Introduction
Inflammation is known to involve a series of
physiological responses to various stimuli, including pathogen,
chemical, and damaged cells of the host (1,2). It
plays a crucial role in various diseases including neuronal
diseases (3-5).
Endotoxin lipopolysaccharide (LPS) can initiate inflammatory
responses. Once macrophages are stimulated and activated by LPS,
they can produce pro-inflammatory mediators and cytokines including
cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS),
interleukin-1beta (IL-1β), IL-6, tumor necrosis factor-α (TNF-α),
and reactive oxygen species (ROS) (6-12).
ROS also play a critical rule in the inflammatory diseases. High
levels of ROS can induce macrophage activation, eventually leading
to the production of cytokines and the activation of inflammation
signaling pathways (11,12).
Mitogen activated protein kinases (MAPKs) and
nuclear factor-kappaB (NF-κB) signaling pathways are involved in
LPS-induced inflammation (1,13).
NF-κB plays a central role in inflammatory response through
expression of inflammatory factors (14,15).
In addition, MAPK family members activated by LPS are associated
with the production of inflammatory factors (16,17).
Thus, inhibiting MAPK and NF-κB activation might have potential as
a therapeutic strategy to ameliorate inflammatory diseases.
Thioredoxin 1 (Trx1), a redox regulating protein,
plays crucial roles in cell growth, cell protection against
neurotoxicity, and inhibition of apoptosis (18,19).
Recently, Wu et al (20)
have reported that Trx1 overexpression can suppress methamphetamine
(METH)-induced inflammation and oxidative stress in mice spleen and
suggested that Trx1 might be an important therapeutic target for
METH-induced immune dysfunction. Chen et al (21) have shown that overexpression of Trx1
in transgenic mice can increase survival from sepsis by suppressing
ER stress induced NF-κB inflammatory signaling, suggesting that
Trx1 might be a potential drug target for treating sepsis. Although
Trx1 plays beneficial roles in the immune system, the precise
mechanism involved in the effect of Trx1 on inflammation remains
unclear. In this study, we examined effects of Trx1 on inflammatory
responses in macrophages and an animal model using cell permeable
Tat-Trx1 fusion protein.
Materials and methods
Materials
We obtained LPS and TPA from Sigma-Aldrich.
Antibodies were provided by Cell Signaling Technology. All other
chemicals were used of high quality reagent grade.
Cell culture, protein treatment and
western blotting
DMEM was used in the culture of Raw 264.7 cells as
described previously (22,23). Tat-Trx1 protein was purified using a
Ni2+-NTA and PD-10 column chromatography and removed
endotoxins from purified protein as described previously (24). Briefly, this protein was treated
with Detoxi-Gel™ (Pierce) according to manufacturer's instruction
and the proteins (<0.03 EU/ml) were confirmed using a Limulus
amoebocyte lysate assay (BioWhitaker).
For concentration- and time-dependent transduction
of Tat-Trx1, Raw 264.7 cells were treated with different
concentrations (0.1-1 µM) of Tat-Trx1 for 1 h or with time periods
(15-60 min) of Tat-Trx1 (1 µM). After transduction, the cells were
further incubated and intracellular stability of Tat-Trx1 was
determined by western blotting using an anti-histidine antibody.
Western blotting was performed as described previously (22,25).
Western blot analysis
Equal amounts of sample proteins were separated with
15% SDS-PAGE and transferred to a nitrocellulose membrane. The
membrane was blocked with 5% nonfat dry milk in TBST buffer (25 mM
Tris-HCl, 140 mM NaCl, 0.1% Tween-20, pH 7.5) for 1 h. The
membranes were immunoblotted with the indicated primary and
HRP-conjugated secondary antibodies as recommended by the
manufacturer. The protein bands were detected using enhanced
chemiluminescent reagents (Amersham).
ROS staining
ROS staining was performed as described previously
(25,26). As the optimization period of the ROS
production and DNA fragmentation by LPS in Raw 264.7 cells is
different, the time of ROS staining and TUNEL staining are
different, respectively. Therefore, we performed the staining of
ROS and TUNEL at the optimal time by LPS. To determine the optimal
ROS staining time, we performed ROS staining over various times,
and we confirm that the optimal time is 3 h under this experimental
condition (data not shown). Tat-Trx1 (1 µM) pre-treated for 1 h and
LPS (1 µg/ml) exposed for 3 h. Cells were then washed twice with
PBS and stained with DCF-DA (20 µM) for 30 min. Fluorescent images
were obtained by fluorescence microscopy (Nikon eclipse 80i) and
the fluorescence intensity was detected with excitation at 485 nm
and emission at 538 nm using a Fluoroskan ELISA plate reader
(Labsystems Oy).
TUNEL staining
TUNEL staining was performed as described previously
(25,26). As the optimization period of the ROS
production and DNA fragmentation by LPS in Raw 264.7 cells is
different, the time of ROS staining and TUNEL staining are
different, respectively. Therefore, we performed the staining of
ROS and TUNEL at the optimal time by LPS. To determine the optimal
TUNEL staining time, we performed TUENL staining over various
times, and we confirm that the optimal time is 12 h under this
experimental condition (data not shown). Tat-Trx1 (1 µM)
pre-treated for 1 h and LPS (1 µg/ml) exposed for 12 h. TUNEL
staining was performed using a Cell Death Detection kit (Roche
Applied Science, Basel, Switzerland). Fluorescent images were
obtained by fluorescence microscope (Nikon eclipse 80i).
Fluorescence intensity levels were measured using a Fluoroskan
ELISA plate reader (Labsystems Oy) at 485 nm excitation and 538 nm
emission.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Raw 264.7 cells (105 cells/well) were
grown overnight in 12-well plate and total RNA extracted using
easy-BLUE according to the manufacturer's instructions (iNtRON).
Also, mouse skin tissue (50 mg) was used for total RNA extraction
using TRIzol reagent (Life Technologies). The cDNA synthesis was
performed using 2 µg of total RNA with cDNA Reverse Transcription
Kit (Omniscript® RT Kit) as per the manufacturer's
instructions (Qiagen). The mRNA levels were quantified using a SYBR
Premix Ex Kit (Bio-Rad) with a CFX Real time PCR Detection Systems
(Bio-Rad). The relative gene expression levels were normalized to
GAPDH and calculated using the 2-ΔΔCq method (27).
The primer sequences for PCR were as follows: COX-2
(sense: 5'-CCAGCACTTCACCCATCAGTT-3'; antisense:
5'-AAGGCGCAGTTTATGTTGTCTGT-3'), iNOS (sense:
5'-GGCTGCCAAGCTGAAATTGAAT-3'; antisense:
5'-CGTGATAGCGCTTCTGGCTCTT-3'), IL-1β (sense:
5'-TGTGTTTTCCTCCTTGCCTC-3'; antisense: 5'-TGCTGCCTAATGTCCCCTTG-3'),
IL-6 (sense: 5'-AAGGAGTGGCTAAGGACCAAGAC-3'; antisense:
5'-AGTGAGGAATGTCCACAAACTGATA-3'), TNF-α (sense:
5'-CTTGTTGCCTCCTCTTTTGC TTA-3'; antisense:
5'-CTTTATTTCTCTCAATGACCCGTAG-3'), GAPDH (sense:
5'-CTTTGGCATTGTGGAAGGGCTC-3'; antisense:
5'-GCAGGGATGATGTTCTGGGCAG-3').
