Introduction
Psoriasis is a chronic immune-mediated
dermatological condition with potential systemic impact,
predominantly associated with skin and joint damage (1). It affects about 2% of the world's
population and can have a serious impact on the patient's quality
of life. It consists of an abnormal inflammatory response
characterized by an increase in proinflammatory cytokines
consequent to keratinocyte hyperproliferation (2,3).
Proper treatment can be selected according to disease severity.
Psoriasis can develop into a debilitating disease that strongly
impacts the quality of life and significantly contributes to health
care costs (4).
In the case of mild and moderate forms, first-line
treatment consists of topical corticosteroids, vitamin D3 analogues
or a combination of the two. For more severe cases, phototherapy
can be utilized. Systemic therapies, such as methotrexate or
cyclosporine, may also be required (5). Phototherapy based on radiation in the
UVB spectrum leads to apoptosis of T lymphocytes and
immunosuppressive effects resulting in clinical improvement of
immunologic skin diseases. Patients diagnosed with psoriasis may
obtain clearance using the excimer laser technology rather than
narrow-band UVB (3,5).
Currently, due to the long-lasting characteristic of
the disease and frustration with conventional medical therapies,
some psoriasis-diagnosed patients seek complementary and
alternative treatments to help manage their symptoms (6). Some factors such as smoking, high body
mass index (BMI), alcohol consumption, trauma, endocrine disorders,
and of course, drugs are known to trigger psoriasis (7).
Certain drugs prescribed for other comorbidities are
considered in the literature to be associated with the exacerbation
of psoriasis; these include lithium, nonsteroidal anti-inflammatory
agents, synthetic antimalarial drugs and β-blockers (8). Understanding the pathophysiology can
provide clues to the treatment and management of drug-induced and
drug-aggravated psoriasis, which may be indistinguishable from
idiopathic psoriasis. Tumor necrosis factor (TNF)α inhibitors
themselves can also trigger psoriasis, which leads to an
interesting discussion (9). Since
there is no standard therapeutic consensus for patients with
moderate to severe forms of psoriasis, the benefits and risks of
systemic therapy or phototherapy must be carefully assessed for
each patient, and the treatment should be individualized
accordingly (10).
It has been noted that patients with chronic
inflammatory conditions such as psoriasis, psoriatic arthritis,
Crohn's disease and rheumatoid arthritis (RA) may have an increased
risk of malignancy due to impaired immune support and stress
management (immunosurveillance) resulting from the effects of
chronic inflammation and immunosuppressive agents (11-13).
Due to exposure to immunosuppressive agents, methotrexate (MTX),
biologic therapies, cyclosporine, and UV light therapies, there may
be an increased cancer risk in patients with psoriasis (14,15).
MTX is a structural analogue of folic acid that reversibly inhibits
dihydrofolate reductase, thereby preventing DNA synthesis. Its
mechanism of action in psoriasis has not been fully understood yet,
but MTX is believed to act primarily as an immunosuppressant and
reduces the rate of epidermal proliferation in psoriasis (16).
A retrospective study by Scott et al
(17) on RA and inflammatory bowel
disease (IBD) patients with non-melanoma skin cancer (NMSC) history
showed that the MTX use was associated with an increased risk of a
second NMSC in RA. The risk particularly increased with an exposure
duration longer than one year or when other medications for other
diseases were associated (17,18).
The addition of an anti-TNFα agent caused a risk increase, but
there was no increased risk with rituximab or abatacept (17). An analysis of 130,315 RA patients
suggested no significantly increased risk of melanoma in patients
treated with TNFα inhibitors. There was also no significant risk in
this same group of patients for tocilizumab and abatacept exposure
(19).
Small-molecule (apremilast, tofacitinib) and
biologic therapies such as anti-TNFα (infliximab, etanercept
adalimumab), anti-IL-12/23 (ustekinumab), anti-IL-17 (secukinumab,
ixekizumab, brodalumab) and anti-IL-23 (guselkumab, tildrakizumab)
can be effective in severe forms of psoriasis or psoriatic
arthritis, but they can have significant side effects and require
close follow-up (5,20). Biologic therapies are a powerful
treatment option for those with moderate to severe psoriasis.
According to current guidelines regarding the use of biologics,
they are contraindicated in those patients with proven malignancy
or premalignancy states, which means that adequately treated NMSC,
as well as malignancies diagnosed and treated earlier than 20 years
previously should be excluded (21). Patients with psoriasis are at a far
greater risk of malignancy as they receive UV therapy such as
311-nm narrowband phototherapy, PUVA (Psoralen plus UVA), and
excimer laser therapy (3,22). Furthermore, some of the patients
suffering from psoriasis receive retinoids (which increase their
sensitivity to UV radiation) and MTX further increasing the risk of
skin cancer occurrence (23).
