Introduction
Bovine mastitis is one of the most prevalent
diseases in dairy cows, and reduces milk production and quality
(1). The results of bovine mastitis
include lowered milk production, increased veterinary drug cost and
high culling rate. In 2020, the economic cost of bovine was ~$2
billion in the US (1-3).
Escherichia coli (E. coli) is a crucial factor for
bovine mastitis that can result in intramammary infection. Frequent
use of antibiotics to treat such infections contributes to the
emergence of antibiotic resistance (2,3).
Lipopolysaccharide (LPS), the primary cell membrane component of
gram-negative bacteria, including E. coli, promotes
intracellular signaling via Toll-like receptor 4 proteins,
activating NF-κB and augmenting the secretion of inflammatory
cytokines, which may further promote the progression of bovine
mastitis (4-6).
Mammary gland epithelial tissue is a crucial barrier against
pathogens that serves an important role in milk synthesis and
secretion (7). Therefore,
investigation of the potential mechanisms employed by bovine
epithelial cells against pathogens is of importance to understand
and prevent inflammation (8).
MicroRNAs (miRNAs/miRs) are small, endogenous,
non-coding RNAs that are 18-22 nucleotides in length (9). miRNAs serve an important role in
post-transcriptional control, regulation and modification, for
example, repressing gene expression (10). Increasing evidence suggests that
miRNAs serve a predominant role in the pathogenesis of numerous
diseases, including bovine mastitis, via regulating cell
proliferation, apoptosis, and immune and defense responses
(11,12). For instance, bovine miR-146a
enhances the immune response of mammary epithelial cells (13). Moreover, miR-92a serves as a
housekeeping gene to analyze the miRNA involved in bovine mastitis
by reverse transcription-quantitative PCR (RT-qPCR) (14). A previous study has also
demonstrated that miR-142-5p is upregulated in bovine mastitis
(15). However, the underlying
mechanisms have not been fully elucidated.
In the present study, the possible roles of
miR-142-5p in bovine mastitis and the mechanisms underlying
miR-142-5p-mediated regulation of LPS-treated MAC-T cell
proliferation, apoptosis and immune responses were investigated.
The present study may provide a potential target for novel
treatment of bovine mastitis.
Materials and methods
Cell culture
Bovine mammary epithelial MAC-T cells were obtained
from ATCC were incubated in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 and humidity.
Transfection
miR-142-5p mimics, miR-142-5p mimics negative
controls (NC), miR-142-5p inhibitors, miR-142-2-5p inhibitors
negative control (NC), pcDNA3.1 and pcDNA3.1-Bcl-2 associated
athanogene 5 (BAG5) were purchased from Shanghai GenePharma Co.,
Ltd. Cells were seeded in a 6-well plate with a density of
105 cells/ml and pre-incubated for 12 h at 37˚C, before
being transfected with 50 nM miR-142-5p mimics or miR-142-5p mimics
NC, 2 µg pc DNA3.1 or pcDNA3.1-BAG5, 50 nM miR-142-5p inhibitors or
miR-142-5p inhibitors NC with 20 nmol/l Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) then incubated at
37˚C with 5% CO2 for 48 h. Cells were harvested 48 h
after transfection for subsequent experiments. The sequences of the
miRNA constructs were as follows: miR-142-5p mimics NC,
5'-GUGUAACACGUCUAUACGCCCA-3'; miR-142-5p mimic,
5'-CAUAAAGUAGAAAGCACUACU-3'; miR-142-5p inhibitors NC,
5'-UCACAACCUCCUAGAAAGAGU-3'; miR-142-5p inhibitors,
5'-AGUAGUGCUUUCUACUUUAUG-3'.
RT-qPCR
Total RNA was extracted from MAC-T cells using
TRIzol® (Invitrogen, Thermo Fisher Scientific, Inc).
Reverse transcription was performed using a reverse transcription
kit (Applied Biosystems, Thermo Fisher Scientific, Inc.) at 72˚C
for 12 min. qPCR was performed using a SYBR qPCR Master Mix (Vazyme
Biotech Co., Ltd.) conducted using the ABI 7900HT real-time PCR
system (Applied Biosystems, Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95˚C for 5 min, denaturation at 95˚C for 15 sec and annealing
and extension at 60˚C for 45 sec, for 40 cycles. Relative miRNA and
mRNA expression levels were quantified using the 2-ΔΔCq
method (16) and normalized to the
internal reference genes U6 and GAPDH, respectively. The sequences
of the primers used were as follows: miR-142-5p forward (F),
5'-GAAGATCTCCAGCCACCTGTTTCACA-3' and reverse (R),
5'-CCGCTCGAGTAGTCCTTCACTTCATG-3'; U6 F, 5'-GCGCGTCGTGAAGCGTTC-3'
and R, 5'-GTGCAGGGTCCGAGGT-3'; BAG5 F, 5'-AGGTGTCCCCGGGTTTAG-3' and
R, 5'-GATGTTGGTTTCCCATATCCA-3'; GAPDH F,
5'-ATGGAAATCCCATCACCATCTT-3' and R, 5'-CGGCCCACTTGATTTTGG-3'.
