1. Introduction
In recent years, neurotrophins (NTs), particularly
brain-derived neurotrophic factor (BDNF), have achieved varying
degrees of success in various diseases, such as spinal cord
injuries, neurodegenerative diseases and tumors (1-4).
NTs are also effective against immune system diseases and are
considered a novel therapeutic strategy to manage allergies or
inflammation.
BDNF, a prominent member of the NT family, has been
extensively studied for autoimmune inflammatory diseases. Previous
studies have indicated that circulating BDNF was mainly produced
from lymphocytes, macrophages, vascular endothelial cells and other
immune cells (5,6). BDNF promotes the survival and
proliferation of lymphocytes by binding to cell membrane receptors
in an autocrine or paracrine manner (7,8). Of
note, the production of BDNF varies among different T lymphocyte
subpopulations (5). BDNF has been
linked to systemic lupus erythematosus (SLE) (9), experimental autoimmune
encephalomyelitis (EAE) (10),
multiple sclerosis (MS) (11) and
other autoimmune inflammatory diseases. SLE involves dysregulation
of T and B lymphocyte networks (12). A previous study by our group
reported on decreased circulatory levels of BDNF in SLE, which
correlated with the number of CD4+ T and CD8+
T lymphocytes (9). So far, the
pathogenesis of SLE has remained elusive. A general consensus is
that the interaction of susceptible genes with certain
environmental factors disrupts the regulatory networks of T and B
lymphocytes, which promotes the production of characteristic
pathogenic antibodies and immune complexes. In addition, T helper
(Th) and T follicular helper (Tfh) cells are excessively produced,
while the production of regulatory T cells (Treg) and inactivation
of cytotoxic T cells are suppressed (12). The balance of inflammatory
cytokines becomes disturbed and interleukin-6 (IL-6), IL-8, IL-10,
IL-17, interferon-γ (IFN-γ) and other cytokines are significantly
upregulated (13). The
antigen-presenting cell-mediated balance between pro-inflammatory
and anti-inflammatory cytokines is vital for the differentiation of
CD4+ T-cell lineages (including Th1, Th2, Th17, Tfh and
Treg), which is also closely linked to the pathogenesis of SLE
(14). It may be speculated that
BDNF affecting the immune function of lymphocytes is a major factor
in the pathogenesis of SLE.
2. BDNF and its receptors
BDNF, which exists as a dimer, was first thought to
only be present in the central nervous system (CNS) and synthesized
by astrocytes; however, it has now also been detected in peripheral
blood. BDNF dimers non-covalently bind to the membrane receptors to
exert their biological effects. In general, both pre- and mature
BDNF are able to bind and signal, albeit using two different
families of receptors, namely the low-affinity p75 NT receptor
(p75NTR) ‘panneurotrophic factor’ receptors (15-17)
and the high-affinity tropomyosin associated kinase (TRK)
receptors. BDNF binds to the high-affinity TrkB (tyrosine kinase B)
receptor of the TRK family (18),
while p75NTR belongs to the tumor necrosis factor (TNF) receptor
superfamily (17). p75NTR is
thought to be a co-receptor for TrkB (19); however, its function has remained
to be fully elucidated (20).
Studies suggest that p75NTR participates in pro-apoptotic processes
during cell development (21),
mediates the migration of Schwann cells (22) and determines the fate of certain
non-neural cells (23). p75NTR,
which was previously known for its weak binding, binds with high
affinity to BDNF precursor (pro-BDNF), and subsequently, the
resulting heterodimer binds to Sortilin, causing neuronal apoptosis
and inhibition of axonal growth (24).
The other receptor, TrkB, is a transmembrane
receptor with a tyrosine kinase domain encoded by the Trk
proto-oncogene, which exists as either the full-length gp145TrkB or
the truncated gp95TrkB (18). TrkB
activation by BDNF stimulates neuronal activity, which is necessary
for memory development and maintenance. The full-length gp145TrkB
has a complex structure, including an extracellular binding domain
for BDNF, a transmembrane region and a cytosolic tyrosine-kinase
domain essential for BDNF signaling (Fig. 1). The full-length gp145TrkB, upon
autophosphorylation, exposes substrate binding sites for SHC,
growth factor receptor-bound protein 2, ATP and phospholipase C
(PLC)γ. In general, gp145TrkB activation triggers three downstream
tyrosine kinase-mediated pathways, PLCγ1/PKC, MAPK-ERK and PI3/Akt,
which regulate cell survival and differentiation (25). On the contrary, the truncated
gp95TrkB, devoid of the tyrosine kinase domain, has a negative
effect, which binds and internalizes BDNF without
autophosphorylation (26). Of
note, truncated gp95trkB may still mediate BDNF-induced cell
proliferation but the mechanism has remained elusive. In this
manner, TrkB affects both cell survival and BDNF production.
