(D. Don) Benn is a perennial herbaceous plant of the
Simaroubaceae DC family that grows in Korea, China, Japan and
Nepal. P. quassioides is a widely used Asian traditional
medicine and is officially recorded in the Korea and Chinese
Pharmacopoeia (ed. 2020) (1). The
dried branches and leaves of P. quassioides may be ingested
or used as an externally applied medicine. According to concepts of
Korean/Asian medicine, the P. quassioides flavor is bitter
and cold with little poison, and the meridian tropism involves the
lung and large intestine. The functions and indications for use
include ‘removing heat or dampness’ (concepts of Korean/Asian
medicine) and detoxification. Thus, this Asian traditional medicine
may be used for wind-heat cold (treatments aimed at expelling out
heat and cooling the body), sore throat, diarrhea and eczema
(2). P. quassioides is also
used to treat rabies and snake bite (3).
Although most β-carboline alkaloids are extracted
from natural plants, a small number of them have been synthesized
chemically. β-carboline alkaloids have a planar tricyclic ring
system consisting of indolopyridine carboline rings. These
β-carboline alkaloids are the most representative alkaloids in
P. quassioides. This alkaloid type has various chemical
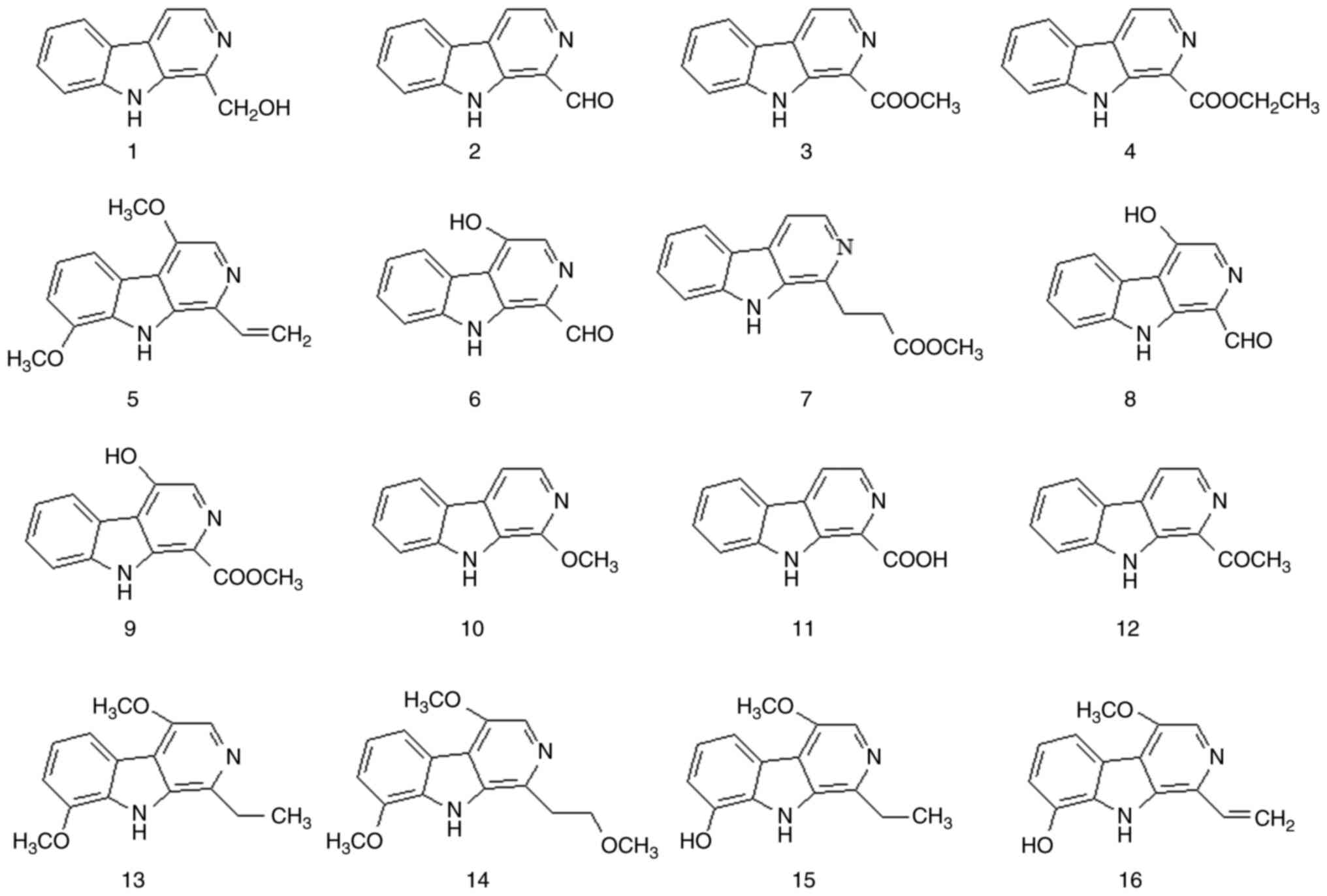
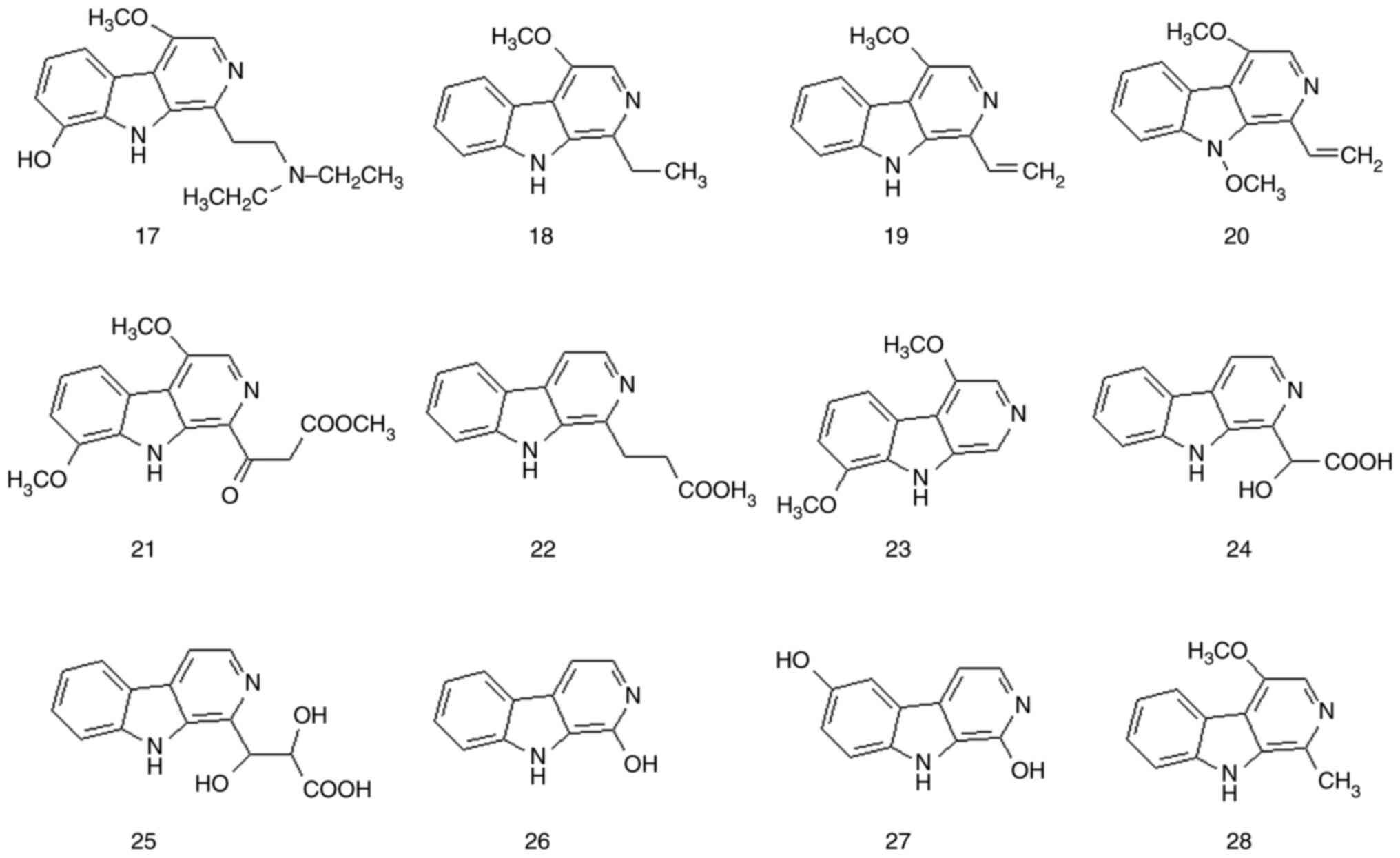
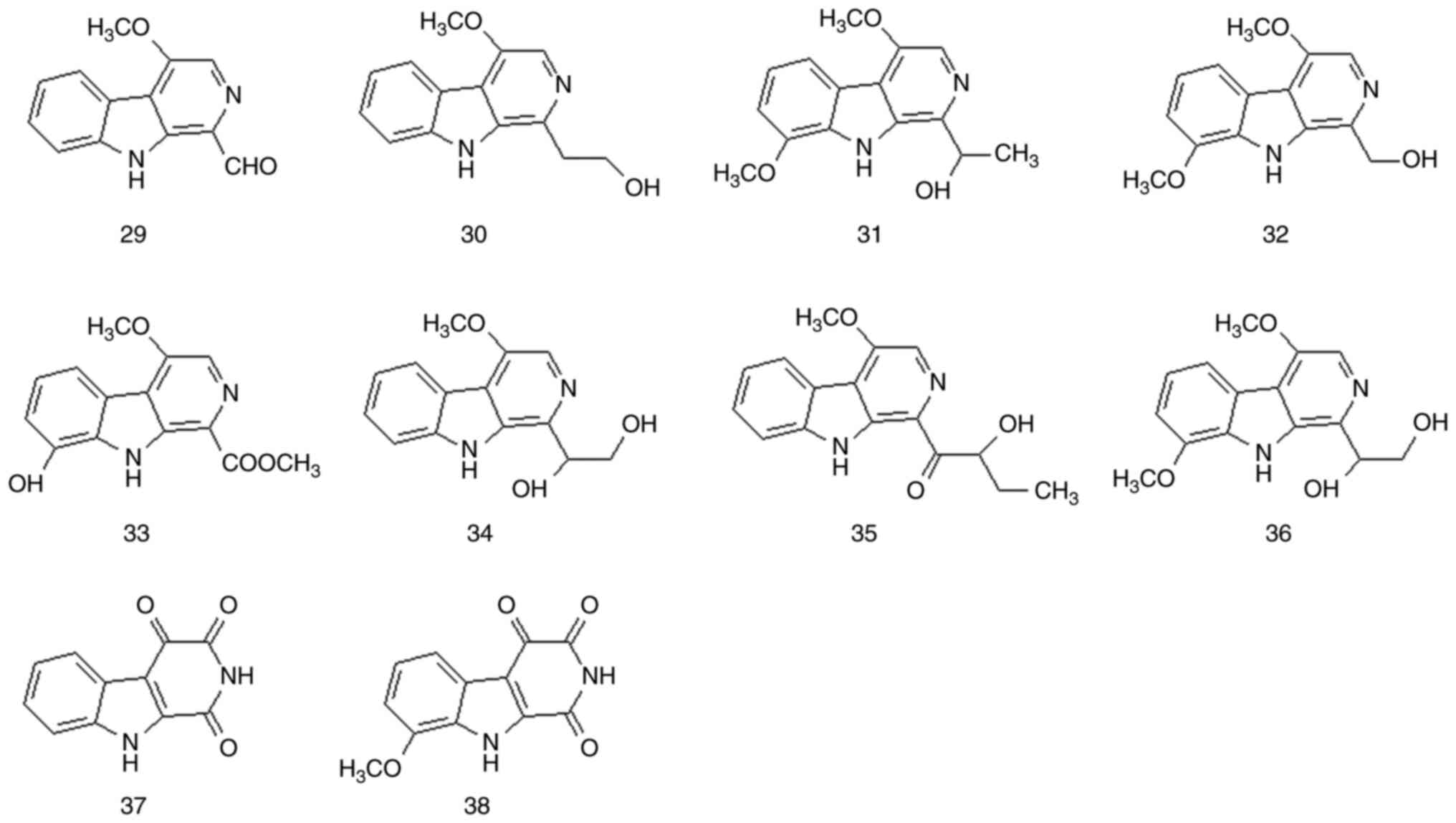
structures and a wide range of biological activities. A total of 38
β-carboline alkaloids have been isolated from P. quassioides
(compounds 1-38). The structures and names of these compounds are
presented in Fig. 1, Fig. 2 and Fig. 3 and Table I and Fig. S1.
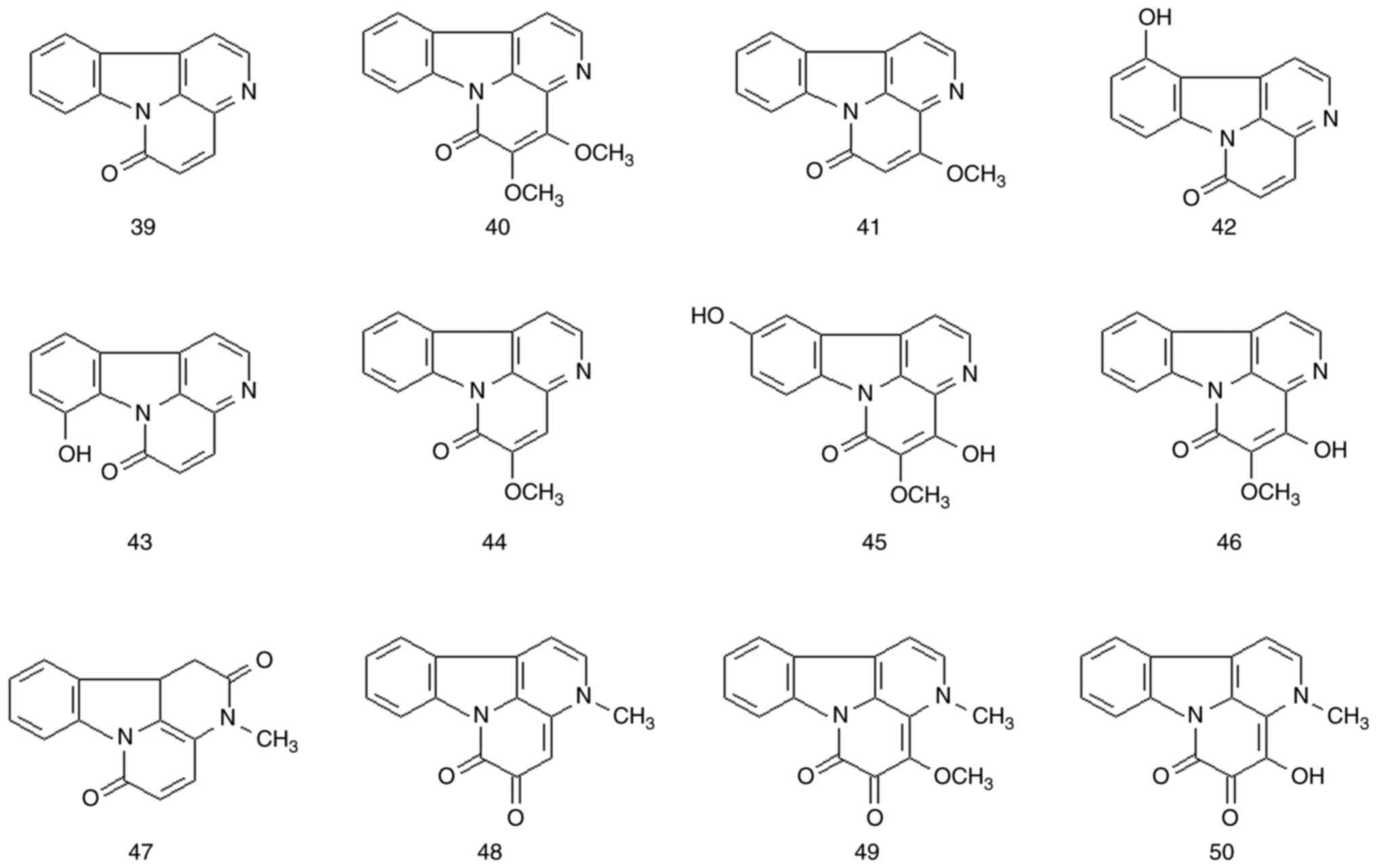
Similar to β-carboline alkaloids, carthinone
alkaloids are polycyclic compounds containing a carboline ring. The
entire molecule is a highly conjugated system. All carthinone types
share a common canthin-6-one backbone, i.e., the basic structure is
based on a canthin-6-one backbone. A total of 12 carthinone
alkaloids were isolated from P. quassioides (compounds
39-50). The structures and names of these compounds are presented
in Fig. 4 and Table II and Fig. S1.