Western blotting
MAC-T cells were harvested and lysed with RIPA lysis
buffer (Beyotime Institute of Biotechnology). Total protein was
extracted from MAC-T cells. The concentration of total protein was
determined using the BCA assay (Abcam). Each protein (20 µg
protein/lane) was isolated using a 12% SDS-PAGE gel. The separated
proteins were then transferred onto PVDF membranes, which were
blocked with 5% skimmed milk overnight at 4˚C. The membranes were
incubated at 37˚C for 2 h with primary antibodies targeted against
the following: P21 (1:1,000; cat. no. ab109199; Abcam), P27
(1:1,000; cat. no. ab75908; Abcam), Cyclin D1 (1:200; cat. no.
ab16663; Abcam), BAG5 (1:1,000; cat. no. ab182658; Abcam),
Caspase-3 (1:5,000; cat. no. ab32351; Abcam), cleaved-Caspase-3
(1:500; cat. no. ab13847; Abcam), cleaved-Caspase-9 (1:1,000; cat.
no. ab2324; Abcam), Caspase-9 (1:1,000; cat. no. ab184786; Abcam),
Bcl-2 (1:1,000; cat. no. ab32124; Abcam), Bax (1:2,000; cat. no.
ab32503; Abcam), AKT (1:10,000; cat. no. ab179463; Abcam),
phosphorylated (p)-AKT (1:1,000; cat. no. ab38449; Abcam), p65
(1:5,000; cat. no. ab32536; Abcam), p-p65 (1:1,000; cat. no.
ab28856; Abcam), GAPDH (1:10,000; cat. no. ab181602; Abcam). Then
the membranes were washed with PBS plus 0.1% Tween 20 at room
temperature for three times and incubated with HRP-conjugated
secondary antibodies (1:5,000; cat. no. ab6721; Abcam) at room
temperature for 1 h. Protein bands were visualized using an ECL kit
(GE Healthcare) and protein expression was semi-quantified using
ImageJ software (version 1.6; National Institutes of Health). GAPDH
was used as a loading control.
Dual-luciferase reporter assay
BAG5 was predicted to be a target gene of miR-142-5p
based on analysis with TargetScan (http://www.targetscan.org, v7.2), which yielded a
context++ score (The context++ score for a specific site is the sum
of the contribution of a series of features). The 3'-untranslated
region (3'UTR) of BAG5 was amplified via PCR from bovine mammary
epithelial MAC-T cell cDNA and then inserted into the multiple
cloning site downstream of the luciferase reporter gene in the
pMIR-REPORT™ luciferase plasmid (Thermo Fisher Scientific, Inc.) to
construct the luciferase reporter plasmid [BAG5 3'UTR wild-type
(WT)]. MAC-T cells were transfected with 1 µg 3'UTR WT or mutant
(MUT) constructed by chemical synthesis (General Biosystems Co.,
Ltd.) and 50 nM miR-142-5p mimics or miR-142-5p mimics NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
transfected BAG5 3'UTR WT or MUT plasmid served as blank group.
After 24 h incubation at 37˚C, the cells were lysed by lysis buffer
as supplied by the Dual-Luciferase Detection kit (Beyotime
Institute of Biotechnology) on ice and luciferase activity was
measured using a Dual-Lumi II Luciferase Reporter Gene Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Firefly luciferase activities were
normalized to Renilla luciferase activities.
Cell Counting Kit-8 (CCK-8) assay
At 48 h post-transfection, MAC-T cells were plated
into 96 well plates (2x103 cells/well). MAC-T cells were
then treated with 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) at 37˚C for 2 h and the cell viability detected at
24, 48 and 72 h. The absorbance rate of each compound was measured
at a wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.). Each experiment was performed in
triplicate.
Flow cytometry assay
Transfected MAC-T cells were seeded into 24-well
plates (5x104 cells/well) and treated with 500 µl
binding buffer, with 5 µl propidium iodide (PI) and Annexin V-FITC
from Annexin V-APC/PI Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd.) in the dark for 15 min at room temperature. The
stained MAC-T cells were analyzed using flow cytometry (FACScan; BD
Biosciences) and the apoptotic rate was determined using FlowJo
7.6.1 software (Tree Star, Inc.).
