Introduction
The history of the treatment of vesicular lithiasis
pathology has undergone one of the fastest medical technology
developments in all surgical disciplines, experiencing the fastest
transition from traditional to modern techniques. In 1985, Erich
Mühe, a radiologist by profession, performed the first endoscopic
cholecystectomy using a tube-shaped instrument (1). Only four years later, following the
Mühe breakthrough, the US reached a 90% rate of laparoscopically
performed cholecystectomy (2).
Despite the constant and consistent technological
advances, iatrogenic lesions during laparoscopic cholecystectomies
have not decreased. Moreover, the lesion profile has acquired
increasingly severe aspects with long-term postoperative results
confirming the significant impact of this pathology. During the
laparoscopic cholecystectomy (LC) of lesions directly diagnosed
intraoperatively and repaired in the same intervention by the
surgeon, only 17% have a favorable path (3). Recent research shows a case study of
27 patients with significant bile duct injuries, in which the
primary repair was conducted by the same team, with a recorded
failure rate of 95% (4), a worrying
percentage rate. In addition, in a retrospective analysis of 151
cases of malpractice following hepatobiliary laparoscopic surgery,
researchers suggest a 42% rate for main biliary pathway (MBP)
lesions (5,6). Therefore we propose a guide for
managing and recognizing different cases of iatrogenic lesions
during laparoscopic cholecystectomies. Such a guide for direct
intraoperative detection or early postoperative detection of
iatrogenic lesions can constitute real support for any surgical
department that addresses such a pathology.
Patients and methods
Our study is a multicenter, analytical, controlled
research based on a 12-year retrospective analysis between January
2008 to December 2020. We selected 108 patients with various
intraoperative iatrogenic lesions on the extrahepatic bile ducts
with or without associated major vascular injuries. The selected
patients were enrolled from 16,559 surgeries performed entirely
laparoscopically or laparoscopically debuted and converted to the
classical approach for benign vesicular pathology during the
abovementioned period. The research took place at two clinics
considered primary centers (CF1 Witting Clinical Hospital and CF2
Clinical Hospital in Bucharest, Romania) that received patients
from four other centers in the province, considered secondary in
this study. Classification of primary and secondary centers is
based on the level of hepato-biliary reconstructive surgical
experience.
Cases of iatrogenic lesions determined in the
primary clinics and those transferred for complete diagnosis
(etiological and staging) and definitive surgical repair in
secondary centers were recorded. For this batch, the following
parameters were considered: The percentage of iatrogenic lesions
recorded, the variability of the main bile duct (MBD) in
relationship with main adjacent important anatomical landmarks, the
anatomical and physiopathological characteristics of the pathology
that determined the respective surgical interventions, factors
related to laparoscopic surgical technique, the surgical technique
used to repair the recorded lesions, the overall survivability and
rate of complications. Starting from the previously mentioned
parameters, we created a study to identify the profiles and precise
intraoperative situations that present the maximum risk of
iatrogenic lesions. We also aimed to quantify the actual impact of
these iatrogenic lesions based on the analysis of hospitalization
costs, the number of required reinterventions, the period of
admission, and clinical evolution.
Our research study resulted in a surgical conduct
guide based on a decision-tree algorithm. We believe that we
created a guide easy to use both intraoperatively and in the
immediate postoperative period that can serve as a model for
solving issues in regards to these iatrogenic lesions. Our guide is
also a model applicable in secondary surgical services that do not
benefit from the immediate help of reference centers in corrective
surgery of iatrogenic lesions of the hepato-biliary tree.
For the present study aim, a new internal
classification method was adopted, obtained by re-organizing the
classical systems for reporting bile duct lesions, focusing more on
describing lesion severity and less on full details of the lesions.
Thus, we categorized the patients into three lesion groups, from
Group A (the simplest) to Group C (the most severe). In Group A, we
included bile losses from the cystic stump, lesions of the Lutschka
duct, and damage to the right accessory hepatic duct (RAHD). Group
B recorded cases of more advanced lesions of bile ducts, with no
vascular damage, and Group C included complex vasculo-biliary
combined lesions.
The equivalence between this internal case reporting
system and traditional classification systems is documented in
Table I. The Neuhaus and
Strasberg-Bismuth classifications are not part of Group C because
these systems cannot encode vascular lesions.
 | Table ICorrespondence between our
classification system and the most cited classifications regarding
vascular and bile duct lesions during cholecystectomies. |
Table I
Correspondence between our
classification system and the most cited classifications regarding
vascular and bile duct lesions during cholecystectomies.
| | Lesional Group |
|---|
| Classification
system | Group A | Group B | Group C |
|---|
| Hannover | A1, A2 | B1, B2, C1, C2, C3,
C4, D1, D2, D3, D4 | C2d, D2d, D3d, D3dpv,
D3pv, D4c, D4d, D4dpv |
| Neuhaus | A1, A2 | B1, B2, C1, C2, D1,
D2, E1, E2, E3 | n/a |
| Stewart-Way | I | II, III | IV |
|
Strasberg-Bismuth | A | B, C, D, E1, E2, E3,
E4, E5 | n/a |
| Siewert | I | II, IIIa, IIIb | IVa, IVb |
Primary statistical processing was performed through
standard tools in Microsoft Excel 365, and advanced processing was
performed with IBM SPSS® version 24 (IBM Corp.) and CDC
Epi Info™ version 7.2.2.1 (https://www.cdc.gov/epiinfo/pc.html). Other
statistical analysis methods consisted of tests that interpret
continuous variables such as The Wilcoxon-Mann-Whitney (U-test) and
the Kruskal-Wallis test (H-Test, a NOVA test), both of which are
non-parametric. For assessing the accuracy of statistical
processing, the P-value was set to 0.05.
