1. Introduction
Asthma is a heterogeneous disorder characterized by
chronic airway inflammation, airway hyperresponsiveness,
bronchoconstriction and mucus hypersecretion, which manifests with
respiratory symptoms and limited expiratory airflow that vary in
intensity and duration (1). Asthma
is the most common chronic respiratory disease, affecting ~339
million people worldwide in 2018(2). Despite a reduction by ~half in
asthma-associated disability and mortality reported in a number of
higher income countries, such as the United States of America,
Canada, United Kingdom, France, Germany and Japan from 2001-2005 to
2011-2015(1), which is primarily
attributed to the use of inhaled corticosteroid (ICS) therapy, an
extensive worldwide disparity remains in years of life lost because
of asthma (3).
While most patients with asthma achieve good control
of the disease in terms of both the symptom burden (asthma
symptoms, sleep disturbance and limited ability to exercise) and
the risk of poor outcomes (limited lung function, frequent
exacerbation, mortality and medication side-effects) (4,5),
5-10% experience severe symptoms and recurrent exacerbations
despite maximum dose treatment with inhaled glucocorticoids and
long-acting β2-agonists (LABA) (6). These patients are defined as having
severe asthma and are under consideration for alternative
therapies. In severe asthma, stepped care approaches in therapy are
frequently ineffective, most likely reflecting asthma heterogeneity
and indicating that a treatment strategy of ‘one size fits all’ is
not efficient in maintaining optimum control of the disease. The
characteristics of severe asthma result in decreased responsiveness
to usual medication and require alternative therapies with higher
specificity that target the mechanisms underlying the increased
severity (7).
To achieve good control of asthma, novel treatments
should preferably target the pathophysiological mechanism
responsible for controlling disease severity and altering the
responsiveness to usual therapies in most patients (4). Several pathways have garnered
attention and led to the development of novel targeted
interventions. Of interest has been the use of monoclonal
antibodies targeting components of type 2 (T2) airway inflammation
(6). To ensure a good selection of
patients for biological therapy trials, a careful phenotype
assessment is required with consideration to differentiate the two
major phenotypes: T2-high and -low airway inflammation (8,9),
based on the degree of T2 inflammation. Type 2 inflammation
involves Type 2 (Th2) lymphocytes (CD4+) and the innate
lymphoid cells group 2 (ILC2) that secrete proteins, such as IL-4,
-5, -13, and IgE, resulting in the recruitment of cells, such as
eosinophils, basophils and mast cells into the airways (8). Non-T2 asthma is defined as asthma
without features of T2 asthma. The definition is arbitrary and is
generally based on the presence of neutrophils in sputum, or the
absence or normal levels of eosinophils or other T2 markers in
sputum (paucigranulocytic), airway biopsies or in blood (10). Current approved biological agents
target T2-high severe asthma, which clinically manifests with a
combination of sputum eosinophilia, peripheral eosinophilia and/or
elevated fractional exhaled nitric oxide (FeNO) (11,12).
While relatively novel biological agents, such as mepolizumab,
benralizumab and reslizumab, target IL-5 or IL-5 receptor (IL-5R),
and dupilumab targets IL-4Rα signaling, previous biological agents,
such as omalizumab, target IgE (13).
The present study aimed to review the effectiveness
and safety profile of currently approved biological agents or those
under investigation, and to provide an overview of the assessment
of patients with severe asthma who are potentially suitable for
biological therapy, to help clinicians select the most appropriate
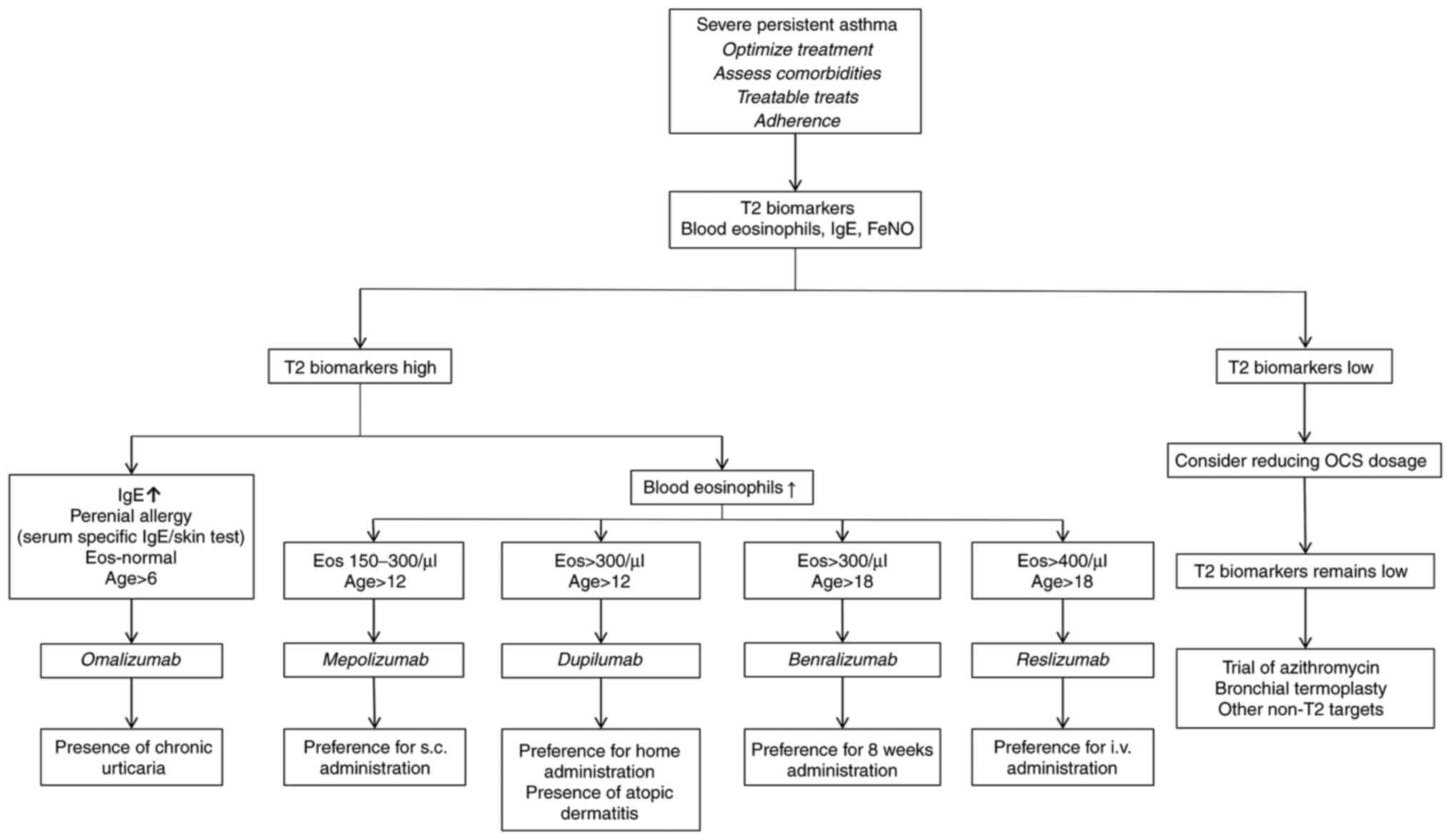
biological agent for managing T2 severe asthma phenotypes (Fig. 1).
2. Pathophysiology of severe asthma
Asthma is a heterogeneous, chronic inflammatory
airway disease with complex pathophysiological mechanisms that
impact clinical outcomes, including drug response (14,15).
Knowledge of the various asthma phenotypes and their different
pathophysiology is continuously growing. Nevertheless, the pathways
and underlying mechanisms of severe asthma pathogenesis are not yet
completely understood.
There are two major groups of asthma phenotypes that
can be differentiated by the inflammatory pathway involved, namely
T2 or T2-high (eosinophilic) and non-T2 or T2-low phenotypes
(16). T2 or T2-high asthma is
characterized by T2 inflammation involving T helper 2 (Th2)
lymphocytes (CD4+), which drives eosinophilic airway
inflammation by producing abundant quantities of proteins such as
IL-4, IL-5, IL-13 and IgE. In the past decade, evidence has
demonstrated that the innate lymphoid cells group 2 (ILC2) plays an
early key role in augmenting T2 inflammatory responses in the
airway (6,8,9,17).
