Introduction
Sepsis is an uncontrolled severe inflammatory
response, and a common complication in patients in the ICU
(1). According to statistics, the
number of severe sepsis-related deaths each year is approximately
3.7 million, and the current mortality rate of sepsis in numerous
countries remains high (2). Despite
significant advancements in the diagnosis and treatment of sepsis
over the past 30 years, sepsis remains the leading cause of death
among patients, with a 30-day mortality rate of 47% (3,4). Acute
organ dysfunction complicated by sepsis is termed sepsis-induced
cardiomyopathy (SIC), and sepsis is aggravated once cardiac
functions are impaired (5).
Approximately 40% of patients with sepsis suffer from myocardial
dysfunction, among which myocardial depression is a serious
clinical symptom with a mortality of 70 to 90%, while the mortality
for sepsis patients without cardiac dysfunction is 20% (6,7).
Devoid of specific clinical symptoms in the early stage, early SIC
is hard to be diagnosed (8). Thus,
the identification of biomarkers involved in the occurrence and
development of SIC is of great significance for the early diagnosis
and prognosis evaluation of SIC.
micro-RNAs (miRNAs or miRs), non-coding RNAs with 21
to 23 nucleotides, not only regulate gene expression, but are also
involved in numerous biological processes such as embryonic
development, organogenesis, and human inflammatory diseases
(9). Most of the studies on sepsis
have centered on the screening of serum miRNA profiles in sepsis
patients, and have identified several miRNAs (including miR-155,
miR-223 and miR-146a) as potential diagnostic markers for severe
sepsis (10,11). miR-132 is an endogenous small RNA
that controls the post transcriptional regulation of gene
expression through controlled degradation or transcriptional
inhibition of mRNA, which can affect the pathogenesis of numerous
diseases (12). miR-223 is widely
involved in the regulation of cellular processes and numerous types
of pathological processes, such as cancer, autoimmune and
inflammatory diseases (13). In a
study by Li et al (14),
hesperidin could alleviate lipopolysaccharide-induced
neuroinflammation in mice by promoting miR-132 expression. In a
previous study (15), serum
miR-146a and miR-223 were markedly reduced in patients with sepsis,
indicating the two can function as new markers for the diagnosis of
sepsis. However, the roles of miR-132 and miR-223 in SIC are not
clear. The myocardial toxicity induced by sepsis is mainly related
to the reduction of cardiac energy, impaired myocardial
contractility, and the process of inflammatory cytokines. The
treatment of sepsis mainly includes optimizing myocardial function
and improving inflammatory response (16). Therefore, markers of myocardial
injury and inflammatory response are essential for detecting the
occurrence and development of SIC.
The present research determined the serum expression
profiles of miR-132 and miR-223 in SIC patients, analyzed the value
of combined serum miR-132 and miR-223 in the early diagnosis and
prognosis prediction of SIC, and investigated the correlation of
miR-132 and miR-223 with myocardial injury and inflammatory
response markers.
Materials and methods
General data
A total of 80 SIC patients admitted to Tianjin
Medical University General Hospital (Tianjin, China) were enrolled
in the research group (RG), including 45 males and 35 females, aged
from 24 to 81 years, with an average age of 58.6±11.3 years. Sixty
healthy participants receiving physical examinations were enrolled
in the control group (CG), including 31 males and 29 females, aged
from 19 to 79 years, with an average age of 56.2±9.6 years. The
guardians of the participants or the patients themselves had full
knowledge of this research and signed the informed consent form and
had complete clinical data. With no violation of ethics and
morality, this research was carried out after obtaining approval
from the Ethics Committee of Tianjin Medical University General
Hospital.
Inclusion and exclusion criteria
The inclusion criteria was as follows: Patients
diagnosed with sepsis according to the diagnostic criteria issued
by the American College of Chest Physicians/Society of Critical
Care Medicine (ACCP/SCCM) (17);
patients aged from 19 to 81 years; patients with complete clinical
data; patients with a white blood cell (WBC) count of over
12x109/l, a heart index of over 3.5 l/(min ·
m2), a systolic blood pressure of <90 mmHg, a mixed
venous oxygen saturation of over 70%, and a mean arterial pressure
of <70 mmHg according to the laboratory tests. The exclusion
criteria were as follows: Patients with a history of cardiac
surgery; patients with autoimmune diseases, congenital heart
disease, acute pulmonary embolism, cerebrovascular disease,
myocarditis, coronary heart disease, acute pulmonary heart disease,
mental illness, acute coronary syndrome, malignant tumors,
congestive heart failure, liver and kidney dysfunction; patients
with an exposure to other myocardial toxic substances such as
doxorubicin, drugs, and alcohol. The aforementioned inclusion and
exclusion criteria were applied to the RG. Participants included in
the CG were healthy individuals.
Detection methods
Elbow venous blood (5 ml) was obtained from
participants within 24 h after admission and placed in a vacuum
blood collection tube. After centrifugation at 1,500 x g and 4˚C
for 15 min, the serum was stored in an EP at -80˚C. Serum
expression profiles of creatine kinase isoenzyme (CK-MB) (cat. no.
D711191), cardiac troponin I (cTnI) (cat. no. D711127), tumor
necrosis factor-α (TNF-α) (cat. no. D721026) and interleukin-6
(IL-6) (cat. no. D711013) were determined by enzyme-linked
immunosorbent assay (ELISA) (18).