Experimental animal
All experiments utilized ICR mice (male, 6-8
week-old, total used animal = 25) obtained from Experimental Animal
Center, Soonchunhyang University. The mice were provided with a
commercial diet and water ad libitum under controlled
temperature, humidity and lighting conditions (light/dark cycle
12:12, and 22±2˚C, 55±5%). All experimental protocols were approved
by the Administrative Panel on Laboratory Animal Care of
Soonchunhyang University (permit no. SCH 15-0002-3). All possible
efforts were made to avoid suffering of the rats and distress, and
minimize the number used during the experiments. The euthanasia for
all experimental animals was conducted utilizing general inhalants
methods with Carbon dioxide (CO2) under the guideline of
our Laboratory Animal Care Permits. During the experiments of in
vivo study, all animal health, discomforts and behavior
condition were monitored minimum 1 time per each day until animal
euthanasia during in vivo animal experiments to follow
ARRIVE checklist (https://www.nc3rs.org.uk/arrive-guidelines). There is
no found the death for all animals during this study.
TPA-induced ear edema
TPA-induced skin animal models were produced as
described elsewhere (22,24). TPA is known as the strongest
promoter of skin inflammation and well described the
characteristics of TPA in skin inflammations (28). To assess the effects of Tat-Trx1
against TPA-induced ear edema, the mice were divided into five
groups (n=5 per group) as follows: control group; TPA-treated
group; Tat-Trx1-treated group; Trx1-treated group; and Tat
peptide-treated group. TPA (1.0 µg) dissolved in 20 µl of acetone
was applied to the inner and outer surfaces of the ears of the mice
every day for 3 days. Tat-Trx1 (10 µg) protein was topically
applied to the ears of mice every day 1 h after TPA treatment.
Twenty-four hours after the final treatment with TPA and Tat-Trx1
(experimental duration=4 days), all mice (n=25) were euthanized
utilizing general inhalants methods in the euthanasia chamber for
fill rate of 30-70% of the chamber volume per minute with Carbon
dioxide (CO2) according AVMA Guidelines for the
Euthanasia of Animals (29). Then,
the ear biopsies were obtained. Ear thicknesses were measured using
a digital thickness gauge (Mitutoyo Corporation). Ear weights were
measured after 5 mm diameter ear biopsies were obtained from each
group using a punch (Kai Industries).
Immunohistochemistry
For histological analysis, ear biopsies were fixed
in 4% paraformaldehyde, embedded in paraffin, sectioned at a
thickness of 5 µm, and then stained with hematoxylin and eosin
(22,24).
Statistical analysis
Data are expressed as the mean ± SEM of three
experiments. The statistical significance analyzed using one-way
ANOVA followed by a Bonferroni post hoc test. P-value < 0.05 was
considered as significant.
Results
Transduction of purified Tat-Trx1 into
Raw 264.7 cells
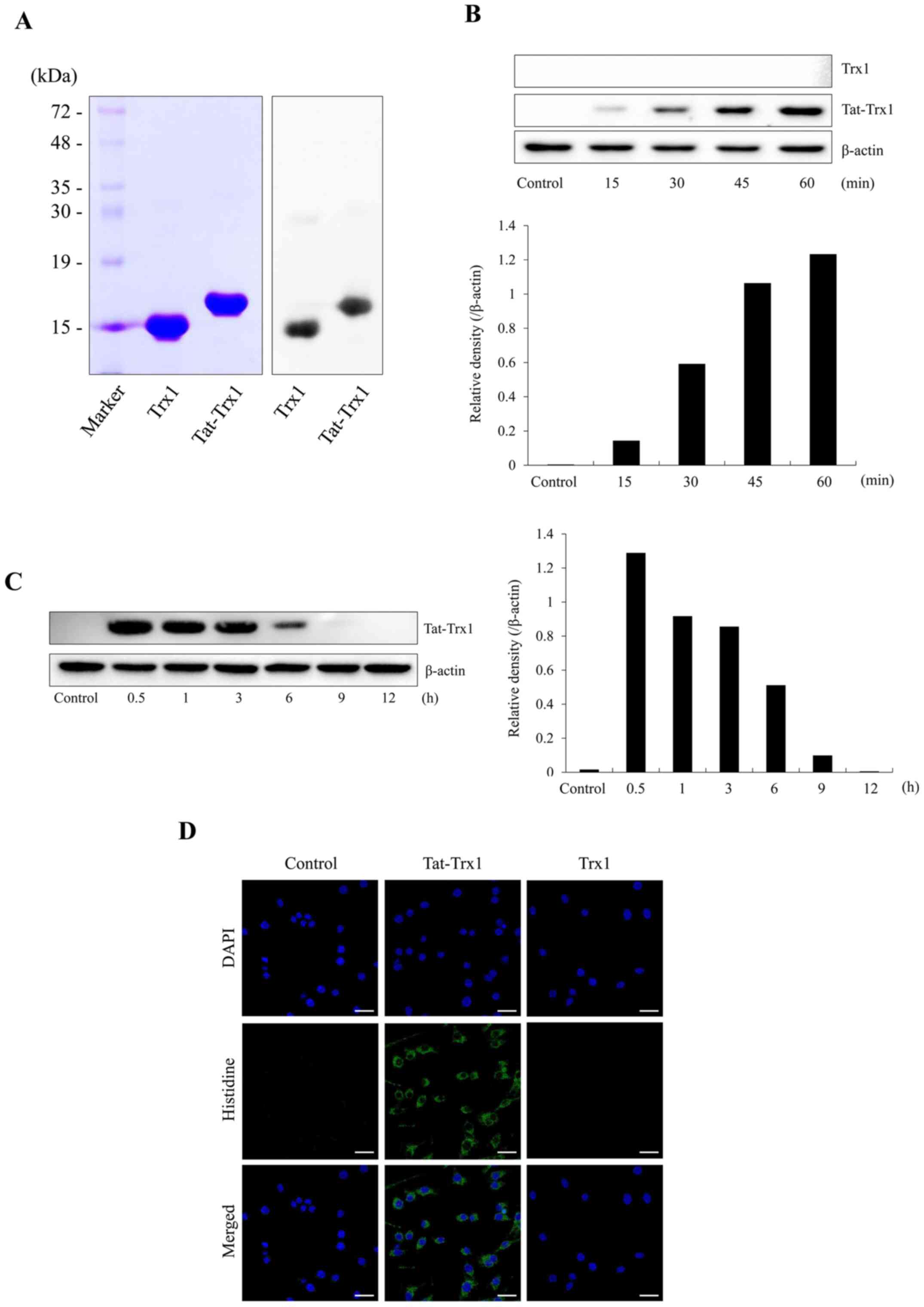
We constructed a fusion protein Tat-Trx1 and
purified it. As expected, one single band was detected for the
purified protein (Fig. 1A). As
shown in Fig. 1B and C, Tat-Trx1 transduced into Raw 264.7 cells
time-dependently and its level was maintained for 6 h. However,
Trx1 did not transduce into Raw 264.7 cells. Transduced Tat-Trx1
was distributed in the cytoplasm and nucleus (Fig. 1D).
