Introduction
Lower extremity deep vein thrombosis (DVT) is a
common peripheral vascular disease, which can lead to venous valve
insufficiency and concurrent pulmonary embolism (1). The incidence of thrombosis in the left
lower extremity is much higher than that in its right counterpart
(2), and primary iliac-femoral vein
thrombosis is particularly common. DVT of the lower extremity can
also extend to the inferior vena cava, or even block the renal vein
to cause renal failure and threaten life (3). There are ~500,000 cases of lower
extremity DVT in the United States each year. Among them, 10% of
patients develop a fatal pulmonary embolism (4), 20-50% of patients develop
post-thrombotic syndrome (5) and
5-10% of patients experience severe post-thrombotic syndrome
(6).
Previous studies have shown that inflammation plays
an important role in the formation of DVT in the lower extremity
(7,8). Interleukin-1β (IL-1β) is an
inflammatory cytokine that is a key contributor to the early stage
of systemic and local inflammatory reactions and is a marker of an
early inflammatory response in the body (9). IL-1β is produced by macrophages and
monocytes, and its production can be induced by endotoxin (10). It promotes thrombosis by directly
acting on vascular endothelial cells, impairing endothelial
function and inducing local thrombosis, and is a major mediator of
thrombus formation. IL-1β binds to the IL-1β receptor on
endothelial cells and activates the NF-κB pathway to synthesize
xanthine dehydrogenase (XD). XD may be converted to xanthine
oxidase (XOD), which acts on xanthine and hypoxanthine (HX) to form
superoxide anions (11-14).
Superoxide anions cause endothelial damage, activate tissue factor
(TF), blood clotting and von Willebrand factor and promote platelet
adhesion and fibrin deposition, thereby initiating and aggravating
thrombosis (15,16). However, the mechanism of action by
which IL-1β participates in the process of DVT has not yet been
fully elucidated.
The DVT model has been widely established in rats
(17,18) and is highly reproducible. In this
model, thrombosis is induced in deep veins by reducing but not
completely blocking the flow of blood. Thus, the blood is in a
slow-flowing or stagnant state, leading to thrombosis and mimicking
DVT in humans.
In the present study, a DVT model was established in
rats. The levels of IL-1β, TF, XOD and NF-κB were analyzed. The
mechanism of IL-1β in the process of DVT of the lower extremity was
further analyzed and evaluated.
Materials and methods
Animals
A total of 70 male Sprague-Dawley rats
(pathogen-free; age, 8-12 weeks; 200±20 g body weight) were
purchased from the Animal Center of Xinjiang Medical University.
The housing conditions of all animals included a room temperature
of 20-25˚C, humidity of 50-60%, 12h-light/12h-dark cycle and free
access to food and water. The rats were randomly divided into 7
groups: Control, sham and 5 DVT groups (S2h, S8h, S24h, S48h and
S72h groups). All animal experiments were conducted according to
the ethical guidelines of Xinjiang Medical University. The study
was approved by the Ethics Committee of Xinjiang Medical
University. All efforts were made to minimize animal suffering.
Establishment of the DVT model
The DVT model was established by reducing the blood
flow of the rats as previously described (18). A 1.5-cm incision was made along the
midline of the abdomen 1 cm below the xiphoid process. Then, the
inferior vena cava was exposed. After separating the vena cava, a
4-0 Ethicon suture was used to ligate the inferior vena cava at a
position 1 cm below the right renal vein. The two collateral veins
above the iliac vein were separated and ligated with 5-0 Ethicon
suture. The abdominal contents were then returned to the abdominal
cavity. After injecting 2-3 ml 0.9% sodium chloride, suturing was
performed layer by layer. The rats were fed normally. For rats in
the sham surgery group, the same procedure was performed but the
veins were not ligated. Rats in the control group did not receive
any surgical procedure.
Sample collection
Venous tissues, venous thrombus and whole blood were
collected from the inferior vena cava of each rat at 2, 8, 24, 48
and 72 h after DVT modeling surgery in the respective DVT groups,
and at 2, 8 and 24 h in the control and sham surgery groups.
However, when comparisons were made between the control and sham
groups, the data collected at 2, 8 and 24 h were not statistically
significant. Thus, only one set of data was displayed. Prior to
sample collection at each time point, the rats were anesthetized
with an intraperitoneal injection of 2% pentobarbital sodium (30
mg/kg). Following the induction of anesthesia, the rats were placed
in a closed container and euthanized by hypoxia, induced by
CO2 at a displacement rate of 30% container volume/min.
The animals were monitored for breathing and heartbeat. When the
breathing and heartbeat stopped, the death of the animals was
confirmed. Venous tissues were maintained in liquid nitrogen after
washing. Venous thrombus tissues were fixed in 10% paraformaldehyde
at room temperature. Serum was separated from whole blood by
centrifugation at 1,000 x g for 15 min at 4˚C and kept at 4˚C.
Enzyme-linked immunosorbent assays
(ELISAs)
The serum levels of IL-1β, TF and XOD were detected
using ELISAs. Rat pro-IL1β (cat. no. CSB-E08055r; Cusabio
Technology, LLC), Rat TF (cat. no. CSB-E07914r; Cusabio Technology,
LLC) and Rat XOD ELISA kits (cat. no. CSB-E13614r; Cusabio
Technology, LLC) were used. The procedures were conducted according
to the protocols provided with the kits. The absorbance value at
450 nm was read using an xMark™ microplate reader (Bio-Rad
Laboratories, Inc.).
Flow cytometric analysis
Endothelial cells in the peripheral blood were
counted by flow cytometry. In detail, 100 µl whole blood was mixed
with 65 µl 10% formaldehyde and incubated for 30 min at room
temperature in the presence of 0.1% Triton™ X-100. After
incubation, the cells were washed with PBS and then stained with
anti-CD31-FITC monoclonal antibody (cat. no. 11-0319-42;
eBioscience) at room temperature for 30-60 min. After washing with
PBS, the cells were subjected to flow cytometric analysis on the
FACScan flow cytometer (BD Biosciences) equipped with CellQuest™
software (Version 5.1; BD Biosciences).