Human TNF-α, IL-6, and ELISA kits were manufactured by Sangon
Biotech (Shanghai) Co., Ltd., China and were used according to the
manufacturer's instructions. The optical density (OD) of each well
was measured with a SpectraMax M multi-mode microplate reader
(Shanghai Molecular Devices Co., Ltd.), and the TNF-α and IL-6
levels were determined.
Reverse transcription-quantitative
(RT-q)PCR detection
Total RNA extraction was performed using an TRIzol
kit (cat. no. 10296010; Invitrogen; Thermo Fisher Scientific, Inc.)
in line with the instructions of the manufacturer. The
concentration and purity of RNA were determined by DR6000 UV-Vis
spectrophotometer (Shanghai Hach Water Analysis Instrument Co.,
Ltd.). RNA was reversely transcribed into cDNA according to the
instructions of the PrimeScript RT kit (cat. no. RR047Q; Takara
Bio, Inc.) and then stored at -20˚C. U6 was used as the internal
reference gene. Primer sequences are presented in Table I. The primers were designed and
synthesized by Thermo Fisher Scientific, Inc. The RT-qPCR reaction
was performed on an ABI PRISM 7500 fluorescence quantitative PCR
instrument using SYBR Green Master Mix (cat. no. A46113; Thermo
Fisher Scientific, Inc.). Conditions for the amplification were as
follows: 95˚C for 15 sec, proceeding with 40 cycles at 60˚C for 30
sec, and 72˚C for 30 sec. Amplification data analysis was performed
according to SDS 2.0.1 (Thermo Fisher Scientific Co., Ltd.,
Shanghai, China). The results were expressed using
2-ΔΔCq (19).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|
| miR-132 |
5'-TGGATCCCCCCCAGTCCCCGTCCCTCAG-3' |
5'-TGAATTCGGATACCTTGGCCGGGAGGAC-3' |
| miR-223 |
5'-CAGAAAGCCCAATTCCATCT-3' |
5'-GGGCAAATGGATACCATACC-3' |
| U6 |
5'-CTCGCTTCGGCAGCACA-3' |
5'-AACGCTTCACGAATTTGCGT-3' |
Statistical analysis
Statistical analysis was performed using SPSS 22.0
(IBM Corp.) and data visualization using GraphPad Prism 8 (GraphPad
Software, Inc.). The counting data were represented by [n (%)] and
their intergroup comparison was analyzed by the Chi-square test.
The measurement data were represented by the mean ± standard
deviation and their intergroup comparison was analyzed by the
independent t-test. The value of miR-132 and miR-223 single or
combined detection in SIC diagnosis was analyzed by the logistic
regression equation and the receiver operating characteristic (ROC)
curve. The correlation of serum miR-132 and miR-223 with CK-MB,
cTnI, TNF-α, ad IL-6 was determined by the Pearson correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
General data of the RG and CG
groups
No marked significance was observed between the RG
and the CG with regard to sex ratio, body mass index (BMI), age,
smoking, drinking, place of residence, educational level, alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
