1. Introduction
Osteoarthritis (OA) is a chronic bone and joint
disease characterized by articular cartilage degeneration and joint
inflammation (1). OA is the
commonest form of arthritis, and the major clinical manifestations
of OA are chronic pain and joint activity disorder (1), which severely affect patients'
quality of life. OA is most common in middle-aged and elderly
subjects (2,3). The pathogenesis of OA is complex, and
its etiology remains to be fully elucidated. At present, the
occurrence of OA is assumed to be associated with various risk
factors, including mechanical, genetic and physical factors
(4-6).
A number of these factors, including age, sex, obesity and bone
density, increase the risk of OA, and age has been reportedly
indicated to be an independent risk factor (4-6).
Current treatments for OA aim to alleviate or control pain, delay
or prevent disease progression, improve or reconstruct joint
function, correct deformity and maintain patients' quality of life
(7). OA treatment is based on the
combination of the disease education, sports activity guidance,
medication and, if necessary, surgical intervention. In addition,
an individualized treatment plan may be developed considering the
patient's age, sex, OA location, extent, symptoms and underlying
comorbidities (7). However, the
clinical results obtained by conventional treatments are poor, and
the risk of side effects is high, including postoperative pain,
fractures and dislocations (7).
Therefore, elucidating the pathological mechanism of OA may be
helpful in identifying novel specific biomarkers that may
contribute to the development of effective treatments for managing
OA symptoms.
Circular RNA (circRNA) is a type of non-coding RNA
that is ubiquitous in eukaryotic cells. Unlike standard linear
RNAs, circRNAs form a covalently closed continuous loop without 5'
or 3' polarity (8). They are
usually considered as byproducts of mis-splicing or mRNA processing
(9). In the present review, the
latest research on circRNAs was summarized with respect to the
pathogenesis of OA, to provide a novel direction for the
prevention, diagnosis and treatment of OA.
2. Generation and biological functions of
circRNAs
Sanger et al (10) and Hsu and Coca-Prados (11), using electron microscopy, were the
first to discover circRNAs in plant-based and eukaryotic organisms
in the 1970s. Subsequently, circRNA [in Hepatitis δ virus (HDV)]
was amplified by PCR and sequenced to confirm that it is also
expressed in humans (12). Several
studies have confirmed that circRNAs are differentially expressed
in OA cartilage and are closely related to the pathological
processes of OA, including extracellular matrix (ECM) degradation,
inflammation and apoptosis (12,13).
With the development of RNA sequencing and bioinformatics,
thousands of circRNAs have been discovered in various organisms
including plants, animals and viruses) (13) and each circRNA is considered to
have a regulatory effect on a variety of biological processes.
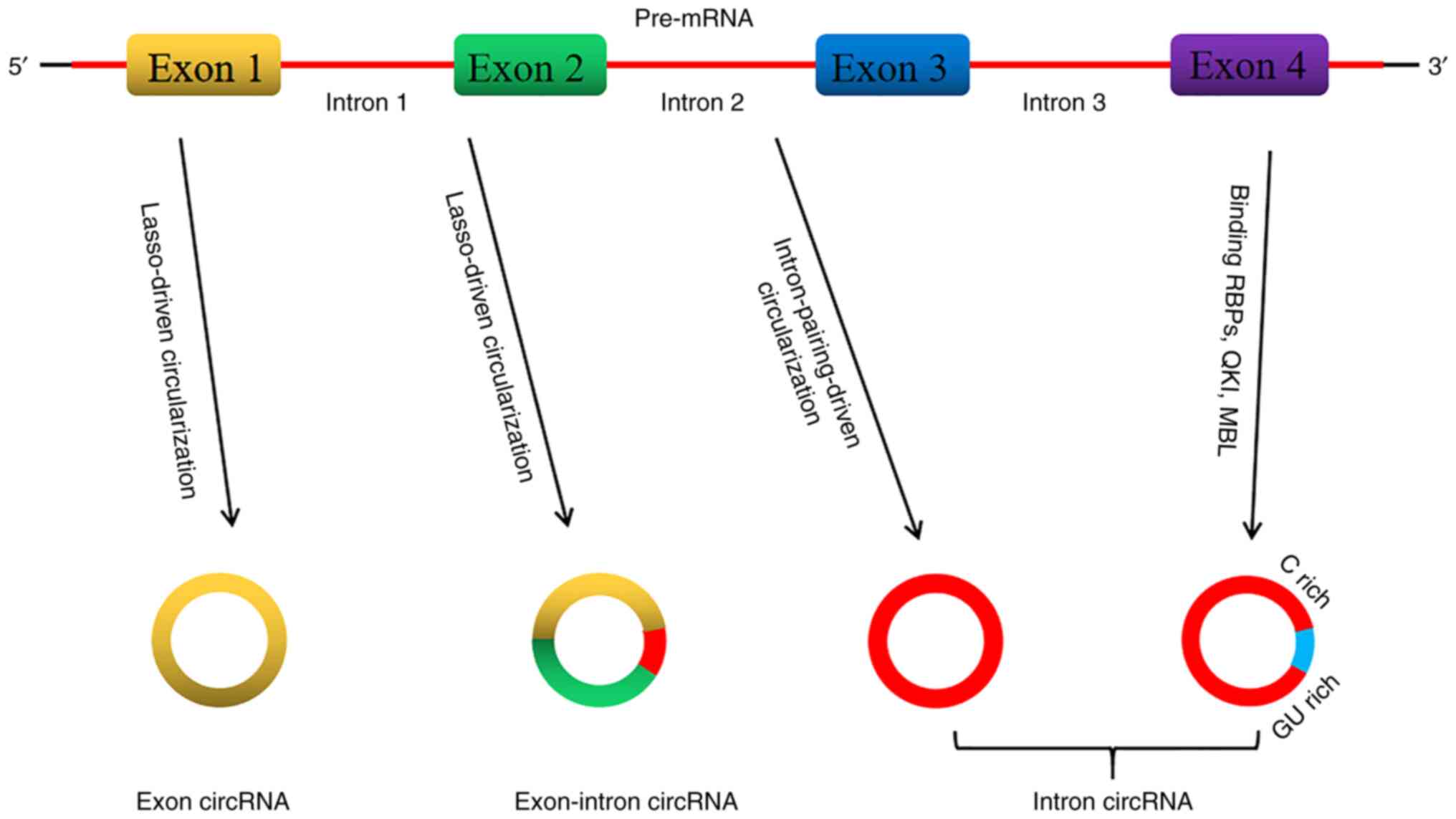
According to the genomic origin and structural
characteristics of circRNAs, they are divided into three types:
Exon circRNA, exon-intron circRNA and intron circRNA (14,15).
