Introduction
Oral submucous fibrosis (OSF) is a potentially
malignant oral condition that has been extensively studied
(1). In particular, Pindborg and
Sirsat were the first to propose that OSF may be a precancerous
condition in 1966(2). The malignant
transformation rate of patients with OSF ranges from 1.2 to 23%
worldwide and according to statistics, OSF has a high tendency to
develop into oral cancer 3-16 years after OSF diagnosis (3). At present, accumulating evidence has
indicated that chewing of areca nut is recognized as one of the
most important risk factors for OSF (4,5).
It has been frequently reported that TGF-β1 induces
the phenotypic transformation of fibroblasts into activated
fibroblasts (6). A previous study
has revealed that TGF-β1 signaling was not only an important
suppressor of inflammation and epithelial cell proliferation, but
also a driver of collagen deposition during lung fibrosis (7). TGF-β was suggested to be the main
trigger for the increased collagen production and decreased
activity of extracellular matrix (ECM) degradation during OSF
(8). An increasing number of
studies have also shown that the aberrant activation of TGF-β1
signaling can led to the development of OSF (9,10).
Pant et al (11) previously
found that chewing of the areca nut induced the JNK/activating
transcription factor 2 (ATF2)/c-Jun signaling axis through
activation of the TGF-β signaling pathway in epithelial cells.
Therefore, it remains a priority to determine the molecular
mechanisms downstream of TGF-β1 signaling in OSF to suppress
TGF-β1-induced collagen production, which has been proposed to be a
viable treatment strategy for OSF (9). The present study established an in
vitro TGF-β1-induced cell model, similar to that reported in
previous studies (12-14),
to investigate the association between collagen deposition and
OSF.
Exosomes are extracellular membrane-enclosed
particles that are ~40-150 nm in size and are of endosomal origin,
which can contain microRNAs (miRNAs/miR), RNAs and proteins
(15,16). Exogenous exosomal molecules are
important mediators of paracrine signaling, which regulate
recipient cell function by transferring information that can
regulate the expression of specific genes or proteins (17,18).
Previous studies have reported the use of adipose-derived
mesenchymal stem cell (ADSC) exosomes (ADSC-Exos) for treating
fibrotic diseases, including liver fibrosis (19,20),
kidney fibrosis (21) and for the
formation of scars during skin wound healing (22). Although OSF is a disease that
appears to be caused by excessive collagen deposition and
dysregulation of ECM remodeling (23), to the best of our knowledge the
effect of ADSC-Exos on OSF remains unclear.
The present study isolated exosomes from human ADSCs
and investigated the possible biological role of ADSC-Exos on oral
mucosal fibroblasts following TGF-β1 stimulation. In particular,
the effects of ADSC-Exos on the expression levels of collagen type
I α 1 chain (COL1A1), collagen type III α 1 chain (COL3A1), matrix
metalloproteinase (MMP)1 and MMP3 in addition to the production of
collagen in TGF-β1-induced oral mucosal fibroblasts were
determined. Furthermore, the present study also explored the
possible underlying mechanism of the role of ADSC-Exos in collagen
metabolism.
Materials and methods
Ethics statement
All individuals provided written informed consent
prior to participation in the present study and the study protocol
was approved by the Ethics Committee of Hunan Xiangya
Stomatological Hospital affiliated with Central South University
(approval no. 20190015; Changsha, China).
Isolation of oral fibroblasts and
ADSCs
Oral mucosal fibroblasts were obtained from the
buccal tissues of patients without OSF during extraction of the
third molar in April-May 2019 at Hunan Xiangya Stomatological
Hospital, Central South University. The inclusion criteria were as
follows: i) Patients agreed to provide tissue samples to be
analyzed in the present study; ii) provided written informed
consent; iii) aged ≥18 years; and iv) no systemic diseases. The
exclusion criteria were as follows: i) Areca-chewers; ii) smokers;
and iii) oral mucosal diseases, including leukoplakia and lichen
planus. The patients were comprised two men and one woman, with an
age 26.00±1.63 years. Fibroblasts were cultured and maintained
according to a previously described method (24,25).
Briefly, the submucosal tissue was obtained from the buccal mucosa
for primary culture. The tissue was washed three times with
ice-cold PBS containing 20% penicillin/streptomycin mixture before
the epithelial tissue was removed under aseptic conditions. The
tissues were then cut into small pieces (0.5x0.5x0.5 mm) and washed
with RPMI-1640 medium (Hyclone; Cytiva) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). In total, five times the
amount of collagenase II (Gibco; Thermo Fisher Scientific, Inc.)
was added to the tissue mass at a concentration of 2 mg/ml and
digested for 30 min in a 37˚C incubator. Subsequently, 3-5 ml
RPMI-1640 complete medium was added to terminate the digestion and
the mixture was incubated at room temperature for 5 min. After
centrifugation at room temperature at 300 x g for 10 min, the
supernatant was discarded and five times the amount of collagenase
II was added to the tissue mass at a concentration of 2 mg/ml for
30 min in a 37˚C incubator. This step was repeated three to four
times. After centrifugation at room temperature 300 x g for 10 min,
the cells were collected cells again by discarding the supernatant
before 5 ml of RPMI-1640 full culture medium was added to the cell
pellet and transferred into T25 cell culture flasks. All cells were
maintained at 37˚C in an incubator containing 5% CO2.
For the selected experiments, fibroblasts were stimulated with 0,
0.1, 1 and 10 ng/ml recombinant TGF-β1 (PeproTech, Inc.) at 37˚C
for 48 h.
ADSCs were obtained from three healthy female
individuals (age, 28.67±1.89 years; range, 25-30 years) during
liposuction procedures performed at the Plastic Surgery Department
of The Xiangya Hospital Central South University in December 2019.
Isolation of ADSCs was performed as previously described (26). Briefly, the adipose tissue was cut
into small pieces and digested with 0.1% collagenase type II
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C water bath and
shaken once every 5 min. After the adipose tissue particles
disappear and the liquid reached a uniform state, an equal volume
of low glucose-DMEM (L-DMEM; HyClone; Cytiva) complete medium (90%
L-DMEM + 10% FBS + 1% penicillin and streptomycin suspension) to
terminate the digestion at room temperature. After centrifugation
at room temperature 300 x g for 5 min, the supernatant was
discarded. The cell pellet was resuspended in 5 ml L-DMEM medium
and transferred into culture flasks. ADSCs were cultured in L-DMEM
supplemented with 10% FBS, cells were maintained at 37˚C in an
incubator containing 5% CO2.
Identification of ADSCs
To assess the capacity (including osteogenesis and
adipogenesis) for ADSCs multilineage differentiation, cells were
cultured under differentiating conditions. For adipocyte
differentiation, ADSCs were cultured in the adipogenesis induction
medium (cat. no. HUXMD-90031; Cyagen Biosciences, Inc.) at 37˚C for
21 days. At the end of the incubation, ADSCs were fixed in 4%
paraformaldehyde at room temperature for 30 min and stained with
Oil Red O (cat. no. G1262; Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 20 min. For
osteoblast differentiation, the ADSCs were cultured in osteogenesis
induction medium (cat. no. HUXMA-90021; Cyagen Biosciences, Inc.)
at 37˚C for 14 days. The osteogenic ADSCs were then fixed in 4%
paraformaldehyde at room temperature for 20 min and stained with 2%
Alizarin red solution (cat. no. C0140; Beyotime Institute of
Biotechnology) at room temperature for 15 min. Stained cells were
visualized using a light microscope at x100 magnification (CX31;
Olympus Corporation). The expression of surface marker proteins
(CD29, CD44, CD73, CD90, CD34 and CD45) on ADSCs was detected by
flow cytometry. The ADSCs were harvested and washed twice with PBS
before being centrifuged at room temperature at 500 x g for 5 min
and the pelleted cells were collected. Then fix the cells in 4% PFA
at room temperature for 20 min. The cells were washed twice with 1
ml washing buffer by centrifugation at room temperature at 500 x g
for 5 min. The cells were then suspended in 1 ml permeabilization
buffer for 10 min at room temperature before being centrifuged at
room temperature 500 x g for 5 min and the pellet was collected.
