1. Introduction
Obesity and iron deficiency are global health
problems affecting billions of people worldwide (1,2).
While overweight and obesity are the key risk factors for many
chronic diseases, such as cardiovascular diseases, diabetes, and
certain cancers (3), iron
deficiency or hypoferremia is the most prevalent single
micronutrient deficiency globally (4). Untreated iron deficiency can cause
iron deficiency anemia, a severe health problem that appears in the
form of tiredness, reduced life productivity, and poor maternal
health, especially among pregnant women (5-8).
Growing evidence supports the existence of an association between
obesity and iron deficiency (9).
This link was observed among children, adolescents, and adults
(10-12).
An observational study of 619 women aged 20-49 years has reported
that iron deficiency was identified in 23.5, 41.9, and 45.6% of
women with normal weight, overweight and obesity, respectively
(13). Another study revealed that
iron deficiency (serum iron <60 µg/dl) was detected in 13.5,
13.6, 23.5, and 21.7% of male adolescents (n=772) with underweight,
normal weight, overweight and obesity, respectively (14). Egwurugwu et al (2018)
reported that mean serum iron was 72.6, 64.2, 59.1, and 54.7 µg/dl
in adult men with normal weight, overweight, grade 1 obesity and
grade 2 obesity, respectively. In contrast, it was 61.2, 52.9,
44.8, and 39.6 µg/dl in the adult women (15). It is believed that hepcidin level
and low-grade chronic inflammation play a central role in the
relationship between obesity and hypoferremia (16). Although obesity is recognized as an
emerging risk factor for iron deficiency, mechanisms of the
relationship are still debatable (17). This review discusses the current
evidence on the relationship between obesity and iron deficiency,
focusing on factors that influence iron homeostasis, such as
hepcidin and low-grade chronic inflammation. Evidence from relevant
published experimental articles on iron deficiency and obesity
published from January 2015 to January 2021 were retrieved by using
Scopus, PubMed and Google Scholar and reviewed. Indeed, few reviews
have been carried out to explore the relationship between obesity
and iron deficiency. However, the previous works have not described
the role of hepcidin and low-grade chronic inflammation in iron
homeostasis.
2. Iron metabolism
Iron is an essential trace element found in many
foods, especially red meats. It is a crucial nutrient for optimal
physical and cognitive development of the human body (18). The adult human body contains two to
four grams of iron, about two-thirds of which is usually stored in
hemoglobin, and the remaining (30-40%) can be found in the