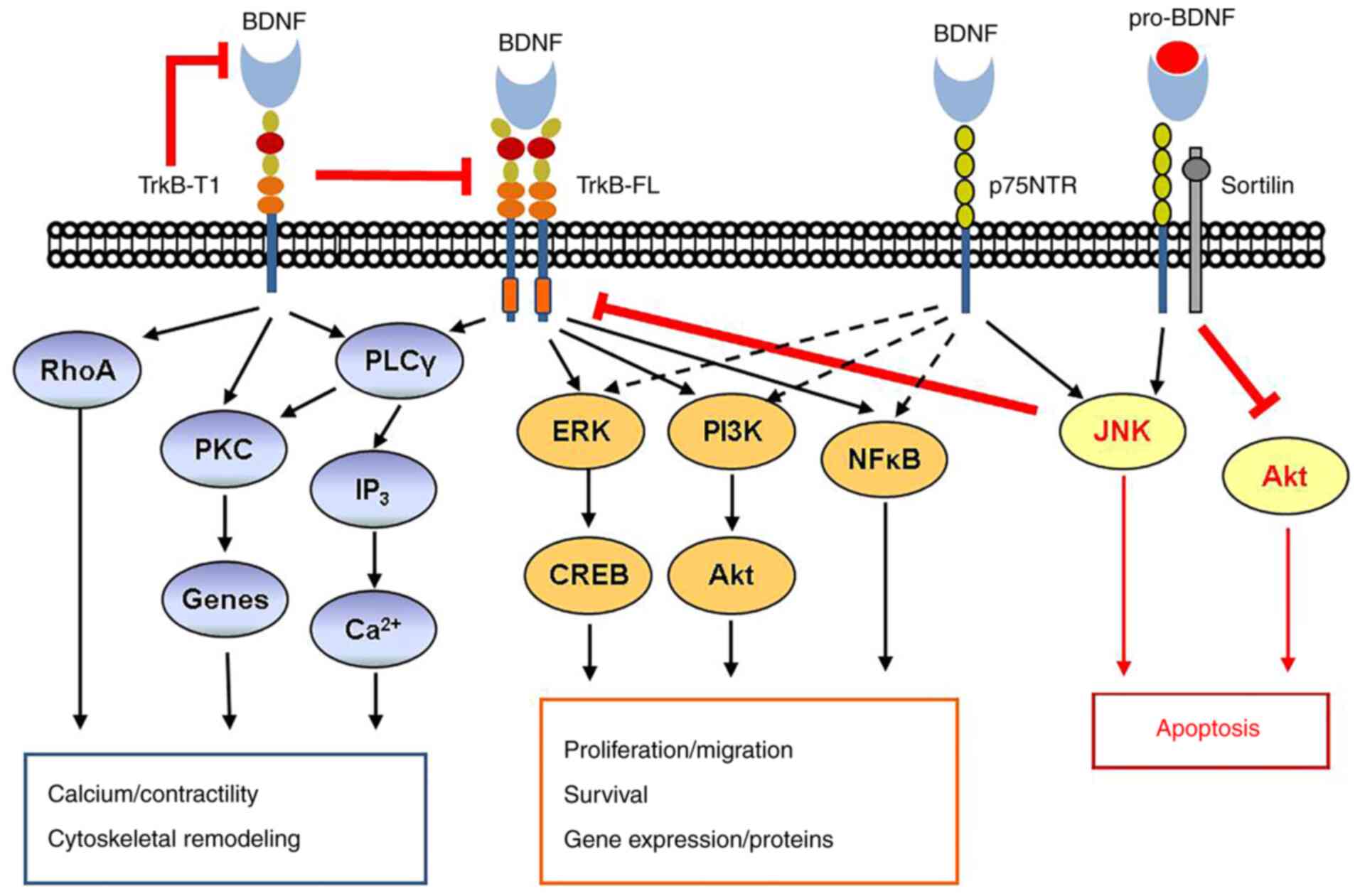 | Figure 1BDNF signaling pathway. BDNF
signaling via TrkB and p75NTR is complex. Activation of TrkB-FL by
mature BDNF classically leads to activation of PLCγ and both the
ERK pathway and the PI3K/Akt pathway promote cell proliferation and
survival. In addition, TrkB may act through the NFκB pathway. In
contrast to TrkB-FL, the truncated TrkB-T1 may be both inhibitory
and activating via RhoA and PKC, resulting in cytoskeletal
remodeling, gene modulation and other effects. The low-affinity
p75NTR, when activated by mature BDNF, typically activates the
pathway including TrkB-FL, but importantly, it may be inhibited via
either the JNK or Akt pathways. This is particularly the case when
p75NTR is activated by pro-BDNF and forms a ternary complex with
the adaptor protein sortilin. BDNF, brain-derived neurotrophic
factor; TrkB-FL, tyrosine kinase B full-length; PLC, phospholipase
C; PKC, protein kinase C; NTR, neurotrophin receptor; CREB,
C-response binding element; RhoA, ras homolog gene family, member
A. |
In the TrkB-p75NTR heterodimer interaction, TrkB
inhibits p75NTR-induced apoptosis, while p75NTR increases the
affinity of BDNF to TrkB, which promotes growth and survival of
nerve cells (27).
