Introduction
Diabetic nephropathy (DN) is one of the most
important and common complications of diabetes. DN is characterized
by glomerular hypertrophy, proteinuria, reduced glomerular
filtration and renal fibrosis, resulting in loss of kidney function
(1). It is known that mesangial
expansion is due to excessive proliferation of mesangial cells and
accumulation of extracellular matrix under the pathogenic condition
of DN (2,3).
Chronic hyperglycemia is the most common feature in
all forms of diabetes mellitus and plays an important role in the
development of diabetes-related complications by accelerating the
induction of aldose reductase and the irreversible formation of
advanced glycation end products (AGEs) (4). Methylglyoxal (MGO), a highly reactive
dicarbonyl compound, is the main precursor of AGEs. MGO reacts
primarily with arginine residues and, to a lesser extent, with
lysine residues, to form MGO-derived AGEs (5,6). It
was well known that AGEs play a pivotal role in the development and
progression of diabetic complications through various mechanisms
(7). Especially, AGEs significantly
contribute to the activation of mesangial cell expansion (8). AGEs increase the expression of the
receptor for AGEs (RAGE) and interaction of AGEs and RAGE leads to
increased oxidative stress by ROS production and NADPH oxidase
(NOX) expression, contributing to the development of DN. It is
known that NOX4 is mainly expressed in the kidney cortex and is
closely related to diabetic nephropathy development (9).
Studies have reported the use of numerous natural
products and their active ingredients in the treatment of diabetes
and diabetes-related complications (10,11).
Psoralea corylifolia Linn. seed (PCS) is a widely used
herbal medicine with various biological activities, including
antitumor, antioxidant, and anti-inflammatory effects (12). It has been extensively used for the
treatment of many pathological conditions, such as skin disorders,
cancer, inflammatory diseases, neurodegenerative diseases, and
kidney disease (12-14).
The major active constituents of PCS are coumarins, flavonoids, and
meroterpenes. In a previous report, we observed the inhibitory
effect of PCS extract on diabetic nephropathy in
streptozotocin-induced diabetic mice (15). However, the detailed mechanism has
not been studied yet. In this study, we investigated the effects of
the PCS extract on the proliferation of mesangial cells, ROS
production, and in the expression of inflammatory and fibrotic
factors.
Materials and methods
Preparation of PCS extract
The PCS was artificially propagated and distributed
in accordance with the relevant laws and was purchased from an
oriental drug store (Kwang Myung Dang Co., Ulsan, Korea). The
extraction procedure was performed as described previously
(16). Briefly, the dried seeds
(300 g) were ground into smaller pieces and extracted twice with 3
L of distilled water, under reflux. The extract was stored in a
freezer (-80˚C) for 24 h before it was evaporated in vacuo to
produce a dark brownish residue.
Preparation of AGEs
The AGEs preparation was performed with a slight
modification of a previously reported method (17). AGEs were prepared under sterile
conditions by incubating BSA (100 mg/ml, MP Biomedicals) and MGO (1
mol/l, Sigma-Aldrich; Merck KGaA) in phosphate-buffered saline
(PBS, pH 7.4) containing 0.02% sodium azide (pH 7.4), at 37˚C for 7
days. Aminoguanidine (AG, 1 mM) was used as a positive control.
Control-BSA was prepared using similar incubation conditions but
without MGO. After incubation, unreacted carbonyls were removed by
extensive dialysis against ammonium bicarbonate buffer (30 mmol/l,
pH 7.9, 4˚C). AGEs and control-BSA preparations were further
filter-sterilized. AGEs concentrations were determined using the
bicinchoninic acid (BCA) protein assay (Pierce; Thermo Fisher
Scientific, Inc.) with BSA as a standard before the experimental
assay (18). Samples were stored in
a freezer at -20˚C until use. The concentration of AGEs and
control-BSA was expressed as the concentration of BSA protein added
to culture medium.
Cell culture
SV40 MES 13 cells were cultured as per the
manufacture's guidelines (CRL-1927, ATCC). The base medium for this
cell line was 3:1 mixture of DMEM (Welgene) and Ham's F12 medium
(Gibco; Thermo Fisher Scientific, Inc.), with 14 mM HEPES. To
prepare the complete growth medium, FBS (Gibco; Thermo Fisher
Scientific, Inc.) was added to the base medium to a final
concentration of 5%. The cells were maintained under standard
culture conditions (37˚C in a humidified 5% CO2
atmosphere). The SV40 MES 13 cells were seeded and incubated for
attachment overnight. The cells were treated with different
concentrations of the PCS extract with 10 µg/ml AGEs for 24 h, as
previously describe (19,20).
Cell viability assay
To determine the cytotoxicity of the PCS extract,
SV40 MES 13 cells were seeded in 96-well plates (1x104
cells/well) and incubated overnight for attachment. The cells were
then treated with various concentrations of the PCS extract for 24
h. To confirm the inhibitory effect of the PCS extract on
AGEs-induced mesangial cell proliferation, the cells were seeded in
96-well plates (1x104 cells/well) and incubated
overnight for attachment. Subsequently, the cells were treated with
various concentrations of the PCS extract with 10 µg/ml AGEs, for
24 h. The D-Plus™ CCK cell viability assay kit (Dongin
LS) was used to measure cell viability. The absorbance was measured
at 450 nm using microplate reader (Synergy™ 2 Multi-Mode
Microplate Reader; BioTek Instruments, Inc.).
Cell cycle analysis
The SV40 MES 13 cells were cultured in 6-well plates
(5x104/well). After overnight incubation to promote
attachment, the cells were treated with 200 µg/ml PCS extract with
AGEs (10 µg/ml). After 24 h of treatment, the cells were fixed in
70% ethanol for 30 min at 4˚C. After washing twice with ice-cold
PBS, the cells were centrifuged again and stained with 1 µg/ml
propidium iodide (PI) staining solution (Sigma-Aldrich; Merck
KGaA). Flow cytometry analysis was performed using a FACSCalibur
flow cytometer (Becton Dickinson).