Results
The iatrogenic lesion rate incidence of laparoscopic
cholecystectomy (LC) cases in our 12-year range study was different
between the two types of clinics, ranging from 0.73% for primary
clinics (80 cases out of 10,959 interventions practiced) to 0.5%
(28 cases out of 5,600 interventions) for secondary clinics. The
overall incidence rate of laparoscopic bile duct injuries (LBDI) in
our study was 0.65%.
The demographic data analysis revealed a ratio of
2.7:1 in favor of female patients with an average age of 47 years,
with limits between 20 and 85 years. Most patients (83%, 90 cases)
came from urban areas. No specific analysis was used to identify
the statistical relevance of these parameters.
Of the 108 cases in our study, 80 came from primary
clinics and 28 from secondary clinics. In addition, from the total
number of cases, 32 (30.2%) lesions were diagnosed
intraoperatively, and 76 lesions were established postoperatively
(69.8%). Of the 32 lesions directly identified intraoperatively,
40% (13 cases) were identified only by dedicated imaging, with the
remaining 60% (20 cases) directly observed. The imaging used
consisted of intraoperative cholangiography (97%) and endsocopic
retrograde cholagiopancreatography (ERCP) in only 3% of cases.
Cholangiography was used in its trans-choledochal variant (7 cases,
59%), followed by the transcystic variant (17%, 2 cases) and in
only 1 case in its trans-vesicular variant, which was a last resort
solution. The diagnosis in the postoperative stage was based mainly
on imaging investigations (92%), including ERCP (60%, 46 cases),
cholagio-MRI (20%, 16 cases), trans-abdominal ultrasound (10%, 8
cases) and cholangio-CT (2%). In only 8% of the cases, the
diagnosis was based on clinical and direct observation (signs of
choleperitoneum and change in the peritoneal drainage aspect).
The vesicular pathology that established the
laparoscopic approach surgical indication was 31.5% (34 cases) of
acute diagnoses, such as acute lithiasic cholecystitis, acute
lithiasic hydropiocholecystitis, vesicular hydrops, acute chronic
cholecystitis (lithiasic or not). The remaining 74 cases (68.5%)
were represented by chronic diagnoses such as chronic cholecystitis
(with or without lithiasis), and vesicular polyposis.
The conversion from laparoscopic intervention to
open approach was recorded in 34 cases (31.5%) and was based on
several reasons, such as the need for staging the lesion and a
correct and complete mapping through additional imaging tests or
the attempt to repair the iatrogenic lesion during the same
procedure.
An analysis of the distribution of the three groups
revealed that 29% of the cases (31 patients) presented with the
simplest lesions (Group A), 56% of the cases (61 patients) present
with Group B lesions, and 15% of the cases (16 patients) presented
with the most severe lesions of Group C. As mentioned, our analysis
consisted of many parameters, resulting in the following detailed