3. Peripheral blood BDNF
It is becoming increasingly clear that BDNF has more
functions than that of a growth factor. It is intricately
intertwined with immunity, inflammation and numerous other
regulatory pathways. In recent years, numerous studies have
indicated that, apart from the CNS, BDNF also exists in peripheral
blood, where it affects the activities of non-neuronal cells.
Lymphocytes, monocytes and vascular endothelial cells are
considered to be the major sources of circulatory BDNF (5,6). In
addition, platelets may contain BDNF (28). BDNF is expressed by both
CD4+ (Th1 and Th2) and CD8+ T cells.
Activated lymphocytes further increase the production of BDNF. IL-6
and TNF-α may specifically enhance monocytes to secrete BDNF
(29). BDNF is known to promote
the maturation, proliferation and activation of T and B lymphocytes
and have an anti-apoptotic effect on T lymphocytes (7,8). In
addition to the expression in peripheral lymphocytes (8,30),
TrkB is also expressed in all thymocytes (5). Of note, TrkB mRNA and protein levels
have an inverse correlation with the maturation and differentiation
of thymocytes; TrkB expression is upregulated in
CD4-CD8- immature thymocytes and then
progressively declines in CD8+ and CD4+
single-positive and CD4+CD8+ mature
thymocytes. The BDNF-TrkB interaction stimulates TrkB
autophosphorylation, which in turn upregulates the c-fos gene in
CD4-CD8- cells to improve thymocyte survival
(5). Functionally deficient TrkB
mice were revealed to have an increased number of pyknotic nuclei
in the thymus on the 15th postnatal day, suggesting an increase in
apoptotic lymphocytes, particularly in the cortical area. Likewise,
Schuhmann et al (31)
demonstrated that BDNF-deficient mice had significantly lower B
lymphocytes in the blood, spleen and bone marrow. This development
was specific to the pre-BII stage of bone marrow B cells. In
addition, the total number of thymus cells decreased. Another study
indicated that exogenous addition of BDNF to a serum-free mature
B-cell culture reduced cell apoptosis by 15% (30). In line with this, Garcia-Suarez
et al (32) reported
massive lymphocyte apoptosis in the thymus of TrkB-deficient mice,
while an improvement in the survival rate of thymic precursor cells
was observed after treatment with BDNF (33). Furthermore, it was reported that
cytokines secreted by Th2 are able to promote BDNF synthesis, which
in turn downregulates the secretion of interferon and IL-22 by Th1
without affecting the secretion of IL-4, IL-10 from Th2. Likewise,
IL-6 and TNF-α are able to enhance BDNF secretion from peripheral
blood monocytes (29). Overall,
peripheral blood BDNF has a complex regulatory network involving
lymphocytes and anti- and pro-inflammatory cytokines and performs a
broad spectrum of activities in non-neural tissues, apart from its
regulatory role in inflammation and autoimmune demyelination in the
CNS (33-38).
Based on these results, it is indicated that BDNF is a key factor
in autoimmune and inflammatory diseases.
4. BDNF in autoimmune inflammatory
diseases
Emerging evidence suggests that BDNF, as an immune
function regulator, is linked to numerous autoimmune and
inflammatory diseases, which is discussed in the following
subsections (Table I).
 | Table IBDNF and autoimmune inflammatory
diseases. |
Table I
BDNF and autoimmune inflammatory
diseases.
| Autoimmune
disease | Serum or
tissue | BDNF level in
disease | (Refs.) |
|---|
| Experimental
autoimmune encephalomyelitis; multiple sclerosis | T cells in the
CNS | Strong expression
of BDNF in T cells near demyelinating lesions, suggesting that BDNF
inhibited further parenchymal injury and participated in
neuroinflammatory responses during repair. However, BDNF partially
resists activation of T lymphocyte apoptosis, which may be the
nature of chronic inflammatory processes. | (11,39,40) |
| CD | Local intestinal
lesions | Local intestinal
lesions in patients with CD with strong expression of BDNF and
TrkB, and BDNF attenuated the apoptosis of glial cells to a small
extent, protecting the integrity of the bowel. | (41-43) |
| Ulcerative
colitis | Local intestinal
lesions | Massive
inflammation was correlated with decreased neurotrophin
immunoreaction in nerve structures and there was a tendency toward
increased neurotrophin production in lamina propria cells. | (44) |
| Pulmonary
sarcoidosis | T cells in
bronchoalveolar lavatory fluid | NGF and BDNF in
CD4+ and CD8+ T lymphocytes in the lung were
increased in pulmonary sarcoidosis. Expression of TrkB receptors
was increased. There was a significant correlation between
lymphocytosis, radiological stage and CD4 or CD8 NT
expression. | (45,46) |
| SSc | Serum | Low serum BDNF
level, particularly in diffuse SSc and in patients with pulmonary
arterial hypertension or anti-Scl-70 antibodies. | (47) |
| pSS | Serum | Decreased BDNF
level in pSS with ILD. BDNF was correlated with the activation of B
and T cells. | (49,50) |
| RA | Plasma and synovial
tissue/SF | Higher plasma BDNF
level in RA, BDNF level in synovial tissue/SF was not correlated
with the number of inflammatory cells or TNF-α or ESR, but plasma
BDNF level was decreased 14 weeks after the initiation of anti-TNF
therapy. | (51) |
| SpA | Plasma and synovial
tissue/SF | mRNA transcripts of
all NTs and receptors were highly expressed in the inflamed
synovium, correlating with vascularity and lymphoid aggregates.