Hematoxylin and eosin (H&E)
staining
H&E staining was used to observe the venous
thrombotic tissue. The staining was performed according to the
protocols provided with the H&E staining kit (Beijing Solarbio
Science & Technology Co., Ltd.). Briefly, the sections were
dewaxed, stained with H&E at room temperature for 3 min,
dehydrated, transparentized and mounted. Morphological features
were observed under a light microscope (Olympus BX51; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNAs were extracted from the thrombus using an
Animal Tissue Total RNA Extraction kit (Tiangen Biotech Co., Ltd.).
Subsequently, RNA was reverse transcribed into cDNA with TIANScript
II RT Kit (Tiangen Biotech Co., Ltd.). The temperature protocol was
as follows: 65˚C for 5 min, 42˚C for 60 min, 85˚C for 5 min, and
4˚C for 10 min. qPCR assays were performed using a SuperReal PreMix
Plus (SYBR Green; Tiangen Biotech Co., Ltd.) to determine the mRNA
levels of IL-1β, TF, XOD and
NF-κB. The primer sequences used are listed in
Table I. GAPDH was used as
an internal reference gene. The primers were synthesized by Sangon
Biotech Co., Ltd. The qPCR thermocycling conditions were 95˚C for 1
min followed by 40 cycles of 95˚C for 10 sec and 60˚C for 34 sec,
and a final extension at 60˚C for 1 min. The comparative
2-ΔΔCq method was used for relative quantification
(19).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Primer | Sequence
(5'-3') |
|---|
| IL-1β | Forward |
CCTGTGTGATGAAAGACGGC |
| | Reverse |
TATGTCCCGACCATTGCTGT |
| NF-κB | Forward |
TGACGGGAGGGGAAGAAATC |
| | Reverse |
TGAACAAACACGGAAGCTGG |
| TF | Forward |
CAAAACGGTCAAATGGTGCG |
| | Reverse |
ACCTCCAGAAATGGCCTTGA |
| XOD | Forward |
AATGCGGACCCTGAAACAAC |
| | Reverse |
TTTCCTATGCCTTCCACGGT |
Western blotting
The inferior vena cava tissues were homogenized and
lysed for 30 min with RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology). After centrifugation at 29,880 x g for
5 min at 4˚C, total proteins were isolated and the protein
concentration was quantified using a BCA 200 Protein Quantification
kit (Beijing Solarbio Science & Technology Co., Ltd.). A total
of 40 µg protein sample/lane was then electrophoresed by 12%
SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked with 5% skimmed milk for 1 h at room temperature and then
incubated with primary antibodies against NF-κB (1:1,000; cat. no.
10745-1-AP; Proteintech Group, Inc.) and GAPDH (1:5,000; cat. no.
10494-1-AP; Proteintech Group) overnight at 4˚C. After rinsing with
TBST (0.1% Tween-20), the membranes were incubated with the
HRP-conjugated secondary antibody (1:10,000; cat. no. BA1054;
Boster Biological Technology, Ltd.) for 1 h at room temperature.
Finally, an ECL kit (Pierce™ Fast Western Blot kit; Thermo Fisher
Scientific, Inc.) was used for visualization of the protein bands.
The densities of the bands were measured using Quantity-one
software (version 4.6.6; Bio-Rad Laboratories, Inc.). The gray
ratio of NF-κB to GAPDH was used to determine the relative
expression level of NF-κB.
Statistical analysis
All assays were performed three times to ensure
reproducibility. All results are expressed as the mean ± SD and
statistical analysis was performed using SPSS statistical software
(version 13.0; SPSS, Inc.). One-way ANOVA was used to compare
differences among groups followed by Tukey's test for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of IL-1β, TF and XOD levels
in serum
The levels of IL-1β, TF and XOD in the serum were
determined using ELISAs. The levels of IL-1β, TF and XOD in the
rats with inferior venous thrombosis exhibited an increasing trend
from 2 to 24 h, reaching a peak at 24 h and then gradually
decreasing from 48 to 72 h (Fig.
1). As shown in Fig. 1A, the
levels of IL-1β in the S8h, S24h and S48h inferior venous
thrombosis groups were significantly higher compared with those in
the control and sham surgery groups (P<0.05). As shown in
Fig. 1B, the levels of TF in the
S8h and S24h inferior venous thrombosis groups were significantly
higher compared with those in the control and sham surgery groups
(P<0.05). However, the S48h and S72h inferior venous thrombosis
groups exhibited no difference in TF levels compared with those in
the control and sham surgery groups. As shown in Fig. 1C, the level of XOD in the S24h
inferior venous thrombosis group was significantly higher compared
with those in the control and sham surgery groups (P<0.05),
while the S2h, S8h and S48h inferior venous stenosis groups
exhibited no difference in XOD levels compared with those in the
control and sham surgery groups. Furthermore, no significant
differences between the control and sham surgery groups were
detected. These results show that DVT of the lower extremities
induced changes in the serum levels of IL-1β, TF and XOD. The
levels of the three indicator proteins peaked at 24 h in the DVT
groups, and exhibited statistically significant differences
compared with the control and sham surgery groups, indicating that
the inflammatory factor IL-1β and the associated markers TF and XOD
may promote thrombosis.