Measurement of ROS production
The SV40 MES 13 cells were seeded in a 96-well black
plate (1x104 cells/well). After overnight incubation for
attachment, the cells were treated with various concentrations of
the PCS extract with AGEs (10 µg/ml) for 24 h. The cells were
stained by 10 µM CM-H2DCFDA (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at 37˚C, after fixed in 10% neutral
buffered formalin for 10 min at 25˚C. After wash twice with PBS
containing Ca2+ and Mg2+, fluorescence was
detected immediately at an excitation/emission wavelength of
495/527 nm by fluorometer (Synergy™ 2 Multi-Mode
Microplate Reader).
Western blotting
The SV40 MES 13 cells were seeded in 6-well plates
(5x104/well). After overnight incubation for attachment,
the cells were treated with different concentrations of the PCS
extract (50, 100 and 200 µg/ml) with 10 µg/ml AGEs for 24 h. The
cells were harvested, and total protein was extracted using
M-PER™ Mammalian Protein Extraction Reagent (Thermo
Fisher Scientific, Inc.) containing a protease inhibitor cocktail
and a phosphatase inhibitor cocktail (both from Sigma-Aldrich;
Merck KGaA). The same amount of total protein was separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) using a Mini-PROTEAN Tetra Cell (Bio-Rad Laboratories,
Inc.) and transferred to a nitrocellulose membrane (GE Healthcare
Life Science) using Tetra Blotting Module (Bio-Rad Laboratories,
Inc.). The membrane was then blocked with 5% skim milk (BioShop
Canada Inc.) or 5% BSA and then incubated with specific primary
antibodies and horseradish peroxidase (HRP)-conjugated secondary
antibodies (Bethyl Laboratories). Antibodies against β-actin,
p-NF-κB p65, and TGF-β1 were purchased from Cell Signaling
Technology, Inc. Antibodies against RAGE, fibronectin, and collagen
(Col1A1) were purchased from Santa Cruz Biotechnology Inc.
Αntibodies against NADPH oxidase 4 (NOX4) were purchased from
Abcam. Chemiluminescent was developed by Immobilon®
Western (EMD Millipore). Detected on the ChemiDoc XRS+ system using
the Image Lab software (Bio-Rad Laboratories, Inc.).
Statistical analyses
Data are expressed as mean ± standard error.
Statistical analysis was done using two-way ANOVA with Tukey's
multiple comparisons test in GraphPad Prism 7 software (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation of AGEs
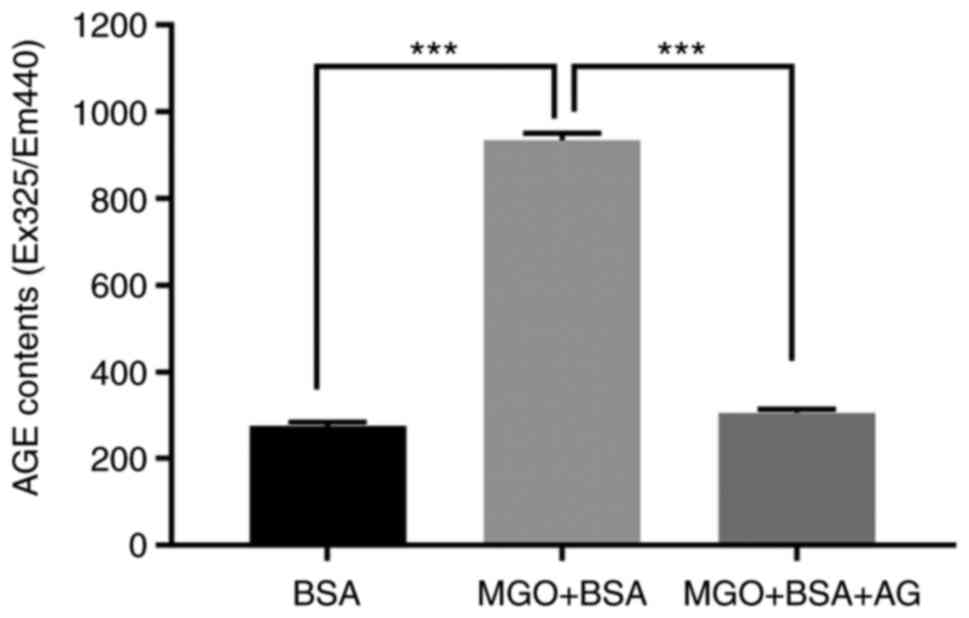
To obtain AGEs for the experiment, AGEs formation
was induced by co-incubation of MGO and BSA at 37˚C for 1 week. To
confirm the formation of AGEs, fluorescence was measured at
excitation and emission wavelengths of 360 and 420 nm,
respectively. Aminoguanidine (AG), an inhibitor of AGEs formation,
was used as a negative control. When MGO and BSA were incubated
together, the observed fluorescence was about 3.4 times higher than
that observed with BSA alone, and it was confirmed that this
increased fluorescence was inhibited by AG (Fig. 1). This result indicated that the
AGEs formation occurred appropriately. The obtained AGEs were
quantified after dialysis and then used in the subsequent
experiments.