high-density lipoprotein cholesterol (HDL-C), and low-density
lipoprotein cholesterol (LDL-C) (P>0.05; Table II).
 | Table IIGeneral information of the RG and CG
[n(%)]/(mean ± SD). |
Table II
General information of the RG and CG
[n(%)]/(mean ± SD).
| Factor | RG (n=80) | CG (n=60) | t/χ2 | P-value |
|---|
| Sex | | | 0.290 | 0.590 |
|
Male | 45 (56.25) | 31 (51.67) | | |
|
Female | 35 (43.75) | 29 (48.33) | | |
| BMI
(kg/m2) | 22.68±2.05 | 22.76±2.16 | 0.223 | 0.824 |
| Age (years) | 58.6±11.3 | 56.2±9.6 | 1.325 | 0.187 |
| Smoking | | | 0.364 | 0.546 |
|
Yes | 32 (40.00) | 21 (35.00) | | |
|
No | 48 (60.00) | 39 (65.00) | | |
| Drinking | | | 0.713 | 0.399 |
|
Yes | 35 (43.75) | 22 (36.67) | | |
|
No | 45 (56.25) | 38 (63.33) | | |
| Place of
residence | | | 0.182 | 0.669 |
|
Urban
area | 63 (78.75) | 49 (81.67) | | |
|
Rural
area | 17 (21.25) | 11 (18.33) | | |
| Educational
level | | | 4.796 | 0.187 |
|
Primary
school | 3 (3.75) | 2 (3.33) | | |
|
Middle
school | 9 (11.25) | 15 (25.00) | | |
|
College | 38 (47.50) | 22 (36.67) | | |
|
University | 30 (37.50) | 21 (35.00) | | |
| ALT (U/l) | 24.53±11.05 | 23.05±10.96 | 0.787 | 0.433 |
| AST (U/l) | 26.14±12.78 | 24.63±13.52 | 0.675 | 0.501 |
| HDL-C (mmol/l) | 1.58±0.25 | 1.64±0.31 | 1.267 | 0.207 |
| LDL-C (mmol/l) | 2.31±0.57 | 2.25±0.36 | 0.715 | 0.476 |
| Hospitalization
time (days) | 25.8±16.7 | - | - | - |
| Primary infection
site | | | - | - |
|
Abdominal
cavity | 45 (56.25) | - | | |
|
Respiratory
system | 29 (36.25) | - | | |
|
Blood
system | 1 (1.25) | - | | |
|
Skin and
soft tissue | 5 (6.25) | - | | |
Expression of serum miR-132 and
miR-223 in RG and CG and their diagnostic value for SIC
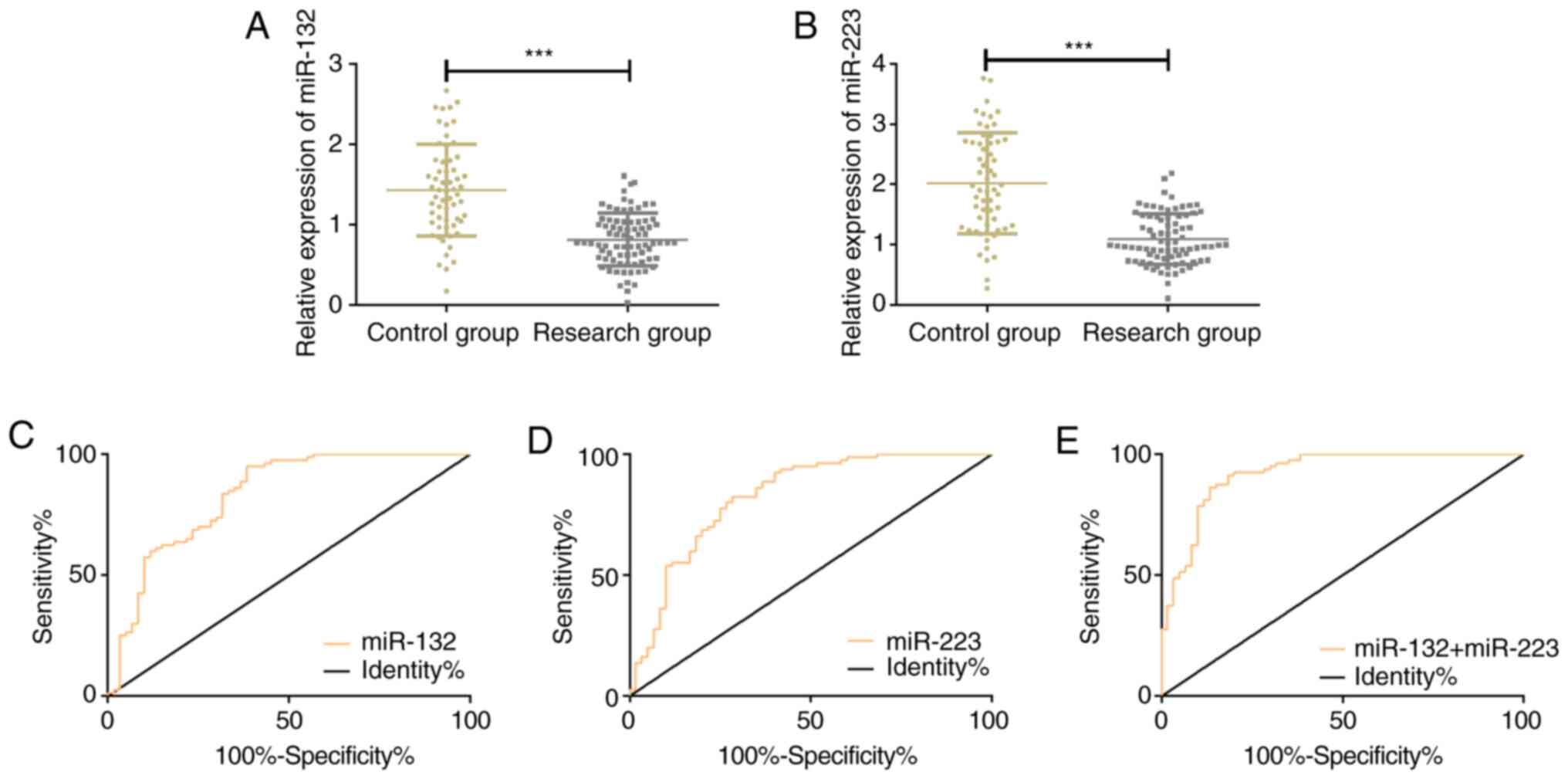
The relative levels of serum miR-132 and miR-223 in
the CG and the RG were (1.43±0.57), (0.82±0.33) and (2.02±0.83),
(1.09±0.42) respectively. The relative expression of miR-132 and
miR-223 in the RG was significantly lower than that in the CG
(P<0.001). According to the ROC curves demonstrating the
diagnosis of SIC by serum miR-132 and miR-223, the AUC of SIC
diagnosis of miR-132 and miR-223 was 0.827 and 0.831, respectively;
the sensitivity of miR-132 and miR-223 was 82.50 and 95.00%,
respectively; the specificity of miR-132 and miR-223 was 71.67 and
61.67%, respectively; the optimal cut-off value was 1.08 for
miR-132 and 1.74 for miR-223. Using miR-132 and miR-223 as
independent variables, the binomial logistic regression analysis
was performed to obtain a logistic regression model: Logit
(P)=7.555+-3.133 miR-132+-2.655 miR-223. In the diagnosis of SIC by
this model, the AUC was 0.922, the sensitivity was 86.25%, the
specificity was 86.67%, and the cut-off value was 0.63 (Table III; Fig. 1).