The relationship between psoriasis and malignancy is
not very well established; there are few studies that have led to
conflicting results (24). Studies
referring to psoriasis patients highlighted an increased risk of
(NMSCs and lymphoma, whereas the results regarding other solid
malignancies are inconsistent (25). Chiesa Fuxench et al (26) suggested that there is an overall
small increased risk of neoplasia in patients with psoriasis
treated with phototherapy or systemic medications, and that they
were shown to have a higher risk for malignant neoplasms compared
with controls. Future studies should be focused on a better
knowledge of the disease severity effect and exposure to treatment
separately on cancer risk in this population.
Dermatologists who treat patients with psoriasis
should consider appropriate cancer screening guidelines and
counseling in their daily practice (26-28).
Another study also confirmed that were was no increased risk for
melanoma, but non-melanoma skin cancer was associated with PUVA,
cyclosporine and anti-TNFα treatment in psoriasis. It also suggests
taking into consideration the incidence of cancer in a patient's
history before using biologic or immunosuppressive therapy because
of the lack of studies on these patient groups (29). Pérez Ramírez et al presented
one psoriasis patient suffering from metastatic melanoma with
spontaneous regression without any treatment. His psoriatic disease
was associated with HLA Cw6 (human leukocyte antigen). The authors
draw their attention on a possible relationship between psoriasis,
HLA Cw6, and spontaneous melanoma regression, and that psoriasis is
considered an immune-mediated disease that can play a protective
role against melanoma (30).
There is limited data regarding the association
between systemic treatments for psoriasis and cancer recurrence,
since patients with a history of malignancy are usually excluded
from participating in clinical trials, and dermatologists hesitate
to initiate immunosuppressive agents in cancer survivors. The
possibility of collision skin lesions, nevi located at the border
of psoriatic plaques, or even nevi found within psoriatic plaques
is considered to be worth exploring. The potential effects of
treatments in such situations and the existence of possible
differential diagnoses, such as the Meyerson phenomenon are also
worth studying. The Meyerson phenomenon is described as the
development of a halo or eczematous patch over another skin lesion.
Topical treatment with corticosteroids is effective in many cases
(31). Some authors suggest that
melanoma may occur as a component of Meyerson phenomenon, and that
careful dermoscopic examination is useful to differentiate between
pigmented lesions with peripheral erythematous halo (32). Nevi located within a psoriasis
plaque may appear to decrease in pigment. This aspect, however,
should not necessarily be interpreted as regression. Some nevi may
be observed to lighten as they are covered by psoriatic scale, and
will subsequently darken when exposed to sunlight.
Case report
We present the case of a 31-year old male patient,
non-smoker, non-alcoholic, who was referred to the Dermatology
Department of ‘St. Parascheva’ Clinical Infectious Diseases
Hospital of Galati in the Fall of 2019 due to the presence of
widespread, sharply demarcated erythemato-squamous, irregularly
shaped lesions, ranging from 2 to 4 cm, located on the trunk and
lower extremities. The patient reported the onset of cutaneous
lesions as early as childhood. He underwent various topical
treatments, including topical corticosteroid creams, ointments and
calcipotriol without significant changes. The initial management
with topical agents and narrow-band UVB phototherapy did not prove
to be of any success. Laboratory tests were within the normal
range. A punch skin biopsy was taken from a plaque situated on the
trunk. The histological examination revealed parakeratosis, marked
hyperkeratosis Munro's microabscesses, agranulocytosis, and
acanthosis.