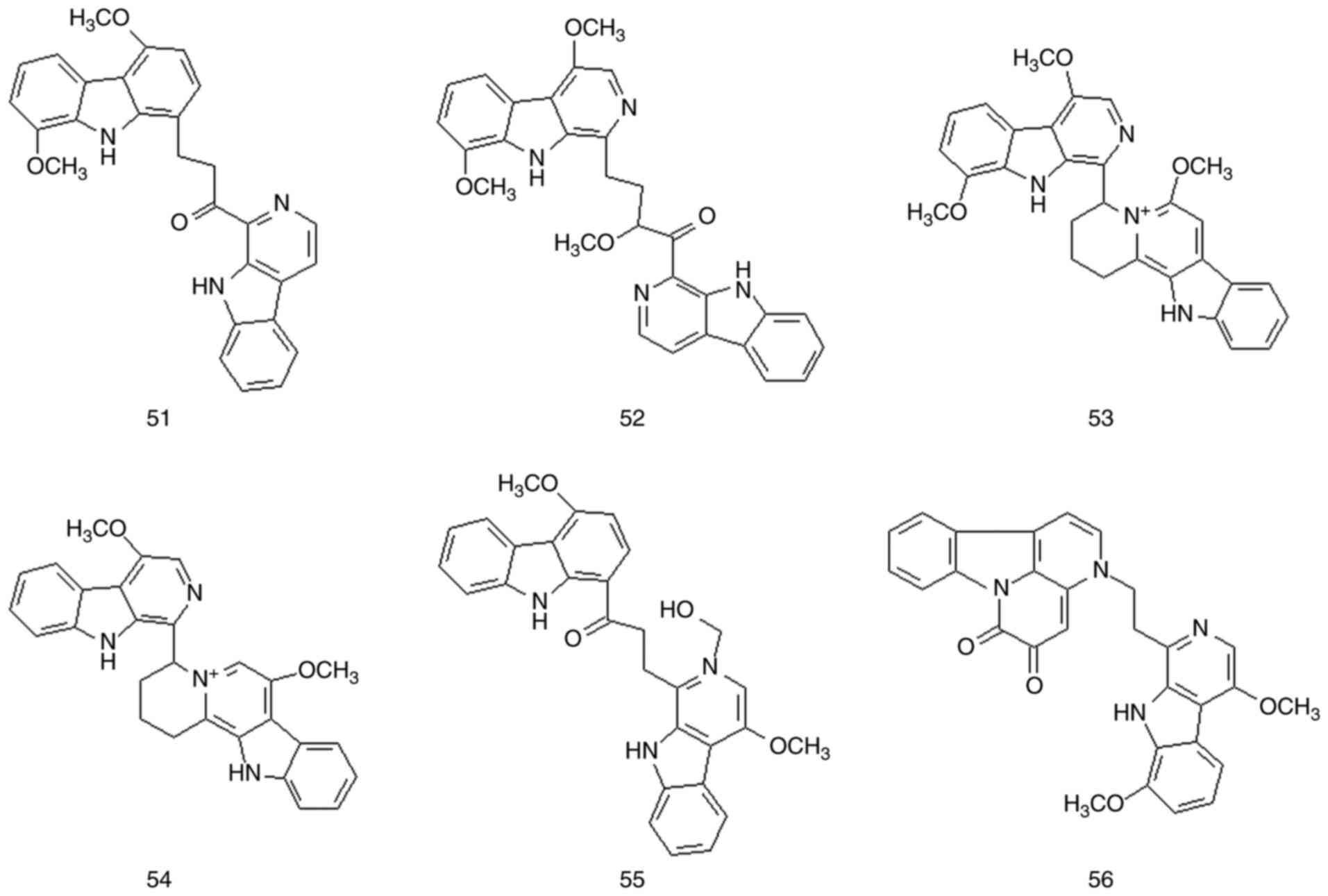
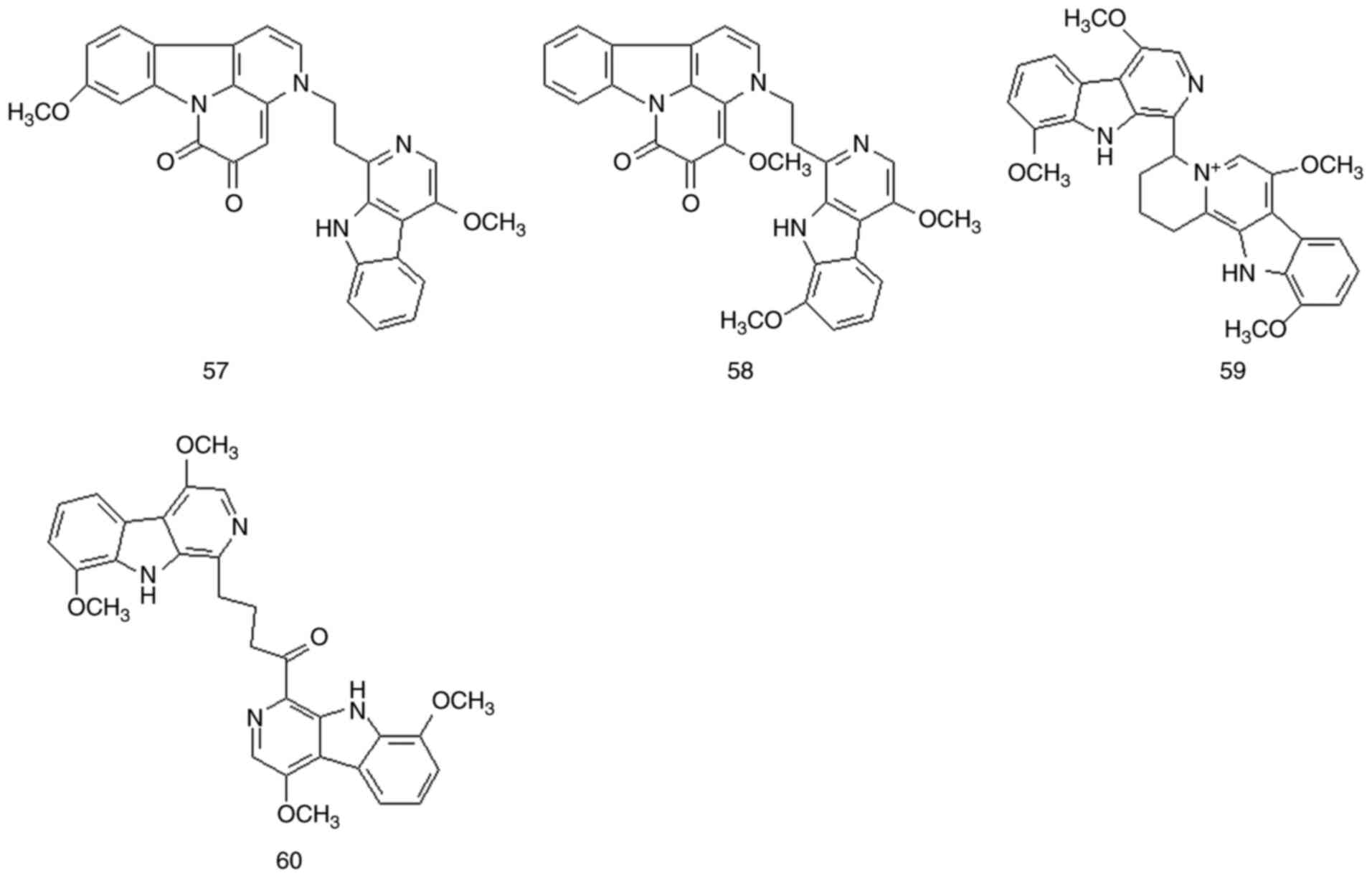
Bis-β-carboline alkaloids are bimolecular compounds
formed by two indole alkaloids joined by chemical bonds. These
compounds are important alkaloid components in Picrasma BL species
and may have biological activities similar to those of β-carboline
alkaloids. A total of 10 bis-β-carboline alkaloids have been
isolated from P. quassioides (compounds 51-60). The
structures and names of these compounds are presented in Figs. 5 and 6, Table
III and Fig. S1.