BDNF level was higher in the SF of patients with SpA than in
OA. | (52,53) |
|
SLE | Serum and
plasma | Serum/plasma BDNF
levels in SLE are correlated with SLEDAI scores and clinical
parameters: C3, C4 and T-cell subsets. Serum BDNF levels may be
decreased in active SLE. | (9,55,59,60) |
BDNF in EAE and MS
EAE is histologically and clinically similar to MS,
which was established in an animal model to study autoimmune
demyelination (11,39). Myelin-reactive T cells of the CNS
produce and release BDNF to promote post-traumatic tissue repair
(39). In both EAE and MS, T cells
near demyelinating lesions significantly increase the expression of
BDNF to inhibit the progression of neuron injury (39). By contrast, BDNF binding to
TrkB-expressing T cells leads to evasion of T-lymphocyte apoptosis,
which is a key event of the chronic inflammatory process in which T
cells are involved (39).
Therefore, it is worthwhile determining whether T cell-derived BDNF
promotes neuronal recovery or promotes the persistence of
inflammation. A study reported that mice deficient in immune cells
producing BDNF displayed attenuated immune response in the acute
phase of EAE, which reduced further brain parenchymal injury but
enhanced axonal loss in the chronic phase progressing into
disability. Of note, transfection of T cells expressing BDNF
strongly reduced brain damage in EAE and protected axons (40). Elevated serum BDNF levels have also
been associated with nerve repair in patients with MS (11).
BDNF in inflammatory bowel diseases
and pulmonary sarcoidosis
Steinkamp et al (41) reported increased expression of BDNF
and TrkB in local intestinal lesions of Crohn's disease (CD). In
CD, enteric glial cells (EGCs) maintain the integrity of the bowel,
while a loss of EGCs causes severe inflammation of the intestine.
BDNF attenuates apoptosis of glial cells, while BDNF-neutralizing
antibodies markedly increase apoptosis. Therefore, it is speculated
that BDNF protects from CD by decreasing the apoptosis of EGCs.
Glial-derived neurotrophic factor promoting an anti-apoptotic loop
in EGCs has a similar role to that in CD (42,43).
Johansson et al (44)
reported that NTs may also be involved in ulcerative colitis, in
which massive inflammation exhibited a strong correlation with
decreased NT immunoreaction. In addition, the production of NT
increased in lamina propria cells. In pulmonary sarcoidosis, nerve
growth factor (NGF) expression in alveolar macrophages increased
with the increase in expression of NGF and BDNF in CD4+
and CD8+ T cells. An increased expression of TrkA, TrkB
and TrkC receptors was also noticed. Furthermore, there was a
significant correlation between the expression of NTs in CD4 or CD8
cell populations and the CD4:CD8 ratio, lymphocyte number, and
radiological staging (45,46). Overall, these results suggested
that increased levels of NTs in bronchoalveolar lavage fluid
modulate the functions of immune cells in pulmonary
sarcoidosis.
BDNF in connective tissue
diseases
The role of BDNF in autoimmune diseases has been
studied extensively. In systemic sclerosis (SSc), which is a
microvascular disease, serum BDNF levels are decreased (47), particularly in diffuse SSc and in
SSc with pulmonary arterial hypertension or anti-Scl-70 antibodies.
Of note, BDNF and TrkB are also synthesized by capillary and
arterial endothelial cells (48).
Therefore, decreased BDNF levels in SSc may also be linked to
microvascular disease and oxidative stress. Fauchais et al
(49) and Li et al
(50) studied serum BDNF levels in
patients with primary Sjogren's syndrome (pSS). They detected high
serum BDNF levels in pSS, which were correlated with the extent of
systemic involvement. NGF and BDNF levels also correlated with the
activation of B and T cells; specifically, the BDNF concentration
was correlated with CD4+ T-cell activation assessed as
human leukocyte antigen DR expression. Li et al (50) reported that, compared with patients
with simple pSS or healthy controls, patients with pSS with
interstitial lung disease (ILD) had lower serum BDNF levels, which
may be used as a potential biomarker of ILD secondary to pSS. In
rheumatoid arthritis (RA), plasma BDNF levels were increased;
however, the concentration did not correlate with the number of
inflammatory cells, the concentration of TNF-α, erythrocyte
sedimentation rate or white blood cell counts in synovial tissue
(51). Of note, plasma BDNF levels
decreased after 14 weeks of anti-TNF therapy. In spondyloarthritis
(SPA), RA and osteoarthritis (OA), mRNA transcripts of all NTs and
receptors were expressed in the inflamed synovium. At the protein
level, BDNF was significantly higher in the synovial fluid of
patients with SPA than in those with OA. Immunohistochemistry
demonstrated that TrkA and NGF were highly expressed in the
inflamed synovium of patients with SPA, correlating with
vascularity and lymphoid aggregates. Additionally, the
immunoreactivity of all receptors was significantly decreased after
infliximab treatment (52,53).