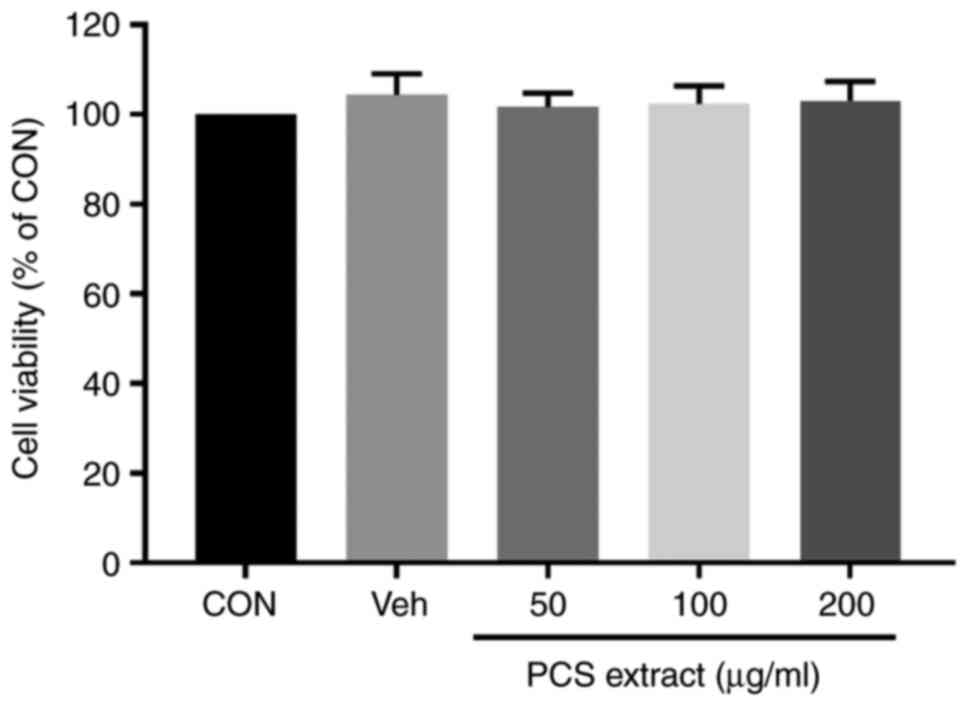
PCS extract does not show cytotoxicity
to SV40 MES 13 cells
Before examining the effect of the PCS extract on
the proliferation of mesangial cells by AGEs, the cytotoxicity of
the PCS extract was first examined by cell counting kit-8 (CCK-8)
assay. Treatment of SV40 MES 13 cells with various concentrations
of the PCS extract (50, 100 and 200 µg/ml) for 24 h did not show
any cytotoxic effect on SV40 MES 13 cells at all the tested
concentrations (Fig. 2).
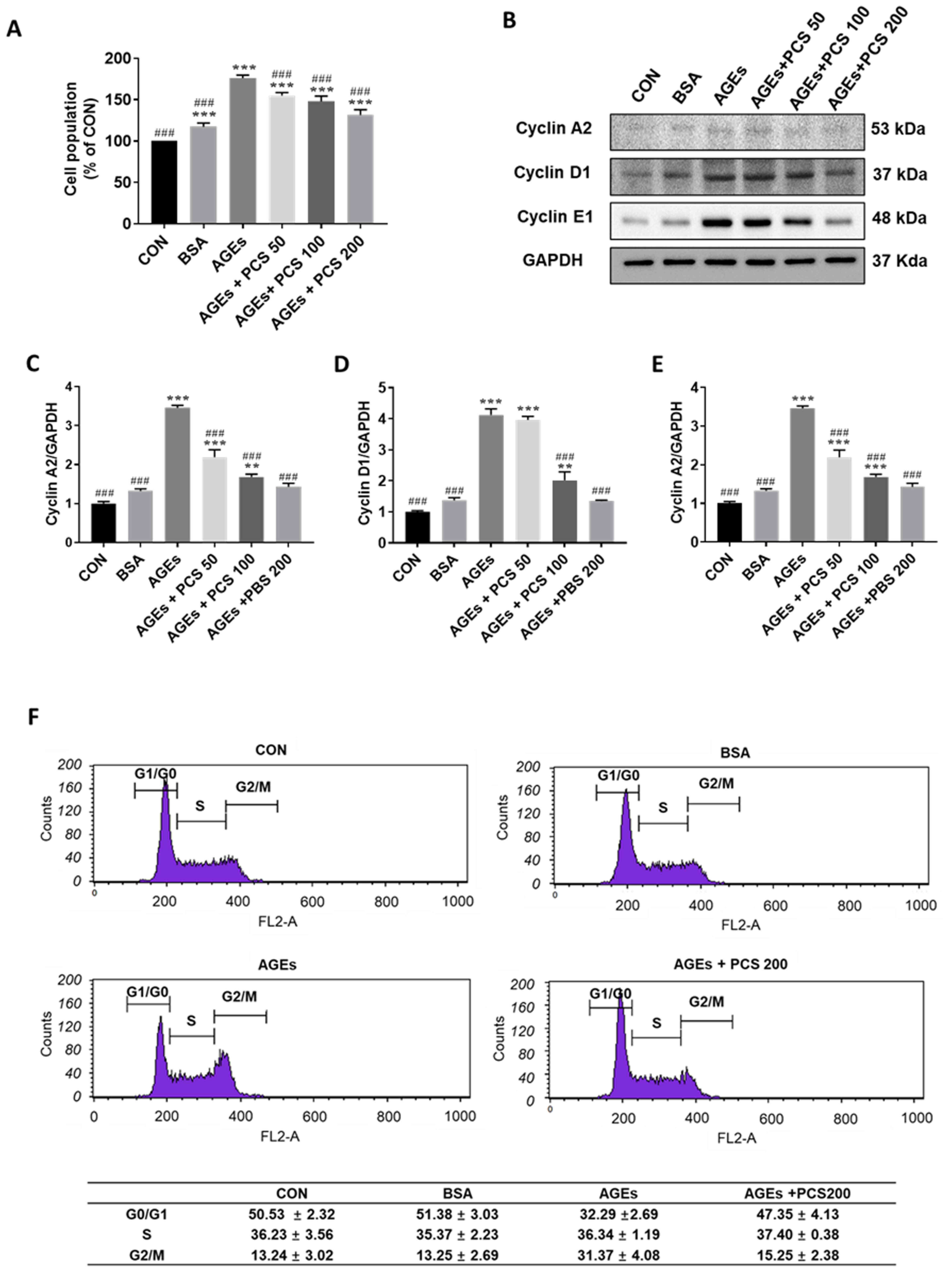
PCS extract inhibited AGEs-induced
proliferation of SV40 MES 13 cells
To investigate the effect of the PCS extract on
AGEs-induced mesangial proliferation, SV40 MES 13 cells were
treated with various concentrations of the PCS extract with AGEs
for 24 h. AGEs treatment significantly increased the cell
proliferation and PCS extract inhibited AGEs-induced mesangial cell
proliferation in a concentration-dependent manner (Fig. 3A). The cell cycle is tightly
regulated by cyclins such as cyclin A2, cyclin D1, and cyclin E in
the kidney (21). We then checked
whether PCS extract affects the expression of these cyclin proteins
by western blotting. In parallel with the results of SV40 MES 13
cell proliferation, AGEs treatment significantly increased the
expression of cyclin A2, cyclin D1, and cyclin E1, and PCS extract
treatment inhibited this increase of expression of cyclin proteins
in a concentration dependent manner.