The cells were washed again for two times with PBS to the cells
before being centrifuged again at room temperature 500 x g for 5
min. The cells were then incubated with 3% BSA (Beyotime Institute
of Biotechnology) in PBS for 30 min at room temperature. In total,
1x106 per aliquot of cells in 100 µl blocking buffer
were incubated with the following primary antibodies for 45 min at
room temperature: Mouse anti-Human CD29 (cat. no. 65191-1-Ig; 1:10;
Proteintech Group, Inc.), mouse anti- Human CD44 (cat. no.
65063-1-Ig; 1:10; Proteintech Group, Inc.), mouse anti-Human CD73
(cat. no. 65162-1-Ig; 1:10; Proteintech Group, Inc.), rabbit
anti-Mouse CD90 (cat. no. FITC-65088; 1:10; Proteintech Group,
Inc), mouse anti-CD34 (cat. no. 60180-1-Ig; 1:10; Proteintech
Group, Inc.) and mouse anti-Human CD45 (cat. no. 60287-1-Ig; 1:10;
Proteintech Group, Inc.). If using FITC-conjugated primary
antibodies, no secondary antibody incubation was used. The cells
were then incubated with diluted secondary antibodies (Alexa
Fluor® 488-conjugated goat anti-mouse IgG; cat. no.
ab150117; 1:25; Abcam) and incubated for 45 min at room temperature
in the dark. A separate secondary antibody (Alexa Fluor®
488-conjugated goat anti-mouse IgG or goat anti-rabbit IgG; cat.
no. ab150077; 1:25; Abcam) incubation was used as a negative
control. Using 5% BSA as the flow cleaning solution, unbound
fluorescence and impurities were washed off and the cells were
loaded into the BD FACSCanto II (BD Biosciences) flow cytometer for
measurements. The results were analyzed using the Flowjo VX10
software (FlowJo LLC).
ADSC-Exos extraction
Exosome extraction was performed as previously
described with a number of modifications (22). Briefly, ADSCs were cultured until
cells reached 80% confluence. The medium was then replaced with
serum-free L-DMEM at 37˚C for 24 h. The conditioned medium was
collected and centrifuged at 300 x g for 10 min at 4˚C and then at
2,000 x g for 30 min at 4˚C to remove dead cells and cellular
debris. After further centrifugation at 2,000 x g for 30 min at
4˚C, the supernatant containing exosomes was collected. Thereafter,
the exosome suspension was filtered through a 0.22-µm filter (EMD
Millipore). A total of 15 ml exosome suspension was added to an
Amicon® Ultra-15 Centrifugal Filter device unit (100
kDa; EMD Millipore) and the supernatant was collected after
centrifugation at 4,000 x g for 1 h at 4˚C. To recover the
concentrated solute, a pipettor was inserted into the bottom of the
filter device to withdraw the sample using a side-to-side sweeping
motion into ensure total recovery. The volume of the
ultrafiltration liquid was reduced from 15 to ~1 ml, before 1/5
volume of Exoquick Exosome Precipitation solution (System
Bioscience, LLC) was added to the exosome suspension for 12 h at
4˚C. On the next day, the mixture was centrifuged at 4˚C at 1,500 x
g for 30 min and the supernatant was removed, before the yellow or
beige precipitate at the bottom of the tube was identified as
exosomes. Finally, an appropriate amount of PBS (300-500 ml) was
added to resuspend the precipitate and the exosome suspension was
obtained after the pipetting gun was blown evenly. Exosome
concentration was determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology).
Identification of ADSC-Exos
The morphology of the exosomes was observed by
transmission electron microscopy (TEM), as previously described
(27). ADSC-Exos pellets (~5 µl)
were fixed with 2.5% glutaraldehyde at room temperature for ≥2 h,
washed three times with 0.1 M PBS for 15 min each time and then
fixed with 1% osmium acid at room temperature for 1-2 h. The
pellets were then dehydrated sequentially in 70, 90 and 100%
acetone for 10 min each time. Pure acetone and embedding solution
[Embed812; Head (Beijing) Biotechnology, Co., Ltd.] was mixed 1:1
and then added to the exosomes at 37˚C for 12 h, followed by
overnight at 37˚C and 24 h at 60˚C for curing. Finally, the
exosomes underwent double staining with 3% uranyl acetate and lead
nitrate at room temperature for 20-30 min. ADSC-Exos were observed
using a Hitachi H-7650 transmission electron microscope (H-7650H;
Hitachi, Ltd.). The size distribution of the ADSC-Exos was measured
by dynamic light scattering with a Malvern Nano instrument (ZS90;
ZEN3690; Malvern Instruments). Expression levels of the
characteristic surface marker proteins on the exosomes were
extracted using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.) and analyzed by western blotting.
Exosome uptake assay
ADSC-Exos were labeled with a green, fluorescent
3,3'-dioctadecyloxacarbocyanine perchlorate (DiO) dye (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 1 µl DiO dye (200 µg/ml; Thermo Fisher Scientific, Inc.)
was added to 20 µl exosome suspension and incubated for 20 min at
37˚C. The reaction was stopped by the addition of an equivalent
volume of exosome-depleted BSA (Sigma-Aldrich; Merck KGaA), before
DiO-labeled exosomes were obtained.
Fibroblasts were washed twice with PBS and seeded at
a density of 2x105 into six-well plates for 48 h at
37˚C. Next, 50 ng/µl DiO-labeled exosomes were added to the
fibroblasts and incubated at 37˚C in the dark for 6 h before being
subsequently fixed with 4% paraformaldehyde at room temperature for
15 min. The cells were then incubated with 0.5% Triton X-100 at
room temperature for 15 min and blocked with 10% BSA at 37˚C for 30
min. The fibroblasts were then stained with 2.5% phalloidin (cat.
no. 40734ES75; Shanghai Yeasen Biotechnology Co., Ltd.) at room
temperature for 20 min. After washing with PBS, the nuclei were
then stained with DAPI at room temperature for 3 min (0.5 µg/ml;
Invitrogen; Thermo Fisher Scientific, Inc.). The fluorescent cells
were visualized using a Zeiss LSM 800 confocal microscope at x100
magnification (Zeiss GmbH).
Immunofluorescence assay
Immunofluorescence was used to detect the expression
of vimentin under normal condition or following TGF-β1 (10 ng/ml)
treatment at 37˚C for 2 days. The experiment was divided into two
groups, the control group (PBS) and the experimental group
(TGF-β1). Briefly, fibroblasts were first fixed with 4%
paraformaldehyde for 15 min at room temperature. After
permeabilization with 0.5% Triton X-100/PBS for 15 min at room
temperature, non-specific binding was blocked with 5% BSA for 1 h
at 37˚C. Cells were subsequently incubated with a rabbit
anti-vimentin monoclonal antibody (cat. no. 9782; 1:1,000; Cell
Signaling Technology, Inc.) at 4˚C overnight. Following the primary
antibody incubation, cells were incubated with an Alexa
Fluor® 488-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. ab150077; 1:2,000; Abcam) for 1 h at 37˚C. The
nuclei were counterstained with DAPI at room temperature for 3 min
(0.5 µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.). Stained
cells were visualized using a Leica DMI6000B fluorescence
microscope at x100 magnification (Leica Microsystems GmbH).