Tat-Trx1inhibits LPS-induced ROS
production and DNA fragmentation
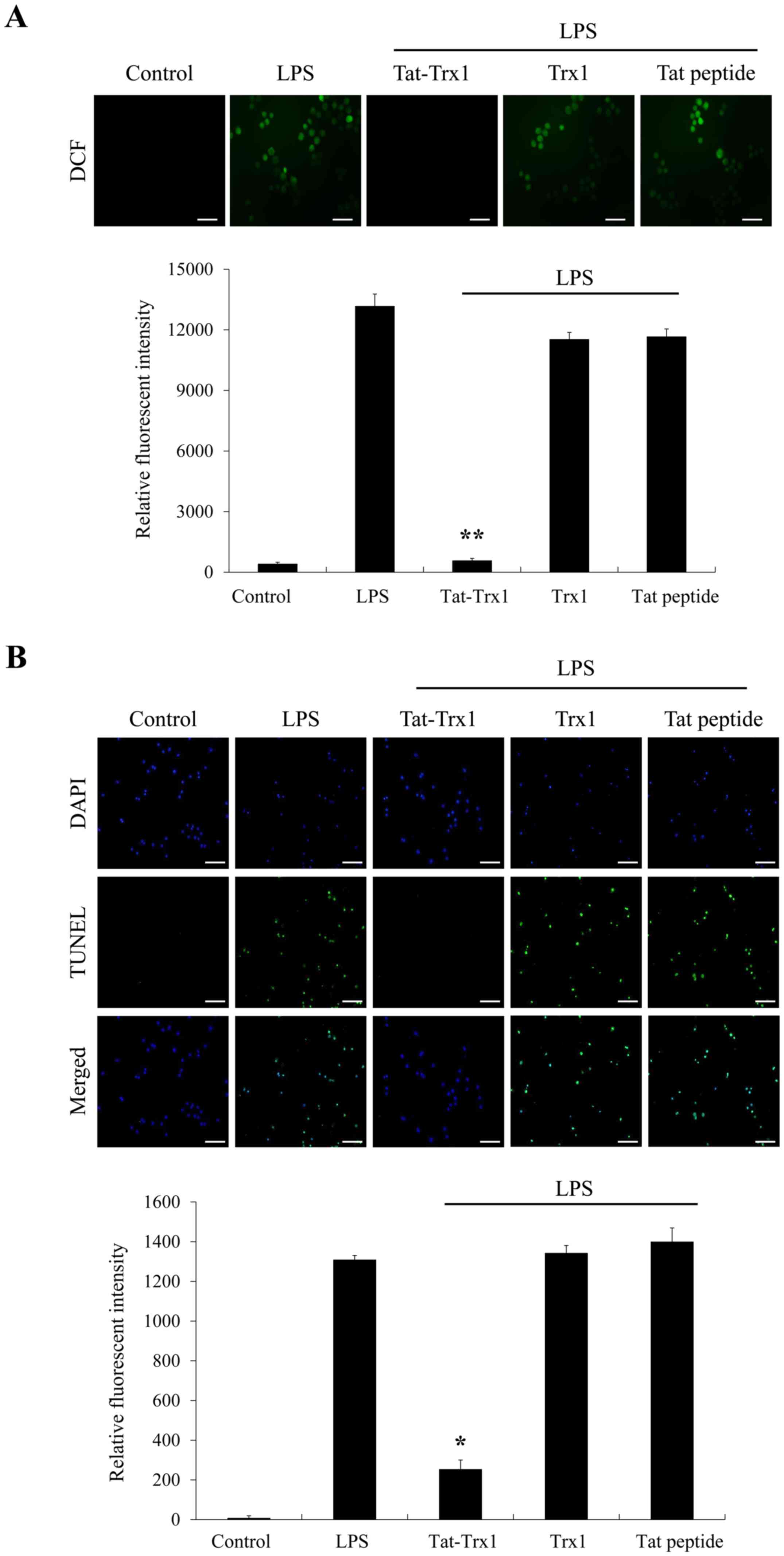
It is known that LPS can ROS production and DNA
fragmentation and lead to cell death (11,12).
Thus, we determined effects of Tat-Trx1 on ROS production and DNA
fragmentation. As shown in Fig. 2A
and B, ROS production and DNA
fragmentation levels were increased in Raw 264.7 cells treated with
LPS only. However, Tat-Trx1 markedly prevented such increases of
ROS production and DNA fragmentation, whereas ROS production and
DNA fragmentation levels showed no significant difference between
Trx1 and Tat peptide treated cells.
Tat-Trx1 inhibits LPS-induced
activation of MAPK and NF-κB signaling
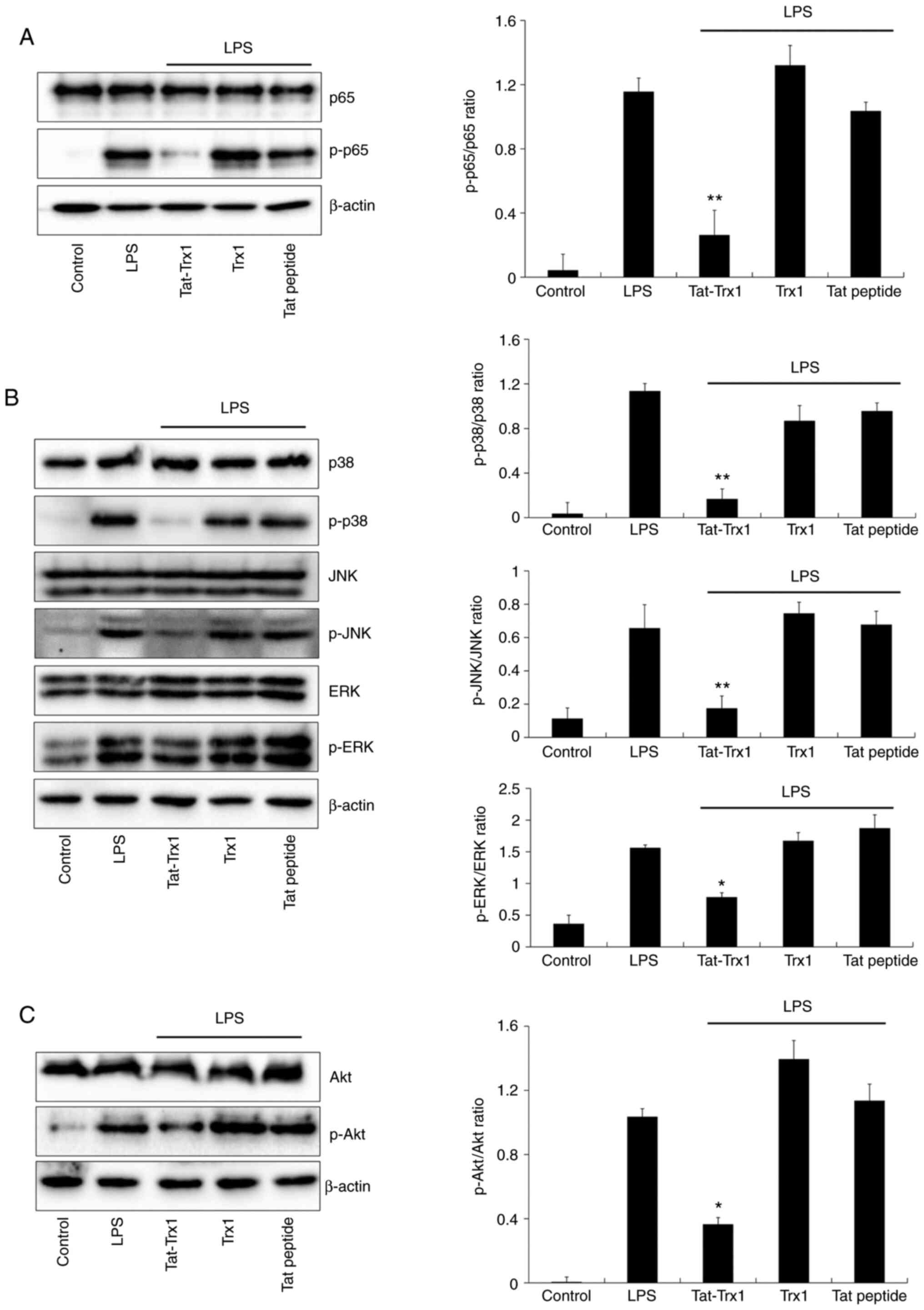
Suppressing MAPK (p38, JNK, and ERK) and NF-κB
activation is important for inhibiting inflammation in
LPS-activated Raw 264.7 cells (30-32).
Thus, we examined whether Tat-Trx1 could inhibit the activation of
MAPK and NF-κB in LPS-treated cells. As shown in Fig. 3A and B, NF-κB (p65) and MAPK expression levels
were markedly increased in LPS-exposed Raw 264.7 cells, whereas
Tat-Trx1 significantly reduced expression levels of p65 and MAPK in
LPS-exposed Raw 264.7 cells. The expression pattern of Akt after
LPS treatment was similar to that of MAPK (Fig. 3C). However, there was no significant
difference the expression of NF-κB, MAPK, and Akt in cells treated
with Trx1 or Tat peptide.