Flow cytometry for cell cycle analysis was performed
after PI staining. AGEs reduced the Percentages of cells in the
G0/G1 phase but increased cells in the G2/M phase, indicating that
AGEs could promote cell cycle progression (Fig. 3F). However, co-treatment with the
PCS extract increased the proportion of cells in the G1 phase and
decreased that in the G2/M phase (Fig.
3F). These results indicate that the PCS extract blocked
AGEs-induced cell cycle progression.
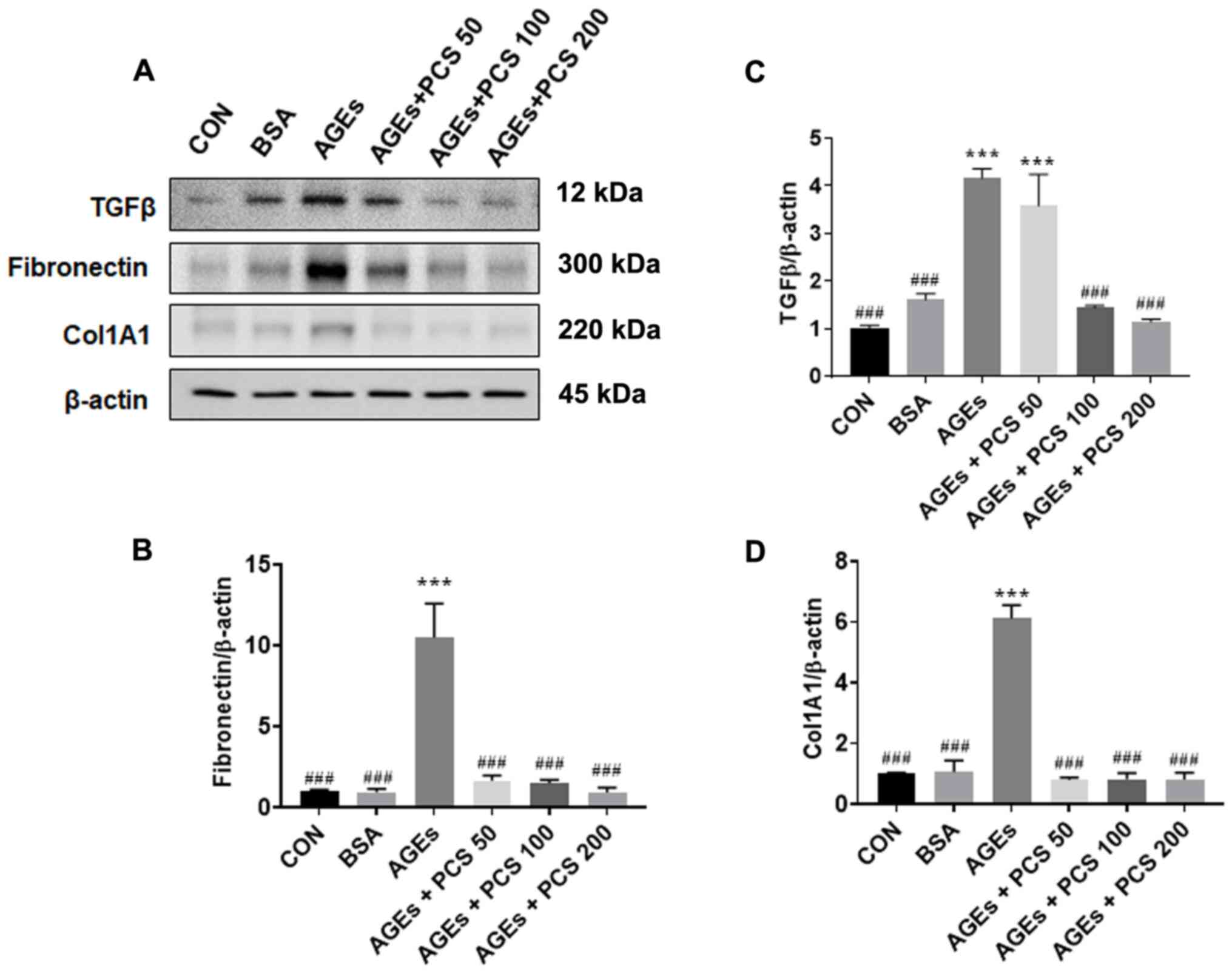
PCS extract inhibited AGEs-induced
expression of fibrotic factors in SV40 MES 13 cells
To investigate the effect of the PCS extract on
AGEs-induced fibrotic factor expression, SV40 MES 13 cells were
treated with various concentrations of the PCS extract in the
presence of AGEs for 24 h. The level of protein expression of
fibrotic factor and collagen was examined by western blotting. The
expression of TGF-β1 protein, which is a central mediator of
fibrogenesis, was increased by AGEs, and PCS extract treatment
inhibited this increase. Similarly, the expression of fibronectin
and collagen (COL1A1), which are extracellular matrix (ECM)
proteins leading to fibrosis, also increased by AGEs, and PCS
extract treatment inhibited this increase (Fig. 4). These results indicate that the
PCS extract could inhibit AGEs-induced mesangial fibrosis.