description of the assigned groups (Table II).
 | Table IIOverall characteristics of the
lesional groups in the study. |
Table II
Overall characteristics of the
lesional groups in the study.
| | Group | |
|---|
| | Group A | Group B | Group C | |
|---|
| Parameter | No. | % | No. | % | No. | % | P-value |
|---|
| Total | 31 (29%) | 61 (56%) | 16 (15%) | - |
| Women | 19 | 61% | 47 | 77% | 10 | 62% | - |
| Men | 12 | 39% | 14 | 23% | 6 | 38% | 0.328 |
| Average age
(years) | 46.2 | 44.9 | 54.5 | 0.130 |
| Median
hospitalization time (days) | 1.6 | 8.8 | 11.8 | <0.001 |
| Median positive
diagnosis time (days) | 2.5 | 3.0 | 2.5 | 0.796 |
| Global rate of
complications | 1 | 3.2% | 8 | 10.8% | 5 | 30% | 0.107 |
| Late stenosis | 0 | 0 | 17 | 28% | 4 | 25% | 0.109 |
| Mortality rates | 0 | 0 | 0 | 0 | 0 | 0 | 0.030 |
| Medium costs
(Lei) | 3075.69 Lei | 11472.32 Lei | 17131.59 Lei | <0.001 |
| Medium costs
(US/EUR) | 750 USD/ 630 EUR | 2,860 USD/ 2,400
EUR | 4,180 USD/ 3,506
EUR | |
The treatment methods used to repair the lesions,
regardless of the lesion group, consisted of stenting (ST), simple
peritoneal drainage (SD), simple suture (SS) of the bile lesion
(laparoscopic or after conversion), bilio-digestive anastomosis
(choledoco-jejunal anastomosis, choledoco-choledochal anastomosis,
hepatico-jejunal anastomosis) and hepatic resection (HR). Without
addressing the group in which they were practiced, their share was
56% (61 cases) for hepato-jejunal anastomoses (HJA), simple sutures
in 16% (17 cases), stenting in 14% (15 cases), simple drainage in
6% (7 cases), liver resection (LR) in 5% (5 cases) and 1% for
choledochal-jejunal anastomosis (CJA) and choledoco-choledochal
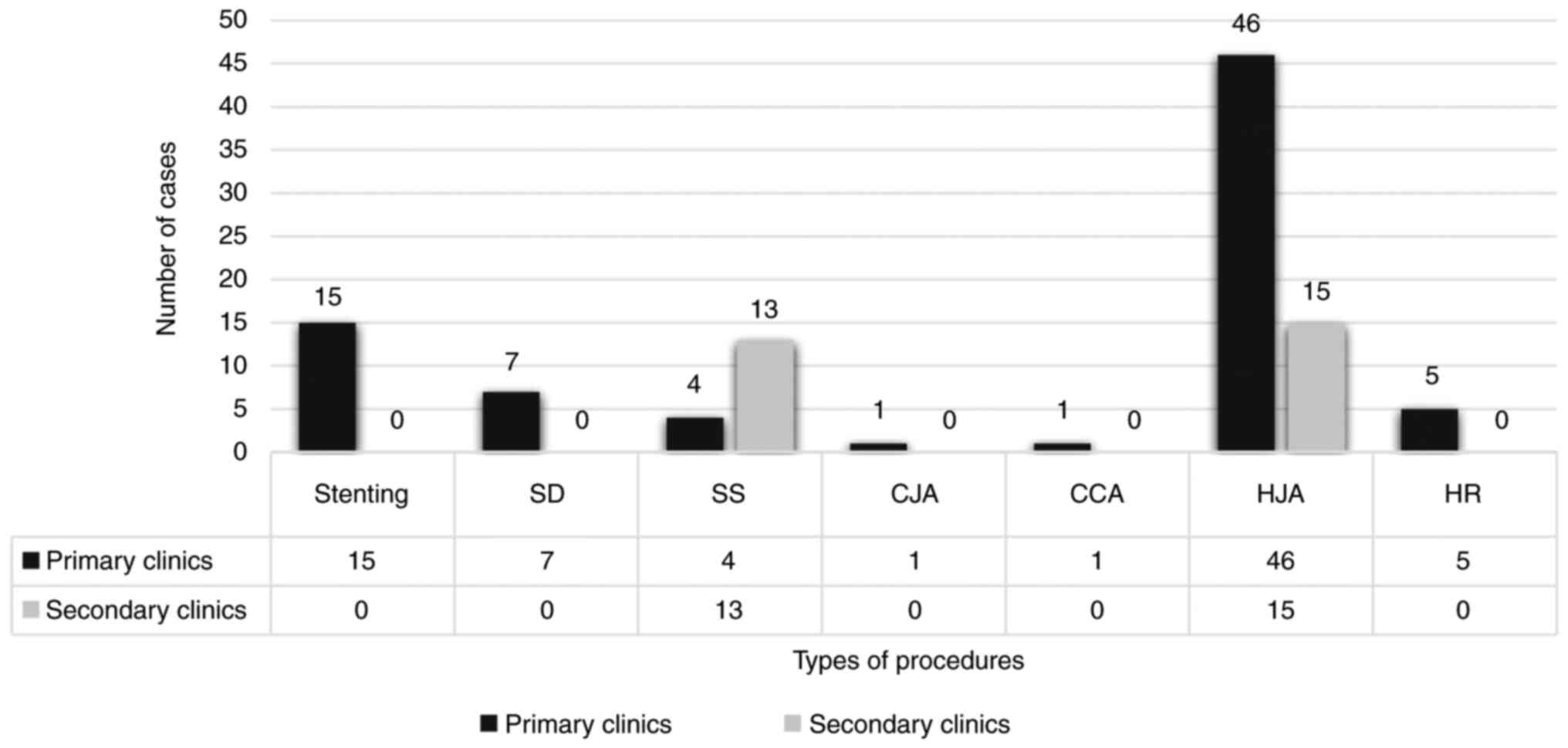
anastomosis (CCA) (Fig. 1).
Regarding the attempted repair performed in the same
surgical center that also determined LBDI, 28 patients were
remitted from the secondary, less experienced in reconstructive
surgery, clinics, to primary ones (26% of all cases). However, the
treatment methods chosen for LBDI repair varied between the primary
and secondary centers; this was further correlated with a different
clinical course for the patients. In the primary clinics, the
following procedures were performed: HJA (46 cases), ST (15 cases),
SD (7 cases) and SS (4 cases), while in the secondary clinics only
HJA (15 cases) and SS (13 cases) were performed. Note that all
cases of hepatic (HR) (5 patients) were fully practiced in the
primary clinics (Fig. 1).
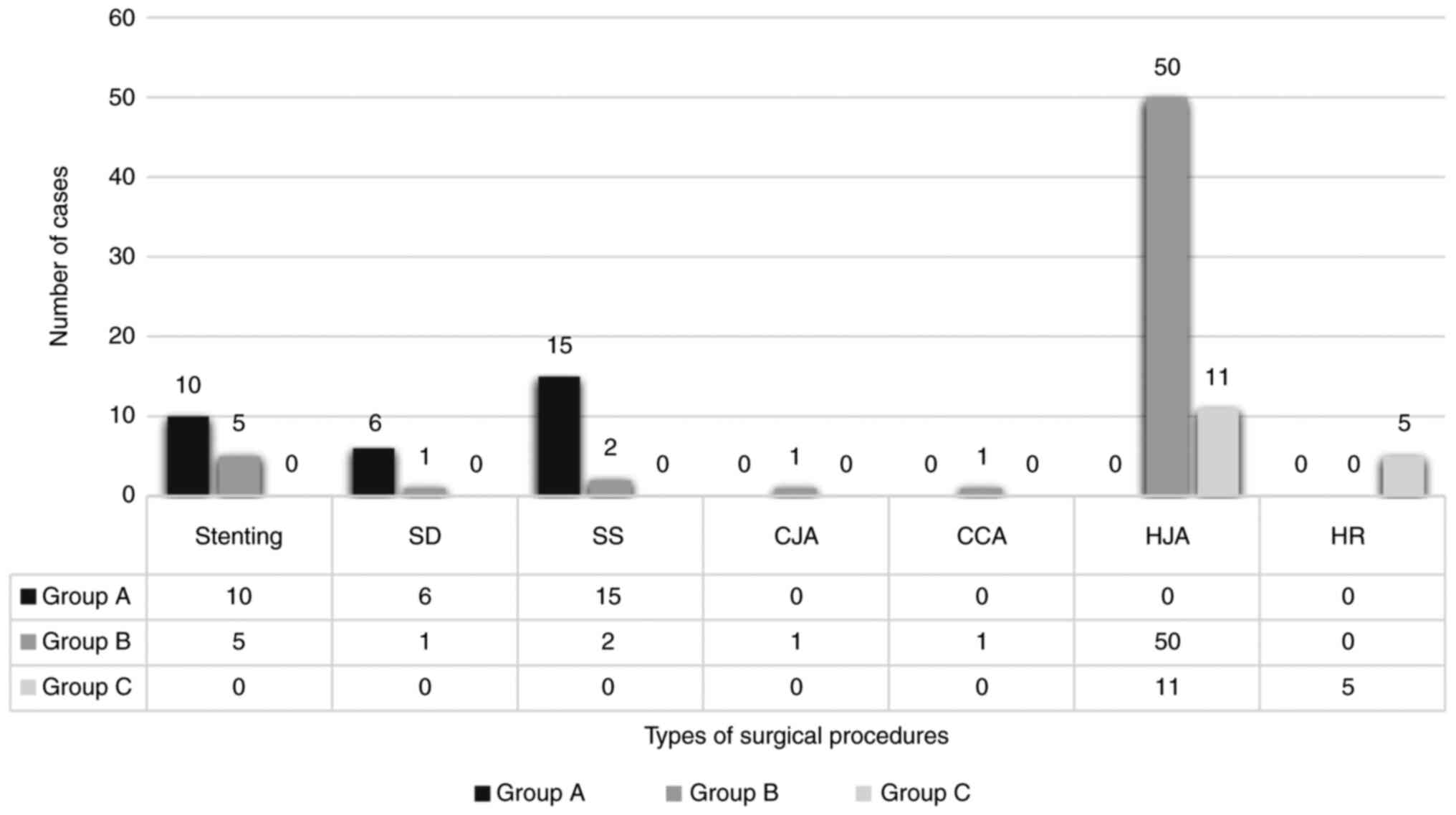
Regarding the treatment methods used in relation to
the groups, it was noted that, for Group A, with the most benign
lesions, SS was the most commonly practiced (15 cases) procedure,
followed by ST (10 cases) and SD (6 cases). The rest of the
treatment methods were not applied, as their complexity was not
justified for such minor injuries. For Group B, with more advanced