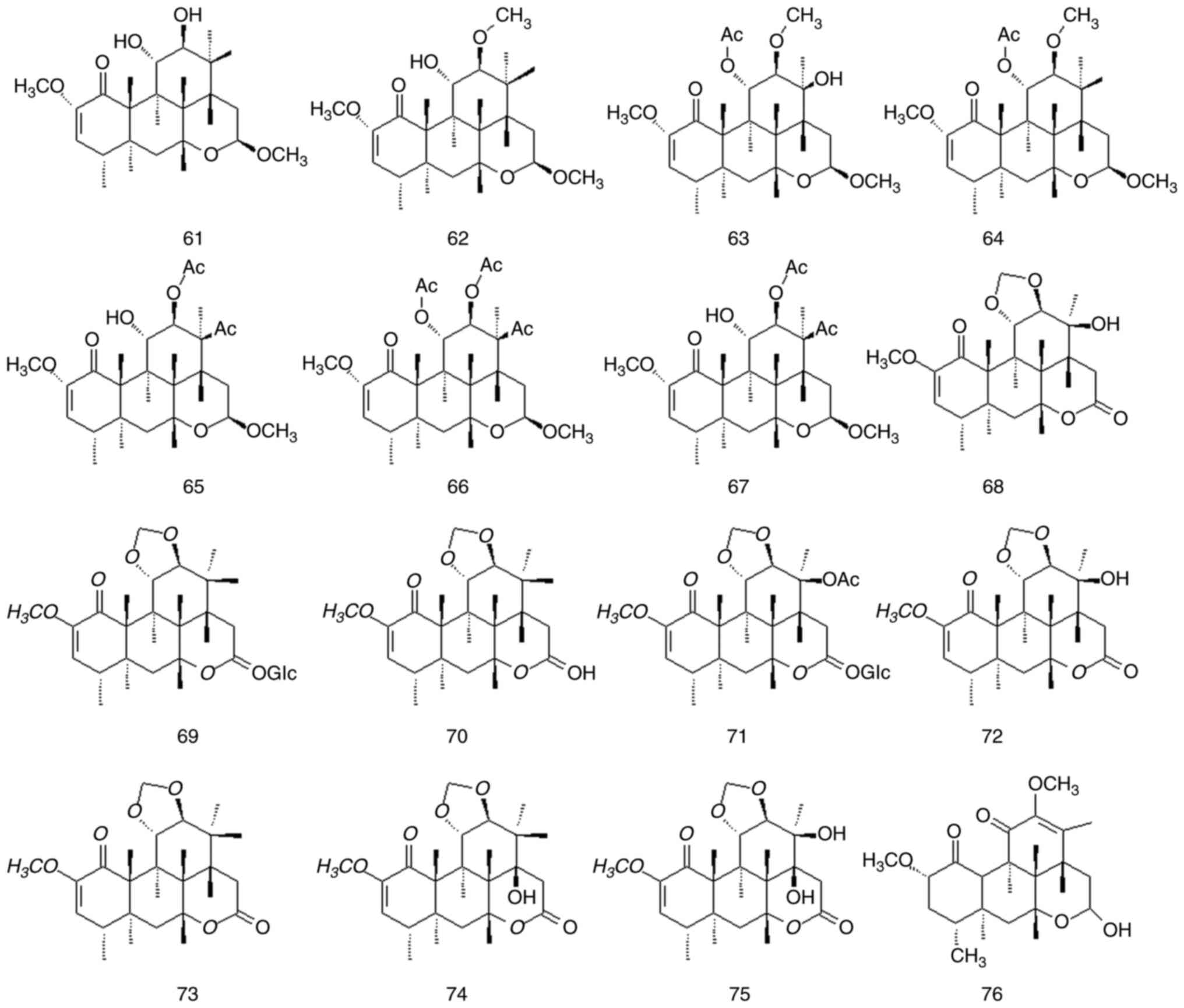
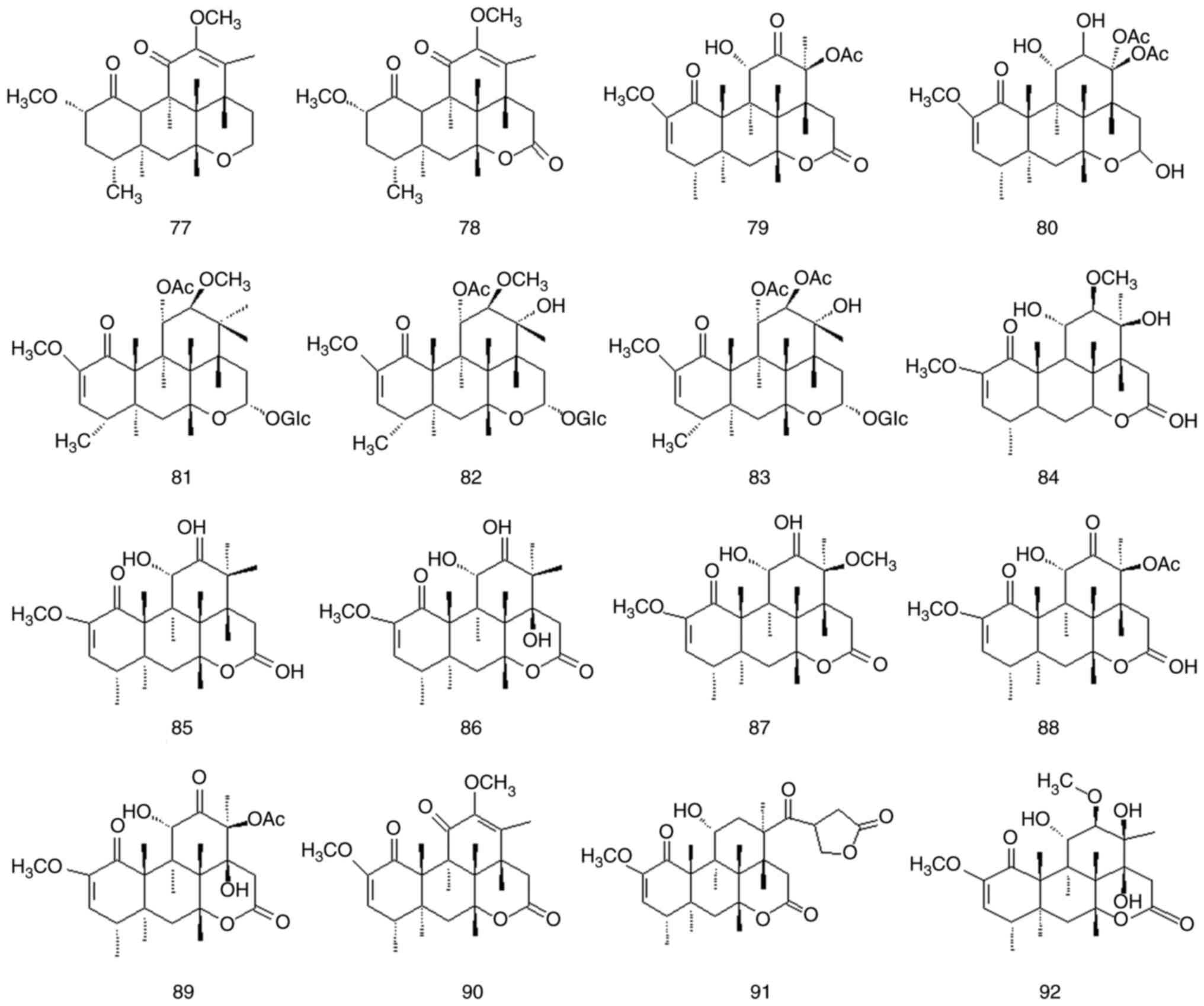
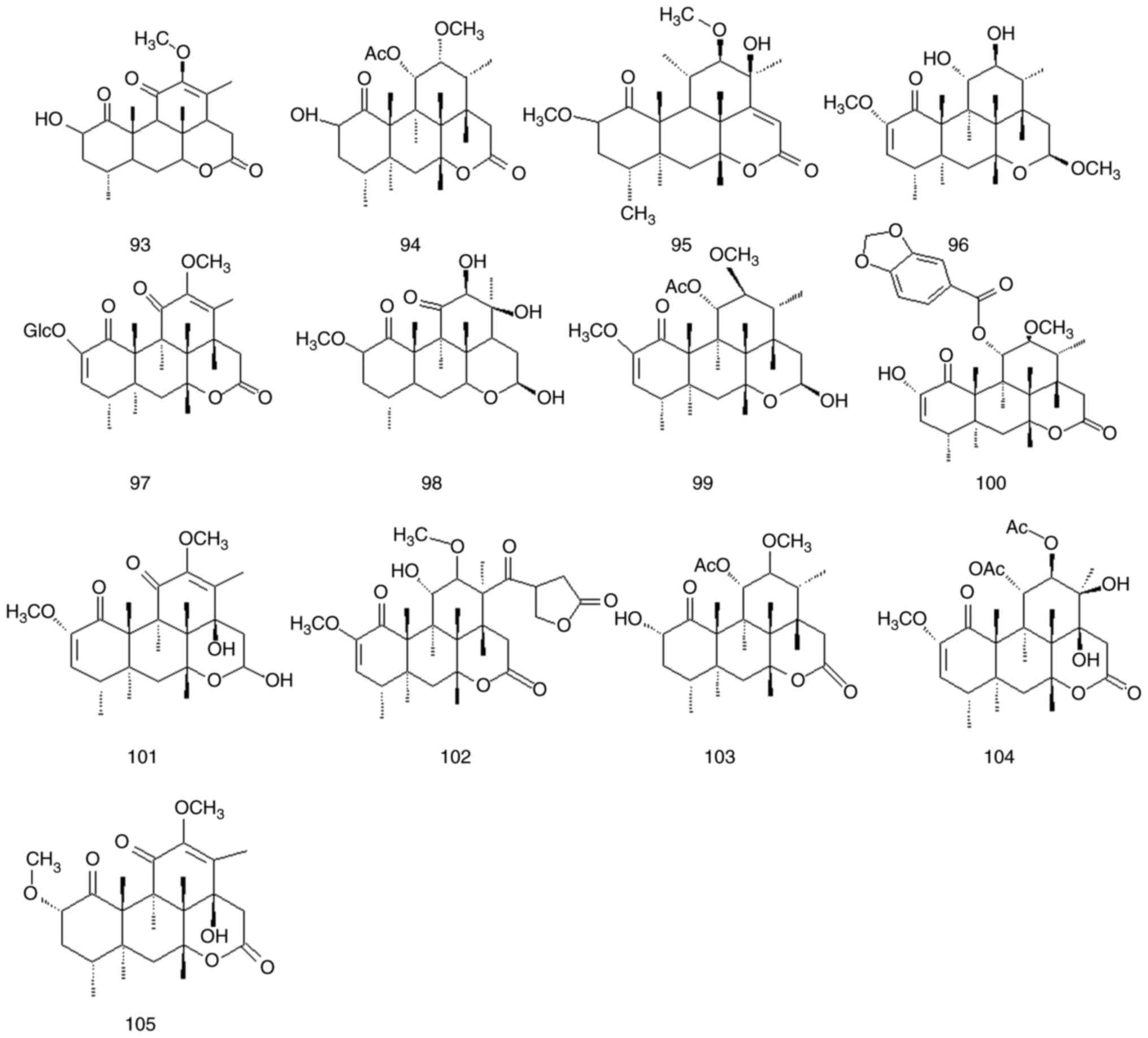
Quassinoids are characteristic components of
Simaroubaceae DC species, and its parent nuclear structure is
mainly composed of nigakihemiacetal and nigakilactone. A total of
45 quassinoids have been isolated from P. quassioides
(compounds 61-105); their names structures are presented in
Fig. 7, Fig. 8 and Fig. 9 and Table IV and Fig. S1.