CircRNAs are produced through a highly complex process that
involves different cyclization mechanisms.
Usually, the spliceosome operates for eukaryotic
pre-messenger RNA (pre-mRNA), which removes introns and junction
exons to produce linear RNA transcripts with 5' or 3' polarity
(16). Most circRNAs are produced
through the reverse splicing process, which constitutes the
difference between circRNA and linear RNA standard splicing; the
resultant sequence does not follow the 5'-3' order (16,17).
In the same pre-mRNA, the exons located between the downstream
5'-splice site (splice donor) and the upstream 3'-splice site
(splice acceptor) are produced without end structures (such as 5'
cap structure or poly A tail) to yield the ring product (14,18,19).
Two different exon cyclization mechanism models were proposed by
Jeck et al (14) in 2013.
The first model is the lasso-driven circularization or exon
skipping. The characteristic of this model is that the original
non-adjacent exons come nearer by partially folded pre-mRNA
transcripts and are close to other exons, leading to exon skipping
and formation of overlapping regions, thereby creating an area
containing exons and an internal lasso intermediate. The introns in
the lasso are removed, resulting in exon circRNA (14,20).
Usually, the introns located between the circularized exons are
spliced, and in certain cases the exon-intron circRNA is not
spliced (20). The second model
refers to cyclization driven by intron pairing or direct reverse
splicing. The model involves complementarity that spans flanking
introns or other RNA secondary structure base assignments and
connects downstream splice donors with upstream splice acceptors to
form a loop structure (20). The
intron circRNA produced by the intron lasso is able to resist the
degradation of debranching enzymes (14,20).
The intron circRNA contains a unique 3'-5' connection, while the
exon circRNA lacks this unique connection. The 5'-splice site that
is rich in 7 nucleotides GU and the sequence close to the C-rich
branch of 11 nucleotides form the junction site (Fig. 1) (21).
Studies confirmed the circRNA biogenesis model
through RNA binding proteins (RBPs) (22,23).
In this model, two flanking introns combine with quaking protein
and muscleblind-like protein 1 to form two flanking intron
sequences that form a bridging point and promote cyclization to
produce circRNA (22,23). This mechanism is similar to the
circularization pathway driven by intron pairing, except that RBP
regulates adjacent splice sites rather than the direct base pairing
between complementary motifs seen in the intron pairing-driven
model.