Immunocytochemistry
Immunocytochemistry was used to detect the
expression of α-SMA under normal condition and following TGF-β1 (10
ng/ml) treatment at 37˚C for 2 days. The experiment was divided
into two groups, the control group (PBS) and the experimental group
(TGF-β1). Briefly, fibroblasts were fixed with 4% paraformaldehyde
at room temperature for 15 min and then permeabilized with 0.5%
Triton X-100/PBS for 15 min at room temperature. Afterwards, 25 µl
1% donkey serum (cat. no. SL050; Beijing Solarbio Science &
Technology Co., Ltd.) to each sample at room temperature for 20 min
for blocking. Cells were incubated with the rabbit anti-α smooth
muscle actin (SMA) antibody (cat. no. ab124964; 1:2,000; Abcam) for
2 h at 37˚C. Enhanced enzyme-labeled goat anti-mouse/rabbit IgG
polymer (cat. no. PV-9000; ZSGB-BIO; OriGene Technologies, Inc.)
was then added and incubated at room temperature for 30 min. DAB
(cat. no. DA1015; Beijing Solarbio Science & Technology Co.,
Ltd.) color developing solution was added and incubate for 2 min at
room temperature. Hematoxylin (Beijing Solarbio Science &
Technology Co., Ltd.) counterstain was then used for 1 min at room
temperature. Stained cells were visualized using light microscopy
at x100 magnification (CX31; Olympus Corporation).
Western blotting
Firstly, the fibroblasts were divided into four
concentration groups: 0, 0.1, 1 and the 10 ng/ml TGF-β1 groups. In
addition, the fibroblasts were divided into three groups: 10 µg/ml
TGF-β1 + 100 µg/ml ADSC-Exos, negative control (PBS) and positive
control group (TGF-β1). The fibroblasts were also divided into four
groups and incubated for 30 min at 37˚C: 10 ng/ml TGFβ1 + 100 µg/ml
ADSC-Exos; 10 ng/ml TGF-β1 + 100 µg/ml ADSC-Exos + 25 µg/ml
Anisomycin (Cell Signaling Technology, Inc.); 10 ng/ml TGF-β1 and
10 ng/ml TGFβ1 + 25 µg/ml Anisomycin.
Total protein was extracted from fibroblasts and
exosomes using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.). The protein was lysed on ice for 30 min,
centrifuged at 12,000 x g at 4˚C for 10 min and the supernatant was
removed. A 20 µl sample was taken for protein concentration
determination by BCA method. Proteins (40 µg per lane) were
separated using 10% SDS-PAGE. The separated proteins were
subsequently transferred onto polyvinylidene fluoride membranes
(EMD Millipore) and blocked with 5% non-fat milk or 5% BSA at room
temperature for 60 min. Membranes were then incubated with the
following primary antibodies at 4˚C overnight: Mouse
anti-phosphorylated (p)-p38 (cat. no. sc-7973; 1:200; Santa Cruz
Biotechnology, Inc.), rabbit anti-p38 (cat. no. sc-535; 1:200;
Santa Cruz Biotechnology, Inc.), mouse anti-CD63 (cat. no. sc-5275;
1:500; Santa Cruz Biotechnology, Inc.), rabbit anti-collagen I
(cat. no. 14695-1-AP; 1:1,000; ProteinTech Group, Inc.), rabbit
anti-tumor susceptibility 101 (TSG101; cat. no. 14497-1-AP;
1:1,000; ProteinTech Group, Inc.), rabbit anti-collagen III (cat.
no. C7805; 1:2,000; Sigma-Aldrich; Merck KGaA) and rabbit
anti-GAPDH (cat. no. GB11002; 1:2,000; Wuhan Servicebio Technology
Co., Ltd.). Following the primary antibody incubation, the
membranes were incubated with HRP-conjugated anti-rabbit IgG (cat.
no. 7074; 1:5,000; Cell Signaling Technology, Inc.) or anti-mouse
IgG (cat. no. 7076; 1:5,000; Cell Signaling Technology, Inc.)
secondary antibodies at 37˚C for 1 h. Protein bands were visualized
using enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.) and analyzed using the Image Lab V3.0 (Bio-Rad, Laboratories,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
After 2 days of 100 µg/ml ADSC-Exos intervention at
37˚C, fibroblasts were extracted and detected to detect the
expressions of collagen-synthesis-related genes COL1A1 and COL3A1
and metalloproteinase genes MMP1 and MMP3. Total RNA was extracted
from fibroblasts using TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as previously described (28). RNA concentration was detected using
a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific,
Inc.) at wavelengths of 260 and 280 nm. In total, 1 µg RNA from
each sample was reverse transcribed into cDNA using a Revert Aid
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.). The temperature protocol used was
pre-denaturation at 70˚C for 5 min, followed by 25˚C for 5 min,
42˚C for 60 min and inactivation at 70˚C for 5 min. qPCR was
subsequently performed using SYBR® Premix Ex Taq™ II
system (cat. no. DRR820A; Takara Bio, Inc.) on an ABI
PRISM® 7900HT system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for the qPCR: 95˚C pre-denaturation 60 sec, followed by 40 cycles
at 95˚C denaturation 15 sec, 60˚C annealing 15 sec and 72˚C
extension 45 sec. The following primer sequences (Sangon Biotech
Co., Ltd.) were used for the qPCR: COL1A1 forward,
5'-ATCAACCGGAGGAATTTCCGT-3' and reverse, 5'-CAC
CAGGACGACCAGGTTTTC-3'; COL3A1 forward, 5'-GCC
AAATATGTGTCTGTGACTCA-3' and reverse, 5'-GGGCGA GTAGGAGCAGTTG-3';
MMP1 forward, 5'-GGCTGAAAG TGACTGGGAAACC-3' and reverse,
5'-TGCTCTTGGCAAA TCTGGCGTG-3'; MMP3 forward, 5'-CTGGACTCCGACAC
TCTGGA-3' and reverse, 5'-CAGGAAAGGTTCTGAAGTG ACC-3' and GAPDH
forward, 5'-GGCACAGTCAAGGCTG AGAATG-3' and reverse,
5'-ATGGTGGTGAAGACGCCA GTA-3'. Relative gene expression was
calculated using the 2-ΔΔCq method (29). GAPDH was used as the housekeeping
gene for normalization.
Statistical analysis
Data analysis was performed using the SPSS 20.0
software (IBM Corp.). Data are presented as the mean ± SD.
Statistical differences between two groups were determined using an
unpaired Student's t-test and comparisons among multiple groups
were performed using a one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TGF-β1 induces collagen production in
oral mucosal fibroblasts
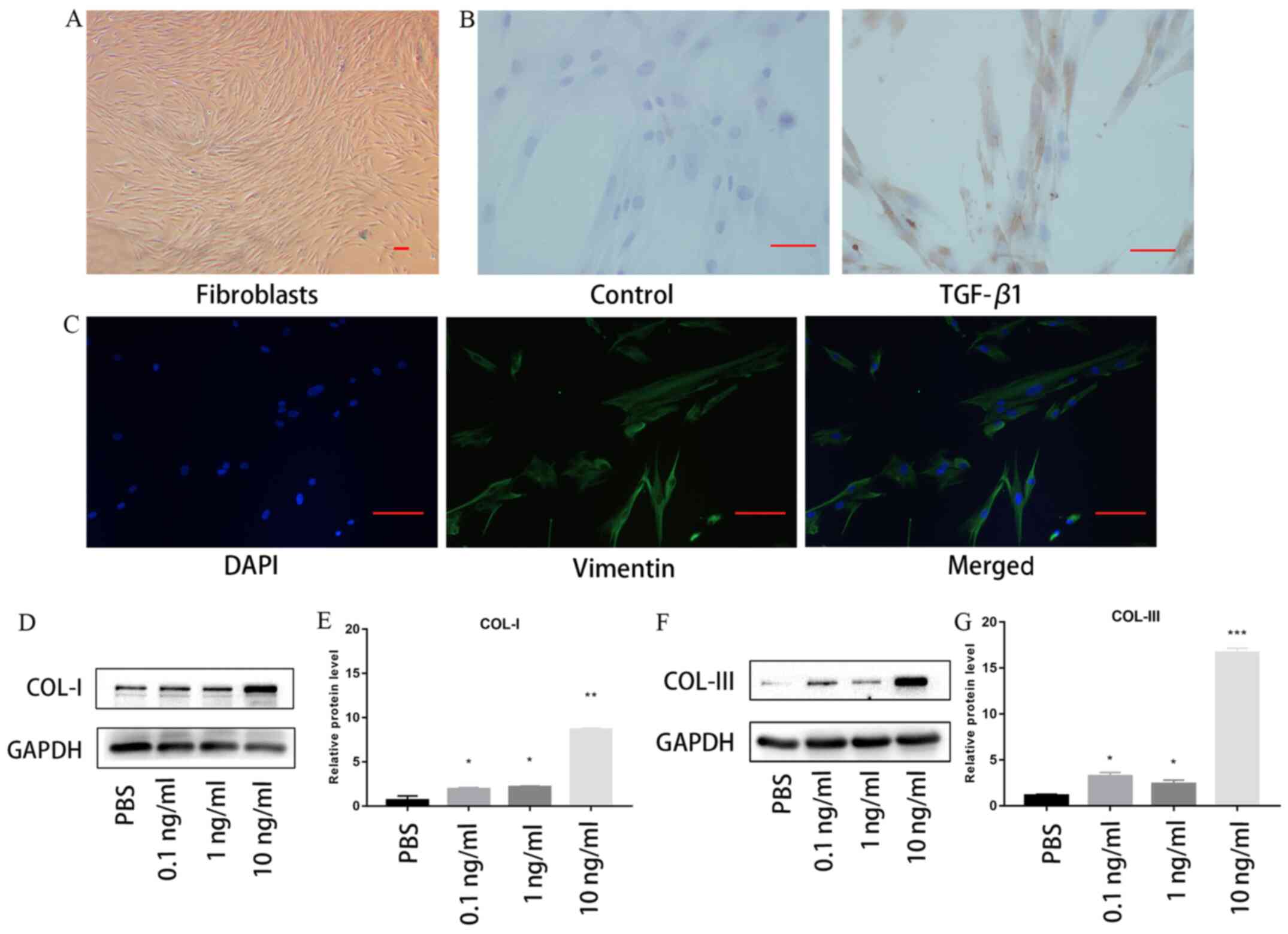
Oral mucosal fibroblasts were found to exhibit a
spindle-like morphology without TGF-β1 treatment (Fig. 1A). Immunocytochemistry staining
revealed that positive staining for α-SMA, a marker of
myofibroblasts, was markedly increased following 10 ng/ml TGF-β1
stimulation (Fig. 1B).