 | Table IIIDiagnostic value of serum miR-132 and
miR-223 for SIC between the research and control groups. |
Table III
Diagnostic value of serum miR-132 and
miR-223 for SIC between the research and control groups.
| Factor | miR-132 | miR-223 | miR-132 +
miR-223 |
|---|
| AUC | 0.827 | 0.831 | 0.922 |
| 95% CI | 0.754-0.899 | 0.760-0.903 | 0.876-0.968 |
| Cut-off | 1.08 | 1.74 | 0.63 |
| Sensitivity
(%) | 82.50 | 95.00 | 86.25 |
| Specificity
(%) | 71.67 | 61.67 | 86.67 |
Serum expression profiles of miR-132
and miR-223 in the survivor group (SG) and deceased group (DC) and
their prognostic value for SIC
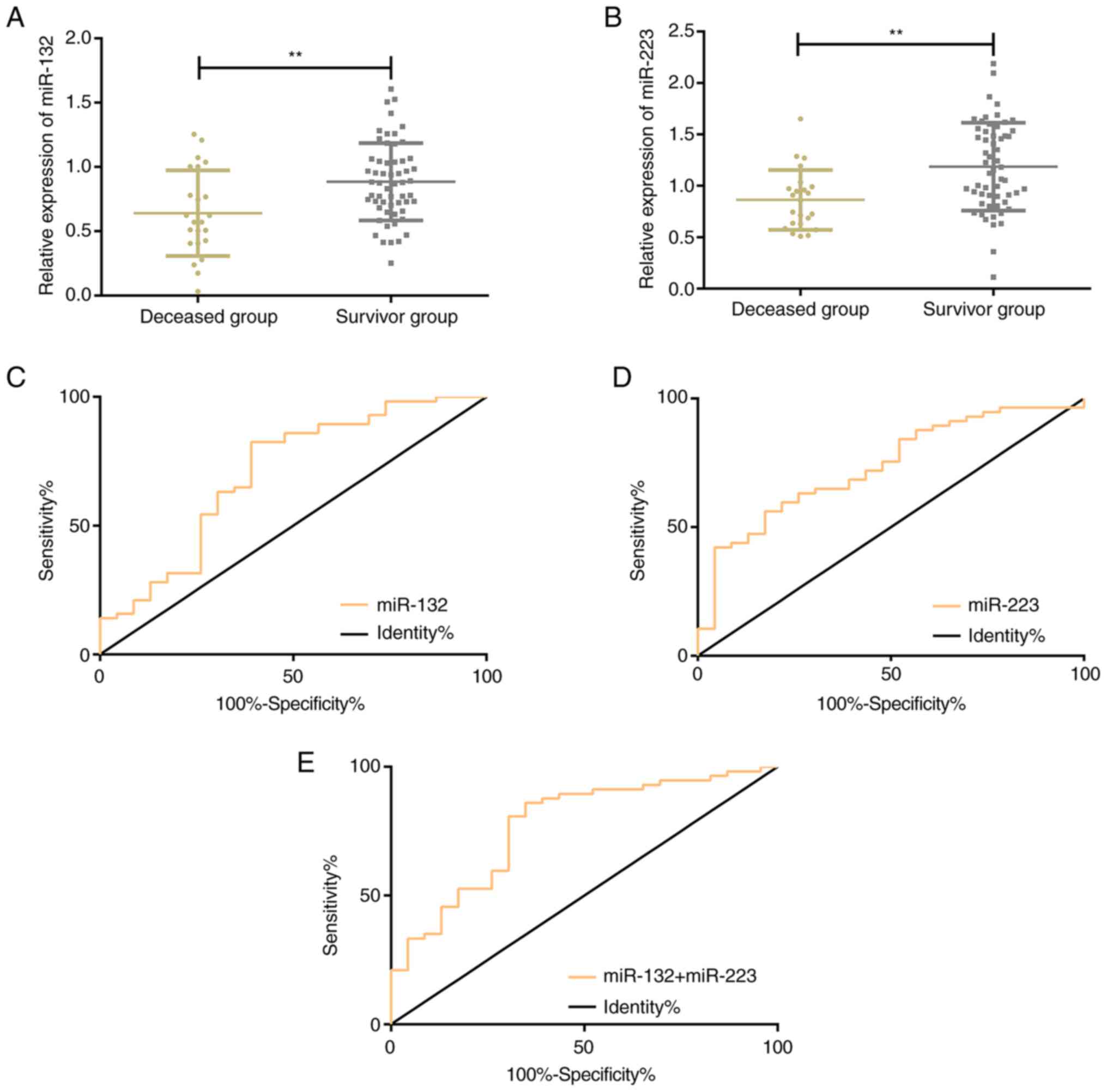
According to the 30-day survival of SIC patients, RG
was divided into the SG (n=57) and the DG (n=23). Serum miR-132 and
miR-223 levels were (0.89+0.33) and (1.19+0.43) in the SG and
(0.64+0.33) and (0.86+0.29) in the DG, respectively. Serum miR-132
and miR-223 levels were significantly higher in the SG than in the
DG (P<0.001). According to the ROC curves demonstrating the
prognosis prediction of SIC by serum miR-132 and miR-223, the AUC
of prognosis prediction of miR-132 and miR-223 was 0.703 and 0.735,
respectively; the sensitivity of miR-132 and miR-223 was 82.46 and
56.14%, respectively; the specificity of miR-132 and miR-223 was
60.87 and 82.61%, respectively; the optimal cut-off value was 0.64
for miR-132 and 1.06 for miR-223. Using miR-132 and miR-223 as
independent variables, the binomial logistic regression analysis
was performed to obtain a logistic regression model: Logit
(P)=-2.725++2.124 miR-132++1.970 miR-223. In the diagnosis
prediction of SIC by this model, the AUC was 0.773, the sensitivity
was 85.96%, the specificity was 65.22%, and the cut-off value was
0.62 (Table IV; Fig. 2).