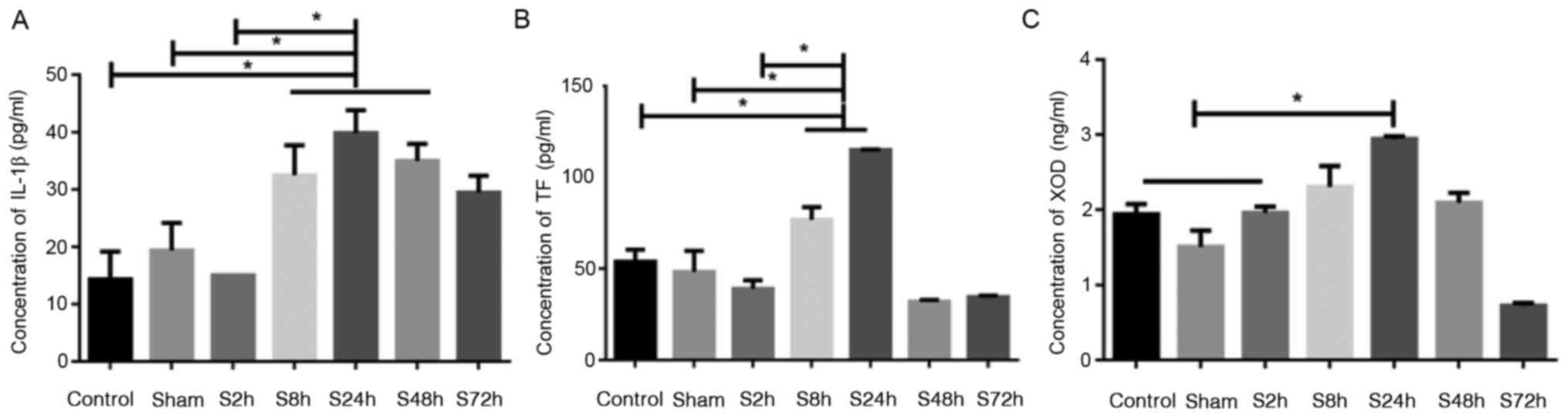 | Figure 1Comparison of IL-1β, TF and XOD levels
in serum. Rats were randomly divided into 7 groups: Control, sham
and 5 DVT groups, namely S2h, S8h, S24h, S48h and S72h, which were
examined 2, 8, 24, 48 and 72 h after DVT modeling surgery,
respectively. ELISAs were used to analyze the levels of (A) IL-1β,
(B) TF and (C) XOD in the serum. *P<0.05 as
indicated. IL-1β, interleukin-1β; TF, tissue factor; XOD, xanthine
oxidase; DVT, deep vein thrombosis. |
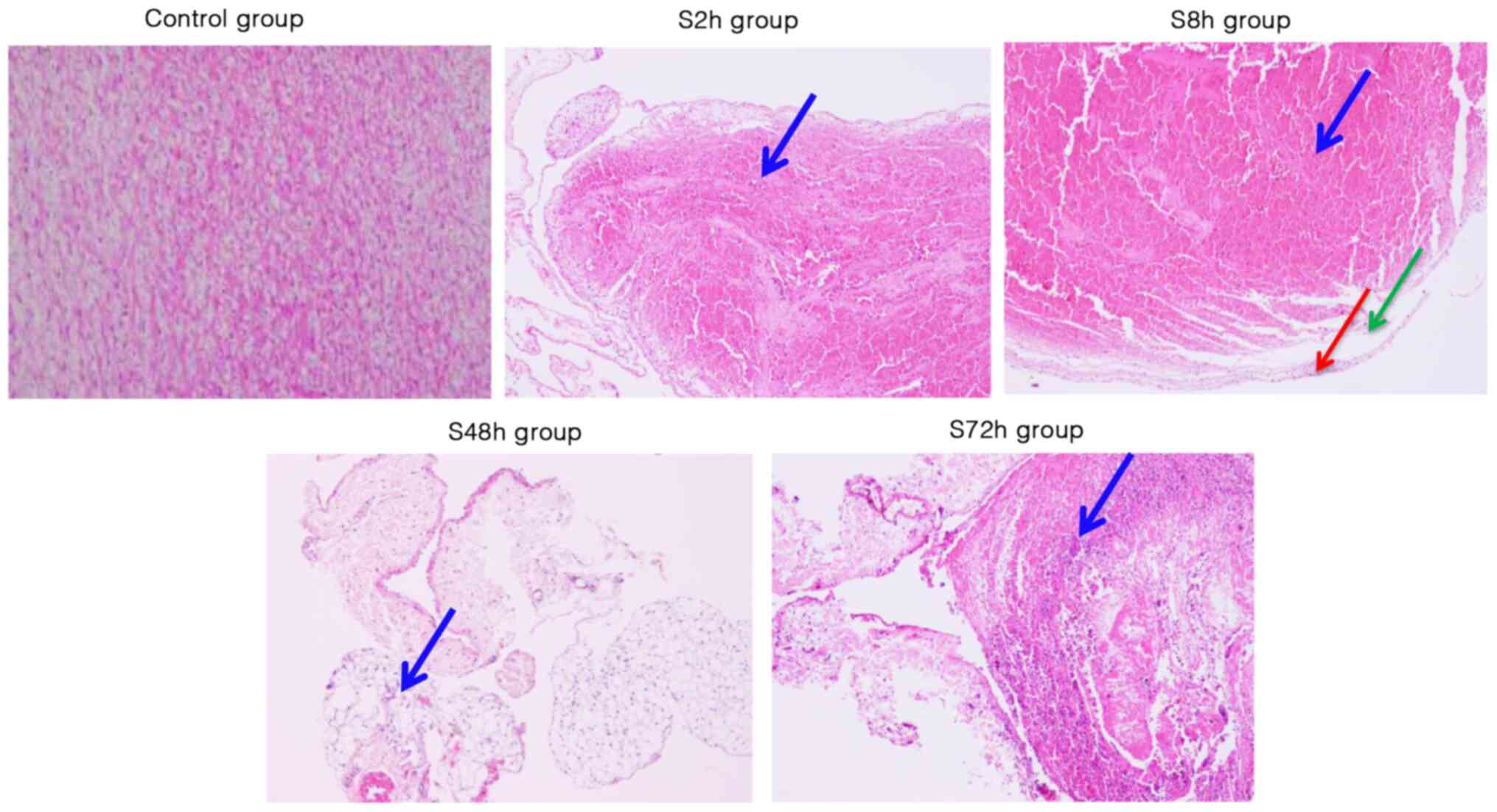
Histopathological changes of venous
thrombosis
To analyze the morphological changes associated with
venous thrombosis, H&E staining was performed. The staining
revealed that in the control group, the surface of the intima was
smooth, the endothelial cells were evenly arranged and no thrombus
was present (Fig. 2). However, at 2
h after surgery, thrombus filled the venous lumen, no granulation
tissue was visible in the thrombus, and no adhesion of the thrombus
to the vascular wall was observed. In addition, a few vascular
endothelial cells were detached and inflammatory cells infiltrated
around the vein; there were numerous red blood cells but few
infiltrative inflammatory cells (Fig.