Immunofluorescence staining demonstrated that vimentin was
expressed by the fibroblasts without TGF-β1 treatment (Fig. 1C). Fibroblasts were also treated
with 0, 0.1, 1 or 10 ng/ml TGF-β1 for 48 h. As shown in Fig. 1D and E, the expression levels of collagen I and
III were significantly upregulated by TGF-β1 in the oral mucosal
fibroblasts in a concentration-dependent manner, compared with
those in cells treated with PBS. Notably, the expression levels of
collagen I and III were the highest following stimulation with 10
mg/ml TGF-β1. These results indicated that TGF-β1 may increase the
synthesis of collagen in vitro. Therefore, 10 ng/ml TGF-β1
was used to induce the oral fibroblasts for the following
experiments.
Characterization of ADSCs and
ADSC-Exos
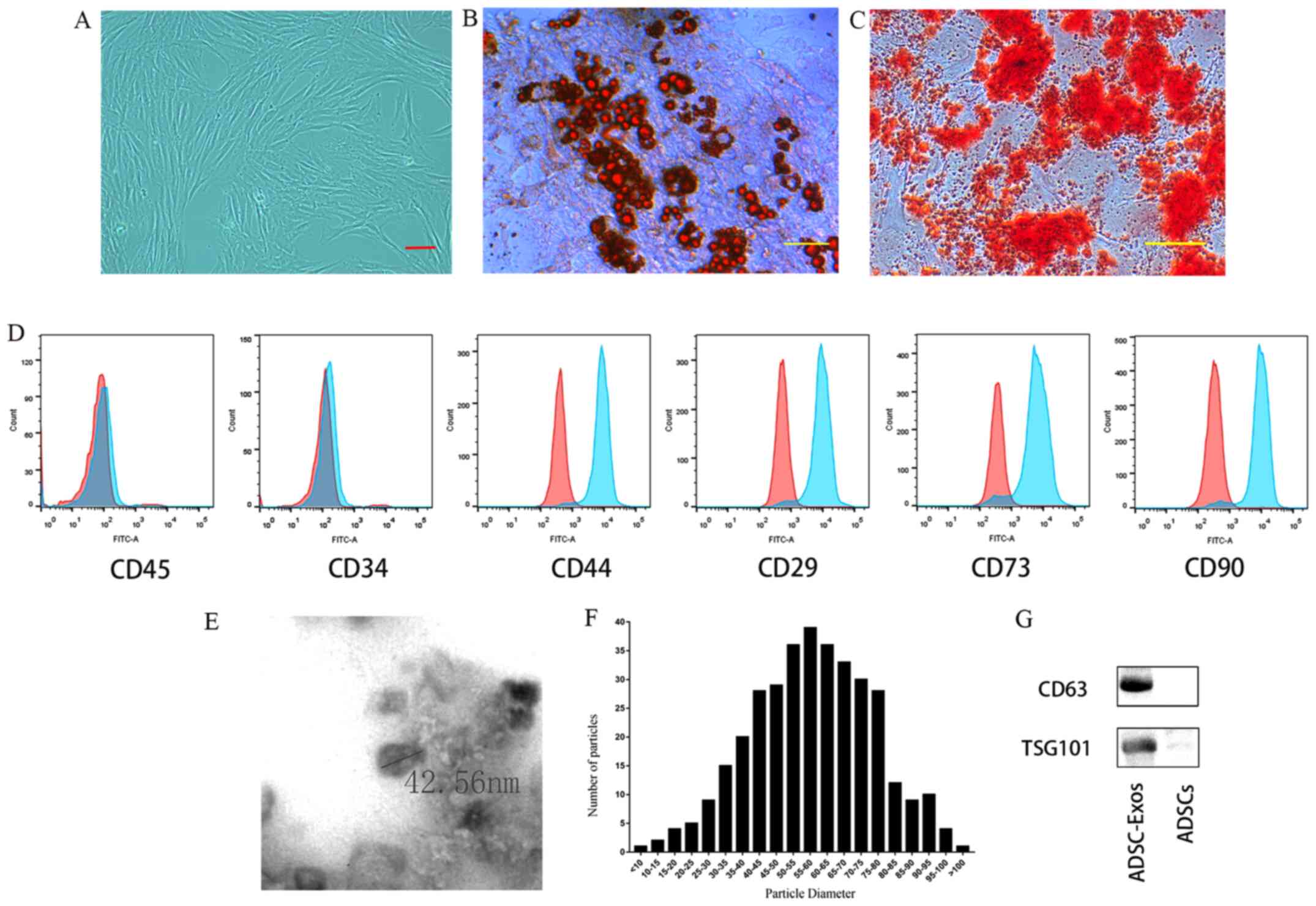
ADSCs were found to exhibit a spindle-like
morphology (Fig. 2A). Following
osteogenic or adipogenic medium incubations, the ADSCs were able to
differentiate into osteoblasts or adipocytes, as demonstrated by
Alizarin Red S and Oil Red O staining, respectively (Fig. 2B and C). Flow cytometry analysis revealed that
ADSCs were highly positive for MSC surface markers (17), including CD29, CD44, CD73 and CD90,
but negative for CD34 and CD45 (Fig.
2D). TEM and western blotting were used to characterize the
exosomes derived from ADSCs. The vesicles exhibited a cup- or
sphere-shaped morphology (Fig. 2E),
with a mean diameter of 58.01±16.17 nm (Fig. 2F). Western blot analysis revealed
that the expression levels of the exosomal marker proteins, CD63
and TSG101(17), were enriched in
ADSC-Exos (Fig. 2G).
ADSC-Exos inhibit TGF-β1-induced
collagen production in oral mucosal fibroblasts
As shown in Fig. 3A,
the DiO-labeled ADSC-Exos (green, fluorescent dye) were
incorporated mainly to the perinuclear regions of
phalloidin-labeled fibroblasts (red fluorescent dye). The
expression levels of collagen I and III were significantly
downregulated in the fibroblasts in the 10 ng/ml TGF-β1 + 100 µg/ml
ADSC-Exos group compared with those in the fibroblasts incubated
with TGF-β1 alone (Fig. 3B and
C).