 | Table IVPrognostic value of serum miR-132 and
miR-223 for SIC between the survivor and deceased groups. |
Table IV
Prognostic value of serum miR-132 and
miR-223 for SIC between the survivor and deceased groups.
| Factor | miR-132 | miR-223 | miR-132 +
miR-223 |
|---|
| AUC | 0.703 | 0.735 | 0.773 |
| 95% CI | 0.567-0.840 | 0.619-0.852 | 0.657-0.888 |
| Cut-off | 0.64 | 1.06 | 0.62 |
| Sensitivity
(%) | 82.46 | 56.14 | 85.96 |
| Specificity
(%) | 60.87 | 82.61 | 65.22 |
Correlation of serum miR-132 and
miR-223 with markers for myocardial injury and inflammatory
response
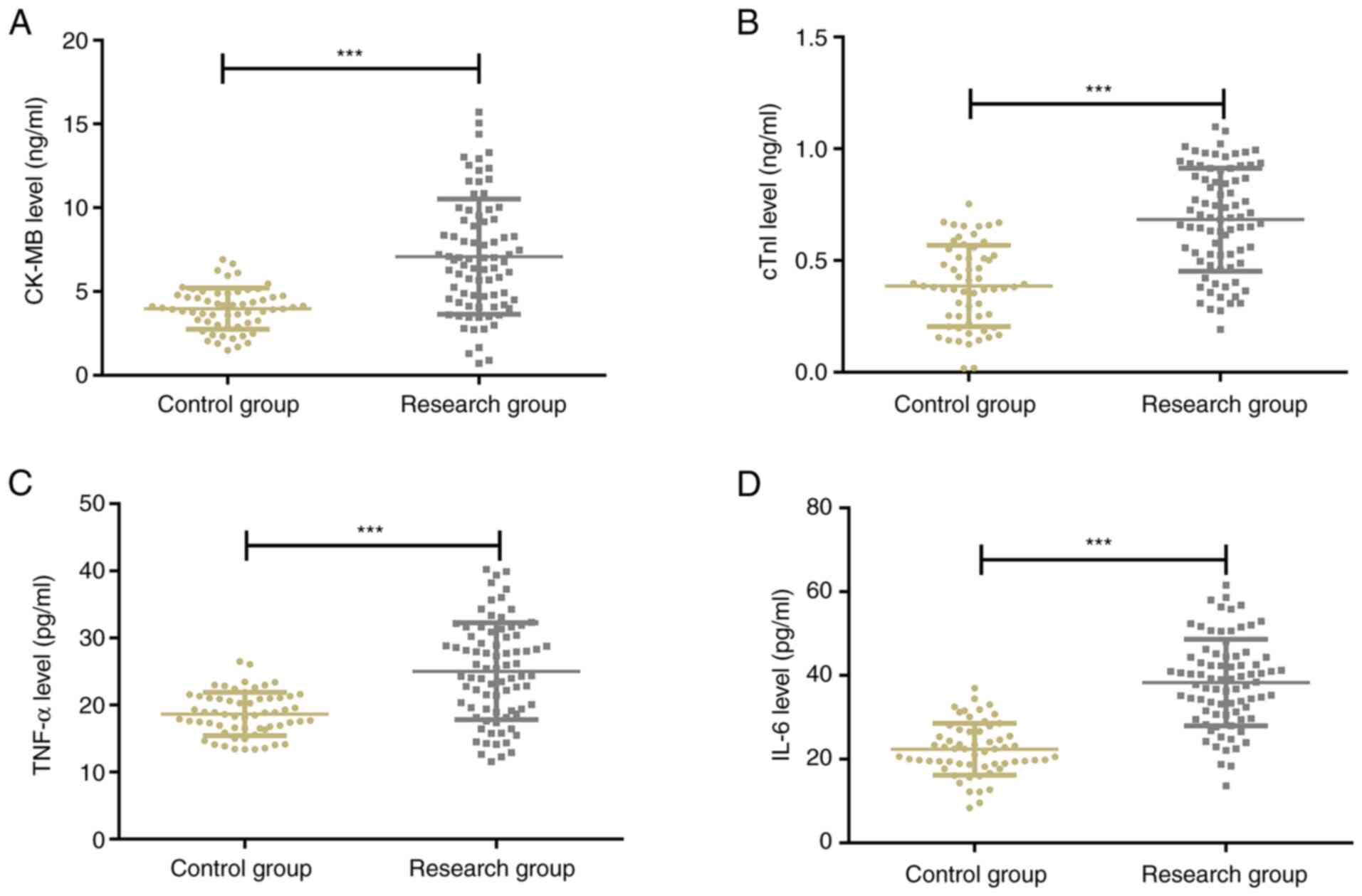
The serum levels of CK-MB, cTnI, TNF-α, and IL-6
were significantly higher in the RG than in the CG (P<0.001).
