Introduction
Heart failure (HF) is the end-stage of numerous
heart diseases, presenting a substantial clinical and economic
burden on public health on a global scale. Due to an increase in
the proportion of the aging population, the prevalence of HF is
likely to increase in the future. Though numerous efforts have been
made in cardiovascular research, current therapeutic approaches do
not improve the mortality and readmission rates of patients with HF
(1). In Chinese clinical practice,
formulations from traditional Chinese medicine that have been
demonstrated to be effective in improving heart function are used
extensively. However, the mechanisms underlying these improvements
has yet to be fully elucidated.
Various mechanisms have been demonstrated to be
involved in the pathophysiological development of HF, including
neuro-endocrine, inflammatory and structural remodeling mechanisms
(2-4).
The energy starvation hypothesis for HF has attracted particular
attention (5). Recently, the
Sigma-1 receptor (Sigma-1R), a molecular chaperone located on the
endoplasmic reticulum (ER) membrane, was suggested to have a
cardioprotective effect, potentially through energy metabolism
improvement (6,7). Sigma receptors were first reported in
the central nervous system (CNS) as a subclass of opioid receptors.
However, pharmacological and behavioral studies have revealed that
sigma receptors are distinct from all previously characterized
mammalian proteins. Sigma receptors are now recognized as
non-opioid receptors located at the ER-mitochondrion interface,
known as the mitochondrion-associated ER membrane (MAM) (8). While Sigma-1Rs have been extensively
studied in the CNS, they are also found in multiple tissues,
including the cardiovascular system. In fact, the protein levels of
Sigma-1R have been revealed to be more highly expressed in the
heart tissue than the brain tissue in rats (9). In the first study on Sigma-1R function
in the heart, Sigma-1R stimulation was found to increase the
contractility of cultured cardiomyocytes (10). Sigma-1R is responsible for
mitochondrial metabolic regulation by regulating Ca2+
signaling from the ER into the mitochondrion (11). Tagashira et al (12) demonstrated that the upregulation of
Sigma-1R by agonists in vivo may rescue cardiac dysfunction
in mice with transverse aortic constriction-induced myocardial
dysfunction, as well as in abdominal aortic constriction-induced
cardiac hypertrophy ovariectomized female rats (13). The mechanisms of cardioprotection by
Sigma-1Rs are the Sigma-1R-mediated Akt-eNOS pathway stimulation
and IP3R-mediated mitochondrial ATP production recovery
(12,13).
In traditional Chinese medicine the formulation Yiqi
Huoxue (YQHX), is used to improve symptoms in patients with HF
(14,15). YQHX is thought to act through the
regulation of mitochondrial function and energy metabolism
(16). Qiliqiangxin is a widely
used YQHX formula that has been reported to increase the ATP
content in the heart tissue of HF rats (17). Similarly, Sigma-1R has been revealed
to exert cardioprotective functions, including enhanced
mitochondrial ATP production. However, whether Sigma-1R is also
regulated by YQHX during HF treatment, in addition to its
involvement in the enhancement of ATP in HF patients, requires
further study. In the present study, a YQHX formulation comprising
the following six types of Chinese herbs was used: Astragalus,
Codonopsis pilosula, Salvia miltiorrhiza,
Ligusticum wallichii, red Paeonia and carthami flowers
(18). To investigate the role of
Sigma-1R in the treatment of HF using YQHX, rats with myocardial
infarction (MI)-induced HF and cultured H9c2 cells with angiotensin
II (Ang II)-induced hypertrophy were used to investigate the effect
of YQHX on MI-induced myocardial dysfunction and Ang II-induced
hypertrophy.
Materials and methods
Materials
YQHX was composed of 40 g astragalus, 40 g
Codonopsis pilosula, 20 g Salvia miltiorrhiza, 20 g
Ligusticum wallichii, 20 g red Paeonia and 20 g carthami
flowers. Slices of the herbs were purchased from Beijing Tong Ren
Tang Chinese Medicine Co., Ltd. The herbs were boiled in water for
1 h and aliquots at a final concentration of 3 g herbs/ml were
stored at 4˚C. Fluvoxamine (Flv), an agonist of Sigma-1R, was
purchased from Abbott Healthcare Products Ltd. Ang II was purchased
from Chinese Peptide Company Ltd. Sigma-1R siRNA was provided by
Sangon Biotech Co., Ltd.
Determination of YQHX components by
ultra-high-performance liquid chromatography-mass spectrometry
(UHPLC-MS)
Aliquots of 0.02 g of lyophilized, pulverized, and
homogenized herbs were extracted using 5 ml methanol-water
(v-v=1:1) for 20 min at room temperature. The extract was filtered
through a 0.22-µm membrane. Next, 3 µl extract was injected into an
analytical column (Shimadzu UHPLC system; Shimadzu Corporation),
equipped with an LC-30AD solvent delivery system, an SIL-30AC
autosampler, a CTO-30A column oven, a DGU-20A3 degasser, and a
CBM-20A controller, for analysis. The separation of the compounds
was performed using a Phenomenex Kinetex C18 (2.6 µm, 100x2.1 mm;
Phenomenex Inc.) at 35˚C. The mobile phase, which consisted of 0.1%
formic acid in water (A) and methanol (B), was delivered at a flow
rate of 0.4 ml/min under a gradient program. The gradient system
was as follows: 0-1 min, 5% B; 1-35 min, 5-60% B; 35-40 min, 60-90%
B; 40-45 min, 90% B; 45.0-45.1 min, 90-5% B; 45.1-50 min, 5% B. The
diode-array detector was set at 254 nm and the online UV spectra
were recorded in a scanning range of 190-400 nm.
The mass spectra were acquired using a TripleTOF™
4600 system with a Duo Spray source (SCIEX; AB Sciex Pte. Ltd.) in
negative and positive ESI mode. The optimized parameters for the
negative and positive modes were as follows: Ion spray voltage,
5,500 (positive ion mode) and -4,500 V (negative ion mode); Turbo V
spray temperature, 600˚C; nebulizer gas (Gas 1), 50 psi; heater gas
(Gas 2), 60 psi; collision gas, medium; curtain gas, 30 psi;
declustering potential, 80 (positive ion mode) and -80 V (negative
ion mode). The collision energy was set at 35 (positive ion mode)
and -35 V (negative ion mode) with a spread of 15 V for the MS-MS
experiments. The data were acquired with the information-dependent
acquisition method. For TOF-MS and TOF-MS-MS analysis, the spectra
covered the range from m-z 70 to 1,000 Da and 50-1,000 Da. The data
were analyzed in Peak View Software™ 2.2 (SCIEX).
Animal models with MI and
treatments
The present study was approved by the Experimental
Animal Ethics Committee of Dongzhimen Hospital, Beijing University
of Chinese Medicine (ethical approval no. 16-24). Male adult
Sprague-Dawley (SD) rats (age, 6-7 weeks; weight, 190-210 g) were
obtained from Beijing Vital River Laboratory Animal Technology Co.,
Ltd. All the rats were kept in the barrier environment animal room
of the Chinese Medicine Pharmacology Laboratory, Dongzhimen
hospital [license no. SYXK(Beijing)2015-0001]. The animals were fed
a standard laboratory diet and given free access to tap water. The
cages were kept in a room with controlled temperature of 22±1˚C,
relative humidity of 65-70% and on a 12 h light-dark cycle. A total
of 40 rats were used in the study. All animals underwent a
myocardial infarction operation or sham operation, as previously
described (18). The rats in the
control group underwent a sham operation with no left anterior
descending coronary artery ligation. Following the surgical
operation, 4 rats died and the surviving MI rats were divided
randomly into three groups (9 rats in each group): MI (5 ml/kg/d
normal saline by gavage); MI + YQHX (5 ml/kg/d by gavage) and MI +
Flv (1 mg/kg/d by gavage). Rats that had undergone a sham operation
were administered with 5 ml/kg/d normal saline by gavage.
