1. Introduction
‘Big data’, large datasets that are difficult to
record, store and analyze with conventional data management
systems, has been accumulating in various fields in recent years
with regard to the development of communication and sensor
technology. The advances in technology regarding big data have
emerged that the use of big data is expected to create new avenues
of research. However, the overall trend of big data is difficult to
understand based on general information processing by humans; thus,
information processing by artificial intelligence (AI) has also
attracted attention (1). In
general, industries have succeeded in improving sales and work
efficiency and decreasing costs using big data and AI (2).
In healthcare, the creation of new knowledge and the
improvement in diagnostic and therapeutic outcomes are expected
through the utilization of big data pertaining to life science
information and medical data (3).
In fact, the implementation of AI in healthcare has been actively
investigated; however, it has not been used in a widespread manner
due to a number of problems (4).
The present review looks back at the history of AI
and AI-based applications, compares the advantages and the issues
of conventional healthcare and AI-based healthcare, and considers
the future development of AI-based applications.
2. Historical view of the clinical
application of computational support
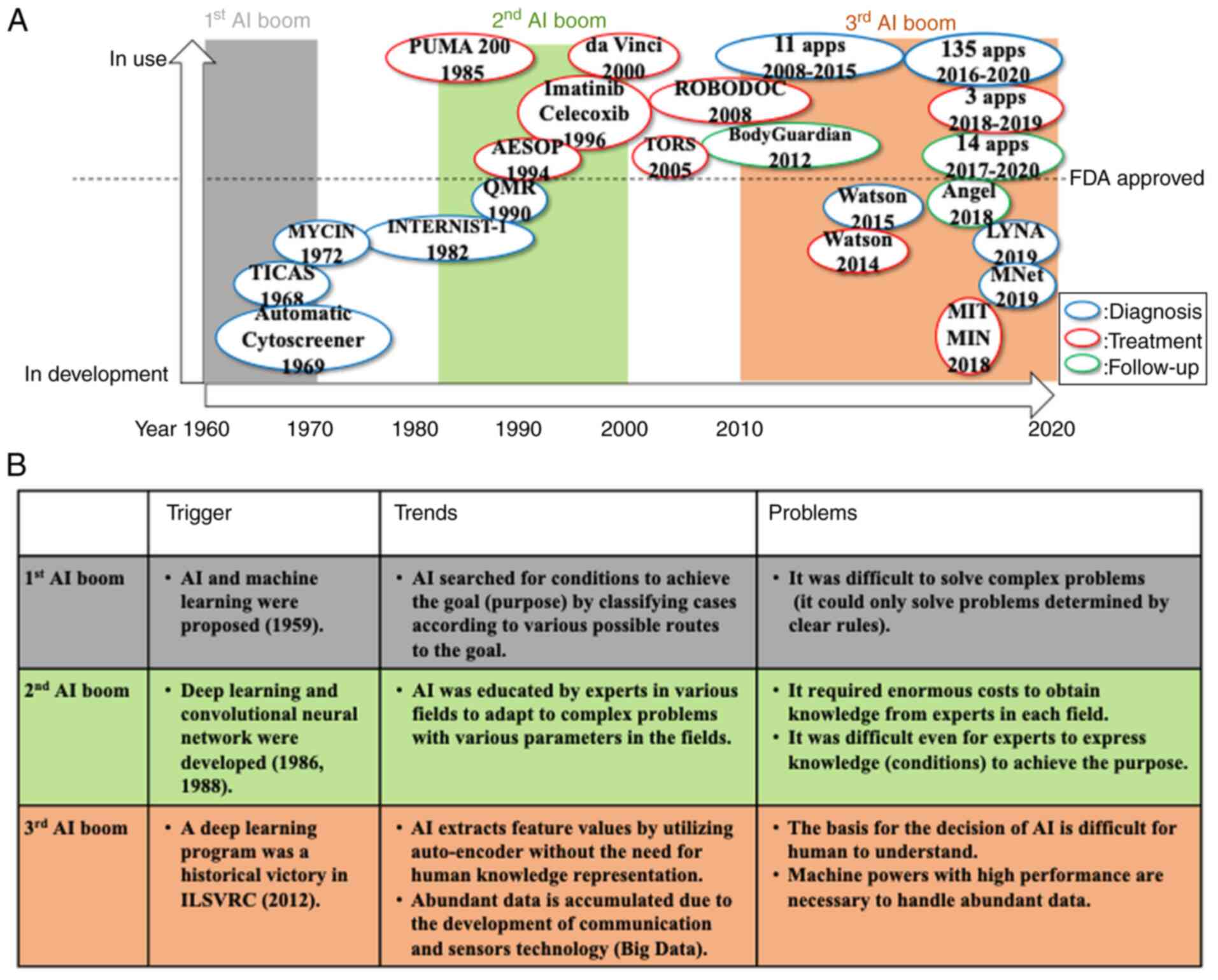
In the 1950s, McCarthy et al (5) proposed AI as a prediction machine
(hardware or software that exhibits behavior which appears
intelligence by predicting associations between variables). Samuel
(6) developed machine learning in
1959, which triggered the first AI boom (Fig. 1). In this period, the
discrimination of cells in microscopic images started to be
investigated using machine learning (7,8). In
the 1970s, progress with AI was temporarily halted, as the AI was
only able to solve simple problems. By contrast, in the same
period, expert systems consisting of knowledge bases and inference
engines were invented, and tools for diagnosis in specific fields
such as MYCIN and INTERNIST-1 were developed (9,10).
Subsequently, deep learning was proposed by Dechter (11) in 1986 and a convolutional neural
network was proposed by LeCun et al (12) in 1988, leading to the second AI
boom. In this boom, to allow adaptation to real-world problems,
experts in various fields educated AI using parameters, including
marketing, healthcare and life science data. In addition, surgical
robots began to flourish during this period. Among them, PUMA 200
was developed to automatically identify the appropriate location of
lesions in computed tomography-guided brain tumor biopsies and was
the first robot used for assisting human neurosurgery (13). AESOP was a breakthrough in robotic
surgery when introduced in 1994, as it was the first laparoscopic
camera holder to be approved by the FDA (14). Moreover, in 2000, the da Vinci
Surgical System obtained FDA approval for use in general
laparoscopic procedures and became the first operative surgical
robot in the US (15). In 2005, a
surgical technique for the da Vinci Surgical System was documented
in canine and cadaveric models called transoral robotic surgery;
this was the only FDA-approved robot to perform head and neck
surgery at the time (16).
In addition, medications based on a computational
analysis of the crystal structure of molecules were developed
(17,18). The ROBODOC Surgical System was
introduced and revolutionized orthopedic surgery by being able to
assist with hip replacement surgeries. This was the first surgical
robot to be approved for use in humans by the FDA in 2008(19).
Thus, during the second AI boom, several tools were
successfully developed. However, it was difficult for humans to
provide the information that an AI needs to solve complex problems,
and it was difficult for the machines of that time to learn the
vast level of information available.
With the advent of deep learning in 2006 and the
development of computers and communication equipment, the interest
in AI was renewed (20,21). In particular, the historical
victory of a deep learning program by utilizing a convolutional
neural network, a deep learning method in image recognition, in an
image recognition contest called the ImageNet Large Scale Visual
Recognition Challenge (ILSVRC) triggered the third AI boom
(22). As a result, image
recognition has become the most applied AI-based technology in the
clinical setting. When considering clinical applications based on
image recognition using AI, IB Neuro is a diagnostic software used
to detect brain tumors by MRI, and this was approved by the FDA in
2008 as the first AI-based application in humans (23). In addition, IntelliSite was
approved by the FDA as the first whole-slide imaging system in 2017
(24,25). Thus, a number of AI-based
applications were approved by the FDA in the third AI boom. The
number of approved applications for diagnostics, mainly image
recognition, increased from 11 in 2008-2015 to 135 in 2016-2020
(Table I) (23,26).