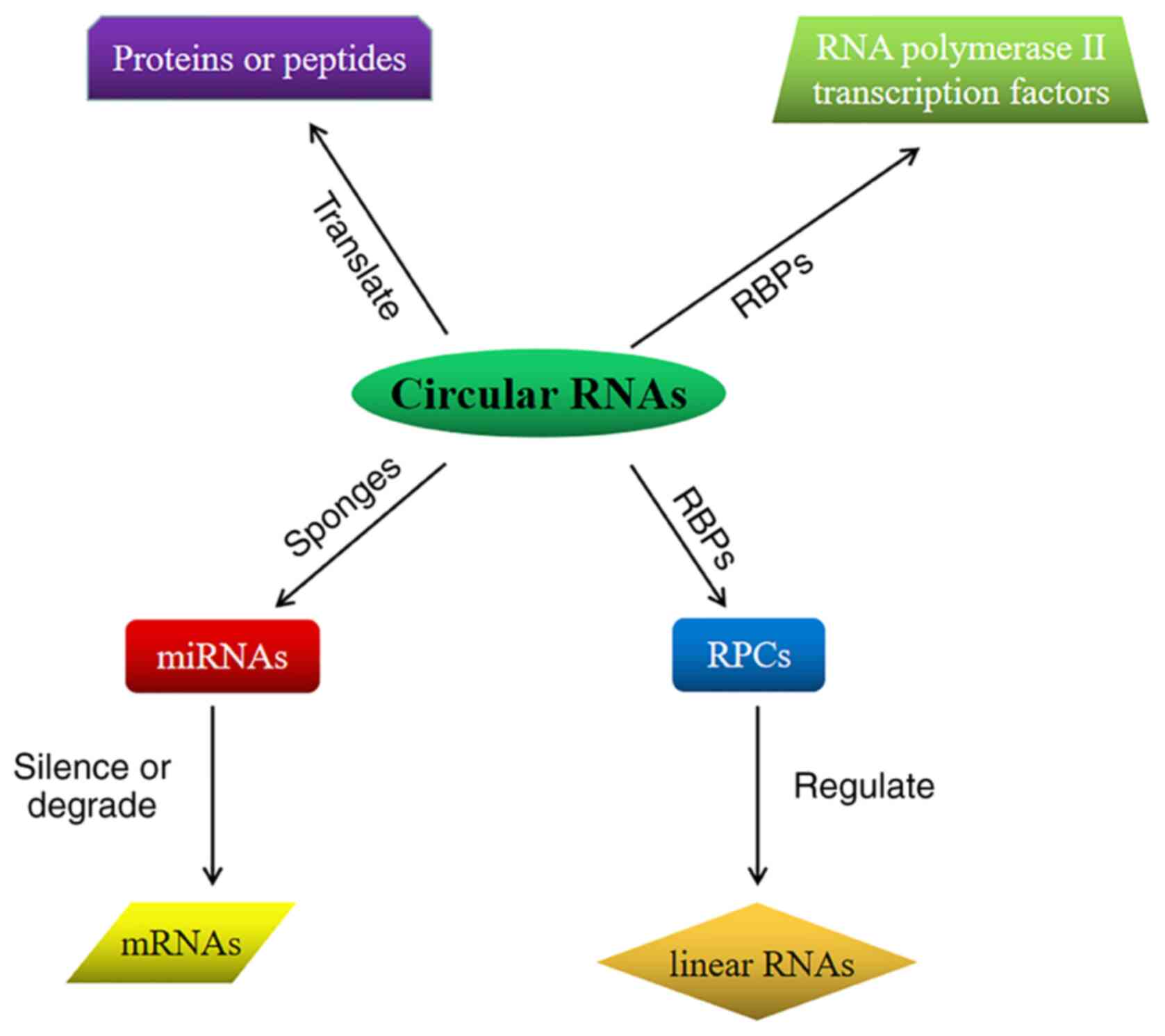
Several studies have confirmed that circRNAs may act
as microRNA (miRNA/miR) sponges, interact with RBP and regulate
gene transcription, and certain circRNAs may be translated into
proteins or peptides (14,20,21,24-29).
Therefore, as illustrated in Fig.
2, the major functions of circRNAs may be summarized as
follows: i) miRNA sponge-as a type of linear non-coding RNA, miRNAs
may silence or degrade target mRNAs through base pairing, thereby
regulating the pathological and physiological processes of
organisms (30). CircRNAs may be
used as competing endogenous RNAs (ceRNAs) that bind to miRNAs,
inhibits the binding of miRNAs to the target and thereby inhibits
mRNA translation (31,32); ii) combination with RBPs-circRNA
binds to RBP to form an RNA-protein complex, which regulates RBP
and interacts with linear RNA to perform biological functions
(31,32); iii) regulation of gene
transcription-numerous circRNAs are present in the nucleus, which
are able to bind to RBPs, particularly with transcription-related
factors, such as RNA polymerase II, and transcription factors, and
recruit transcription-related factors into the parental genes,
thereby affecting the expression of the parental genes and
regulating the transcription process (33); and iv) translation into protein or
peptide-evidence suggests that certain circRNAs may be also
translated into proteins. When the synthetic circular RNA contains
an efficiently translated internal ribosome entry site sequence,
the circular RNA directly binds to the ribosome and is translated
in eukaryotic cells (22,34). Another study confirmed that
methylated adenosine N6 may drive natural eukaryotic endogenous
circRNA protein translation, which indicates that certain circRNAs
exhibit a protein-encoding function (35,36).
3. Role of circRNAs in OA
CircRNAs have been reportedly involved in the
development and process of several human diseases, such as
diabetes, cardiovascular disease, cancer and Alzheimer's disease
(37,38). However, the role of circRNAs in the
pathogenesis of OA remains to be fully elucidated. Previous studies
have indicated that certain circRNAs are abnormally expressed in
human OA articular cartilage compared with cartilage tissue of
healthy controls.
Li et al (39) used whole-transcriptome sequencing
and determined that 42 types of circRNAs may be differentially
expressed in OA cartilage tissue. Another recent study identified
122 circRNAs that were differentially expressed in OA through RNA
sequencing; five downregulated circRNAs and an upregulated circRNA
were confirmed by reverse transcription-quantitative (RT-q)PCR
(40). Xiao et al (41) used the Illumina sequencing platform
to detect the expression of circRNA in the OA knee joint and
identified a total of 197 differentially expressed circRNAs,
including hg38_circ_0007474 and hg38_circ_0000118, and RT-qPCR
confirmed that three circRNAs were related to OA (hsa_circ_0002485,
hsa_circ_0005567 and hsa_circ_0045714). Another previous study
suggested that 71 circRNAs were differentially expressed in OA
cartilage (42). Furthermore, 16
and 55 of these circRNAs were upregulated and downregulated in OA
cartilage tissues, respectively. These differentially expressed
circRNAs were indicated to be involved in the pathological process
of OA in cartilage tissues (42).