Treatments were administered on the day following surgery once a
day for 4 weeks.
Echocardiography and measurement of
cardiac hypertrophy
Non-invasive echocardiographic measurements were
performed in the rats using a Sino-Japanese Joint AloCa5000
ultrasound system equipped with a 7-15 MHz real-time
microvisualization scan head probe. In brief, after 4 weeks of
treatment, each rat was anesthetized with sodium pentobarbital (50
mg/kg body weight), and their hearts were imaged in two-dimensional
parasternal long-axis view. The left ventricular end-diastolic
volume (LVEDV), left ventricular end-systolic volume (LVESV), left
ventricular ejection fraction (LVEF), and left ventricular
fractional shortening (LVFS) were measured. To determine the extent
of cardiac hypertrophy, the hearts of each rat were weighed and the
ratio of heart weight-to-body weight (HW/BW) was calculated.
Transmission electron microscopy
(TEM)
Following heart perfusion with 4% paraformaldehyde,
the heart tissues were minced into 1-2 mm3 cubes and
fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer for 2 h at
room temperature. Tissues were post-fixed with 2% osmium tetroxide
for 1 h at 4˚C, and then rinsed three times with cold PBS. The
tissues were then progressively dehydrated using a series of
ethanol washes (50, 70, 90 and 100% EtOH), followed by dehydration
with propylene oxide and infiltration with 1:1 propylene oxide:Epon
with 1.5% DMP-30 for 1 h. Finally, the tissues were cut into 70-nm
sections using an ultramicrotome, placed on TEM grids, stained with
lead citrate, and imaged using a transmission electron microscope
(Hitachi Ltd.; model H-7650).
Measurement of ATP content in heart
tissue
Heart tissues were collected 4 weeks post-surgery
and homogenized on ice prior to assaying for ATP content. The ATP
content was determined using an ATP colorimetric-fluorometric assay
kit (cat. no. MAK1900; Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocol. In brief, heart tissue (10 mg) was
homogenized in 100 µl ATP assay buffer and then deproteinized using
a 10-kDa MWCO spin filter. ATP standards and fluorometric reaction
mixtures were prepared according to the manufacturer's protocol.
Next, fluorometric reaction mixtures with standards or samples were
incubated in the plate at room temperature for 30 min in the dark.
The fluorescence (FLU; λex = 535 nm and λem =
587 nm) was measured using a microplate reader and the amount of
ATP in the samples was calculated using an ATP standard curve.
Western blot analysis
Western blot analysis was performed as previously
described (18). The heart tissues
were homogenized on ice in RIPA buffer with complete protease
inhibitor (Roche Diagnostics GmbH). Following centrifugation for 20
min, the protein concentration of the resulting clear supernatant
was determined using the BCA method before use for western
blotting. In brief, 40-100 µg proteins were loaded and separated by
10 or 8% SDS-PAGE and transferred onto nitrocellulose membranes.
Membranes were blocked in Tris-buffered saline-Tween-20 (TBST) with
5% skimmed milk at room temperature for 1 h, and then incubated
with primary antibodies overnight at 4˚C. Following rinsing, the
membranes were incubated with peroxidase conjugated secondary
antirabbit (dilution, 1:5,000; cat. no. BA1054; Boster Biological
Technology Co., Ltd.) or antimouse antibodies (dilution, 1:5,000;
cat. no. BA1050; Boster Biological Technology Co., Ltd.) at room
temperature for 1 h and evaluated by enhanced chemiluminescence
(Super ECL Plus; Applygen Technologies) using a chemiluminescence
image analysis system (Tanon 4600; Tanon Science and Technology
Co., Ltd). Quantification was performed with densitometric analysis
using NIH ImageJ software (version 1.29; National Institutes of
Health). The antibodies used included anti-Sigma-1 receptor
antibody (dilution, 1:1,000; cat. no. 61994; Cell Signaling
Technology, Inc.), anti-inositol-1,4,5-trisphosphate type 2
receptor (IP3R2) antibody (dilution,1:500; cat. no.
sc-398434; Santa Cruz Biotechnology, Inc.) and anti-GAPDH antibody
(1:5,000; cat. no. ab181602; Abcam).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent, according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.). Next, reverse transcription was
performed using 1 µg total RNA and RevertAid Reverse Transcriptase
and oligo(dT)18 (cat. no. K1622; Thermo Fisher
Scientific, Inc.) at the following thermal profile: 42˚C for 60 min
and 70˚C for 5 min. PCR reactions were performed using SYBR green
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
a PCR machine (MxPro-Mx3000P; Agilent Technologies, Inc.) using the
following specific primer pairs: Calcineurin A (forward,
5'-CGATTCTCCGACAGGAAAAA-3' and reverse,
5'-AAGGCCCACAAATACAGCAC-3'); brain natriuretic peptide (BNP;
forward, 5'-CAGAAGCTGCTGGAGCTGATA-3' and reverse,
5'-TCCGGTCTATCTTCTGCCCA-3'); and GAPDH (forward,
5'-AGTTCAACGGCACAGTCAAG-3' and reverse,
5'-TACTCAGCACCAGCATCACC-3'). The thermocycling conditions were as
follows: Denaturation at 95˚C for 10 min, followed by 40 cycles of
95˚C for 30 sec 55˚C for 30 sec and 72˚C for 20 sec. Data analysis
was performed using MxPro-Mx3000P software, and was calculated as
the change in the cycle threshold (ΔCT) for the target gene
relative to the ΔCT for GAPDH (control gene) (19).
H9c2 cell culture and small
interfering RNA (siRNA) transfection
The H9c2 cell line was obtained from China National
Infrastructure of Cell Line Resource and maintained in plastic
25-cm2 flasks in Dulbecco's modified Eagle's medium
(DMEM-F12; Biological Industries), containing 10% fetal bovine
serum (FBS). The culture flasks were kept in a 95% air and 5%
CO2 humidified environment at 37˚C. At confluence, the
cells were washed and maintained in serum-free DMEM without
supplements for 24 h prior to the subsequent experiments. The cells
were then cultured with Ang II (10-7 mol/l) or YQHX
(freeze-dried powder of YQHX dissolved in DMEM, 0.1 mg/ml) for 72 h
prior to being harvested. To silence Sigma-1R expression, H9c2
cells were transfected with Sigma-1R siRNA (sense,
5'-ACACGTGGATGGTGGAGTA-3' and anti-sense,
5'-TACTCCACCATCCACGTGT-3'). The negative control siRNA (sense,
5'-TTCTCCGAACGTGTCACGT-3' and anti-sense,
5'-ACGTGACACGTTCGGAGAA-3'; Sangon Biotech Co., Ltd.) was used as
control. In brief, according to manufacturer's protocols, the
cultured cells were plated onto 6-well culture plates with 2.5 ml
medium per well. Next, 4.6 µl Sigma-1R siRNA (20 µM) was added to
125 µl DMEM and 7.5 µl Lipofectamine 2000 (GeneCopoeia Inc.) was
added to 125 µl DMEM. The two solutions were incubated separately
at room temperature for 5 min prior to mixing and incubating at
room temperature for 15 min. The resulting solution was added to
cells, which were incubated at 37˚C in a 5% CO2
atmosphere for 6 h to initiate transfection. The medium was
replaced with complete medium and the transfected cells were
collected for detection at 24 h or 72 h after transfection. In the
control group, the H9c2 cells were transfected with a negative
control siRNA for 6 h at 37˚C in a 5% CO2 atmosphere
using Lipofectamine 2000.