PCS extract inhibited AGEs-induced
RAGE signaling pathway in SV40 MES 13 cells
It has been known that AGEs promote ROS production
through RAGE, causing oxidative stress (22). AGEs treatment significantly
increased the expression of RAGE. However, treatment with the PCS
extract led to a concentration-dependent decrease in AGEs-induced
RAGE expression (Fig. 5A and
B). PCS extract treatment without
AGEs also decreased the expression of RAGE protein, which was
significantly decreased compared to the control when 200 µg/ml PCS
extract was added (Fig. 5E and
F). The expression level of NOX4,
which is known to play a major role in ROS production in the
kidneys (23), was also
significantly increased by AGEs induction, this effect, however,
was concentration-dependently decreased upon treatment with the PCS
extract (Fig. 5A and C). Therefore, we checked intracellular ROS
level. We found that ROS levels were also significantly increased
by AGEs treatment, and this increase was inhibited by treatment
with PCS extract (Fig. 5F). These
results suggest that the PCS extract could inhibit RAGE expression
and thereby inhibit NOX4 expression and ROS production. ROS is
known to activate a number of transcription factors, which include
various inflammation factors such as NF-κB (24). When we checked the expression of
phosphorylation of NF-κB in SV40 MES 13 cells, AGEs treatment
significantly increased phosphorylation of NF-κB and PCS extract
treatment significantly inhibited this increase (Fig. 5A and D). These results suggest that the PCS
extract could inhibit AGEs-induced RAGE-ROS-NF-κB signaling
pathways.
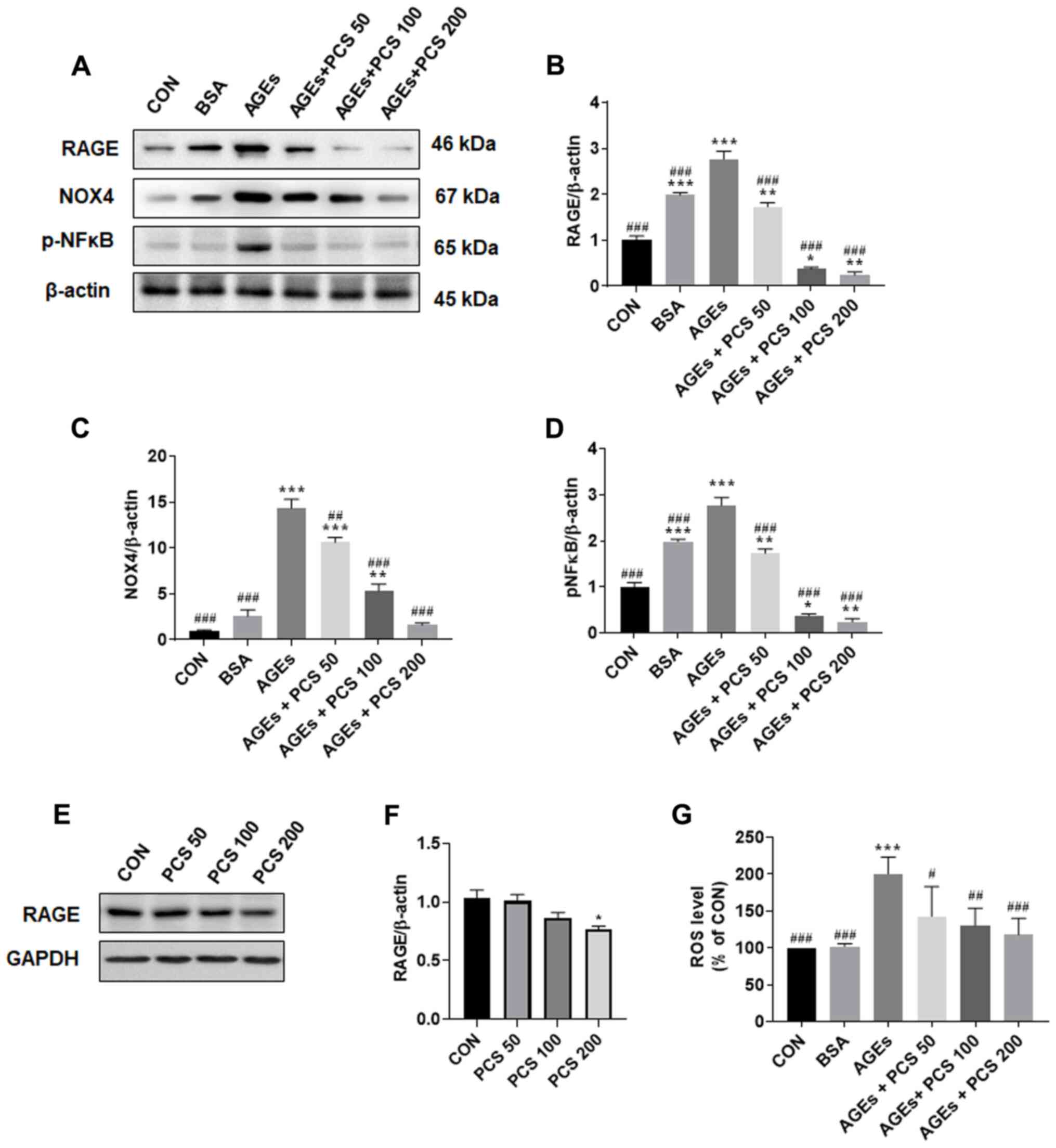 | Figure 5PCS extract treatment inhibited
AGEs-induced ROS production and expression of RAGE, NOX4 and p-NFκB
in SV40 MES 13 cells. The SV40 MES 13 cells were treated with
various concentrations of the PCS extract (50, 100 and 200 µg/ml)
in the presence AGE (10 µg/ml) for 24 h. (A) After cells were
harvested, the expression of RAGE, NOX4 and p-NFκB p65 was examined
by western blotting. Representative blots are presented. (B-D)
Quantification of the results from Fig. 5A. After treating cells
with PCS extract as aforementioned, (E) cells were harvested, and
the expression of RAGE was determined by western blotting. The
representative blots are presented. (F) Quantification of the
results from Fig 5E. (G) ROS levels were measured by staining with
10 µM CM-H2DCFDA. Fluorescence was detected at an
excitation/emission wavelength of 495/527 nm. Data are presented as
the mean ± standard error (n=3; independent experiments).