For cell cycle analysis, cells were fixed with 70%
ethanol at 4˚C overnight. The cells were treated with 10 mg/ml
RNase A (Beijing Solarbio Science & Technology Co., Ltd.), 400
mg/ml PI (Beyotime Institute of Biotechnology) and 0.1% Triton
X-100 (Beijing Solarbio Science & Technology Co., Ltd.) in the
dark for 30 min at 37˚C. The results were evaluated via flow
cytometry (Cytoflex; Beckman Coulter, Inc.) and quantified by BD
CellFIT software (v 7.6.2; BD Biosciences).
Clone formation assay
MAC-T cells were seeded into 6-well plates
(2x103 cells/well) and the culture was terminated when
clonal cell cluster became visible to the naked eye and contained
>50 cells. Following this, cells were fixed with 10%
formaldehyde and 1% crystal violet for 30 min at 25˚C. Colony
formation was determined using a light microscope in 10 randomly
chosen fields and quantified by ImageJ software v 1.5.3 (National
Institutes of Health).
ELISA
MAC-T cells were seeded into a 24-well plate
(5x104 cells/well). The levels of cytokines was
determined using TNF-α (TWp024586); IL-1β (TWp023753); IL-6
(TWp023756); IL-8 (TWp023757) and IL-10 (TWp002476) ELISA kits
(Shanghai Tongwei Industrial Co., Ltd.) according to the
manufacturer's protocol.
5-Ethynyl-2'-deoxyuridine (EdU)
assay
An EdU assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to determine MAC-T cell viability. Cells
were plated into a 24-well plate (5x104 cells/well).
Each well was supplemented with 50 µM EdU for 2 h at 37˚C.
Subsequently, MAC-T cells were stained with DAPI (Beyotime
Institute of Biotechnology) for 5 min in the dark at room