lesions, the distribution was different, with 50 cases of HJA, 5
cases of ST, 2 cases with SS and 1 case of CJA and CCA. For Lesion
Group C, with the most advanced lesions, the surgical methods used
were predominantly complex ones, with 11 cases of HJA and 5 cases
of HR (Fig. 2).
The most common long-term complication in
reconstructive surgery of extrahepatic bile ducts was, of course,
stenosis. Thus our study remotely followed this complication
through the following directions: the overall percentage of
stenosis, stenosis rate correlated with lesional groups, surgical
procedures chosen for repair and establishment of what types of
surgical procedures triggered stenosis and their origin (performed
in primary clinics or secondary ones). This study recorded stenosis
according to Bismuth's classic 4-point classification system
(7) which is still valid today
despite being described in the days of open-approach only and even
if in our study stenosis was the result of lesions caused by
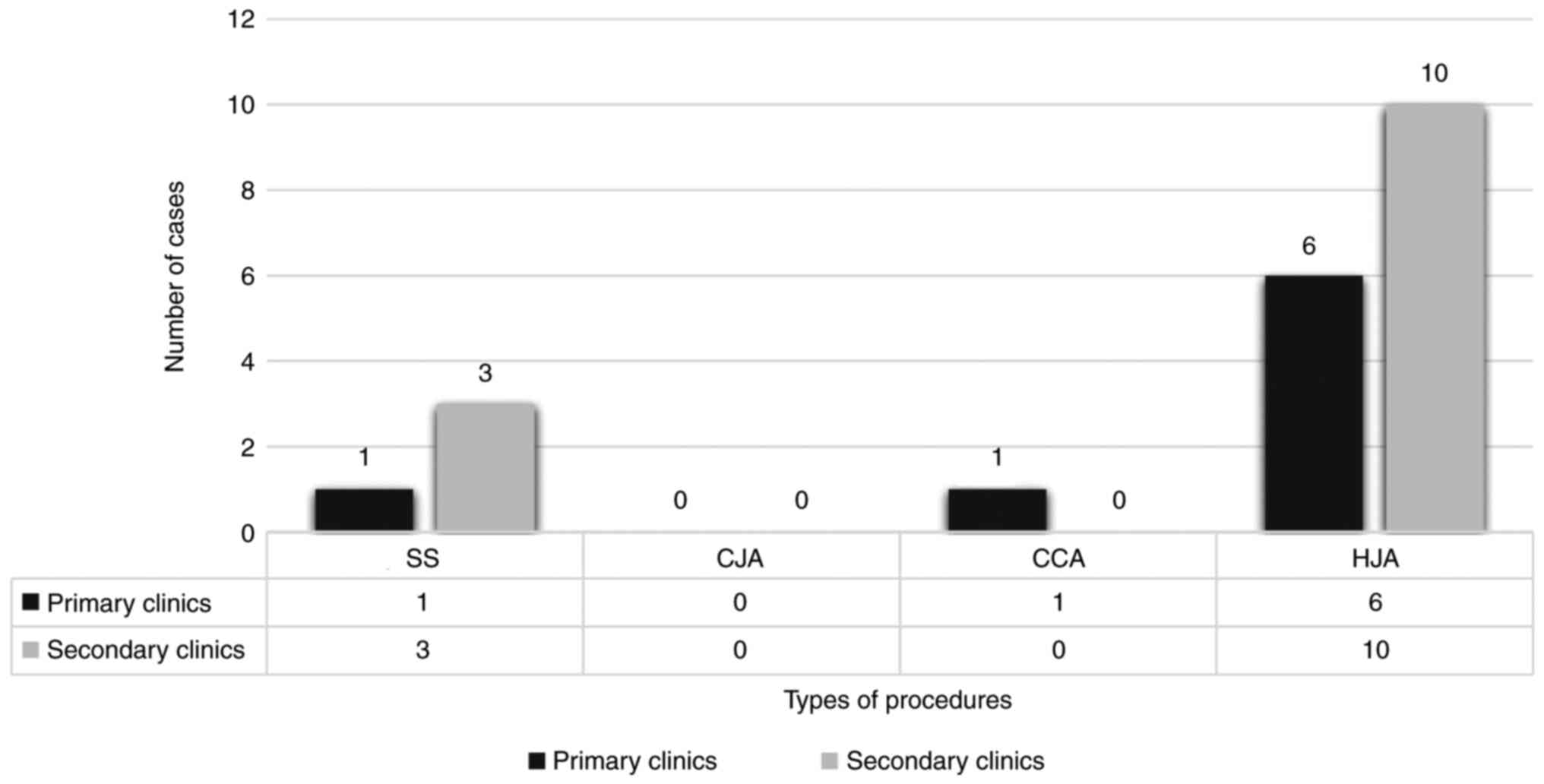
laparoscopic surgery. The overall stenosis rate in the batch was 21
cases. These came exclusively from groups B and C (81%, 17 and 19%,
4 cases, respectively). Group A did not cause any stenosis. Most
stenosis developed from patients from secondary clinics (62%, 13
cases) compared to primary clinics that caused a stenosis rate of
38% (8 cases). Disregarding the centers where the interventions
were performed, the analysis of surgical procedures chosen for LBDI
repair that determined late-term stenosis revealed that CCA was
credited with 100% stenosis rate followed by HJA with 26% and the
last being SS, with 24%. The procedure that did not cause any
stenosis was CJA. Suppose we factor in the type of center where
those surgical procedures were performed. In that case, we note
that for HJA the highest number of cases with stenosis came from
secondary clinics (10 case), compared to only 6 HJA performed in
primary centers. The same situation was evident for SS in which 3
cases in secondary centers determined stenosis and only 1 case was
present in primary centers (Fig.
3).
Analysis of the average length of the hospital stay
showed a steady increase from 1.6 days for Group A to 8.8 days for
Group B and to 11.8 days for Group C (Table II).
As we previously mentioned, the study considered
both bile duct lesions and associated vascular lesions. Vascular
lesions associated with LBDI, i.e., cases corresponding only to
Group C, were recorded for 20 cases (19% of the total). From a
topographical viewpoint, these lesions were most often located at
the level of the right hepatic artery (RHA), with 12 cases (60% of
Group C and 12% of the total), followed by the common hepatic
artery (CHA) with 3 cases (15% of Group C and 3% of the total). The
rarest vascular injuries were left hepatic artery (LHA) lesions
with 1 case (5% of Group C, 1% of the total) and 3 cases (15% of