In addition to applications for diagnosis, applications for
treatment are also being approved by the FDA, with three AI-based
applications to support treatment processes such as radiotherapy
being approved in 2018-2019 (23,26).
 | Table IFDA approved AI-based
applications. |
Table I
FDA approved AI-based
applications.
| Applications | Company | Purpose | Medical
specialty | FDA Cleared |
|---|
| IB Neuro | Imaging Biometrics,
LLC | Diagnosis | Neuroradiology | 2008 |
| Pathwork Tissue of
Origin Test Kit-FFPE | Pathwork
Diagnostics, Inc. | Diagnosis | Pathology | 2010 |
| DeltaView Model
2.1 | Riverain
Technologies | Diagnosis | Radiology | 2011 |
| AlphaPoint Imaging
Software | RadLogics,
Inc. | Diagnosis | Radiology | 2012 |
| BodyGuardian Remote
Monitoring System | Preventice | Follow-up | Cardiology | 2012 |
| ClearRead
+Confirm | Riverain
Technologies | Diagnosis | Radiology | 2012 |
| Temporal
Comparison | Riverain
Technologies | Diagnosis | Radiology | 2012 |
| cvi42 | Circle
Cardiovascular Imaging, Inc. | Diagnosis | Radiology | 2014 |
| Ahead 100 | BrainScope | Diagnosis | Neurology | 2014 |
| AliveCor | AliveCor | Diagnosis | Cardiology | 2014 |
| Lung Density
Analysis | Imbio LLC | Diagnosis | Radiology | 2014 |
| Vitrea CT Lung
Density Analysis Software | Vital Images,
Inc. | Diagnosis | Radiology | 2015 |
| Stroke VCAR | GE Medical
Systems | Diagnosis | Neuroradiology | 2016 |
| QbCheck | QbTech AB | Diagnosis | Psychiatry | 2016 |
| PixelShine | AlgoMedica | Diagnosis | Radiology | 2016 |
| Steth IO | Stratoscientific,
Inc. | Diagnosis | General
medicine | 2016 |
| ClearRead CT | Riverain
Technologies | Diagnosis | Radiology | 2016 |
| Arterys Cardio
DL | Arterys Inc | Diagnosis | Radiology | 2016 |
| CT CoPilot | ZepMed, LLC. | Diagnosis | Neuroradiology | 2016 |
| ClearView cCAD | ClearView
Diagnostics Inc. | Diagnosis | Oncology | 2016 |
| Arterys Cardio
DL | Arterys Inc. | Diagnosis | Radiology | 2017 |
| Cantab Mobile | Cambridge
Cognition, Ltd. | Diagnosis | Neurology | 2017 |
| Lung Nodule
Assessment and Comparison Option | Philips Medical
Systems | Diagnosis | Radiology | 2017 |
| EnsoSleep | EnsoData, Inc. | Diagnosis | Neurology | 2017 |
| AmCAD-US | AmCad BioMed
Corporation | Diagnosis | Radiology | 2017 |
| QuantX | Quantitative
Insights, Inc. | Diagnosis | Radiology | 2017 |
| NeuroQuant | Cortechs.ai | Diagnosis | Neuroradiology | 2017 |
| LesionQuant | Cortechs.ai | Diagnosis | Neuroradiology | 2017 |
| Arterys Oncology
DL | Arterys Inc | Diagnosis | Radiology | 2017 |
| Rooti Rx System ECG
Event Recorder, Rooti Link APP Software | Rooti Labs,
Ltd. | Diagnosis | Cardiology | 2017 |
| BioFlux | Biotricity,
Inc. | Diagnosis | Cardiology | 2017 |
| CNeuro cMRI | Combinostics
Oy | Diagnosis | Neuroradiology | 2018 |
| Idx | IDx LLC | Diagnosis | Ophthalmology | 2018 |
| WAVE Clinical
Platform | Excel Medical
Electronics, LLC | Follow-up | Hospital
monitoring | 2018 |
| Insight BD | Siemens
Healthineers | Diagnosis | Radiology | 2018 |
| Viz LVO
(ContaCT) | Viz. AI, Inc. | Diagnosis | Neuroradiology | 2018 |
| DM-Density | Densitas, Inc. | Diagnosis | Oncology | 2018 |
| OsteoDetect | Imagen
Technologies, Inc. | Diagnosis | Radiology | 2018 |
| Quantib Brain | Quantib BV | Diagnosis | Neuroradiology | 2018 |
| Guardian Connect
System | Medtronic | Diagnosis | Endocrinology | 2018 |
| PowerLook Density
Assessment Software | ICAD Inc. | Diagnosis | Radiology | 2018 |
| Viz CTP | Viz. ai, inc. | Diagnosis | Neuroradiology | 2018 |
| NeuralBot | Neural Analytics,
Inc. | Diagnosis | Radiology | 2018 |
| OsteoDetect | Imagen
Technologies | Diagnosis | Radiology | 2018 |
| EchoMD Automated
Ejection Fraction Software | Bay Labs, Inc. | Diagnosis | Radiology | 2018 |
| MindMotion GO | MindMaze SA | Follow-up | Orthopedics | 2018 |
| LungQ | Thirona
Corporation | Diagnosis | Radiology | 2018 |
| HealthCCS | Zebra Medical
Vision Ltd. | Diagnosis | Radiology | 2018 |
| EchoMD Automated
Ejection Fraction Software | Bay Labs, Inc. | Diagnosis | Cardiology | 2018 |
| DenSeeMammo | Statlife | Diagnosis | Oncology | 2018 |
| DreaMed | DreaMed Diabetes,
Ltd | Follow-up | Endocrinology | 2018 |
| ProFound™ AI
Software V2.1 | iCAD, Inc | Diagnosis | Radiology | 2018 |
| BriefCase- ICH | Aidoc Medical,
Ltd. | Diagnosis | Neuroradiology | 2018 |
| AmCAD-UT Detection
2.2 | AmCAD BioMed
Corporation | Diagnosis | Endocrinology | 2018 |
| Arterys MICA | Arterys, Inc. | Diagnosis | Radiology | 2018 |
| ECG App | Apple, Inc. | Follow-up | Cardiology | 2018 |
| Volpara Imaging
Software | Volpara Health
Technologies Limited | Diagnosis | Oncology | 2018 |
| AI-ECG
Platform | Shenzhen Carewell
Electronics, Ltd. | Diagnosis | Cardiology | 2018 |
| FibriCheck | Qompium NV | Follow-up | Cardiology | 2018 |
| Irregular Rhythm
Notification Feature | Apple, Inc. | Diagnosis | Cardiology | 2018 |
| RightEye Vision
System | RightEye, LLC | Diagnosis | Ophthalmology | 2018 |
| Accipiolx | MaxQ-Al, Ltd. | Diagnosis | Radiology | 2018 |
| icobrain | Icometrix NV | Diagnosis | Radiology | 2018 |
| FluoroShield™ | Omega Medical
Imaging, LLC | Treatment | Radiology | 2018 |
| Vitrea CT Brain
Perfusion | Vital Images,
Inc. | Diagnosis | Neuroradiology | 2018 |