Pearson correlation analysis revealed negative correlations of
serum miR-132 and miR-223 levels with serum levels of CK-MB, cTnI,
TNF-α, and IL-6 (r=-0.633, P<0.001; r=-0.532, P<0.001;
r=-0.483, P<0.001; r=-0.496, P<0.001; r=-0.603, P<0.001;
r=-0.616, P<0.001; r=-0.568, P<0.001; r=-0.511, P<0.001)
(Figs. 3 and 4).
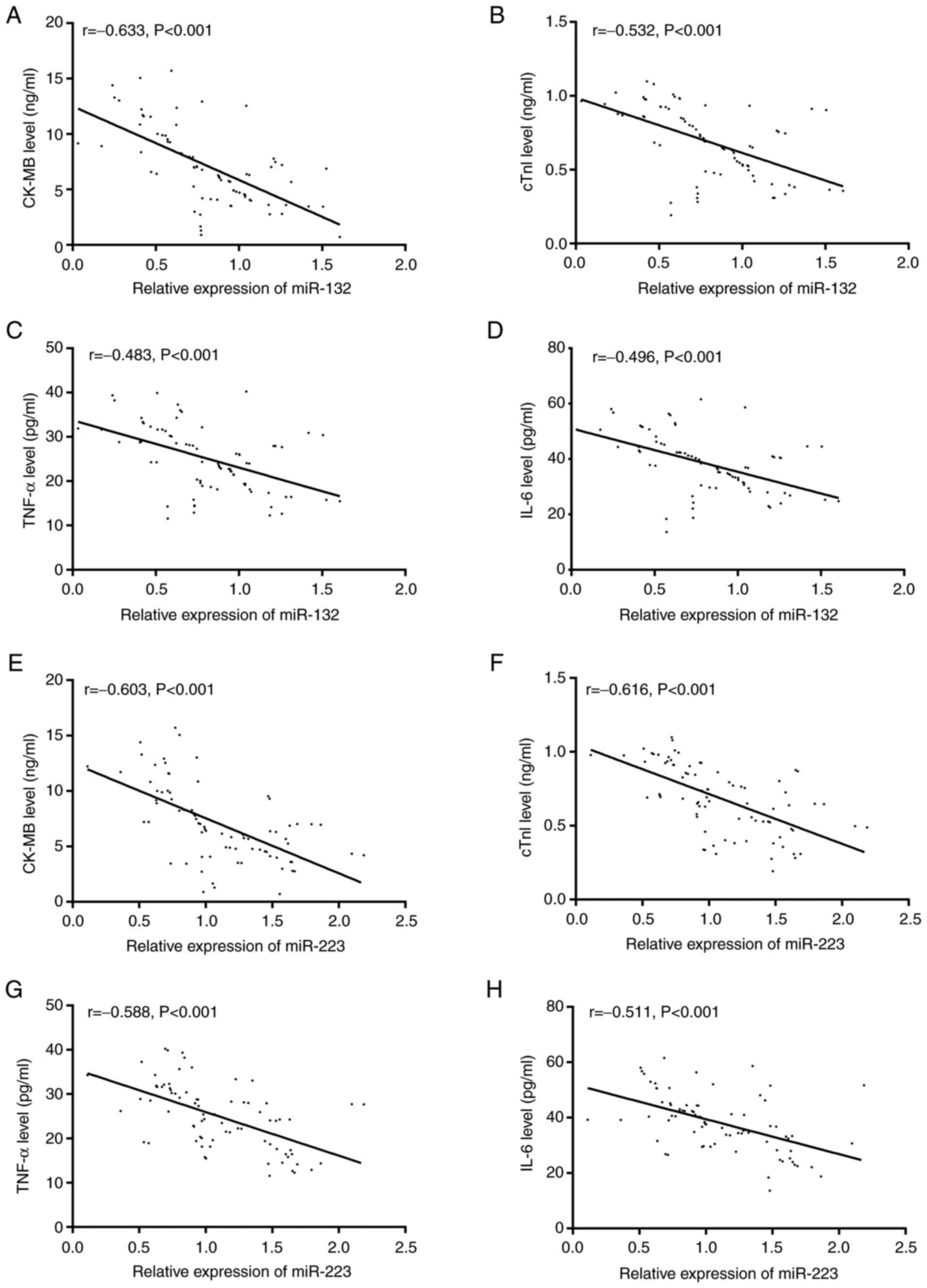 | Figure 4Correlation of serum miR-132 and
miR-223 levels with serum levels of CK-MB, cTnI, TNF-α, and IL-6 in
the research group. (A-D) Serum miR-132 level was negatively
correlated with CK-MB, cTnI, TNF-α, and IL-6 levels (r=-0.633,
P<0.001; r=-0.532, P<0.001; r=-0.483, P<0.001; r=-0.496,
P<0.001). (E-H) Serum miR-223 level was negatively correlated
with CK-MB, cTnI, TNF-α, and IL-6 levels (r=-0.603, P<0.001;
r=-0.616, P<0.001; r=-0.568, P<0.001; r=-0.511, P<0.001).
miR, microRNA; CK-MB, creatine kinase-MB; cTnI, cardiac troponin I;
TNF-α, tumor necrosis factor α; IL, interleukin. |
Discussion
SIC is a common myocardial complication in patients
with sepsis, and its severity poses a threat to human health
(20). Research has revealed that
numerous miRNAs, including miR-146a, miR-223 and miR-21-3p, are
valuable in the critical regulation of sepsis combined with cardiac
dysfunction, although their mechanisms have yet to be elucidated
(21).