Cell surface area measurement
To establish the cell surface area, H9c2 cells were
stained with rhodamine-conjugated phalloidin (Beijing Solarbio
Science & Technology Co., Ltd.). According to the
manufacturer's protocols, the cultured cells were washed twice with
37˚C PBS and then fixed in 4% formaldehyde for 10 min at room
temperature. Following permeabilizing in 0.1% Triton X-100 in PBS
for 5 min at room temperature, the cells were incubated with
rhodamine-conjugated phalloidin (100 nM) at room temperature for 30
min in the dark. Next, the nuclei were stained with DAPI for 30 sec
and images of H9c2 cells were captured using a laser confocal
microscope at 400x magnification (TCS SP8X; Leica Microsystems,
Inc.). The surface areas were then analyzed using Image-Pro Plus
6.0 software (Media Cybernetics, Inc.). Each group of cells was
counted in six wells, and at least 100 cells were measured in each
well. The cell surface area rate was given as the rate in the
control group.
Measurement of ATP content in H9c2
cells
The ATP content in the H9c2 cells was measured using
an enhanced ATP detection kit (Beyotime Institute of
Biotechnology). According to the manufacturer's protocols, the
cultured cells were washed twice with PBS and lysed. After 5 min of
centrifugation at 12,000 x g at 4˚C, the resulting supernatant was
used for detection. Next, 100 µl ATP detection fluid was added to
the detection platform. The plate was left to stand at room
temperature for 5 min to allow all the background ATP to be
consumed. Next, 20 µl the sample or standard was added to each
detection platform and mixed quickly. Finally, the mixture was
analyzed using a luminometer (Varioscan Flash; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All of the statistical tests were performed using
SPSS 16.0 (SPSS, Inc.). All values are expressed as the mean ±
standard deviation. The statistical significance of the differences
between the groups was tested using one-way analysis of variance,
followed by the Tukey's post hoc test. Two group comparisons were
performed using independent sample t-tests. Comparisons of
non-normal distribution data were followed by analysis with the
Kruskal-Wallis method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of YQHX using liquid
chromatography-mass spectrometry (LC-MS)
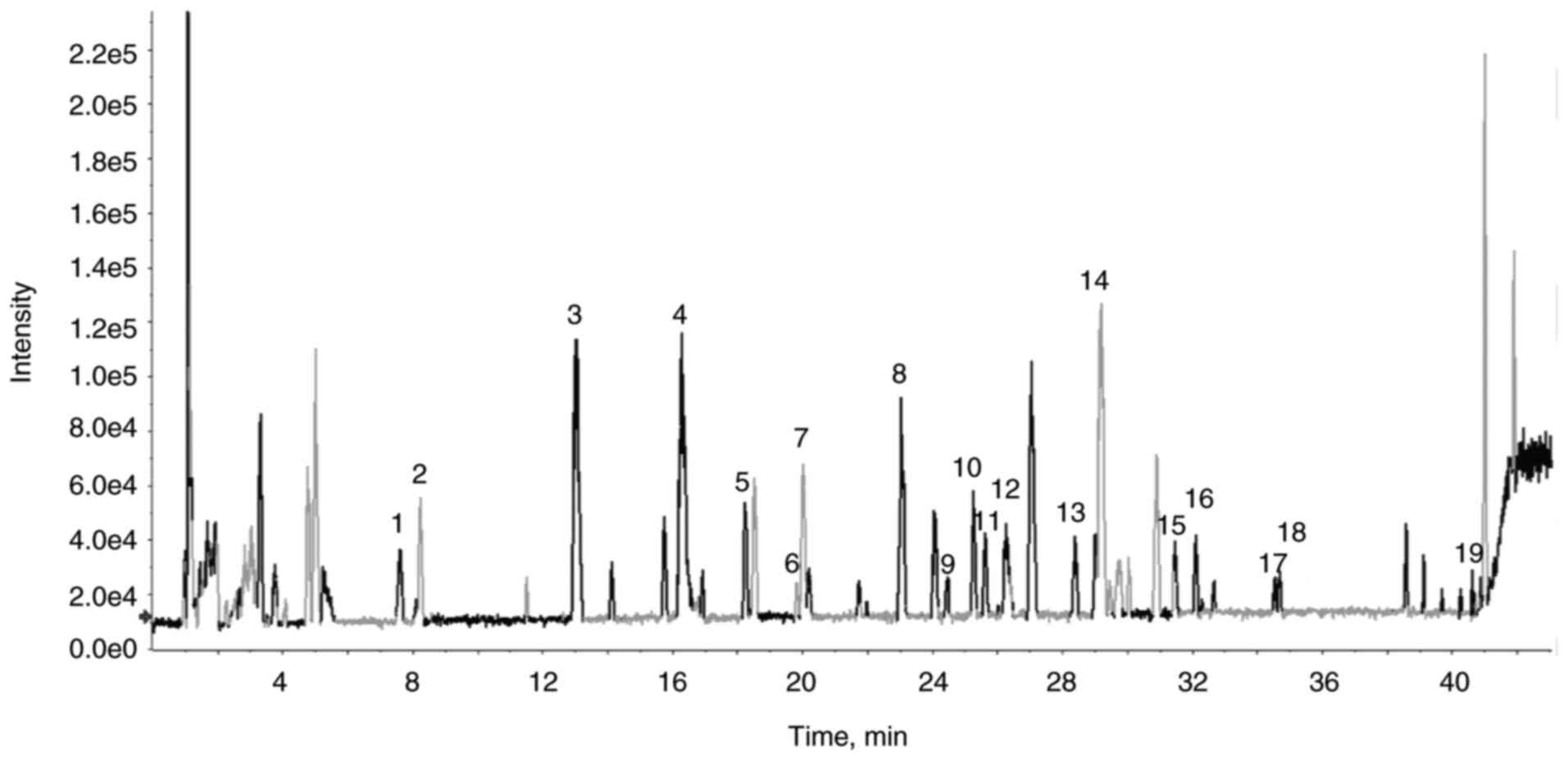
The main components of YQHX were analyzed by LC-MS.
The typical base peak chromatograms of the components of YQHX are
shown in Fig. 1. Approximately 21
major compounds were detected (Table
I), including paeoniflorin, calycosin and tanshinone.
 | Table IComponents identified from YQHX
extracts. |
Table I
Components identified from YQHX
extracts.
| Peak no. | Retention time,
min | Assigned
identity | Molecular
formula |
|---|
| 1 | 7.6 |
Dihydrokaempferol-3-O-glucoside |
C21H22O11 |
| 2 | 8.2 | Tryptophan |
C11H12N2O2 |
| 3 | 13 | Hydroxysafflor
yellow A |
C27H32O16 |
| 4 | 16.3 | Paeoniflorin |
C23H28O11 |
| 5 | 18.44 | Ferulic acid |
C10H10O4 |
| 6 | 19.6 | Senkyunolide J |
C12H18O4 |
| 7 | 20.1 |
Calycosin-7-O-β-D-glucoside |
C22H22O10 |
| 8 | 23.1 |
4-hydroxy-3-butylphthalide |
C12H14O3 |
| 9 | 24.4 | Senkyunolide F |
C12H14O3 |
| 10 | 25.6 | Ononin |
C22H22O9 |
| 11 | 25.7 | Lobetyolin |
C20H28O8 |
| 12 | 26.2 | Salvianlic acid
B |
C36H30O16 |
| 13 | 28.3 |
3-butylidenephthalide |
C12H12O2 |
| 14 | 29.1 | Calycosin |
C16H12O5 |
| 15 | 31.5 |
benzoylpaeoniflorin |
C30H32O12 |
| 16 | 32.1 | Z-Ligustilide |
C12H14O2 |
| 17 | 34.5 | Senkyunolide A |
C12H16O2 |
| 18 | 34.6 | Formononetin |
C16H12O4 |
| 19 | 40.6 | Levistolide A |
C24H28O4 |
| 20 | 40.9 | Tanshinone I |
C18H12O3 |
| 21 | 41.9 | Tanshinone IIA |
C19H18O3 |
YQHX preserves heart function and
alleviates myocardial hypertrophy in MI rats
To observe the effect of YQHX on the heart function,
non-invasive echocardiographic measurements were taken 4 weeks
post-treatment. The results demonstrated that 4 weeks after MI
surgery, the rats developed heart dysfunction, as shown by
increased LVEDV and LVESV and decreased LVEF and LVFS (all
P<0.01 vs. sham; Table II).