| SubtlePET | Subtle Medical,
Inc. | Diagnosis | Neuroradiology | 2018 |
| FerriSmart Analysis
System | Resonance Health
Analysis Service Pty, Ltd. | Diagnosis | Radiology | 2018 |
| Embrace | Empatica Srl | Follow-up | Neurology | 2018 |
| Quantib ND | Quantib BV | Diagnosis | Neuroradiology | 2018 |
| iSchemaView
RAPID | iSchemaView,
Inc. | Diagnosis | Radiology | 2018 |
| Study Watch | Verily Life
Sciences LLC | Follow-up | Cardiology | 2019 |
| cmTriage | CureMetrix,
Inc. | Diagnosis | Oncology | 2019 |
| Thoracic VCAR with
GSI Pulmonary Perfusion | GE Medical
Systems | Diagnosis | Radiology | 2019 |
| KardiaAI | AliveCor, Inc | Follow-up | Cardiology | 2019 |
| Loop System | Spry Health,
Inc. | Follow-up | Hospital
monitoring | 2019 |
|
RhythmAnalytics | Biofourmis
Singapore Pte, Ltd. | Follow-up | Cardiology | 2019 |
| Bone Vcar | GE Medical
Systems | Diagnosis | Radiology | 2019 |
| Aidoc Briefcase-
ICH and PE triage | Aidoc Medical,
Ltd. | Diagnosis | Radiology | 2019 |
| Deep Learning Image
Reconstruction | GE Medical Systems,
LLC. | Diagnosis | Radiology | 2019 |
| eMurmer ID | CSD Labs GmbH | Diagnosis | Cardiology | 2019 |
| HealthPNX | Zebra Medical
Vision Ltd. | Diagnosis | Radiology | 2019 |
| Aidoc BriefCase-
CSF triage | Aidoc Medical,
Ltd. | Diagnosis | Radiology | 2019 |
| ReSET-O | Pear Therapeutics,
Inc. | Treatment | Psychiatry | 2019 |
| HealthICH | Zebra Medical
Vision Ltd. | Diagnosis | Neuroradiology | 2019 |
| Advanced
Intelligent Clear-IQ Engine (AiCE) | Canon Medical
Systems Corporation | Diagnosis | Radiology | 2019 |
| Koios DS | Koios Medical,
Inc | Diagnosis | Oncology | 2019 |
| DeepCT | Deep01 Limited | Diagnosis | Neuroradiology | 2019 |
|
iNtuition-Structural Heart Module | TeraRecon,
Inc. | Diagnosis | Radiology | 2019 |
| AI-Rad Companion
(Pulmonary) | Siemens
Healthineers | Diagnosis | Radiology | 2019 |
| ACR | LAB Urine
Analysis Test System | Healthy.io,
Ltd. | Diagnosis | Urology | 2019 |
| Current Wearable
Health Monitoring System | Current Health,
Ltd. | Follow-up | Hospital
monitoring | 2019 |
| physIQ Heart Rhythm
and Respiratory Module | physIQ, Inc | Diagnosis | Cardiology | 2019 |
| RayCare 2.3 | RaySearch
Laboratories AB | Treatment | Radiology | 2019 |
| Critical Care
Suite | GE Medical
Systems | Diagnosis | Radiology | 2019 |
| Biovitals Analytics
Engine | Biofourmis
Singapore Pte. Ltd | Follow-up | Cardiology | 2019 |
| Caption
Guidance | Caption Health,
Inc. | Diagnosis | Radiology | 2019 |
| AI-Rad Companion
(cardiovascular) | Siemens
Healthineers | Diagnosis | Radiology | 2019 |
| SubtleMR | Subtle Medical,
Inc. | Diagnosis | Radiology | 2019 |
| StoneChecker | Imaging Biometrics,
LLC | Diagnosis | Radiology | 2019 |
| BrainScope TBI | BrainScope Company,
Inc | Diagnosis | Neurology | 2019 |
| ProFound AI
Software V2.1 | ICAD Inc. | Diagnosis | Oncology | 2019 |
| KOALA | IB Lab GmbH | Diagnosis | Radiology | 2019 |
| EchoGo Core | Ultromics,
Ltd. | Diagnosis | Cardiology | 2019 |
| RSI-MRI+ | HealthLytix | Diagnosis | Radiology | 2019 |
| HealthCXR | Zebra Medical
Vision, Ltd. | Diagnosis | Radiology | 2019 |
| icobrain | Icometrix NV | Diagnosis | Neuroradiology | 2019 |
| QyScore
Software | Qynapse | Diagnosis | Neuroradiology | 2019 |
| Aidoc BriefCase-
LVO | Aidoc Medical,
Ltd. | Diagnosis | Neuroradiology | 2019 |
| AutoMIStar | Apollo Medical
Imaging Technology Pty, Ltd. | Diagnosis | Neuroradiology | 2019 |
| TransparaTM | Screenpoint Medical
B.V. | Diagnosis | Radiology | 2019 |
| ADAS 3D | Galgo Medical
S.L | Diagnosis | Radiology | 2020 |
| QuantX | Quantitative
Insights, Inc. | Diagnosis | Radiology | 2020 |
| Eko Analysis
Software | Eko Devices,
Inc. | Follow-up | Cardiology | 2020 |
| densitas
densityai | Densitas, Inc. | Diagnosis | Radiology | 2020 |
| red dot | Behold.AI
Technologies, Ltd. | Diagnosis | Radiology | 2020 |
| icobrain-ctp | Icometrix NV | Diagnosis | Neuroradiology | 2020 |
| Broncholab | Fluidda, Inc. | Diagnosis | Radiology | 2020 |
| Transpara | ScreenPoint Medical
B.V. | Diagnosis | Oncology | 2020 |
| Al-Rad Companion
(Musculoskeletal) | Siemens
Healthineers | Diagnosis | Radiology | 2020 |
| Hepatic VCAR | GE Medical
Systems | Diagnosis | Radiology | 2020 |
| MammoScreen | Therapixel | Diagnosis | Oncology | 2020 |
| RAPID ICH | iSchemaView,
Inc. | Diagnosis | Neuroradiology | 2020 |
| AIMI-Triage CXR
PTX | RadLogics,
Inc. | Diagnosis | Radiology | 2020 |
| CuraRad-ICH | Keya Medical | Diagnosis | Neuroradiology | 2020 |
| NinesAI | Nines, Inc. | Diagnosis | Neuroradiology | 2020 |
| HealthVCF | Zebra Medical
Vision, Ltd. | Diagnosis | Radiology | 2020 |
| Syngo.CT
CaScoring | Siemens
Healthineers | Diagnosis | Radiology | 2020 |
| MEDO ARIA | Medo.AI | Diagnosis | Orthopedics | 2020 |
| Auto 3D Bladder
Volume Tool | Butterfly Network,
Inc. | Diagnosis | Urology | 2020 |
| AI-Rad Companion
Brain MR | Siemens
Healthineers | Diagnosis | Neuroradiology | 2020 |
| qER | Qure.ai
Technologies | Diagnosis | Neuroradiology | 2020 |
| BriefCase-IFG | Aidoc Medical,
Ltd. | Diagnosis | Radiology | 2020 |
| CINA | AVICENNA.AI | Diagnosis | Neuroradiology | 2020 |
| Rapid ASPECTS | iSchemaView
Inc. | Diagnosis | Neuroradiology | 2020 |