These studies suggested that abnormal expression of circRNAs in
human OA cartilage is involved in the pathogenesis of OA. These
differentially expressed circRNAs can either promote or inhibit the
occurrence and development of OA.
4. Promotion of OA pathogenesis
It has been indicated that the increased catabolism
of ECM articular cartilage accelerates the development of OA
(43). Type II collagen (Col II)
and aggrecan are important components of the ECM. The major
pathological feature of OA is the decrease of Col II and aggrecan.
The main enzymes responsible for ECM degradation are disintegrin
and metalloproteinase with thrombospondin-like motifs (ADAMTS) and
matrix metalloproteinases (MMPs) (44). In addition, numerous MMPs are
highly upregulated in OA cartilage and knocking down ADAMTS may
reduce cartilage destruction (44). Further evidence has indicated that
inflammation is another important driving factor of OA cartilage
pathology (45,46). The inflammatory process of OA
involves traditional pro-inflammatory mediators, including IL-1β
and TNF-α and other chemokines, such as nicotinamide
phosphoribosyltransferase (47).
It has been indicated that the decrease in the number of
chondrocytes induced by apoptosis is an important factor leading to
the degeneration of OA cartilage (9).
As circRNAs have a wide range of biological
functions, they may have key regulatory roles in the occurrence and
development of OA by regulating cartilage matrix metabolism and
inflammation, along with chondrocyte proliferation, differentiation
and apoptosis (44-47).
CircRNA-CER is a special type of ECM-related
chondrocyte circRNA (48). Through
network analysis of circRNA-miRNA-mRNA interactions, five miRNAs
with potential binding sites for circRNA-CER were identified:
miR-636, miR-665, miR-217, miR-646 and miR-136. Further
investigation indicated that after miR-136 was combined with
circRNA 3'-UTR, the expression of circRNA-CER and the MMP13 gene
was jointly regulated. Application of small interfering (si)RNA to
knockdown circRNA-CER (si-circRNA-CER) in OA chondrocytes
significantly reduced the mRNA expression of MMP13. Co-transfection
with miR-136 inhibitor and si-circRNA-CER reduced MMP13 expression
and increased the mRNA expression of Col II and proteoglycan (such
as cartilage proteoglycan) compared with cartilage tissue of
healthy controls (42). This
indicated that circRNA-CER may be used as a ceRNA to regulate the
expression of MMP13 and participate in the degradation of
chondrocyte ECM (42). Therefore,
inhibiting circRNA-CER is likely a novel treatment strategy for
stimulating ECM regeneration and delaying joint degeneration.
CircRNA-MSR is another special type of
cartilage-related circRNA. It has been indicated that circRNA-MSR
is highly expressed in the damaged cartilage area in OA (49). At the same time, its expression
also notably increases under the mechanical stress of chondrocytes.
Therefore, it is also called circRNA related to mechanical stress
(49). Following knockdown of
circRNA-MSR, the expression of TNF-α was inhibited, while that of
Col II and aggrecan was increased, indicating that circRNA-MSR is
able to promote ECM degradation. Further studies have confirmed
that the 3'-UTR of circRNA-MSR and TNF-α were complementary to
miR-875, which suggested that the common target miRNA of
circRNA-MSR and TNF-α is miR-875(49). Knockdown of circRNA-MSR may inhibit
ECM degradation and reduce inflammation.
Wu et al (50) confirmed that hsa_circ_0005105
contains the binding site of miR-26a. It was demonstrated that
hsa_circ_0005105 was highly expressed in IL-1β-induced OA
chondrocytes, while the expression of miR-26a was downregulated,
indicating that hsa_circ_0005105 likely participates in the
biological process of OA through miR-26a (50). Hsa_circ_0005105 has been indicated
to inhibit the expression of Col II and aggrecan and promote the
expression of MMP13 and ADAMTS4, whereas miR-26a exhibited the
opposite effect (50).
Hsa_circ_0005105 promoted ECM degradation by regulating the
expression of miR-26a. Therefore, the study by Wu et al
(50) suggested that
hsa_circ_0005105 promoted the development and process of OA.
Zhou et al (51) discovered a circRNA from the mouse
Atp9b locus, which was named circRNA_Atp9b (circ_15898). After
stimulation with IL-1β, the expression of circRNA_Atp9b in mouse
chondrocytes was markedly increased. Knockout of circRNA_Atp9b
promoted the expression of Col II while inhibiting the production
of MMP13, cyclooxygenase-2 and IL-6. Their study also indicated
that circRNA_Atp9b acts as miR-138-5p ceRNA in IL-1β-induced
chondrocytes (51). CircRNA_Atp9b
directly targets miR-138-5p and knockdown of miR-138-5p reversed
the effect of circRNA_Atp9b on ECM degradation and inflammation
(51). In summary, these results
indicated that circRNA_Atp9b, which functions as a sponge for
miR-138-5p, promoted the progression of OA by regulating ECM
catabolism and chondrocyte inflammation.