iron-binding proteins, such as ferritin and transferrin (19). Iron is mainly utilized in the bone
marrow to synthesize the heme part of the hemoglobin to produce red
blood cells in a process called erythropoiesis (20). The principal source of iron in this
process comes from the recycling of heme iron from ageing red blood
cells, which is mediated by macrophage. This process provides about
90% of the body iron requirement (21). The remaining 10% should be acquired
from the daily diet to replace usual body iron losses (22). The absorption of dietary iron
mainly occurs in the intestinal enterocytes in the duodenum and
proximal jejunum (23).
Ferroportin is the only known iron transporter found in human
intestinal enterocytes (24). It
transports iron across the basolateral membrane of intestinal
enterocytes into the bloodstream, where iron is carried by
transferrin and circulated throughout the body (22). Iron-requiring body cells, including
red blood cells, express transferrin receptor on their surface to
obtain the iron they need. When iron is required, cellular
transferrin receptor transcription is elevated to increase iron
uptake and vice versa (25). In
healthy individuals, the body stores excess iron in ferritin in the
liver that can be released into circulation when needed (21).
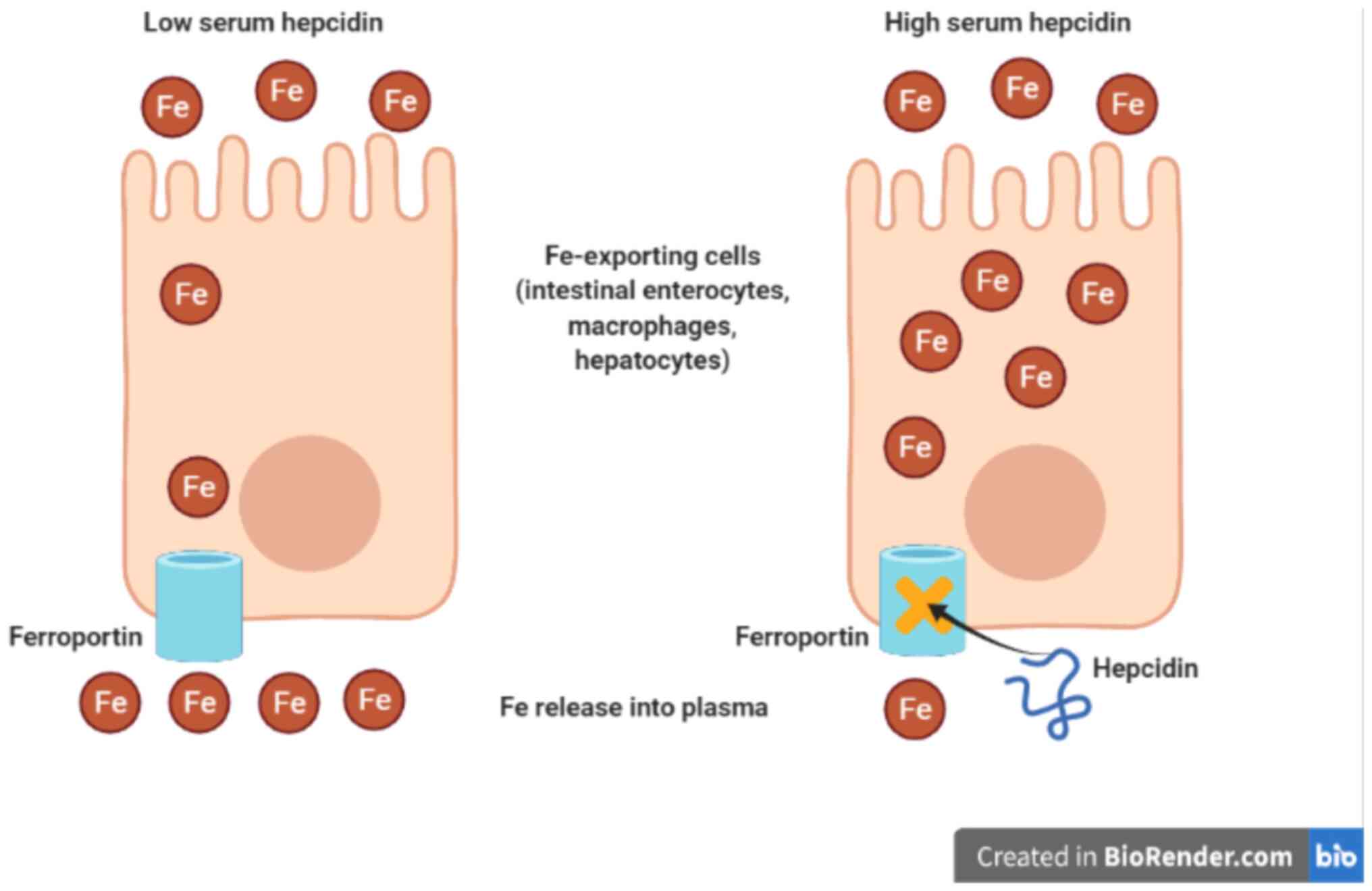
3. Hepcidin and iron homeostasis
Hepcidin is a small peptide hormone that is
considered a key regulator for body iron homeostasis (26). Hepcidin is synthesized mainly in
the liver and produced at low levels in other organs like adipose
tissue (27). Hepcidin regulates
plasma iron level by binding to ferroportin leading to
internalization and degradation of ferroportin through blockage of
cellular iron transport (28).
Consequently, dietary iron absorption from the small intestine is
downregulated, and thus, serum iron concentration is dropped
(29). In addition, hepcidin slows
down the release of recycled iron by macrophages to peripheral and
iron mobilization from iron stores in the liver or spleen (30) (Fig.
1).
4. Obesity and low-grade chronic
inflammation
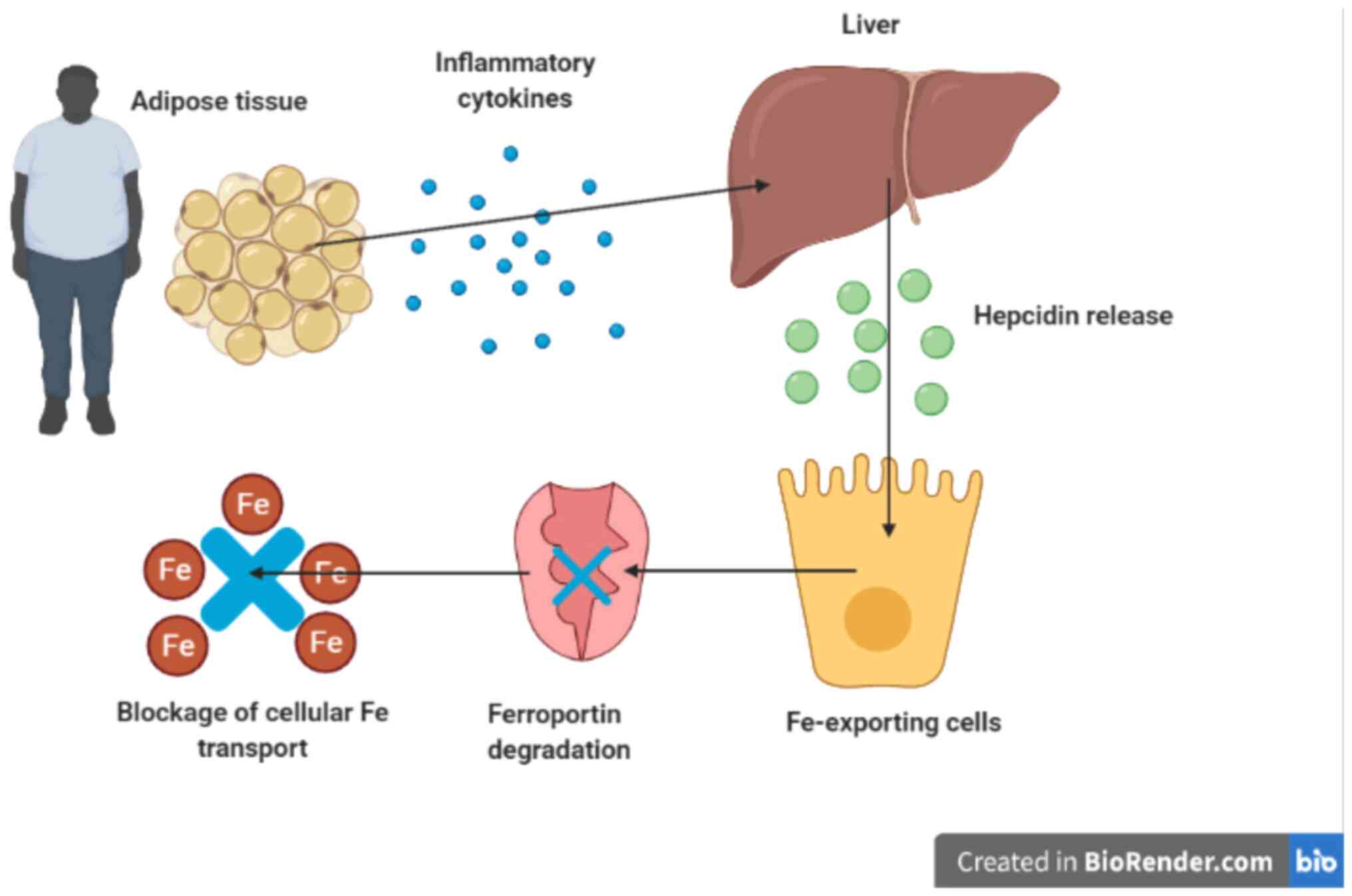
Obesity is associated with low-grade chronic
inflammation (31). Several
pro-inflammatory cytokines are secreted by adipose tissues,
including interleukin-6 and tumor necrosis factor alpha. Indeed,
about one-third of interleukin-6 in the circulation is released
from adipose tissue (32). The
principal mechanism that links obesity and iron deficiency is
low-grade systemic inflammation, observed in people with obesity
(33). In people with overweight
and obesity, serum hepcidin and serum interleukin-6 are
significantly higher than those with normal weight (12,34).