2). At 8 h, no adhesion between the thrombus and vascular wall
was evident. In addition, fibroblasts were observed at the margin
of the thrombus, the number of neutrophils was markedly increased,
a small number of endothelial cells were detached from the valve
and the number of surrounding inflammatory cells was increased
(Fig. 2). At 48 h after the DVT
modeling surgery, granulation tissue was clearly observed, the
thrombus was partially adherent to the vessel wall, and the
infiltration of inflammatory cells around the vein was evident. In
addition, the endothelium was detached from the valve and the valve
was damaged (Fig. 2). At 72 h after
the surgery, a large amount of granulation tissue was present, and
adhesion between the thrombus and vessel wall was apparent. In
addition, inflammatory cells had infiltrated around the vein and
the structure of the valve was destroyed (Fig. 2). These results demonstrate the
changes associated with the formation of DVT in the lower
extremities at different time points.
Changes in the number of circulating
endothelial cells (CECs) in the peripheral blood
To analyze the relationship between the number of
endothelial cells in the peripheral blood circulation and
thrombosis, the flow cytometric analysis of CD31-FITC-H was
performed in the 7 groups of rats. As shown in Fig. 3, the level of CD31 positivity in the
blood of rats with inferior venous stenosis exhibited an upward
trend from 8 to 72 h. The levels of CD31 in the S2h and S8h
inferior venous stenosis groups exhibited no significant
differences compared with those in the control and sham surgery
groups. However, the CD31 levels in the S24h, S48h and S72h
inferior venous stenosis groups were significantly higher compared
with those in the control and sham surgery groups (P<0.05). In
addition, the level of CD31 exhibited significant differences among
the 24, 48 and 72 h inferior venous stenosis groups (P<0.05),
but no significant difference was observed between the control and
sham surgery groups. These results indicate that the degree of
venous endothelial cell damage is associated with the number of
CECs in the peripheral blood.
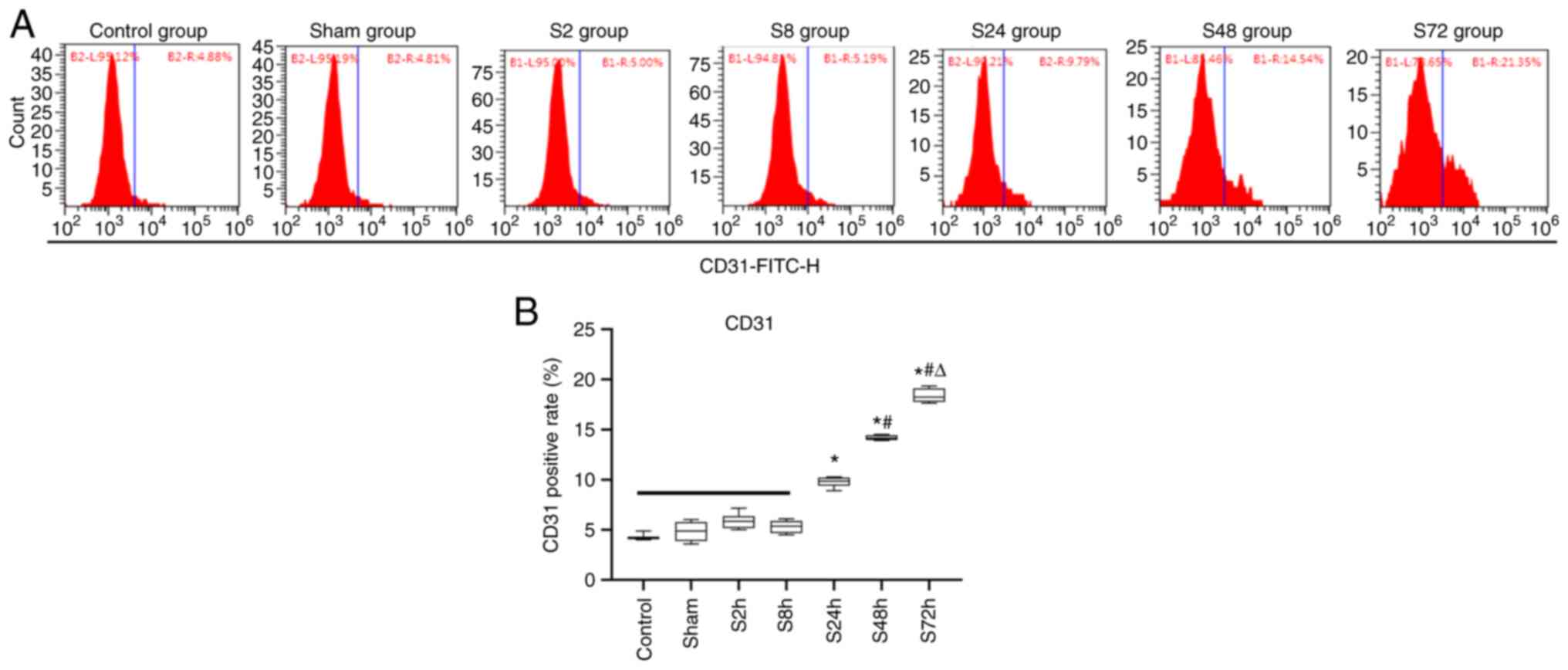 | Figure 3Changes in the expression rate of CD31
in the peripheral blood of rats. Rats were randomly divided into
the control, sham surgery and S2h, S8h, S24h, S48h and S72h groups,
which were examined 2, 8, 24, 48 and 72 h after deep vein
thrombosis modeling surgery, respectively. Flow cytometry was used
to analyze the positive rate of the peripheral blood endothelial
cell marker CD31. (A) Representative flow cytometry plots for each
group. (B) Statistical analysis of the CD31 positive rate in each
group. *P<0.05 vs. Control, sham, 2, and 8 h groups;
#P<0.05 vs. 24 h group; ΔP<0.05 vs. 48
h group. |
Differential expression of IL-1β, TF,
XOD and NF-κB mRNA
RT-qPCR was used to detect the mRNA levels of
IL-1β, TF, XOD and NF-κB in the
control, sham surgery and inferior venous stenosis groups. The mRNA
levels of IL-1β (Fig. 4A),
NF-κB (Fig. 4B),
TF (Fig. 4C) and XOD
(Fig. 4D) exhibited no significant
differences between the control and sham surgery groups. However,
the levels of these mRNAs in the S24h inferior venous stenosis
group were significantly higher compared with those in the control
and sham surgery groups (P<0.05). These results demonstrate that
IL-1β, NF-κB, TF and XOD mRNA
expression levels were elevated in the thrombus tissues of the DVT
model rats.