Another study suggested that in the cartilage tissue
of an OA mouse model treated with IL-1β, circRNA.33186 was markedly
upregulated (52). After knocking
down circRNA.33186, the expression of Col II increased and the
expression of MMP-13 decreased. At the same time, knockdown of
circRNA.33186 promoted the proliferation of chondrocytes treated
with IL-1β and inhibited apoptosis (52). Further investigation demonstrated
that circRNA.33186 could directly bind to and inhibit miR-127-5p,
increase the expression of MMP-13 and promote OA (52). These results suggested that
circRNA.33186 is a potential drug target for OA treatment.
Chen et al (53) reported that circRNA-UBE2G1 and
hypoxia-inducible factor (HIF)-1α were overexpressed in OA
cartilage tissues, while the expression level of miR-373 was
downregulated compared with healthy cartilage tissues. Further
research indicated that circRNA-UBE2G1 acts as a ceRNA to bind to
miR-373 and HIF-1α is the target of miR-373. Thus, circRNA-UBE2G1
promotes OA progression by regulating the miR-373/HIF-1α axis
(53).
Another recent study suggested that circ_0136474 is
involved in the pathological process of OA (54). RT-qPCR detection revealed that
circ_0136474 and DNA methyltransferase 3A (DNMT3A) were both
overexpressed in OA cartilage tissue, while the expression of
miR-766-3p was reduced compared with healthy cartilage tissues
(54). Further research indicated
that DNMT3A is the target of miR-766-3p. Inhibiting the expression
of circ_0136474 was able to reduce oxidative damage in OA
chondrocytes by regulating the miR-766-3p/DNMT3A axis (54).
Ni et al (55) indicated that circPSM3 is highly
expressed in OA chondrocytes. Further research suggested that
circPSM3 inhibits the proliferation and differentiation of OA
chondrocytes by targeting miRNA-296-5p, while knocking down
circPSM3 promoted the proliferation and differentiation of OA
chondrocytes. CircPSM3 may be a potential therapeutic target for OA
treatment. Zhang et al (56) indicated that circRNA-CDR1as was
markedly upregulated in OA chondrocytes compared with control
chondrocytes and negatively correlated with miR-641. Furthermore,
circRNA-CDR1as promoted cartilage ECM metabolism and inflammation
by inhibiting the expression of miR-641(56). Zhu et al (57) demonstrated that circGCN1L1 and
TNF-α are upregulated in OA, while miR-330-3p is downregulated
compared with healthy cartilage tissues. A further study indicated
that circGCN1L1 promoted the expression of TNF-α by inhibiting the
expression of miR-330-3p, while overexpression of TNF-α promoted
cell inflammation and reduced the anabolism of ECM (57) (Table
I).
 | Table IRoles of circRNAs in the promotion of
the pathogenesis of osteoarthritis. |
Table I
Roles of circRNAs in the promotion of
the pathogenesis of osteoarthritis.
| CircRNA | Target | Biological
function | Refs. |
|---|
| circRNA-CER | miR-136 | Degradation of ECM,
promotion of inflammation | (42) |
| circRNA-MSR | miR-875 | Degradation of ECM,
promotion of inflammation | (49) |
|
hsa_circ_0005105 | miR-26a | Degradation of
ECM | (50) |
| circRNA_Atp9b | miR-138-5p | Degradation of ECM,
promotion of inflammation | (51) |
| circRNA.33186 | miR-127-5p | Degradation of
ECM | (52) |
| circRNA-UBE2G1 | miR-373 | Degradation of ECM,
promotion of apoptosis | (53) |
| circ_0136474 | miR-766-3p | Degradation of ECM,
promotion of apoptosis | (54) |
| circPSM3 | miR-296-5p | Inhibition of cell
proliferation and differentiation | (55) |
| circRNA-CDR1as | miR-641 | Degradation of ECM,
promotion of inflammation | (56) |
| circGCN1L1 | miR-330-3p | Reduction of ECM
generation, promotion of inflammation | (57) |
These studies demonstrated that circRNAs serve an
important role in the development of OA. CircRNAs act on different
signaling molecules to promote chondrocyte apoptosis, ECM
degradation and inflammation, and accelerate the process of OA.
Inhibiting the expression of these signaling molecules may provide
a novel direction for the treatment of OA.
5. Inhibition of OA pathogenesis
Although numerous circRNAs are known to promote the
pathogenesis of OA, studies have also suggested that circRNAs such
as ciRS-7, circSERPINE2 and hsa_circ_0045714 are able to inhibit
the pathogenesis of OA.
Zhou et al (58) found that the expression of ciRS-7
in blood samples of patients with OA was decreased, while the
expression of miR-7 was increased compared with blood from healthy
controls. After IL-1β treatment of chondrocytes, cell proliferation
was inhibited, and the release of inflammatory factors was promoted
(58). The expression of ciRS-7
was downregulated in chondrocytes treated with IL-1β, while the
expression of miR-7 was upregulated. Knockdown of ciRS-7 and
upregulation of miR-7 promoted inflammation and apoptosis of OA
chondrocytes, which indicated that ciRS-7 exhibits a protective
effect, while miR-7 promotes the development of OA (58). It was suggested that ciRS-7, a
ceRNA, inhibits the expression of miR-7 and reduces apoptosis and
inflammatory response of OA chondrocytes (58). This may provide novel insights for
the treatment of OA.