YQHX administration for 4 weeks attenuated the impairment of LVEF
and LVFS (all P<0.01 vs. MI; Table
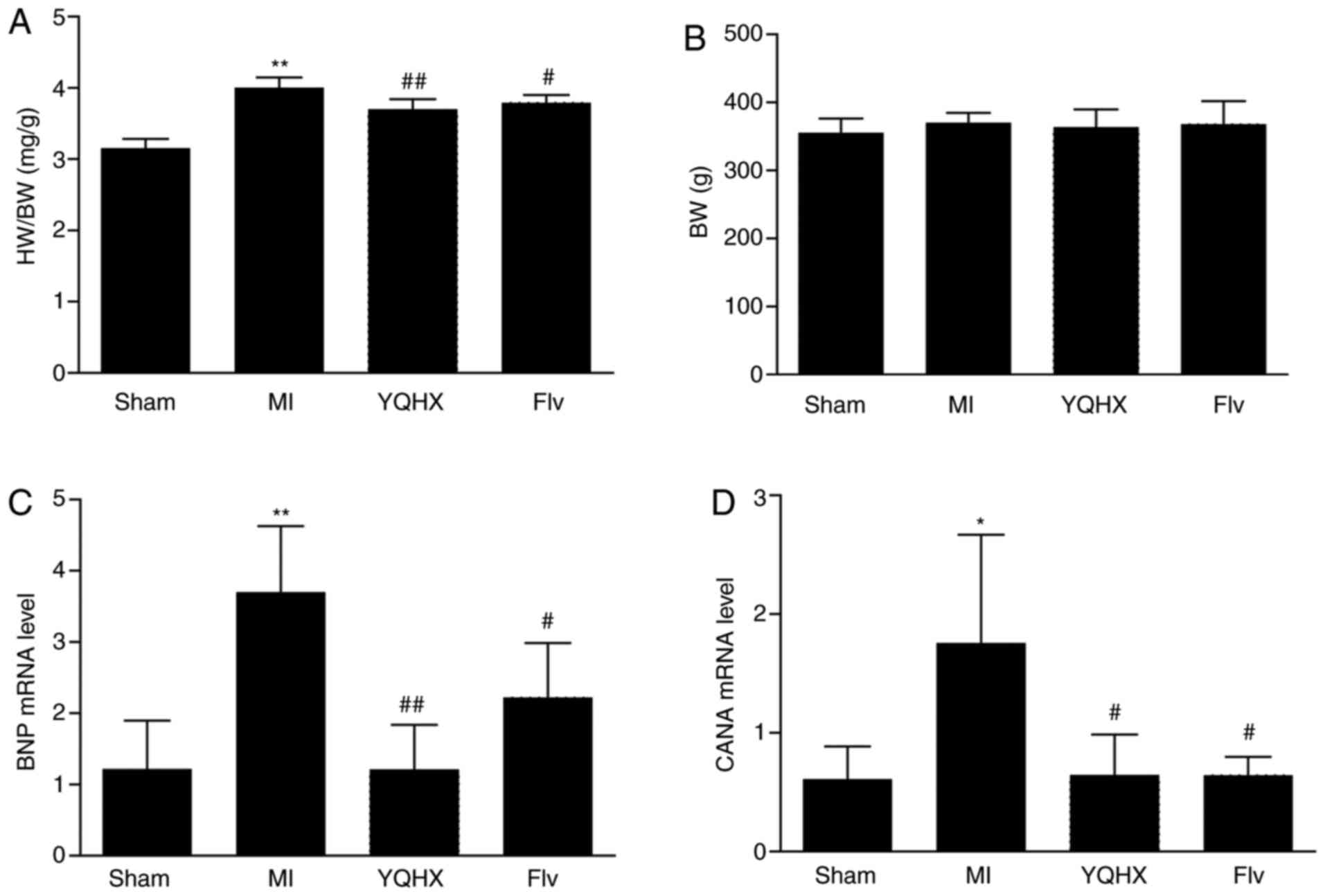
II) but had no effects on LVEDV and LVESV (Table II). Hypertrophy markers, including
the ratio of HW/BW, and the levels of calcineurin A and BNP mRNA
were also detected. The ratio of HW/BW was found to increase in the
MI group, compared with the sham-operated controls (P<0.01 vs.
sham; Fig. 2A). YQHX treatment
significantly decreased the rise in the HW/BW ratio (P<0.01 vs.
sham; Fig. 2A), and a similar
effect was observed in Flv (Fig.
2A). The calcineurin A and BNP mRNA levels were significantly
increased in the MI group, and YQHX and Flv treatment antagonized
the increase in calcineurin A and BNP mRNA levels (Fig. 2C and D).
 | Table IIEchocardiographic data for rats 4
weeks after MI with treatments, including YQHX. |
Table II
Echocardiographic data for rats 4
weeks after MI with treatments, including YQHX.