Based on the clinical and histological findings, the
diagnosis of psoriasis vulgaris was made. After a thorough
clinical-biological assessment, the patient was deemed eligible for
systemic therapy, and MTX treatment starting with 15 mg per week
was proposed. After about 4 weeks, the response to the treatment
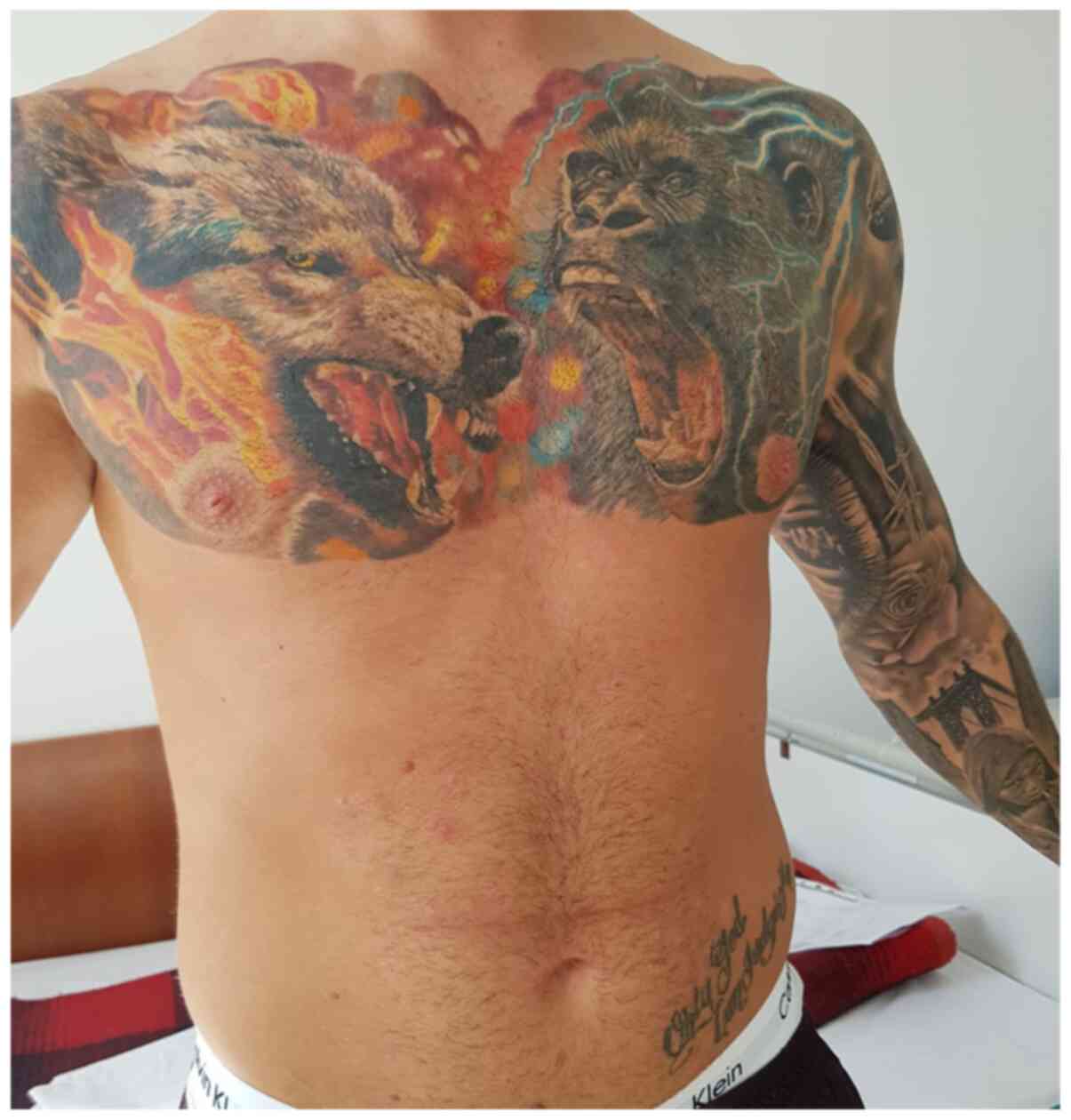
was encouraging with clearance of the skin, but the outcome was
inadequate, despite adherence to treatment (Fig. 1).
Unfortunately, after a 4-month treatment with MTX,
the lesions reappeared, becoming refractory to treatment;
therefore, MTX treatment would later prove to be unsuitable. Given
his recalcitrant disease covering up to 15% of his body surface,
the patient's desire to have a clear skin, and based on the fact
that the patient was naïve to biologic therapy, he was started on
secukinumab 300 mg monthly. Secukinumab, a fully human monoclonal
IL-17A antibody which binds to IL-17A inhibiting the inflammatory
cascade, has been shown in several clinical trials to be safe and
effective for the treatment of psoriasis. The patient is being
followed-up for clinical and biological parameters, with complete
skin clearance. He also presented some 1-3 mm brown and black
macules that involved the arms and trunk. He recalls that they were
not present at birth, but that he has had them for many years. Our
hypothesis was that they appeared after the patient's repeated sun
exposure during the summer holidays in order to obtain a clear
skin.