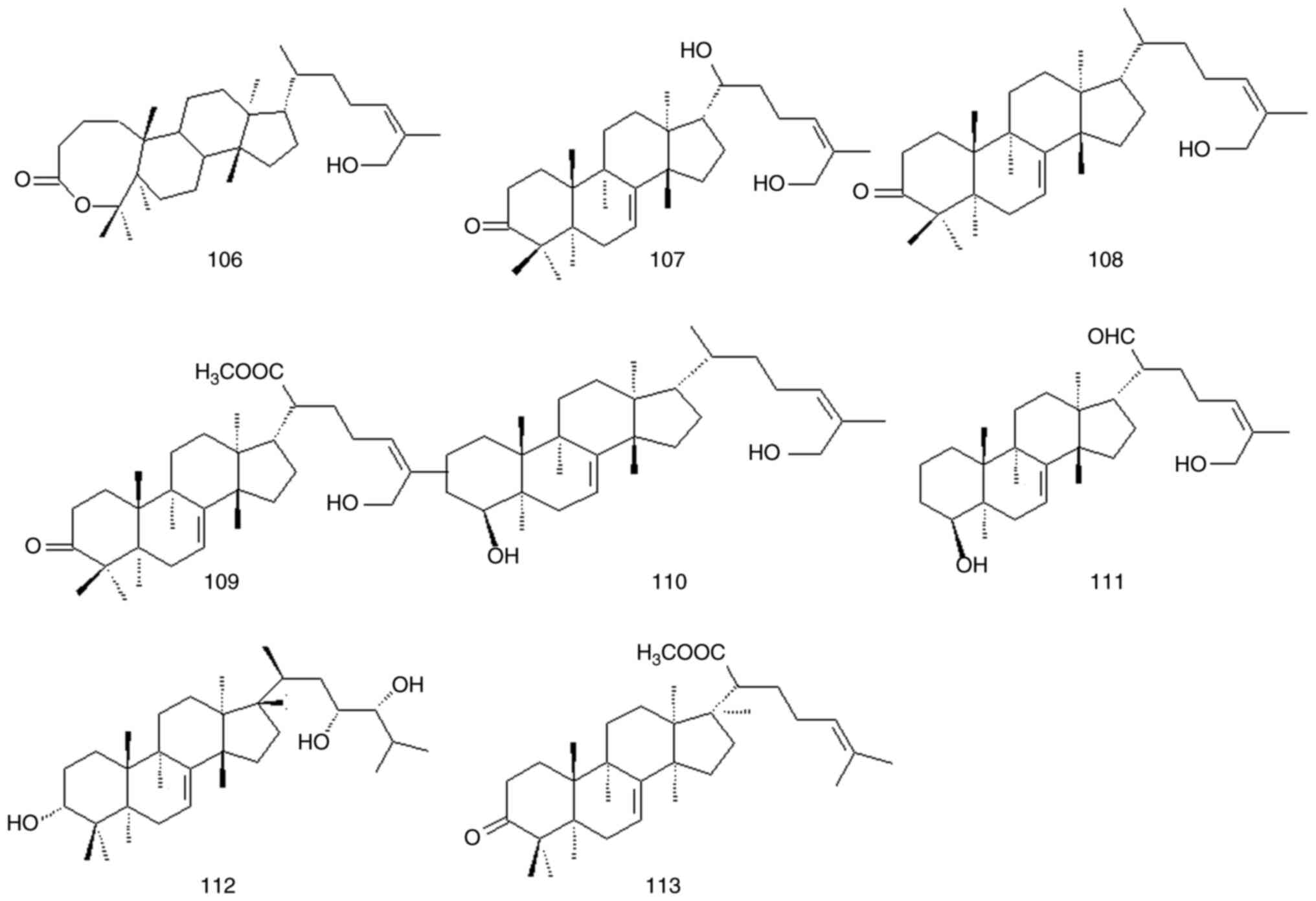
Although triterpenoids account for a small
proportion of the active compounds in P. quassioides, these
molecules have anti-inflammatory and anticancer effects. A total of
eight triterpenoids have been isolated from P. quassioides
(compounds 106-113). The structures of these compounds are
presented in Fig. 10 and Table V and Fig. S1.
NO is an important inflammatory factor. Therefore,
inhibiting NO production is an effective method for treating and
preventing inflammation-related diseases. TNF-α is a polypeptide
cytokine that regulates other inflammatory factors and proteases,
thereby regulating inflammation (23). IL-6 is another circulating cytokine
that regulates immune cell activation, T and B cell proliferation
and differentiation, as well as inflammatory responses (24). Of note, ILs are divided into
proinflammatory (IL-1, -6 and -8) and anti-inflammatory (IL-4 and
-10) factors. COX, also called prostaglandin G/H synthase, has two
isoforms, COX-1 and COX-2, which have key roles in inflammation and
are targeted by nonsteroidal anti-inflammatory drugs (25).
Cancer is caused by the continuous proliferation and
abnormal differentiation of cells. Worldwide, cancer is the second
major cause of death in humans. Of note, cancer is a complex,
multi-factorial disease, which makes treatment difficult and poses
several challenges for survival (45,46).
In recent years, cancer awareness has markedly improved, and
treatments have also been developed. In spite of efforts regarding
the early detection and timely treatment of cancer,
cancer-associated mortality is at an all-time high (47). The currently available clinical
treatments for cancer mainly include surgical treatment,
radiotherapy and chemotherapy (48). Early cancer detection generally
leads to surgical treatment, whereas chemotherapy is mainly used
for advanced cancer. Commonly used chemotherapy drugs include
5-fluorouracil, cisplatin, paclitaxel and doxorubicin (49). However, the toxic side effects of
chemotherapy drugs affect patient health (50). Natural Chinese herbal medicine has
become a hot topic in anticancer research in recent years due to
low toxicity and reduced side effects of various herbal
formulations (51).
Several studies indicated that the crude extracts or
compounds derived from Chinese herbal medicines effectively
inhibited the proliferation of liver, gastric, lung, breast and
colon cancer cells and induced cancer cell apoptosis (50-54).