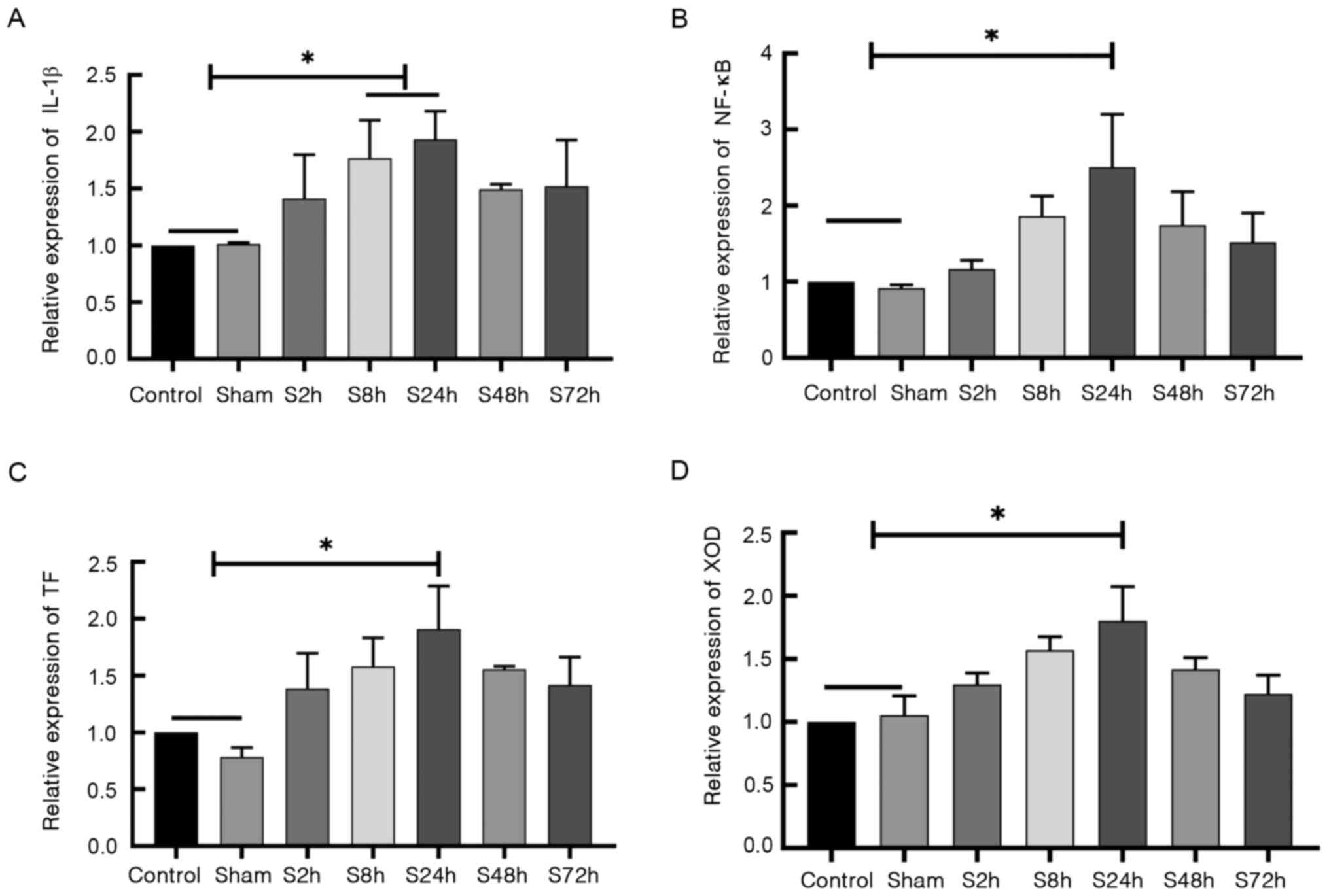 | Figure 4Expression of IL-1β,
TF, XOD and NF-κB mRNA. Rats were randomly
divided into the control, sham surgery and S2h, S8h, S24h, S48h and
S72h groups, which were examined 2, 8, 24, 48 and 72 h after deep
vein thrombosis modeling surgery, respectively. Reverse
transcription-quantitative PCR was used to detect the mRNA levels
in thrombus tissues. The mRNA levels of (A) IL-1β, (B)
NF-κB, (C) TF and (D) XOD are shown.
