Introduction
Cardiovascular disease is one of the leading causes
of human mortality worldwide, which is an important social and
economic burden (1). The loss of
cardiomyocytes during various ischemic heart diseases is considered
to be irreversible, thereby contributing to permanent cardiac
dysfunction (2). Apoptosis is a
type of cell death that is characterized by prominent changes in
caspase activation and serves an important role in cardiac injury
(3). Therefore, the amelioration of
apoptosis and improving cardiomyocyte viability are of particular
research interest.
Autophagy is the phagocytosis and subsequent
degradation of intracellular proteins or organelles for metabolism
(4). Autophagy has been implicated
in numerous physiological and pathological processes, including
survival, differentiation, cancer, aging and heart disease
(5). Emerging evidence has
indicated that autophagy exerts a biphasic role in cardiac
physiology, whereby excessive or reduced autophagy levels can both
result in cardiomyocyte apoptosis (6,7).
However, the exact mechanism underlying this remains unclear. mTOR
is considered to be a key signaling component among the several
proteins that have been reported to regulate autophagy (8). mTOR can directly or indirectly
integrate cell signals, such as ATP and hypoxia, to regulate
autophagy induction (9,10). In addition, as an important
regulatory enzyme of metabolism, increased phosphorylation of
5'-AMP-activated protein kinase (AMPK) was previously found to
inhibit autophagy (11).
Isoflavones are particularly abundant in legumes
(such as soy, chickpea and red clover) and can also be found in
various traditional Chinese medicines (such as Astragali Radix and
Puerariae Lobatae Radix) (12,13).
Previous studies have reported the protective effects of isoflavone
in neuron injury, where it conferred antioxidant and
anti-inflammatory properties (14,15).
Ononin (ON) is a bioactive isoflavone of legumes, however, to the
best of our knowledge, the specific functions and mechanisms of ON
in cardiomyocytes have not been previously reported. Therefore, the
present study aimed to assess the potential role of ON in
H2O2-induced H9C2 cell injury and investigate
how it could modulate the AMPK/mTOR/autophagy signaling pathway.
Additionally, ON was studied in a rat myocardial infarction model
to study its possible role in cardiac function.
Materials and methods
Drugs
ON (MedChemExpress), chloroquine (CQ;
MedChemExpress) and Compound C (CC; Selleck Chemicals) were
dissolved in DMSO (Sigma-Aldrich; Merck KGaA) and diluted with
PBS.
Cell culture and treatment
The rat cardiomyocyte H9C2 cell line was purchased
from the American Type Culture Collection. Cells were cultured in
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Beyotime Institute of Biotechnology) and
1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) in an
atmosphere containing 5% CO2 at 37˚C. The cells were
subsequently randomly divided into the following treatment groups:
i) Control group; ii) H2O2 group, which
involved culturing in serum-free DMEM and exposed to
H2O2 (400 µmol/l) to stimulate cardiomyocyte
injury for 4 h at 37˚C; iii) H2O2 + ON group,
which was treated with H2O2 and ON (10
µmol/l) simultaneously for 4 h at 37˚C; iv)
H2O2 + ON + CC group, which was treated with
H2O2, ON and CC (10 µM) simultaneously for 4
h at 37˚C; and (v) H2O2 + ON + CQ group,
which was treated with H2O2, ON and CQ (10
µM) simultaneously for 4 h at 37˚C.
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to evaluate cell viability. A total of
1x104 H9C2 cells were plated onto 96-well plates and
after treatment with H2O2 (400 µmol/l), 10 µl
CCK-8 solution was added to each well and incubated at 37˚C for 2
h. Finally, the optical density absorbance of each well was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.) to calculate the relative viability.
Flow cytometry assay
The FITC Annexin V Apoptosis Detection Kit (BD
Biosciences) was used to evaluate H9C2 cell apoptosis, and the
experimental procedures were conducted according to the
manufacturer's protocols. After H2O2
treatment (400 µmol/l), H9C2 cells were collected, washed twice
with PBS and transferred into a culture tube (5x104
cells/tube)before 5 µl Annexin V-FITC and 5 µl PI were added to the
cells and incubated for 15 min at room temperature in the dark. BD
FACSCalibur™ flow cytometer (BD Biosciences) and Cell Quest
software version 3.1 were used to perform the subsequent apoptosis
analysis.
Western blot analysis
Total protein from H9C2 cells or rat heart tissue
was lysed using RIPA buffer (Cell Signaling Technology, Inc.)
supplemented with protease and phosphatase inhibitors (Cell
Signaling Technology, Inc.). Protein concentration was determined
using a BCA kit (Beyotime Institute of Biotechnology). Protein
lysate samples of 30 µg were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore). The membranes were
blocked with 8% non-fat milk at room temperature for 60 min, before
being incubated with the following primary antibodies overnight at
4˚C: Cleaved-caspase-3 (1:500; Cell Signaling Technology, Inc.,
cat. no. 9664), caspase 3 (1:1,000; Cell Signaling Technology,
Inc., cat. no. 9662), Bcl2 (1:800; Cell Signaling Technology, Inc.,
cat. no. 3869), Bax (1:1,000; Cell Signaling Technology, Inc., cat.
no. 2772), GAPDH (1:10,000, Abcam, cat. no. ab9385), p62 (1:1,000;
Cell Signaling Technology, Inc., cat. no. 5114), LC3-I (1:1,000;
Cell Signaling Technology, Inc., cat. no. 4108), LC3-II (1:1,000;
Cell Signaling Technology, Inc., cat. no. 4108), actin (1:10,000;
Abcam, cat. no. ab20272), phosphorylated (p-) AMPK (1:1,000; Cell
Signaling Technology, Inc., cat. no. 4186), AMPK (1:1,000; Cell
Signaling Technology, Inc., cat. no. 5832), p-mTOR (1:1,000; Cell
Signaling Technology, Inc., cat. no. 5536) and mTOR (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 2972). After washing with PBS
with 0.1% Tween®-20 three times at 10 min each, the
membranes were incubated with the corresponding HRP-conjugated
secondary antibodies of goat anti-rabbit IgG (1:3,000; Cell
Signaling Technology, Inc., no. 7074) and goat anti-mouse IgG
(1:3,000; Cell Signaling Technology, Inc., no. 56970) for 1 h at
room temperature. Finally, the blots were visualized using an ECL
kit (EMD Millipore) and quantified using the Image Lab software
version 4.0 (Bio-Rad Laboratories, Inc.) with GAPDH used as the
loading control of Cleaved-caspase-3 and actin used as the loading
control of the other proteins.