A particularity observed in this patient was his
strong desire for cosmesis that led him to seek out tattooing as a
solution to mask his psoriatic lesions. Tattooing has been
identified as a possible trigger for the Koebner phenomenon
occurring in psoriasis patients (33). Stress provoked by a negative
self-image can be both an initiating and sustaining factor in
psoriasis through the increased release of cytokines, hormones, and
neuropeptides that combine to cause overall proinflammatory and
immunomodulatory effects in what is now referred to as the
Brain-Skin connection (34).
The Ethics approval was obtained from the Ethics
Commission of the ‘St. Parascheva’ Clinical Hospital of Infectious
Diseases, Galati (approval no. 24/26.02.2021) and written informed
consent was obtained from the patient.
Discussion
In summary, the risk of new or recurrent systemic
malignancies is similar between patients with biologic and
non-biologic treatments. The risk of additional non-melanoma skin
cancer occurrences in patients with a history may be increased, and
data concerning additional primary melanomas and melanoma
recurrence are inconclusive in melanoma survivors. Despite evidence
suggesting the short-term efficacy and safety of biologic therapy
as compared to classic conventional systemic therapies, there are
concerns regarding the long-term risk of developing cancer in
patients treated with biologic therapy as compared to those treated
by conventional systemic therapies (35-37).
Based on high-level evidence, therapies for psoriasis appear to be
safe. Additional long-term data are warranted for newer treatments
and for their use in cancer survivors (38).
Long-term studies focused on safety guidance are
still lacking for these newer treatments, including biologic agents
targeting IL-12/23 (ustekinumab), IL-23 (guselkumab), IL-17
(ixekizumab, secukinumab), phosphodiesterase-4 (apremilast), and
small-molecule inhibitors of Janus kinase (tofacitinib), as well as
data for their use in cancer patients and cancer survivors. An
increased risk of squamous cell carcinoma (SCC) has been confirmed
by several studies, but there is conflicting evidence regarding the
risk for melanoma (39,40).
Nevi are considered to be an independent marker of
overall melanoma risk. Most nevi in adults range from 2 to 6 mm and
are estimated to be composed of thousands of melanocytes depending
on the size and type of the nevus (41). They have a uniform color and
clinically symmetric architecture. They are classified into one of
three major categories: Junctional (melanocytes confined to the
epidermis only), intradermal (confined to the dermis only) and
compound (both a dermal and an epidermal component) (42). Microscopically, nevi are well
circumscribed, symmetric, and are composed of melanocytes with a
banal cytology. They have two main histopathological features:
nesting and maturation (43). The
clinical importance of dysplastic nevi resides in their association
with melanoma risk. In these patients, the risk of melanoma
increases with the presence of melanoma in their personal or
familial history and with the number of nevi (44,45).
Patients with multiple atypical lesions are known to
have an increased risk of developing melanoma. It is considered
that periodic cutaneous assessment is a generally accepted
procedure (46). Dermoscopy, also
called epiluminescence microscopy, is an in vivo
non-invasive technique that aids visualization of the otherwise
invisible morphology of a pigmented lesion, thereby improving the
clinical diagnosis. Currently, dermoscopy is considered to be one
of the most efficient tools for the early diagnosis of melanoma. It
reduces the frequency of having to biopsy benign lesions (as
excisional biopsy is difficult to perform on each and every lesion
in patients who develop multiple melanocytic lesions) and is
efficient for monitoring nevi. Biopsy should be performed when
differential diagnoses are difficult (47,48).
Thorough skin examination, meaning clinical and dermoscopic
evaluation of melanocytic lesions, must be encouraged in patients
treated with systemic therapies such as biologic agents. Melanoma
is a skin cancer with high immunogenicity. There are concerns for
patients treated with TNFα inhibitors, since the melanoma risk is
increased when the suppression of the immune system occurs
(49,50).
There are limited data concerning the relationship
between melanocytic lesions, psoriasis and melanoma. Immunologic
pathways implicated in psoriasis induce a reduction in the number
of melanocytic nevi. Nevertheless, little is known about the
association of melanocytic nevi with psoriasis (51). Di Cesare et al demonstrated
that psoriatic patients have fewer melanocytic nevi than control
subjects without psoriasis, which suggests that the immune
pathogenic background of psoriasis may play a protective role
against the development of melanocytic lesions. It was also
observed that patients treated with biologic agents are more likely
to have more nevi than patients treated with other methods, and
that no cases of melanoma development were reported during
biological treatment (52). The use
of sun protective creams was significantly reduced in patients with
psoriasis probably because of the ‘therapeutic’ use of UV exposure
by the patients. Therefore, it is not surprising that more
psoriatic patients than control subjects displayed solar lentigines
(52). Cengiz et al
developed a study to investigate the number of melanocytic nevi in
psoriatic patients as compared with control subjects, and whether
there is any relationship between the disease severity and the type
of treatment. They detected a very low proportion of clinically
atypical nevi and no cases of melanoma in either patients or
controls. In addition, they found that psoriatic patients had
significantly fewer nevi than the control group (53) and their results are consistent with
other studies in the literature (52,54).