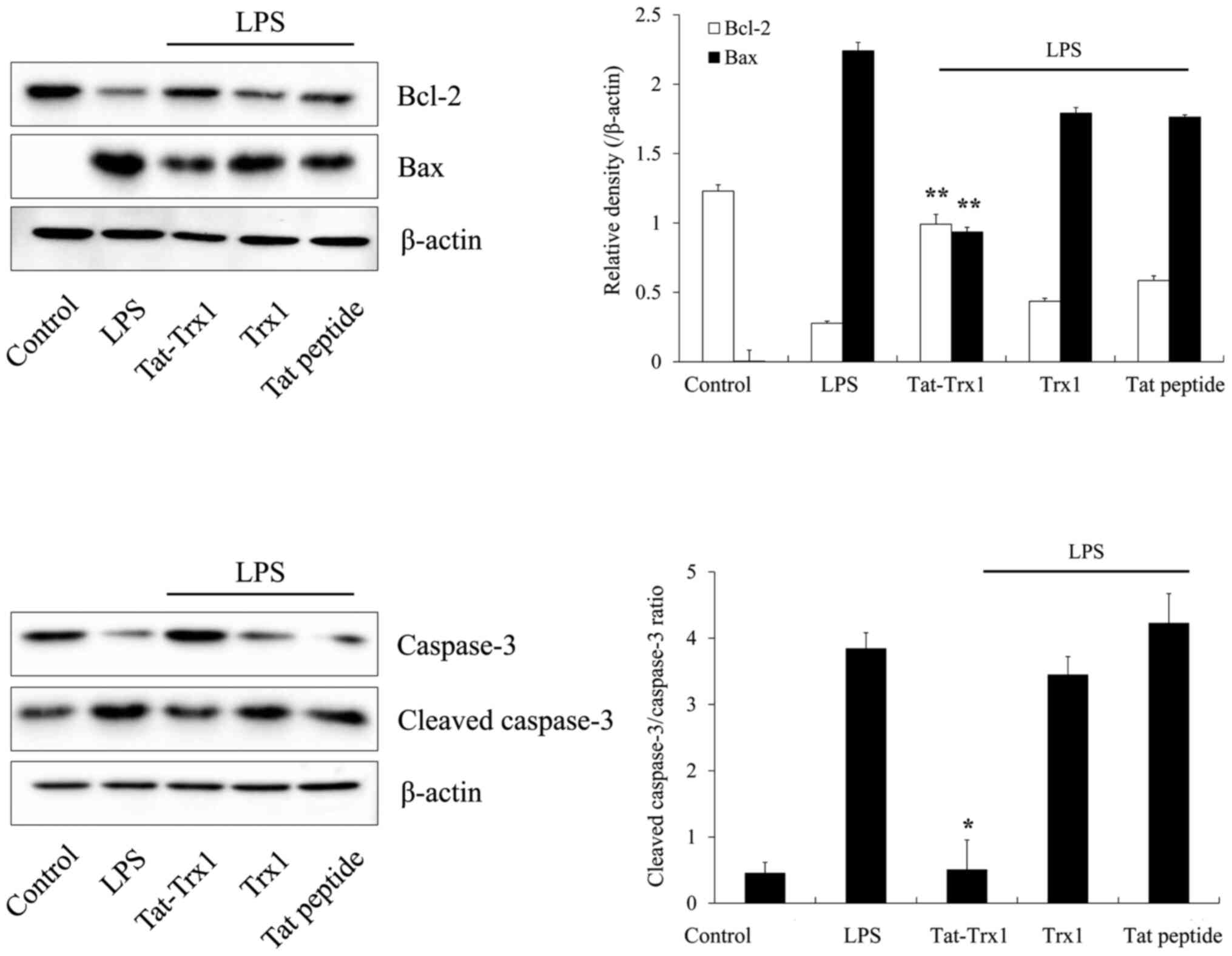
We also determined levels of Bcl-2, Bax, caspase-3,
and cleaved caspase-3 proteins. Bax and cleaved caspase-3 protein
expression levels were increased in LPS-treated Raw 264.7 cells,
whereas Tat-Trx1 reduced these protein expression levels in
LPS-treated cells. In contrast, Tat-Trx1 markedly increased Bcl-2
and caspase-3 protein expression levels in LPS-treated cells,
whereas there was no significant difference the expression of
Bcl-2, Bax, caspase-3, and cleaved caspase-3 proteins in cells
treated with Trx1 or Tat peptide (Fig.
4).
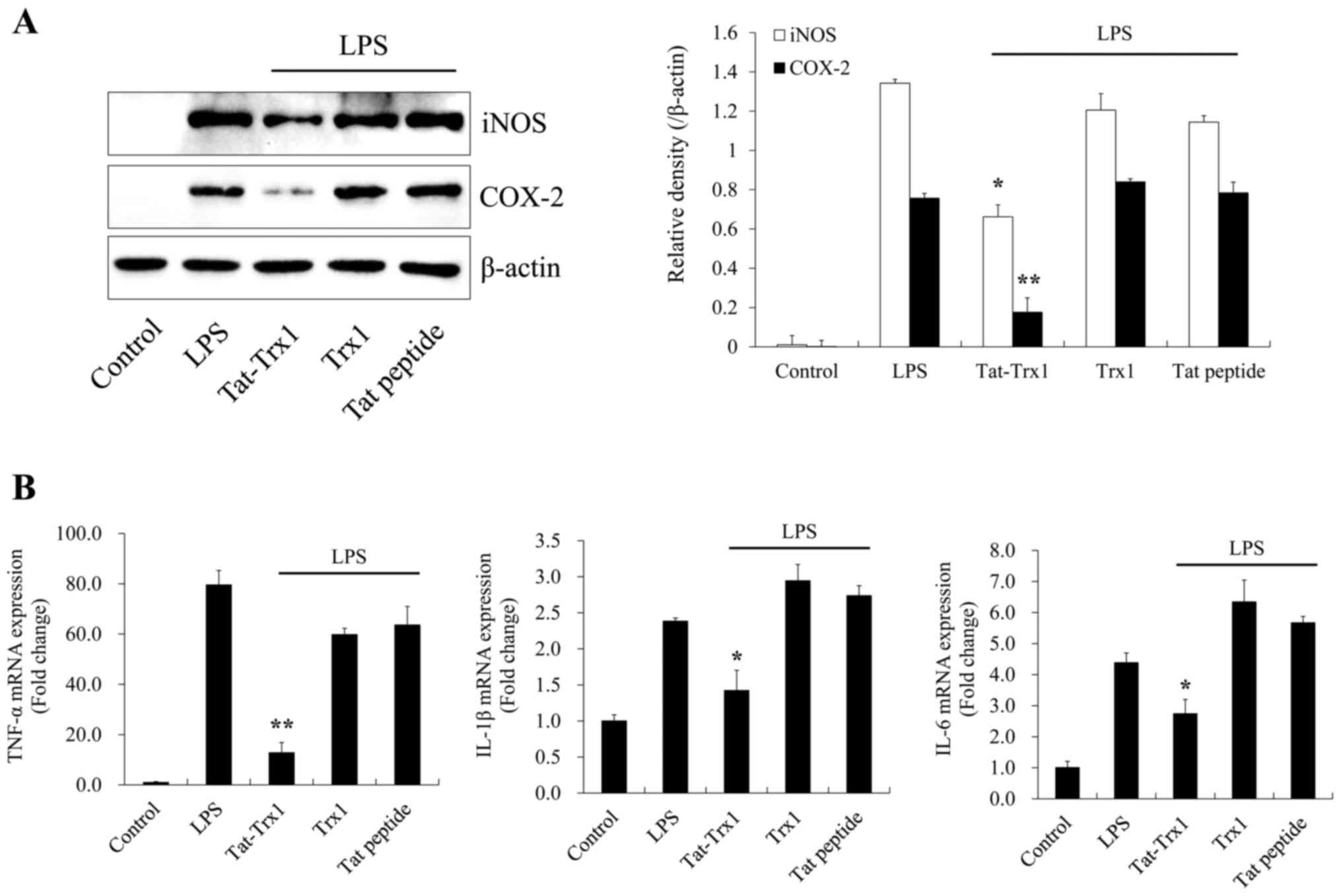
Tat-Trx1 inhibits LPS-induced
inflammation
We further investigated whether Tat-Trx1 inhibited
LPS-induced inflammatory responses in Raw 264.7 cells. As shown in
Fig. 5A and B, LPS drastically increased the expression
of pro-inflammatory mediator proteins (iNOS and COX-2) and cytokine
genes (IL-1β, IL-6, and TNF-α) in Raw 264.7 cells. In contrast,
Tat-Trx1 significantly reduced these inflammation factors in cells.