Hepcidin which is synthesized in the liver is stimulated by
pro-inflammatory cytokines such as interleukin-6(35) (Fig.
2). A recent study reported that overweight and obese women
with central adiposity demonstrated higher serum hepcidin, higher
inflammation level, lower iron status, and lower iron absorption
when fed with supplemental iron (36).
In response to infection and inflammation, the acute
phase restricts the iron availability to pathogens by sequestering
iron within macrophages resulting in iron deficiency (37). A vital component of the immune
system called toll-like receptors (TLR) can identify
pathogen-associated molecular patterns (PAMP). Activation of TLR
causes iron deficiency primarily through increasing the hepatic
hepcidin (38). This situation
induces ineffective erythropoiesis, which leads to anemia of
inflammation, a kind of anemia linked to infectious diseases,
cancer, and chronic kidney disease (39). Several studies have proven that
hypoferremia activates transcription of the osteocyte-secreted
protein fibroblast growth factor 23 (FGF23) (40). FGF23, a phosphate and vitamin D
homeostasis regulator produced from bone (41), is secreted as pro-inflammatory
cytokines in response to the activation of TLR4, followed by
up-regulation of hepcidin and down-regulation of erythropoietin
expression in addition to reduced serum iron and transferrin
saturation (42). Contrarily,
inhibition of FGF23 signalling alleviates hypoferremia and
attenuates dysregulation of erythropoiesis in acute inflammation
(43). The link between FGF23 and
inflammation is bound to two mechanisms; i) indirect pathway
through elevated hepcidin production, resulting in iron
sequestration in macrophages and subsequent functional iron
deficiency; ii) direct pathway by stimulating HIF1α transcription
leading to increased HIF1α, which together with HIF1β will bind to
HIF response element on the FGF23 promoter inducing its
transcription (40). Overall,
inflammation, iron status, and erythropoietin are known to regulate
FGF23 reciprocally (42,43).
5. Association between overweight and
obesity, and iron status
The first report of a potential connection between
people with obesity and iron status appeared in the early 1960s
(44). Four decades later, a
cross-sectional study conducted in 2003 described a higher
prevalence of iron deficiency in overweight and obese children and
adolescents (10). Using data from
the National Health and Nutrition Examination Survey (NHANES III),
a study confirmed that American children with overweight were twice
as likely to be iron deficient than children with normal weight
(11). Similar findings were also
reported among adults. Lecube et al (2006) found that
postmenopausal women with obesity had higher soluble transferrin
receptor than non-obese postmenopausal women (45). Similarly, Yanoff et al
(2007) reported an increase in the prevalence of iron deficiency in
obese adults with significantly lower serum iron level and higher
soluble transferrin receptor level than non-obese adults (12). In another study, Menzie et
al (2008) found a significantly lower level of serum iron and
transferrin saturation in adults with obesity when compared to
non-obese adults. In addition, fat mass was found as a significant
negative predictor of serum iron concentration (46).
Most studies that explore the relationship between
obesity and iron deficiency assessed mainly specific hematological
and biochemical markers, such as serum iron, hemoglobin,
hematocrit, and ferritin, to evaluate iron status. On the other
hand, inflammatory markers and serum hepcidin, which have an
essential role in iron homeostasis, are less commonly investigated
(47). Paknahad et al
(2008) found that serum hemoglobin and hematocrit increased
significantly across body mass index (BMI) quartiles in adult
Iranian women (48). However, no
significant difference in BMI was reported among lactating Kenyan
women with different levels of iron depletion (as defined by
ferritin), despite hemoglobin was shown to increase with increasing
BMI (49). In another report,
hematocrit increased with increasing BMI and waist-hip ratio in
Nigerian women (50). Aderibigbe
et al (2011) showed that waist circumference and waist-hip
ratio increased with increasing ferritin concentration in South
African women, while serum iron decreased with increasing BMI in
women (51). In another study, a
lower odds ratio of anemia was reported in women with overweight
and obesity than women with normal weight from Egypt and Peru but
not from Mexico (52).