The pathways that induce cell death include the intrinsic pathway,
extrinsic pathway and the endoplasmic reticulum (ER) stress pathway
(47). The intrinsic pathway is
also called the mitochondrial pathway. During apoptosis, various
mitochondrial components integrate cell death signals and mediate
the progression of apoptosis (55). The extrinsic pathway is activated
by cell surface death receptors, such as Fas and TNF receptor
(56). ER stress causes caspase-12
activation and induces apoptosis (57). ER stress may also promote DNA
damage and autophagy-induced cell death (58). It is possible that chemotherapy
drugs directly act on genes or proteins to stimulate the activation
of downstream signaling pathways, involving B-cell lymphoma-2,
MAPK, phosphatidylinositol 3-kinase/protein kinase B and
recombinant glycogen synthase kinase 3 beta, to induce cell death
(59-62).
Neurological diseases cause physical damage to the
nervous system, impairing human health and well-being. Such
diseases include Alzheimer's disease (75). Through the isolation and extraction
of plants and the ELISA test, it was determined that the drug
resistance of certain bitter wood extracts was related to the
deposition of neuroinhibitors and reduction of Aβ142. Further
exploration of the structure-activity relationship of alkaloids and
molecular docking experiments suggested that certain active
components of P. quassioides (D. Don) Benn extracts may have
efficacy for treating neurodegenerative diseases. In addition, the
β-carboline alkaloids of sorrel wood extracts as benzodiazepine
antagonists are able to effectively control social anxiety,
convulsions and other types of behavior in mouse models (76,77).
As major diseases in humans, neurological diseases are accompanied
by changes in the corresponding enzymes monoamine oxidase (MAO)-A
and MAO-B (78,79); furthermore, the levels of ROS
increased in cells and damage to mitochondria occurred (80-82).
Chemical studies on plants of the Picrasma BL family
indicated that alkaloids and quassinoids may be the major
components contained in P. quassioides. Most of these
alkaloids have parent β-carboline and canthin rings. Quassin may be
a tetracyclic diterpene lactone component. In general, P.
quassioides extracts and isolated compounds have good
anti-inflammatory, antibacterial, antitumor and neuroprotective
activities, while also having beneficial effects on the digestive
system, heat removal and detoxification.
Not applicable.
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Education (grant no. 2020R1I1A2052417) and The
Korean Research Institute of Bioscience and Biotechnology Research
Information System (grant no. RBM0112112).
Not applicable.
JL, YXG, HNS and TK conceptualized the study,
performed the literature search, collected and analyzed data and
wrote the manuscript. HJ, HS and DPX performed the literature
search and analyzed data. HNS and TK performed the literature
review and revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
National Pharmacopoeia Committee.
Pharmacopoeia of the people's Republic of China, volume I (2010
edition), pp186, 2010.
|
|
2
|
Jiao WH, Gao H, Zhao F, Lin HW, Pan YM,
Zhou GX and Yao XS: Anti-inflammatory alkaloids from the stems of
Picrasma quassioides BENNET. Chem Pharm Bull (Tokyo).
59:359–364. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liang WF: Anti-snake bite action of
Picrasma quassioides. Zhong Yao Tong Bao. 12(54)1987.PubMed/NCBI(In Chinese).
|
|
4
|
Mohd Jamil MDH, Taher M, Susanti D, Rahman
MA and Zakaria ZA: Phytochemistry, traditional use and
pharmacological activity of Picrasma quassioides: A critical
reviews. Nutrients. 12(2584)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jung YS, Eun CS, Jung YT, Kim HJ and Yu
MH: Anti-inflammatory effects of Picrasma quassioides (D.
DON) BENN leaves extracts. J Life Sci. 23:629–636. 2013.
|
|
6
|
Liu C, Cheng RR, Yang L, Song ZC and Wang
ZT: Inhibition of CYP450 enzymes by quassinoids from Picrasma
quassioides leaves. Phytochem Lett. 30:138–142. 2019.
|
|
7
|
Jiao WH, Gao H, Li CY, Zhou GX, Kitanaka
S, Ohmura A and Yao XS: Beta-carboline alkaloids from the stems of
Picrasma quassioides. Magn Reson Chem. 48:490–495.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sung Y, Koike K, Nikaido T, Ohmoto T and
Sankawa U: Inhibitors of cyclic AMP phosphodiesterase in
Picrasma quassioides Bennet, and inhibitory activities of
related beta-carboline alkaloids. Chem Pharm Bull (Tokyo).
32:1872–1877. 1984.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao WY, Song XY, Zhao L, Zou CX, Zhou WY,
Lin B, Yao GD, Huang XX and Song SJ: Quassinoids from Picrasma
quassioides and their neuroprotective effects. J Nat Prod.
82:714–723. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu J, Xiao D, Lin QH, He JF, Liu WY, Xie
N, Feng F and Qu W: Cytotoxic tirucallane and apotirucallane
triterpenoids from the stems of Picrasma quassioides. J Nat
Prod. 79:1899–1910. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aggarwal NR, King LS and D'Alessio FR:
Diverse macrophage populations mediate acute lung inflammation and
resolution. Am J Physiol Lung Cell Mol Physiol. 306:L709–L725.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fahy JV: Type 2 inflammation in
asthma-present in most, absent in many. Nat Rev Immunol. 15:57–65.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lontchi-Yimagou E, Sobngwi E, Matsha TE
and Kengne AP: Diabetes mellitus and inflammation. Curr Diab Rep.
13:435–444. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Agita A and Alsagaff MT: Inflammation,
immunity, and hypertension. Acta Med Indones. 49:158–165.
2017.PubMed/NCBI
|
|
15
|
Mason A, Holmes C and Edwards CJ:
Inflammation and dementia: Using rheumatoid arthritis as a model to
develop treatments? Autoimmun Rev. 17:919–925. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Taleb S: Inflammation in atherosclerosis.
Arch Cardiovasc Dis. 109:708–715. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fonceca AM, Zosky GR, Bozanich EM, Sutanto
EN, Kicic A, McNamara PS, Knight DA, Sly PD, Turner DJ and Stick
SM: Accumulation mode particles and LPS exposure induce TLR-4
dependent and independent inflammatory responses in the lung.
Respir Res. 19(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olmos-Ortiz A, Déciga-García M,
Preciado-Martínez E, Bermejo-Martínez L, Flores-Espinosa P,
Mancilla-Herrera I, Irles C, Helguera-Repetto AC, Quesada-Reyna B,
Goffin V, et al: Prolactin decreases LPS-induced inflammatory
cytokines by inhibiting TLR-4/NFκB signaling in the human placenta.
Mol Hum Reprod. 25:660–667. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu
XF, Zhou QG, Chen YY, Guo AZ and Hu CM: Indirubin inhibits
LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB
and MAPK signaling pathways. Inflammation. 40:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu Q, Zeng K, Ma X, Song F, Jiang Y, Tu P
and Wang X: Resokaempferol-mediated anti-inflammatory effects on
activated macrophages via the inhibition of JAK2/STAT3, NF-κB and
JNK/p38 MAPK signaling pathways. Int Immunopharmacol. 38:104–114.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kwon MY, Park J, Kim SM, Lee J, Cho H,
Park JH and Han IO: An alpha-lipoic acid-decursinol hybrid compound
attenuates lipopolysaccharide-mediated inflammation in BV2 and
RAW264.7 cells. BMB Rep. 52:508–513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang M, Wang X, Yuan Y, Zhou Q, Tong C
and Jiang W: Different effect of glutamine on macrophage tumor
necrosis factor-alpha release and heat shock protein 72 expression
in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai).
41:171–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Floros T and Tarhini AA: Anticancer
cytokines: Biology and clinical effects of interferon-α2,
interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol.
42:539–548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nagayama M, Niwa K, Nagayama T, Ross ME
and Iadecola C: The cyclooxygenase-2 inhibitor NS-398 ameliorates
ischemic brain injury in wild-type mice but not in mice with
deletion of the inducible nitric oxide synthase gene. J Cereb Blood
Flow Metab. 19:1213–1219. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shin NR, Shin IS, Jeon CM, Hong JM, Oh SR,
Hahn KW and Ahn KS: Inhibitory effects of Picrasma
quassioides (D. Don) Benn. On airway inflammation in a murine
model of allergic asthma. Mol Med Rep. 10:1495–1500.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of beta-carboline
alkaloids, isolated from Picrasma quassioides, through
inhibition of the iNOS pathway. Planta Med. 78:1906–1911.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of acute and chronic intestinal inflammation. Nat
Protoc. 12:1295–1309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao W, He J, Zhang Y, Ito Y, Su Q and Sun
W: Preparative isolation and purification of alkaloids from
Picrasma quassioides (D. Don) Benn. By high-speed
countercurrent chromatography. J Liq Chromatogr Relat Technol.