temperature and then cultured with 1X ApolloR reaction cocktail
supplied with EdU assay kit (Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at room temperature, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature. Cell viability was
determined using a fluorescent microscope.
Immunofluorescence staining
Cells were seeded into a 24-well plate
(5x104 cells/well) containing slides and incubated for
24 h at 37˚C with 5% CO2, then the slides were taken
out. Cells were fixed with 4% paraformaldehyde for 30 min at room
temperature, blocked with 5% BSA for 30 min at room temperature and
incubated with an anti-BAG5 primary antibody (1:1,000; cat. no.
ab182658; Abcam) overnight at 4˚C, followed by incubation with a
secondary goat-anti-rabbit antibody (1:5,000; cat. no. ab6721;
Abcam) at room temperature for 1 h in the dark. Cells were then
stained with DAPI for 5 min at room temperature (Beyotime Institute
of Biotechnology). Images were captured using a fluorescent
microscope (Nikon Corporation) with Zen imaging software 2.3 (Carl
Zeiss AG).
Statistical analysis
Data are presented as the mean ± SD of at least
three independent experiments. Statistical analyses were performed
using SPSS 19.0 (IBM Corp.). Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
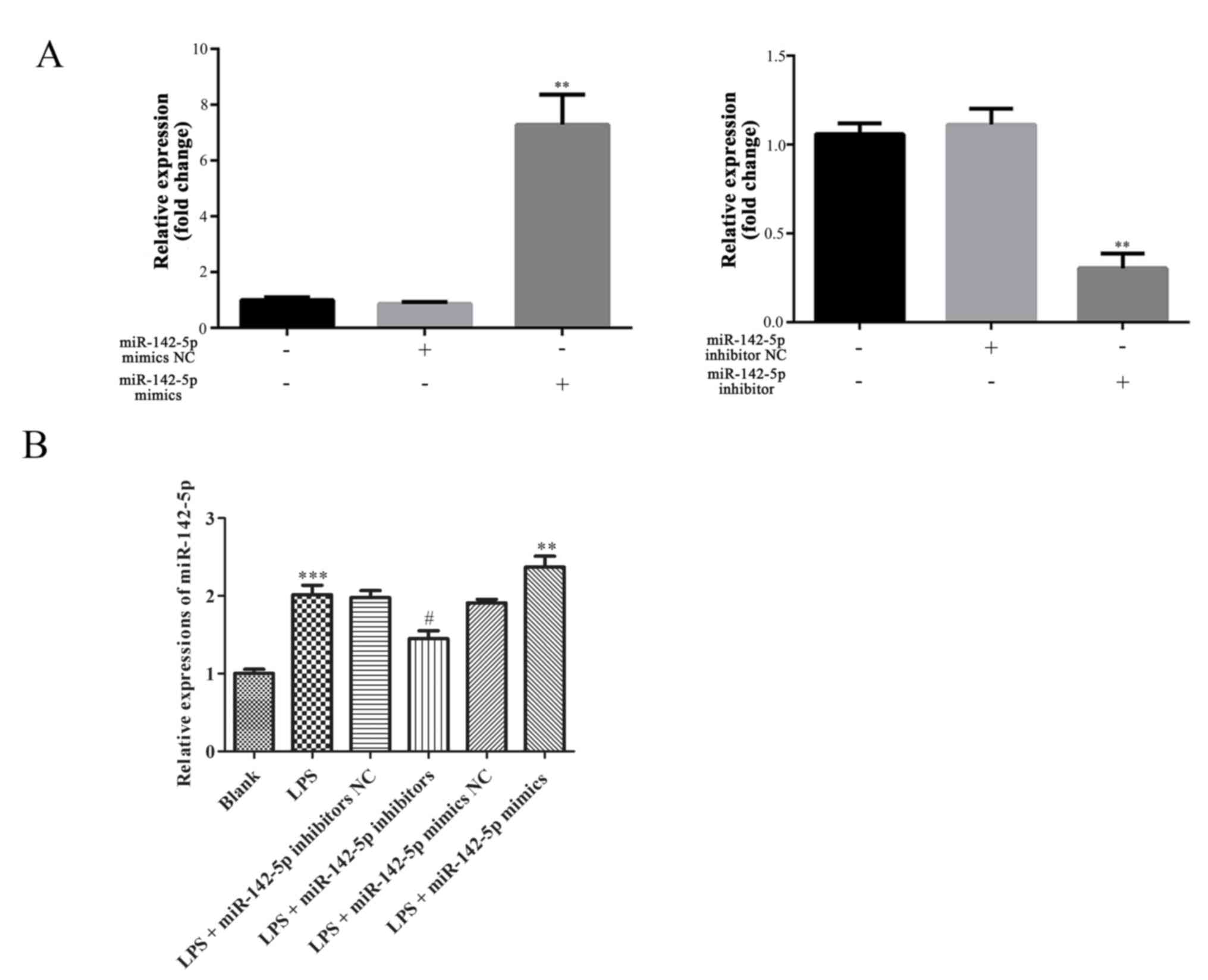
Expression of miR-142-5p
The expression of miR-142-5p was significantly
upregulated or downregulated following transfection with miR-142-5p
mimics or miR-142-5p inhibitors compared with other treatments,
respectively (Fig. 1A).
Additionally, compared with the blank groups, the fluorescence