Surprisingly, Kordas et al (2013) investigated the
association between iron deficiency, anemia, and weight status
among nonpregnant Colombian women and reported that women with
overweight and obesity had a lower likelihood of anemia (53).
A better understanding of the relationship between
obesity and iron deficiency has been gained when researchers
started to measure inflammatory markers and serum hepcidin in
addition to various iron status markers (54). Zimmermann et al (2008)
studied the relationship between obesity and iron deficiency among
healthy premenopausal women from transition countries using iron
isotope labelled test meals and hypothesized that the mechanism
might be mediated by inflammation. In their study, 22% of the women
were considered overweight, and 20% were iron deficient. They
measured the iron status indicators (hemoglobin and serum ferritin)
and C-reactive protein (CRP) at pre-meal and two-week post-meal. It
was observed that fractional iron absorption was negatively
correlated with CRP and BMI (55).
Alam et al (2015) reported that serum iron and transferrin
saturation were significantly lower in individuals with obesity
than those with normal body weight, whilst BMI was positively
correlated with serum CRP (56).
Similarly, Kaner et al (2016) investigated the relationship
between iron deficiency and obesity in Turkish women with
overweight and obesity and reported that the iron deficiency risk
could be more likely to occur in obesity due to elevated
inflammation level (13). Another
study reported a positive association between obesity and
inflammation and mild changes in iron markers (57). Additionally, Tussing-Humphreys
et al (2010) measured serum hepcidin in obese and non-obese
premenopausal adult women and observed a higher hepcidin level
among women with obesity (58).
Another study evaluated serum hepcidin level and iron status and
their association with BMI in a sample of children with obesity
from Egypt and reported lower hemoglobin and ferritin levels and
higher hepcidin level among obese children (59). Conversely, a study that
investigated iron, hepcidin, and inflammation levels in young women
with overweight and obesity concluded that iron deficiency was not
linked to inflammation or hepcidin (60). Most of these reports suggested that
obesity may negatively affect the iron status and cause iron
deficiency due to increased serum levels of low-grade chronic
inflammation and/or hepcidin.
6. Effect of weight loss on iron status
The traditional way to treat iron deficiency is by
providing an iron supplement. However, several studies found that
the efficacy of iron supplementation was significantly lower when
used among individuals with overweight and obesity compared to
those with normal body weight (55,61).
This could be caused by a reduction in iron absorption associated
with elevated serum hepcidin levels observed in people with
overweight and obesity (36).
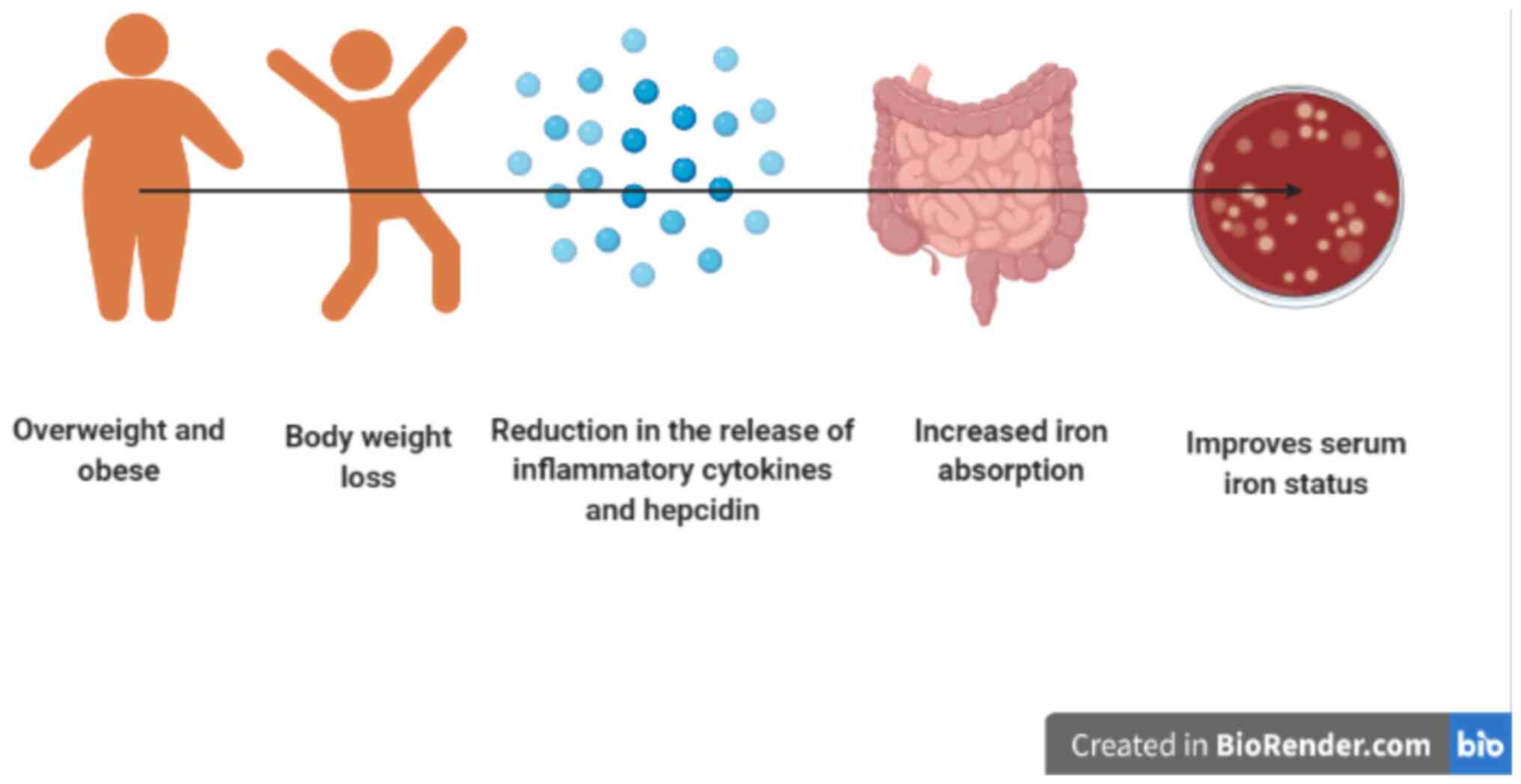
Weight loss that is induced by an energy-restricted diet and/or
exercise may improve obesity-related hypoferremia and help to
restore iron homeostasis in individuals with overweight and obesity
(62). In addition, a reduction in
adipose tissue is associated with alterations in the levels of
pro-inflammatory cytokines, which may lead to diminished hepcidin
release and improved iron status in people with overweight and
obesity (63,64) (Fig.