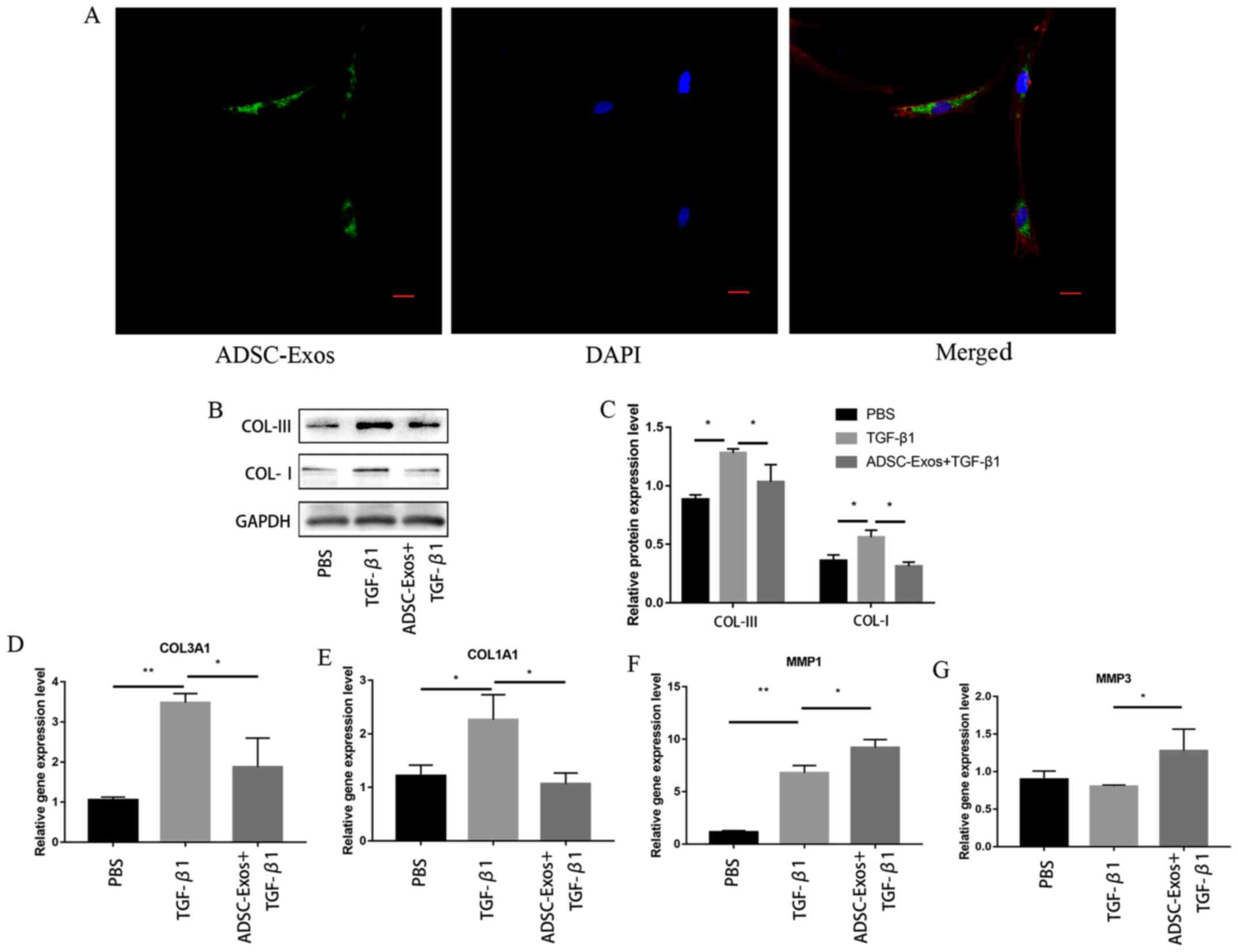 | Figure 3ADSC-Exos inhibit TGF-β1-induced
collagen production. (A) Confocal microscopy images of the uptake
of DiO-labeled ADSC-Exos by fibroblasts. Green, DiO; red,
phalloidin; blue, DAPI. Scale bar, 20 µm. (B) ADSC-Exos
downregulated the expression levels of collagen I and collagen III
in TGF-β1-induced oral fibroblasts, (C) which was quantified. The
mRNA expression levels of (D) COL3A1, (E) COL3A1, (F) MMP1 and (G)
MMP3 were analyzed using reverse transcription-quantitative PCR.
The results are presented as the mean ± SD; n=3 for each group.
*P<0.05 and **P<0.01. ADSC-Exos,
adipose-derived mesenchymal stem cells exosomes; DiO,
3,3’-dioctadecyloxacarbocyanine perchlorate; COL1A1, collagen type
I α 1 chain; COL3A1, collagen type III α 1 chain; MMP, matrix
metalloproteinase. |
The expression levels of COL1A1 and COL3A1 mRNA were
also found to be significantly downregulated in fibroblasts
stimulated with TGF-β1 + ADSC-Exos compared with those in
fibroblasts stimulated with TGF-β1 alone (Fig. 3D and E). In addition, when the fibroblasts were
stimulated with TGF-β1 + ADSC-Exos, the expression levels of MMP1
and MMP3 were found to be significantly upregulated compared with
those in fibroblasts incubated with TGF-β1 alone (Fig. 3F and G).
ADSC-Exos downregulate collagen
expression by inhibiting the p38 MAPK signaling pathway
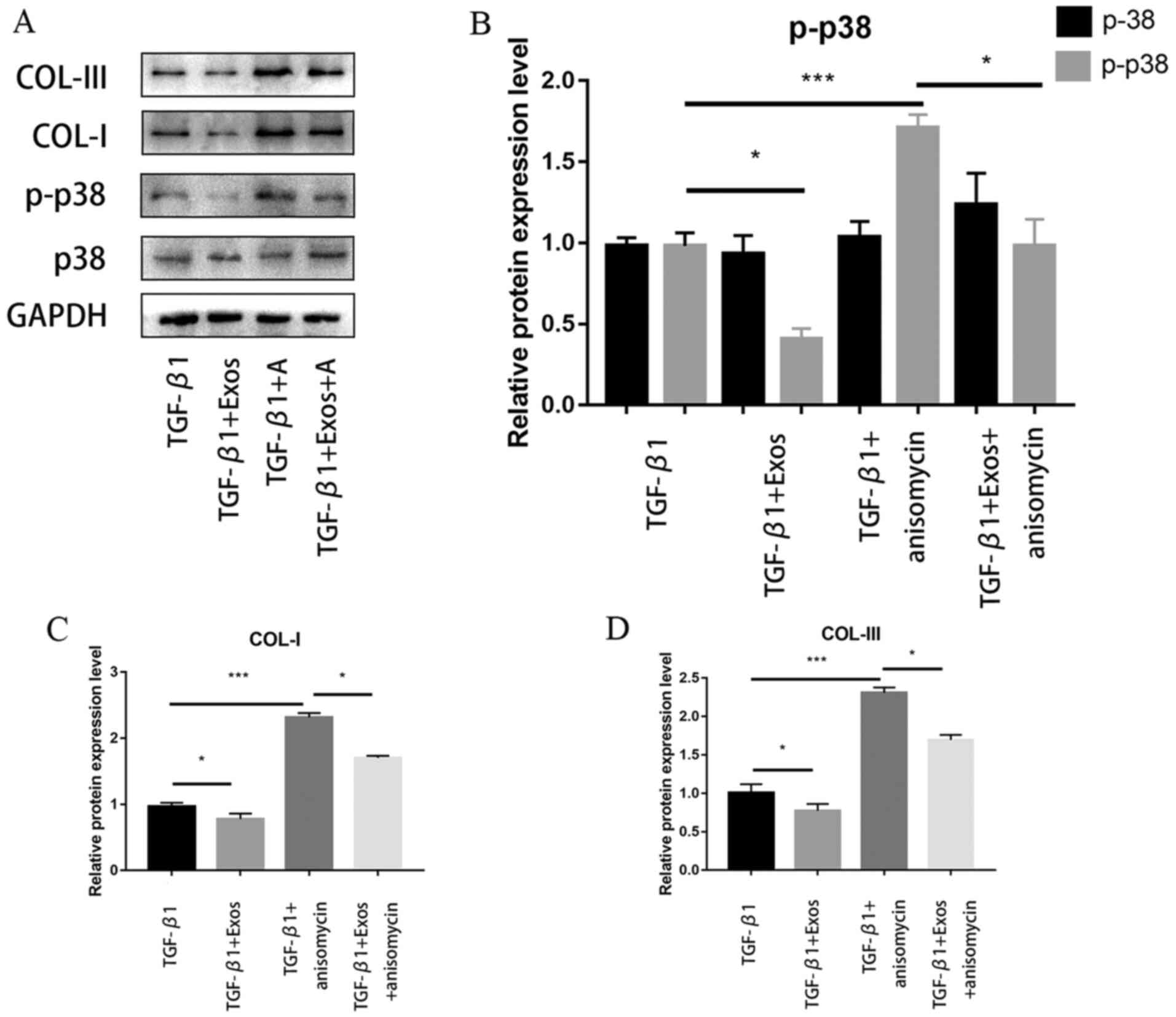
As shown in Fig. 4A,
western blotting analysis demonstrated that ADSC-Exos downregulated
the phosphorylation levels of p38. The expression levels of
collagen I and III were also discovered to be downregulated
concomitantly (Fig. 4A).