| EyeArt | Eyenuk, Inc. | Diagnosis | Ophthalmology | 2020 |
| InferRead Lung
CT.AI | Beijing Infervision
Technology Co., Ltd. | Diagnosis | Radiology | 2020 |
| Rapid LVO 1.0 | iSchemaView,
Inc. | Diagnosis | Neuroradiology | 2020 |
| HealthMammo | Zebra Medical
Vision, Ltd. | Diagnosis | Oncology | 2020 |
| Caption
Interpretation Automated Ejection Fraction Software | Caption Health | Diagnosis | Cardiology | 2020 |
| AI-Rad Companion
Prostate MR | Siemens
Healthineers | Diagnosis | Radiology | 2020 |
| FractureDetect
(FX) | Imagen
Technologies | Diagnosis | Radiology | 2020 |
| VIDA|vision | VIDA Diagnostics,
Inc. | Diagnosis | Radiology | 2020 |
| Accipiolx | MaxQ Al, Ltd. | Diagnosis | Neuroradiology | 2020 |
| Aidoc BriefCase for
iPE Triage | Aidoc Medical,
Ltd. | Diagnosis | Radiology | 2020 |
| Aview 2.0 | Coreline Soft Co.,
Ltd. | Diagnosis | Radiology | 2020 |
| AVA (Augmented
Vascular Analysis) | See-Mode
Technologies Pte, Ltd. | Diagnosis | Cardiology | 2020 |
| THINQ | CorticoMetrics
LLC | Diagnosis | Neuroradiology | 2020 |
| Cleerly Labs
V2.0 | Cleerly, Inc. | Diagnosis | Radiology | 2020 |
| Syngo.CT Neuro
Perfusion | Siemens
Healthineers | Diagnosis | Neuroradiology | 2020 |
| Quantib
Prostate | Quantib BV | Diagnosis | Radiology | 2020 |
| AVIEW LCS | Coreline Soft Co.,
Ltd. | Diagnosis | Radiology | 2020 |
| Liver Surface
Nodularity (LSN) | Imaging Biometrics,
LLC | Diagnosis | Radiology | 2020 |
| WRDensity | Whiterabbit.ai
Inc. | Diagnosis | Oncology | 2020 |
| Neuro.AI
Algorithm | TeraRecon,
Inc. | Diagnosis | Neuroradiology | 2020 |
| FastStroke, CT
Perfusion 4D | GE Medical
Systems | Diagnosis | Neuroradiology | 2020 |
| PROView | GE Medical
Systems | Diagnosis | Radiology | 2020 |
| Genius AI
Detection | Hologic, Inc. | Diagnosis | Radiology | 2020 |
| HALO | NiCo-Lab B.V. | Diagnosis | Neuroradiology | 2020 |
| HealthJOINT | Zebra Medical
Vision, Ltd. | Diagnosis | Radiology | 2020 |
| HepaFat-AI | Resonance Health
Analysis Service Pty, Ltd. | Diagnosis | Radiology | 2020 |
| SQuEEZ
Software | Cardiowise,
Inc. | Diagnosis | Radiology | 2020 |
| EchoGo Pro | Ultromics,
Ltd. | Diagnosis | Cardiology | 2020 |
| AI Metrics | AI Metrics,
LLC | Diagnosis | Radiology | 2020 |
| BrainInsight | Hyperfine Research,
Inc. | Diagnosis | Neuroradiology | 2021 |
| HearFlow
Analysis | HeartFlow,
Inc. | Diagnosis | Radiology | 2021 |
| uAI
EasyTriage-Rib | Shanghai United
Imaging Intelligence Co., Ltd. | Diagnosis | Radiology | 2021 |
| Visage Breast
Density | Visage Imaging
GmbH | Diagnosis | Oncology | 2021 |
| CLEWICU System | CLEW Medical,
Ltd. | Diagnosis | Hematology | 2021 |
| qp-Prostate | Quibim | Diagnosis | Radiology | 2021 |
| Lvivo Software
Application | DiA Imaging
Analysis, Ltd. | Diagnosis | Cardiology | 2021 |
| Veolity | MeVis Medical
Solutions AG | Diagnosis | Radiology | 2021 |
| NinesMeasure | Nines, Inc. | Diagnosis | Radiology | 2021 |
| Optellum Virtual
Nodule Clinic, Optellum Software, Optellum Platform | Optellum, Ltd. | Diagnosis | Radiology | 2021 |
| Imbio RV/LV
Software | Imbio LLC | Diagnosis | Radiology | 2021 |
| Vbrain | Vysioneer,
Inc. | Diagnosis | Neuroradiology | 2021 |
| Viz ICH | Viz. AI, Inc. | Diagnosis | Neuroradiology | 2021 |
| syngo.CT Lung CAD
(VD20) | Subtle Medical,
Inc. | Diagnosis | Radiology | 2021 |
| Saige-Q | DeepHealth | Diagnosis | Oncology | 2021 |
| MEDO-Thyroid | Medo.AI | Diagnosis | Endocrinology | 2021 |
| CINA CHEST | AVICENNA.AI | Diagnosis | Radiology | 2021 |
| Overjet Dental
Assist | Overjet, Inc. | Diagnosis | Radiology | 2021 |
Moreover, AI-based applications for follow-up of
treatment progress are also being developed. In 2012, the
BodyGuardian Remote Monitoring System was the first AI-based
application for follow-up to be approved by the FDA (23). Subsequently, applications for
follow-up are being actively developed and 13 applications were
approved by the FDA in 2017-2020 (23,26).
Similarly, computational support continues to contribute to drug
development, such as methylenetetrahydrofolate dehydrogenase 2
targeting one carbon metabolism (27).
AI is being applied to various processes in the
medical field. Current AI and AI-based applications are often
specialized for each field; however, applications that are widely
available, such as IBM Watson (ibm.com/watson), have been also developed (28-30).
These applications have been developed during each of the AI booms,
each of which exhibited their own trends and problems. AI in the
current boom is more developed than before, but there are also
problems such as the lack of knowledge regarding the information
that AI recognizes. The resolution of such problems is expected to
further develop AI. Big data and AI will continue to support
healthcare in numerous ways.
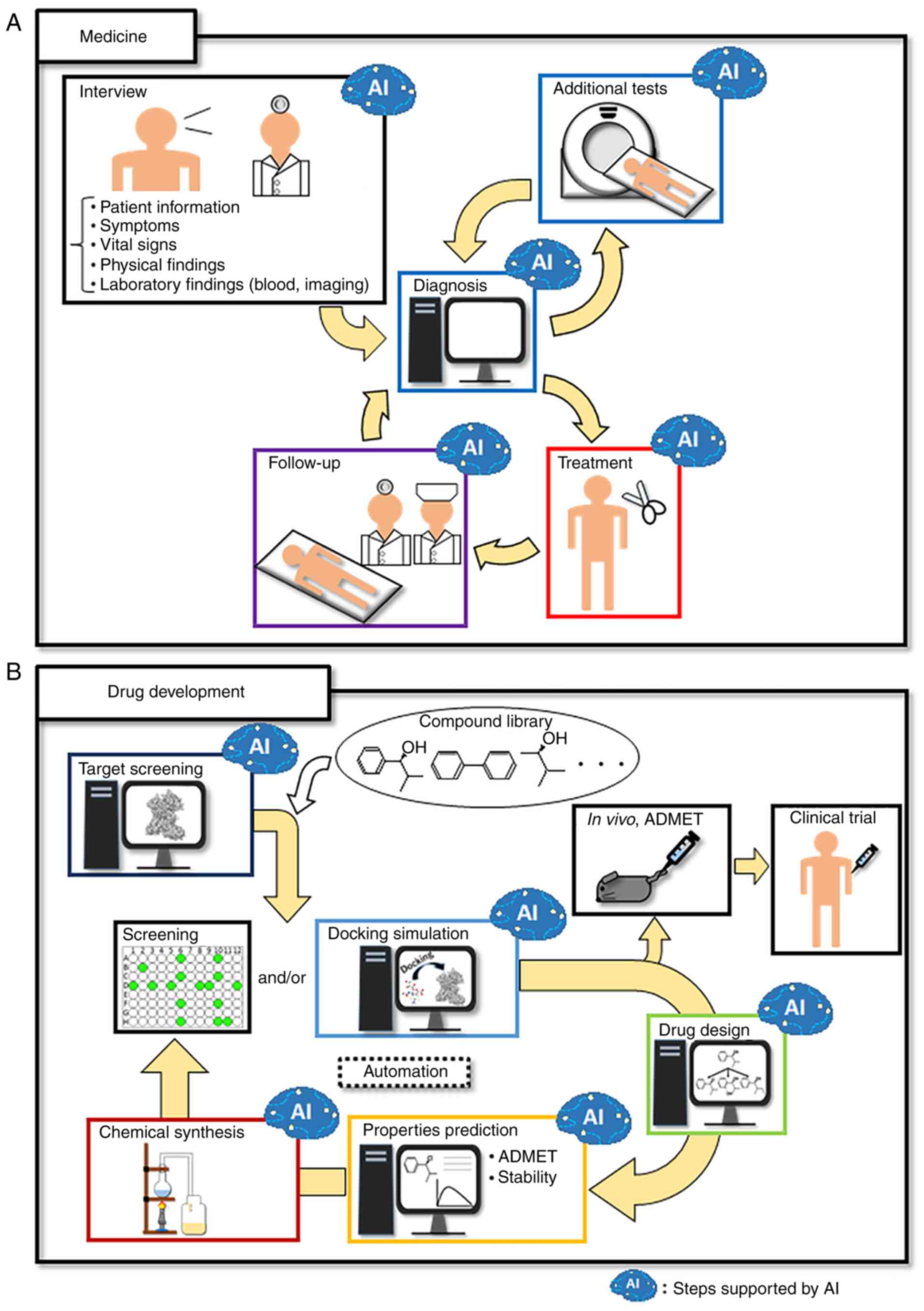
3. Current AI applied in medicine
Diagnosis
Diagnosis requires the ability to process different
types of information about patients and detect abnormalities with
high accuracy and reproducibility. In conventional diagnosis,
physicians process various pieces of patient information using
their own knowledge and/or experience, and detect abnormalities in
patients using their own senses or through diagnostic equipment.