Studies on miR-132 and miR-223 in sepsis are
numerous. In a study by Liu et al (22), miR-132 inhibited inflammation
responses in lung injury models induced by sepsis. Moreover, the
transfection of miR-132 mimics into macrophages pre-processed with
acetylcholine inhibited the pro-inflammatory response following
lipopolysaccharide challenge by inhibiting NF-κB and STAT3
pathways, and the transfection of miR-132 inhibitors into
macrophages pre-processed with acetylcholine promoted the
production of TNF-α, IL-1β, and IL-6. A study by Essandoh and Fan
(23) reported that miR-223 levels
were markedly lower in surviving sepsis patients than in
non-surviving sepsis patients, suggesting that a lower miR-223
expression level may result in septic death. miR-132 and miR-223
are potentially important in SIC. Previous studies have focused
mostly on the mechanism of action of miR-132 and miR-223 in sepsis
(24,25). The diagnostic value of the
combination of miR-132 and miR-223 in SIC is unclear. In the
present study, the serum levels of miR-132 and miR-223 were
significantly downregulated in SIC patients. The combination of the
two revealed a good diagnostic value. A previous study (26) discovered that the loss of miR-223
double-stranded (5p and 3p) aggravated the inflammatory response
and myocardial dysfunction of multiple sepsis, causing higher
mortality. In the present study it was hypothesized that miR-132
and miR-223 may affect the prognosis of SIC. The results revealed
that miR-132 combined with miR-223 had a predictive value in the
survival of SIC patients. Thus, they are both significant in the
early diagnosis and prognosis prediction of SIC.
Inflammatory cytokines including TNF-α and IL-6 that
are excessively released in sepsis patients are associated with
cardiac dysfunction (27). CK-MB
and cTnI are markers and indicators of myocardial damage, and they
can rapidly increase after myocardial damage (28). In a study by Qin et al
(29), the myocardial enzyme
indexes (including CK-MB and cTnI) and inflammation indexes
(including TNF-α and IL-6,) of a rat model of myocardial injury
induced by sepsis were markedly increased, and the use of
erythropoietin protected the heart from sepsis-induced myocardial
injury. Markers of myocardial injury and inflammatory response play
an important role in SIC. In the present study, the levels of serum
CK-MB, cTnI, TNF-α and IL-6 were markedly increased in SIC
patients, and miR-132 and miR-223 were both negatively correlated
with CK-MB, cTnI, TNF-α, and IL-6. In a previous study (30), the decrease in miR-132 expression
increased the levels of TNF-α, IL-1β, and IL-6 and promoted cell
apoptosis. In addition, the overexpression of miR-132 played a
protective role in rat models with cerebral hemorrhage. A previous
study (31) revealed that the high
miR-223 level in WT-exosomes was transmitted to cardiomyocytes and
in turn led to a decrease in TNF-α, IL-1β, and IL-6, protecting
septic patients from myocardial damage induced by mesenchymal stem
cells. Hence, the downregulation of miR-132 and miR-223 in SIC may
be related to myocardial injury and upregulation of inflammatory
factors, but the specific mechanism remains to be elucidated.
The present study confirmed the favorable role of
miR-132 and miR-223 combined detection in the early diagnosis and
prognosis evaluation of SIC. miR-132 and miR-223 exhibited an
inhibitory gene effect in SIC, however, in vivo experiments
were not conducted. These two miRNAs and their target targets may
function in SIC, however the specific regulatory mechanism remains
to be elucidated. Furthermore, the present research included
individuals undergoing health checkups instead of sepsis in the CG.
These shortcomings warrant improvement in future studies to better
support the results.
In summary, the combined detection of miR-132 and
miR-223 can be used for early diagnosis and prognostic evaluation
of SIC, and these two miRNAs were revealed to be correlated with
CK-MB, cTnI, TNF-α, and IL-6 levels.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and YC designed the study and drafted the
manuscript. YL, BL, MY, JZ and YY were responsible for the
collection and analysis of the experimental data. JZ, YY and YC
revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China). Patients who participated in this research, signed the
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sato R, Kuriyama A, Takada T, Nasu M and
Luthe SK: Prevalence and risk factors of sepsis-induced
cardiomyopathy: A retrospective cohort study. Medicine (Baltimore).
95(e5031)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang F, Zhao LN, Sun Y and Chen Z:
Levosimendan as a new force in the treatment of sepsis-induced
cardiomyopathy: Mechanism and clinical application. J Int Med Res.
47:1817–1828. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dombrovskiy VY, Martin AA, Sunderram J and
Paz HL: Rapid increase in hospitalization and mortality rates for
severe sepsis in the United States: A trend analysis from 1993 to
2003. Crit Care Med. 35:1244–1250. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Russell JA and Walley KR: Update in sepsis
2012. Am J Respir Crit Care Med. 187:1303–1307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Semeraro N, Ammollo CT, Semeraro F and
Colucci M: Sepsis, thrombosis and organ dysfunction. Thromb Res.