Thus, the terminology has changed from ‘Th2-high’ to ‘T2-high’,
reflecting the role of innate immunity, along with CD4+
cells, in the pathophysiology of asthma. T2-high asthma encompasses
several subtypes in both children and adults, such as early-onset
allergic and late-onset eosinophilic asthma, and
aspirin-exacerbated respiratory disease (18).
Non-T2 or T2-low asthma is less well characterized
(10); Th1 and Th17 cells,
neutrophils and proteins, such as IL-1β, IL-6, IL-8, IL-17A/F,
TNF-α and IFN-γ, are involved in its pathobiology. In addition to
inflammatory biomarkers, non-T2 asthma may also be driven by
irregular neuronal activation as well as structural abnormalities
involving airway smooth muscle (10). Non-T2 asthma is characterized by
neutrophilic or paucigranulocytic inflammation and a lack of
response to corticosteroid therapy (10,18).
Severe neutrophilic asthma is associated with chronic infection
with atypical bacteria, smoking, obesity and poorly understood
underlying smooth muscle abnormalities (18).
This division of the patterns of inflammation is
mostly arbitrary and it is likely that mixtures of both pathways
exist, at least to some degree, in most patients (7). Exposure to different triggers, such
as allergens, viruses or other irritants, activates airway
epithelial cells, which leads to secretion of specific cytokines
[thymic stromal lymphopoietin (TSLP), IL-25 and IL-33] that
initiate an inflammatory cascade resulting in asthma symptoms
(8,19). The distinct inflammatory pathway
initiated differs depending on the particular trigger, patient
genotype, and the subtype of immune cells stimulated and their
specific secreted mediators (19).
In the T2 immune response, Th2 cells and ILC2 are
activated, and the secretion of the cytokines IL-4, IL-5 and IL-13
is stimulated (20,21). Allergens trigger a direct,
immediate bronchoconstrictor response by activating mast cell
mediator release. Mast cells are also a potential source of T2
cytokines (6). IL-4 induces Th0
cells to differentiate into Th2 cells and Ig class switch,
resulting in IgE production by B cells. Subsequently, IgE binds to
mast cells and triggers the release of toxic granules (6,8,19).
IL-5 is the primary regulator of eosinophil proliferation,
migration, activation and survival. IL-13 stimulates IL-4-induced
IgE production by B cells, mucus production by goblet cells and
goblet cell metaplasia; it may also have a direct effect on airway
smooth muscle, increasing airway hyperresponsiveness (6).
T2 innate lymphoid cells and activation by the
innate immune system of structural cells, such as the airway
epithelium, are involved in the T2 inflammatory pathway independent
of exogenous allergens, without atopy and with normal levels of
serum IgE. This asthma pattern is associated with nasal polyposis
and aspirin sensitivity (6,8,22).
ILC2 cells produce more IL-5 and IL-13 compared with CD4 Th2 cells
independent of allergen exposure (22). Various harmful external triggers
(microbes, air pollutants or glycolipids) cause respiratory
epithelial damage and increase epithelial cell production of the
alarmins IL-25, IL-33 and TSLP. Alarmins bind to the receptors on
T2 ILC2 and activate them to produce T2 cytokines (6,22,23).
Production of leukotriene E4 and prostaglandin D2 by recruited and
activated eosinophils and mast cells may also stimulate ILC2 cells,
leading to a continuous cycle of T2 inflammatory response (6), in which eosinophils serve a key role
in two major events: Hyperresponsiveness and remodeling of the
airways. Persistent eosinophilic inflammation maintained by these
pathways leads to constant damage at the bronchial level. This
airway damage is attributed to the degranulation of eosinophils and
the release of toxic proteins, such as major basic protein,
eosinophil peroxidase (EPO), eosinophil cationic protein and
eosinophil-derived neurotoxin (6,8). In
addition, the regeneration process of the airway can have harmful
consequences, including goblet cell hyperplasia, smooth muscle
hypertrophy and deposition of extracellular matrix proteins, which
causes membrane thickening and fibrosis (24), resulting in airway mucus plugging
and airway wall edema. Recently, a cross-sectional analysis of CT
scans of the lungs indicated that mucus plugging may be a
particularly important mechanism in severe asthma, and identified
EPO-associated changes in mucin structure resulting in abnormally
sticky mucus as another key pathophysiological mechanism in severe
asthma (6,25). Therefore inhaled corticosteroids
and bronchodilators may become less effective, thus requiring novel
specific therapies for patients with severe asthma.
3. Targeting T2 severe asthma
phenotypes-currently approved treatments and biological drugs under
investigation
Targeting IgE
Currently approved biological therapies for severe
asthma are shown in Table I.
Omalizumab [Xolair®] is a humanized monoclonal antibody
that binds to IgE and prevents it combining with the IgE receptor
on plasma cells (such as mast cells and basophils) (6,7).
This blocking effect impedes mast cell activation and the
subsequent production of inflammatory mediators when IgE is
activated by allergens (7).
Omalizumab for subcutaneous use was initially approved by the
United States Food and Drug Administration (FDA) in 2003 as a
biological therapy for severe allergic asthma in patients >12
years of age, and in 2016 received approval in patients >6 years
of age who have moderate to severe persistent allergic asthma
(26). Eligibility criteria for
this biological agent are poor control using conventional asthma
treatment, and allergic components revealed by a positive skin test
or in vitro reactivity to a perennial aero-allergen, such as
house dust mites, pollen and fungal mould (6,26).
Furthermore, the therapeutic regime depends on levels of total IgE
and patient weight [either once/month or every 2 weeks,
subcutaneous (s.c.)].
 | Table ICurrently approved biological
therapies for severe asthma. |
Table I
Currently approved biological
therapies for severe asthma.
| Drug | Target | Administration | Patient age,
years | Indications | Side effects | Peculiarities |
|---|
| Omalizumab | Circulating
IgE | Every 2-4 weeks,
s.c. | ≥6 | Moderate to severe
persistent asthma; positive skin test or in vitro reactivity
to perennial aeroallergen; symptoms inadequately controlled with
ICS | Injection site
reaction, fever, nosebleeds, joint pain, bone fractures, arm or leg
pain, generalized pain, nausea, vomiting, stomach pain, headache,
earache, dizziness, fatigue | IgE level and
weight-based dosing |
| Reslizumab | IL-5 ligand | Every 4 weeks,
i.v. | ≥12 | Severe eosinophilic
asthma that remains uncontrolled despite maximal therapy with ICS
plus another controller | Headache, asthma
worsening, nasopharyngitis, upper respiratory tract infection,
sinusitis, injection site reactions | Weight-based
dosing |
| Mepolizumab | IL-5 ligand | Every 4 weeks,
s.c. | ≥12 | Severe eosinophilic
asthma that remains uncontrolled with ICS | Respiratory tract
infection, bronchitis, worsening of asthma, headache, injection
site reaction | |
| Benralizumab | IL-5 receptor
α | Every 4 weeks,
s.c. | ≥12 | Severe eosinophilic
asthma (≥300 blood eosinophils/µl) inadequately controlled with
ICS | Upper respiratory
tract infection, worsening asthma, injection site reaction | Administration
decreased to every 8 weeks after 3 doses |
| Dupilumab | IL-4 receptor
α | Every 2 weeks,
s.c. | ≥12 | Moderate to severe
eosinophilic asthma or OCS-dependent asthma (FDA). Severe asthma
with type 2 inflammation (increased blood eosinophils and/or raised
FeNO levels) that remain inadequately controlled despite high-dose
ICS plus another controller (EMA) | Allergic
conjunctivitis, conjunctivitis, injection site reaction, ophthalmic
inflammation, eye irritation | |
Numerous clinical studies, and real-world
observational studies, have demonstrated that omalizumab can
decrease the frequency of exacerbations and decrease the need for
additional medication, including ICS, but shows little improvement
in lung function compared with high-dose combined ICS/LABA therapy
(27-31).
In a large study of severe asthma, Hanania et al (28) evaluated omalizumab in patients
requiring high-dose combined ICS/LABA treatment, but still
presenting with symptoms and ongoing exacerbations. The
introduction of omalizumab in addition to high-dose ICS/LABA
decreased asthma exacerbations (loss of asthma control requiring
systemic corticosteroids) by 25%. Other trials with omalizumab have
shown a significant decrease in asthma exacerbations of 35-60%, an
88% decrease in asthma-associated hospitalization in pediatric
patients, as well as a decrease of ICS doses by >50% in a
significant number of patients (27,29-31).