Group C, 3% of the total) with portal vein (PV) lesions.
Discussion
As the statistical analysis shows, of the 28 cases
in which a primary repair was attempted at the same center where
the lesion they was induced (the situation of secondary clinics),
patients had a long-term progress worse than those operated on in
centers with higher experience in hepato-biliary repair surgery
(OR: 7.0, 95% CI: 2.5-19.6; P<0.01). General unfavorable
progress is dictated by the induction of stenosis, as a highly
unfavorable complication, especially of bilio-digestive
anastomoses, but also of simple sutures performed as a means to
solve laparoscopic bile duct injuries (LBDI). Regarding stenosis,
as a specific complication, this claim is supported by the higher
chance of stenosis (OR:7.0, 95% CI; P=0.02) for the group of
patients operated per primam in secondary centers compared
to those operated and treated in primary centers. By comparing the
tendency of stenosis of the two subgroups of patients, a clear
difference is clearly observed to the detriment of patients who
underwent primary repair attempted by the same team that caused the
iatrogenic lesion; among the 4 SS that caused late stenosis, 3 were
carried out at primary clinics, respectively, and of the 16
hepato-jejunal anastomoses (HJA) which resulted in stenosis only 6
came from the two clinics experienced in hepato-biliary surgery.
Although, in absolute numbers, the number of cases are smaller
compared to wider international studies, the conclusions are
similar and fully support this hypothesis, being consistent with
publications by prestigious surgeons, such as studies of Stewart
and Way (3) and Lillemoe et
al (8).
The average length of hospitalization time
increased, as expected, in proportion to the severity of the
lesion; thus, cases in Group C required the longest period of
admission, with an average of 11.8 days (P=0.001).
The statistical analysis of the costs recorded
compared among the 3 lesion groups revealed a clear differentiation
as well as an obvious growth trend directly proportional to the
severity of the lesion (P<0.001). In this regard, comparing
Group B with Group A had a P-value of 0.002 and comparing Group C
with Group B also has a P-value of 0.002.
The risk of requiring invasive surgery as a
definitive method of treatment compared to the need for
minimal-invasive approach also increased with advancement in the
lesion groups (P<0.001). The difference between Group B compared
to Group A was 27.7% (95% CI: 5.5-138.9, P<0.001), in favor of
invasive interventions. However, if we translate this analysis
between Group C compared to Group B (OR: 1.8, 95% CI: 0.4-9.0;
P=0.450), no statistical significance was found. Therefore, the
difference was no longer obvious between these classes, suggesting
that only Group A required mainly minimally invasive interventions
that no longer were useful in the context of complex injuries such
as those found in Groups B and C.