| Parameter | Sham | MI | MI+YQHX | MI+Flv |
|---|
| LVEDV | 220.1±43.3 |
415.4±67.5a |
445.1±61.1a |
403.5±114.5a |
| LVESV | 24.8±8.9 |
286.7±54.5a |
242.3±54.6a |
227.8±90.1a |
| LVEF | 90.4±3.3 |
31.2±4.6a |
46.1±6.5a,b |
45.7±11a,b |
| LVFS | 63.2±5.2 |
15.5±2.5a |
24.2±4.1a,b |
24.1±7.2a,b |
YQHX improves the ATP content and
mitochondrial ultrastructure of myocardial tissue in MI rats
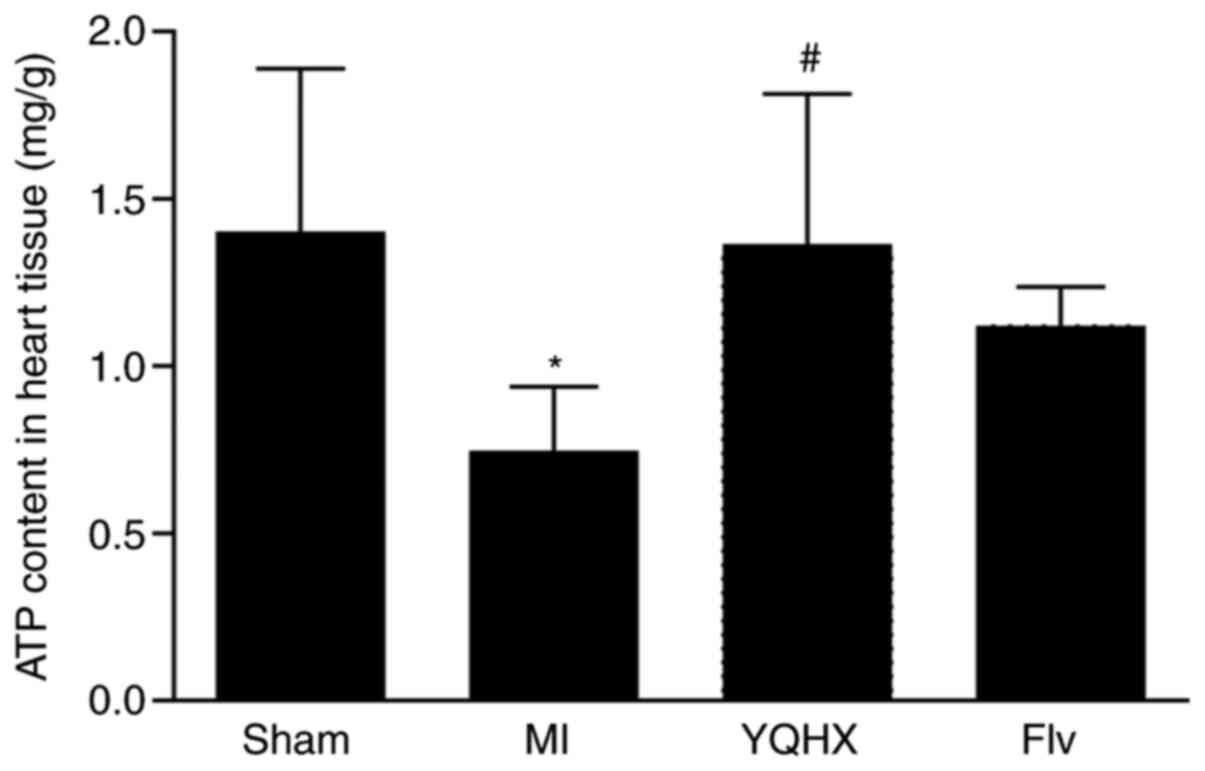
To observe the effect of YQHX on energy metabolism
in HF, the ATP content was detected in the hearts of the different
groups. The results demonstrated that 4 weeks after MI surgery, the
ATP content in the rats was found to decrease to 53% in the heart
tissues, compared with the sham controls (P<0.05; Fig. 3). YQHX administration increased the
ATP content in the heart tissues to nearly normal levels
(P<0.05; Fig. 3). Flv treatment
exhibited a tendency to increase the ATP content in heart failure
(P>0.05; Fig. 3).
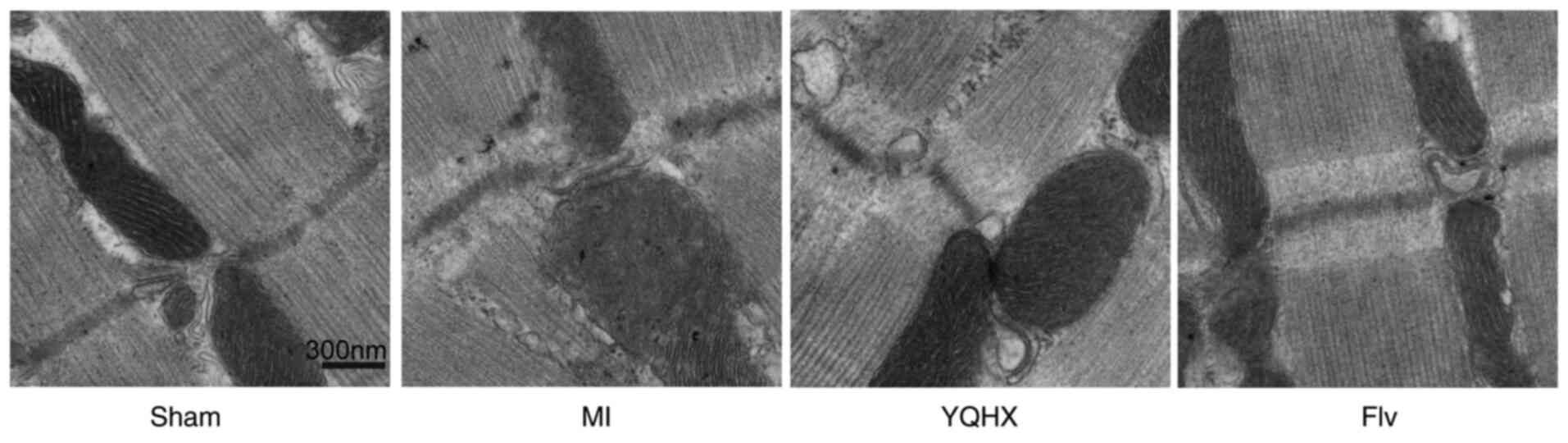
Approximately 90% of ATP is synthesized inside the
mitochondria. The function of mitochondria is based on its
structure. Therefore, the mitochondrial ultrastructure of the
surrounding infarction areas of left ventricular (LV) myocardial
tissues from the different groups was investigated using TEM.
Representative micrographs are provided in Fig. 4. The mitochondria in the heart
tissues of the sham group showed complete membranes and neatly
arranged internal ridges. By contrast, in the MI rats, most of the
mitochondrial membranes disappeared and the internal ridges were
ruptured and indistinct. Subsequently, YQHX and Flv treatment were
revealed to improve the mitochondrial ultrastructure of heart
tissues following MI surgery (Fig.
4).
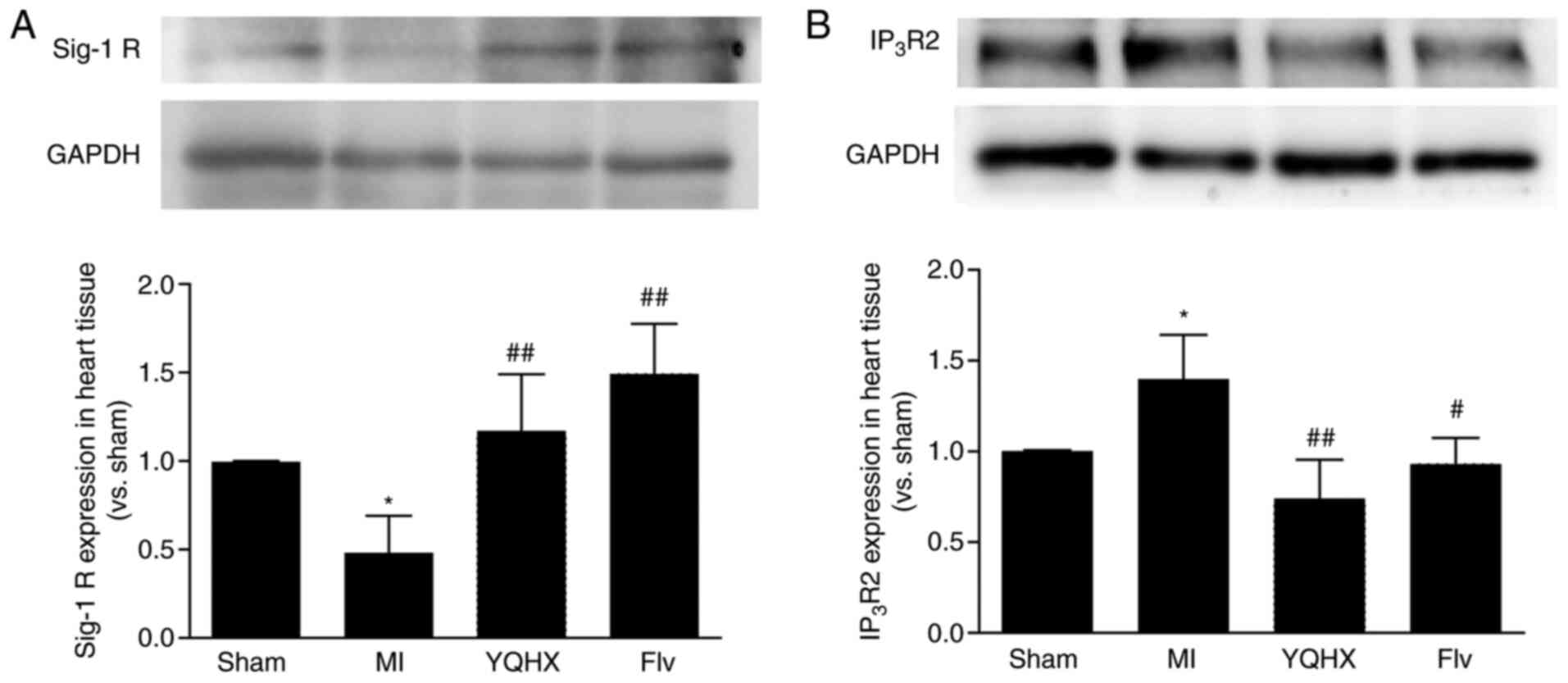
YQHX administration attenuates the
change in Sigma-1R and IP3R2 expression in the LV of MI
rats
To evaluate the role of Sigma-1R in the curative
effect of YQHX on HF, Sigma-1R and Ca2+
transporter-IP3R2 expression was detected in the heart
tissues of rats by western blotting. The data showed that Sigma-1R
expression in the heart tissue decreased significantly 4 weeks
post-MI surgery in rats (P<0.05 vs. sham) and was be reversed by
treatment with the YQHX and Sigma-1R agonist Flv (P<0.01 vs. MI;
Fig. 5A). IP3R2 was
upregulated 4 weeks post-MI surgery. However, the administration of
YQHX and Flv eliminated this MI-induced upregulation of
IP3R2 (Fig. 5B).