*P<0.05 as indicated. IL-1β, interleukin-1β; TF,
tissue factor; XOD, xanthine oxidase. |
Differences in NF-κB protein
expression in tissues
The protein expression levels of NF-κB in the venous
tissues of the control, sham surgery and inferior venous stenosis
groups were detected by western blotting (Fig. 5). The protein expression of NF-κB
exhibited no significant difference between the control and sham
surgery groups; however, NF-κB expression levels in the 2-72 h
inferior venous stenosis groups were significantly higher compared
with those in the control and sham surgery groups (P<0.05).
These results indicate that the expression of NF-κB is elevated in
the venous tissues of DVT model rats.
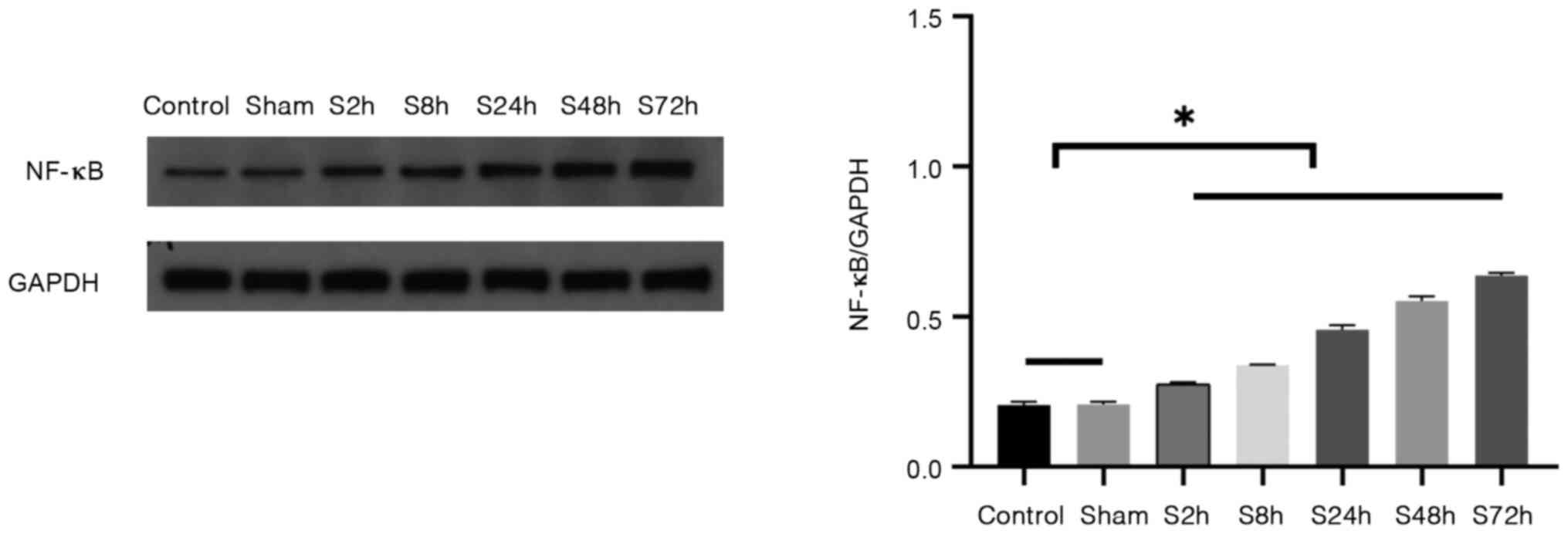 | Figure 5Expression of NF-κB protein in venous
tissues. Rats were randomly divided into the control, sham surgery
and S2h, S8h, S24h, S48h and S72h groups, which were examined 2, 8,
24, 48 and 72 h after deep vein thrombosis modeling surgery,
respectively. Western blotting was used to detect the NF-κB protein
levels in each group. Representative western blots and quantitative
results are shown in the left and right panels, respectively.
*P<0.05 as indicated. |
Discussion
CD31, also known as platelet/endothelial cell
adhesion molecule 1, is a transmembrane glycoprotein with a
molecular mass of 130 kDa. It is expressed at high levels at the
junctions of vascular endothelial cells and is a member of the
immunoglobulin superfamily. CD31 promotes platelet adhesion and
thrombosis (20) and is mainly
expressed on the surface of cells such as endothelial cells,
platelets and monocytes (21). It
is used as a specific marker of vascular endothelial cells. In the
present study, flow cytometric analysis revealed that the
expression level of CD31 was increased in rats with inferior venous
stenosis and increased significantly from 8 to 72 h after DVT
surgery.
CECs are present in peripheral blood following
vascular endothelium damage. The number of CECs is closely
associated with the degree of vascular endothelial injury;
therefore, it can be used as an index to evaluate the degree of
vascular injury (22). In the
present study, flow cytometry indicated that the number of CECs in
the peripheral blood of the rats 24-72 h following the induction of
DVT was significantly higher than that of rats in the control
group. In addition, the expression levels of NF-κB and IL-1β were
positively associated with the number of CECs in the peripheral
blood. These findings indicate that inflammation occurred in the
rats with inferior venous stenosis due to endothelial cell damage,
and the high expression of IL-1β causes oxidative stress and may
promote thrombus formation.
IL-1β is an inflammatory cytokine that plays an
important role in the early stages of systemic and local
inflammatory reactions, and is a marker of early inflammatory
response in the body (9). IL-1β is
generated by macrophages and monocytes and its production may be
induced by endotoxin (10). It acts
directly on vascular endothelial cells, where it impairs
endothelial function and promotes local thrombosis (23). Therefore, IL-1β is important in
thrombus formation. The present study demonstrated that the levels
of IL-1β in the peripheral blood and tissues of DVT model rats were
higher than those in the control and sham surgery groups. As the
severity of the thrombus increased, the level of IL-1β also
increased, suggesting that IL-1β may be involved in thrombus
formation. Therefore, IL-1β may be used as an indicator to judge
the occurrence and severity of DVT.