Myocardial infarction model and ononin
treatment
All animal procedures were conducted according to
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health and were approved by the
Animal Care and Use Committee of Wenzhou Medical University (Cixi,
China). Male Sprague-Dawley rats (250-300 g, 6-8 weeks) were
obtained from Experimental Animal Center of Wenzhou Medical
University. Rats were maintained in climate- and light-controlled
room (24±1˚C, relative humidity of 65±15%, 12/12-h light/dark
cycle) with ad libitum access to food and water for at least
1 week prior to the experiments. Myocardial infarction (MI) was
surgically induced as previously described (16). A total of 18 rats were anesthetized
via an intraperitoneal injection of pentobarbital sodium (50 mg/kg)
and ventilated with a rodent ventilator. Rats were divided into
three groups (n=6/group): i) Sham; ii) MI and iii) MI + ON. The
heart was then exposed, and the left anterior descending coronary
artery was ligated permanently with a 6-0 silk suture in the MI
group. The heart was exposed but the left anterior descending
coronary artery was not ligated in the sham group. Rats in the
treatment groups were intragastric injection of ON (20 mg/kg)
(13) daily, whilst the MI group
received the same volume of laboratory animal drinking water daily
until 28 days after surgery.
Cardiac function assessment
Heart function was assessed using transthoracic
echocardiography 28 days after MI induction. Left ventricular
ejection fraction (LVEF) and left ventricular fraction shortening
(LVFS) were analyzed using Vevo® 2100 workstation
software version 3.1.0 (VisualSonics, Inc.).
Masson's trichrome staining
A total of 28 days post-surgery, all rats were
euthanized with 20% CO2 for histological analysis. All
heart tissues were quickly excised and dehydrated in a 30% sucrose
solution. The hearts were then embedded in Tissue-Tek®