This method sometimes fails to detect abnormalities in patients or
results in the wrong decisions being made. In addition, the
diagnostic ability is dependent on the experience of the physician.
Therefore, AI is expected to have diagnostic performance with
reproducibility and accuracy equal to or better than that of
skilled physicians, and to compensate for differences in physician
experience. Current AI for diagnosis is actively being developed to
perform diagnostic imaging with computed tomography and tissue
sections. In particular, convolutional neural networks perform well
in the ILSVRC every year; therefore, convolutional neural networks
are the most used for diagnostic imaging and perform as well as or
better than skilled physicians (31). Moreover, systems have been
developed to predict radiation or anticancer drug sensitivity using
convolutional neural networks (32,33).
In addition to diagnostic imaging, AI is also being developed to
diagnose diseases such as cancer via machine learning of blood
components (34). However,
limitations in measurement sensitivity and technical artifacts such
as noise are barriers to diagnosis using blood components. The
development of improved measurement technology and/or more advanced
machine learning models would be required for a diagnosis that
applies machine learning of blood components (35).
As aforementioned, the current application of AI for
diagnosis mainly improves the accuracy of each test. By contrast,
for the identification of a disease from various symptoms in a
patient and the results of tests, a wide range of knowledge, not
specific knowledge, and advanced information processing is
necessary. To meet this demand, AI assistants such as Watson are
also being developed that can learn the literature on a subject by
enabling the processing of natural language, and can make complex
decisions using expert systems (28,36).
Thus, for diagnosis, AI is mainly developed to
improve the accuracy of each test and make appropriate decisions
using the large quantity of related literature available, and
different algorithms suitable for each process are applied.
Treatment
For treatment, surgical robots are mainly being
developed. Surgical robots are suitable for detailed work with
precise movements that is beyond the reach of human hands. In
conventional surgical robot algorithms, classification and
detection of objects required during surgery are performed by the
developer manually creating features of the region of interest;
however, with the advent of deep learning, convolutional neural
networks are being applied for the classification and detection of
objects (37,38). In addition, real-time predictions
are also being made with recurrent neural networks (39). Conventional surgical robots are
operated by a physician; however, surgical robots that work
automatically without operation by a physician are also being
developed (40). Thus, the
development of AI has also led to the development of robots that
support physicians during surgery.
In addition, in drug therapy, AI-based applications
are being introduced in diagnosis and follow-up rather than in the
treatment process. Various AI applications have been introduced in
the drug development process to develop therapeutic drugs. In the
drug development process, developers need to process an enormous
amount of information to discover just a few promising compounds
from millions to tens of millions of candidate compounds (41). Various types of AI play active
roles in processing this information, as described in detail later
in this review.
Thus, in the treatment process, surgical robots are
mainly being developed to make the operation more accurate and
reduce the burden on the physician, while in the drug development
process, AI is being used to process large quantities of
information.
Follow-up
In medicine, no matter what type of disease or what
type of treatment is given, follow-up is more or less always
necessary. In addition, life expectancy in the world has increased
by 20 years in the last 50 years, and as the population ages, the
risk of having chronic diseases increases. Against this background,
wearable devices equipped with AI that can constantly monitor
health conditions and immediately detect any abnormality in wearers
are actively being developed. In particular, wearable devices are
expected to be used in cardiology, where the condition of the
patient may change rapidly and there is a direct link with
mortality status. In fact, more than half of the applications for
follow-up approved by the FDA in 2017-2020, including the ECG app
on the Apple™ Watch, are wearable cardiology devices (23,26).
A number of the algorithms in wearable devices are applied
artificial neural networks or adaptive algorithms (42).
In addition to the wearable devices, automated
communication systems have also being introduced for follow-up. For
example, Pharmabot was a chatbot developed in 2015 to assist in
medication education for pediatric patients and their parents using
a Left-Right parsing algorithm and Care Angel, which applied an
automated voice dialogue system to check on the condition of the
person requiring care, to detect abnormalities and to alert the
caregiver to changes if necessary (43,44).
As aforementioned, AI-based applications are
contributing to medicine in numerous ways. The characteristics of
conventional medicine and AI-based medicine are summarized in
Table II. In the current section,
some examples of the applied AI-based applications have been
introduced; however, it is difficult for AI-based medicine to
classify diseases and symptoms that are still difficult to define,
and to obtain the sense of security that is felt upon meeting a
doctor or nurse directly. It would be possible to realize better
medical care by solving the disadvantages of both and integrating
the advantages of both.
 | Table IIComparison between conventional and
computational medicine. |
Table II
Comparison between conventional and
computational medicine.
| Medicine | Conventional | Computational |
|---|
| Diagnosis | Doctors meet
patients; patients are diagnosed using diagnostic equipment at the
hospital; diagnostic accuracy depends on the experience of a
doctor | AI can detect
lesions with the same or better accuracy than a skilled doctor; AI
support is used to avoid misdiagnoses; AI supports the decision of
the doctor by processing of the medical literature |
| Treatment | Doctors perform
surgery directly; doctors or nurses administer medications | Robotics support
doctors in surgery; devices automatically administer medications
based on time and symptoms |
| Follow-up | Doctors or nurses
meet patients; patients are diagnosed using diagnostic equipment at
a hospital | Devices, such as
those that are wearable, can detect abnormalities at a very early
stage; the AI can consult with patients about their medical
conditions and medications |
| Advantages | Patients can meet
their doctors and nurses; abstract expression is possible | AI decreases the
burden on doctors and nurses; AI responds quickly in an
emergency |
| Problems | The burden on
doctors and nurses is heavy; sometimes it is not possible to
respond immediately in an emergency | The process of
outputting the data is incomprehensible to humans. Application cost
is high. AI cannot take responsibility for mistakes. |
4. AI in drug development as a foundation
for drug therapy
Medications used in pharmacotherapy are discovered
from among millions to tens of millions of candidate compounds
after long years of research and after a huge amount of money has
been expended. To evaluate each candidate compound, a number of key
pieces of information are required for evaluation, such as in
vitro and in vivo pharmacological activity, safety,
target specificity, pharmacokinetics, physical properties such as
molecular weight and solubility, stability in the body and during
storage, and development cost. Therefore, drug development sites
always generate enormous amounts of information. Developers need to
extract the important information from this in order to discover
optimal compounds. Therefore, it is expected that AI would improve
the accuracy and efficiency of developers in drug development. AI
was introduced to the field of drug development earlier than to the
medical field (45). In
conventional drug development, developers propose hypotheses for
the treatment of a disease and focus on a target that can be used
to develop a therapeutic drug within the hypothesis; this is
followed by drug development. However, this process might miss
crucial therapeutic targets and drugs for various diseases. In
AI-based drug development, AI can propose, and lead to the
development of, important targets and candidate drugs for disease
therapy (Table III). In
particular, Watson is able to identify connections and
relationships among diseases, drugs, genes and other factors, and
can generate novel hypotheses by mining the scientific literature
(28). This tool is useful not
only for drug development, but also for drug repurposing. In
addition, Watson is constantly and automatically updated. Automatic
learning in AI is important, not only to decrease the effort
involved, but also to create better methods to meet unmet needs in
life sciences and medicine.
 | Table IIIComparison between conventional and
AI-based drug development. |
Table III
Comparison between conventional and
AI-based drug development.
| Drug
development | Conventional | AI-based |
|---|
| Driving factor | Target-driven | Data-driven |
| Targets | Easily druggable
targets with known structure and interactions in cells | Meaningful targets
extracted by machine learning using big data |
| Advantages | It is easy for
humans to understand identified targets and compounds. | Saves time and
money by predicting the activities and properties of compounds
before synthesis; compounds that target un-druggable molecules may
be identified |
| Problems | Targets are limited
by the complex and/or un-known structures and interactions; the
identification of promising compounds is time consuming (numerous
synthesis-evaluation cycles). | A large amount of
accurate data is necessary for learning; AI cannot understand
whether compounds are meaningful for humans |
AI-based drug development can save time and money.