XOD is a molybdenum-containing flavin protease
present in various organisms (24,25),
which can catalyze the conversion of HX to xanthine. XOD is derived
from XD by the oxidation of cysteine residues; when activated XOD
produces superoxide radicals that induce oxidative damage (26), directly activate the inflammatory
signaling cascade and promote an immune response. Oxidative
stress-mediated damage primarily promotes an inflammatory response
by upregulating the expression of pro-inflammatory cytokines
(27). The present study showed
that the levels of IL-1β in the peripheral blood and tissues of the
DVT model rats were positively associated with those of XOD. As the
severity of the thrombus increased, the level of IL-1β increased
gradually and the level of XOD also increased, suggesting that
IL-1β promotes the expression of XOD and exacerbates tissue
damage.
TF is the only transmembrane glycoprotein expressed
on cell surfaces in the coagulation system and it initiates major
exogenous coagulation pathways in the body (28,29).
When blood vessels are damaged, endothelial cells and damaged
vascular endothelial fibroblasts express TF to initiate the
coagulation process (30).
Inflammation is an important component of thrombotic diseases.
Inflammatory responses induce endothelial cells and monocytes to
express TF, synthesize and release various adhesion molecules,
inflammatory mediators and chemokines, expand the inflammatory
response and promote thrombosis (31). The present study demonstrated that
the expression levels of TF in the peripheral blood and tissues of
the rat model of DVT were significantly increased compared with
those in sham surgery and control groups, suggesting that TF
participates in the thrombosis formation process, and is positively
associated with IL-1β. IL-1β possibly promotes thrombosis by
inducing the expression of TF.
When cells are stimulated by ischemia, hypoxia,
viral microbes, cytokines and inflammation, NF-κB dissociates from
IκB and enters the nucleus where it regulates gene expression
(32). The effect of activated
NF-κB on cells is manifested by the inhibition of apoptosis or
promotion of the expression of inflammation-associated proteins
(33). The present study revealed
that NF-κB was only weakly expressed in the control and sham
surgery groups. However, NF-κB expression in the venous blood
vessels was significantly increased after DVT; the expression of
NF-κB mRNA began to increase at 2 h after surgery,
peaked at 24 h and then gradually decreased while the expression of
NF-κB exhibited an increasing trend from 2 to 72 h. This indicates
that NF-κB is closely associated with thrombosis.
Inflammation and thrombosis are associated by an
intricate network in the body (34-36).
Inflammatory factors can promote the secretion of blood coagulation
factors, making the blood hypercoagulable and more likely to form a
thrombus. Furthermore, initiation of the coagulation cascade can
induce the release of inflammatory factors, aggravating
inflammation (37-39).
The present study indicated that the high expression of IL-1β in
the DVT model is positively associated with the expression of TF,
XOD and NF-κB, and closely associated with the extent of the
endothelial cell damage, which together promote thrombosis.
In conclusion, the present study suggests that
IL-1β, TF, XOD and NF-κB play important roles in the development of
DVT. These findings may provide a theoretical basis for the
clinical diagnosis and treatment of DVT by suggesting relevant
indices for detection.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XG designed the study. RZP and QF collected the
data. RZP, QF and GT performed the statistical analysis. RZP and XG
interpreted the data. RZP prepared the manuscript. BZ performed
H&E staining. RZP, QF and GT confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of Xinjiang Medical University and conducted according to
the ethical guidelines of Xinjiang Medical University. All efforts
were made to minimize animal suffering.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Crop MJ, Siemes C, Berendes P, van der
Straaten F, Willemsen S and Levin MD: Influence of C-reactive
protein levels and age on the value of D-dimer in diagnosing
pulmonary embolism. Eur J Haematol. 92:147–155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ibrahim NA, Hassan FM, Elgari MM and
Abdalla SE: Risk factors for deep vein thrombosis of lower
extremities in sudanese women. Vasc Health Risk Manag. 14:157–164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Wei WF, Lin SM, Liu ZJ, Huang HC and
Qiu WL: Expression of inflammatory factors in pregnant women with
acute venous thrombosis and its relationship with the degree of
vascular injury. Guangdong Med J. 35:558–560. 2014.(In
Chinese).
|
|
4
|
Alexander P and Giangola G: Deep venous
thrombosis and pulmonary embolism: Diagnosis, prophylaxis, and
treatment. Ann Vasc Surg. 13:318–327. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kahn SR, Shrier I, Julian JA, Ducruet T,
Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J,
et al: Determinants and time course of the postthrombotic syndrome
after acute deep venous thrombosis. Ann Intern Med. 149:698–707.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kahn SR: The post thrombotic syndrome.
Thromb Res. 127 (Suppl 3):S89–S92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang T, Li Q, Wang L and Li G: Expression
variations and clinical significance of MMP-1, MMP-2 and
inflammatory factors in serum of patients with deep venous
thrombosis of lower extremity. Exp Ther Med. 17:181–186.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou J, Pang L, Zuo B and Yan B:
Discussion on the clinical application of deep vein thrombosis risk
assessment module. Attend Pract and Res. 11:117–118. 2015.(In
Chinese).
|
|
9
|
Torbicki A, Perrier A, Konstantinides S,
Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D,
Janssens U, et al: Guidelines on the diagnosis and management of
acute pulmonary embolism: The task force for the diagnosis and
management of acute pulmonary embolism of the European society of
cardiology (ESC). Eur Heart J. 29:2276–2315. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Goekoop RJ, Steeghs N, Niessen RW, Jonkers
GJ, Dik H, Castel A, Gelder LW, Vlasveld LT, van Klink RC, Planken
EV and Huisman MV: Simple and safe exclusion of pulmonary embolism
in outpatients using quantitative D-dimer and wells' simplified
decision rule. Thromb Haemostasis. 97:146–150. 2007.PubMed/NCBI
|
|
11
|
Deem TL and Cook-Mills JM: Vascular cell
adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix
metalloproteinases: Role of reactive oxygen species. Blood.