Subsequently, the cultured fibroblasts were treated with 25 µg/ml
anisomycin, a p38 activator, in the presence of TGF-β1. As shown in
Fig. 4B, anisomycin treatment
(TGF-β1 + A) could upregulate the phosphorylation levels of p38
compared with those in fibroblasts stimulated with TGF-β1 alone.
The expression levels of collagen I and III were also upregulated
following the addition of anisomycin to the medium compared with
those in fibroblasts stimulated with TGF-β1 alone (Fig. 4B and C). Notably, ADSC-Exos treatment (TGF-β1 +
Exos + A) significantly reversed the effects of anisomycin on p38
phosphorylation and collagen I and III expression.
Discussion
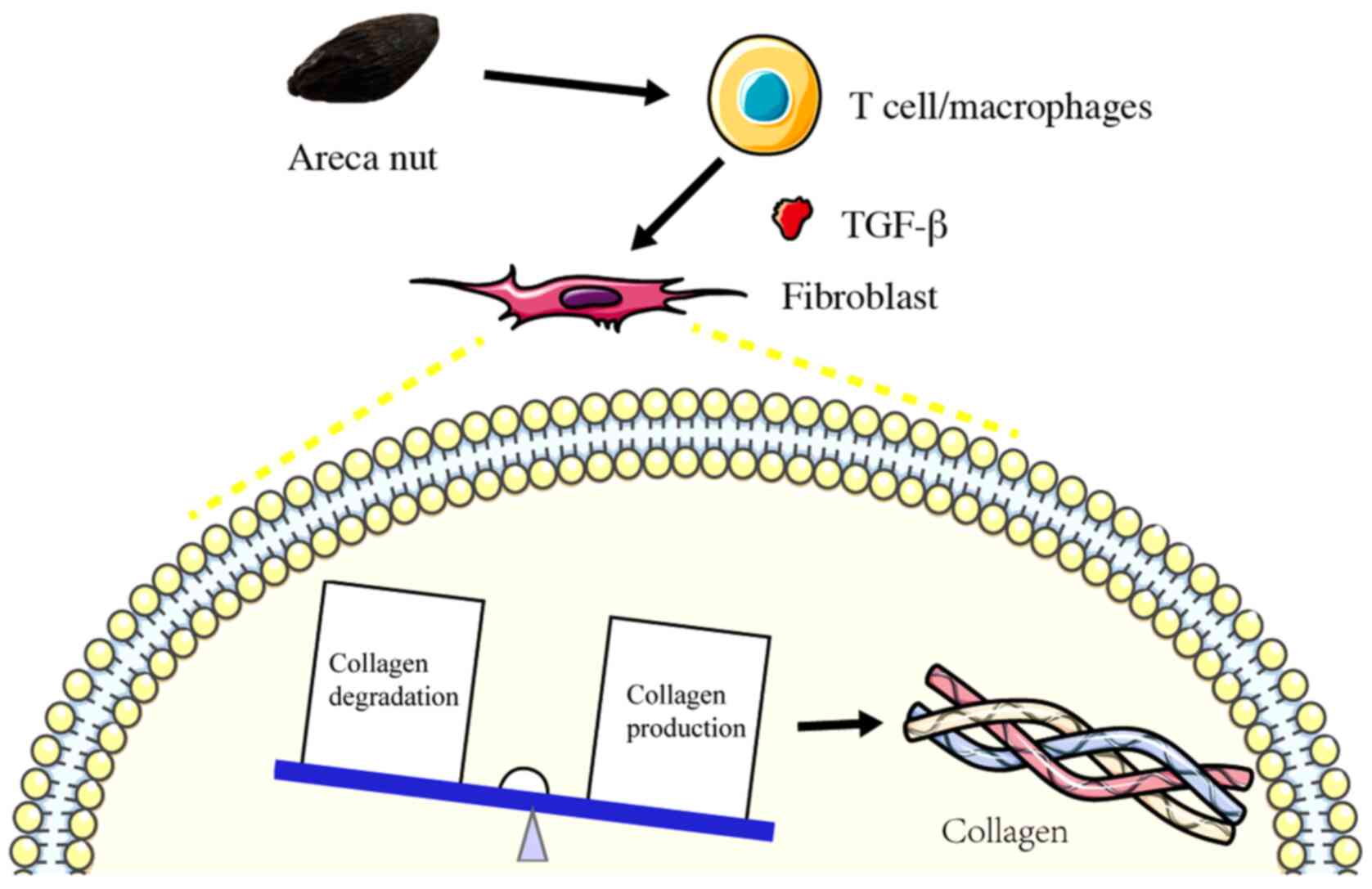
OSF is a multifactorial disease that is caused by
the chewing of areca nuts (Fig. 5).
According to reports, in 1970 ~10% of the world's population
habitually chewed areca nuts (30),
but this percentage increased to 10-20% in 2002(31). In 1996, worldwide estimates
indicated that ~2.5 million individuals were affected by OSF
(32). Excessive collagen
accumulation and hyalinization in the lamina propria, submucosa and
superficial muscle layers of the connective tissue are
characteristics of OSF (33). Since
it has been recognized as one of the oral potentially malignant
disorders (5,34), it is important to prevent malignant
transformation in patients diagnosed with OSF.
Kondaiah et al (35) revealed that areca nut can induce and
activate TGF-β in oral epithelial cells, leading to a sustained
expression of this profibrotic cytokine, which acts on the
fibroblasts and results in OSF. Previous studies have revealed that
the aberrant expression of TGF-β1 can lead to the development of a
variety of fibrotic diseases, including liver, cardiac and
pulmonary fibrosis (36-38).
The fibrotic process underlying OSF also involves excessive
collagen deposition and dysregulation in the ECM remodeling process
(23). ECM components, such as
collagen, have been suggested to hold potential as markers for
evaluating the severity of OSF (39). Therefore, it can be hypothesized
that therapeutically targeting the TGF-β1 signaling pathway and
inhibiting the active secretion of collagens may represent a
promising approach. Notably, a number of antifibrotic strategies
have been previously attempted to target the activation,
proliferation and/or recruitment of fibroblasts, including
Pirfenidone (40) and Salvia
miltiorrhiza Bunge (41). The
findings of the present study revealed that the expression levels
of α-SMA were upregulated in TGF-β1-induced oral fibroblasts, which
is a similar finding to that observed in patients with clinical OSF
(35). In addition, the expression
levels of collagen I and III were upregulated in cells stimulated
with TGF-β1 in a dose-dependent manner. These findings suggested
that TGF-β1 can significantly promote the production of collagen in
oral fibroblasts.