However, Trx1 or Tat peptide did not change the expression of
pro-inflammatory mediators or cytokines.
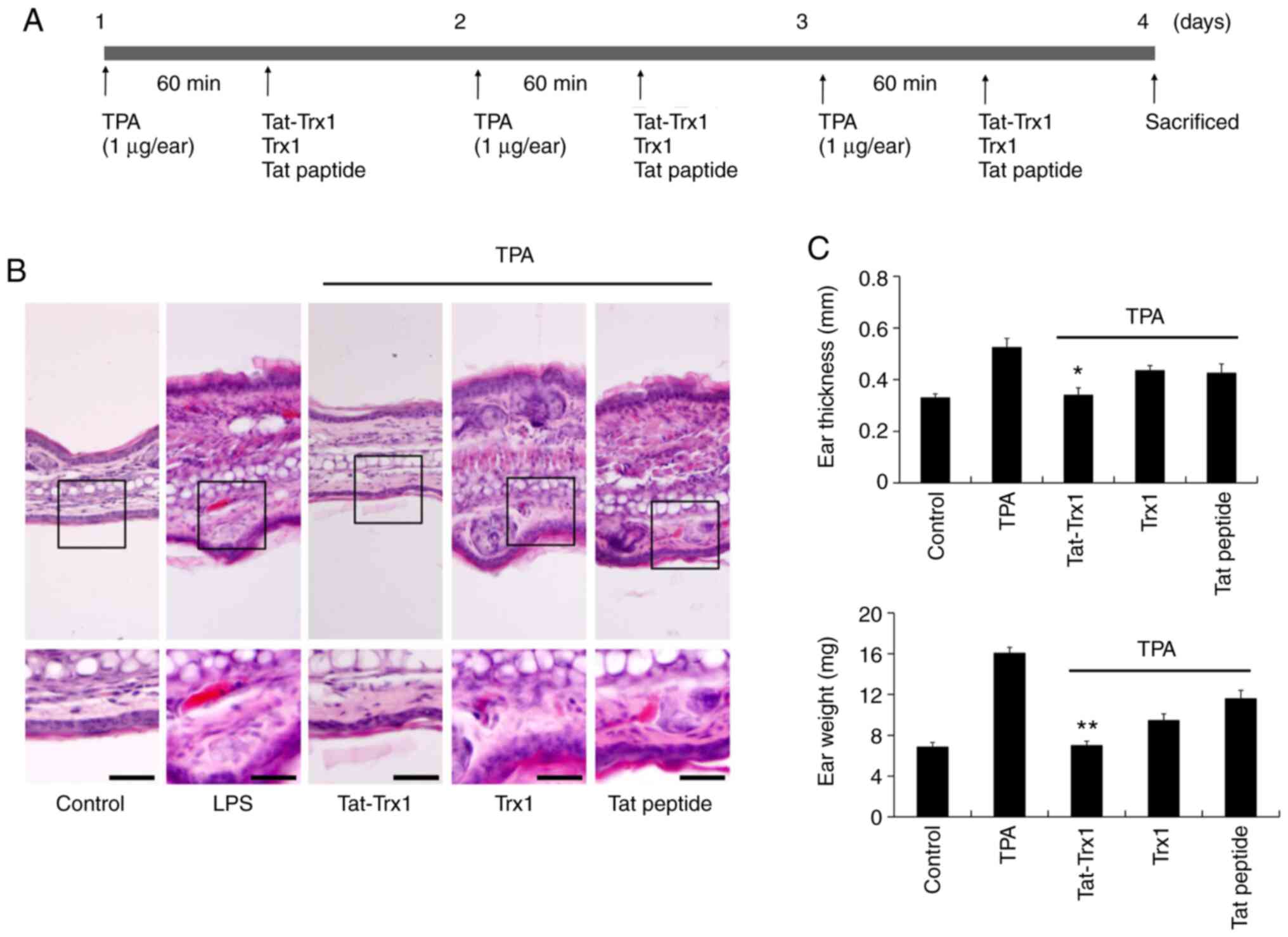
Effect of Tat-Trx1 in TPA-induced
animal model
TPA-induced ear edema animal model is generally used
for skin inflammation study. Thus, we examined the
anti-inflammatory effect of Tat-Trx1 in TPA-induced ear edema
animal model. We designed the experiment to induced mice ear edema
using TPA. As shown in Fig. 6A, TPA
(1 µg/ear) was topically applied to mouse ears 1 h prior to
Tat-Trx1 treatment and then evaluate the effect of Tat-Trx1 on
TPA-induced ear edema model. TPA markedly increased ear thickness
and weight of mouse showing skin inflammation. However, Tat-Trx1
significantly inhibited skin inflammation, ear thickness, and
weight (Fig. 6B and C).