This suggests a protective role of the cytokines involved in
psoriatic disease and an increased secretion of IL-17, TNFα, and
IL-6 against the development of melanocytic lesions (52).
It is believed that immunosuppression may induce
melanocyte-stimulating hormone or melanoma growth-stimulatory
activity, two endogenous growth factors specific for melanocytes.
Therefore, the growth and development of melanocytes could be
stimulated under these circumstances. Genetic factors may also be
involved. The role of immuno-surveillance in tumoral genesis is
therefore essential, and can prevent the appearance of malignant
lesions and malignant transformation of benign lesions (55).
Melanocytic proliferation may be benign, like in
eruptive melanocytic nevi, or malignant, as in melanoma (56). The literature available suggests
that patients undergoing biologic treatment should be encouraged to
monitor their pre-existing nevi and to observe the appearance of
new ones (56-58).
Paradoxically, exacerbation of psoriasis has been observed with the
introduction of monoclonal antibody therapies such as ipilimumab,
nivolumab and pembrolizumab for advanced stage melanoma (59).
We discovered a case report of a young lady with
arthritis who developed halo nevi at the site of every nevus while
being treated with tocilizumab. When describing the development of
halo nevi, vitiligo and diffuse alopecia areata in this patient,
cellular and humoral immunity were considered to be causative
factors. Tocilizumab blocks IL-6R, leading to an increase in serum
IL-6 that can have direct effects on melanocytes. The presented
case describes the pathogenesis and development of halo nevi,
diffuse alopecia areata and vitiligo associated with tocilizumab
therapy. The regression of melanocytes during the treatment with
tocilizumab provides evidence for IL-6 as a potential future target
in the treatment of melanoma (60,61).
Continuous monitoring for invasive features of pigmented lesions is
a reasonable alternative to excision (62,63).
The newer biologic and non-biologic agents appear to
be promising and effective, but additional studies are needed to
evaluate the malignancy risk in these agents. We should also remind
patients of the importance of prophylaxis and the use of sunscreen
products among patients of this group.
To conclude, the risk of new or recurrent systemic
malignancies is similar between patients on biologic and
non-biologic treatments. Recent research concerning the development
of new melanocytic lesions in patients under immunosuppressive
therapy showed that the treatment with biologic agents was
associated with increased nevi count and the appearance of
dermoscopic changes in existing nevi, but none of the changes, or
any of the subsequently excised nevi, were malignant. Based on
high-level evidence, psoriasis therapies appear to be safe.
Any clinical or dermoscopic changes in existing
melanocytic nevi in patients undergoing biological treatment or
other immunosuppressive therapies should be carefully monitored as
alternative to excision. As in other dermatological conditions,
temporization and follow-up with both clinical and dermoscopic
monitoring of pigmented lesions are an alternative to surgical
excision. Additionally, reflectance confocal microscopy or optical
coherence tomography could be used. Further long-term data are
warranted for novel treatments and for their use in patients with
malignancies.
Acknowledgements
Not applicable.
Funding
The present study was supported by ‘Dunarea de Jos’ University
of Galati [Multidisciplinary Integrated Center of Dermatological
Interface Research MIC-DIR (internal grant no.
RF3668/01.10.2021)].
Availability of data and materials
Further information regarding the case study can be
obtained from the corresponding author upon reasonable request. All
information in the short review is documented by relevant
references.
Authors' contributions
ALT contributed to the conception, design, and
drafting the study. VA, CB, DSJ, SAM and MC, contributed to the
interpretation of the data, and to the revision of the manuscript
critically for important scientific content. FCB, LB and DSR made
substantial contributions to the conception and design of the work
and were responsible for the clinical management of the patient,
while TN, LCN and EN supervised and substantially revised this
work. All authors agreed on the final manuscript and contributed
equally in all the stages of the study. They reached an agreement
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics approval was obtained from the Ethics
Commission of the ‘St. Parascheva’ Clinical Hospital of Infectious
Diseases, Galati (approval no. 24/26.02.2021) and written informed
consent was obtained from the patient prior to publication.