In conventional drug development, screening is performed using
millions to tens of millions of compound libraries, followed by
synthetic development based on the candidate compounds obtained
from the screening and the re-evaluation of their activities to
identify those with promise. However, numerous identified compounds
do not exhibit physical properties and safety profiles that are
suitable for pharmaceutical applications; therefore, other
compounds are often re-synthesized. To avoid such time and money
loss, AI-based drug development can predict the activity, physical
properties and safety of each compound using computers. Various
AI-based applications have been developed to predict these
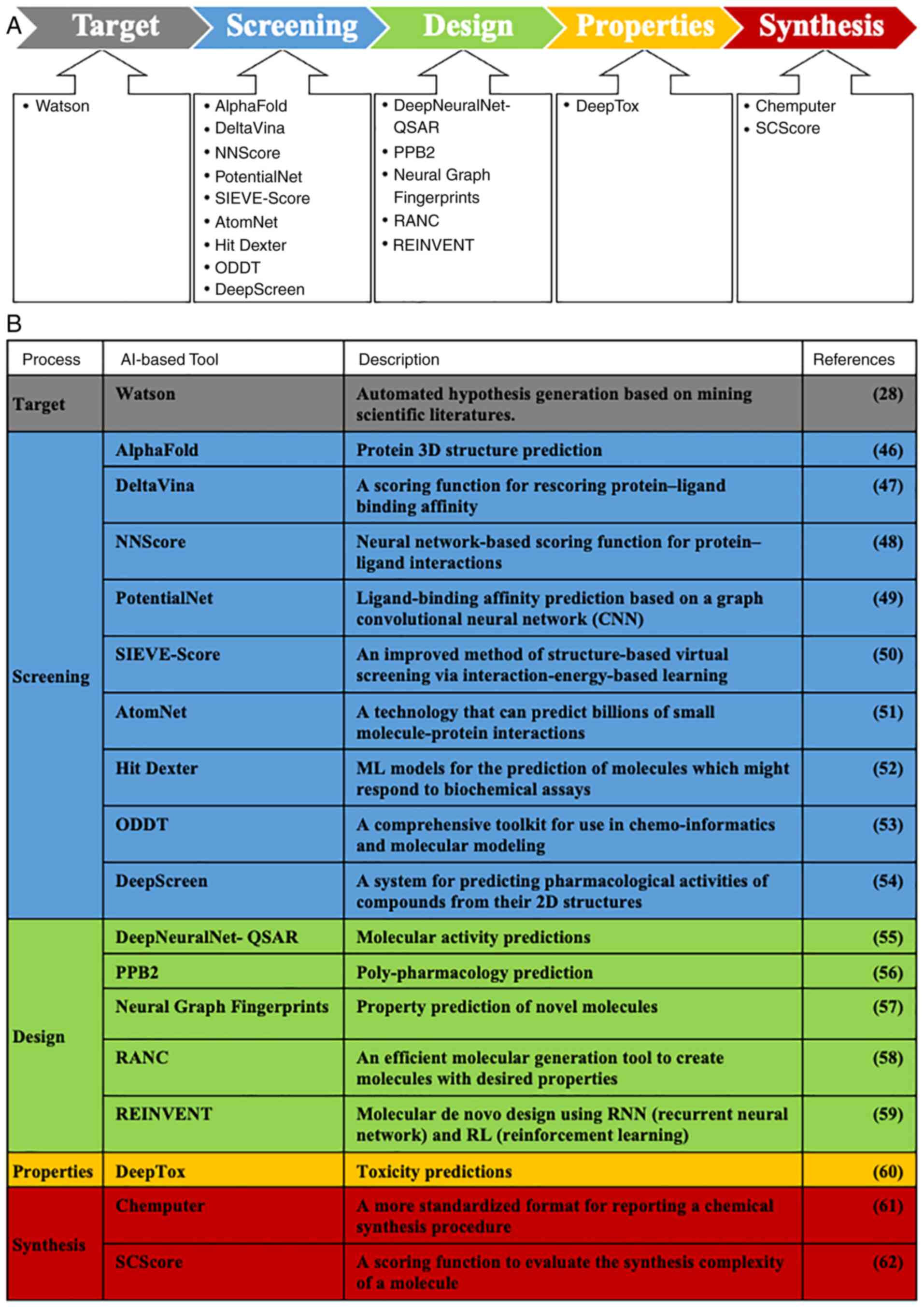
parameters (Figs. 2 and 3) (28,46-62).
Furthermore, applications have been developed that predict not only
the properties of individual compounds, but also suitable routes of
synthesizing pharmacological reagents or therapeutic compounds
(61,62). The cost of drug development has
been decreased by these applications; however, the accuracy of the
AI prediction of the compound properties is not sufficient and
further improvement of this factor is necessary. In particular,
AI-based applications have been actively developed as screening
steps, which require time and money. However, it is difficult to
predict the affinities between a target protein and compounds, for
the following reasons: i) Difficulties in predicting protein
flexibility; ii) ambiguity regarding the complexity of a protein in
an actual environment, and iii) difficulties in assessing the
solvent effects of an actual environment (63,64).
To solve these problems, a number of experiments and new algorithms
would be necessary.
One of the most difficult steps in the process of
drug development is the prediction of adverse effects. It has been
reported that computational modeling using machine learning is
useful for predicting adverse effects (65). Moreover, it is possible to
manufacture synthetic patients and data artificially by analyzing
existing data using machine learning techniques (66,67).
As there are no ethical concerns regarding the privacy and costs of
using synthetic data, this would be a powerful tool for clinical
studies that require a large number of patients and may be an
effective alternative for preparing training data for machine
learning algorithms. These fields of research could be further
enriched by AI in the near future and would also contribute to the
realization of personalized precision medicine.
5. Perspectives for AI-based medicine and
drug development
AI has been used for clinical purposes and drug
development. However, the current AI-based applications are only
being developed for specific applications at each stage of
pharmacological or medical applications. In particular, AI-based
applications with a high accuracy for diagnosis have actively been
developed. However, a diagnosis is not determined based only on the
result of one diagnostic method, and should be performed by
comprehensively combining various types of information, such as
chief complaints and physical findings; a system that integrates
the various specific diagnostic data would be necessary in the
future (Fig. 3A). If the disease
remained unclear based only on the acquired information, the system
would be a present a diagnostic method to determine the condition.
The system would avoid missing information and improve the accuracy
of the diagnosis. In addition, the system would not only be used
for diagnosis, but also for monitoring the progress of treatment
after surgery and drug therapy. At present, almost no AI-based
application has been developed that predicts the therapeutic effect
or proposes a therapeutic method. However, a system has been
reported in basic research that predicts the sensitivity of
anticancer agents and radiation therapy based on phase contrast
image information (32,33). In the near future, AI may be able
to predict the therapeutic effects of various therapeutic methods
in advance and suggest an appropriate therapeutic method. Thus,
clinical AI in the future should be a system that interprets and
integrates various types of clinical information and considers the
changes caused by each treatment, to promote the best flow toward
the complete cure of the disease. In addition, by constantly
collecting information on complaints and physical status, both
inside and outside the hospital, AI systems should always support
the ability of a patient to live without aggravating their
condition.
In drug development, numerous specific AI-based
applications are already in use. In particular, a number of
applications for the simulation of the docking of compounds to
target proteins have been developed (47-53).
Moreover, in addition to having an excellent specific score (such
as affinity for a target protein), a candidate compound needs to
have comprehensive excellent safety and pharmacokinetics.
Therefore, a system for identifying promising compounds by
considering various characteristics, as well as learning specific
scores, will be required in the future (Fig. 3B). Furthermore, in drug
development, clinical trials impose a heavy financial and time
burden. It is necessary to improve the efficiency of clinical
trials by using portal devices, which configure and manage devices
remotely over the network or via USB connection, and collecting and
selecting applicable patients.
The most important key to solving the problems
facing AI-based applications is making it possible for people to
understand the judgment process of AI. AI-based clinical
applications would be utilized in important aspects of future
treatment decisions, such as the diagnosis and evaluation of
treatment effectiveness. If the AI determination process is
unclear, the medical staff could not evaluate the validity of the
AI determination, which would lead to the distrust of AI. To solve
the uncertainty of AI, ‘Explainable AI’ has been actively
researched (68). The development
of Explainable AI would be essential for the widespread use of
AI-based applications in healthcare.