In addition, it has been shown that omalizumab did not affect the
frequency of exacerbations in patients with asthma receiving
maintenance oral corticosteroid (OCS) treatment. Biomarkers, such
as blood eosinophils and FeNO, have been reported to be
representative of T2 inflammation, and high levels of these
biomarkers [FeNO ≥19.5 parts per billion (ppb), peripheral blood
eosinophils ≥260/µl or periostin ≥50 ng/ml] may indicate patients
with the greatest potential clinical benefit from anti-IgE
treatment (29). However,
real-world studies suggested that eosinophil levels are not a good
predictor of the potential decrease in exacerbations seen with
omalizumab (31,32).
Previous studies have identified novel potential
mechanisms of action of omalizumab in association with decreased
viral-induced exacerbations of asthma by increasing viral clearance
(33,34). The frequency of exacerbations
occurring in spring and fall was shown to be significantly
decreased by omalizumab in school-age children with moderate to
severe asthma and a history of exacerbations (33). The effect of viral-induced
exacerbations may be mediated by the increased production of IFN-α
by plasmacytoid dendritic cells (6,33,34).
Omalizumab was developed to target IgE in allergic asthma and there
have been few, limited studies on omalizumab in non-allergic asthma
(6,34).
The most common adverse events (AEs) of omalizumab
include injection site reaction, fever, nosebleeds, joint pain,
bone fractures, arm or leg pain, generalized pain, nausea,
vomiting, stomach pain, headache, earache, dizziness and fatigue
(26,29-37).
Risk of anaphylaxis may occur up to 24 h after any dose and
treatment should be discontinued if severe hypersensitivity
reaction occurs (36,37). Malignant neoplasms have been
reported, with a rate of 0.5% compared with a rate of 0.2% in
controls in clinical trials (29-32).
It is recommended to monitor patients at high risk for geohelminth
infection while taking omalizumab, as well as for eosinophilia,
vasculitic rash, neuropathy and/or cardiac complications,
especially upon decreasing OCS dose (26,37).
Omalizumab is also indicated for the treatment of
patients >12 years of age with chronic spontaneous urticaria
(CSU) who remain symptomatic despite H1 antihistamine treatment
(37). The association of moderate
to severe persistent allergic asthma and CSU may be a strong
argument for choosing the treatment with omalizumab, compared with
other treatments (Fig. 1)
(36).
Long-term studies of omalizumab demonstrate that it
is efficient and generally well-tolerated, and is recommended as
initial anti-T2 treatment of choice for patients (both adults and
children >6 years old) with severe asthma and underlying
allergic sensitization (35-37).
Targeting T2 cytokines. Anti-IL-5 and
IL-5Rα blockers
Anti-IL-5 and anti-IL-5R are two currently available
and effective interventional strategies to regulate eosinophil
involvement in airway inflammation and asthma symptoms (5,6,35,36,38).
Anti-IL-5R may also have an inhibitory effect on basophils, as
these cells also express IL-5R (5). IL-5 is produced by Th2 lymphocytes,
ILC2 and mast cells, regulates final differentiation of
eosinophils, and participates in the recruitment of eosinophils to
the airway and their activation (5,6).
Mepolizumab and reslizumab are monoclonal antibodies directed
against IL-5 that prevent activation of IL-5R on eosinophils.
Benralizumab is also a monoclonal antibody but is directed against
IL-5R where antibody-dependent cell-mediated cytotoxicity (ADCC)
induces cell apoptosis (5,6). Both treatment strategies efficiently
and safely decrease levels of circulating and airway eosinophils
(7,35,36,38).
Mepolizumab [Nucala®] is a recombinant,
humanized monoclonal anti-IL-5 antibody (IgG1κ) specifically
targeting the α subunit, thereby blocking the interaction between
the α-subunit and IL-5R on the eosinophil cell surface. Inhibiting
the binding of IL-5 to eosinophils results in inactivation of
eosinophil maturation, activation and growth (35,36,39).
Mepolizumab has been approved as an add-on treatment for patients
≥12 (FDA) or ≥6 years old [European Medicines Agency (EMA)] with
severe refractory eosinophilic asthma that has not been well
controlled with previous treatments (38,39).
Mepolizumab has a standard dose of 100 mg administered every 4
weeks, s.c. In the first randomized controlled trial (RCT) in
patients with mild allergic asthma, Leckie et al (38) demonstrated that a single dose of
mepolizumab (10 mg/kg) decreased blood eosinophil count for 16
weeks and sputum eosinophil count for ≤4 weeks. Furthermore,
mepolizumab prevented increased blood eosinophil levels during the
late-phase response following allergen exposure. However,
mepolizumab showed no effect on lung function, airway response to
allergen or airway responsiveness.
In the Dose Ranging Efficacy and Safety with
Mepolizumab severe asthma (DREAM) phase II RCT study of 616
patients with eosinophilic asthma, the efficacy and safety of
administration of different doses of mepolizumab [75, 250 and 750
mg by intravenous (i.v.) injection] were assessed. Patients with a
history of ≥2 exacerbations requiring systemic corticosteroids in
the last 12 months, and those with evidence of eosinophilic airway
inflammation at the study entry or documented within the previous
year were used. Evidence to demonstrate eosinophilic inflammation
of the airways included: i) Blood eosinophil count of 300 cells/µl;
ii) sputum eosinophil count ≥3%; iii) FeNO 50 ppb or iv) prompt
deterioration following ≤25% decrease in inhaled or oral
corticosteroid (CS) maintenance dose (40). The trial demonstrated a decrease in
asthma exacerbation by ~50% in response to all doses of
mepolizumab. Only blood eosinophilia and history of recurrent
asthma exacerbation were associated with a beneficial effect
following treatment. A blood eosinophil count of >150 cells/µl
was associated with a beneficial effect on decreasing further
asthma attacks, but mepolizumab exhibited no significant effect on
symptoms, FeNO, quality of life or lung function. Similar results
regarding the risk of asthma exacerbation were reported in a phase
III trial in patients with severe eosinophilic asthma (41). The efficacy of 100 mg mepolizumab
dose given monthly via s.c. injection was demonstrated in patients
with severe asthma, blood eosinophil count of >150 cells/µl
(and/or >300 cells/µl within the previous year) and ≥2
exacerbations in the previous year. The study both confirmed
earlier results on exacerbation and also demonstrated a beneficial
effect on lung function, asthma symptoms and quality of life
(41). In the Steroid Reduction
with Mepolizumab Study phase III trial, which included patients
with severe asthma requiring daily maintenance OCS therapy,
mepolizumab decreased the required OCS dose by 50% while
maintaining symptom control, and decreased the rate of asthma
exacerbation by 32% compared with placebo (P<0.05) (42).
Through combined analysis of the aforementioned
studies (38,40-42),
mepolizumab has demonstrated a positive long-term safety profile.
The rate of AEs has been reported to be low over the trial period
or when compared with previous placebo-controlled studies (38,40-43).
Respiratory tract infection (67%), bronchitis (21%), worsening of
asthma (27%), headache (29%) and injection site reactions (12%)
were the most common AEs reported. Hypersensitive or systemic
allergic reactions were recorded in ~2% of patients and <1%
experienced a non-allergic systemic reaction; there were no reports
of anaphylaxis (associated with mepolizumab therapy) (16,39,43),
and no malignancy risk associated with mepolizumab is known in
humans (39).
Reslizumab [Cinqair(R)] is a recombinant
humanized monoclonal antibody (IgG4κ) that, like mepolizumab,
targets IL-5 to prevent its binding with IL-5R (36). Reslizumab has been approved by the
FDA (44) and EMA (45) as an add-on treatment for patients
≥18 years with severe eosinophilic asthma that is still
uncontrolled despite patients receiving maximal therapy with ICS
and another controller (16).
Reslizumab is used to treat patients with peripheral blood
eosinophils of ≥400 cells/µl and ≥3 asthma exacerbations during the
past 12 months; it is administered by i.v. injection once every 4
weeks at a dose of 3 mg/kg (16,36,44,45).