This series of cases revealed a rate of 20% LBDI
associated with vascular injury, which is consistent with most
published international studies (9). The consistency of this value leads us
to believe that the seriousness of this possible complication is
undervalued by most surgeons, especially in the context where
laparoscopic cholecystectomy tends to be regarded as a trivial
intervention with minimal risks to the patient (9,10). The
analysis of vascular lesions associated with ductal bile lesions
reveals, predictably, that the most frequently injured in a
traumatic event include RHA (in our study rated with 60% of lesions
of LBDI with concomitant vascular interest, respectively 11% of the
total LBDI in the study). Although the percentage itself is higher
than that reported in the literature [12% by authors such as Singh
et al (11) and Deziel et
al (12)], this study is
relatively narrow in representation and includes cases that have
also been submitted from less experienced centers, compared to the
cited studies that enrolled top-rated reference surgical
centers.
The statistical analysis allowed us also to note
that the risk of vascular injury significantly increased the closer
the LBDI was with the main biliary convergence (P=0.01). We
expected that the vascular-associated lesions, interpreted as a
whole, would lead to a severe increase in the incidence of late
stenosis. However, this expectation was contradicted by the
statistical analysis (P=0.4), which showed only a marginal increase
in this risk. When isolating lesions on CHA from the entire group
of vascular lesions, there was clearly an increased risk of
stenosis of the procedures chosen to restore the hepatobiliary
continuity. Furthermore, we can see that vascular lesions, being
the main criterion for Group C classification, which, in turn,
generated significantly higher LBDI case management costs, are
therefore a significant risk factor that directly influences the
socio-economic impact on these surgical cases.
The overall incidence rate of iatrogenic lesions
recorded in this analysis was 0.65%, a higher level than the 0.5%
threshold cited in most international studies (13-15).
In conclusion, the present study confirms that the
incidence ceiling of LBDI during laparoscopic cholecystectomy (LC)
did not fall below the threshold of 0.5%, which is double than the
incidence rate reported by the most significant studies analyzing
the incidence of open vs. laparoscopic incidence rates during LC
(14). It is obvious that there is
still much effort to be put into significantly lowering this
threshold (15,16). One way towards reaching this goal
should be that all surgeons strictly adhere to the basic concept of
always obtaining the critical view of safety advocated by Strasberg
(17) and outlined by many studies
published over the years (18,19).
Following the analysis of surgical treatment options
(both open and laparoscopic), factoring in the clinical results
obtained (immediate and remote) for each method, we developed a
guide with treatment options for the LBDI diagnosed during surgery
or in the immediate postoperative period. The choices are presented
according to levels: first intention, 2nd line, 3rd line, and so
on.
Although our study has resorted to the creation of
an internal classification system, it has minimal relevance to
well-established main bile duct (MBD) injury reporting systems;
thus, the recommendation guide will consider the most widely
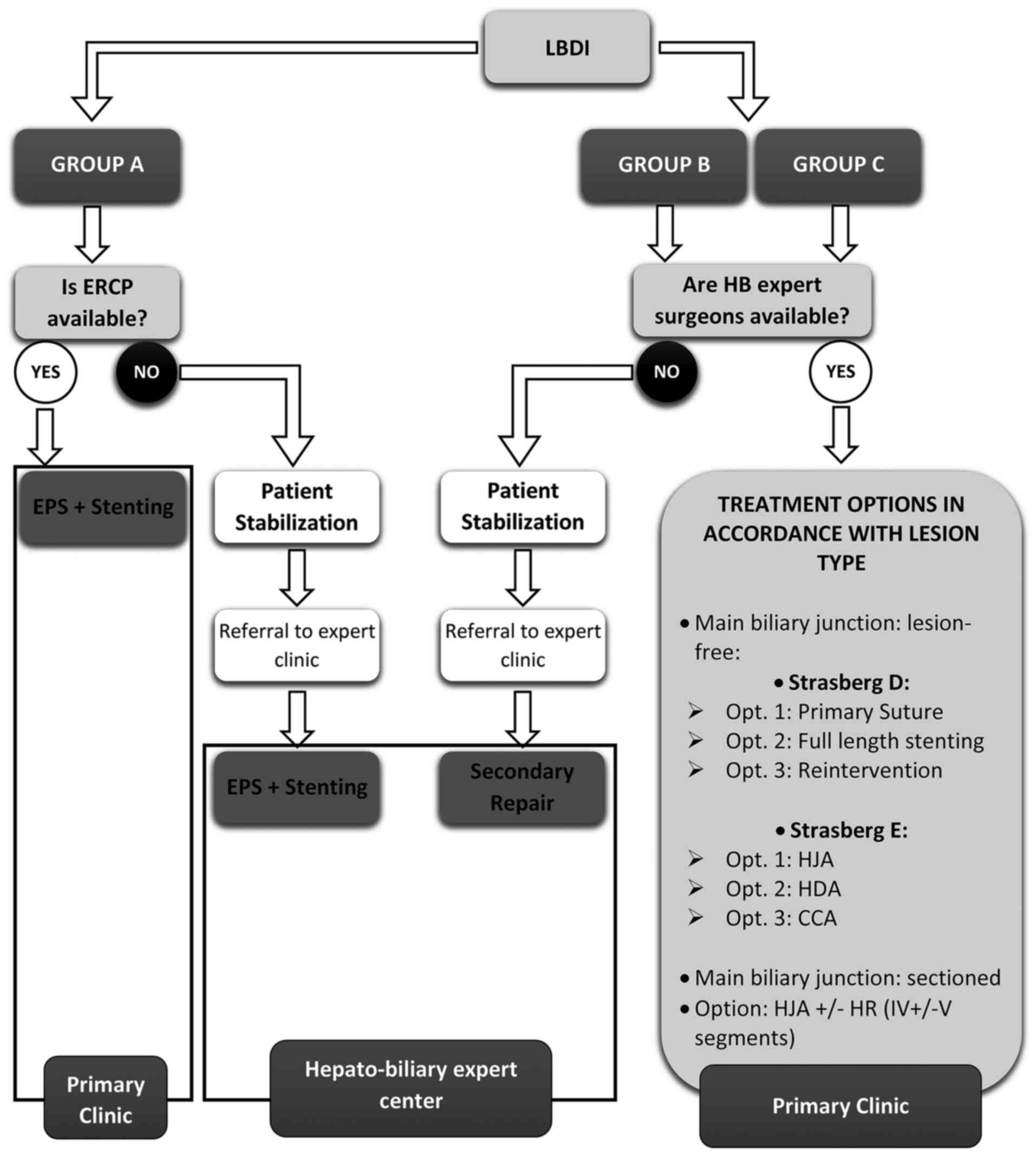
recognized Strasberg classification (Fig. 4).