104:2385–2393. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Erusalimsky JD: Vascular endothelial
senescence: From mechanisms to pathophysiology. J Appl Physiol
(1985). 106:326–332. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fan LM, Douglas G, Bendall JK, McNeill E,
Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, et
al: Endothelial cell-specific reactive oxygen species production
increases susceptibility to aortic dissection. Circulation.
129:2661–2672. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wakefield TW, Myers DD and Henke PK:
Mechanisms of venous thrombosis and resolution. Arterioscler Thromb
Vasc Biol. 28:387–391. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kanaji N, Sato T, Nelson A, Wang X, Li Y,
Kim M, Nakanishi M, Basma H, Michalski J, Farid M, et al:
Inflammatory cytokines regulate endothelial cell survival and
tissue repair functions via NF-κB signaling. J Inflam Res.
4:127–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Phan SH, Gannon DE, Varani J, Ryan US and
Ward PA: Xanthine oxidase activity in rat pulmonary artery
endothelial cells and its alteration by activated neutrophils. Am J
Pathol. 134:1201–1211. 1989.PubMed/NCBI
|
|
17
|
Ma J, Li X, Wang Y, Yang Z and Luo J:
Rivaroxaban attenuates thrombosis by targeting the NF-κB signaling
pathway in a rat model of deep venous thrombus. Int J Mol Med.
40:1869–1880. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Humphries J, Gossage JA, Modarai B,
Burnand KG, Sisson TH, Murdoch C and Smith A: Monocyte
urokinase-type plasminogen activator up-regulation reduces thrombus
size in a model of venous thrombosis. J Vasc Surg. 50:1127–1134.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Feng YM, Chen XH and Zhang X: Roles of
PECAM-1 in cell function and disease progression. Eur Rev Med
Pharmacol Sci. 20:4082–4088. 2016.PubMed/NCBI
|
|
21
|
Woodfin A, Voisin MB and Nourshargh S:
PECAM-1: A multi-functional molecule in inflammation and vascular
biology. Arterioscler Thromb Vasc Biol. 27:2514–2523.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B,
Arikan H and Koc M: Circulating endothelial cell number and markers
of endothelial dysfunction in previously preeclamptic women. Am J
Obstet Gynecol. 213(533)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van de Veerdonk FL and Netea MG: New
insights in the immunobiology of IL-1 family members. Front
Immunol. 4(167)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Moriwaki Y, Yamamoto T and Higashino K:
Distribution and pathophysiologic role of molybdenum-containing
enzymes. Histol Histopathol. 12:513–524. 1997.PubMed/NCBI
|
|
25
|
Zeki S, Miura S, Suzuki H, Watanabe N,
Adachi M, Yokoyama H, Horie Y, Saito H, Kato S and Ishii H:
Xanthine oxidase-derived oxygen radicals play significant roles in
the development of chronic pancreatitis in WBN/Kob rats. J
Gastroenterol Hepatol. 17:606–616. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lassegue B, Martin AS and Griendling KK:
Biochemistry, physiology, and pathophysiology of NADPH oxidases in
the cardiovascular system. Circ Res. 110:1364–1390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nomura J, Busso N, Ives A, Matsui C,
Tsujimoto S, Shirakura T, Tamura M, Kobayashi T, So A and Yamanaka
Y: Xanthine oxidase inhibition by febuxostat attenuates
experimental atherosclerosis in mice. Sci Rep.
4(4554)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Breitenstein A, Tanner FC and Luscher TF:
Tissue factor and cardiovascular disease: Quo vadis? Circ J.
74:3–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yao XJ, Zhao ZM and Xia T: Inflammation
and Thrombosis. Chinese J Thromb Hemostasis. 21:190–192. 2015.(In
Chinese).
|
|
30
|
Lopez-Vilchez I, Galan AM, Hernandez MR,
Caballo C, Roque M, Diaz-Ricart M, White JG and Escolar G:
Platelet-associated tissue factor enhances platelet reactivity and
thrombin generation in experimental studies in vitro. Thromb Res.
130:e294–e300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li J and Yan W: Relationship between
neutrophil activation and pathological progression of venous
thrombosis. Chinese J Trad Med Traumatology Orthopedics. 17:70–72.
2009.(In Chinese).
|
|
32
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Beg AA and Baldimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Blix K, Jensvoll H, Brækkan SK and Hansen
JB: White blood cell count measured prior to cancer development is
associated with future risk of venous thromboembolism-the tromsø
study. PLoS One. 8(e73447)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Boyle S, White RH, Brunson A and Wun T:
Splenectomy and the incidence of venous thromboembolism and sepsis
in patients with immune thrombocytopenia. Blood. 121:4782–4790.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Malgor RD and Labropoulos N: Re-modelling
of venous thrombosis. Phlebology. 28 (Suppl 1):S25–S28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Deatrick KB, Obi A, Luke CE, Elfline MA,
Sood V, Upchurch GR Jr, Jaffer F, Wakefield TW and Henke PK: Matrix
metalloproteinase-9 deletion is associated with decreased mid-term
vein wall fibrosis in experimental stasis DVT. Thromb Res.
132:360–366. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ripplinger CM, Kessinger CW, Li C, Kim JW,
McCarthy JR, Weissleder R, Henke PK, Lin CP and Jaffer FA:
Inflammation modulates murine venous thrombosis resolution in vivo:
Assessment by multimodal fluorescence molecular imaging.
Arterioscler Thromb Vasc Biol. 32:2616–2624. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rodriguez AL, Wojcik BM, Wrobleski SK,
Myers DD Jr, Wakefield TW and Diaz JA: Statins, inflammation and
deep vein thrombosis: A systematic review. J Thromb Thrombolysis.
33:371–382. 2012.PubMed/NCBI View Article : Google Scholar
|