BDNF in SLE
Recently, serum BDNF levels in SLE have been
receiving increasing attention (54-59)
and have been linked to various clinical parameters, suggesting the
involvement of BDNF in the pathogenesis and progression of SLE.
Fauchais et al (58)
indicated that the serum levels of BDNF and the SLE disease
activity index (SLEDAI) were not correlated. Although BDNF levels
were reduced after treatment, they remained higher in patients with
SLE than in healthy controls. They speculated that BDNF levels were
independent of the Th1 and Th2 profile. Furthermore, BDNF levels
were the lowest in a subgroup of lupus anticoagulant-positive
patients, which may have been due to antiphospholipid
antibody-induced vascular lesions and oxidative stress, as in SSc
(47). Ikenouchi et al
(56) also reported that serum
BDNF levels did not correlate with SLEDAI. However, significantly
increased BDNF levels exhibited a good correlation with psychiatric
symptoms, including acute state of confusion, anxiety disorder,
cognitive dysfunction, mood disorder and psychosis in
neuropsychiatric syndrome of SLE (NPSLE). Ikenouchi-Sugita et
al (54) and Ikenouchi et
al (56) suggested that plasma
BDNF levels and the catecholamine metabolites have a higher
predictive value regarding the severity of psychotic symptoms in
SLE, offering an alternate diagnosis to steroid-induced psychosis.
Of note, Tamashiro et al (55) demonstrated that increased plasma
BDNF levels did not correlate with CNS lesions, which contradicts
previous studies. In addition, they proved a negative correlation
between plasma BDNF levels and SLEDAI and a positive correlation
with the levels of complements and the numbers of circulatory
lymphocytes. Zheng et al (59) suggested that decreased serum BDNF
levels aggravated depression, while increased BDNF improved
depression in SLE. The serum BDNF levels exhibited an increase in
the stable stage of SLE and a decrease in the active stage. A study
by our group indicated that the BDNF concentration decreased after
repeated thawing of blood samples, suggesting poor stability of
BDNF protein (9). In this study,
blood samples were collected during the same period for ELISA
analysis within three months. However, in the study by Tamashiro
et al (55), the blood
samples of the control group were from the serum banks of clinical
hospitals, which may have had lower BDNF protein levels due to
degradation during long-term preservation. This notion is in
agreement with the study by Zuccato et al (60), who also demonstrated a
time-dependent change in serum BDNF levels due to different sample
storage conditions. The study by our group indicated that BDNF
levels were correlated with the SLEDAI, levels of complements and
the numbers of circulatory lymphocytes (9). In addition, it was demonstrated that
serum BDNF levels in SLE were decreased and were the lowest in
NPSLE. Another study suggested that plasma BDNF levels were
consistently lower in patients with NPSLE with irreversible organic
brain damage than in healthy controls and the level increased with
the improvement in the disease (57). Thus, elevated BDNF levels may also
be used to track the recovery of brain damage in NPSLE.
5. Conclusion
In summary, an altered tissue or circulatory
concentration of BDNF may be linked to autoimmune and inflammatory
diseases. Studies in transgenic and knockout mice, particularly in
adult surviving animals, revealed hitherto unknown roles of BDNF,
which may be key to understanding immune disease pathologies. BDNF
regulates both immune and neuroimmune interactions. Managing BDNF
levels may be a potential therapeutic strategy, particularly in
diseases with dysregulated production of BDNF.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
BT and NW analyzed the literature and prepared the
manuscript. All authors read and approved the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blesch A and Tuszynski MH: Spontaneous and
neurotrophin-induced axonal plasticity after spinal cord injury.
Prog Brain Res. 137:415–423. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iarikov DE, Kim BG, Dai HN, McAtee M, Kuhn
PL and Bregman BS: Delayed transplantation with exogenous
neurotrophin administration enhances plasticity of corticofugal
projections after spinal cord injury. J Neurotrauma. 24:690–702.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Benussi L, Binetti G and Ghidoni R: Loss
of neuroprotective factors in neurodegenerative dementias: The end
or the starting point? Front Neurosci. 11(672)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Palasz E, Wysocka A, Gasiorowska A,
Chalimoniuk M, Niewiadomski W and Niewiadomska G: BDNF as a
promising therapeutic agent in Parkinson's disease. Int J Mol Sci.