Patient consent for publication
Written informed consent was obtained from the
patient for the use of all data and graphical images for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Griffiths CE and Barker JN: Pathogenesis
and clinical features of psoriasis. Lancet. 370:263–271.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gudjonsson JE and Elder JT: Psoriasis:
Epidemiology. Clin Dermatol. 25:535–546. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ardeleanu V, Sabina Radaschin D and Tatu
AL: Excimer laser for psoriasis treatment: A case report and short
review. Exp Ther Med. 20:52–55. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kruger G, Koo J, Lebwohl M, Menter A,
Stern RS and Rolstad T: The impact of psoriasis on quality of life:
Results of a 1998 national psoriasis foundation patient-membership
survey. Arch Dermatol. 137:280–284. 2001.PubMed/NCBI
|
|
5
|
Menter A, Gottlieb A, Feldman SR, Van
Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA,
Korman NJ, et al: Guidelines of care for the management of
psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis
and guidelines of care for the treatment of psoriasis with
biologics. J Am Acad Dermatol. 58:826–850. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nwabudike LC and Tatu AL: Using
complementary and alternative medicine for the treatment of
psoriasis: A step in the right direction. JAMA Dermatol.
155(636)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Basavaraj KH, Ashok NM, Rashmi R and
Praveen TK: The role of drugs in the induction and/or exacerbation
of psoriasis. Int J Dermatol. 49:1351–1361. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tatu AL, Elisei AM, Chioncel V, Miulescu M
and Nwabudike LC: Immunologic adverse reactions of β-blockers and
the skin. Exp Ther Med. 18:955–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li SJ, Perez-Chada LM and Merola JF: TNF
inhibitor-induced psoriasis: Proposed algorithm for treatment and
management. J Psoriasis Psoriatic Arthritis. 4:70–80.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Naldi L: Malignancy concerns with
psoriasis treatments using phototherapy, methotrexate, cyclosporin,
and biologics: Facts and controversies. Clin Dermatol. 28:88–92.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen YJ, Chang YT, Wang CB and Wu CY: The
risk of cancer in patients with rheumatoid arthritis: A nationwide
cohort study in Taiwan. Arthritis Rheum. 63:352–358.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goldacre MJ, Wotton CJ, Yeates D,
Seagroatt V and Jewell D: Cancer in patients with ulcerative
colitis, Crohn's disease and coeliac disease: Record linkage study.
Eur J Gastroenterol Hepatol. 20:297–304. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen YJ, Wu CY, Chen TJ, Shen JL, Chu SY,
Wang CB and Chang YT: The risk of cancer in patients with
psoriasis: A population-based cohort study in Taiwan. J Am Acad
Dermatol. 65:84–91. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McKenna KE, Patterson CC, Handley J,
McGinn S and Allen G: Cutaneous neoplasia following PUVA therapy
for psoriasis. Br J Dermatol. 134:639–642. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Murdaca G, Colombo BM, Cagnati P, Gulli R,
Spanò F and Puppo F: Update upon efficacy and safety of TNF-α
inhibitors. Expert Opin Drug Saf. 11:1–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel RV, Clark LN, Lebwohl M and Weinberg
JM: Treatments for psoriasis and the risk of malignancy. J Am Acad
Dermatol. 60:1001–1017. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scott FI, Mamtani R, Brensinger CM, Haynes
K, Chiesa-Fuxench ZC, Zhang J, Chen L, Xie F, Yun H, Osterman MT,
et al: Risk of nonmelanoma skin cancer associated with the use of
immunosuppressant and biologic agents in patients with a history of
autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol.
152:164–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nwabudike LC and Tatu AL: Response
to-chronic exposure to tetracyclines and subsequent diagnosis for
non-melanoma skin cancer in a large Mid-Western US population. J
Eur Acad Dermatol Venereol. 32(e159)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mercer LK, Askling J, Raaschou P, Dixon
WG, Dreyer L, Hetland ML, Strangfeld A, Zink A, Mariette X, Finckh
A, et al: Risk of invasive melanoma in patients with rheumatoid
arthritis treated with biologics: Results from a collaborative
project of 11 European biologic registers. Ann Rheum Dis.
76:386–391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sbidian E, Chaimani A, Garcia-Doval I, Do
G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, et
al: Systemic pharmacological treatments for chronic plaque
psoriasis: A network meta-analysis. Cochrane Database Syst Rev.
12(CD011535)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Smith CH, Anstey AV, Barker JN, Burden AD,
Chalmers RJ, Chandler D, Finlay AY, Griffiths CE, Jackson K, McHugh
NJ, et al: British association of dermatologists guidelines for use
of biological interventions in psoriasis 2005. Br J Dermatol.