In a phase III RCT designed to establish the cut-off
level of eosinophils that should be used to select patients with
asthma for reslizumab treatment, Corren et al (46) showed that, in patients with ≥400
eosinophils/µl, treatment led to significant improvements in
symptom control, lung function and the need for rescue medication.
Another phase III trial demonstrated that the administration of
reslizumab as an add-on therapy to ICS with or without other
controllers significantly decreased the rate of asthma exacerbation
compared with placebo (by 34%; P<0.0001) (47). Murphy et al (48), in an open-label study evaluating
the efficacy and safety of reslizumab for up to 24 months, showed
that both patients receiving reslizumab before the study started
and reslizumab-naive patients reported improvement in asthma
control and lung function through the whole study period. The most
common AEs in both categories of patients were asthma worsening,
upper respiratory tract infection, headache and injection site
reactions. Parasitic and opportunistic infections and anaphylaxis
were not reported. Reslizumab differs from mepolizumab in two ways:
It is administered by i.v. injection based on weight (3 mg/kg/4
weeks) and it has a higher cut-off value for eosinophils (≥400
cells/µl).
Benralizumab [Fasenra®] is a humanized
afucosylated monoclonal antibody against IL-5Rα. Unlike mepolizumab
and reslizumab, it induces eosinophil apoptosis via ADCC involving
natural killer cells (49,50), resulting in a more profound and
potentially earlier onset of eosinophil depletion (6). However, it is difficult to estimate
whether there is a clinical benefit (36). A potential advantage of the rapid
decrease in circulating eosinophil number may be observed in
patients who present with acute severe exacerbation associated with
eosinophilia (6,36).
Benralizumab has been approved as an add-on therapy
for inadequately controlled severe eosinophilic asthma in subjects
>12 (FDA) or 18 years old (EMA) with ≥300 blood eosinophils/µl
(51,52) and 30 mg/4 weeks (s.c.) is
administered for the first 3 months and then every 8 weeks. In two
pivotal phase III RCTs: i) Efficacy and safety of benralizumab for
patients with severe asthma uncontrolled with high-dosage inhaled
corticosteroids and long-acting β2-agonists (SIROCCO) (53) and ii) benralizumab, an anti-IL-5
receptor α monoclonal antibody, as add-on treatment for patients
with severe, uncontrolled, eosinophilic asthma (CALIMA) (54), benralizumab (30 mg/4 or 8 weeks)
was administered as an add-on therapy to a large number of patients
with severe asthma and compared with a placebo. A significant
decrease in asthma exacerbation rate of 45-51% was observed in
patients with peripheral blood eosinophil count ≥300 cell/µl and
the forced expiratory volume in one second (FEV1) values were also
significantly improved (by up to 159 ml) compared with the control
(53). Another phase III RCT, the
ZONDA trial (Efficacy and Safety Study of Benralizumab to Reduce
OCS Use in Patients With Uncontrolled Asthma on High Dose Inhaled
Corticosteroid Plus LABA and Chronic OCS Therapy) (55) investigated the effects of
benralizumab therapy for 24 weeks on OCS necessity in patients with
OCS-dependent asthma. The median reduction in OCS dose was 75% for
patients treated with benralizumab compared with 25% following
treatment with a placebo. In addition, compared with the placebo,
the annual exacerbation rate was decreased by 55% but there was no
improvement in lung function. In the the safety trial for
benralizumab performed by Busse et al (56), the authors showed that the
efficiency, safety and tolerability profile of benralizumab
maintained for 2 years was similar to that observed over 1 year in
previous RCTs (53,54). The AEs that were most frequently
reported included upper respiratory tract infection (14-16%) and
worsening asthma (7-10%). Parasitic infection was not reported, and
the hypersensitivity reactions were similar between study groups
(53,54,56).
IL-4Rα blockers
Dupilumab [Dupixent®] is a fully human
monoclonal antibody directed against IL-4Rα, which is common to
both IL-4 and IL-13, and therefore able to inhibit the signaling of
both of these ILs (6,16). Dupilumab is approved by the FDA
(57) as an add-on therapy for
patients aged ≥12 years with moderate to severe eosinophilic asthma
or asthma that is dependent on OCS, and by the EMA (58) as an add-on treatment for patients
with severe asthma aged ≥12 years with T2 inflammation
characterized by increased blood eosinophils and/or raised FeNO
levels that remain inadequately controlled despite high-dose ICS
plus another controller. Dupilumab (s.c.) can be administered at an
initial dose of 600 mg (two 300 mg injections) followed by 300 mg/2
weeks (recommended only for patients with OCS-dependent asthma), or
at an initial dose of 400 mg (two 200 mg injections) followed by
200 mg/2 weeks. Wenzel et al (59) in a phase II dose-ranging trial
involving patients with severe uncontrolled asthma, reported that
all types of administration scheme (200 or 300 mg every 2 or 4
weeks) resulted in improved asthma control and FEV1, and fewer
severe exacerbations compared with placebo. Although these results
were reported in all patients, those with ≥300 eosinophils/µl
showed the greatest decline in annual severe exacerbation rate and
improvement in lung function.
Liberty Asthma Quest phase III RCT (60) confirmed the efficacy and safety
profile of add-on dupilumab (200 or 300 mg/2 weeks for 52 weeks;
s.c.) in patients aged ≥12 years with moderate to severe
uncontrolled asthma and a history of exacerbations. Similar to the
aforementioned study, the greatest benefits, such as decreased
exacerbation rate, and rapid and maintained improvement in FEV1,
were observed in patients with elevated T2 biomarkers at baseline
(blood eosinophils ≥150 cells/µl or FeNO ≥25 ppb), although the
potential efficacy of dupilumab in T2-low disease was not
confirmed. This study also demonstrated a favorable safety profile
of dupilumab. The AE rates were similar across the intervention
groups (81.0%). The most common serious AE was pneumonia (8.2% in
the treatment group and 8.4% in the placebo group). The most
frequent AE was injection site reaction (15.2% in the low-dose
dupilumab group, 18.4% in the high-dose dupilumab group vs. 5.4 and
10.3%, respectively, in the matched placebo groups). Allergic
conjunctivitis, conjunctivitis, injection site reaction, ophthalmic
inflammation and eye irritation may occur during therapy with
dupilumab (59,60).
Liberty Asthma Venture phase III RCT (61) demonstrated the efficiency of
dupilumab in decreasing the rate of asthma exacerbations (by 59.3%)
and the need for OCS, while maintaining asthma control and
improving lung function. The rate of AEs during the study period
was similar in the two groups (62% in the treatment arm; 64% in the
placebo arm). According to post hoc analysis of phase II trials,
treatment with dupilumab at 200/300 mg/2 weeks was associated with
a significant improvement in asthma symptom control, decreased rate
of severe exacerbation and improved lung function compared with
placebo, regardless of the exacerbation history of the patient
(62).
Another recent post hoc analysis of the Liberty
Asthma Quest study (63)
demonstrated that dupilumab at 200/300 mg/2 weeks significantly
decreased the rate of severe exacerbation and improved asthma
control and lung function in patients with uncontrolled, moderate
to severe asthma with evidence of allergic asthma, and in patients
without an allergic component compared with the placebo
(P<0.01).
Dupilumab is also indicated for the treatment of
patients aged ≥6 years with moderate-to-severe atopic dermatitis
whose disease is not adequately controlled with topical
prescription therapies or when those therapies are not advisable
(64). As shown in Fig. 1, a patient with moderate to severe
asthma who also has moderate to severe atopic dermatitis would
likely benefit most from dupilumab therapy (36).
Anti-IL-13
In a RCT involving patients with moderate to severe
asthma, lebrikizumab, a monoclonal antibody targeting
IL-13(65), demonstrated only a
small improvement in lung function (66). According to the result of the phase
IIb study conducted by Hanania et al (67), the greatest benefit from
lebrikizumab therapy was observed in patients with high serum
periostin concentration (known biomarker of increased IL-13
activity within the airway). The treatment with lebrikizumab
reduced the rate of asthma exacerbations, which was more pronounced
in the periostin-high patients (all doses: 60% reduction) than in
the periostin-low patients (all doses: 5% reduction); no
dose-response was evident. Lung function showed a modest
improvement, with greatest increase in FEV1 in periostin-high
patients (all doses: 9.1% placebo adjusted improvement) compared
with periostin-low patients (all doses: 2.6% placebo-adjusted
improvement).