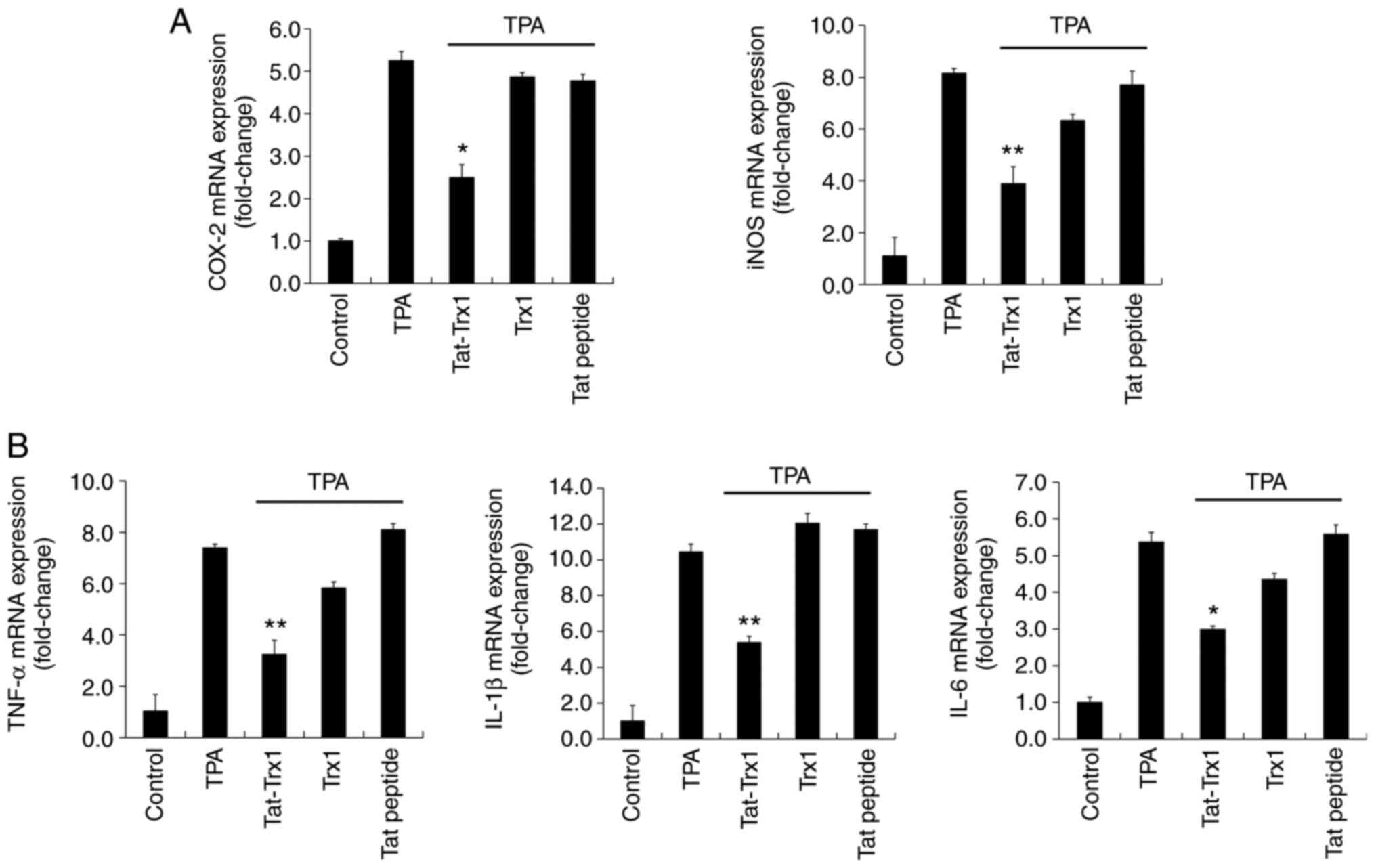
We also determined the effect of Tat-Trx1 in
TPA-exposed mouse model (Fig. 7).
In TPA treated mouse ears, iNOS, COX-2, IL-1β, IL-6, and TNF-α mRNA
expression levels were markedly increased. However, Tat-Trx1
significantly reduced these mRNA expression levels in the animal
model. However, iNOS, COX-2, IL-1β, IL-6, and TNF-α mRNA expression
levels showed no significant difference in Trx1 or Tat peptide
treated group compared to those in the TPA treated group.
Discussion
Inflammatory response plays an important role in the
defense against external stimuli including microbial pathogens and
chemicals. Also, inflammatory response can lead to various diseases
including neurodegenerative diseases (33-36).
It is well known that Trx1 is a redox regulating protein with a
critical function in cell growth, gene expression, and apoptosis
(37,38). In addition, Trx1 exerts beneficial
role in cell survival from oxidative stress due to its antioxidant
and anti-inflammatory effects (39-41).
Therefore, we investigated the anti-inflammatory effect of Trx1 in
macrophages and an animal model. Since Tat peptide can transduce
into cells cross the cellular membrane due to its potential for
membrane permeability with safety and nontoxic effect, it is widely
used in protein delivery into cells (42-44).
In this study, we fused a Tat peptide with human Trx1 gene to
produce a cell permeable Tat-Trx1 protein. Many reports have shown
a beneficial effect of therapeutic transducible Tat fused proteins
on cell survival both in vitro and in vivo (22,25,45-50).
LPS plays a crucial role in inducing inflammatory
responses, leading to various inflammatory diseases (51). LPS can also increase intracellular
ROS levels in macrophages. High levels of ROS can induce
inflammatory responses (43,44).
Previous studies have revealed that LPS can induce high levels of
ROS and that antioxidants play a beneficial role against
LPS-induced inflammation (52,53).
It has been reported that Trx1 can protect cells against damage
caused by oxidative stress in various diseases due to its
anti-oxidant function (21,54-56).
In the present study, cell permeable fusion protein Tat-Trx1
transduced into macrophages and drastically reduced LPS-induced ROS
generation and DNA damage, suggesting that Tat-Trx1 could play a
beneficial role in cell survival against LPS-induced damage.
Many reports have shown that LPS can induce NF-κB
activation and its upstream regulator, MAPK. MAPKs pathway can
regulate inflammatory responses. Activated p65 can lead to the
production of pro-inflammatory mediators and cytokines (14-17,57-61).
Thus, it is widely believed that NF-κB and MAPKs signaling is a
promising target for treating inflammatory diseases. Here, we
showed that Tat-Trx1 reduced NF-κB and MAPK activation caused by
LPS, indicating that Tat-Trx1 could inhibit inflammation by
regulating NF-κB and MAPKs signaling.
It is well described that TPA, a promotor of skin
tumorigenesis, can induce inflammatory symptom and cancer
development. In general, TPA-induced mouse model can be used to
understand the inflammatory mechanism (28,62-65).
Several studies have reported that TPA is a phorbol ester that
promotes a direct activation of protein kinase C pathway, which
results in elevated levels of prostaglandin E2 and
leukotriene B4 in applied to the skin. Also, TPA
promotes the activation of MAPK pathway that lead to the expression
of several important molecules for the inflammatory responses such
as adhesion molecules, chemokines, and inflammatory mediators
(66-69).
In addition, other studies have shown that production levels of
pro-inflammatory mediators and cytokines are markedly increased in
TPA-induced inflammation model (63,70,71).
He et al (72) have shown
that inflammatory responses in animal model can be quantified by
measuring ear weight and ear thickness after treatment with natural
compounds (Qi-Wei-Xiao-Yan-Tang: XYT). XYT can significantly
ameliorate inflammatory responses in an animal model, suggesting
that it can be used as a therapeutic agent (72). Topical application of various
anti-inflammatory therapeutic agents can also significantly inhibit
inflammation by suppressing inflammatory responses in a TPA-induced
mouse model (73-75).
Results of the present study revealed that Tat-Trx1
markedly reduced TPA-induced ear thickness, weight, and
inflammatory responses. Taken together, our results indicate that
Tat-Trx1 can significantly prevent inflammation in macrophages and
in an animal model by inhibiting the production of pro-inflammatory
mediators and cytokines as well as NF-κB and MAPK activation.
Therefore, Tat-Trx1 can be used as a therapeutic agent for treating
diseases induced by inflammatory damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by Basic Science Research
Program (grant no. 2019R1A6A1A11036849) through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Education.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EJY, MJS, WSE and SYC were involved in the
conceptualization of the study. HJY, YJC, EJS, LRL and HJC
performed the experiments, and acquired and analyzed the data. HJK,
YHY, DWK and DSK produced the methodology of the animal model and
concurrently conducted animal studies. SHL, SL, JP and KHH were
responsible for data analysis and interpretation, and participated
in the drafting of the manuscript. EJY and SYC confirmed the
authenticity of all the raw data. WSE, MJS and SYC were involved in
the writing and editing of the manuscript and provided final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The care of animals conformed to the Guide for the
Care and Use of Laboratory Animals of the National Veterinary
Research and Quarantine Service of Korea and the present study was
approved by the Institutional Animal Care and Use Committee of
Soonchunhyang University Cheonan, Chungcheongnam, Republic of Korea
(approval no. SCH 15-0002-3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Nathan C and Ding A: Nonresolving
inflammation. Cell. 140:871–882. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Straub RH and Schradin C: Chronic
inflammatory systemic diseases: An evolutionary trade-off between
acutely beneficial but chronically harmful programs. Evol Med
Public Health. 2016:37–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Corriveau CC and Danner RL: Endotoxin as a
therapeutic target in septic shock. Infect Agents Dis. 2:35–43.