Mesenchymal stem cells, which have the capacity for
self-renewal and differentiation into chondrocytes, osteoblasts and
adipocytes (21), can be isolated
and expanded from a variety of tissues, including adipose tissues,
bone marrow, the umbilical cord and dental pulps (42). Due to their minute size, exosomes
confer high hemocompatibility, good stability and do not obstruct
the vasculature as a therapeutic agent (43,44).
The use of exosomes has been reported to be an effective strategy
for immune rejection to kill tumor cells and change the
microenvironment for cancer development (45,46).
In addition, the role of exosomes in prostate cancer progression
and metastasis has been attracting the attention of researchers,
with a previous study reporting their potential application as
biomarkers and therapeutic targets (47). The present study isolated ADSCs from
human adipose tissues, which were able to differentiate into
osteocytes and adipocytes under in vitro conditions. In the
presence of TGF-β1, treatment with ADSC-Exos significantly
downregulated the expression levels of collagen I and III.
Additionally, the present study suggested that ADSC-Exos also
downregulated the expression levels of COL1A1 and COL3A1 mRNA,
whilst upregulating those of MMP1 and MMP3 in TGF-β1-induced
fibroblasts. These results suggest that ADSC-Exos may not only
inhibit collagen expression, but may also promote the degradation
of collagen.
Exosomes can contain both mRNA and miRNA and be
delivered to other cells in a new location to regulate their
function (48). For example, Qu
et al (20) previously found
that ADSC-Exos containing miR-181-5p can increase autophagy and
reduce TGF-β1-induced liver fibrosis in hepatic stellate cells in a
carbon tetrachloride-induced liver fibrosis mouse model. Pant et
al (11) found that chewing of
the areca nut induced the JNK/ATF2/c-Jun axis through activation of
the TGF-β signaling pathway in OSF. Illeperuma et al
(49) found that the TGF-β1
expression levels were upregulated in 42 patients with OSF compared
with those in the normal oral mucosa and reported that
dysregulations in both collagen deposition and degradation occurred
during the fibrosis process. In addition, ADSC-Exos have been
reported to be internalized by fibroblasts, leading to the
upregulation of the gene expression levels of N-cadherin, cyclin
D1, collagen I and collagen III, thereby increasing wound healing
(50). Another previous study has
also reported the promising effects of applying ADSC-Exos for
accelerating wound healing, where they deliver miR-21 to the
fibroblasts and downregulate TGF-β1 protein expression, thereby
reducing the formation of scars (51). Therefore, there is experimental
evidence that ADSC-Exos can regulate the expression of collagen,
though the specific underlying mechanism remain a topic for further
research.
The p38 MAPK signaling pathway has been closely
associated with collagen synthesis, which promotes ECM production
(52). Li et al (52) found that ADSC-conditioned medium can
decrease collagen deposition of fibroblasts by regulating the
p38/MAPK signaling pathway. Similarly, Chai et al (53) also reported that ADSC-conditioned
medium can decrease collagen deposition and suppress scar formation
by inhibition of the p38 MAPK signaling pathway. Consistent with
these previous findings, results from the present study
demonstrated that ADSC-Exos can downregulate the levels of p38
phosphorylation and collagen I and III expression in oral
fibroblasts following TGF-β1 stimulation. However, following the
treatment with anisomycin, a p38 activator, the phosphorylation
levels of p38 and the expression levels of collagen I and III were
upregulated. These observations suggested that inhibition of the
p38 MAPK signaling pathway may serve a key role in the antifibrotic
properties of ADSC-Exos.
From the present study, the underlying mechanism
through which ADSC-Exos exert their effects on the fibroblasts
requires further investigation. A limitation of present study is
that the effect of ADSC-Exos was not investigated using in
vivo experiments and the lack of investigations in the effects
potentially mediated by TGF-β2 and 3, both of which also warrant
further investigation.
In conclusion, findings of the present study
demonstrated that ADSC-Exos can inhibit TGF-β1-induced collagen
synthesis in oral mucosal fibroblasts in vitro by regulating
the p38 MAPK signaling pathway. Therefore, the application of
ADSC-Exos may represent a promising strategy for OSF treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from The Hunan 2018
Key R & D Project of China (grant no. 2018SK2104), China Hunan
Provincial Science and Technology Program (grant no. 2017GK2265)
and The Fundamental Research Funds for the Central Universities of
Central South University (grant no. 2018zzts918).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and FL performed all the experiments and analyzed
the data. BL, ZY, LL, YW and GL contributed to data acquisition and
performed statistical analysis. LP revised the manuscript for
important intellectual content, made substantial contributions to
conception and design, gave final approval of the version to be
published, and agreed to be accountable for all aspects of the
work. JH designed the study. JL and JH confirm the authenticity of
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Scientific Research
Projects Approval Determination of Independent Ethics Committee of
Hunan Xiangya Stomatological Hospital Central South University,
affiliated with Central South University (Changsha, China). Written
informed consent was obtained from all patients before the samples
were collected during surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang PY, Chen YT, Wang YH, Su NY, Yu HC
and Chang YC: Malignant transformation of oral submucous fibrosis
in Taiwan: A nationwide population-based retrospective cohort
study. J Oral Pathol Med. 46:1040–1045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pindborg JJ and Sirsat SM: Oral submucous
fibrosis. Oral Surg Oral Med Oral Pathol. 22:764–779.
1966.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shen YW, Shih YH, Fuh LJ and Shieh TM:
Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic
Mechanisms, and Treatments. Int J Mol Sci. 21(7231)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arakeri G, Rai KK, Hunasgi S, Merkx MA,
Gao S and Brennan PA: Oral submucous fibrosis: An update on current
theories of pathogenesis. J Oral Pathol Med. 46:406–412.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoithapprabhunath TR, Maheswaran T,
Dineshshankar J, Anusushanth A, Sindhuja P and Sitra G:
Pathogenesis and therapeutic intervention of oral submucous
fibrosis. J Pharm Bioallied Sci. 5 (Suppl 1):S85–S88.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hashimoto S, Gon Y, Takeshita I, Matsumoto
K, Maruoka S and Horie T: Transforming growth Factor-beta1 induces
phenotypic modulation of human lung fibroblasts to myofibroblast
through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir
Crit Care Med. 163:152–157. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wei Y, Kim TJ, Peng DH, Duan D, Gibbons
DL, Yamauchi M, Jackson JR, Le Saux CJ, Calhoun C, Peters J, et al:
Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung
and tumor fibrosis. J Clin Invest. 127:3675–3688. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Tilakaratne WM, Klinikowski MF, Saku T,
Peters TJ and Warnakulasuriya S: Oral submucous fibrosis: Review on
aetiology and pathogenesis. Oral Oncol. 42:561–568. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rajalalitha P and Vali S: Molecular
pathogenesis of oral submucous fibrosis - a collagen metabolic
disorder. J Oral Pathol Med. 34:321–328. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kale AD, Mane DR and Shukla D: Expression
of transforming growth factor β and its correlation with
lipodystrophy in oral submucous fibrosis: An immunohistochemical
study. Med Oral Patol Oral Cir Bucal. 18:e12–e18. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pant I, Rao SG and Kondaiah P: Role of
areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β
pathway in precancerous Oral Submucous Fibrosis. Sci Rep.
6(34314)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hsieh YP, Chen HM, Lin HY, Yang H and
Chang JZ: Epigallocatechin-3-gallate inhibits
transforming-growth-factor-β1-induced collagen synthesis by
suppressing early growth response-1 in human buccal mucosal
fibroblasts. J Formos Med Assoc. 116:107–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Zhao TT, Zhang P, Xu CJ, Rong ZX,
Yan ZY and Fang CY: Autophagy mediates oral submucous fibrosis. Exp
Ther Med. 11:1859–1864. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang JZ, Yang WH, Deng YT, Chen HM and
Kuo MY: EGCG blocks TGFβ1-induced CCN2 by suppressing JNK and p38
in buccal fibroblasts. Clin Oral Investig. 17:455–461.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tkach M and Théry C: Communication by
Extracellular Vesicles: Where We Are and Where We Need to Go. Cell.