O.C.T.™ Compound (Sakura Finetek USA, Inc.) and cut into 7-µm
slices. Modified Masson's Trichrome Stain kit (Beijing Solarbio
Science & Technology Co., Ltd.) was used to examine infarct
size according to the manufacturer's instructions. A light
microscope (Leica Microsystems GmbH) was used to capture images (x1
magnification). Following Masson's staining, myocardial fibers
appear red, while collagen fibers appear blue. The proportion of
the infarct area was quantified using Imaging Pro Plus software 6.0
(Media Cybernetics, Inc.) and calculated as follows: Infarct heart
area (%)=(endocardial length + epicardial length of the infarcted
area/endocardial length + epicardial length of whole left
ventricle) x100%.
Statistical analysis
All data are presented as the mean ± SEM. All the
cell experiments were repeated ≥ three times. GraphPad Prism 6.0
software (GraphPad Software, Inc.) was used for graph generation
and data analysis. Student's t-test was performed to analyze
differences between two groups. Differences among > three groups
were analyzed using one-way ANOVA followed by Tukey's multiple
comparisons post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ON improves cell viability in
H2O2-induced H9C2 cardiomyocyte injury
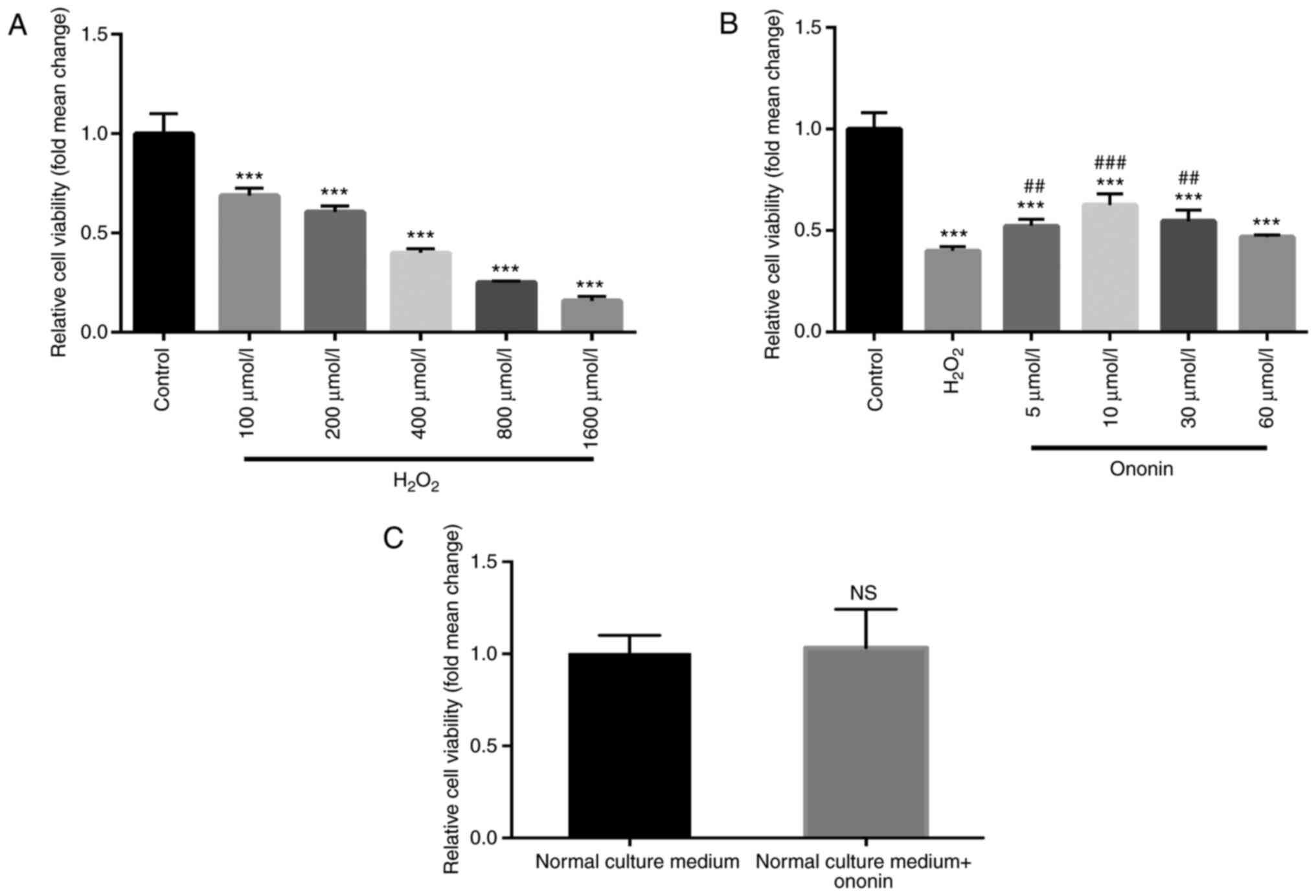
As indicated by the results of CCK-8 assays,
H2O2 induced a concentration-dependent
reduction in H9C2 cardiomyocyte viability compared with that in the
control group (Fig. 1A). Due to its
ability to induce a 50% decrease in cell viability, the 400 µmol/l
dose of H2O2 was selected for subsequent
experiments. Treatment with suitable concentrations of ON (5, 10 or
30 µmol/l) significantly ameliorated the
H2O2-induced reductions in H9C2 cell
viability (Fig. 1B) with 10 µmol/l
ON exerting the optimal protective effects. Therefore, 10 µmol/l ON
was chosen for subsequent experiments. An excess of ON (60 µmol/l)