intensity of GFP notably increased or decreased following
transfection with miR-145-5p mimics or miR-142-5p inhibitors,
respectively (Fig. 1B).
Furthermore, the expression of miR-142-5p in cells treated with LPS
was significantly increased, which was significantly increased
following transfection with miR-142-5p mimics. By contrast,
compared with the LPS group, miR-142-5p expression was
significantly lower following transfection with miR-142-5p
inhibitors (Fig. 1C).
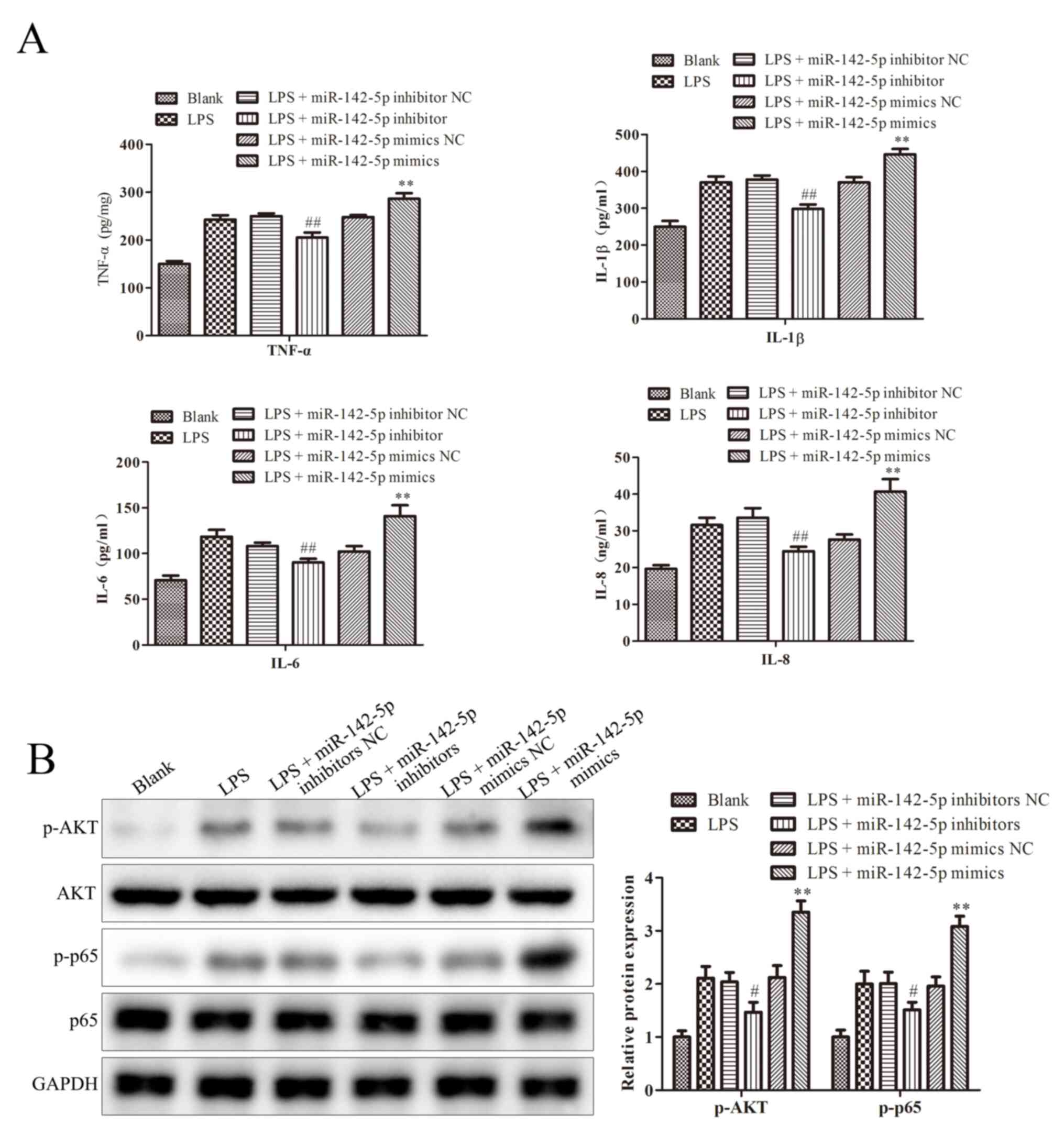
miR-142-5p regulates the expression of
cytokines and NF-κB signaling pathway-related proteins
Cytokines have previously been shown to contribute
to the progression of bovine mastitis. Sun et al reported
that blocking NF-κB pathway significantly relieves bovine mastitis
(17). Wang et al reported
that TNF-α, IL-1β and IL-6 and IL-8 levels are imported indicators
in bovine mastitis (18). As shown
in Fig. 2A, compared with the LPS
group, miR-142-5p mimics significantly increased TNF-α, IL-1β and
IL-6 and IL-8 levels, whereas the opposite effect was observed in
cells transfected with miR-142-5p inhibitors. Meanwhile, compared
with the LPS + miR-142-5p mimics NC group, the protein expression
levels of AKT and p65 in cells treated with miR-142-5p mimics + LPS
were significantly upregulated, but miR-142-5p inhibitors
transfection significantly downregulated LPS-induced protein
expression levels, compared to LPS + miR-142-5p inhibitor NC group
(Fig. 2B). These results were
consistent with the western blotting results for p-AKT and p-p65
protein expression levels (Fig.
2B).