Situations falling within Group A
For situations falling within Group A: i) If
endsocopic retrograde cholagiopancreatography (ERCP) is available,
then it is preferable to perform it together with the endoscopic
papilosphincerotomy (EPS) on a comfortable length followed by
placement of a bile stent with appropriate characteristics
(caliber, material, anchorage system). In this way the evolution is
the closest to being considered ideal and the chances for favorable
resolution are maximum. ii) If ERCP is not possible, than it is
preferable to fully stabilize the case and subsequently to refer
it, as soon as the general condition permits, to a clinic or center
of excellence in hepato-biliary surgery where EPS with stenting can
be performed. Stabilization measures should include placement of a
peritoneal polyethylene drainage tube in the proximity juxta of
biliary lesions (usually in subhepatic space), i.v.
hydroelectrolytic and volemic support, nasogastric probe,
antibiotic medication with tropism on the bile ducts, non-steroidal
anti-inflammatory, antispastic and antalgic medication.
Situations falling within Groups B and
C
For situations falling within Groups B and C: i) If
the clinic in which the surgical team that determined the LBDI also
has the necessary experience in the repair of the hepato-biliary
tree, then it is preferable that the case be taken over by that
team. The advantages are, of course, multiple, primarily logistical
in nature. The solutions for repair are in close connection with
the type of injury as follows:
For Strasberg type D injuries
i) Primary option: If we have a type D lesion
without associated vascular damage and the diameter of the defect
is between 3 and 5 mm, the first option included primary suture
with an 4.0/5.0 absorbable monofilament surgical thread and close
proximity drainage for monitoring. This solution can be performed
laparoscopically (especially if the lesion was immediately
identified intraoperatively) but also openly. ii) Secondary option:
If the site of the ductal lesion has been deprived of vascular
support (on a variable length) by a concomitant vascular
impairment, then the primary suture is considered as prohibited
because bile leaks and consecutive choleperitoneum will most likely
be recorded at this level, starting with the very first week
postoperatively. Thus, in these situations, an endoscopic
radiological-guided stenting is indicated. iii) Third option: If
the initial lesion has evolved from class D to class E, then open
surgical reintervention is the highly recommended, the options
available being those discussed in the section below.
For Strasberg type E injuries
If the main biliary convergence is free: i)
Option 1: HJA should be carried out and has undeniable advantages,
such as a good vascular inflow at the level of the bile duct, from
the intestinal wall, but also a reduced mounting tension, due to
the mobility of the jejunal loop, which can be properly ascended in
such a way as not to endanger the anastomosis; bilio-digestive
derivation with a free loop, in the absence of Oddian sphincter
obstacles, is not subject to such a pressure regime, since the
higher pressure gradient in the main bile duct and lower at the
level of the loop will cause a completely free transanastomotic
leakage of the bile. ii) Option 2: Hepatico-duodenal anasatomosis
(HAD), which confers the advantage of an immediate proximity
between the two structures as well as being less technically
challenging. Other advantages include easy construction of the
anastomosis of at least 1 cm in diameter; benefits for the duodenal
wall in regards to good vascularization; installation of axial
drainage with trans-ligamentary externalization and; fnally, remote
revision of the anastomosis by means of upper digestive endoscopy
(UDE), an advantage that no other bilio-digestive anastomosis has.
Of course, the main disadvantage is the tendency of the duodenum to
descend, especially during heavy food intake, exerting a higher
tension in the anastomotic assembly and thus having an increased
risk of fistulization, even if an extensive Kocher maneuver is
performed for mobilizing the duodenal frame. In addition, duodenum
anastomoses present a higher risk of biliary contamination, with
all consecutive shortcomings: repeated episodes of cholangitis or
even liver abscesses. iii) Option 3: Choledoco-choledochal
anastomosis (CCA), if the following conditions are met: minimal
distance between the two resulting biliary stumps, no tension
whatsoever in the resulting final anastomosis, no associated
vascular lesion is recorded, the use of monofilament surgical wires
with fine round needle tips and with the knots executed outside the
lumen. However, the inconveniences of this method of repair are
multiple. The diameters of the two stumps are narrow with an
opening of less than 10 mm, thus making an end-to-end (E-E)
anastomosis with separate stiches almost impossible. It is also
difficult to assess the degree of desiccation of the bile ductal
wall (even in the absence of a clear vascular lesion) induced by
the electrosurgical unit during the LC. This method must therefore
be viewed with caution. For the reasons exposed, many authors
consider as mandatory the use of a prosthesis inside the T-T
anastomosis with a trans-ligamentary externalized axial drainage,
left in place for at least 6 months or the use of a biliary stent.
These methods are intended to calibrate the anastomosis, thus
preventing late stenosis, but contributing to the reduction of
endoluminal pressure on stiches because, of all methods, the high
endoluminal pressure regime in these situations is much higher than
in any other type of bilio-digestive derivation.