*P<0.05, **P<0.005 and
***P<0.001 vs. CON; #P<0.05,
##P<0.005 and ###P<0.001 vs. AGEs. PCS,
Psoralea corylifolia L. seed; AGEs, advanced glycation end
products; ROS, reactive oxygen species; RAGE, receptor of AGEs;
NOX4, NADPH oxidase 4; p, phosphorylated; CON, control; BSA, bovine
serum albumin. |
Discussion
The mesangial cells are specialized kidney cells
that make up the mesangium of the glomerulus and constitute up to
30-40% of the total glomerular cell population (25). The mesangial cells can synthesize
and secrete many protein factors that regulate the structure and
function of the glomerulus (26).
Alteration in the mesangial cell function is a key factor that
results in the progression of glomerular disease in numerous models
of chronic renal failure, such as DN. The expansion of the
mesangial matrix is one of the hallmarks of DN; it is caused by the
proliferation of mesangial cells and the increased deposition of
extracellular matrix proteins, such as fibronectin and collagen,
into the mesangium (2,3).
In chronic hyperglycemia, AGEs are actively produced
and accumulate in the circulating blood and various tissues,
resulting in vascular complications in diabetes (27). Several studies have reported that
AGEs induce mesangial cell expansion by increasing mesangial cell
proliferation and ECM production, and blocking AGEs or AGEs-RAGE
signaling can inhibit mesangial cell expansion (28,29).
In this study, we demonstrated that PCS extract treatment
attenuated AGEs-induced mesangial cell expansion by inhibition of
cyclin protein expression and attenuated the expression of fibrotic
factors by decrease of NOX4 expression and NF-κB activation.
In order to obtain AGEs, we proceeded with AGEs
formation through co-incubation of BSA and MGO (30). MGO, which is a kind of the
dicarbonyl intermediates, is known to be a very reactive precursor
of AGEs. Co-incubation of BSA and MGO showed significantly
increased of AGEs formation and used for this study.
We found that AGEs treatment clearly increased SV40
MES 13 mesangial cell proliferation and PCS extract inhibited this
increase of cell proliferation. The major factors that positively
regulate the G1 phase are cyclins D1 and E1(31). Cyclin A2 plays a critical role
during the S phase, which is the somatic form of cyclin A (32). It is well known that cyclin A2, E1,
and D1 are closely related to the regulation of renal mesangial
cell proliferation (33,34). Therefore, the decrease of protein
expression of cyclin A2, E1 and D1 by PCS extract might contribute
to the inhibition of proliferation of mesangial cells by
attenuating the cell cycle.
TGF-β1 is a critical mediator of glomerulosclerosis
and fibrosis, leading to end stages renal disease and has a key
role in the progression of chronic kidney diseases (35). Increased expression of TGF-β1 mRNA
and protein is observed in patients with fibrotic kidney disease,
including DN (35). TGF-β1
stimulates mesangial cell proliferation (32). In addition, TGF-β1 induces the
production of ECM protein, such as fibronectin and collagen
production in various renal cells, including glomerular mesangial
cells, renal fibroblasts, and renal tubular epithelial cells
(36). PCS extract significantly
inhibited AGEs-induced expression of TGF-β1, fibronectin and
collagen. Therefore, the decreased of TGF-β1 expression by PCS
extract contributed to the inhibition of cell proliferation and
reduction of ECM protein expression.
It is ideal to conduct experiments with a treatment
time optimized for each experiment. However, the experiment was
conducted by selecting the treatment conditions for 24 h, as
previously describe (19,20), evaluate the change induced by the
PCS extract.
AGEs bind to RAGE and activate NAD(P)H oxidase (NOX)
pathway, leading to the generation of ROS (22). Diabetic condition elevates NOX4
expression in renal mesangial cells, and NOX4 mediates mesangial
cell hypertrophy and extracellular matrix accumulation (37). NOX4 is constitutively active and
produces mainly hydrogen peroxide (H2O2) as
the prevalent ROS detected rather superoxide radical anion
(O2·-) in kidney (38).
Even if superoxide radical anion is produced, it spontaneously
undergoes dismutation to form another ROS, hydrogen peroxide
(39). It was well known that
hydrogen peroxide increases extracellular matrix through
TGF-β1(40). It is ideal to conduct
experiments with a treatment time optimized for each experiment.
However, the experiment was conducted by selecting the treatment
conditions for 24 h, as previously describe (19,20),
evaluate the change induced by the PCS extract. Although there was
no experiment of time point to confirm the change of NOX4
expression and ROS level due to the initial reaction of AGEs-RAGE,
even after 24-h treatment, PCS extract inhibited the AGEs-induced
RAGE expression and subsequently decreased the expression of NOX4
and ROS production. These beneficial effects of PCS extracts might
contribute to the prevents of DN.
An increased level of intracellular ROS is known to
activate NF-κB and trigger an inflammatory response (41). Our result showed that phosphorylated
NF-κB expression, which was induced by AGEs, was also significantly
inhibited by PCS extract treatment. Therefore, it is speculated
that treatment with the PCS extract suppressed ROS production,
thereby downregulating the activation the NF-κB signaling pathway.
NF-κB is a transcription factor, which regulates the expression of
numerous inflammatory response-related genes during kidney injury
(42). Upon activation, the
phosphorylated NF-κB translocate into the nucleus and triggers the
expression of its target genes, including TGF-β1 and further
results in ECM accumulation (43).
It was also known that NF-κB signal transduction pathway is related
with glomerular mesangial cell proliferation through cyclin D
(44). Therefore, treatment with
the PCS extract inhibited the AGEs-induced NF-κB activation,
resulting in the reduction of the expression of fibrotic factors,
such TGF-β, fibronectin, and collagen, and cell proliferation.