As a member of the serine protease inhibitor
superfamily, SERPINE2 is able to modify the activity of MMPs and
maintain ECM homeostasis (59-61).
Exons 2-4 of the SERPINE2 gene may produce circSERPINE2 through
reverse splicing, which mainly includes the protein-coding sequence
of SERPINE2 mRNA (59-61).
Shen et al (62) reported
that circSERPINE2 was low in the diseased cartilage tissue of
patients with OA, while miR-1271 was highly expressed. miR-1271 was
reported to be negatively correlated with the expression of SOX9,
aggrecan and COL2A1(62). The
binding site of miR-1271 within ETS-related gene (ERG) is highly
conserved, and miR-1271 is able to inhibit the expression of ERG
(62). ERG serves an important
role in inducing the permanent differentiation of chondrocytes into
articular cells (63). Therefore,
lack of ERG expression may increase the susceptibility of joints to
damage. Inhibition of miR-1271 induces ERG overexpression and
exhibited a protective role in OA (62). The results suggested that
circSERPINE2 acts as a miR-1271 sponge that is able to inhibit
chondrocyte apoptosis, promote ECM synthesis and prevent ECM
degradation, thereby delaying the OA process (62). The circSERPINE2-miR-1271-ERG axis
provides a novel direction for OA therapy.
Liu et al (42) indicated that hsa_circ_0045714 may
have miR-193b binding sites, therefore, hsa_circ_0045714 may serve
a role in OA by regulating miR-193b. Insulin-like growth factor
(IGF)1 receptor (IGF1R), a member of the IGF family, is able to
activate the PI3K and MAPK signaling pathways after binding to IGF1
and IGF2, and regulate cell proliferation, differentiation and
apoptosis through autophosphorylation (64). It has been demonstrated that the
division and proliferation of chondrocytes and the synthesis of Col
II and proteoglycan are inseparable from IGF1R (65), which indicates that IGF1R serves a
key role in chondrocyte differentiation. A dual-luciferase reporter
gene assay confirmed that IGF1R is the key target gene of miR-193b.
It has been indicated that overexpression of hsa_circ_0045714 is
able to inhibit the transcriptional activity of miR-193b, thereby
upregulating the expression of IGF1R. IGF1R has been indicated to
exhibit a similar function to hsa_circ_0045714 (65,66).
IGF1R promotes the expression of Col II and proteoglycans and
upregulated the proliferation of chondrocytes (65,66).
miR-193b exhibits the opposite effect to IGF1R, as miR-193b
inhibited the expression of Col II and proteoglycan, downregulated
the proliferation of chondrocytes and promoted apoptosis (66). These results have suggested that
hsa_circ_0045714, a ceRNA of miR-193b, inhibits the expression of
miR-193b and promotes the expression of the miR193b target gene
IGF1R, thereby promoting the synthesis of ECM and the proliferation
of chondrocytes and inhibiting the apoptosis of chondrocytes. These
results provide a novel direction for molecular targeted therapies
of OA (Table II).
 | Table IIRoles of circRNAs in inhibiting the
pathogenesis of osteoarthritis. |
Table II
Roles of circRNAs in inhibiting the
pathogenesis of osteoarthritis.
| CircRNA | Target | Biological
function | Refs. |
|---|
| ciRS-7 | miR-7 | Inhibition of
apoptosis and inflammation | (58) |
| circSERPINE2 | miR-1271 | Inhibition of
apoptosis, promotion of ECM synthesis | (62) |
|
hsa_circ_0045714 | miR-193b | Promotion of cell
proliferation | (66) |
These studies suggest that circRNAs play an
important role in the development of OA. CircRNAs act on different
signaling molecules to promote the proliferation of chondrocytes,
inhibit ECM degradation, reduce inflammation and delay the process
of OA. Promoting the expression of these signaling molecules may
provide a novel direction for delaying the progression of OA.
6. Conclusions and perspectives
OA is one of the most common degenerative joint
diseases, with pain and activity limitation being the major
symptoms. It seriously affects the quality of life of patients and
places a heavy economic burden on families and society (7). Despite extensive research on OA, its
pathogenesis remains to be fully elucidated and therefore, OA
cannot yet be completely cured. The roles of circRNAs as regulatory
molecules in the pathogenesis of OA have attracted increasing
attention from scientists. These differentially expressed circRNAs
provide a novel direction for the diagnosis and treatment of
OA.
In addition, various signaling pathways are involved
in the pathological process of OA, including PI3K/Akt and MAPK/ERK
signaling pathways (64). CircRNAs
exhibit a wide range of biological roles and most of their targets
are essential molecules in cell signaling. Identification of novel
circRNA-related signaling molecules will aid in gaining a deeper
understanding of the role of circRNAs in OA, in addition to
providing a theoretical basis for the targeted therapy of
circRNAs.
Collectively, the present review provided a
comprehensive resource that explains the important role of circRNAs
in the pathogenesis of OA and reveals the interaction among various
circRNAs and miRNAs in OA. Taken together, circRNAs serve a
potential role in cell signaling and the pathogenesis of OA.
