ: Low doses of vanadate and trigonella synergistically
regulate Na+/K+ -ATPase activity and GLUT4
translocation in alloxan-diabetic rats. Mol Cell Biochem.
285:17–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sun HJ, Cao L, Zhu MY, Wu ZY, Shen CY, Nie
XW and Bian JS: DR-region of Na+/K+-ATPase is
a target to ameliorate hepatic insulin resistance in obese diabetic
mice. Theranostics. 10:6149–6166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pirkmajer S and Chibalin AV: Na,K-ATPase
regulation in skeletal muscle. Am J Physiol Endocrinol Metab.
311:E1–E31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pirkmajer S and Chibalin AV: Hormonal
regulation of Na+-K+-ATPase from the
evolutionary perspective. Curr Top Membr. 83:315–351.
2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Catalán RE, Martínez AM, Aragonés MD,
Fernández I and Miguel BG: Inhibitory effect of insulin and
cytoplasmic factor(s) on brain (Na(+) + K+) ATPase. Neurosci Res.
13:139–145. 1992.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Banday AA, Asghar M, Hussain T and
Lokhandwala MF: Dopamine-mediated inhibition of renal Na,K-ATPase
is reduced by insulin. Hypertension. 41:1353–1358. 2003.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sowers JR: Effects of insulin and IGF-I on
vascular smooth muscle glucose and cation metabolism. Diabetes. 45
(Suppl 3):S47–S51. 1996.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Silva CID, Gonçalves-De-Albuquerque CF,
Moraes BPT, Garcia DG and Burth P: Na/K-ATPase: Their role in cell
adhesion and migration in cancer. Biochimie. 185:1–8.
2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lu S, Cai S, Peng X, Cheng R and Zhang Y:
Integrative transcriptomic, proteomic and functional analysis
reveals ATP1B3 as a diagnostic and potential therapeutic target in
hepatocellular carcinoma. Front Immunol. 12(636614)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Vigneri R, Goldfine ID and Frittitta L:
Insulin, insulin receptors, and cancer. J Endocrinol Invest.
39:1365–1376. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Vigneri R, Sciacca L and Vigneri P:
Rethinking the relationship between insulin and cancer. Trends
Endocrinol Metab. 31:551–560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Belfiore A, Frasca F, Pandini G, Sciacca L
and Vigneri R: Insulin receptor isoforms and insulin
receptor/insulin-like growth factor receptor hybrids in physiology
and disease. Endocr Rev. 30:586–623. 2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Vella V, Sciacca L, Pandini G, Mineo R,
Squatrito S, Vigneri R and Belfiore A: The IGF system in thyroid
cancer: New concepts. Mol Pathol. 54:121–124. 2001.PubMed/NCBI View Article : Google Scholar
|