Another IL-13 monoclonal antibody, tralokinumab, has
showed unimpressive effects compared with other biological agents
(68,69), and this class of biological drugs
has not received regulatory approval.
Targeting alarmins (TSLP, IL-25,
IL-33)
Alarmins are key cytokines involved in both T2 and
non-T2 mechanisms of airway inflammation in asthma. Currently,
several trials are evaluating different molecules targeting these
cytokines (6).
Tezepelumab is a human monoclonal antibody directed
against TSLP produced by epithelial cells in response to injury to
dendritic cells, CD4 T cells, CD8 T cells, mast cells, B cells,
eosinophils, basophils and ILC. Tezepelumab impairs the interaction
of TSLP with TSLP receptor and prevents its downstream effects
(6,36). TSLP is responsible for activating
innate immune, dendritic, T and B cells, and induces production of
cytokines by antigen-specific Th2 cells (36,70).
In a phase IIb study by Corren et al (70), the effect of tezepelumab was
evaluated in adult patients aged 18-75 years with uncontrolled
asthma despite appropriate treatment with medium- to high-dose ICS
plus LABA and a history of ≥2 asthma exacerbations requiring
systemic glucocorticoids in the year before the start of the study.
The patients were not selected based on specific markers of atopy,
such as eosinophilia or high levels of total or specific IgE. The
patients received either placebo or tezepelumab, administered
subcutaneously, at a dose of 70 or 210 mg/4 weeks, or 280 mg/2
weeks. All dosage regimens showed a decrease in Th2 biomarkers
(blood eosinophil count, FeNO and serum IgE), demonstrating an
anti-inflammatory effect and suggesting inhibition of T2 cytokine
production. Treatment with tezepelumab also showed a large decrease
in the annualized asthma exacerbation rate and improvement in FEV1
compared with placebo. This effect on exacerbation was observed in
patients with low FeNO and blood eosinophil levels, suggesting the
involvement of TSLP in non-T2 airway inflammation.
Regarding drug-associated serious AEs, one patient
who received low-dose tezepelumab reported pneumonia and stroke,
while another patient who received medium-dose treatment reported
Guillain-Barre syndrome. No anaphylactic reaction or identification
of neutralizing antibodies was reported (70). The efficacy and safety of
tezepelumab in severe, uncontrolled asthma patients are being
investigated in a number of ongoing phase III RCTs, such as
NAVIGATOR (ClinicalTrials.gov identifier,
NCT03347279), SOURCE (ClinicalTrials.gov identifier, NCT03406078) and
DESTINATION (ClinicalTrials.gov identifier, NCT03706079) (71-73).
IL-33 is also a potential target for biological
therapy in asthma. IL-33, one of the members of the IL-1
superfamily, is an alarmin cytokine promoting inflammatory
responses (74). IL-33 promotes
the Th2-mediated immune response and further production of many
pro-inflammatory cytokines, such as IL-4, IL-5 and IL-13.
Furthermore, IL-33 promotes bronchial remodeling and lung fibrosis,
causing further advancement of asthma (75). At phase 2 of the asthma clinical
trial, the anti-IL-33 receptor monoclonal antibody is being
investigated in subjects with moderately severe asthma and compared
to the placebo fluticasone propionate/salmeterol combination and
fluticasone propionate (ClinicalTrials.gov identifier, NCT03207243). Another
clinical trial involving anti-IL-33 antibody in asthma patients is
in a phase 1 clinical trial, and compares it to the placebo
Dupilumab and fluticasone propionate (ClinicalTrials.gov identifier, NCT03112577).
Several biological agents against IL-33, such as
GSK3772847 and REGN3500, are currently in different stages of
development (76,77). A phase II asthma clinical trial
(ClinicalTrials.gov identifier,
NCT03207243) is investigating monoclonal anti-IL-33R in subjects
with moderate and severe asthma compared with placebo, fluticasone
propionate or fluticasone propionate/salmeterol combination
(76). Another phase I clinical
trial in patients with asthma is comparing anti-IL-33 antibody with
placebo, fluticasone propionate and dupilumab (ClinicalTrials.gov identifier, NCT03112577) (77).
4. Discussion
Along with other types of chronic respiratory
disease, asthma, primarily the severe form, remains one of the most
important health problems globally (1,78-81).
The management of severe asthma has significantly changed during
the past decade (1). In severe
asthma, conventional treatments based on non-specific drugs, such
as CS and bronchodilators, are often inefficient and should be
replaced by personalized medicine based on the effective
identification of different asthma phenotypes (1,6,36).
The currently approved biological agents and those in development
target these specific phenotypes (36). At present, there is no biological
drug that is significantly more efficient than others for most
patients with asthma (36).
Therefore, selecting the adequate monoclonal antibody should be
carefully individualized to each patient (Fig. 1).
Before starting biological therapy, it is necessary
to correctly diagnose severe asthma, optimize and improve adherence
to the current treatment, assess comorbidities and exclude
potential confounding pathologies that mimic some of the symptoms
of asthma, such as chronic obstructive pulmonary disease, laryngeal
dyskinesia, bronchiectasis and hypersensitivity pneumonitis
(16,82,83).
The most common comorbidities associated with asthma, which require
adequate treatment, are rhinosinusitis, gastroesophageal reflux
disease, aspiration, cardiovascular comorbidities, obstructive
sleep apnea and upper respiratory tract infection (16).
According to GINA recommendations, if available and
affordable, an add-on biologic T2 agent, such as anti-IgE,
anti-IL-5/5R or anti-IL-4R, should be considered in patients with
severe asthma who show typical biomarkers of T2 airway inflammation
(1). The T2-high phenotype is
characterized by eosinophilic airway inflammation (8,16),
while the T2-low phenotype is characterized by neutrophilic or
paucigranulocytic airway inflammation (10,18).
The most common biomarkers used for non-invasive assessment of
severe asthma include peripheral blood eosinophil count, serum IgE
and FeNO (1,8,16).
The only evidence-based biomarker of the T2-low phenotype is sputum
neutrophil count (6). To select
the most effective and appropriate biological agent to treat severe
asthma, not only the disease characteristics, and patient age and
preference, should be considered, but also the indications and
posology for biological therapies approved by international
agencies and/or national guidelines, as well as biomarkers (as
predictors of response) (1,36).
Each biological agent has unique dosing features, which can be a
key factor in determining which agent is best to use (36). For example, dosing of omalizumab is
based on serum IgE levels and weight. Reslizumab and mepolizumab
are administered every 4 weeks, while benralizumab, following an
initial loading period, is administered every 8 weeks. Certain
patients may prefer i.v. administration and therefore would rather
use reslizumab. Dupilumab can be self-administered at home by the
patient, but dosing is every 2 weeks; while this may be suitable
for patients who are physically active and able to correctly
self-administer the treatment, others who are less active or less
confident in self-administration may require treatment from a
health care professional (36).
At present, due to a lack of direct comparison
trials, there are no recommendations for choosing among the
currently approved IL-5 pathway-targeting biological agents.
Therefore, in the absence of head-to-head RCTs, several network
meta-analyses have been performed (84-86).
A recent matching-adjusted indirect comparison meta-analysis
suggested that mepolizumab and benralizumab have similar efficiency
profiles (84). Another network
meta-analysis indicated that reslizumab was more effective than
benralizumab in patients with moderate to severe eosinophilic
asthma with a history of ≥2 exacerbations in the previous year
(85), whereas other indirect
treatment comparisons indicated that, in comparison with reslizumab
and benralizumab, mepolizumab improved disease control and
decreased risk of asthma exacerbations regardless of the blood
eosinophil threshold (86). A
recent meta-analysis suggested that although all current biological
agents were effective in improving lung function and decreasing the
asthma exacerbation risk when compared with placebo, dupilumab was
significantly more efficient compared with omalizumab in decreasing
the risk of exacerbation, while there was no difference between
reslizumab, mepolizumab, benralizumab and dupilumab. Furthermore,
dupilumab was revealed to be significantly more effective than
omalizumab, benralizumab and mepolizumab at improving FEV1, whereas
omalizumab, benralizumab, mepolizumab and reslizumab showed similar
improvements in FEV1(87). By
contrast, another arm-based network meta-analysis showed no
significant difference between monoclonal antibodies regarding the
effect on the rate of asthma exacerbation (88).