did not induce significant protective effects (Fig. 1B). To rule out the possibility that
ON may increase the viability of the cardiomyocytes without
H2O2 treatment, ON (10 µmol/l) was added to
the H9C2 cell culture medium but did not affect cell viability
(Fig. 1C). These results indicate
the cardioprotective effects of ON against
H2O2-induced H9C2 cell injury.
ON alleviates apoptosis in
H2O2-induced H9C2 cardiomyocytes
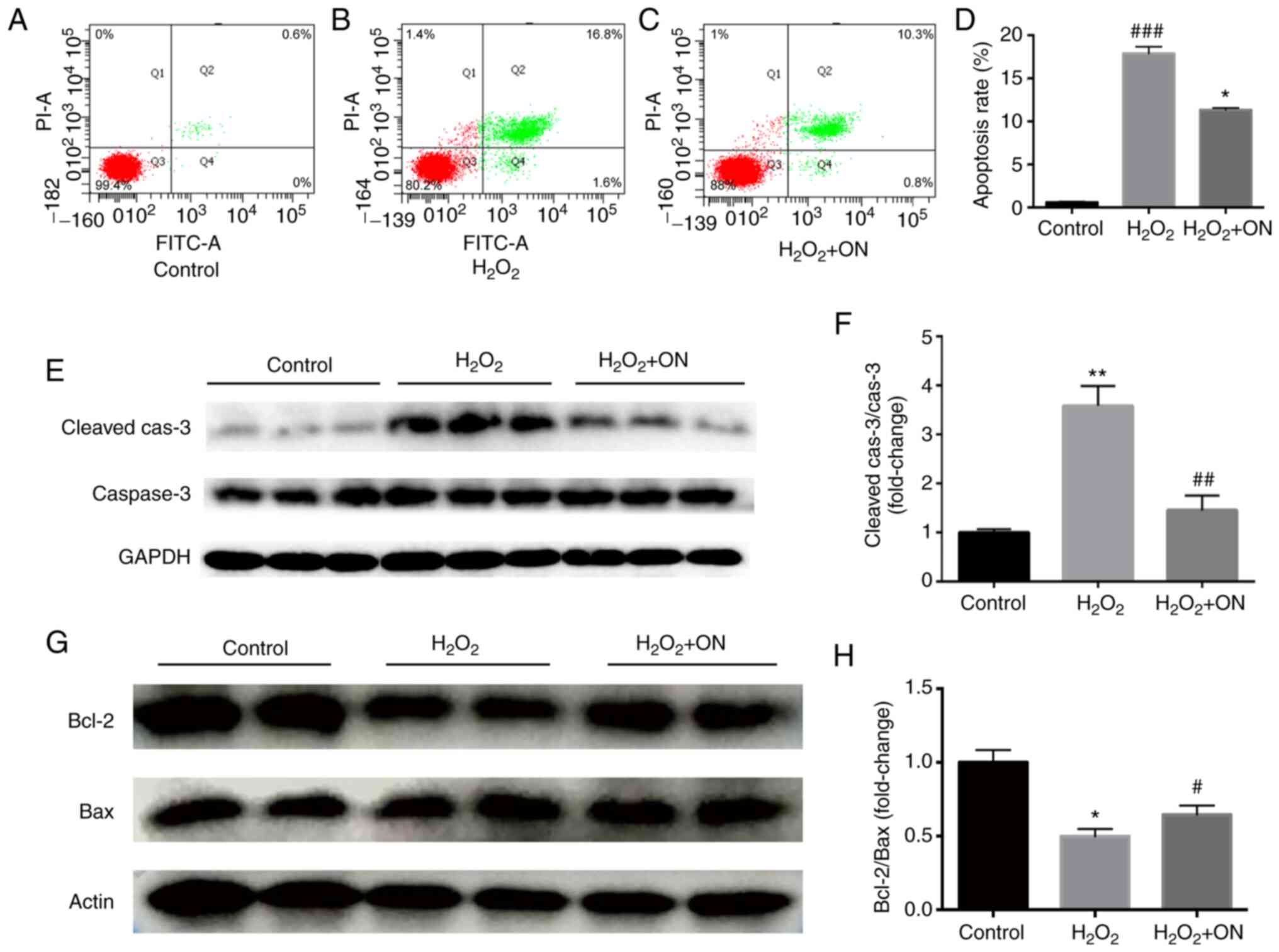
Flow cytometry assays (Fig. 2A, B
and D) demonstrated that
H2O2 (400 µmol/l) could induce apoptosis in
H9C2 cardiomyocytes. The apoptosis-promoting effect of
H2O2 was confirmed by results from the
western blot assays, which was evidenced by the significant
increase in cleaved-caspase 3 levels (Fig. 2E and F) and significant downregulation in the
Bcl2/Bax ratio (Fig. 2G and
H) compared with those in the
control group. After ON administration (10 µmol/l), compared with
those in the H2O2 group, both the number of
apoptotic cells (Fig. 2C and
D) and the level of
apoptosis-associated protein cleaved-caspase 3 were significantly
decreased (Fig. 2E and F), whilst the Bcl2/Bax ratio was
significantly increased (Fig. 2G
and H). These results suggest that
ON may alleviate apoptosis to promote cardiomyocyte viability.
Ononin suppresses
H2O2-induced H9C2 cardiomyocyte apoptosis by
enhancing autophagy
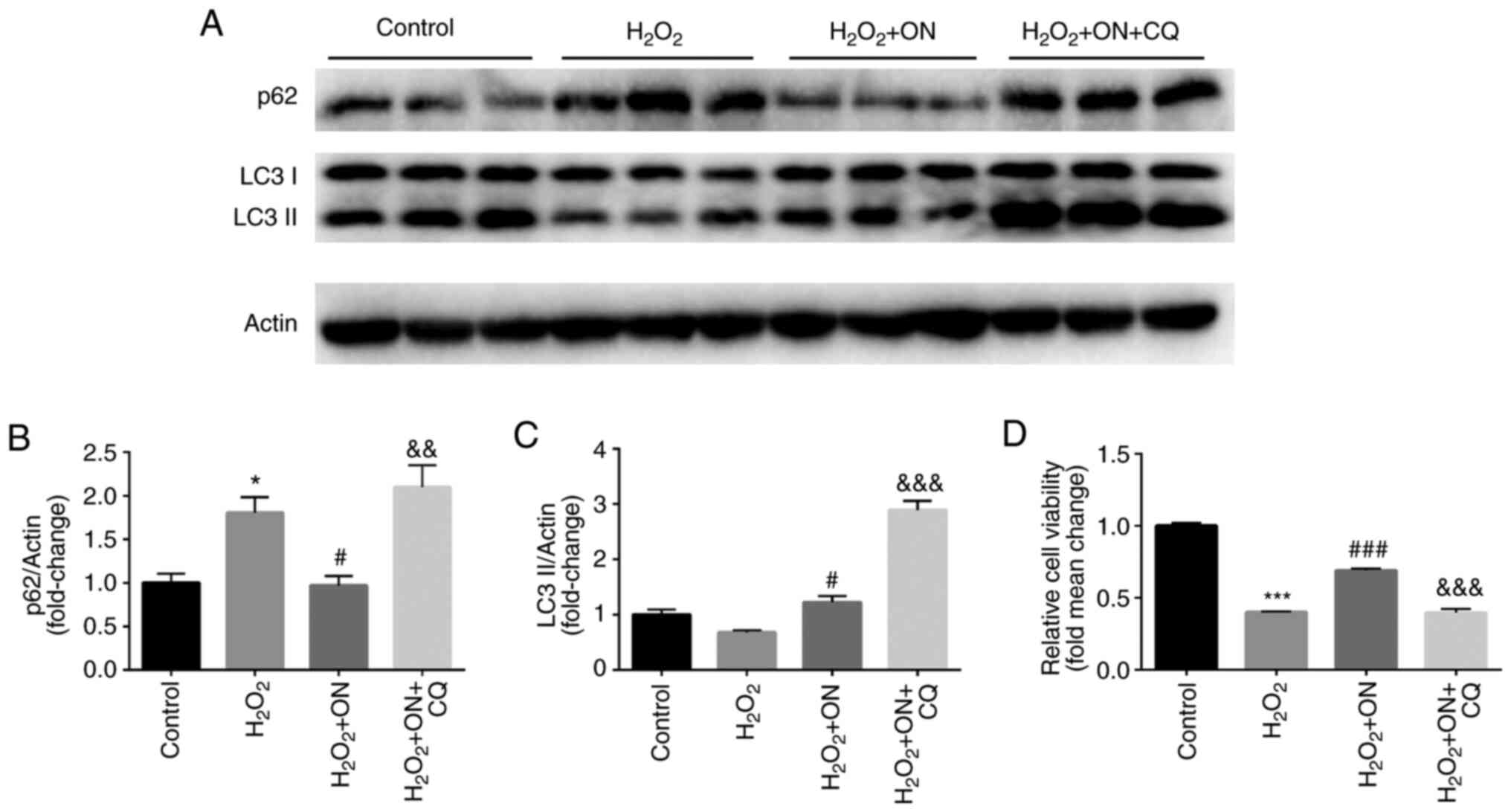
Autophagy serves an important role in cardiomyocyte
injury and restoration in several cellular models (17). To determine the effects of ON on
autophagy in H2O2-treated H9C2
cardiomyocytes, the expression of autophagy-associated proteins
LC3-II and p62 was estimated after ON treatment. Compared with that
in the H2O2 group, the
H2O2 + ON group showed a significant decrease
in p62 expression (Fig. 3A and
B) and a significant increase in
LC3-II expression (Fig. 3A and
C), suggesting that ON can enhance
autophagy in H2O2-treated H9C2 cells.