3).
A few studies investigated the effect of weight loss
on iron status, low-grade chronic inflammation, and/or hepcidin
release in individuals with overweight and obesity (62). Amato et al (2010) examined
the effect of BMI reduction on serum hepcidin levels and iron
status in obese children. They observed a significant decrease in
hepcidin and leptin levels and a significant increase in iron
absorption (65). Similarly, Gong
et al (2014) reported an improvement of iron status with
unchanged serum ferritin concentrations and an increase in
transferrin saturation after the intervention, along with an
improvement of inflammatory markers (66). Coimbra et al (2017) studied
the effect of an eight-month physical exercise programme on
hepcidin, inflammation, and iron status in overweight and obese
children and adolescents. The results showed a decrease in BMI
z-score, body fat mass, CRP, interleukin-6, ferritin, hepcidin, and
soluble transferrin receptor, in addition to an increase in serum
iron concentration (67).
Cepeda-Lopez et al (2016) evaluated the effects of fat loss
after bariatric surgery on inflammation, serum hepcidin, and iron
absorption. After six months of follow-up, they reported that the
total body fat, interleukin-6, and hepcidin were significantly
lower, whereas the iron absorption increased by 28% in
iron-deficient subjects (68).
Another study conducted six- and twelve-month weight loss programs
(high protein diet or low protein diet: 5600 kJ/day) among young
women (18-25 years) and revealed that an initial body weight loss
of more than 10% could be associated with an improved iron status
regardless of the diet type and hepcidin levels (69). Recently, Kaner et al (2019)
reported changes in body weight and iron parameters of
premenopausal Turkish women with overweight and obesity who
participated in a weight loss program. They found a statistically
significant relationship between body weight loss and CRP levels
and concluded that weight loss helps to improve blood iron
parameters due to its positive effect on low-grade chronic
inflammation (70).
7. Future research and clinical
implications
The high prevalence of obesity in combination with
iron deficiency incidence observed in different age and gender
groups suggesting a connection between obesity and iron status.
However, few studies investigate the cause-effect relationship
between them. This review highlights the importance of
well-controlled randomized clinical trials to confirm the causality
relationship between obesity and iron deficiency. In spite of
recent evidence showing iron disturbances with obesity, outcomes
from the routine iron marker, such as hemoglobin and serum
ferritin, appear to be not enough to understand the causal
relationship between obesity and iron status. Analysis of
additional biochemical markers, including hepcidin and
pro-inflammatory cytokines such as CRP and interleukin-6, should be
considered. Designing energy-restricted diets that deliver
sufficient iron to meet recommended intakes for subjects is
essential in weight loss trials. Therefore, it would be crucial in
the clinical setting to conduct a continuous assessment for dietary
iron intake in subjects who received medical nutrition therapy for
weight management. Moreover, physical activity levels during the
weight loss trial need to be monitors and controlled. Successful
weight loss of at least 5-10% of initial weight may be needed to
positively impact iron, inflammation and hepcidin levels and ensure
the effect of diet-induced weight loss on these markers. A strict
selection criterion applied at the recruitment stage is also
essential to minimize confounding effects on the weight and iron
outcomes. To avoid results bias, certain groups of the target
population should be excluded, such as patients with certain
medical conditions, pregnant and lactating mothers, vegetarians,
smokers, and those who have recently supplemented iron. Further
research is needed to examine the possible connections between
iron, hepcidin, and other comorbid conditions, including
cardiovascular disease, diabetes and metabolic syndrome. The
function of body fat amount and distribution (android vs. gynoid
fat), especially concerning gender differences, should be
investigated further. Finally, the link between obesity and iron
deficiency is an emerging research field and one of the current
limitation in the studies reviewed is the lack of formula between
iron markers and obesity to predict iron deficiency.