Regardless of the chosen biological therapy, a
4-month trial should be performed before asthma control assessment
(4). In the case of a failure of
biologic therapy associated with an inadequate biological effect of
the treatment, a strong case could be made for switching biologic
(4,6). There are several ongoing studies on
biological drug switching in patients with severe asthma that is
not well controlled (89-91).
The results of the OSMO (Omalizumab Switch to MepOlizumab) study a
multicenter, open-label, 32-week trial, demonstrated that patients
with uncontrolled severe asthma receiving omalizumab showed an
improvement in asthma control after switching to mepolizumab
(89). A 24-week, multicenter
prospective, open-label pilot study showed that patients with
severe eosinophilic asthma and inadequate response to omalizumab
reported a significantly improved asthma control after switching to
reslizumab (90). Other
preliminary findings also indicated decreased OCS maintenance dose
and improved quality of life scores after switching to benralizumab
in patients with sub-optimal response with mepolizumab (91).
To the best of our knowledge, only one study has
investigated the effect of biological agent withdrawal in patients
with severe asthma (92). The
study showed the recurrence of severe eosinophilic asthma within
3-6 months of mepolizumab cessation following 12 months of
continuous therapy. The first signal to return was elevated blood
eosinophil levels, followed by sputum eosinophils and then asthma
exacerbations. However, how and when to switch or withdraw
established biological agents and the duration of the treatment at
which the patient should be characterized as either a responder or
non-responder to biological agents are yet to be adequately
determined. Clinical trials of biological agents targeting IL-6,
IL-17 or IL-33 in patients with asthma with T2-low phenotypes are
ongoing, but current data suggest that these patients should be
treated with chronic macrolide, imatinib or bronchial thermoplasty
(16).
5. Conclusion
The advent of biological therapy has revolutionized
the management of severe asthma. The success of biological agents
is primarily based on adequate selection of patients. Individual
assessment of patients for allergic vs. eosinophilic asthma is
possible by identifying measurable biomarkers that are predictive
of treatment efficacy. The clinical effects of currently approved
monoclonal antibodies are consistent, with a significant decrease
in asthma exacerbation rate, and a less pronounced improvement in
symptom control and lung function.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
APF, CO and DT contributed to the conception and
design of the study. RC, IP, ET and ACI performed the literature
review. APF wrote the first draft of the manuscript. RMR revised
the manuscript. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Initiative for Asthma. Global
strategy for asthma management and prevention, 2021. Available
from: https://ginasthma.org/gina-reports/. Accessed August
12, 2021.
|
|
2
|
The Global Asthma Report 2018. Auckland,
New Zealand: Global asthma network. Available from: https://theunion.org/technical-publications/the-global-asthma-report-2018
http://globalasthmanetwork.org/.
Accessed December 13, 2020.
|
|
3
|
D'Amato G, Vitale C, Molino A, Stanziola
A, Sanduzzi A, Vatrella A, Mormile M, Lanza M, Calabrese G,
Antonicelli L and D'Amato M: Asthma-related deaths. Multidiscip
Respir Med. 11(37)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
GINA Pocket Guide 2019. Difficult to treat
and severe asthma in adults and adolescents. Available from:
https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf.
Accessed September 10, 2020.
|
|
5
|
Munteanu LA, Fildan AP, Tudorache E,
Fira-Mladinescu O, Frandes M, Timar B, Oancea C and Tofolean DE:
Inhaler technique errors in Romanian patients with asthma-a
multicenter study. Patient Prefer Adherence. 13:1401–1414.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pavord ID, Shrimanker R and Hanania NA:
Biologics targeting type 2 inflammation. In: Severe Asthma (ERS
Monograph). Chung KF, Israel E and Gibson PG (eds). European
Respiratory Society, Sheffield, pp285-303, 2019.
|
|
7
|
Busse WW: Biological treatments for severe
asthma: A major advance in asthma care. Allergol Int. 68:158–166.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Robinson D, Humbert M, Buhl R, Cruz AA,
Inoue H, Korom S, Hanania NA and Nair P: Revisiting type 2-high and
type 2-low airway inflammation in asthma: Current knowledge and
therapeutic implications. Clin Exp Allergy. 47:161–175.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stokes JR and Casale TB: Characterization
of asthma endotypes: Implications for therapy. Ann. Allergy Asthma
Immunol. 117:121–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sze E, Bhalla A and Nair P: Mechanisms and
therapeutic strategies for non-T2 asthma. Allergy. 75:311–325.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McGregor MC, Krings JG, Nair P and Castro
M: Role of biologics in asthma. Am J Respir Crit Care Med.
199:433–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Krings JG, McGregor MC, Bacharier LB and
Castro M: Biologics for severe asthma: Treatment-specific effects
are important in choosing a specific agent. J Allergy Clin Immunol
Pract. 7:1379–1392. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tan R, Liew MF, Lim HF, Leung BP and Wong
WSF: Promises and challenges of biologics for severe asthma.
Biochem Pharmacol. 179(114012)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eger KA and Bel EH: The emergence of new
biologics for severe asthma. Curr Opin Pharmacol. 46:108–115.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pavord ID, Beasley R, Agusti A, Anderson
GP, Bel E, Brusselle G, Cullinan P, Custovic A, Ducharme FM, Fahy
JV, et al: After asthma: Redefining airways diseases. Lancet.
391:350–400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rogliani P, Calzetta L, Matera MG, Laitano
R, Ritondo BL, Hanania NA and Cazzola M: Severe asthma and
biological therapy: When, which, and for whom. Pulm Ther. 6:47–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Ferrari L, Chiappori A, Bagnasco D,
Riccio AM, Passalacqua G and Canonica GW: Molecular phenotyping and
biomarker development: Are we on our way towards targeted therapy
for severe asthma? Expert Rev Respir Med. 10:29–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kuruvilla ME, Lee FE and Lee GB:
Understanding asthma phenotypes, endotypes, and mechanisms of
disease. Clin Rev Allergy Immunol. 56:219–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bernstein JA and Panettieri R Jr:
Treatment of severe, uncontrolled eosinophilic asthma: Where we are
heading. J Asthma. 56:459–472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Voehringer D, Reese TA, Huang X, Shinkai K
and Locksley RM: Type 2 immunity is controlled by IL-4/IL-13
expression in hematopoietic non-eosinophil cells of the innate
immune system. J Exp Med. 203:1435–1446. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang YJ, Kim HY, Albacker LA, Baumgarth
N, McKenzie AN, Smith DE, Dekruyff RH and Umetsu DT: Innate
lymphoid cells mediate influenza-induced airway hyper-reactivity
independently of adaptive immunity. Nat Immunol. 12:631–638.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
King GG, James A, Harkeness L and Warki P:
Pathophysiology of severe asthma: We've only just started.
Respirology. 23:262–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brusselle GG, Maes T and Bracke KR:
Eosinophils in the spotlight: Eosinophilic airway inflammation in
nonallergic asthma. Nat Med. 19:977–979. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bakakos A, Loukides S and Bakakos P:
Severe eosinophilic asthma. J Clin Med. 8(1375)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dunican EM, Elicker BM, Gierada DS, Nagle
SK, Schiebler ML, Newell JD, Raymond WW, Lachowicz-Scroggins ME, Di
Maio S, Hoffman EA, et al: Mucus plugs in patients with asthma
linked to eosinophilia and airflow obstruction. J Clin Invest.
128:997–1009. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schulman ES: Development of a monoclonal
anti-immunoglobulin E antibody (omalizumab) for the treatment of
allergic respiratory disorders. Am J Respir Crit Care Med.
164:S6–S11. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Busse WW, Morgan WJ, Gergen PJ, Mitchell
HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic
JA, et al: Randomized trial of omalizumab (anti-IgE) for asthma in
inner-city children. N Engl J Med. 364:1005–1015. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hanania NA, Alpan O, Hamilos DL, Condemi
JJ, Reyes-Rivera I, Zhu J, Rosen KE, Eisner MD, Wong DA and Busse
W: Omalizumab in severe allergic asthma inadequately controlled
with standard therapy: A randomized trial. Ann Intern Med.
154:573–582. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hanania NA, Wenzel S, Rosén K, Hsieh HJ,
Mosesova S, Choy DF, Lal P, Arron JR, Harris JM and Busse W:
Exploring the effects of omalizumab in allergic asthma: An analysis
of biomarkers in the EXTRA study. Am J Respir Crit Care Med.