If the main biliary convergence is
affected
If the main biliary convergence is affected by the
transection line of the LBDI, HJA is the only viable option, even
when sometimes a partial liver resection (LR) of the IV and Vth
segment must be practiced in order to gain access here for the
jejunal loop. If the clinic in which LBDI was registered does not
have an expert or group of surgeons experienced in this type of
hepato-biliary repair surgery, then the recommendation is for
complete stabilization of the case (under the same conditions as in
the previous situation, with the difference that, this time, given
the increased complexity of the ductal lesion with or without an
associated vascular lesion, the patient's supportive measures are
more laborious) and then transfer of the case to an expert center
where it will be re-evaluated in order to choose the optimal
treatment option.
The iatrogenic lesions of the main biliary pathways
are far from being completely clarified and still represent a
difficult surgical situation during open and laparoscopic
surgeries. The outcome of these situations is intricately linked
with the actual moment of discovery of the lesion and the surgical
methods for repairing such defects and implies many options that an
experienced surgeon must be aware of.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data sets that this study has been drafted upon
are available from the corresponding author through E-mail request
in a reasonable manner.
Authors' contributions
CM drafted the overall study guidelines. CM, DC, and
FDG were responsible for designing the surgical strategy. GG and ER
performed the statystical analyses. All authors read and approved
the final manuscript for publication.
Ethics approval and consent to
participate
Since this was a retrospective analysis, no ethical
approval or consent was needed from the supporting institution or
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Reynolds W Jr: The first laparoscopic
cholecystectomy. JSLS. 5:89–94. 2001.PubMed/NCBI
|
|
2
|
Berci G: Laparoscopic cholecystectomy
viewed from the USA. Aust N Z J Surg. 61:249–250. 1991.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stewart L and Way LW: Bile duct injuries
during laparoscopic cholecystectomy. Factors that influence the
results of treatment. Arch Surg. 130:1123–1129. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nuzzo G, Giuliante F, Giovannini I,
Murazio M, D'Acapito F, Ardito F, Vellone M, Gauzolino R,
Costamagna G and Di Stasi C: Advantages of multidisciplinary
management of bile duct injuries occurring during cholecystectomy.
Am J Surg. 195:763–769. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
James RH, Brigstocke JR, Shields DA and
Scurr JH: Medicolegal claims following laparoscopic cholecystectomy
in the UK and Ireland. Ann R Coll Surg Engl. 92:286–291.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grigoriu ME, Costea RV, Grigoriu CI and
Furtunescu FL: Endoscopic management of choledocolithiasis related
to periampullary duodenal diverticula. Med Surg J. 122:102–108.
2018.
|
|
7
|
Bismuth H: Postoperative strictures of the
bile ducts. In: The Biliary Tract V. Blumgart LH (ed).
Churchill-Livingstone, New York, NY, pp209-218, 1982.
|
|
8
|
Lillemoe KD, Martin SA, Cameron JL, Yeo
CJ, Talamini MA, Kaushal S, Coleman J, Venbrux AC, Savader SJ,
Osterman FA and Pitt HA: Major bile duct injuries during
laparoscopic cholecystectomy. Follow-up after combined surgical and
radiologic management. Ann Surg. 225:459–471. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nuzzo G, Giuliante F, Giovannini I, Ardito
F, D'Acapito F, Vellone M, Murazio M and Capelli G: Bile duct
injury during laparoscopic cholecystectomy: Results of an Italian
national survey on 56 591 cholecystectomies. Arch Surg.
140:986–992. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tzovaras G and Dervenis C: Vascular
injuries in laparoscopic cholecystectomy: An underestimated
problem. Dig Surg. 23:370–374. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh K, Singh R and Kaur M: Clinical
reappraisal of vasculobiliary anatomy relevant to laparoscopic
cholecystectomy. J Minim Access Surg. 13:273–279. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Deziel DJ, Millikan KW, Economou SG,
Doolas A, Ko ST and Airan MC: Complications of laparoscopic
cholecystectomy: A national survey of 4,292 hospitals and an
analysis of 77,604 cases. Am J Surg. 165:9–14. 1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lalisang TJM, Situmorang I, Ibrahim F,
Widianto P and Marbun VMG: Management of post-cholecystectomy bile
duct injuries without operative mortality at Jakarta tertiary
hospital in Indonesia-a cross-sectional study. Ann Med Surg (Lond).
62:211–215. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jajja MR, Laboe A, Hashmi S, Nadeem SO,
Sayed BA and Sarmiento JM: Standardizing diagnostic and surgical
approach to management of bile duct injuries after cholecystectomy:
Long-term outcomes of patients treated at a high-volume HPB center.
J Gastrointest Surg: Feb 2, 2021 (Epub ahead of print).
|
|
15
|
Ray S, Sanyal S, Das S, Jana K, Das AK and
Khamrui S: Outcomes of surgery for post-cholecystectomy bile duct
injuries: An audit from a tertiary referral center. J Visc Surg.
157:3–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barauskas G, Paškauskas S, Dambrauskas Z,
Gulbinas A and Pundzius J: Referral pattern, management, and
long-term results of laparoscopic bile duct injuries: A case series
of 44 patients. Medicina (Kaunas). 48:138–144. 2012.PubMed/NCBI
|
|
17
|
Strasberg SM: Biliary injury in
laparoscopic surgery: Part 1. Processes used in determination of
standard of care in misidentification injuries. J Am Coll Surg.
201:598–603. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sgaramella LI, Gurrado A, Pasculli A, de
Angelis N, Memeo R, Prete FP, Berti S, Ceccarelli G, Rigamonti M,
Badessi FGA, et al: The critical view of safety during laparoscopic
cholecystectomy: Strasberg yes or no? An Italian multicentre study.
Surg Endosc. 35:3698–3708. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Strasberg SM and Helton WS: An analytical
review of vasculobiliary injury in laparoscopic and open
cholecystectomy. HPB (Oxford). 13:1–14. 2011.PubMed/NCBI View Article : Google Scholar
|