1993.PubMed/NCBI
|
|
7
|
Won AN, Kim SA, Ahn JY, Han JH, Kim CH,
Lee JH and Kim DI: HO-1 induction by Selaginella tamariscina
extract inhibits inflammatory response in
lipopolysacchraride-stimulated RAW 264.7 macrophages. Evid
Complement Alternat Med. 2018(7816923)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: Roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duffield JS: The inflammatory macrophage:
A story of Jekyll and Hyde. Clin Sci (Lond). 104:27–38.
2003.PubMed/NCBI
|
|
10
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luedde T and Schwabe RF: NF-κB in the
liver - linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee IT, Shih RH, Lin CC, Chen JT and Yang
CM: Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1
expression induced by lipopolysaccharide in human renal mesangial
cells. Cell Commun Signal. 10(33)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim HJ, Lee HS, Chong YH and Kang JL: p38
Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB
activation in the development of lung injury and RAW 264.7
macrophages. Toxicology. 225:36–47. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zucoloto AZ, Manchope MF,
Staurengo-Ferrari L, Pinho-Ribeiro FA, Zarpelon AC, Saraiva ALL,
Cecílio NT, Alves-Filho JC, Cunha TM, Menezes GB, et al: Probucol
attenuates lipopolysaccharide-induced leukocyte recruitment and
inflammatory hyperalgesia: Effect on NF-кB activation and cytokine
production. Eur J Pharmacol. 809:52–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scherle PA, Jones EA, Favata MF, Daulerio
AJ, Covington MB, Nurnberg SA, Magolda RL and Trzaskos JM:
Inhibition of MAP kinase kinase prevents cytokine and prostaglandin
E2 production in lipopolysaccharide-stimulated
monocytes. J Immunol. 161:5681–5686. 1998.PubMed/NCBI
|
|
18
|
Loftis JM, Choi D, Hoffman W and Huckans
MS: Methamphetamine causes persistent immune dysregulation: A
cross-species, translational report. Neurotox Res. 20:59–68.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bai J, Nakamura H, Kwon YW, Tanito M, Ueda
S, Tanaka T, Hattori I, Ban S, Momoi T, Kitao Y, et al: Does
thioredoxin-1 prevent mitochondria- and endoplasmic
reticulum-mediated neurotoxicity of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine? Antioxid Redox
Signal. 9:603–608. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu XL, Li X, Li Y, Kong LP, Fang JL, Zhou
XS, Li M, Jia JJ and Bai J: The overexpression of Thioredoxin-1
suppressing inflammation induced by methamphetamine in spleen. Drug
Alcohol Depend. 159:66–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen G, Li X, Huang M, Li M, Zhou X, Li Y
and Bai J: Thioredoxin-1 increases survival in sepsis by
inflammatory response through suppressing endoplasmic reticulum
stress. Shock. 46:67–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yeo HJ, Shin MJ, You JH, Kim JS, Kim MY,
Kim DW, Kim DS, Eum WS and Choi SY: Transduced Tat-CIAPIN1 reduces
the inflammatory response on LPS- and TPA-induced damages. BMB Rep.
52:695–699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Youn GS, Park JK, Lee CY, Jang JH, Yun SH,
Kwon HY, Choi SY and Park J: MicroRNA-22 negatively regulates
LPS-induced inflammatory responses by targeting HDAC6 in
macrophages. BMB Rep. 53:223–228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shin MJ, Kim DW, Choi YJ, Cha HJ, Lee SH,
Park J, Han KH, Eum WS and Choi SY: PEP-1-GLRX1 protein exhibits
anti-inflammatory effects by inhibiting the activation of MAPK and
NF-κB pathways in Raw 264.7 cells. BMB Rep. 53:106–111.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shin MJ, Kim DW, Lee YP, Ahn EH, Jo HS,
Kim DS, Kwon OS, Kang TC, Cho YJ, Park J, et al: Tat-glyoxalase
protein inhibits against ischemic neuronal cell damage and
ameliorates ischemic injury. Free Radic Biol Med. 67:195–210.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim SJ, Shin MJ, Kim DW, Yeo HJ, Yeo EJ,
Choi YJ, Sohn EJ, Han KH, Park J, Lee KW, et al: Tat-biliverdin
reductase A exerts a protective role in oxidative stress-induced
hippocampal neuronal cell damage by regulating the apoptosis and
MAPK signaling. Int J Mol Sci. 21(2672)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Stanley PL, Steiner S, Havens M and
Tramposch KM: Mouse skin inflammation induced by multiple topical
applications of 12-O-tetradecanoylphorbol-13-acetate. Skin
Pharmacol. 4:262–271. 1991.PubMed/NCBI View Article : Google Scholar
|
|
29
|
American Veterinary Medical Association:
AVMA Guidelines for the Euthanasia of Animals: 2020 Edition.
https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
Accessed March 2020.
|
|
30
|
Shou J, Kong X, Wang X, Tang Y, Wang C,
Wang M, Zhang L, Liu Y, Fei C, Xue F, et al: Tizoxanide inhibits
inflammation in LPS-activated RAW264.7 macrophages via the
suppression of NF-κB and MAPK activation. Inflammation.
42:1336–1349. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barton GM and Medzhitov R: Toll-like
receptor signaling pathways. Science. 300:1524–1525.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Langford MP, McGee DJ, Ta KH, Redens TB
and Texada DE: Multiple caspases mediate acute renal cell apoptosis
induced by bacterial cell wall components. Ren Fail. 33:192–206.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tsaryk R, Peters K, Barth S, Unger RE,
Scharnweber D and Kirkpatrick CJ: The role of oxidative stress in
pro-inflammatory activation of human endothelial cells on Ti6Al4V
alloy. Biomaterials. 34:8075–8085. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Booze ML, Hansen JM and Vitiello PF: A
novel mouse model for the identification of thioredoxin-1 protein
interactions. Free Radic Biol Med. 99:533–543. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Susanti D, Wong JH, Vensel WH, Loganathan
U, DeSantis R, Schmitz RA, Balsera M, Buchanan BB and Mukhopadhyay
B: Thioredoxin targets fundamental processes in a methane-producing
archaeon, Methanocaldococcus jannaschii. Proc Natl Acad Sci
USA. 111:2608–2613. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nadeau PJ, Charette SJ, Toledano MB and
Landry J: Disulfide Bond-mediated multimerization of Ask1 and its
reduction by thioredoxin-1 regulate H(2)O(2)-induced c-Jun
NH(2)-terminal kinase activation and apoptosis. Mol Biol Cell.
18:3903–3913. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ueda S, Masutani H, Nakamura H, Tanaka T,
Ueno M and Yodoi J: Redox control of cell death. Antioxid Redox
Signal. 4:405–414. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Couchie D, Vaisman B, Abderrazak A,
Mahmood DFD, Hamza MM, Canesi F, Diderot V, El Hadri K,
Nègre-Salvayre A, Le Page A, et al: Human plasma thioredoxin-80
increases with age and in ApoE-/- mice induces
inflammation, angiogenesis, and atherosclerosis. Circulation.
136:464–475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cerrato CP, Pirisinu M, Vlachos EN and
Langel Ü: Novel cell-penetrating peptide targeting mitochondria.