According to the findings of this review, there is
emerging evidence to support the link between obesity and iron
deficiency. Increased hepcidin levels mediated by chronic
inflammation are thought to be the cause of this link. The findings
emphasize the significance of closely monitoring and treating iron
deficiency in people with overweight and obesity. In those
patients, routine iron status markers such as hemoglobin, serum
ferritin, and serum iron appear to be a sound indicator of iron
status. In patients with class 2 obesity and above (BMI ≥35),
caution is advised when using ferritin because inflammatory-related
iron disturbances may be more noticeable. Thus, examining
additional biochemical markers such as soluble transferrin receptor
and hepcidin should be considered to evaluate iron status
accurately. Furthermore, while attempting to determine iron status
in individuals with overweight and obesity, potential confounding
from higher inflammation should be considered. This could involve
measuring acute phase proteins like CRP, or, at least, avoiding
iron testing during periods of apparent infection. Initial
assessment for iron status in patients with overweight and obesity
seeking medical nutrition therapy for weight loss might be
advantageous in the clinical setting. This is especially important
for women who have a higher iron requirement, such as those with a
history of iron insufficiency, heavy or protracted menses, or low
iron intake. It is critical to design energy-restricted diets that
supply sufficient iron to meet the dietary needs of overweight and
obese patients.
8. Conclusions
This review describes the association between
obesity and iron deficiency. The mechanisms responsible for this
relationship remains undefined. However, growing evidence
highlights a principal role for elevation in low-grade chronic
inflammation and hepcidin levels associated with obesity in
disturbances in iron homeostasis, which may lead to hypoferremia.
Because of the high overweight and obesity prevalence globally, it
is important to develop public health strategies to manage
obesity-related hypoferremia. Individuals with overweight and
obesity should be targeted by periodical screening for iron status,
particularly in population groups at high risk for iron deficiency
anemia, including children, adolescents, and women of childbearing
age. Common clinical signs and symptoms of anemia are usually not
shown in patients at the early stage of iron deficiency anemia.
Therefore, periodical monitoring of iron markers would be
beneficial for patients with overweight and obesity. In addition,
designing effective weight loss programs via an energy-restricted
diet and/or exercise can help individuals with overweight and
obesity to lose excessive body weight and restore iron homeostasis.
Finally, future studies with robust, well-designed randomized
clinical trials, relevant adjustment for important confounders, and
use of various markers related to the iron status, inflammation,
and hepcidin are needed to examine the putative causal relationship
between obesity and iron deficiency.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NMA, AA and HANAJ acquired the resources by
searching Scopus, PubMed and Google Scholar, collected the data
from retrieved articles and drafted the initial manuscript. WMRWH
reviewed and edited the manuscript for important intellectual
content. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chooi YC, Ding C and Magkos F: The
epidemiology of obesity. Metabolism. 92:6–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cappellini MD, Musallam KM and Taher AT:
Iron deficiency anaemia revisited. J Intern Med. 287:153–170.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gregg EW and Shaw JE: Global health
effects of overweight and obesity. N Engl J Med. 377:80–81.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Camaschella C: New insights into iron
deficiency and iron deficiency anemia. Blood Rev. 31:225–233.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Percy L, Mansour D and Fraser I: Iron
deficiency and iron deficiency anaemia in women. Best Pract Res
Clin Obstet Gynaecol. 40:55–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Halib H, Muda WM, Dam PC and Mohamed HJ:
Prevalence of iron deficiency and its associated risk factors among
primary school children in Kelantan. J Fundam Appl Sci. 9:397–412.
2017.
|
|
7
|
Bah F, Harith S and Farisni TN: Food
knowledge and practices related to anemic conditions among pregnant
women in Kuala Terengganu, Malaysia. Indonesian J Pub Health.
7:19–28. 2020.
|
|
8
|
Zani H, Shahril MR, Wan Abdul Rahman WN,
Mukhali HB, Ismail R and Mohd Yusop Y: Anaemia-related knowledge
amongst pregnant women in Kuala Terengganu, Malaysia. Asian J Med
Biomed. 4:1–9. 2020.
|
|
9
|
Zhao L, Zhang X, Shen Y, Fang X, Wang Y
and Wang F: Obesity and iron deficiency: A quantitative
meta-analysis. Obes Rev. 16:1081–1093. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pinhas-Hamiel O, Newfield RS, Koren I,
Agmon A, Lilos P and Phillip M: Greater prevalence of iron
deficiency in overweight and obese children and adolescents. Int J
Obes Relat Metab Disord. 27:416–418. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nead KG, Halterman JS, Kaczorowski JM,
Auinger P and Weitzman M: Overweight children and adolescents: A
risk group for iron deficiency. Pediatrics. 114:104–108.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yanoff LB, Menzie CM, Denkinger B, Sebring
NG, McHugh T, Remaley AT and Yanovski JA: Inflammation and iron
deficiency in the hypoferremia of obesity. Int J Obes (Lond).
31:1412–1419. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaner G, Pekcan G, Pamuk G, Pamuk B and
Amoutzopoulos B: Is iron deficiency related with increased body
weight? A cross-sectional study. Prog Nutr. 18:102–110. 2016.
|
|
14
|
Huang YF, Tok TS, Lu CL, Ko HC, Chen MY
and Chen SC: Relationship between being overweight and iron
deficiency in adolescents. Pediatr Neonatol. 56:386–392.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Egwurugwu JN, Ekweogu CN, Nwamkpa P,
Ohamaeme MC, Ugwuezumba PC and Ogunnaya FU: Association between
serum phosphate and iron concentrations with body mass index in a
population of adults in Orlu, Imo State, Nigeria. Niger J Exp Clin
Biosci. 6:1–7. 2018.
|
|
16
|
D'angelo G: Role of hepcidin in the
pathophysiology and diagnosis of anemia. Blood Res. 48:10–15.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aigner E, Feldman A and Datz C: Obesity as
an emerging risk factor for iron deficiency. Nutrients.