35:1597–1606. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu P, Li H, Luan R, Huang G, Liu Y, Wang
M, Chao Q, Wang L, Li D, Fan H, et al: Identification of
β-carboline and canthinone alkaloids as anti-inflammatory agents
but with different inhibitory profile on the expression of iNOS and
COX-2 in lipopolysaccharide-activated RAW 264.7 macrophages. J Nat
Med. 73:124–130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fan H, Qi D, Yang M, Fang H, Liu K and
Zhao F: In vitro and in vivo anti-inflammatory effects of
4-methoxy-5-hydroxycanthin-6-one, a natural alkaloid from
Picrasma quassioides. Phytomedicine. 20:319–323.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao W, Yu J, Su Q, Liang J, Zhao L, Zhang
Y and Sun W: Antihypertensive effects of extract from Picrasma
quassioides (D. Don) Benn. In spontaneously hypertensive rats.
J Ethnopharmacol. 145:187–192. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu JF, Shao M, Zhai DW, Liu K and Wu LJ:
Protective effect of 4-methoxy-5-hydroxycanthin-6-one, a natural
alkaloid, on dextran sulfate sodium-induced rat colitis. Planta
Med. 75:142–145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Noldin VF, de Oliveira Martins DT,
Marcello CM, da Silva Lima JC, Delle Monache F and Cechinel Filho
V: Phytochemical and antiulcerogenic properties of rhizomes from
Simaba ferruginea St. Hill. (Simaroubaceae). Z Naturforsch C J
Biosci. 60:701–706. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
de Souza Almeida ES, Filho VC, Niero R,
Clasen BK, Balogun SO and de Oliveira Martins DT: Pharmacological
mechanisms underlying the anti-ulcer activity of methanol extract
and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models.
J Ethnopharmacol. 134:630–636. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sasaki T, Li W, Higai K and Koike K:
Canthinone alkaloids are novel protein tyrosine phosphatase 1B
inhibitors. Bioorg Med Chem Lett. 25:1979–1981. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ono H: Molecular mechanisms of
hypothalamic insulin resistance. Int J Mol Sci.
20(1317)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Legrand N, Bretscher CL, Zielke S, Wilke
B, Daude M, Fritz B, Diederich WE and Adhikary T: PPARβ/δ recruits
NCOR and regulates transcription reinitiation of ANGPTL4. Nucleic
Acids Res. 47:9573–9591. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jiao WH, Gao H, Li CY, Zhao F, Jiang RW,
Wang Y, Zhou GX and Yao XS: Quassidines A-D, bis-beta-carboline
alkaloids from the stems of Picrasma quassioides. J Nat
Prod. 73:167–171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao S, Kanno Y, Li W, Sasaki T, Zhang X,
Wang J, Cheng M, Koike K, Nemoto K and Li H: Identification of
picrasidine C as a subtype-selective PPARα agonist. J Nat Prod.
79:3127–3133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao S, Kanno Y, Li W, Wakatabi H, Sasaki
T, Koike K, Nemoto K and Li H: Picrasidine N Is a subtype-selective
PPARβ/δ agonist. J Nat Prod. 79:879–885. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chong HC, Chan JS, Goh CQ, Gounko NV, Luo
B, Wang X, Foo S, Wong MT, Choong C, Kersten S and Tan NS:
Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and
enhances angiogenesis to accelerate wound healing in diabetic mice.
Mol Ther. 22:1593–1604. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Georgiadi A, Wang Y, Stienstra R,
Tjeerdema N, Janssen A, Stalenhoef A, van der Vliet JA, de Roos A,
Tamsma JT, Smit JW, et al: Overexpression of angiopoietin-like
protein 4 protects against atherosclerosis development.
Arterioscler Thromb Vasc Biol. 33:1529–1537. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao F, Chen L, Bi C, Zhang M, Jiao W and
Yao X: In vitro anti-inflammatory effect of picrasmalignan A by the
inhibition of iNOS and COX-2 expression in LPS-activated macrophage
RAW 264.7 cells. Mol Med Rep. 8:1575–1579. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907.
2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Subramani R, Nandy SB, Pedroza DA and
Lakshmanaswamy R: Role of growth hormone in breast cancer.
Endocrinology. 158:1543–1555. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Takano T: Natural history of thyroid
cancer (Review). Endocr J. 64:237–244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pramesh CS, Mistry RC and Laskar SG:
Neoadjuvant chemoradiotherapy in resectable oesophageal cancer.
Lancet Oncol. 6:824–826. 2005.
|
|
49
|
Siddiqui NS, Godara A, Byrne MM and Saif
MW: Capecitabine for the treatment of pancreatic cancer. Expert
Opin Pharmacother. 20:399–409. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Suroowan S and Mahomoodally MF: Herbal
medicine of the 21st century: A focus on the chemistry,
pharmacokinetics and toxicity of five widely advocated
phytotherapies. Curr Top Med Chem. 19:2718–2738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sun W, Yu J, Gao H, Wu X, Wang S, Hou Y,
Lu JJ and Chen X: Inhibition of lung cancer by
2-Methoxy-6-Acetyl-7-methyljuglone through induction of necroptosis
by targeting receptor-interacting protein 1. Antioxid Redox Signal.
31:93–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Q, Mo J, Zhao C, Huang K, Feng M, He
W, Wang J, Chen S, Xie Z, Ma J and Fan S: Raddeanin A suppresses
breast cancer-associated osteolysis through inhibiting osteoclasts
and breast cancer cells. Cell Death Dis. 9(376)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zang M, Hu L, Zhang B, Zhu Z, Li J, Zhu Z,
Yan M and Liu B: Luteolin suppresses angiogenesis and vasculogenic
mimicry formation through inhibiting notch1-VEGF signaling in
gastric cancer. Biochem Biophys Res Commun. 490:913–919.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang S, Li X, Dou H, Hu Y, Che C and Xu D:
Sesamin induces A549 cell mitophagy and mitochondrial apoptosis via
a reactive oxygen species-mediated reduction in mitochondrial
membrane potential. Korean J Physiol Pharmacol. 24:223–232.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ni Y, Zhang H and Li Z and Li Z:
Connective tissue growth factor (CCN2) inhibits TNF-α-induced
apoptosis by enhancing autophagy through the Akt and Erk pathways
in osteoblasts. Pharmazie. 75:213–217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang HF, Wang ZQ, Ding Y, Piao MH, Feng
CS, Chi GF, Luo YN and Ge PF: Endoplasmic reticulum stress
regulates oxygen-glucose deprivation-induced parthanatos in human
SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci
Ther. 24:29–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Matt S and Hofmann TG: The DNA
damage-induced cell death response: A roadmap to kill cancer cells.
Cell Mol Life Sci. 73:2829–2850. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Liao NC, Shih YL, Chou JS, Chen KW, Chen
YL, Lee MH, Peng SF, Leu SJ and Chung JG: Cardamonin induces cell
cycle arrest, apoptosis and alters apoptosis associated gene
expression in WEHI-3 mouse leukemia cells. Am J Chin Med.
47:635–656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Su D, Zhou Y, Hu S, Guan L, Shi C, Wang Q,
Chen Y, Lu C, Li Q and Ma X: Role of GAB1/PI3K/AKT signaling high
glucose-induced cardiomyocyte apoptosis. Biomed Pharmacother.
93:1197–1204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang X, Tang S, Li D, Yu X, Wang F and
Xiao X: DIDS inhibits overexpression BAK1-induced mitochondrial
apoptosis through GSK3β/β-catenin signaling pathway. J Cell
Physiol. 233:5070–5077. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lee HE, Choi ES, Shin JA, Kim LH, Cho NP
and Cho SD: Apoptotic effect of methanol extract of Picrasma
quassioides by regulating specificity protein 1 in human
cervical cancer cells. Cell Biochem Funct. 32:229–235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gong YX, Liu Y, Jin YH, Jin MH, Han YH, Li
J, Shen GN, Xie DP, Ren CX, Yu LY, et al: Picrasma
quassioides extract elevates the cervical cancer cell apoptosis
through ROS-mitochondrial axis activated p38 MAPK signaling
pathway. In Vivo. 34:1823–1833. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xie DP, Gong YX, Jin YH, Ren CX, Liu Y,
Han YH, Jin MH, Zhu D, Pan QZ, Yu LY, et al: Anti-tumor properties
of Picrasma quassioides extracts in H-RasG12V
liver cancer are mediated through ROS-dependent mitochondrial
dysfunction. Anticancer Res. 40:3819–3830. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Xiao X, Si X, Tong X and Li G: Ultrasonic
microwave-assisted extraction coupled with high-speed
counter-current chromatography for the preparation of nigakinones
from Picrasma quassioides (D. Don) Benn. Phytochem Anal.