Moreover, CQ (10 µM), an autophagy inhibitor, significantly
increased the levels of p62 and LC3 II compared with those in the
H2O2 + ON group (Fig. 3A-C). Furthermore, compared with that
in the H2O2 + ON group and the
H2O2 group, CQ significantly reversed the
anti-apoptotic effects of ON (Fig.
3D), further suggesting the autophagy modulatory role of ON in
the cardiomyocytes following H2O2
challenge.
ON promotes autophagy via the
AMPK/mTOR pathway
The AMPK/mTOR signaling pathway serves an important
role in autophagy (18). Therefore,
it was hypothesized that ON may also engage autophagy through this
pathway. CC, a specific AMPK inhibitor, was used to test the
present hypothesis in H9C2 cells. Compared with that in the
H2O2 group, ON treatment significantly
increased the p-AMPK/AMPK ratio (Fig.
4A and B) whilst significantly
diminishing the p-mTOR/mTOR ratio (Fig.
4A and C). After treatment with
CC (10 µM) in the H2O2 group, compared with
those in the H2O2 + ON group, the effects of
ON on p-AMPK/AMPK (Fig. 4A and
B), p-mTOR/mTOR (Fig. 4A and C), p62 (Fig.
4A and D) and LC3-II (Fig. 4A and E) were significantly reversed. However,
the protective effects of ON on cell viability against
H2O2 were lost after the AMPK/mTOR pathway
was inhibited by CC (Fig. 4F).
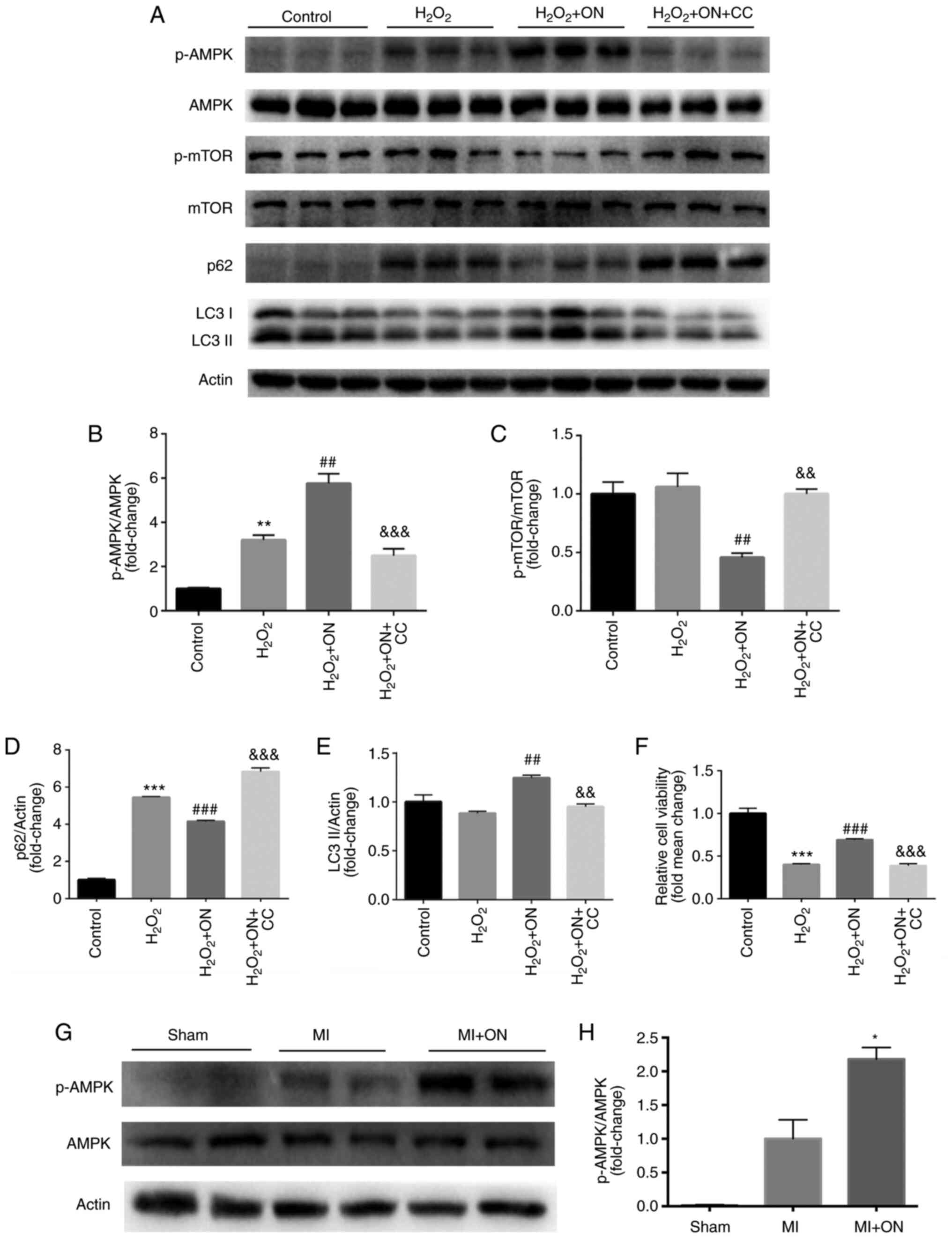 | Figure 4ON promotes autophagy via the
AMPK/mTOR signaling pathway. (A) Representative western blotting
images of p-AMPK, AMPK, p-mTOR, mTOR, p62, LC3-I and LC3-II in the
control, H2O2, H2O2 +
ON and H2O2 + ON + CC groups. Quantification
of (B) p-AMPK/AMPK, (C) p-mTOR/mTOR, (D) p62 and (E) LC3-II. (F)
Cell Counting Kit-8 viability assays in the four different groups
were assessed. **P<0.01 and ***P<0.001
vs. Control; ##P<0.01 and ###P<0.001
vs. H2O2; &&P<0.01 and
&&&P<0.001 vs. H2O2
+ ON. (G) Representative western blotting images of p-AMPK and AMPK
in rat heart tissues isolated from the sham, MI and MI + ON groups,
which were (H) quantified. *P<0.05 vs. MI group. The
in vitro western blots were repeated 3 times. n=3
animals/group for in vivo western blots. ON, ononin; p-,
phosphorylated; CC, Compound C; MI, myocardial infarction; AMPK, 5'
AMP-activated protein kinase. |
Subsequently, the p-AMPK/AMPK ratio was also
measured in the rat heart tissues. Compared with that in the MI
group, ON significantly increased the p-AMPK/AMPK ratio, consistent
with the in vitro results (Fig.