21(1170)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ziemssen T, Kümpfel T, Schneider H,
Klinkert WE, Neuhaus O and Hohlfeld R: Secretion of brain-derived
neurotrophic factor by glatiramer acetate-reactive T-helper cell
lines: Implications for multiple sclerosis therapy. J Neurol Sci.
233:109–112. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakahashi T, Fujimura H, Altar CA, Li J,
Kambayashi J, Tandon NN and Sun B: Vascular endothelial cells
synthesize and secrete brain-derived neurotrophic factor. FEBS
Lett. 470:113–117. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'Onofrio M, de Grazia U, Morrone S, Cuomo
L, Spinsanti P, Frati L, Gulino A and Ragona G: Expression of
neurotrophin receptors in normal and malignant B lymphocytes. Eur
Cytokine Netw. 11:283–291. 2000.PubMed/NCBI
|
|
8
|
Skaper SD: The biology of neurotrophins,
signalling pathways, and functional peptide mimetics of
neurotrophins and their receptors. CNS Neurol Disord Drug Targets.
7:46–62. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tian B, Yang C, Wang J, Hou X, Zhao S, Li
Y and Yang P: Peripheral blood brain-derived neurotrophic factor
level and tyrosine kinase B expression on T lymphocytes in systemic
lupus erythematosus: Implications for systemic involvement.
Cytokine. 123(154764)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lühder F, Gold R, Flügel A and Linker RA:
Brain-derived neurotrophic factor in neuroimmunology: Lessons
learned from multiple sclerosis patients and experimental
autoimmune encephalomyelitis models. Arch Immunol Ther Exp (Warsz).
61:95–105. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu ZL, Luo C, Hurtado PR, Li H, Wang S, Hu
B, Xu JM, Liu Y, Feng SQ, Hurtado-Perez E, et al: Brain-derived
neurotrophic factor precursor in the immune system is a novel
target for treating multiple sclerosis. Theranostics. 11:715–730.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kleczynska W, Jakiela B, Plutecka H,
Milewski M, Sanak M and Musial J: Imbalance between Th17 and
regulatory T-cells in systemic lupus erythematosus. Folia Histochem
Cytobiol. 49:646–653. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoshio T, Okamoto H, Kurasawa K, Dei Y,
Hirohata S and Minota S: IL-6, IL-8, IP-10, MCP-1 and G-CSF are
significantly increased in cerebrospinal fluid but not in sera of
patients with central neuropsychiatric lupus erythematosus. Lupus.
25:997–1003. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao
G, Wang W, Qi W and Kong K: Multiple roles of the p75 neurotrophin
receptor in the nervous system. J Intl Med Res. 37:281–288.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Blöchl A and Blöchl R: A cell-biological
model of p75NTR signaling. J Neurochem. 102:289–305.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao C, Zhang L, Sun D, Li J, Yao X, Zhou H
and Wang Y: Roles of p75NTR in maintaining brain hemostasis and the
implications for p75NTR-targeted Therapies. Curr Alzheimer Res.
14:554–561. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deinhardt K and Chao MV: Trk receptors.
Handb Exp Pharmacol. 220:103–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meier S, Alfonsi F, Kurniawan ND, Milne
MR, Kasherman MA, Delogu A, Piper M and Coulson EJ: The p75
neurotrophin receptor is required for the survival of neuronal
progenitors and normal formation of the basal forebrain, striatum,
thalamus and neocortex. Development. 146(dev181933)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roux PP and Barker PA: Neurotrophin
signaling through the p75 neurotrophin receptor. Prog Neurobiol.
67:203–233. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kendall SE, Goldhawk DE, Kubu C, Barker PA
and Verdi JM: Expression analysis of a novel p75(NTR) signaling
protein, which regulates cell cycle progression and apoptosis. Mech
Dev. 117:187–200. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Enomoto M, Bunge MB and Tsoulfas P: A
multifunctional neurotrophin with reduced affinity to p75NTR
enhances transplanted Schwann cell survival and axon growth after
spinal cord injury. Exp Neurol. 248:170–182, 2013. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Caroleo MC, Costa N, Bracci-Laudiero L and
Aloe L: Human monocyte/macrophages activate by exposure to LPS
overexpress NGF and NGF receptors. J Neuroimmunol. 113:193–201.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang CR, Zhang XY, Liu Y, Du JY, Liang R,
Yu M, Zhang FQ, Mu XF, Li F, Zhou L, et al: Antidepressant drugs
correct the imbalance between proBDNF/p75NTR/Sortilin and mature
BDNF/TrkB in the brain of mice with chronic stress. Neurotox Res.
37:171–182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ohira K and Hayashi M: A new aspect of the
TrkB signaling pathway in neural plasticity. Curr Neuropharmacol.