187:804–811. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ledford D, Busse W, Trzaskoma B, Omachi
TA, Rosén K, Chipps BE, Luskin AT and Solari PG: A randomized
multicenter study evaluating Xolair persistence of response after
long-term therapy. J Allergy Clin Immunol. 140:162–169.e2.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Humbert M, Taillé C, Mala L, Le Gros V,
Just J and Molimard M: STELLAIR investigators. Omalizumab
effectiveness in patients with severe allergic asthma according to
blood eosinophil count: The STELLAIR study. Eur Respir J.
51(1702523)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Casale TB, Luskin AT, Busse W, Zeiger RS,
Trzaskoma B, Yang M, Griffin NM and Chipps BE: Omalizumab
effectiveness by biomarker status in patients with asthma: Evidence
from PROSPERO, a prospective real-world study. J Allergy Clin
Immunol Pract. 7:156–164.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Esquivel A, Busse WW, Calatroni A, Togias
AG, Grindle KG, Bochkov YA, Gruchalla RS, Kattan M, Kercsmar CM,
Khurana Hershey G, et al: Effects of omalizumab on rhinovirus
infections, illnesses, and exacerbations of asthma. Am J Respir
Crit Care Med. 196:985–992. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gill MA, Liu AH, Calatroni A, Krouse RZ,
Shao B, Schiltz A, Gern JE, Togias A and Busse WW: Enhanced
plasmacytoid dendritic cell antiviral responses after omalizumab. J
Allergy Clin Immunol. 141:1735–1743.e9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Busse W: Biological treatments for severe
asthma: Where do we stand? Curr Opin Allergy Clin Immunol.
18:509–518. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mavissakalian M and Brady S: The current
state of biologic therapies for treatment of refractory asthma.
Clinic Rev Allerg Immunol. 59:195–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xolair® Full Prescribing
Information. Genentech. March 21, 2014. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf.
Accessed August 12, 2021.
|
|
38
|
Leckie MJ, ten Brinke A, Khan J, Diamant
Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF,
Djukanovic R, et al: Effects of an interleukin-5 blocking
monoclonal antibody on eosinophils, airway hyper-responsiveness,
and the late asthmatic response. Lancet. 356:2144–2148.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nucala® Full Prescribing
Information. GlaxoSmithKline. September 2019. Available from:
https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF.
Accessed August 12, 2021.
|
|
40
|
Pavord ID, Korn S, Howarth P, Bleecker ER,
Buhl R, Keene ON, Ortega H and Chanez P: Mepolizumab for severe
eosinophilic asthma (DREAM): A multicentre, double-blind,
placebo-controlled trial. Lancet. 380:651–659. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ortega HG, Liu MC, Pavord ID, Brusselle
GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey
SW, et al: Mepolizumab treatment in patients with severe
eosinophilic asthma. N Engl J Med. 371:1198–1207. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bel EH, Wenzel SE, Thompson PJ, Prazma CM,
Keene ON, Yancey SW, Ortega HG and Pavord ID: SIRIUS Investigators.
Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic
asthma. N Engl J Med. 371:1189–1197. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lugogo N, Domingo C, Chanez P, Leigh R,
Gilson MJ, Price RG, Yancey SW and Ortega HG: Long-term efficacy
and safety of mepolizumab in patients with severe eosinophilic
asthma: A multi-center, open-label, phase III study. Clin Ther.
38:2058–2070.e1. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
US Food, Drug Administration: CINQAIR
(reslizumab) Injection, for Intravenous Use. Reference ID: 3906489,
2016.
|
|
45
|
European Medicines Agency.
EMA/450050/2016. EMEA/H/C/003912. EPAR summary for the public.
CINQAERO. INN - reslizumab. https://www.ema.europa.eu/en/documents/overview/cinqaero-epar-summary-public_en.pdf,
Accessed May 19, 2021.
|
|
46
|
Corren J, Weinstein S, Janka L, Zangrilli
J and Garin M: Phase 3 study of reslizumab in patients with poorly
controlled asthma: Effects across a broad range of eosinophil
counts. Chest. 150:799–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Castro M, Zangrilli J, Wechsler ME,
Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O'Brien C
and Korn S: Reslizumab for inadequately controlled asthma with
elevated blood eosinophil counts: Results from two multicentre,
parallel, double-blind, randomised, placebo-controlled, phase 3
trials. Lancet Respir Med. 3:355–366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Murphy K, Jacobs J, Bjermer L, Fahrenholz
JM, Shalit Y, Garin M, Zangrilli J and Castro M: Long-term safety
and efficacy of reslizumab in patients with eosinophilic asthma. J
Allergy Clin Immunol Pract. 5:1572–1581.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kolbeck R, Kozhich A, Koike M, Peng L,
Andersson CK, Damschroder MM, Reed JL, Woods R, Dall'acqua WW,
Stephens GL, et al: MEDI-563, a humanized anti-IL-5 receptor alpha
mAb with enhanced antibody-dependent cell-mediated cytotoxicity
function. J Allergy Clin Immunol. 125:1344–1353.e2. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pham TH, Damera G, Newbold P and Ranade K:
Reductions in eosinophil biomarkers by benralizumab in patients
with asthma. Respir Med. 111:21–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
US Food, Drug Administration: FASENRA
(benralizumab) injection, for subcutaneous use. Reference ID:
4181236, 2019.
|
|
52
|
European Medicines Agency: EMEA/H/C/4433,
2019. www.ema.europa.eu/en/documents/overview/fasenra-epar-medicine-overview_en.pdf.
Accessed 10 May 2021.
|
|
53
|
Bleecker ER, FitzGerald JM, Chanez P, Papi
A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M,
Werkström V, et al: Efficacy and safety of benralizumab for
patients with severe asthma uncontrolled with high-dosage inhaled
corticosteroids and long-acting β2-agonists (SIROCCO): A
randomised, multicentre, placebo-controlled phase 3 trial. Lancet.
388:2115–2127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
FitzGerald JM, Bleecker ER, Nair P, Korn
S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule
S, et al: Benralizumab, an anti-interleukin-5 receptor α monoclonal
antibody, as add-on treatment for patients with severe,
uncontrolled, eosinophilic asthma (CALIMA): A randomised,
double-blind, placebo-controlled phase 3 trial. Lancet.
388:2128–2141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Nair P, Wenzel S, Rabe KF, Bourdin A,
Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S and Goldman
M: ZONDA Trial Investigators. Oral glucocorticoid-sparing effect of
benralizumab in severe asthma. N Engl J Med. 376:2448–2458.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Busse WW, Bleecker ER, FitzGerald JM,
Ferguson GT, Barker P, Sproule S, Olsson RF, Martin UJ and Goldman
M: BORA study investigators. Long-term safety and efficacy of
benralizumab in patients with severe, uncontrolled asthma: 1-year
results from the BORA phase 3 extension trial. Lancet Respir Med.
7:46–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
US Food, Drug Administration. DUPIXENT
(dupilumab) injection, for subcutaneous use, 2017. Available from:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf.
Accessed December 11, 2020.
|
|
58
|
European Medicines Agency. Dupinex:
EMEA/H/C/004390, 2018. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent.
Accessed December 1, 2020.
|
|
59
|
Wenzel S, Castro M, Corren J, Maspero J,
Wang L, Zhang B, Pirozzi G, Sutherland ER, Evans RR, Joish VN, et
al: Dupilumab efficacy and safety in adults with uncontrolled
persistent asthma despite use of medium-to-high-dose inhaled
corticosteroids plus a long-acting β2 agonist: A randomised
double-blind placebo-controlled pivotal phase 2b dose-ranging
trial. Lancet. 388:31–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Castro M, Corren J, Pavord ID, Maspero J,
Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, et al:
Dupilumab efficacy and safety in moderate-to-severe uncontrolled
asthma. N Engl J Med. 378:2486–2496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rabe KF, Nair P, Brusselle G, Maspero JF,
Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al:
Efficacy and safety of dupilumab in glucocorticoid-dependent severe
asthma. N Engl J Med. 378:2475–2485. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Corren J, Castro M, Ford LB, Bernstein JA,
Jayawardena S, Maroni J, Rowe P, Amin N, Pirozzi G, Graham NMH, et
al: Dupilumab improves asthma outcomes irrespective of frequency of
previous asthma exacerbation history. Ann Allergy Asthma Immunol.