In addition, as AI-based healthcare would accumulate
a greater amount of medical information than the current healthcare
system, it would be necessary to prepare an infrastructure and
security systems to handle large amounts of information. Futuristic
clinical AI might monitor not only medical information, but also
the tasks of patients and medical staff, and might forecast
workflow bottlenecks.
As aforementioned, the implementation of AI could
facilitate more accuracy and greater efficiency in various fields
of healthcare; however, it has some issues and limitations. Both
medical staff and developers would need to understand the issues
and limitations, and then the coexistence of humans and AI could
lead to better healthcare.
6. Conclusion
AI has evolved with the times and has been utilized
in applications in drug development and healthcare. These
applications are steadily producing results, and the use of AI is
becoming established. The implementation of AI in society will need
to overcome issues such as how to develop leading companies and
train data scientists. However, company development may still face
some obstacles, such as implementing AI, employment and cost. In
the future, to improve human health, we should not only develop AI,
but also think about the coexistence of humans with AI.
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology (grant nos. 19K22658, 19K09172,
19K07688, 20H00541, 221S0002 and 21K19526) and the Japan Agency for
Medical Research and Development (grant no. 16cm0106414h0001).
Partial support was received from the Princess Takamatsu Cancer
Research Fund (2019).
Availability of data and materials
Not applicable.
Authors' contributions
HI, MT and AV conceptualized the study. AA, MK and
HI wrote the manuscript. All authors have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Institutional endowments were received partially
from Hirotsu Bio Science Inc. (Tokyo, Japan), Kinshu-kai Medical
Corporation (Osaka, Japan), IDEA Consultants, Inc. (Tokyo, Japan),
Kyowa-kai Medical Corporation (Hyogo, Japan), Unitech Co., Ltd.
(Chiba, Japan), Chugai Co., Ltd. (Tokyo, Japan), Yakult Honsha Co.,
Ltd. (Tokyo, Japan) and Ono Pharmaceutical Co., Ltd. (Osaka,
Japan).
References
|
1
|
Santos DP and Baeßler B: Big data,
artificial intelligence, and structured reporting. Eur Radiol Exp.
2(42)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akyazi T, Goti A, Oyarbide A, Alberdi E
and Bayon F: A guide for the food industry to meet the future
skills requirements emerging with industry 4.0. Foods.
9(492)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Favaretto M, Shaw D, De Clercq E, Joda T
and Elger BS: Big data and digitalization in dentistry: A
systematic review of the ethical issues. Int J Environ Res Public
Health. 17(2495)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Etienne H, Hamdi S, Le Roux M, Camuset J,
Khalife-Hocquemiller T, Giol M, Debrosse D and Assouad J:
Artificial intelligence in thoracic surgery: Past, present,
perspective and limits. Eur Respir Rev. 29(200010)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McCarthy J, Minsky ML, Rochester N and
Shannon CE: A Proposal for the Dartmouth Summer Research Project on
Artificial Intelligence, August 31, 1955. AI Magazine.
27(12)2006.
|
|
6
|
Samuel AL: Some studies in machine
learning using the game of checkers. IBM J Res Dev. 3:210–229.
1959.
|
|
7
|
Wied GL, Bahr GF, Oldfield DG and Bartels
PH: Computer-assisted identification of cells from uterine
adenocarcinoma. A clinical feasibility study with TICAS. I.
Measurements at wavelength 530 nm. Acta Cytol. 12:357–370.
1968.PubMed/NCBI
|
|
8
|
Ishiyama T, Tsubura E, Hirao F, Yamamura
Y, Takeda S, Tateisi K, Yamamoto M, Uemura M, Yaida K, Hayakawa F,
et al: A study of the automation of cytodiagnosis. Med Biol Eng.
7:297–306. 1969.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shortliffe EH, Axline SG, Buchanan BG,
Merigan TC and Cohen SN: An artificial intelligence program to
advise physicians regarding antimicrobial therapy. Comput Biomed
Res. 6:544–560. 1973.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miller RA, Pople HE and Myers JD:
INTERNIST-1, An experimental computer-based diagnostic consultant
for general internal medicine. N Engl J Med. 307:478–486.
1982.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dechter R: Learning while searching in
constraint-satisfaction-problems. In: Proceedings of the Fifth
National Conference on Artificial Intelligence. American
Association for Artificial Intelligence, Philadelphia, pp178-183,
1986.
|
|
12
|
LeCun Y, Boser B, Denker JS, Henderson D,
Howard RE, Hubbard W and Jackel LD: Backpropagation applied to
handwritten zip code recognition. Neural Comput. 1:541–551.
1989.
|
|
13
|
Kwoh YS, Hou J, Jonckheere EA and Hayati
S: A robot with improved absolute positioning accuracy for CT
guided stereotactic brain surgery. IEEE Trans Biomed Eng.
35:153–160. 1988.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Jacobs LK, Shayani V and Sackier JM:
Determination of the learning curve of the AESOP robot. Surg
Endosc. 11:54–55. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sung GT and Gill IS: Robotic laparoscopic
surgery: A comparison of the DA Vinci and Zeus systems. Urology.
58:893–898. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weinstein GS, O'malley BW Jr and Hockstein
NG: Transoral robotic surgery: Supraglottic laryngectomy in a
canine model. Laryngoscope. 115:1315–1319. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurumbail RG, Stevens AM, Gierse JK,
McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM,
Penning TD, Seibert K, et al: Structural basis for selective
inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature.
384:644–648. 1996.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Molnár L and Losonczy H: Tyrosine kinase
inhibitor STI571: New possibility in the treatment of chronic
myeloid leukemia. Orv Hetil. 143:2379–2384. 2002.PubMed/NCBI(In Hu).
|
|
19
|
Lanfranco AR, Castellanos AE, Desai JP and
Meyers WC: Robotic surgery: A current perspective. Ann Surg.
239:14–21. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hinton GE, Osindero S and The YW: A fast
learning algorithm for deep belief nets. Neural Comput.
18:1527–1554. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hinton GE and Salakhutdinov RR: Reducing
the dimensionality of data with neural networks. Science.
313:504–507. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Komura D and Ishikawa S: Machine learning
approaches for pathologic diagnosis. Virchows Arch. 475:131–138.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
American College of Radiology. Cleared AI
Algorithms. 2021. https://models.acrdsi.org. Accessed July 1, 2021.
|
|
24
|
U.S. Food and Drug Administration: FDA
allows marketing of first whole slide imaging system for digital
pathology. 2017. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology.
Accessed July 1, 2021.
|
|
25
|
Mills AM, Gradecki SE, Horton BJ,
Blackwell R, Moskaluk CA, Mandell JW, Mills SE and Cathro HP:
Diagnostic efficiency in digital pathology: A comparison of optical
versus digital assessment in 510 surgical pathology cases. Am J
Surg Pathol. 42:53–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
The Medical Futurist: FDA-approved
A.I.-based algorithms. 2021. https://medicalfuturist.com/fda-approved-ai-based-algorithms/.
Accessed July 1, 2021.
|
|
27
|
Asai A, Koseki J, Konno M, Nishimura T,
Gotoh N, Satoh T, Doki Y, Mori M and Ishii H: Drug discovery of
anticancer drugs targeting methylenetetrahydrofolate dehydrogenase
2. Heliyon. 4(e01021)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Spangler S, Wilkins AD, Bachman BJ,
Nagarajan M, Dayaram T, Haas P, Regenbogen S, Pickering CR, Comer
A, Myers JN, et al: Automated hypothesis generation based on mining
scientific literature. KDD'14: Proceedings of the 20th ACM SIGKDD
international conference on Knowledge discovery and data mining:
1877-1886, 2014.
|
|
29
|
Doyle-Lindrud S: Watson will see you now:
A supercomputer to help clinicians make informed treatment
decisions. Clin J Oncol Nurs. 19:31–32. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tojo A: Clinical sequencing in leukemia
with the assistance of artificial intelligence. Rinsho Ketsueki.