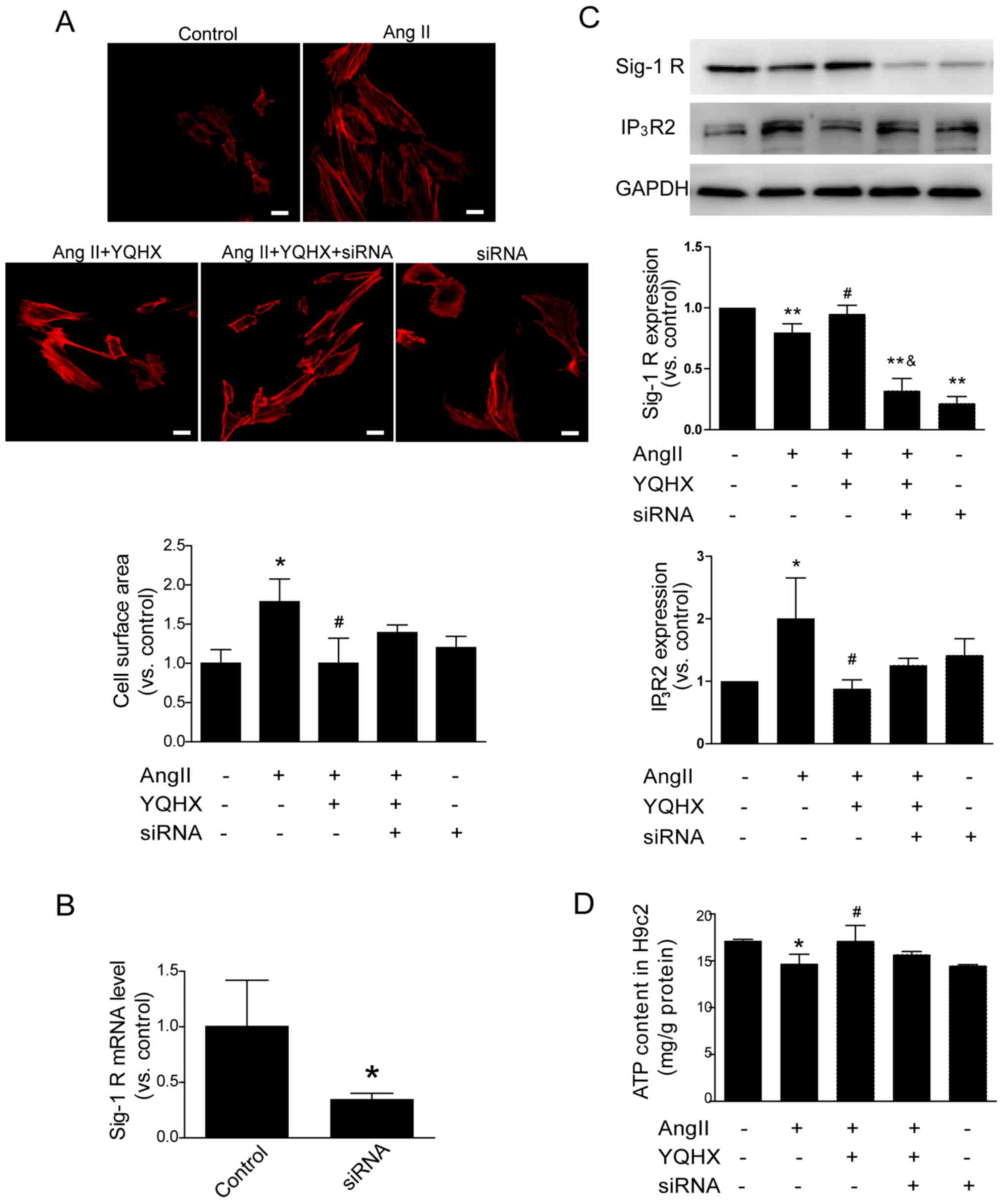
YQHX attenuates cell hypertrophy and
decreases ATP induced by Ang II in H9c2 by stimulating
Sigma-1R
Ventricular hypertrophy is a compensatory attempt by
the heart to enhance its performance. However, it may become
maladaptive when the heart is persistently exposed to an increased
load. In cultured H9c2 cells, cell hypertrophy was induced by
incubating cells with Ang II. Using rhodamine-conjugated phalloidin
staining, cell hypertrophy was investigated. The results revealed
that the cell surface area in Ang II-treated cells was increased
significantly compared with control cells (P<0.01). YQHX
treatment attenuated Ang II-induced hypertrophy (Fig. 6A). Using western blotting, Sigma-1R
expression was revealed to be downregulated following treatment
with Ang II (Fig. 6C), and was the
same as reported in a previous study (12). When Ang II-induced H9c2 cells were
co-incubated with YQHX, decreased Sigma-1R expression was restored
(Fig. 6C). The protein levels of
IP3R2 were also evaluated by western blotting. The
results demonstrated that IP3R2 levels were increased in
Ang II-incubated cells compared with the control cells and were
partially antagonized by YQHX (Fig.
6C). ATP starvation is a pathophysiological characteristic in
HF (5). Our previous in vivo
experiments demonstrated that YQHX improved heart energy metabolism
in HF rats, indicated by an increased ATP content in heart tissues
(Fig. 3). Next, the effect of YQHX
on ATP content was investigated in cultured H9c2 cells and it was
demonstrated that cells incubated with Ang II had a decreased ATP
content (Fig. 6D), in addition to
an increased cell surface area (Fig.
6A).
To determine whether Sigma-1R was the key regulator
in the anti-hypertrophy effect of YQHX, H9c2 cells were transfected
with a specific inhibitor siRNA of Sigma-1R. As demonstrated in
Fig. 6B, the levels of Sigma-1R
mRNA expression were downregulated by 65% in cells incubated with
Sigma-1R siRNA (P<0.05).
These results demonstrated that YQHX treatment
attenuated Ang II-induced hypertrophy in Ang II-treated cells.
Furthermore, Sigma-1R siRNA was revealed to partially neutralize
this effect (Fig. 6A).