Although experiments have demonstrated the prospective value of
circRNAs in the treatment of OA, further research is required,
focusing on their potential widespread use as biomarkers for OA
diagnosis and on developing novel therapeutic targets for OA. The
goal of this research will be to use these targets to develop novel
drugs, delay the progression of OA and improve the quality of life
of patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JW and YZ drafted and revised the manuscript. BY,
CW, YG and XJ contributed to manuscript conception. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taruc-Uy RL and Lynch SA: Diagnosis and
treatment of osteoarthritis. Prim Care. 40:821–836. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Felson DT, Naimark A, Anderson J, Kazis L,
Castelli W and Meenan RF: The prevalence of knee osteoarthritis in
the elderly. The framingham osteoarthritis study. Arthritis Rheum.
30:914–918. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oliveria SA, Felson DT, Reed JI, Cirillo
PA and Walker AM: Incidence of symptomatic hand, hip, and knee
osteoarthritis among patients in a health maintenance organization.
Arthritis Rheum. 38:1134–1141. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Murphy L, Schwartz TA, Helmick CG, Renner
JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G and Jordan JM:
Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum.
59:1207–1213. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang W: Risk factors of knee
osteoarthritis-excellent evidence but little has been done.
Osteoarthritis Cartilage. 18:1–2. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu CX and Sun S: An emerging role for
circular RNAs in osteoarthritis. Yonsei Med J. 59:349–355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Kos A, Dijkema R, Arnberg AC, van der
Meide PH and Schellekens H: The hepatitis delta (delta) virus
possesses a circular RNA. Nature. 323:558–560. 1986.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7(e30733)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vicens Q and Westhof E: Biogenesis of
circular RNAs. Cell. 159:13–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y and Wang Z: Efficient backsplicing
produces translatable circular mRNAs. RNA. 21:172–179.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen I, Chen CY and Chuang TJ: Biogenesis,
identification, and function of exonic circular RNAs. Wiley
Interdiscip Rev RNA. 6:563–579. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Conn SJ, Pillman KA, Toubia J, Conn VM,
Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA and
Goodall GJ: The RNA binding protein quaking regulates formation of
circRNAs. Cell. 160:1125–1134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Huang S, Yang B, Chen BJ, Bliim N,
Ueberham U, Arendt T and Janitz M: The emerging role of circular
RNAs in transcriptome regulation. Genomics. 109:401–407.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen X, Han P, Zhou T, Guo X, Song X and
Li Y: circRNADb: A comprehensive database for human circular RNAs
with protein-coding annotations. Sci Rep. 6(34985)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Granados-Riveron JT and Aquino-Jarquin G:
The complexity of the translation ability of circRNAs. Biochim
Biophys Acta. 1859:1245–1251. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ebert MS and Sharp PA: MicroRNA sponges:
Progress and possibilities. RNA. 16:2043–2050. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abdelmohsen K, Kuwano Y, Kim HH and
Gorospe M: Posttranscriptional gene regulation by RNA-binding
proteins during oxidative stress: Implications for cellular
senescence. Biol Chem. 389:243–255. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin QF, Yang L, Zhang Y, Xiang JF, Wu YW,
Carmichael GG and Chen LL: Long noncoding RNAs with snoRNA ends.
Mol Cell. 48:219–230. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen CY and Sarnow P: Initiation of
protein synthesis by the eukaryotic translational apparatus on
circular RNAs. Science. 268:415–417. 1995.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of circRNAs. Mol
Cell. 66:9–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, function
and role in human diseases. Front Mol Biosci. 4(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-New member of noncoding RNA with novel functions. Exp Biol Med
(Maywood). 242:1136–1141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li H, Yang HH, Sun ZG, Tang HB and Min JK:
Whole-transcriptome sequencing of knee joint cartilage from
osteoarthritis patients. Bone Joint Res. 8:288–301. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xiang S, Li Z, Bian Y and Weng X: RNA
sequencing reveals the circular RNA expression profiles of
osteoarthritic synovium. J Cell Biochem. 120:18031–18040.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xiao K, Xia Z, Feng B, Bian Y, Fan Y, Li
Z, Wu Z, Qiu G and Weng X: Circular RNA expression profile of knee
condyle in osteoarthritis by illumina HiSeq platform. J Cell
Biochem. 120:17500–17511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J
and Ao Y: Circular RNA related to the chondrocyte ECM regulates
MMP13 expression by functioning as a MiR-136 ‘Sponge’ in human
cartilage degradation. Sci Rep. 6(22572)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafari M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Marchev AS, Dimitrova PA, Burns AJ, Kostov
RV, Dinkova-Kostova AT and Georgiev MI: Oxidative stress and
chronic inflammation in osteoarthritis: Can NRF2 counteract these
partners in crime? Ann NY Acad Sci. 1401:114–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sun MM, Beier F and Pest MA: Recent
developments in emerging therapeutic targets of osteoarthritis.