58:1913–1917. 2017.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
31
|
Esteva A, Kuprel B, Novoa RA, Ko J,
Swetter SM, Blau HM and Thrun S: Dermatologist-level classification
of skin cancer with deep neural networks. Nature. 542:115–118.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toratani M, Konno M, Asai A, Koseki J,
Kawamoto K, Tamari K, Li Z, Sakai D, Kudo T, Satoh T, et al: A
convolutional neural network uses microscopic images to
differentiate between mouse and human cell lines and their
radioresistant clones. Cancer Res. 78:6703–6707. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yanagisawa K, Toratani M, Asai A, Konno M,
Niioka H, Mizushima T, Satoh T, Miyake J, Ogawa K, Vecchione A, et
al: Convolutional neural network can recognize drug resistance of
single cancer cells. Int J Mol Sci. 21(3166)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tanos R, Tosato G, Otandault A, Al Amir
Dache Z, Pique Lasorsa L, Tousch G, El Messaoudi S, Meddeb R, Assaf
MD, Ychou M, et al: Machine learning-assisted evaluation of
circulating DNA quantitative analysis for cancer screening. Adv Sci
(Weinh). 7(2000486)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Im YR, Tsui DWY, Diaz LA Jr and Wan JCM:
Next-generation liquid biopsies: Embracing data science in
oncology. Trends Cancer. 7:283–292. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Harada Y and Shimizu T: Impact of a
Commercial Artificial Intelligence-Driven Patient Self-Assessment
Solution on Waiting Times at General Internal Medicine Outpatient
Departments: Retrospective Study. JMIR Med Inform.
8(e21056)2020.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Litjens G, Kooi T, Bejnordi BE, Setio AAA,
Ciompi F, Ghafoorian M, van Der Laak JA, van Ginneken B and Sánchez
CI: A survey on deep learning in medical image analysis. Med Image
Anal. 42:60–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Khosravi P, Kazemi E, Imielinski M,
Elemento O and Hajirasouliha I: Deep convolutional neural networks
enable discrimination of heterogeneous digital pathology images.
EBioMedicine. 27:317–328. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Meyer A, Zverinski D, Pfahringer B,
Kempfert J, Kuehne T, Sündermann SH, Stamm C, Hofmann T, Falk V and
Eickhoff C: Machine learning for real-time prediction of
complications in critical care: A retrospective study. Lancet
Respir Med. 6:905–914. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Saeidi H, Opfermann JD, Kam M, Raghunathan
S, Leonard S and Krieger A: A confidence-based shared control
strategy for the smart tissue autonomous robot (STAR). Rep US.
2018:1268–1275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Brown N, Cambruzzi J, Cox PJ, Davies M,
Dunbar J, Plumbley D, Sellwood MA, Sim A, Williams-Jones BI,
Zwierzyna M and Sheppard DW: Big data in drug discovery. Prog Med
Chem. 57:277–356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vélez-Guerrero MA, Callejas-Cuervo M and
Mazzoleni S: Artificial intelligence-based wearable robotic
exoskeletons for upper limb rehabilitation: A review. Sensors
(Basel). 21(2146)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Comendador B, Francisco B, Medenilla J,
Nacion S and Serac T: Pharmabot: A pediatric generic medicine
consultant chatbot. J Automat Control Eng. 3:137–140. 2015.
|
|
44
|
Wong V, Rosenbaum S, Sung W, Kaplan RM,
Bott N, Platchek T, Milstein A and Shah NR: Caring for caregivers:
bridging the gap between family caregiving policy and practice.
NEJM Catal Innov Care Deliv. 2:2021.
|
|
45
|
Paul D, Sanap G, Shenoy S, Kalyane D,
Kalia K and Tekade RK: Artificial intelligence in drug discovery
and development. Drug Discov Today. 26:80–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
AlQuraishi M: AlphaFold at CASP13.
Bioinformatics. 35:4862–4865. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang C and Zhang Y: Improving
scoring-docking-screening powers of protein-ligand scoring
functions using random forest. J Comput Chem. 38:169–177.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Durrant JD and McCammon JA: NNScore 2.0: A
neural-network receptor-ligand scoring function. J Chem Inf Model.
51:2897–2903. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Feinberg EN, Sur D, Wu Z, Husic BE, Mai H,
Li Y, Sun S, Yang J, Ramsundar B and Pande VS: PotentialNet for
molecular property prediction. ACS Cent Sci. 4:1520–1530.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yasuo N and Sekijima M: Improved method of
structure-based virtual screening via interaction-energy-based
learning. J Chem Inf Model. 59:1050–1061. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wallach I, Dzamba M and Heifets A:
AtomNet: A deep convolutional neural network for bioactivity
prediction in structure-based drug discovery. arXiv: 1510.02855,
2015.
|
|
52
|
Stork C, Chen Y, Šícho M and Kirchmair J:
Hit dexter 2.0: Machine-learning models for the prediction of
frequent hitters. J Chem Inf Model. 59:1030–1043. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wójcikowski M, Zielenkiewicz P and
Siedlecki P: Open drug discovery toolkit (ODDT): A new open-source
player in the drug discovery field. J Cheminform.
7(26)2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rifaioglu AS, Nalbat E, Atalay V, Martin
MJ, Cetin-Atalay R and Doğan T: DEEPScreen: High performance
drug-target interaction prediction with convolutional neural
networks using 2-D structural compound representations. Chem Sci.
11:2531–2557. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Xu Y, Ma J, Liaw A, Sheridan RP and
Svetnik V: Demystifying multitask deep neural networks for
quantitative structure-activity relationships. J Chem Inf Model.
57:2490–2504. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Awale M and Reymond JL: Polypharmacology
Browser PPB2: Target prediction combining nearest neighbors with
machine learning. J Chem Inf Model. 59:10–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Duvenaud DK, Maclaurin D, Iparraguirre J,
Bombarell R, Hirzel T, Aspuru-Guzik A and Adams RP: Convolutional
networks on graphs for learning molecular fingerprints. In:
Advances in neural information processing systems 28. NIPS
Foundation, Montreal. 2:2224–2232. 2015.
|
|
58
|
Putin E, Asadulaev A, Ivanenkov Y,
Aladinskiy V, Sanchez-Lengeling B, Aspuru-Guzik A and Zhavoronkov
A: Reinforced adversarial neural computer for de novo molecular
design. J Chem Inf Model. 58:1194–1204. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Olivecrona M, Blaschke T, Engkvist O and
Chen H: Molecular de-novo design through deep reinforcement
learning. J Cheminform. 9(48)2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mayr A, Klambauer G, Unterithiner T and
Hochreiter S: DeepTox: Toxicity prediction using deep learning.
Front Environ Sci. 3(80)2016.
|
|
61
|
Steiner S, Wolf J, Glatzel S, Andreou A,
Granda JM, Keenan G, Hinkley T, Aragon-Camarasa G, Kitson PJ,
Angelone D and Cronin L: Organic synthesis in a modular robotic
system driven by a chemical programming language. Science.
363(eaav2211)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Coley CW, Rogers L, Green WH and Jensen
KF: SCScore: Synthetic complexity learned from a reaction corpus. J
Chem Inf Model. 58:252–261. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Antunes DA, Devaurs D and Kavraki LE:
Understanding the challenges of protein flexibility in drug design.
Expert Opin Drug Discov. 10:1301–1313. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Uehara S and Tanaka S: AutoDock-GIST:
Incorporating thermodynamics of active-site water into scoring
function for accurate protein-ligand docking. Molecules.
21(1604)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Jamal S, Ali W, Nagpal P, Grover S and
Grover A: Computational models for the prediction of adverse
cardiovascular drug reactions. J Transl Med. 17(171)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Walonoski J, Kramer M, Nichols J, Quina A,
Moesel C, Hall D, Duffett C, Dube K, Gallagher T and McLachlan S:
Synthea: An approach, method, and software mechanism for generating
synthetic patients and the synthetic electronic health care record.
J Am Med Inform Assoc. 25:230–238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Choi E, Biswal S, Malin B, Duke J, Stewart
WF and Sun J: Generating multi-label discrete patient records using
generative adversarial networks. PMLR. 68:286–305. 2017.
|
|
68
|
Linardatos P, Papastefanopoulos V and
Kotsiantis S: Explainable AI: A Review of Machine Learning
Interpretability Methods. Entropy (Basel). 23(18)2021.PubMed/NCBI View Article : Google Scholar
|