We previously reported that PCS extract inhibited
AGEs formation in vitro and in vivo (45). In the present study, we found that
PCS extract downregulated the expression of the AGEs receptor,
RAGE, and its sub-signaling molecules. In particular, the
expression of RAGE protein lower than the CON level by the PCS
extraction treatment was observed not only in the AGEs induction
but also in the absence of the AGES induction. Further studies are
definitely required to elucidate the mechanism underlying the
downregulation of RAGE expression by PCS extract. In a previous
report, we reported the protective effect of PCS extract and
coumarins on diabetic nephropathy in streptozotocin-induced type 1
diabetic mice and psoralen and isopsoralen, which are coumarins and
the major components of the PCS extract, improved markers related
to mesangial cell damage and fibrosis caused by high glucose
(15). In addition, it has been
reported that Hydrangea paniculata-derived coumarins have
beneficial effects on diabetic nephropathy (46,47).
Therefore, it is possible that coumarins in the PCS extract may
play a role in inhibiting AGEs-induced hyperproliferation of
mesangial cells and the downregulation of RAGE and fibrotic factor
expression.
In summary, we demonstrated that the PCS extract
inhibited the AGEs-induced mesangial cell proliferation by
inhibition of the expression of cyclin A2, D1, and E1. In addition,
PCS extract inhibited the AGEs-induced ROS production and the
expression of RAGE and fibrotic factors. Our findings suggest that
treatment with the PCS extract blocks RAGE-ROS-NF-κB-TGF-β1
signaling pathways contributing to the protection against diabetic
nephropathy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korea
government (MSIT) (grant no. 2019R1A2B5B02070355), and the Gachon
University research fund of 2019 (grant no. GCU-2019-0320).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSJ conceived and designed the current study, and
wrote the manuscript. HC and ES contributed to the design of the
present study, performed the experiments and wrote the manuscript.
All authors read and approved the final manuscript and all authors
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhattacharjee N, Barma S, Konwar N,
Dewanjee S and Manna P: Mechanistic insight of diabetic nephropathy
and its pharmacotherapeutic targets: An update. Eur J Pharmacol.
791:8–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tung CW, Hsu YC, Shih YH, Chang PJ and Lin
CL: Glomerular mesangial cell and podocyte injuries in diabetic
nephropathy. Nephrology (Carlton). 23 (Suppl 4):S32–S37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kawanami D, Matoba K and Utsunomiya K:
Signaling pathways in diabetic nephropathy. Histol Histopathol.
31:1059–1067. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huebschmann AG, Regensteiner JG, Vlassara
H and Reusch JE: Diabetes and advanced glycoxidation end products.
Diabetes Care. 29:1420–1432. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bellier J, Nokin MJ, Larde E, Karoyan P,
Peulen O, Castronovo V and Bellahcène A: Methylglyoxal, a potent
inducer of AGEs, connects between diabetes and cancer. Diabetes Res
Clin Pract. 148:200–211. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rehman S, Faisal M, Alatar AA and Ahmad S:
Physico-chemical changes induced in serum proteins,
immunoglobulin-G and fibrinogen by reactive carbonyl species,
Methylglyoxal. Curr Protein Pept Sci. 21:916–923. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ott C, Jacobs K, Haucke E, Navarrete
Santos A, Grune T and Simm A: Role of advanced glycation end
products in cellular signaling. Redox Biol. 2:411–429.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gruden G, Perin PC and Camussi G: Insight
on the pathogenesis of diabetic nephropathy from the study of
podocyte and mesangial cell biology. Curr Diabetes Rev. 1:27–40.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thallas-Bonke V, Thorpe SR, Coughlan MT,
Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME and
Forbes JM: Inhibition of NADPH oxidase prevents advanced glycation
end product-mediated damage in diabetic nephropathy through a
protein kinase C-alpha-dependent pathway. Diabetes. 57:460–469.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rios JL, Francini F and Schinella GR:
Natural products for the treatment of type 2 diabetes mellitus.
Planta Med. 81:975–994. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choudhury H, Pandey M, Hua CK, Mun CS,
Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, et al: An
update on natural compounds in the remedy of diabetes mellitus: A
systematic review. J Tradit Complement Med. 8:361–376.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seo E, Oh YS, Kim D, Lee MY, Chae S and
Jun HS: Protective role of Psoralea corylifolia L. seed
extract against hepatic mitochondrial dysfunction induced by
oxidative stress or aging. Evid Based Complement Alternat Med.
2013(678028)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heo J: Donguibogam. Namsandang, Seoul,
1980 (original work published, 1610) (In Korean).
|
|
14
|
Khushboo PS, Jadhav VM, Kadam VJ and Sathe
NS: Psoralea corylifolia Linn.-‘Kushtanashini’. Pharmacogn
Rev. 4:69–76. 2010.
|
|
15
|
Seo E, Kang H, Oh YS and Jun HS:
Psoralea corylifolia L. seed extract attenuates diabetic
nephropathy by inhibiting renal fibrosis and apoptosis in
streptozotocin-induced diabetic mice. Nutrients.
9(828)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Seo E, Oh YS and Jun HS: Psoralea
corylifolia L. seed extract attenuates nonalcoholic fatty liver
disease in high-fat diet-induced obese mice. Nutrients.
8(83)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adisakwattana S, Thilavech T and Chusak C:
Mesona Chinensis Benth extract prevents AGE formation and
protein oxidation against fructose-induced protein glycation in
vitro. BMC Complement Altern Med. 14(130)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharaf H, Matou-Nasri S, Wang Q, Rabhan Z,
Al-Eidi H, Al Abdulrahman A and Ahmed N: Advanced glycation
endproducts increase proliferation, migration and invasion of the
breast cancer cell line MDA-MB-231. Biochim Biophys Acta.
1852:429–441. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chiang CK, Wang CC, Lu TF, Huang KH, Sheu
ML, Liu SH and Hung KY: Involvement of endoplasmic reticulum
stress, autophagy, and apoptosis in advanced glycation end
products-induced glomerular mesangial cell injury. Sci Rep.