Curr Opin Rheumatol. 29:96–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Laiguillon MC, Houard X, Bougault C,
Gosset M, Nourissat G, Sautet A, Jacques C, Berenbaum F and Sellam
J: Expression and function of visfatin (Nampt), an adipokine-enzyme
involved in inflammatory pathways of osteoarthritis. Arthritis Res
Ther. 16(R38)2014.PubMed/NCBI View
Article : Google Scholar
|
|
48
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9(e1003777)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu Q, Zhang X, Hu X, Yuan L, Cheng J,
Jiang Y and Ao Y: Emerging roles of circRNA related to the
mechanical stress in human cartilage degradation of osteoarthritis.
Mol Ther Nucleic Acids. 7:223–230. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu Y, Zhang Y, Zhang Y and Wang JJ:
CircRNA hsa_circ_0005105 upregulates NAMPT expression and promotes
chondrocyte extracellular matrix degradation by sponging miR-26a.
Cell Biol Int. 41:1283–1289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhou ZB, Du D, Huang GX, Chen A and Zhu L:
Circular RNA Atp9b, a competing endogenous RNA, regulates the
progression of osteoarthritis by targeting miR-138-5p. Gene.
646:203–209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhou ZB, Huang GX, Fu Q, Han B, Lu JJ,
Chen AM and Zhu L: circRNA.33186 contributes to the pathogenesis of
osteoarthritis by sponging miR-127-5p. Mol Ther. 27:531–541.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen G, Liu T, Yu B, Wang B and Peng Q:
CircRNA-UBE2G1 regulates LPS-induced osteoarthritis through
miR-373/HIF-1a axis. Cell Cycle. 19:1696–1705. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhu H, Zhu S, Shang X, Meng X, Jing S, Yu
L and Deng Y: Exhausting circ_0136474 and restoring miR-766-3p
attenuate chondrocyte oxidative injury in IL-1β-induced
osteoarthritis progression through regulating DNMT3A. Front Genet.
12(648709)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ni JL, Dang XQ and Shi ZB: CircPSM3
inhibits the proliferation and differentiation of OA chondrocytes
by targeting miRNA-296-5p. Eur Rev Med Pharmacol Sci. 24:3467–3475.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang W, Zhang C, Hu C, Luo C, Zhong B and
Yu X: Circular RNA-CDR1as acts as the sponge of microRNA-641 to
promote osteoarthritis progression. J Inflamm (Lond).
17(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhu H, Hu Y, Wang C, Zhang X and He D:
CircGCN1L1 promotes synoviocyte proliferation and chondrocyte
apoptosis by targeting miR-330-3p and TNF-α in TMJ osteoarthritis.
Cell Death Dis. 11(284)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhou X, Jiang L, Fan G, Yang H, Wu L,
Huang Y, Xu N and Li J: Role of the ciRS-7/miR-7 axis in the
regulation of proliferation, apoptosis and inflammation of
chondrocytes induced by IL-1β. Int Immunopharmacol. 71:233–240.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Boulaftali Y, François D, Venisse L,
Jandrot-Perrus M, Arocas V and Bouton MC: Endothelial protease
nexin-1 is a novel regulator of a disintegrin and metalloproteinase
17 maturation and endothelial protein C receptor shedding via furin
inhibition. Arterioscler Thromb Vasc Biol. 33:1647–1654.
2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pagliara V, Adornetto A, Mammì M, Masullo
M, Sarnataro D, Pietropaolo C and Arcone R: Protease Nexin-1
affects the migration and invasion of C6 glioma cells through the
regulation of urokinase plasminogen activator and matrix
metalloproteinase-9/2. Biochim Biophys Acta. 1843:2631–2644.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rao JS, Kahler CB, Baker JB and Festoff
BW: Protease nexin I, a serpin, inhibits plasminogen-dependent
degradation of muscle extracellular matrix. Muscle Nerve.
12:640–646. 1989.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shen S, Wu Y, Chen J, Xie Z, Huang K, Wang
G, Yang Y, Ni W, Chen Z, Shi P, et al: CircSERPINE2 protects
against osteoarthritis by targeting miR-1271 and ETS-related gene.
Ann Rheum Dis. 78:826–836. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jones SW, Watkins G, Le Good N, Roberts S,
Murphy CL, Brockbank SM, Needham MR, Read SJ and Newham P: The
identification of differentially expressed microRNA in
osteoarthritic tissue that modulate the production of TNF-alpha and
MMP13. Osteoarthritis Cartilage. 17:464–472. 2009.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang Y, Moerkens M, Ramaiahgari S, de
Bont H, Price L, Meerman J and van de Water B: Elevated
insulin-like growth factor 1 receptor signaling induces
antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling
routes. Breast Cancer Res. 13(R52)2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD,
Tang D, Sheu TJ, Li TF, Shi Q and Wang YJ: IGF-1 regulation of type
II collagen and MMP-13 expression in rat endplate chondrocytes via
distinct signaling pathways. Osteoarthritis Cartilage. 17:100–106.
2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Li BF, Zhang Y, Xiao J, Wang F, Li M, Guo
XZ, Xie HB, Xia H and Chen B: Hsa_circ_0045714 regulates
chondrocyte proliferation, apoptosis and extracellular matrix
synthesis by promoting the expression of miR-193b target gene
IGF1R. Hum Cell. 30:311–318. 2017.PubMed/NCBI View Article : Google Scholar
|