23:540–546. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kwon HS, Lee H, Lee JS, Lee K, Choi JH and
Jang DS: Two new β-carboline alkaloids from the stems of
Picrasma quassioides. Arch Pharm Res. 41:513–518.
2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lai ZQ, Liu WH, Ip SP, Liao HJ, Yi YY, Qin
Z, Lai XP, Su ZR and Lin ZX: Seven alkaloids from Picrasma
quassioides and their cytotoxic activities. Chem Nat Compd.
50:884–888. 2014.
|
|
69
|
Kuo PC, Shi LS, Damu AG, Su CR, Huang CH,
Ke CH, Wu JB, Lin AJ, Bastow KF, Lee KH and Wu TS: Cytotoxic and
antimalarial beta-carboline alkaloids from the roots of Eurycoma
longifolia. J Nat Prod. 66:1324–1327. 2003.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Jiang MX and Zhou YJ: Canthin-6-one
alkaloids from Picrasma quassioides and their cytotoxic
activity. J Asian Nat Prod Res. 10:1009–1012. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Daoud A, Song J, Xiao F and Shang J:
B-9-3, a novel β-carboline derivative exhibits anti-cancer activity
via induction of apoptosis and inhibition of cell migration in
vitro. Eur J Pharmacol. 724:219–230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jiao WH, Chen GD, Gao H, Li J, Gu BB, Xu
TT, Yu HB, Shi GH, Yang F, Yao XS and Lin HW: (±)-Quassidines I and
J, two pairs of cytotoxic bis-β-carboline alkaloid enantiomers from
Picrasma quassioides. J Nat Prod. 78:125–130.
2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Yamashita N, Kondo M, Zhao S, Li W, Koike
K, Nemoto K and Kanno Y: Picrasidine G decreases viability of
MDA-MB 468 EGFR-overexpressing triple-negative breast cancer cells
through inhibition of EGFR/STAT3 signaling pathway. Bioorg Med Chem
Lett. 27:2608–2612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zhao WY, Chen JJ, Zou CX, Zhang YY, Yao
GD, Wang XB, Huang XX, Lin B and Song SJ: New tirucallane
triterpenoids from Picrasma quassioides with their potential
antiproliferative activities on hepatoma cells. Bioorg Chem.
84:309–318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Guo E, Hu Y, Du T, Zhu H, Chen L, Qu W,
Zhang J, Xie N, Liu W, Feng F and Xu J: Effects of Picrasma
quassioides and its active constituents on Alzheimer's disease
in vitro and in vivo. Bioorg Chem. 92(103258)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Koe BK and Lebel LA: Contrasting effects
of ethyl beta-carboline-3-carboxylate (beta CCE) and diazepam on
cerebellar cyclic GMP content and antagonism of both effects by Ro
15-1788, a specific benzodiazepine receptor blocker. Eur J
Pharmacol. 90:97–102. 1983.PubMed/NCBI View Article : Google Scholar
|
|
77
|
File SE and Lister RG: Interactions of
ethyl-beta- carboline-3-carboxylate and Ro 15-1788 with CGS 8216 in
an animal model of anxiety. Neurosci Lett. 39:91–94.
1983.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Manzoor S and Hoda N: A comprehensive
review of monoamine oxidase inhibitors as anti-Alzheimer's disease
agents: A review. Eur J Med Chem. 206(112787)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Kumar MJ and Andersen JK: Perspectives on
MAO-B in aging and neurological disease: Where do we go from here?
Mol Neurobiol. 30:77–89. 2004.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Singh A, Kukreti R, Saso L and Kukreti S:
Oxidative stress: A key modulator in neurodegenerative diseases.
Molecules. 24(1583)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Simpson DSA and Oliver PL: ROS generation
in microglia: Understanding oxidative stress and inflammation in
neurodegenerative disease. Antioxidants (Basel).
9(743)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kerr JS, Adriaanse BA, Greig NH, Mattson
MP, Cader MZ, Bohr VA and Fang EF: Mitophagy and Alzheimer's
disease: Cellular and molecular mechanisms. Trends Neurosci.
40:151–166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Reniers J, Robert S, Frederick R, Masereel
B, Vincent S and Wouters J: Synthesis and evaluation of β-carboline
derivatives as potential monoamine oxidase inhibitors. Bioorg Med
Chem. 19:134–144. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sasaki T, Li W, Ohmoto T and Koike K:
Evaluation of canthinone alkaloids as cerebral protective agents.
Bioorg Med Chem Lett. 26:4992–4995. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zhu C, Deng G and Lin C: Study on chemical
constituents of Picrasma quassioides. Zhongguo Zhong Yao Za
Zhi. 36:886–890. 2011.PubMed/NCBI(In Chinese).
|
|
86
|
Matsuzaki T, Fukamiya N, Okano M, Fujita
T, Tagahara K and Lee KH: Picrasinoside H, a new quassinoid
glucoside, and related compounds from the stem wood of Picrasma
ailanthoides. J Nat Prod. 54:844–848. 1991.PubMed/NCBI View Article : Google Scholar
|
|
87
|
He C, Wang Y, Yang T, Wang H, Liao H and
Liang D: Quassinoids with insecticidal activity against
diaphorina citri kuwayama and neuroprotective activities
from Picrasma quassioides. J Agric Food Chem. 68:117–127.
2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Houël E, Stien D, Bourdy G and Deharo E:
Quassinoids: anticancer and antimalarial activities. In: Natural
Products: Phytochemistry, Botany and Metabolism of Alkaloids,
Phenolics and Terpenes. Ramawat KG and Mérillon JM (eds). Berlin,
Heidelberg: Springer Berlin Heidelberg, pp3775-3802, 2013.
|
|
89
|
Houël E, Bertani S, Bourdy G, Deharo E,
Jullian V, Valentin A, Chevalley S and Stien D: Quassinoid
constituents of Quassia amara L. leaf herbal tea. Impact on its
antimalarial activity and cytotoxicity. J Ethnopharmacol.
126:114–118. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Niiho Y, Mitsunaga K, Koike K and Ohmoto
T: Studies on the gastric antiulcer components from the woods of
Picrasma quassioides (simaroubaceae). Nat Med. 48:116–121.
1994.
|
|
91
|
Teja Sri K, Bhargavi S, Ushasri S,
Amareswara Reddy B and Geethika Priscilla M: Antiulcer herbal
drugs-A compilation. Int J Uni Pharm Bio Sci. 2:285–297. 2013.
|
|
92
|
Huang X, Su Z, Shen X, Tang Q, Xie Y, Liu
Z and Lai X: Determination of andrographolides and alkaloids in
Xiaoyanlidan tablets by RP-HPLC. Chin Tradit Pat Med. 6:451–454.
2003.
|
|
93
|
Yang N, Xiong A, Wang R, Yang L and Wang
Z: Quality evaluation of traditional Chinese medicine compounds in
Xiaoyan Lidan tablets: Fingerprint and quantitative analysis using
UPLC-MS. Molecules. 21(83)2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Renliu X: TLC identification and
determination of deoxyandrographolide of compound kumuxiaoyan
tablets. Chin Tradit Pat Med, 1992.
|
|
95
|
Saiin C, Rattanajak R, Kamchonwongpaisan
S, Ingkaninan K, Sukontason K, Baramee A and Sirithunyalug B:
Isolation and in vitro antimalarial activity of hexane extract from
Thai Picrasma javanica B1 stembark. Southeast Asian J Trop
Med Public Health. 34 (Suppl 2):S51–S55. 2003.PubMed/NCBI
|
|
96
|
Rahman S, Fukamiya N, Okano M, Tagahara K
and Lee KH: Anti-tuberculosis activity of quassinoids. Chem Pharm
Bull (Tokyo). 45:1527–1529. 1997.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ohmoto T, Nikaido T, Koike K, Kohda K and
Sankawa U: Inhibition of adenosine 3',5'-cyclic monophosphate
phosphodiesterase by alkaloids. II. Chem Pharm Bull (Tokyo).
36:4588–4592. 1988.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Zhao L, Zhao Y, Guo L and Zhang L:
Pharmacokinetic and bioavailability study of
5-hydroxy-4-methoxycanthin-6-one, a typical canthinone alkaloid, in
rats using ultra-high performance liquid
chromatography/electrospray ionization tandem mass spectrometry.
Biomed Chromatogr. 34(e4830)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Xuan YH and Jin Y, Row KH and Jin Y:
Antioxidant and anticancer activities of extracts from Picrasma
quassioides (D. Don) Benn. Asian J Chem. 22:7219–7226.
2010.
|