Additionally, the attenuation of the Ang II-induced decrease in
Sigma-1R levels and increase in IP3R2 levels by YQHX was
inhibited by Sigma-1R siRNA (Fig.
6C), which also attenuated the improvement in the ATP content
in Ang II-treated cells by YQHX (Fig.
6D; P>0.05).
Discussion
In HF, a deficit in energy may account for the
contractile dysfunction observed during maximal exertion (5). In traditional Chinese medicine,
QiShenYiQi and Qiliqiangxin, which are manufactured using YQHX,
have been suggested to ameliorate the symptoms of disorders in
cardiac structure and function, possibly via an increase in the
myocardial ATP content (20-22).
Studies on the major active ingredients of Astragalus, a drug used
in the formulation of YQHX, including Astragaloside IV and
Astragalus polysaccharide, have been proven to regulate
mitochondrial function and energy biosynthesis (23,24).
In the present study, heart dysfunction in MI rats, represented by
decreased LVEF and LVFS, was attenuated after 4 weeks of YQHX
treatment. Myocardial hypertrophy, measured as the ratio of HW/BW,
was also found to be alleviated following treatment. Taken
together, these results indicated that YQHX improved heart function
in MI rats with heart failure, which is consistent with the results
of our previous study (18). To
investigate the effects of YQHX treatment on energy metabolism in
HF, the ATP content and mitochondria ultrastructure were assessed
in the heart tissues of MI rats following euthanasia. While the ATP
content in rats with HF was decreased, it was found that treatment
with YQHX attenuated this effect. Additionally, the impaired
mitochondrial ultrastructure of heart tissues post-MI surgery was
improved following treatment with YQHX.
Sigma-1R has been demonstrated to participate in
numerous diseases, including stroke, dementia and cancer (25), and was recently found to exert
cardioprotective effects (12), as
well as being identified as a receptor chaperone. It is located in
the membrane of MAM, and has been revealed to be expressed
abundantly in whole-cell extracts from LV and RV, compared with
brain and kidney extracts (7). When
Sigma-1R was used as a selective serotonin reuptake inhibitor in a
previous study, it was reported to decrease post-MI morbidity and
mortality in patients with depression (26). The results of the present study
indicated that Sigma-1R expression is decreased in the heart of HF
rats, which is in agreement with the results of a previous study
(12). When treated with YQHX, rats
with MI surgery showed increased Sigma-1R expression in the heart,
as well as increased ATP content and improved heart function. The
same change was observed in rats treated with Flv, an agonist of
Sigma-1R. Studies have demonstrated that Sigma-1R is associated
with the maintenance of mitochondrial structure and function
(11). In earlier
Sigma-1R-/- knockdown experiments in mice, irregularly
shaped, highly fused mitochondrial network with abnormal cristae
and a significantly lower ATP pool were observed in cardiomyocytes
(27). In addition,
Sigmar1-/- hearts showed significant cardiac fibrosis,
collagen deposition and the development of cardiac contractile
dysfunction (26). The results of
the present study suggested that YQHX may improve cardiac function
by increasing Sigma-1R expression and improving mitochondrial
ultrastructure and ATP production.
Several studies have demonstrated that Sigma-1R
serves key roles in heart function improvement by regulating
Akt-eNOS signaling and intracellular Ca2+ (12,28).
Sigma-1R acts as an inter-organelle signaling modulator between the
ER and mitochondria, nuclei and plasma membranes. By regulating
ER-mitochondrion signaling, Sigma-1R influences intra-mitochondrial
Ca2+ homeostasis and cellular bioenergetics (29). Sigma-1R, acting as a molecular
chaperone to IP3R, has been observed to enhance
Ca2+ signaling from the ER into mitochondria, thereby
activating the tricarboxylic acid cycle and increasing ATP
production (8). Studies have
demonstrated that IP3R2 is upregulated and Sigma-1R
downregulated in hypertrophic cardiomyocytes. Sigma-1R agonists
exhibit improved heart function by stabilizing the
Sigma-1R-IP3R complex (28). In the present study, the levels of
IP3R2 in the heart tissues of the different groups were
detected and it was found that YQHX neutralized the increased
IP3R2 expression in MI rats. These results suggested
that YQHX may improve injured heart function and ATP content in the
heart tissue, possibly via Sigma-1R and IP3R2
regulation. To confirm the role of Sigma-1R in the cardioprotective
effect of YQHX, Sigma-1R was knocked down via siRNA transfection in
H9c2 cells in vitro. While YQHX prevented cell hypertrophy
and the reduction in ATP induced by Ang II, these positive effects
were found to be partially inhibited by Sigma-1R inhibition. These
in vitro results suggested that YQHX is able to increase the
ATP content and rescue the cell hypertrophy induced by Ang II,
partly by stimulating Sigma-1R. However, further experiments
focused on intercellular Ca2+ homeostasis regulation and
Sigma-1R-IP3R complex stabilization are required to
confirm these results. Notably, in addition to IP3R
regulation, Sigma-1R binds to another important Ca2+
transporter-the ryanodine receptor (RyR) and regulates its function
(13). Sigma-1R stimulation
inhibits the RyR-induced Ca2+ release from the
sarcoplasmic reticulum, leading to the suppression of
overconstriction (30). In future
studies, the mechanisms underlying regulation of Ca2+
homeostasis by Sigma-1R and how YQHX affects this will be
investigated.
A previous study demonstrated that the expression of
Sigma-1R is regulated by numerous factors, including cold
stimulation, ERS signal molecules and certain chemicals (31). A recent study has demonstrated that
miRNA may also regulate the expression of Sigma-1R (32). In the present study, numerous
monomers in the formulation for YQHX that had effects on the
regulation of mitochondrial bioenergetic- s were investigated
(33-35).
However, how specific components of YQHX regulate the expression of
Sigma-1R requires further study.
In conclusion, these in vivo and in
vitro results demonstrated that YQHX improved heart function by
regulating the heart's energy metabolism and inhibiting cell
hypertrophy. These effects partially relied on the stimulation of
Sigma-1R. The results of the present study provided evidence of
Sigma-1R as a therapeutic target for HF treatment in traditional
Chinese medicine, and the significant therapeutic potential of
Sigma-1R for cardiovascular disease. However, further investigation
is required to fully elucidate the details of Sigma-1R function in
the treatment of HF with YQHX (28).
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant nos. 81403366 and 81570656),
Fundamental Research Funds for the Central Universities (grant no.
2020-JYB-ZDGG-114) and the ‘Xin Ao’ research fund of the Beijing
University of Chinese Medicine (grant no. 2017-XAJLJJ-012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL, CL, JW, AW, TZ, ZM, YZ, QJ and HC performed the
study; LL, LC, DZ and WJL designed the experiments; LL, BN and WJL
analyzed the data; LL and WJL wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were performed in accordance
with the guidelines of the Institutional Animal Care and Use
Committee of the Beijing University of Chinese Medicine, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ambrosy AP, Fonarow GC, Butler J, Chioncel
O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN
and Gheorghiade M: The global health and economic burden of
hospitalizations for heart failure: Lessons learned from
hospitalized heart failure registries. J Am Coll Cardiol.
63:1123–1133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang Y, Bauersachs J and Langer HF:
Immune mechanisms in heart failure. Eur J Heart Fail. 19:1379–1389.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Zhou B and Tian R: Mitochondrial
dysfunction in pathophysiology of heart failure. J Clin Invest.