FASEB J. 29:4589–4599. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Moon JI, Han MJ, Yu SH, Lee EH, Kim SM,
Han K, Park CH and Kim CH: Enhanced delivery of protein fused to
cell penetrating peptides to mammalian cells. BMB Rep. 52:324–329.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Q, Hao X, Zaidi SSA, Guo J, Ren X, Shi
C, Zhang W and Feng Y: Oligohistidine and targeting peptide
functionalized TAT-NLS for enhancing cellular uptake and promoting
angiogenesis in vivo. J Nanobiotechnology. 16(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu H, You C, Chen F, Jiao J, Gao Z, An P,
Sun B and Chen R: Enhanced cellular uptake of near-infrared
triggered targeted nanoparticles by cell-penetrating peptide TAT
for combined chemo/photothermal/photodynamic therapy. Mater Sci Eng
C. 103(109738)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yue LH, Zhao YL, Chen J and Lu DR: Effect
of fusion protein TAT and heme oxygenase-1 on liver sinusoidal
endothelial cells apoptosis during preservation injury. Chin Med J
(Engl). 123:68–73. 2010.PubMed/NCBI
|
|
47
|
Kubo E, Fatma N, Akagi Y, Beier DR, Singh
SP and Singh DP: TAT-mediated PRDX6 protein transduction protects
against eye lens epithelial cell death and delays lens opacity. Am
J Physiol Cell Physiol. 294:C842–C855. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim HR, Kim DW, Jo HS, Cho SB, Park JH,
Lee CH, Choi YJ, Yeo EJ, Park SY, Kim ST, et al: Tat-biliverdin
reductase A inhibits inflammatory response by regulation of MAPK
and NF-κB pathways in Raw 264.7 cells and edema mouse model. Mol
Immunol. 63:355–366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yeo HJ, Shin MJ, Yeo EJ, Choi YJ, Kim DW,
Kim DS, Eum WS and Choi SY: Tat-CIAPIN1 inhibits hippocampal
neuronal cell damage through the MAPK and apoptotic signaling
pathways. Free Radic Biol Med. 135:68–78. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Eum WS, Shin MJ, Lee CH, Yeo HJ, Yeo EJ,
Choi YJ, Kwon HJ, Kim DS, Kwon OS, Lee KW, et al: Neuroprotective
effects of Tat-ATOX1 protein against MPP+-induced
SH-SY5Y cell deaths and in MPTP-induced mouse model of Parkinson's
disease. Biochimie. 156:158–168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820.
2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kim SM, Ha JS, Han AR, Cho SW and Yang SJ:
Effects of α-lipoic acid on LPS-induced neuroinflammation and NLRP3
inflammasome activation through the regulation of BV-2 microglial
cells activation. BMB Rep. 52:613–618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Nishio K, Horie M, Akazawa Y, Shichiri M,
Iwahashi H, Hagihara Y, Yoshida Y and Niki E: Attenuation of
lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and
tocotrienols. Redox Biol. 1:97–103. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang M, Zhu K, Zhang L, Li L and Zhao J:
Thioredoxin 1 protects astrocytes from oxidative stress by
maintaining peroxiredoxin activity. Mol Med Rep. 13:2864–2870.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Stosic-Grujicic S, Stojanovic I,
Maksimovic-Ivanic D, Momcilovic M, Popadic D, Harhaji L, Miljkovic
D, Metz C, Mangano K, Papaccio G, et al: Macrophage migration
inhibitory factor (MIF) is necessary for progression of autoimmune
diabetes mellitus. J Cell Physiol. 215:665–675. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yamamoto M, Yamato E, Toyoda S, Tashiro F,
Ikegami H, Yodoi J and Miyazaki J: Transgenic expression of
antioxidant protein thioredoxin in pancreatic beta cells prevents
progression of type 2 diabetes mellitus. Antioxid Redox Signal.
10:43–49. 2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sharif O, Bolshakov VN, Raines S, Newham P
and Perkins ND: Transcriptional profiling of the LPS induced
NF-kappaB response in macrophages. BMC Immunol. 8(1)2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684.
2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Saccani S, Pantano S and Natoli G:
p38-Dependent marking of inflammatory genes for increased NF-kappa
B recruitment. Nat Immunol. 3:69–75. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
60
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu HT, Du YG, He JL, Chen WJ, Li WM, Yang
Z, Wang YX and Yu C: Tetramethylpyrazine inhibits production of
nitric oxide and inducible nitric oxide synthase in
lipopolysaccharide-induced N9 microglial cells through blockade of
MAPK and PI3K/Akt signaling pathways, and suppression of
intracellular reactive oxygen species. J Ethnopharmacol.
129:335–343. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bald T, Landsberg J, Jansen P, Gaffal E
and Tüting T: Phorbol ester-induced neutrophilic inflammatory
responses selectively promote metastatic spread of melanoma in a
TLR4-dependent manner. OncoImmunology. 5(e1078964)2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kuo DH, Lai YS, Lo CY, Cheng AC, Wu H and
Pan MH: Inhibitory effect of magnolol on TPA-induced skin
inflammation and tumor promotion in mice. J Agric Food Chem.
58:5777–5783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kim MJ, Kim DW, Park JH, Kim SJ, Lee CH,
Yong JI, Ryu EJ, Cho SB, Yeo HJ, Hyeon J, et al: PEP-1-SIRT2
inhibits inflammatory response and oxidative stress-induced cell
death via expression of antioxidant enzymes in murine macrophages.
Free Radic Biol Med. 63:432–445. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sun J, Zhao Y, Jin H and Hu J: Curcumin
relieves TPA-induced Th1 inflammation in K14-VEGF transgenic mice.
Int Immunopharmacol. 25:235–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW,
Lee AY and Kim HK: Anti-inflammatory effects of Asparagus
cochinchinensis extract in acute and chronic cutaneous
inflammation. J Ethnopharmacol. 121:28–34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Murakawa M, Yamaoka K, Tanaka Y and Fukuda
Y: Involvement of tumor necrosis factor (TNF)-alpha in phorbol
ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema
in mice. Biochem Pharmacol. 71:1331–1336. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Song HY, Lee JA, Ju SM, Yoo KY, Won MH,
Kwon HJ, Eum WS, Jang SH, Choi SY and Park J: Topical transduction
of superoxide dismutase mediated by HIV-1 Tat protein transduction
domain ameliorates 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced inflammation in mice. Biochem Pharmacol.
75:1348–1357. 2008.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Xian YF, Hu Z, Ip SP, Chen JN, Su ZR, Lai
XP and Lin ZX: Comparison of the anti-inflammatory effects of
Sinapis alba and Brassica juncea in mouse models of
inflammation. Phytomedicine. 50:196–204. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kundu JK, Shin YK and Surh YJ: Resveratrol
modulates phorbol ester-induced pro-inflammatory signal
transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as
prime targets. Biochem Pharmacol. 72:1506–1515. 2006.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Xian YF, Mao QQ, Ip SP, Lin ZX and Che CT:
Comparison on the anti-inflammatory effect of Cortex
Phellodendri Chinensis and Cortex Phellodendri
Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear
edema in mice. J Ethnopharmacol. 137:1425–1430. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
He XY, Liu QC, Peng W, Huang YL and Wu CJ:
Bioactivities and serum pharmacochemistry of
Qi-Wei-Xiao-Yan-Tang. Pharm Biol. 51:629–634.
2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chang SN, Khan I, Dey DK, Cho KH, Hwang
BS, Bae KB, Kang SC and Park JG: Decursinol angelate ameliorates
12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced NF-κB
activation on mice ears by inhibiting exaggerated inflammatory cell
infiltration, oxidative stress and pro-inflammatory cytokine
production. Food Chem Toxicol. 132(110699)2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Xu XT, Mou XQ, Xi QM, Liu WT, Liu WF,
Sheng ZJ, Zheng X, Zhang K, Du ZY, Zhao SQ, et al:
Anti-inflammatory activity effect of
2-substituted-1,4,5,6-tetrahydrocyclopenta[b]pyrrole on
TPA-induced skin inflammation in mice. Bioorg Med Chem Lett.
26:5334–5339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wu JY, Chen YJ, Bai L, Liu YX, Fu XQ, Zhu
PL, Li JK, Chou JY, Yin CL, Wang YP, et al: Chrysoeriol ameliorates
TPA-induced acute skin inflammation in mice and inhibits NF-κB and
STAT3 pathways. Phytomedicine. 68(153173)2020.PubMed/NCBI View Article : Google Scholar
|