4G and H). These results
suggest that ON can enhance autophagy through the p-AMPK/mTOR
signaling pathway to improve viability and alleviate apoptosis.
ON treatment results in recovery of
cardiac function after MI
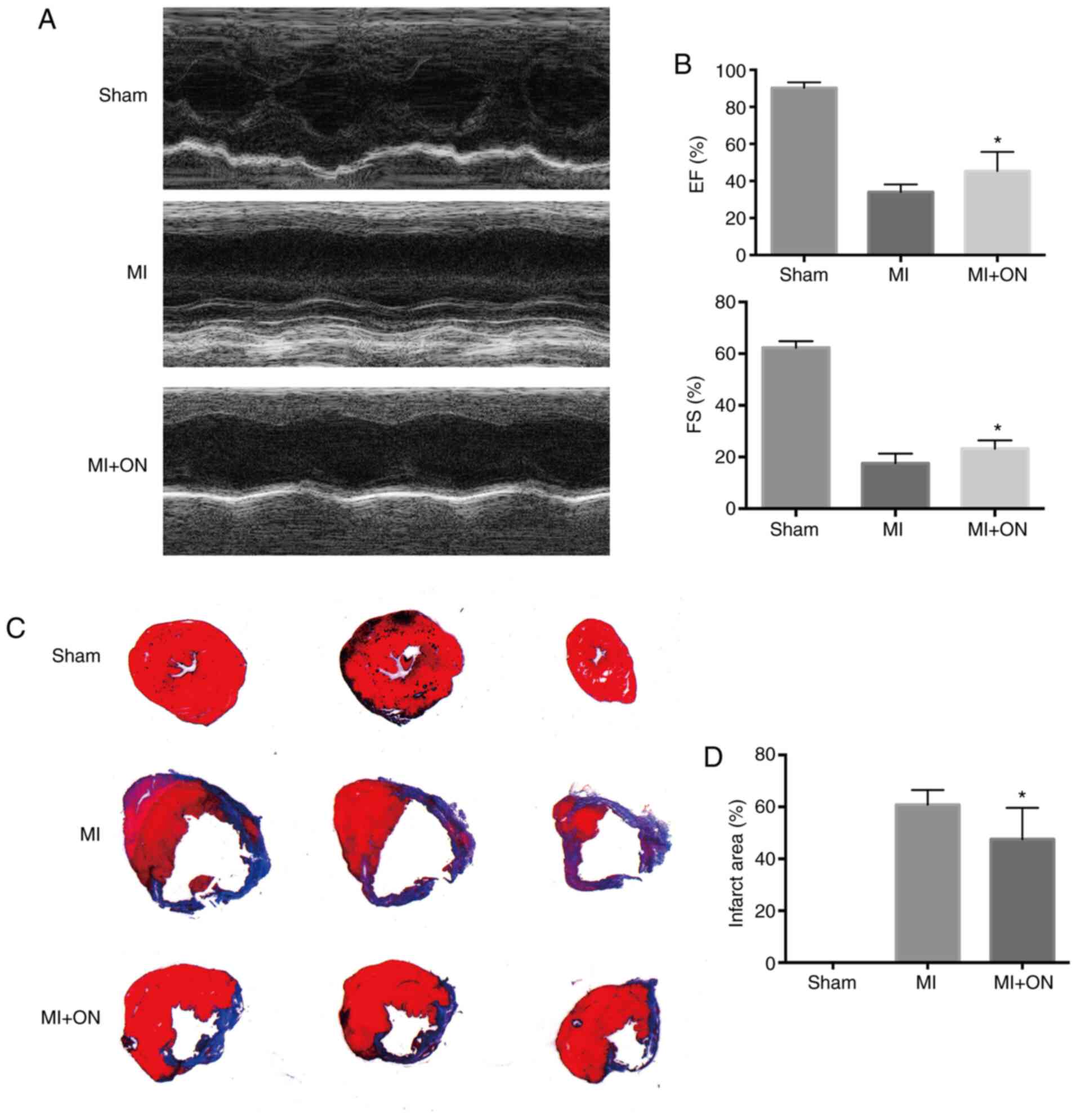
To assess whether the anti-apoptotic effect of ON
in vitro could result in favorable cardiac recovery in
vivo, echocardiography was performed 28 days after MI
induction. Compared with that in the MI group, ON delivery
significantly improved cardiac function, as evidenced by the
significantly improved LVEF and LVFS (Fig. 5A and B). To verify the improvement in cardiac
function induced by ON, the heart tissue sections were examined
histologically on day 28 after MI induction by Masson's trichrome
staining to visualize the infarct size (Fig. 5C). Compared with that in the MI
group, the ON group showed a significantly decreased infarct size
(Fig. 5D). These results supported
the notion that ON treatment could reduce the infarct area to
preserve function.