123:222–224.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Corren J, Castro M, O'Riordan T, Hanania
NA, Pavord ID, Quirce S, Chipps BE, Wenzel SE, Thangavelu K, Rice
MS, et al: Dupilumab efficacy in patients with uncontrolled,
moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract.
8:516–526. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dupixent® Full Prescribing
Information. Regeneron Sanofi Genzyme. January 2021. Available
from: https://www.regeneron.com/downloads/dupixent_fpi.pdf.
Accessed August 12, 2021.
|
|
65
|
Ultsch M, Bevers J, Nakamura G, Vandlen R,
Kelley RF, Wu LC and Eigenbrot C: Structural basis of signaling
blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol.
425:1330–1339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Corren J, Lemanske RF, Hanania NA,
Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC,
Su Z, et al: Lebrikizumab treatment in adults with asthma. N Engl J
Med. 365:1088–1098. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hanania NA, Noonan M, Corren J, Korenblat
P, Zheng Y, Fischer SK, Cheu M, Putnam WS, Murray E, Scheerens H,
et al: Lebrikizumab in moderate-to-severe asthma: Pooled data from
two randomised placebo-controlled studies. Thorax. 70:748–756.
2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Piper E, Brightling C, Niven R, Oh C,
Faggioni R, Poon K, She D, Kell C, May RD, Geba GP and Molfino NA:
A phase II placebo-controlled study of tralokinumab in
moderate-to-severe asthma. Eur Respir J. 41:330–338.
2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Brightling CE, Chanez P, Leigh R, O'Byrne
PM, Korn S, She D, May RD, Streicher K, Ranade K and Piper E:
Efficacy and safety of tralokinumab in patients with severe
uncontrolled asthma: A randomised, double-blind,
placebo-controlled, phase 2b trial. Lancet Respir Med. 3:692–701.
2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Corren J, Parnes JR, Wang L, Mo M, Roseti
SL, Griffiths JM and van der Merwe R: Tezepelumab in adults with
uncontrolled asthma. N Engl J Med. 377:936–946. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Menzies-Gow A, Colice G, Griffiths JM,
Almqvist G, Ponnarambil S, Kaur P, Ruberto G, Bowen K, Hellqvist Å,
Mo M and Garcia Gil E: NAVIGATOR: A phase 3 multicentre,
randomized, double-blind, placebo-controlled, parallel-group trial
to evaluate the efficacy and safety of tezepelumab in adults and
adolescents with severe, uncontrolled asthma. Respir Res.
21(266)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Weschler ME, Colice G, Griffiths JM,
Almqvist G, Skärby T, Piechowiak T, Kaur P, Bowen K, Hellqvist Å,
Mo M and Garcia Gil E: SOURCE: A phase 3, multicentre, randomized,
double-blind, placebo-controlled, parallel group trial to evaluate
the efficacy and safety of tezepelumab in reducing oral
corticosteroid use in adults with oral corticosteroid dependent
asthma. Respir Res. 21(264)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Menzies-Gow A, Ponnarambil S, Downie J,
Bowen K, Hellqvist Å and Colice G: DESTINATION: A phase 3,
multicentre, randomized, double-blind, placebo-controlled,
parallel-group trial to evaluate the long-term safety and
tolerability of tezepelumab in adults and adolescents with severe,
uncontrolled asthma. Respir Res. 21(279)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gabryelska A, Kuna P, Antczak A,
Białasiewicz P and Panek M: IL-33 mediated inflammation in chronic
respiratory diseases-understanding the role of the member of IL-1
superfamily. Front Immunol. 10(692)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Efficacy and safety study of GSK3772847 in
subjects with moderately severe asthma. ClinicalTrials.gov Identifier: NCT03207243,
https://clinicaltrials.gov/ct2/show/NCT03207243.
Accessed August 12, 2020.
|
|
77
|
Study of REGN3500 and dupilumab in
patients with asthma. ClinicalTrials.gov Identifier: NCT03112577,
https://clinicaltrials.gov/ct2/show/NCT03112577.
Accessed August 12, 2020.
|
|
78
|
Dantes E, Fildan AP, Toma CL, Voicu GH and
Oancea C: Respiratory impact in workers exposed to air pollutants
from petroleum refinery. J Environ Prot Ecol. 17:523–531. 2016.
|
|
79
|
Tofolean D, Popescu G, Arghir IA, Frandes
M and Fildan AP: A different aproach of chronic obstructive
pulmonary disease severity and plastic medical devices used for
oxygenotherapy. Mater Plast. 56:295–230. 2019.
|
|
80
|
Barsan M, Rajnoveanu AG, Cocarla A, Bolfa
P, Login CC, Socaciu AI, Decea N and Leucuta DC: A study of
oxidative stress and pulmonary damage after silica instillation in
rats and the effect of curcumin administration. Med Pr. 72:239–247.
2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Trenchea M, Arghir IA, Popescu GG, Rascu
S, Bechir ES, Tofolean DE, Fildan AP, Ion I and Dantes E: The triad
nocturia, smoking and obstructive sleep apnea. Rev Chim.
70:1839–1842. 2019.
|
|
82
|
Chung KF, Wenzel SE, Brozek JL, Bush A,
Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et
al: International ERS/ATS guidelines on definition, evaluation and
treatment of severe asthma. Eur Respir J. 43:343–373.
2014.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hogea Stanca P, Tudorache E, Fildan AP,
Fira-Mladinescu O, Marc M and Oancea C: Risk factors of chronic
obstructive pulmonary disease exacerbations. Clin Respir J.
14:183–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bourdin A, Husereau D, Molinari N, Golam
S, Siddiqui MK, Lindner L and Xu X: Matching-adjusted indirect
comparison of benralizumab versus interleukin-5 inhibitors for the
treatment of severe asthma: A systematic review. Eur Respir J.
52(1801393)2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Casale TB, Pacou M, Mesana L, Farge G, Sun
SX and Castro M: Reslizumab compared with benralizumab in patients
with eosinophilic asthma: A systematic literature review and
network meta-analysis. J Allergy Clin Immunol Pract. 7:122–130.e1.
2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Busse W, Chupp G, Nagase H, Albers FC,
Doyle S, Shen Q, Bratton DJ and Gunsoy NB: Anti-IL-5 treatments in
patients with severe asthma by blood eosinophil thresholds:
Indirect treatment comparison. J Allergy Clin Immunol.
143:190–200.e20. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Calzetta L, Matera MG and Rogliani P:
Monoclonal antibodies in severe asthma: Is it worth it? Expert Opin
Drug Metab Toxicol. 15:517–520. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Edris A, De Feyter S, Maes T, Joos G and
Lahousse L: Monoclonal antibodies in type 2 asthma: A systematic
review and network meta-analysis. Respir Res.
20(179)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Albers F, Liu MC, Chipps BE, Chapman KR,
Muñoz X, Bergna M, Devouassoux G, Azmi J, Price R and Galkin D:
Therapeutic switch from omalizumab to mepolizumab in patients with
uncontrolled severe eosinophilic asthma: Treatment effect by prior
omalizumab treatment duration. J Allergy Clin Immunol. 143 (Suppl
2)(AB102)2019.
|
|
90
|
Pérez de Llano LA, Cosío BG, Domingo C,
Urrutia I, Bobolea I, Valero A, Entrenas Costa LM, Quirce S,
Barranco P, Marina Malanda N, et al: Efficacy and safety of
reslizumab in patients with severe asthma with inadequate response
to omalizumab: A multicenter, open-label pilot study. J Allergy
Clin Immunol Pract. 7:2277–2283.e2. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kavanagh J, Green L, Fernandes M, Roxas C,
Kent BD, Jackson DJ and d'Ancona G: Response to benralizumab after
sub-optimal response to mepolizumab in oral corticosteroid
dependent severe eosinophilic asthma. Am J Respir Crit Care Med.
199(A2675)2019.
|
|
92
|
Haldar P, Brightling CE, Singapuri A,
Hargadon B, Gupta S, Monteiro W, Bradding P, Green RH, Wardlaw AJ,
Ortega H and Pavord ID: Outcomes after cessation of mepolizumab
therapy in severe eosinophilic asthma: A 12-month follow-up
analysis. J Allergy Clin Immunol. 133:921–923. 2014.PubMed/NCBI View Article : Google Scholar
|