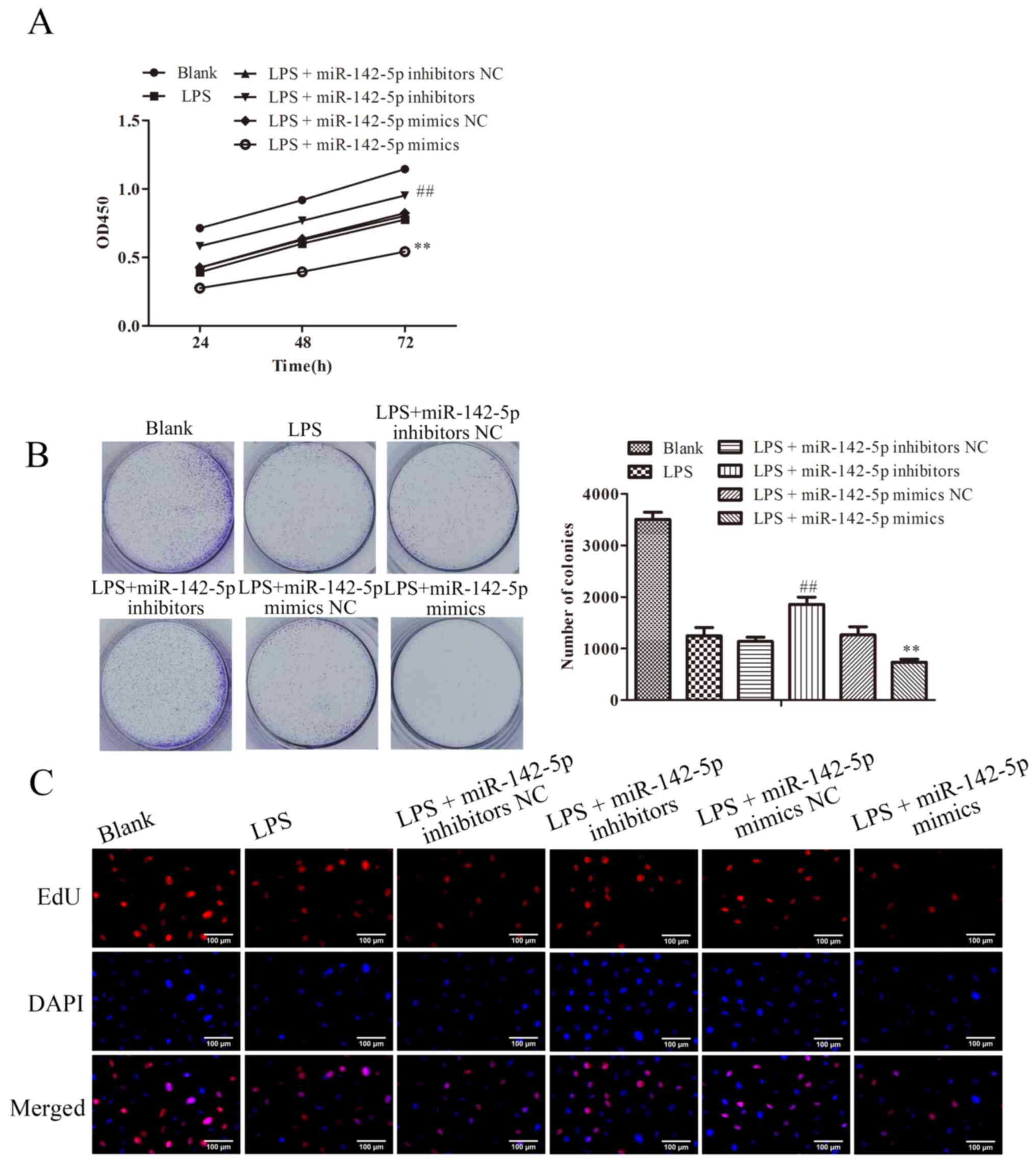
miR-142-5p regulates the progression
of LPS-induced MAC-T cells
To further investigate the potential roles of
miR-142-5p in bovine mastitis, CCK-8, clone formation and EdU
assays were performed to evaluate the effects of miR-142-5p on the
proliferation and cell viability of LPS-treated MAC-T cells. As
shown in Fig. 3A, LPS significantly
suppressed MAC-T cell viability, which was significantly enhanced
in the LPS + miR-142-5p mimics group. By contrast, compared with
the LPS group, MAC-T cell viability was significantly increased
following transfection with miR-142-5p inhibitor. These results
were in line with those from clone formation; compared with blank
group, LPS-treatment suppressed the clone forming ability of cells,
by contrast, compared with the LPS group, the clone forming ability
of cells was increased with miR-142-5p inhibitor transfection.
Similar results were shown by EdU assay (Fig. 3B and C).