6(34167)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang W, Wang R, Liu D, Zuo M, Zhao C,
Zhang T and Li W: Protective effects of kaempferitrin on advanced
glycation end products induce mesangial cell apoptosis and
oxidative stress. Int J Mol Sci. 19(3334)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thomasova D and Anders HJ: Cell cycle
control in the kidney. Nephrol Dial Transplant. 30:1622–1630.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koulis C, Watson AMD, Gray SP and
Jandeleit-Dahm KA: Linking RAGE and Nox in diabetic micro- and
macrovascular complications. Diabetes Metab. 41:272–281.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sedeek M, Callera G, Montezano A, Gutsol
A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM
and Hébert RL: Critical role of Nox4-based NADPH oxidase in
glucose-induced oxidative stress in the kidney: Implications in
type 2 diabetic nephropathy. Am J Physiol Renal Physiol.
299:F1348–F1358. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ha H and Lee HB: Oxidative stress in
diabetic nephropathy: Basic and clinical information. Curr Diab
Rep. 1:282–287. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Scindia YM, Deshmukh US and Bagavant H:
Mesangial pathology in glomerular disease: Targets for therapeutic
intervention. Adv Drug Deliv Rev. 62:1337–1343. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schlöndorff D and Banas B: The mesangial
cell revisited: No cell is an island. J Am Soc Nephrol.
20:1179–1187. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rhee SY and Kim YS: The role of advanced
glycation end products in diabetic vascular complications. Diabetes
Metab J. 42:188–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee EJ, Kang MK, Kim DY, Kim YH, Oh H and
Kang YH: Chrysin inhibits advanced glycation end products-induced
kidney fibrosis in renal mesangial cells and diabetic kidneys.
Nutrients. 10(882)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qiu YY, Tang LQ and Wei W: Berberine
exerts renoprotective effects by regulating the AGEs-RAGE signaling
pathway in mesangial cells during diabetic nephropathy. Mol Cell
Endocrinol. 443:89–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bourajjaj M, Stehouwer CD, van Hinsbergh
VW and Schalkwijk CG: Role of methylglyoxal adducts in the
development of vascular complications in diabetes mellitus. Biochem
Soc Trans. 31:1400–1402. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haberstroh U, Zahner G, Disser M, Thaiss
F, Wolf G and Stahl RA: TGF-beta stimulates rat mesangial cell
proliferation in culture: Role of PDGF beta-receptor expression. Am
J Physiol. 264:F199–F205. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lang S, Hartner A, Sterzel RB and
Schöcklmann HO: Requirement of cyclin D1 in mesangial cell
mitogenesis. J Am Soc Nephrol. 11:1398–1408. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schöcklmann HO, Lang S and Sterzel RB:
Regulation of mesangial cell proliferation. Kidney Int.
56:1199–1207. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sureshbabu A, Muhsin SA and Choi ME: TGF-β
signaling in the kidney: Profibrotic and protective effects. Am J
Physiol Renal Physiol. 310:F596–F606. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu L, Border WA, Huang Y and Noble NA:
TGF-beta isoforms in renal fibrogenesis. Kidney Int. 64:844–856.
2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rajaram RD, Dissard R, Jaquet V and de
Seigneux S: Potential benefits and harms of NADPH oxidase type 4 in
the kidneys and cardiovascular system. Nephrol Dial Transplant.
34:567–576. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Takac I, Schröder K, Zhang L, Lardy B,
Anilkumar N, Lambeth JD, Shah AM, Morel F and Brandes RP: The
E-loop is involved in hydrogen peroxide formation by the NADPH
oxidase Nox4. J Biol Chem. 286:13304–13313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ratliff BB, Abdulmahdi W, Pawar R and
Wolin MS: Oxidant mechanisms in renal injury and disease. Antioxid
Redox Signal. 25:119–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Iglesias-De La Cruz MC, Ruiz-Torres P,
Alcamí J, Díez-Marqués L, Ortega-Velázquez R, Chen S,
Rodríguez-Puyol M, Ziyadeh FN and Rodríguez-Puyol D: Hydrogen
peroxide increases extracellular matrix mRNA through TGF-beta in
human mesangial cells. Kidney Int. 59:87–95. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fukami K, Taguchi K, Yamagishi S and Okuda
S: Receptor for advanced glycation endproducts and progressive
kidney disease. Curr Opin Nephrol Hypertens. 24:54–60.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno
JA, Santamaria B, Ruiz-Ortega M, Egido J and Ortiz A: NF-kappaB in
renal inflammation. J Am Soc Nephrol. 21:1254–1262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang J, Zeng Z, Wu T, Yang Z, Liu B and
Lan T: Emodin attenuates high glucose-induced TGF-β1 and
fibronectin expression in mesangial cells through inhibition of
NF-κB pathway. Exp Cell Res. 319:3182–3189. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang BF, Xu HS, Li YJ, Ye RG, Kong QY and
Yu XQ: Role of Akt/NF-kappa B signal transduction pathway in murine
glomerular mesangial cell proliferation induced by immune complex.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 20:314–318. 2004.PubMed/NCBI(In Chinese).
|
|
45
|
Truong CS, Seo E and Jun HS: Psoralea
corylifolia L. seed extract attenuates methylglyoxal-induced
insulin resistance by inhibition of advanced glycation end product
formation. Oxid Med Cell Longev. 2019(4310319)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sen Z, Weida W, Jie M, Li S, Dongming Z
and Xiaoguang C: Coumarin glycosides from Hydrangea
paniculata slow down the progression of diabetic nephropathy by
targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis.
Phytomedicine. 57:385–395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang
J and Chen X: Skimmin, a coumarin from Hydrangea paniculata,
slows down the progression of membranous glomerulonephritis by
anti-inflammatory effects and inhibiting immune complex deposition.
Evid Based Complement Alternat Med. 2013(819296)2013.PubMed/NCBI View Article : Google Scholar
|