164:1226–1232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo
J, Li HM, Zhang WS, Chen CY and Xie H: Exosomes from human
umbilical cord blood accelerate cutaneous wound healing through
miR-21-3p-mediated promotion of angiogenesis and fibroblast
function. Theranostics. 8:169–184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu
Y, Luo J, Liu YW, Yin H, Huang J, et al: Exosomal DMBT1 from human
urine-derived stem cells facilitates diabetic wound repair by
promoting angiogenesis. Theranostics. 8:1607–1623. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sjoqvist S, Kasai Y, Shimura D, Ishikawa
T, Ali N, Iwata T and Kanai N: Oral keratinocyte-derived exosomes
regulate proliferation of fibroblasts and epithelial cells. Biochem
Biophys Res Commun. 514:706–712. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng
M and Liu Y: MiR-122 modification enhances the therapeutic efficacy
of adipose tissue-derived mesenchymal stem cells against liver
fibrosis. J Cell Mol Med. 21:2963–2973. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M and
Lu L: Exosomes derived from miR-181-5p-modified adipose-derived
mesenchymal stem cells prevent liver fibrosis via autophagy
activation. J Cell Mol Med. 21:2491–2502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu F, Chong Lee Shin OL, Pei G, Hu Z,
Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, et al: Adipose-derived
mesenchymal stem cells employed exosomes to attenuate AKI-CKD
transition through tubular epithelial cell dependent Sox9
activation. Oncotarget. 8:70707–70726. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang L, Hu L, Zhou X, Xiong Z, Zhang C,
Shehada HM, Hu B, Song J and Chen L: Exosomes secreted by human
adipose mesenchymal stem cells promote scarless cutaneous repair by
regulating extracellular matrix remodelling. Sci Rep.
7(13321)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khan I, Kumar N, Pant I, Narra S and
Kondaiah P: Activation of TGF-β pathway by areca nut constituents:
A possible cause of oral submucous fibrosis. PLoS One.
7(e51806)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang SF, Hsieh YS, Tsai CH, Chen YJ and
Chang YC: Increased plasminogen activator inhibitor-1/tissue type
plasminogen activator ratio in oral submucous fibrosis. Oral Dis.
13:234–238. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ni WF, Tsai CH, Yang SF and Chang YC:
Elevated expression of NF-kappaB in oral submucous fibrosis -
evidence for NF-kappaB induction by safrole in human buccal mucosal
fibroblasts. Oral Oncol. 43:557–562. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li X, Xie X, Lian W, Shi R, Han S, Zhang
H, Lu L and Li M: Exosomes from adipose-derived stem cells
overexpressing Nrf2 accelerate cutaneous wound healing by promoting
vascularization in a diabetic foot ulcer rat model. Exp Mol Med.
50:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han P, Lai A, Salomon C and Ivanovski S:
Detection of salivary small extracellular vesicles associated
inflammatory cytokines gene methylation in gingivitis. Int J Mol
Sci. 21(5273)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2 (Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rao AR and Das P: Evaluation of the
carcinogenicity of different preparations of areca nut in mice. Int
J Cancer. 43:728–732. 1989.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gupta PC and Warnakulasuriya S: Global
epidemiology of areca nut usage. Addict Biol. 7:77–83.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cox SC and Walker DM: Oral submucous
fibrosis. A review. Aust Dent J. 41:294–299. 1996.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chang MC, Lin LD, Wu HL, Ho YS, Hsien HC,
Wang TM, Jeng PY, Cheng RH, Hahn LJ and Jeng JH: Areca nut-induced
buccal mucosa fibroblast contraction and its signaling: A potential
role in oral submucous fibrosis - a precancer condition.
Carcinogenesis. 34:1096–1104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chang YC, Tsai CH, Lai YL, Yu CC, Chi WY,
Li JJ and Chang WW: Arecoline-induced myofibroblast
transdifferentiation from human buccal mucosal fibroblasts is
mediated by ZEB1. J Cell Mol Med. 18:698–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kondaiah P, Pant I and Khan I: Molecular
pathways regulated by areca nut in the etiopathogenesis of oral
submucous fibrosis. Periodontol 2000. 80:213–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Han CY, Koo JH, Kim SH, Gardenghi S,
Rivella S, Strnad P, Hwang SJ and Kim SG: Hepcidin inhibits Smad3
phosphorylation in hepatic stellate cells by impeding
ferroportin-mediated regulation of Akt. Nat Commun.
7(13817)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hu C, Dandapat A, Sun L, Khan JA, Liu Y,
Hermonat PL and Mehta JL: Regulation of TGFbeta1-mediated collagen
formation by LOX-1: Studies based on forced overexpression of
TGFbeta1 in wild-type and lox-1 knock-out mouse cardiac
fibroblasts. J Biol Chem. 283:10226–10231. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sheppard D: Transforming growth factor
beta: A central modulator of pulmonary and airway inflammation and
fibrosis. Proc Am Thorac Soc. 3:413–417. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Angadi PV, Kale AD and Hallikerimath S:
Evaluation of myofibroblasts in oral submucous fibrosis:
Correlation with disease severity. J Oral Pathol Med. 40:208–213.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dai JP, Zhu DX, Sheng JT, Chen XX, Li WZ,
Wang GF, Li KS and Su Y: Inhibition of Tanshinone IIA, salvianolic
acid A and salvianolic acid B on Areca nut extract-induced oral
submucous fibrosis in vitro. Molecules. 20:6794–6807.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Teixeira FG, Carvalho MM, Sousa N and
Salgado AJ: Mesenchymal stem cells secretome: A new paradigm for
central nervous system regeneration? Cell Mol Life Sci.
70:3871–3882. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xin H, Li Y and Chopp M: Exosomes/miRNAs
as mediating cell-based therapy of stroke. Front Cell Neurosci.
8(377)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
De Jong OG, Van Balkom BW, Schiffelers RM,
Bouten CV and Verhaar MC: Extracellular vesicles: Potential roles
in regenerative medicine. Front Immunol. 5:608. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xie C, Ji N, Tang Z, Li J and Chen Q: The
role of extracellular vesicles from different origin in the
microenvironment of head and neck cancers. Mol Cancer. 18:83.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sun X, Meng H, Wan W, Xie M and Wen C:
Application potential of stem/progenitor cell-derived extracellular
vesicles in renal diseases. Stem Cell Res Ther. 10:8.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li FX, Liu JJ, Xu F, Lin X, Zhong JY, Wu F
and Yuan LQ: Role of tumor-derived exosomes in bone metastasis.
Oncol Lett. 18:3935–3945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Illeperuma RP, Ryu MH, Kim KY, Tilakaratne
WM and Kim J: Relationship of fibrosis and the expression of
TGF-β1, MMP-1, and TIMP-1 with epithelial dysplasia in oral
submucous fibrosis. Oral Med Pathol. 15:21–28. 2010.
|
|
50
|
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu
R, Huang F, Zhang H and Chen L: Exosomes derived from human adipose
mensenchymal stem cells accelerates cutaneous wound healing via
optimizing the characteristics of fibroblasts. Sci Rep.
6(32993)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cai Y, Li J, Jia C, He Y and Deng C:
Therapeutic applications of adipose cell-free derivatives: A
review. Stem Cell Res Ther. 11(312)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J,
Yang X, He T, Guan H, Zheng Z, et al: Adipose tissue-derived stem
cells suppress hypertrophic scar fibrosis via the p38/MAPK
signaling pathway. Stem Cell Res Ther. 7(102)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chai CY, Song J, Tan Z, Tai IC, Zhang C
and Sun S: Adipose tissue-derived stem cells inhibit hypertrophic
scar (HS) fibrosis via p38/MAPK pathway. J Cell Biochem.
120:4057–4064. 2019.PubMed/NCBI View Article : Google Scholar
|