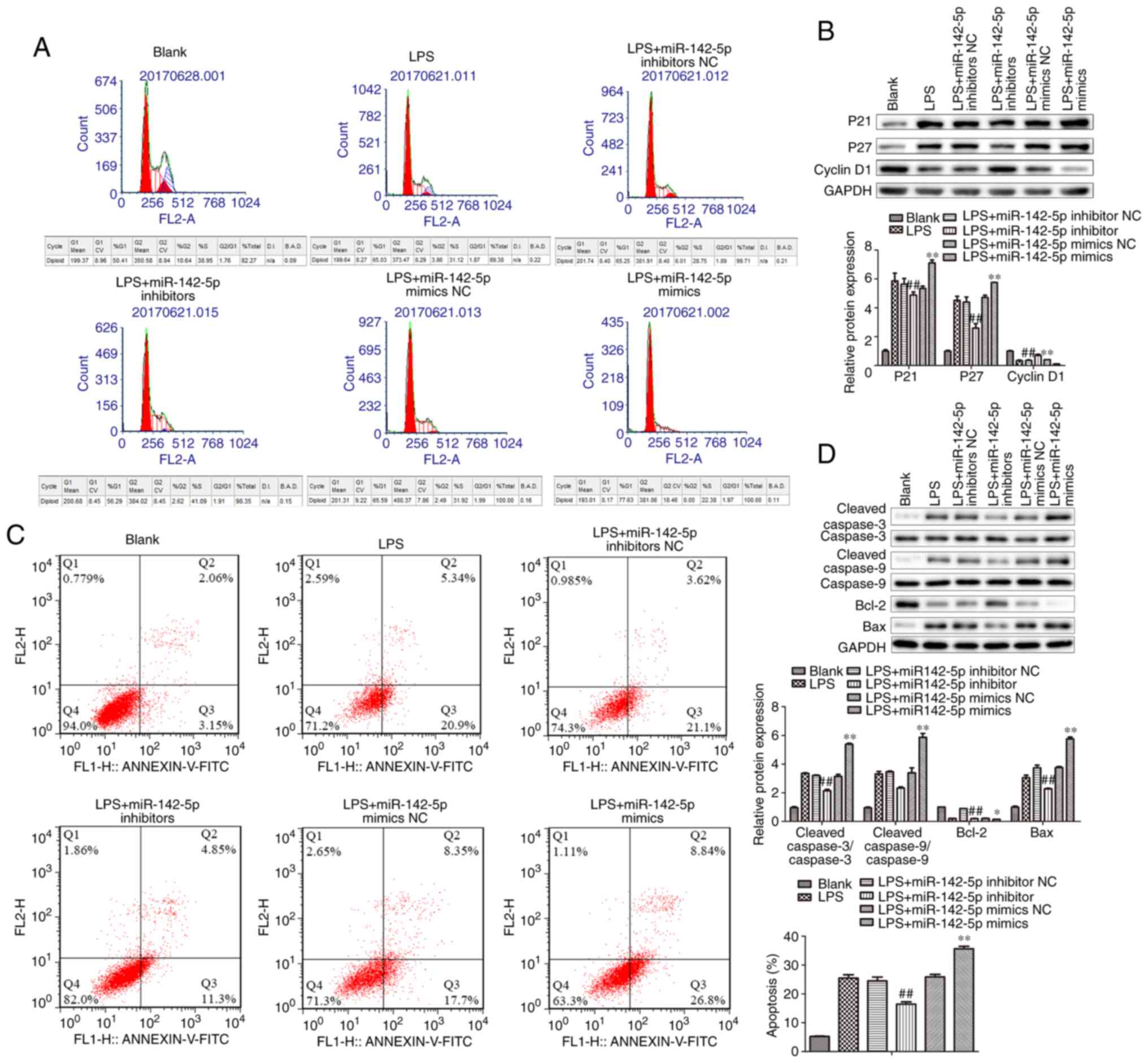
miR-142-5p regulates the cell cycle
and apoptosis of MAC-T cells
Compared with the blank group, a marked increase in
cells arrested in the G0/G1 phase of the cell
cycle was observed in the LPS group, which was notably enhanced in
the LPS + miR-142-5p mimics group, but obviously reversed in the
LPS + miR-142-5p inhibitors group (Fig.
4A). Furthermore, the protein expression levels of P21 and P27
were significantly increased, whereas Cyclin D1 protein expression
levels were significantly decreased in the LPS + miR-142-5p mimics
group compared with the LPS group (Fig.
4B). By contrast, miR-142-5p inhibitors significantly reversed
LPS-mediated effects on protein expression.
To further verify the possible roles of miR-142-5p
in regulating MAC-T cells, the rate of apoptosis was also examined.
As shown in Fig. 4C, miR-142-5p
mimics significantly increased the apoptotic rate in LPS-induced
MAC-T cells, whereas miR-142-5p inhibitors significantly reversed
LPS-induced MAC-T cell apoptosis. Additionally, miR-142-5p mimics
significantly increased the protein expression levels of
cleaved-Caspase-3/Caspase-3, cleaved-Caspase-9/Caspase-9 and Bax,
and significantly decreased Bcl-2 protein expression levels in
LPS-treated MAC-T cells, whereas the opposite effect was observed
following miR-142-5p inhibitors transfection (Fig. 4D).