153:486–497. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wong T, Hsu L and Liao W: Phototherapy in
psoriasis: A review of mechanisms of action. J Cutan Med Surg.
17:6–12. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gross RL, Schwartzman-Morris JS, Krathen
M, Reed G, Chang H, Saunders KC, Fisher MC, Greenberg JD, Putterman
C, Mease PJ, et al: A comparison of malignancy incidence among
psoriatic and rheumatoid arthritis patients in a large US cohort.
Arthritis Rheumatol. 66:1472–1481. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Polachek A, Muntyanu A, Lee KA, Ye JY,
Chandran V, Cook RJ and Gladman DD: Malignancy in psoriatic
disease: Results from prospective longitudinal cohorts. Semin
Arthritis Rheum. 51:144–149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A
and Gelfand JM: The risk of cancer in patients with psoriasis: A
population-based cohort study in the health improvement network.
JAMA Dermatol. 152:282–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nwabudike LC and Tatu AL: Reply to
Gambichler T et al. Altered epigenetic pathways and cell
cycle dysregulation in healthy appearing skin of patients with
koebnerized squamous cell carcinomas following skin surgery. J Eur
Acad Dermatol Venereol. 33:e3–e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rebegea L, Firescu D, Baciu G and Ciubara
A: Psycho-oncology support. BRAIN Broad Res Artif Intell Neurosci.
10:77–88. 2019.
|
|
29
|
Beyaert R, Beaugerie L, Van Assche G,
Brochez L, Renauld JC, Viguier M, Cocquyt V, Jerusalem G, Machiels
JP, Prenen H, et al: Cancer risk in immune mediated inflammatory
diseases (IMID). Mol Cancer. 12(98)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pérez Ramírez S, Parra V, Avilés Izquierdo
JA, Vicario JL, Martín M and Márquez-Rodas I: Metastatic melanoma
with spontaneous regression, psoriasis and HLA-Cw6: Case report and
a hypothesis to explore. Tumori. 100:144e–147e. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Niculet E, Bobeica C and Tatu AL:
Glucocorticoid-induced skin atrophy: The old and the new. Clin
Cosmet Investig Dermatol. 13:1041–1050. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sezer E, Özturk Durmaz E, Çetin E and
Şahin S: Meyerson phenomenon as a component of melanoma in situ.
Acta Dermatovenerol Croat. 24:81–82. 2016.PubMed/NCBI
|
|
33
|
Kluger N, Estève E, Fouéré S,
Dupuis-Fourdan F, Jegou MH and Lévy-Rameau C: Tattooing and
psoriasis: A case series and review of the literature. Int J
Dermatol. 56:822–827. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chapman BP and Moynihan J: The brain-skin
connection: Role of psychosocial factors and neuropeptides in
psoriasis. Expert Rev Clin Immunol. 5:623–627. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kamata M and Tada Y: Safety of biologics
in psoriasis. J Dermatol. 45:279–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cohen BL and Sachar DB: Update on
anti-tumor necrosis factor agents and other new drugs for
inflammatory bowel disease. BMJ. 357(j2505)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Neagu M, Constantin C, Tanase C and Boda
D: Patented biomarker panels in early detection of cancer. Recent
Pat Biomark. 1:10–14. 2011.
|
|
39
|
Geller S, Xu H, Lebwohl M, Nardone B,
Lacouture ME and Kheterpal M: Malignancy risk and recurrence with
psoriasis and its treatments: A concise update. Am J Clin Dermatol.
19:363–375. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Papp K, Gottlieb AB, Naldi L, Pariser D,
Ho V, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W,
et al: Safety surveillance for ustekinumab and other psoriasis
treatments from the psoriasis longitudinal assessment and registry
(PSOLAR). J Drugs Dermatol. 14:706–714. 2015.PubMed/NCBI
|
|
41
|
Karram S, Novy M, Saroufim M, Loya A,
Taraif S, Houreih MA, Rauscher B, Habib RH, Oberkanins C and
Khalifeh I: Predictors of BRAF mutation in melanocytic nevi:
Analysis across regions with different UV radiation exposure. Am J
Dermatopathol. 35:412–418. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ferringer T: Update on
immunohistochemistry in melanocytic lesions. Dermatol Clin.
30:567–579, v. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Clark WH Jr, Elder DE, Guerry D IV,
Epstein MN, Greene MH and Van Horn M: A study of tumor progression:
The precursor lesions of superficial spreading and nodular
melanoma. Hum Pathol. 15:1147–1165. 1984.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Silva JH, Sá BC, Avila AL, Landman G and
Duprat Neto JP: Atypical mole syndrome and dysplastic nevi:
Identification of populations at risk for developing
melanoma-review article. Clinics (Sao Paulo). 66:493–499.