Discussion
Stress-induced apoptosis contributes to
cardiomyocyte loss (19).
Therefore, inhibiting and reversing this process may prove to be an
effective way to treat heart diseases (20,21).
In the present study, the potential effects of ON in
H2O2-induced H9C2 cardiomyocyte apoptosis
were assessed. Increasing attention is being paid on exploring
naturally-occurring extracts for drug development due to minimal
side effects (22). As a dietary
nutrient, isoflavones are widely abundant in legumes and exhibit
antioxidant, anti-inflammatory and immunoregulatory effects
(13). ON is a major bioactive
isoflavone that can regulate oxidative stress, inflammation and
immunity (15,23,24).
Because of the close associations among oxidative stress,
inflammation, immunity and apoptosis, it was speculated that ON can
serve a role in H2O2-induced H9C2 cell injury
(25). In the present study,
H2O2 reliably induced H9C2 cardiomyocyte
apoptosis, which was previously demonstrated (26). Following ON treatment, cell
viability improved, and the apoptosis rate decreased, indicating
the cardioprotective effects of ON in oxidative stress-induced
cardiomyocyte apoptosis. Furthermore, ON increased the ejection
fraction and decreased cardiac fibrosis in rats with MI confirmed
its potential cardioprotective function.
To the best of our knowledge, the anti-apoptotic
role of ON in cardiomyocytes has not been previously reported. It
was speculated that autophagy may be involved in this process.
Autophagy is involved in different models of cell death, including
apoptosis, pyroptosis, necroptosis and necrosis (27,28).
Emerging evidence has indicated that autophagy can regulate the
apoptosis signaling pathway (29).
Although autophagy can initiate apoptosis in cells exposed to an
external stimuli, this does not mean that increased autophagy is
harmful. Reducing autophagic levels can decrease apoptosis whereas
and cell viability can also be improved by increasing autophagy
(30). Therefore, autophagy is
important for the maintenance of homeostasis and is beneficial to
cells at appropriate operation levels. For example, a previous
study reported that increased autophagy can promote nerve cell
survival under nutrient deficiency (31). The present results demonstrated that
ON increased autophagy, as observed by increased expression levels
of the autophagy-associated protein markers p62 and LC3-II.
Moreover, after treatment with the autophagy inhibitor CQ, ON lost
its cardioprotective effects, as indicated by results from the
CCK-8 assays. This suggests that ON alleviated H9C2 cardiomyocyte
apoptosis at least partially by promoting autophagy.
The full signaling pathway profile that can regulate
autophagy remain poorly understood, despite this process being
precisely modulated (32). As a key
molecule, mTOR integrates upstream signaling to inhibit the
induction of autophagy (33).
PI3K/AKT and MAPK/ERK1/2 signaling can activate the mTOR pathway to
inhibit autophagy, whereas AMPK and p53 signaling negatively
regulate mTOR to promote autophagy (34,35).
The increased LC3-II and reduced p62 expression after ON treatment
indicated that autophagy was upregulated, which was caused by the
downregulation of mTOR phosphorylation. The present study also
quantified the expression of upstream signaling components (p-AKT)
of p-mTOR (data not shown). However, only p-AMPK levels were firmly
enhanced after ON treatment. The benefits of p-AMPK stimulation
have been previously observed in many cardiovascular diseases,
including MI and ischemia reperfusion injury (36-38).
In the present study, AMPK activation inhibited the phosphorylation
of mTOR, leading to an increase in autophagy. After treatment with
the p-AMPK inhibitor CC, ON lost its protective effects as
demonstrated by the CCK-8 assays, suggesting that ON can improve
cell viability and reduce apoptosis through the AMPK/mTOR/autophagy
signaling pathway after H2O2-induced
cardiomyocyte injury. However, the protective effect of ON on
cardiomyocytes and its potential mechanisms was only reported in
vitro in the present study. The functional properties of ON
should be studied further in vivo before it can be applied
for clinical use. By developing a MI model and evaluating the role
of ON in cardiac function and myocardial fibrosis, the present
study determined the protective effects of ON further to provide a
novel approach for the clinical treatment of MI.
In conclusion, the present study identified a
protective role of ON on cardiomyocytes and cardiac function. This
effect may be mediated through the AMPK/mTOR/autophagy pathway.
However, the specific mechanisms of ON in regulating cardiac
function in vivo require further exploration.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Zhejiang Medical
Science and Technology Project (grant no. 2020KY290).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RP designed the experiments and wrote the
manuscript. QZ and JW performed the experiments and analyzed the
data. RP and QZ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Committee of Wenzhou Medical University (Cixi, China; approval no.
2019635).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Okwuosa IS, Lewsey SC, Adesiyun T,
Blumenthal RS and Yancy CW: Worldwide disparities in cardiovascular
disease: Challenges and solutions. Int J Cardiol. 202:433–440.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miao C, Lei M, Hu W, Han S and Wang Q: A
brief review: The therapeutic potential of bone marrow mesenchymal
stem cells in myocardial infarction. Stem Cell Res Ther.
8(242)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aljakna A, Fracasso T and Sabatasso S:
Molecular tissue changes in early myocardial ischemia: From
pathophysiology to the identification of new diagnostic markers.