7:276–285. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cao T, Matyas JJ, Renn CL, Faden AI,
Dorsey SG and Wu J: Function and mechanisms of truncated BDNF
receptor TrkB.T1 in neuropathic pain. Cells. 9(1194)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Matusica D, Skeldal S, Sykes AM, Palstra
N, Sharma A and Coulson EJ: An intracellular domain fragment of the
p75 neurotrophin receptor (p75(NTR)) enhances tropomyosin receptor
kinase A (TrkA) receptor function. J Biol Chem. 288:11144–11154.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Burnouf T, Kuo YP, Blum D, Burnouf S and
Su CY: Human platelet concentrates: A source of
solvent/detergent-treated highly enriched brain-derived
neurotrophic factor. Transfusion. 52:1721–1728. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schulte-Herbrüggen O, Nassenstein C,
Lommatzsch M, Quarcoo D, Renz H and Braun A: Tumor necrosis
factor-alpha and interleukin-6 regulate secretion of brain-derived
neurotrophic factor in human monocytes. J Neuroimmunol.
160:204–209. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
De Santi L, Cantalupo L, Tassi M,
Raspadori D, Cioni C and Annunziata P: Higher expression of BDNF
receptor gp145trkB is associated with lower apoptosis intensity in
T cell lines in multiple sclerosis. J Neurol Sci. 277:65–70.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schuhmann B, Dietrich A, Sel S, Hahn C,
Klingenspor M, Lommatzsch M, Gudermann T, Braun A, Renz H and
Nockher WA: A role for brain-derived neurotrophic factor in B cell
development. J Neuroimmunol. 163:15–23. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Garcia-Suarez O, Blanco-Gelaz MA, Lopez
ML, Germana A, Cabo R, Díaz-Esnal B, Silos-Santiago I, Ciriaco E
and Vega JA: Massive lymphocyte apoptosis in the thymus of
functionally deficient TrkB mice. J Neuroimmunol. 129:25–34.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maroder M, Bellavia D, Vacca A, Felli MP
and Screpanti I: The thymus at the crossroad of neuroimmune
interactions. Ann N Y Acad Sci. 917:741–747. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Linker R, Gold R and Luhder F: Function of
neurotrophic factors beyond the nervous system: Inflammation and
autoimmune demyelination. Crit Rev Immunol. 29:43–68.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vega JA, García-Suárez O, Hannestad J,
Pérez-Pérez M and Germanà A: Neurotrophins and the immune system. J
Anat. 203:1–19. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nockher WA and Renz H: Neurotrophins in
inflammatory lung diseases: Modulators of cell differentiation and
neuroimmune interactions. Cytokine Growth Factor Rev. 14:559–578.
2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stadelmann C, Kerschensteiner M, Misgeld
T, Brück W, Hohlfeld R and Lassmann H: BDNF and gp145trkB in
multiple sclerosis brain lesions: Neuroprotective interactions
between immune and neuronal cells? Brain. 125:75–85.
2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tabakman R, Lecht S, Sephanova S,
Arien-Zakay H and Lazarovici P: Interactions between the cells of
the immune and nervous system: Neurotrophins as neuroprotection
mediators in CNS injury. Prog Brain Res. 146:387–401.
2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Frota ER, Rodrigues DH, Donadi EA, Brum
DG, Maciel DR and Teixeira AL: Increased plasma levels of brain
derived neurotrophic factor (BDNF) after multiple sclerosis
relapse. Neurosci Lett. 460:130–132. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Linker RA, Lee DH, Demir S, Wiese S, Kruse
N, Siglienti I, Gerhardt E, Neumann H, Sendtner M, Lühder F and
Gold R: Functional role of brain-derived neurotrophic factor in
neuroprotective autoimmunity: Therapeutic implications in a model
of multiple sclerosis. Brain. 133:2248–2263. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Steinkamp M, Schulte N, Spaniol U, Pflüger
C, Hartmann C, Kirsch J and von Boyen GB: Brain derived
neurotrophic factor inhibits apoptosis in enteric glia during gut
inflammation. Med Sci Monit. 18:BR117–BR122. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Steinkamp M, Gundel H, Schulte N, Spaniol
U, Pflueger C, Zizer E and von Boyen GB: GDNF protects enteric glia
from apoptosis: Evidence for an autocrine loop. BMC Gastroenterol.
12(6)2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Meir M, Flemming S, Burkard N, Wagner J,
Germer CT and Schlegel N: The glial cell-line derived neurotrophic
factor: A novel regulator of intestinal barrier function in health
and disease. Am J Physiol Gastrointest Liver Physiol.
310:G1118–G1123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Johansson M, Norrgård O and Forsgren S:
Study of expression patterns and levels of neurotrophins and
neurotrophin receptors in ulcerative colitis. Inflamm Bowel Dis.