6:3587–3600. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Fartusie FS and Mohssan SN: Essential
trace elements and their vital roles in human body. Indian J Adv
Chem Sci. 5:127–136. 2017.
|
|
19
|
Anderson GJ and Frazer DM: Current
understanding of iron homeostasis. Am J Clin Nutr. 106 (Suppl
6):1559S–1566S. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Milto IV, Suhodolo IV, Prokopieva VD and
Klimenteva TK: Molecular and cellular bases of iron metabolism in
humans. Biochemistry (Mosc). 81:549–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abbaspour N, Hurrell R and Kelishadi R:
Review on iron and its importance for human health. J Res Med Sci.
19:164–174. 2014.PubMed/NCBI
|
|
22
|
Dev S and Babitt JL: Overview of iron
metabolism in health and disease. Hemodial Int. 21 (Suppl
1):S6–S20. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fuqua BK, Vulpe CD and Anderson GJ:
Intestinal iron absorption. J Trace Elem Med Biol. 26:115–119.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Morgan EH and Oates PS: Mechanisms and
regulation of intestinal iron absorption. Blood Cells Mol Dis.
29:384–399. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Handelman GJ and Levin NW: Iron and anemia
in human biology: A review of mechanisms. Heart Fail Rev.
13:393–404. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ganz T and Nemeth E: Hepcidin and iron
homeostasis. Biochim Biophys Acta. 1823:1434–1443. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reichert CO, da Cunha J, Levy D, Maselli
LM, Bydlowski SP and Spada C: Hepcidin: Homeostasis and diseases
related to iron metabolism. Acta Haematol. 137:220–236.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ganz T: Hepcidin-a regulator of intestinal
iron absorption and iron recycling by macrophages. Best Pract Res
Clin Haematol. 18:171–182. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sangkhae V and Nemeth E: Regulation of the
iron homeostatic hormone hepcidin. Adv Nutr. 8:126–136.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chaston T, Chung B, Mascarenhas M, Marks
J, Patel B, Srai SK and Sharp P: Evidence for differential effects
of hepcidin in macrophages and intestinal epithelial cells. Gut.
57:374–382. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fontana L, Eagon JC, Trujillo ME, Scherer
PE and Klein S: Visceral fat adipokine secretion is associated with
systemic inflammation in obese humans. Diabetes. 56:1010–1013.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coimbra S, Catarino C and Santos-Silva A:
The role of adipocytes in the modulation of iron metabolism in
obesity. Obes Rev. 14:771–779. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nemeth E, Rivera S, Gabayan V, Keller C,
Taudorf S, Pedersen BK and Ganz T: IL-6 mediates hypoferremia of
inflammation by inducing the synthesis of the iron regulatory
hormone hepcidin. J Clin Invest. 113:1271–1276. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Wrighting DM and Andrews NC: Interleukin-6
induces hepcidin expression through STAT3. Blood. 108:3204–3209.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stoffel NU, El-Mallah C, Herter-Aeberli I,
Bissani N, Wehbe N, Obeid O and Zimmermann MB: The effect of
central obesity on inflammation, hepcidin, and iron metabolism in
young women. Int J Obes (Lond). 44:1291–1300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gozzelino R and Arosio P: Iron homeostasis
in health and disease. Int J Mol Sci. 17(130)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schmidt PJ: Regulation of iron metabolism
by hepcidin under conditions of inflammation. J Biol Chem.
290:18975–18983. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Weiss G: Iron metabolism in the anemia of
chronic disease. Biochim Biophys Acta. 1790:682–693.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
David V, Martin A, Isakova T, Spaulding C,
Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL
and Wolf M: Inflammation and functional iron deficiency regulate
fibroblast growth factor 23 production. Kidney Int. 89:135–146.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shimada T, Hasegawa H, Yamazaki Y, Muto T,
Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S and Yamashita
T: FGF-23 is a potent regulator of vitamin D metabolism and
phosphate homeostasis. J Bone Miner Res. 19:429–435.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Agoro R, Park MY, Le Henaff C, Jankauskas
S, Gaias A, Chen G, Mohammadi M and Sitara D: C-FGF23 peptide
alleviates hypoferremia during acute inflammation. Haematologica.
106:391–403. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Agoro R, Montagna A, Goetz R, Aligbe O,
Singh G, Coe LM, Mohammadi M, Rivella S and Sitara D: Inhibition of
fibroblast growth factor 23 (FGF23) signaling rescues renal anemia.
FASEB J. 32:3752–3764. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wenzel B, Stults H and Mayer J:
Hypoferraemia in obese adolescents. Lancet. 2:327–328.
1962.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lecube A, Carrera A, Losada E, Hernández
C, Simó R and Mesa J: Iron deficiency in obese postmenopausal
women. Obesity (Silver Spring). 14:1724–1730. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Menzie CM, Yanoff LB, Denkinger BI, McHugh
T, Sebring NG, Calis KA and Yanovski JA: Obesity-related
hypoferremia is not explained by differences in reported intake of
heme and nonheme iron or intake of dietary factors that can affect
iron absorption. J Am Diet Assoc. 108:145–148. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Aderibigbe OR, Pisa PT, Vorster HH and
Kruger SH: The relationship between iron status and adiposity in
women from developing countries: A review. Crit Rev Food Sci Nutr.
54:553–560. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Paknahad Z, Mahboob S, Omidvar N, Ebrahimi
M, Ostadrahimi A and Afiatmilani SH: Body Mass Index and its
relationship with haematological indices in Iranian women. Pak J
Nutr. 7:377–380. 2008.
|
|
49
|
Ettyang GA, van Marken Lichtenbelt WD,
Oloo A and Saris WH: Serum retinol, iron status and body
composition of lactating women in Nandi, Kenya. Ann Nutr Metab.