128:3716–3726. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hein S, Arnon E, Kostin S, Schönburg M,
Elsässer A, Polyakova V, Bauer EP, Klövekorn WP and Schaper J:
Progression from compensated hypertrophy to failure in the
pressure-overloaded human heart: Structural deterioration and
compensatory mechanisms. Circulation. 107:984–991. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Neubauer S: The failing heart-an engine
out of fuel. N Engl J Med. 356:1140–1151. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ehmke H: The Sigma-1 receptor: A molecular
chaperone for the heart and the soul? Cardiovasc Res. 93:6–7.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bhuiyan MS and Fukunaga K: Targeting
Sigma-1 receptor signaling by endogenous ligands for
cardioprotection. Expert Opin Ther Targets. 15:145–155.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayashi T and Su TP: Sigma-1 receptor
chaperones at the ER-mitochondrion interface regulate Ca(2+)
signaling and cell survival. Cell. 131:596–610. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bhuiyan MS, Tagashira H, Shioda N and
Fukunaga K: Targeting Sigma-1 receptor with fluvoxamine ameliorates
pressure-overload-induced hypertrophy and dysfunctions. Expert Opin
Ther Targets. 14:1009–1022. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ela C, Barg J, Vogel Z, Hasin Y and Eilam
Y: Sigma receptor ligands modulate contractility, Ca++
influx and beating rate in cultured cardiac myocytes. J Pharmacol
Exp Ther. 269:1300–1309. 1994.PubMed/NCBI
|
|
11
|
Marriott KS, Prasad M, Thapliyal V and
Bose HS: σ-1 receptor at the mitochondrial-associated endoplasmic
reticulum membrane is responsible for mitochondrial metabolic
regulation. J Pharmacol Exp Ther. 343:578–586. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tagashira H, Bhuiyan S, Shioda N, Hasegawa
H, Kanai H and Fukunaga K: Sigma1-receptor stimulation with
fluvoxamine ameliorates transverse aortic constriction-induced
myocardial hypertrophy and dysfunction in mice. Am J Physiol Heart
Circ Physiol. 299:H1535–H1545. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tagashira H, Bhuiyan MS and Fukunaga K:
Diverse regulation of IP3 and ryanodine receptors by pentazocine
through σ1-receptor in cardiomyocytes. Am J Physiol Heart Circ
Physiol. 305:H1201–H1212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li XQ, He JC, Huang PX and Cao XB: Chinese
medicine syndromes in congestive heart failure: A literature study
and retrospective analysis of clinical cases. Chin J Integr Med.
22:738–744. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin SS, Liu CX, Zhang JH, Wang XL and Mao
JY: Efficacy and safety of oral Chinese patent medicine combined
with conventional therapy for heart failure: An overview of
systematic reviews. Evid Based Complement Alternat Med.
2020(8620186)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen YY, Li Q, Pan CS, Yan L, Fan JY, He
K, Sun K, Liu YY, Chen QF, Bai Y, et al: QiShenYiQi Pills, a
compound in Chinese medicine, protects against pressure
overload-induced cardiac hypertrophy through a multi-component and
multi-target mode. Sci Rep. 5(11802)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Fu M, Wang J, Zhang J, Han X, Song
Y, Fan Y, Hu K, Zhou J and Ge J: Qiliqiangxin improves cardiac
function through regulating energy metabolism via HIF-1α-dependent
and independent mechanisms in heart failure rats after acute
myocardial infarction. Biomed Res Int. 2020(1276195)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lou LX, Wu AM, Zhang DM, Wu SX, Gao YH,
Nie B, Zhao MJ, Lv XY, Jin QS, Zhao YZ, et al: Yiqi Huoxue recipe
improves heart function through inhibiting apoptosis related to
endoplasmic reticulum stress in myocardial infarction model of
rats. Evid Based Complement Alternat Med.
2014(745919)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li J, Guan XK and Liu RX: Role of Chinese
herbal medicines in regulation of energy metabolism in treating
cardiovascular diseases. Chin J Integr Med. 25:307–315.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang J, Wei C, Wang H, Tang S, Jia Z,
Wang L, Xu D and Wu Y: Protective effect of qiliqiangxin capsule on
energy metabolism and myocardial mitochondria in pressure overload
heart failure rats. Evid Based Complement Alternat Med.
2013(378298)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang DX, Zhao HP, Pan CS, Liu YY, Wei XH,
Yang XY, Chen YY, Fan JY, Wang CS, Han JY and Li PP: QiShenYiQi
Pills, a compound Chinese medicine, ameliorates doxorubicin-induced
myocardial structure damage and cardiac dysfunction in rats. Evid
Based Complement Alternat Med. 2013(480597)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu Y, Li S, Wu H, Bian Z, Xu J, Gu C, Chen
X and Yang D: Beneficial effects of astragaloside IV against
angiotensin II-induced mitochondrial dysfunction in rat vascular
smooth muscle cells. Int J Mol Med. 36:1223–1232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luan A, Tang F, Yang Y, Lu M, Wang H and
Zhang Y: Astragalus polysaccharide attenuates isoproterenol-induced
cardiac hypertrophy by regulating TNF-α-PGC-1α signaling mediated
energy biosynthesis. Environ Toxicol Pharmacol. 39:1081–1090.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsai SY, Hayashi T, Mori T and Su TP:
Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med
Chem. 9:184–189. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Taylor CB, Youngblood ME, Catellier D,
Veith RC, Carney RM, Burg MM, Kaufmann PG, Shuster J, Mellman T,
Blumenthal JA, et al: Effects of antidepressant medication on
morbidity and mortality in depressed patients after myocardial
infarction. Arch Gen Psychiatry. 62:792–798. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abdullah CS, Alam S, Aishwarya R, Miriyala
S, Panchatcharam M, Bhuiyan MAN, Peretik JM, Orr AW, James J,
Osinska H, et al: Cardiac dysfunction in the sigma 1 receptor
knockout mouse associated with impaired mitochondrial dynamics and
bioenergetics. J Am Heart Assoc. 7(e009775)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tagashira H, Bhuiyan MS, Shioda N and
Fukunaga K: Fluvoxamine rescues mitochondrial Ca2+
transport and ATP production through σ(1)-receptor in hypertrophic
cardiomyocytes. Life Sci. 95:89–100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Su TP, Hayashi T, Maurice T, Buch S and
Ruoho AE: The Sigma-1 receptor chaperone as an inter-organelle
signaling modulator. Trends Pharmacol Sci. 31:557–566.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kushnir A, Wajsberg B and Marks AR:
Ryanodine receptor dysfunction in human disorders. Biochim Biophys
Acta Mol Cell Res. 1865:1687–1697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rousseaux CG and Greene SF: Sigma
receptors [sigmaRs]: Biology in normal and diseased states. J
Recept Signal Transduct Res. 36:327–388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bao Q, Zhao M, Chen L, Wang Y, Wu S, Wu W
and Liu X: MicroRNA-297 promotes cardiomyocyte hypertrophy via
targeting Sigma-1 receptor. Life Sci. 175:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Hu Y, Qiukai E, Zuo J, Yang L and
Liu W: Salvianolic acid B inhibits mitochondrial dysfunction by
up-regulating mortalin. Sci Rep. 7(43097)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kwan KK, Huang Y, Leung KW, Dong TT and
Tsim KW: Danggui Buxue Tang, a Chinese herbal decoction containing
astragali radix and angelicae sinensis radix, modulates
mitochondrial bioenergetics in cultured cardiomyoblasts. Front
Pharmacol. 10(614)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jin HJ and Li CG: Tanshinone IIA and
cryptotanshinone Prevent mitochondrial dysfunction in
hypoxia-induced H9c2 cells: Association to mitochondrial ROS,
intracellular nitric oxide, and calcium levels. Evid Based
Complement Alternat Med. 2013(610694)2013.PubMed/NCBI View Article : Google Scholar
|