129:290–295. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hochstadt A, Meroz Y and Landesberg G:
Myocardial dysfunction in severe sepsis and septic shock: More
questions than answers? J Cardiothorac Vasc Anesth. 25:526–535.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Romero-Bermejo FJ, Ruiz-Bailen M,
Gil-Cebrian J and Huertos-Ranchal MJ: Sepsis-induced
cardiomyopathy. Curr Cardiol Rev. 7:163–183. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care.
3(48)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
An R, Feng J, Xi C, Xu J and Sun L:
miR-146a attenuates sepsis-induced myocardial dysfunction by
suppressing IRAK1 and TRAF6 via targeting ErbB4 expression. Oxid
Med Cell Longev. 2018(7163057)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sluijter JP and Doevendans PA:
Sepsis-associated cardiac dysfunction is controlled by small RNA
molecules. J Mol Cell Cardiol. 97:67–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zheng G, Pan M, Jin W, Jin G and Huang Y:
MicroRNA-135a is up-regulated and aggravates myocardial depression
in sepsis via regulating p38 MAPK/NF-κB pathway. Int
Immunopharmacol. 45:6–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qian Y, Song J, Ouyang Y, Han Q, Chen W,
Zhao X, Xie Y, Chen Y, Yuan W and Fan C: Advances in roles of
miR-132 in the nervous system. Front Pharmacol.
8(770)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mancuso R, Agostini S, Hernis A,
Zanzottera M, Bianchi A and Clerici M: Circulatory miR-223-3p
discriminates between Parkinson's and Alzheimer's patients. Sci
Rep. 9(9393)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li M, Shao H, Zhang X and Qin B:
Hesperidin alleviates lipopolysaccharide-induced neuroinflammation
in mice by promoting the miRNA-132 pathway. Inflammation.
39:1681–1689. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM,
Wan XJ and Zhu KM: Serum miR-146a and miR-223 as potential new
biomarkers for sepsis. Biochem Biophys Res Commun. 394:184–188.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang H, Bei Y, Shen S, Huang P, Shi J,
Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al: miR-21-3p controls
sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol
Cell Cardiol. 94:43–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hashmat N, Shabbir I, Rahat T, Ijaz F and
Majeed S: Clinical profile and disease outcome of septic patients
at public sector hospital. Pak J Med Res. 54:44–47. 2015.
|
|
18
|
Zhuang YT, Xu DY, Wang GY, Sun JL, Huang Y
and Wang SZ: IL-6 induced lncRNA MALAT1 enhances TNF-α expression
in LPS-induced septic cardiomyocytes via activation of SAA3. Eur
Rev Med Pharmacol Sci. 21:302–309. 2017.PubMed/NCBI
|
|
19
|
Zhao A, Li G, Péoc'h M, Genin C and
Gigante M: Serum miR-210 as a novel biomarker for molecular
diagnosis of clear cell renal cell carcinoma. Exp Mol Pathol.
94:115–120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care.
4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang H, Bei Y, Huang P, Zhou Q, Shi J, Sun
Q, Zhong J, Li X, Kong X and Xiao J: Inhibition of miR-155 protects
against LPS-induced cardiac dysfunction and apoptosis in mice. Mol
Ther Nucleic Acids. 5(e374)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao
N, Huang C, Shao Q, Ding C, Qing C, et al: miR-132 inhibits
lipopolysaccharide-induced inflammation in alveolar macrophages by
the cholinergic anti-inflammatory pathway. Exp Lung Res.
41:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Essandoh K and Fan GC: Role of
extracellular and intracellular microRNAs in sepsis. Biochim
Biophys Acta. 1842:2155–2162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nahid MA, Yao B, Dominguez-Gutierrez PR,
Kesavalu L, Satoh M and Chan EK: Regulation of TLR2-mediated
tolerance and cross-tolerance through IRAK4 modulation by miR-132
and miR-212. J Immunol. 190:1250–1263. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aziz F: The emerging role of miR-223 as
novel potential diagnostic and therapeutic target for inflammatory
disorders. Cell Immunol. 303:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang X, Huang W, Yang Y, Wang Y, Peng T,
Chang J, Caldwell CC, Zingarelli B and Fan GC: Loss of
duplexmiR-223 (5p and 3p) aggravates myocardial depression and
mortality in polymicrobial sepsis. Biochim Biophys Acta.
1842:701–711. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFκB. Int Immunopharmacol. 55:69–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu J, Li J, Tian P, Guli B, Weng G, Li L
and Cheng Q: H2S attenuates sepsis-induced cardiac
dysfunction via a PI3K/Akt-dependent mechanism. Exp Ther Med.
17:4064–4072. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qin YJ, Zhang XL, Yu YQ, Bian XH and Dong
SM: Cardioprotective effect of erythropoietin on sepsis-induced
myocardial injury in rats. World J Emerg Med. 4:215–222.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y, Han B, He Y, Li D, Ma X, Liu Q
and Hao J: MicroRNA-132 attenuates neurobehavioral and
neuropathological changes associated with intracerebral hemorrhage
in mice. Neurochem Int. 107:182–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Gu H, Qin D, Yang L, Huang W,
Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B and Fan GC:
Exosomal miR-223 contributes to mesenchymal stem cell-elicited
cardioprotection in polymicrobial sepsis. Sci Rep.
5(13721)2015.PubMed/NCBI View Article : Google Scholar
|