47:276–283. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Famodu AA and Awodu OA: Anthropometric
indices as determinants of haemorheological cardiovascular disease
risk factors in Nigerian adults living in a semi-urban community.
Clin Hemorheol Microcirc. 43:335–344. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Aderibigbe OR, Pisa PT, Mamabolo RL,
Kruger HS, Vorster HH and Kruger A: Iron status and cardiovascular
disease risk in black South African women: The PURE study. South
African J Clin Nutr. 24:179–185. 2011.
|
|
52
|
Eckhardt CL, Torheim LE, Monterrubio E,
Barquera S and Ruel MT: The overlap of overweight and anaemia among
women in three countries undergoing the nutrition transition. Eur J
Clin Nutr. 62:238–246. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kordas K, Fonseca Centeno ZY, Pachón H and
Jimenez Soto AZ: Being overweight or obese is associated with lower
prevalence of anemia among Colombian women of reproductive age. J
Nutr. 143:175–181. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cheng HL, Bryant C, Cook R, O'Connor H,
Rooney K and Steinbeck K: The relationship between obesity and
hypoferraemia in adults: A systematic review. Obes Rev. 13:150–161.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zimmermann MB, Zeder C, Muthayya S,
Winichagoon P, Chaouki N, Aeberli I and Hurrell RF: Adiposity in
women and children from transition countries predicts decreased
iron absorption, iron deficiency and a reduced response to iron
fortification. Int J Obes (Lond). 32:1098–1104. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Alam F, Memon AS and Fatima SS: Increased
body mass index may lead to hyperferritinemia irrespective of body
iron stores. Pak J Med Sci. 31:1521–1526. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shekarriz R and Vaziri MM: Iron profile
and inflammatory status of overweight and obese women in Sari,
North of Iran. Int J Hematol Oncol Stem Cell Res. 11:108–113.
2017.PubMed/NCBI
|
|
58
|
Tussing-Humphreys LM, Nemeth E, Fantuzzi
G, Freels S, Guzman G, Holterman AX and Braunschweig C: Elevated
systemic hepcidin and iron depletion in obese premenopausal
females. Obesity (Silver Spring). 18:1449–1456. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Abdel Hamed ER, Sallam SF, Hamdy HA, El
Shafie AI, El Kassas GM, Khairy SA and Abdelsalam HM: Serum
hepcidin level and iron status in a sample of obese Egyptian
children. Med Res J. 14:7–11. 2015.
|
|
60
|
Cheng HL, Bryant CE, Rooney KB, Steinbeck
KS, Griffin HJ, Petocz P and O'Connor HT: Iron, hepcidin and
inflammatory status of young healthy overweight and obese women in
Australia. PLoS One. 8(e68675)2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Baumgartner J, Smuts CM, Aeberli I, Malan
L, Tjalsma H and Zimmermann MB: Overweight impairs efficacy of iron
supplementation in iron-deficient South African children: A
randomized controlled intervention. Int J Obes (Lond). 37:24–30.
2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Teng IC, Tseng SH, Aulia B, Shih CK, Bai
CH and Chang JS: Can diet-induced weight loss improve iron
homoeostasis in patients with obesity: A systematic review and
meta-analysis. Obes Rev. 21(e13080)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chang JS, Li YL, Lu CH, Owaga E, Chen WY
and Chiou HY: Interleukin-10 as a potential regulator of hepcidin
homeostasis in overweight and obese children: A cross-sectional
study in Taiwan. Nutrition. 30:1165–1170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sanad M, Osman M and Gharib A: Obesity
modulate serum hepcidin and treatment outcome of iron deficiency
anemia in children: A case control study. Ital J Pediatr.
37(34)2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Amato A, Santoro N, Calabrò P, Grandone A,
Swinkels DW, Perrone L and del Giudice EM: Effect of body mass
index reduction on serum hepcidin levels and iron status in obese
children. Int J Obes (Lond). 34:1772–1774. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gong L, Yuan F, Teng J, Li X, Zheng S, Lin
L, Deng H, Ma G, Sun C and Li Y: Weight loss, inflammatory markers,
and improvements of iron status in overweight and obese children. J
Pediatr. 164:795–800.e2. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Coimbra S, Catarino C, Nascimento H, Inês
Alves A, Filipa Medeiros A, Bronze-da-Rocha E, Costa E,
Rocha-Pereira P, Aires L, Seabra A, et al: Physical exercise
intervention at school improved hepcidin, inflammation, and iron
metabolism in overweight and obese children and adolescents.
Pediatr Res. 82:781–788. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cepeda-Lopez AC, Allende-Labastida J,
Melse-Boonstra A, Osendarp SJ, Herter-Aeberli I, Moretti D,
Rodriguez-Lastra R, Gonzalez-Salazar F, Villalpando S and
Zimmermann MB: The effects of fat loss after bariatric surgery on
inflammation, serum hepcidin, and iron absorption: A prospective
6-mo iron stable isotope study. Am J Clin Nutr. 104:1030–1038.
2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cheng HL, Griffin HJ, Bryant CE, Rooney
KB, Steinbeck KS and O'Connor HT: Impact of diet and weight loss on
iron and zinc status in overweight and obese young women. Asia Pac
J Clin Nutr. 22:574–582. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kaner G, Pekcan AG and Şarer Yürekli BP:
Effect of a weight loss intervention on iron parameters in
overweight and obese Turkish women. Prog Nutr. 21:50–56. 2019.
|