Int J Legal Med. 132:425–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu D, Zhang K and Hu P: The role of
autophagy in acute myocardial infarction. Front Pharmacol.
10(551)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Woodall BP and Gustafsson ÅB: Autophagy-A
key pathway for cardiac health and longevity. Acta Physiol (Oxf).
223(e13074)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Munson MJ and Ganley IG: MTOR, PIK3C3, and
autophagy: Signaling the beginning from the end. Autophagy.
11:2375–2376. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Bari MAA and Xu P: Molecular regulation
of autophagy machinery by mTOR-dependent and -independent pathways.
Ann NY Acad Sci. 1467:3–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sciarretta S, Forte M, Frati G and
Sadoshima J: New insights into the role of mTOR signaling in the
cardiovascular system. Circ Res. 122:489–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jia J, Bissa B, Brecht L, Allers L, Choi
SW, Gu Y, Zbinden M, Burge MR, Timmins G, Hallows K, et al: AMPK, a
regulator of metabolism and autophagy, is activated by lysosomal
damage via a novel galectin-directed ubiquitin signal transduction
system. Mol Cell. 5:951–969. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Messina M: A brief historical overview of
the past two decades of soy and isoflavone research. J Nutr.
140:1350S–1354S. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Luo LY, Fan MX, Zhao HY, Li MX, Wu X and
Gao WY: Pharmacokinetics and bioavailability of the isoflavones
formononetin and ononin and their in vitro absorption in ussing
chamber and caco-2 cell models. J Agric Food Chem. 66:2917–2924.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mu H, Bai YH, Wang ST, Zhu ZM and Zhang
YW: Research on antioxidant effects and estrogenic effect of
formononetin from trifolium pratense (red clover). Phytomedicine.
16:314–319. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dong L, Yin L, Zhang Y, Fu X and Lu J:
Anti-inflammatory effects of ononin on
lipopolysaccharide-stimulated RAW 264.7 cells. Mol Immunol.
83:46–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang
JH, Denoble PJ, Tao H and Sun X: Hydrogen-rich saline protects
myocardium against ischemia/reperfusion injury in rats. Exp Biol
Med (Maywood). 234:1212–1219. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li DL and Hill JA: Cardiomyocyte autophagy
and cancer chemotherapy. J Mol Cell Cardiol. 71:54–61.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, Liu Z, Shu S, Cai J, Tang C and
Dong Z: AMPK/mTOR signaling in autophagy regulation during
cisplatin-induced acute kidney injury. Front Physiol.
11(619730)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ganguly S, Mitra A and Sarkar S: Role of
α-crystallin B in regulation of stress induced cardiomyocyte
apoptosis. Cardiovasc Hematol Agents Med Chem. 12:60–65.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Del Re DP, Amgalan D, Linkermann A, Liu Q
and Kitsis RN: Fundamental mechanisms of regulated cell death and
implications for heart disease. Physiol Rev. 99:1765–1817.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Broughton KM, Wang BJ, Firouzi F,
Khalafalla F, Dimmeler S, Fernandez-Aviles F and Sussman MA:
Mechanisms of cardiac repair and regeneration. Circ Res.
122:1151–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lundstrom K: Unlocking the therapeutic
potential of plant extracts. Future Med Chem. 8:245–248.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo L, Zhou J, Zhao H, Fan M and Gao W:
The anti-inflammatory effects of formononetin and ononin on
lipopolysaccharide-induced zebrafish models based on lipidomics and
targeted transcriptomics. Metabolomics. 15(153)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Choy KW, Murugan D, Leong XF, Abas R,
Alias A and Mustafa MR: Flavonoids as natural anti-inflammatory
agents targeting nuclear factor-Kappa B (NFκB) signaling in
cardiovascular diseases: A mini review. Front Pharmacol.
10(1295)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu MY, Li CJ, Hou MF and Chu PY: New
insights into the role of inflammation in the pathogenesis of
atherosclerosis. Int J Mol Sci. 22(2034)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Xia J, Jiang N, Xian Y, Ju H, Wei Y
and Zhang X: Corin protects H2O2-induced
apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes.
Biomed Pharmacother. 97:594–599. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ryter SW, Mizumura K and Choi AMK: The
impact of autophagy on cell death modalities. Int J Cell Biol.
2014(502676)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chung KW and Chung HY: The effects of
calorie restriction on autophagy: Role on aging intervention.
Nutrients. 11(2923)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Y and Zhang H: Regulation of
autophagy by mTOR signaling pathway. Adv Exp Med Biol. 1206:67–83.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Coward J, Ambrosini G, Musi E, Truman JP,
Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH Jr and
Schwartz GK: Safingol (L-threo-sphinganine) induces autophagy in
solid tumor cells through inhibition of PKC and the PI3-kinase
pathway. Autophagy. 5:184–193. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mrakovcic M and Fröhlich LF: p53-mediated
molecular control of autophagy in tumor cells. Biomolecules.
8(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee SY, Ku HC, Kuo YH, Chiu HL and Su MJ:
Pyrrolidinyl caffeamide against ischemia/reperfusion injury in
cardiomyocytes through AMPK/AKT pathways. J Biomed Sci.
22(18)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lei X, Wu Q, Leng W, Wu M, Chen L and
Liang Z: Exenatide reduces cardiomyocyte apoptosis by stimulating
adiponectin secretion and activating APPL1-AMPK-PPARα axis. Ann
Transl Med. 7(326)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li R, Liu Y, Shan YG, Gao L, Wang F and
Qiu CG: Bailcalin protects against diabetic cardiomyopathy through
keap1/Nrf2/AMPK-mediated antioxidative and lipid-lowering effects.
Oxid Med Cell Longev. 2019(3206542)2019.PubMed/NCBI View Article : Google Scholar
|