2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Marinescu SA, Tatu AL, Mihai IR and
Giuglea C: Correlations between clinics, dermoscopy and
histopathology in a female with two dermatofibromas-a case report.
Rom J Morphol Embryol. 57:323–326. 2016.PubMed/NCBI
|
|
46
|
Kittler H: Use of digital dermoscopy to
monitor melanocytic lesions: Risks and benefits. J Drugs Dermatol.
2:309–311. 2003.PubMed/NCBI
|
|
47
|
Tatu AL: Umbilicated blue-black lesion on
the lateral thorax. J Cutan Med Surg. 21(252)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Marghoob AA and Scope A: The complexity of
diagnosing melanoma. J Invest Dermatol. 129:11–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Passarelli A, Mannavola F, Stucci LS,
Tucci M and Silvestris F: Immune system and melanoma biology: A
balance between immunosurveillance and immune escape. Oncotarget.
8:106132–106142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bastian BC: The molecular pathology of
melanoma: An integrated taxonomy of melanocytic neoplasia. Annu Rev
Pathol. 9:239–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Stefan O, Tudor G, Constantinescu C, Luca
C, Boda D, Caruntu C, Cioplea M, Nichita L and Zurac SA: E-cadherin
and N-cadherin expression pattern in common melanocytic nevi.
Virchows Arch. 475(S28)2019.
|
|
52
|
Di Cesare A, Riitano A, Suppa M, Fidanza
R, Zangrilli A, Esposito M, Fargnoli MC, Chimenti S and Peris K:
Frequency of melanocytic nevi in psoriatic patients is related to
treatment and not to disease severity. J Am Acad Dermatol.
69:947–953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cengiz FP, Emiroglu N, Bahali AG, Ozkaya
DB, Su O and Onsun N: The relationship of psoriasis and melanocytic
nevi. Indian J Dermatol. 61:664–667. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Balato N, Di Costanzo L, Balato A, Patruno
C, Scalvenzi M and Ayala F: Psoriasis and melanocytic naevi: Does
the first confer a protective role against melanocyte progression
to naevi? Br J Dermatol. 164:1262–1270. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Katoulis AC, Kanelleas A, Zambacos G,
Panayiotides I and Stavrianeas NG: Development of two primary
malignant melanomas after treatment with adalimumab: A case report
and review of the possible link between biological therapy with
TNF-alpha antagonists and melanocytic proliferation. Dermatology.
221:9–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Queirós CS, Laureano-Oliveira A,
Lopéz-Presa D and Filipe P: Spitz nevus and infliximab: Association
or coincidence? An Bras Dermatol. 95:615–618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Manganoni AM, Zane C, Pavoni L, Farisoglio
C, Sereni E and Calzavara-Pinton P: Cutaneous melanoma in patients
in treatment with biological therapy: Review of the literature and
case report. Dermatol Online J. 17(12)2011.PubMed/NCBI
|
|
58
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Johnson DB, Sullivan RJ, Ott PA, Carlino
MS, Khushalani NI, Ye F, Guminski A, Puzanov I, Lawrence DP,
Buchbinder EI, et al: Ipilimumab therapy in patients with advanced
melanoma and preexisting autoimmune disorders. JAMA Oncol.
2:234–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nadesalingam K, Goodfield M and Emery P:
Halo naevi, vitiligo and diffuse alopecia areata associated with
tocilizumab therapy. Oxf Med Case Reports.
2016(omw027)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Niculet E, Chioncel V, Elisei AM, Miulescu
M, Buzia OD, Nwabudike LC, Craescu M, Draganescu M, Bujoreanu F,
Marinescu E, Marinescu E, et al: Multifactorial expression of IL-6
with update on COVID-19 and the therapeutic strategies of its
blockade (review). Exp Ther Med. 21(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Koseoglu G, Akay BN, Kucuksahin O and
Erdem C: Dermoscopic changes in melanocytic nevi in patients
receiving immunosuppressive and biologic treatments: Results of a
prospective case-control study. J Am Acad Dermatol. 73:623–629.
2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Giurcaneanu C, Nitipir C, Popa LG, Forsea
AM, Popescu I and Bumbacea RS: Evolution of melanocytic nevi under
vemurafenib, followed by combination therapy with dabrafenib and
trametinib for metastatic melanoma. Acta Dermatovenerol Croat.
23:114–121. 2015.PubMed/NCBI
|