13:398–409. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ricci A, Mariotta S, Saltini C, Falasca C,
Giovagnoli MR, Mannino F, Graziano P, Sciacchitano S and Amenta F:
Neurotrophin system activation in bronchoalveolar lavage fluid
immune cells in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse
Lung Dis. 22:186–194. 2005.PubMed/NCBI
|
|
46
|
Dagnell C, Grunewald J, Kramar M,
Haugom-Olsen H, Elmberger GP, Eklund A and Olgart Höglund C:
Neurotrophins and neurotrophin receptors in pulmonary
sarcoidosis-granulomas as a source of expression. Respir Res.
11(156)2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lise MC, Sparsa A, Marie I, Lalloué F, Ly
K, Martel C, Bezanahary H, Gondran G, Loustaud-Ratti V, Bonnetblanc
JM, et al: Serum neurotrophin profile in systemic sclerosis. PLoS
One. 5(e13918)2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Donovan MJ, Lin MI, Wiegn P, Ringstedt T,
Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S and Hempstead BL:
Brain derived neurotrophic factor is an endothelial cell survival
factor required for intramyocardial vessel stabilization.
Development. 127:4531–4540. 2000.PubMed/NCBI
|
|
49
|
Fauchais AL, Boumediene A, Lalloue F,
Gondran G, Loustaud-Ratti V, Vidal E and Jauberteau MO:
Brain-derived neurotrophic factor and nerve growth factor correlate
with T-cell activation in primary Sjogren's syndrome. Scand J
Rheumatol. 38:50–57. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li YJ, Yang CS, Lei L, Wu KF, Yang PT and
Xiao WG: Serum nerve grow factor and brain-derived neurotrophic
factor profiles in Sjögren's syndrome concomitant with interstitial
lung disease. Clin Rheumatol. 33:1161–1164. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Grimsholm O, Rantapää-Dahlqvist S, Dalén T
and Forsgren S: BDNF in RA: Downregulated in plasma following
anti-TNF treatment but no correlation with inflammatory parameters.
Clin Rheumatol. 27:1289–1297. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rihl M, Kruithof E, Barthel C, De Keyser
F, Veys EM, Zeidler H, Yu DT, Kuipers JG and Baeten D: Involvement
of neurotrophins and their receptors in spondyloarthritis
synovitis: Relation to inflammation and response to treatment. Ann
Rheum Dis. 64:1542–1549. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Barthel C, Yeremenko N, Jacobs R, Schmidt
RE, Bernateck M, Zeidler H, Tak PP, Baeten D and Rihl M: Nerve
growth factor and receptor expression in rheumatoid arthritis and
spondyloarthritis. Arthritis Res Ther. 11(R82)2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ikenouchi-Sugita A, Yoshimura R, Okamoto
T, Umene-Nakano W, Ueda N, Hori H, Katsuki A, Saito K, Tanaka Y and
Nakamura J: Serum brain-derived neurotrophic factor levels as a
novel biological marker for the activities of psychiatric symptoms
in systemic lupus erythematosus. World J Biol Psychiatry.
11:121–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tamashiro LF, Oliveira RD, Oliveira R,
Frota ER, Donadi EA, Del-Ben CM, Teixeira AL and Louzada-Junior P:
Participation of the neutrophin brain-derived neurotrophic factor
in neuropsychiatric systemic lupus erythematosus. Rheumatology
(Oxford). 53:2182–2190. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ikenouchi A, Yoshimura R, Ikemura N,
Utsunomiya K, Mitoma M and Nakamura J: Plasma levels of brain
derived-neurotrophic factor and catecholamine metabolites are
increased during active phase of psychotic symptoms in CNS lupus: A
case report. Prog Neuropsychopharmacol Biol Psychiatry.
30:1359–1363. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ikenouchi-Sugita A, Yoshimura R, Ueda N,
Kodama Y, Umene-Nakano W and Nakamura J: Continuous decrease in
serum brain-derived neurotrophic factor (BDNF) levels in a
neuropsychiatric syndrome of systemic lupus erythematosus patient
with organic brain changes. Neuropsychiatr Dis Treat. 4:1277–1281.
2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fauchais AL, Lise MC, Marget P, Lapeybie
FX, Bezanahary H, Martel C, Dumonteil S, Sparsa A, Lalloué F, Ly K,
et al: Serum and lymphocytic neurotrophins profiles in systemic
lupus erythematosus: A case-control study. PLoS One.
8(e79414)2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zheng Q, Xu MJ, Cheng J, Chen JM, Zheng L
and Li ZG: Serum levels of brain-derived neurotrophic factor are
associated with depressive symptoms in patients with systemic lupus
erythematosus. Psychoneuroendocrinology. 78:246–252.
2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zuccato C, Marullo M, Vitali B, Tarditi A,
Mariotti C, Valenza M, Lahiri N, Wild EJ, Sassone J, Ciammola A, et
al: Brain-derived neurotrophic factor in patients with Huntington's
disease. PLoS One. 6(e22966)2011.PubMed/NCBI View Article : Google Scholar
|