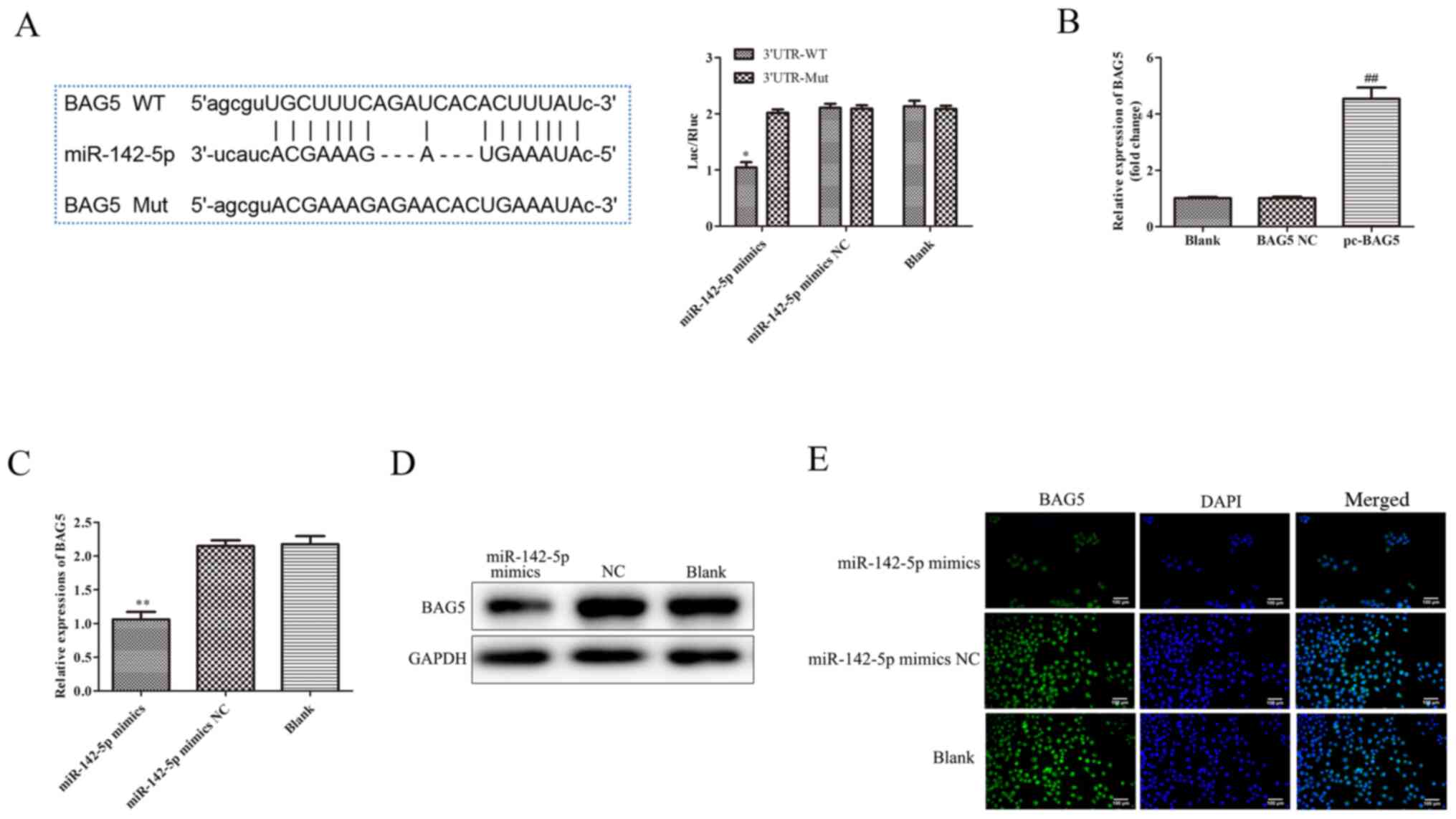
BAG5 is a target of miR-142-5p
Bioinformatics analysis demonstrated BAG5 was a
target of miR-142-5p. TargetScan (http://www.targetscan.org/vert_72/) predicted that
BAG5 was a target of miR-142-5p, possessing a relatively high
Context++ score. The binding sites of miR-142-5p on BAG5 are
presented in Fig. 5A. The
luciferase reporter assay results suggested that, compared with the
miR-142-5p mimics NC group, the luciferase activity of MAC-T cells
was significantly reduced after co-transfection with miR-142-5p
mimics and BAG5 3'UTR WT, there was no significant difference
between the blank group (transfected BAG5 3'UTR WT or MUT plasmid)
and miR-142-5p mimics NC groups, but following co-transfection with
miR-142-5p mimics and BAG5 3'UTR MUT, the levels of luciferase
activities did not change significantly.
RT-qPCR and western blotting were performed to
determine the mRNA and protein expression levels of BAG5,
respectively. BAG5 mRNA expression was significantly increased by
transfection with Lv BAG5 compared with BAG5 NC (Fig. 5B). As shown in Fig. 5C, the mRNA expression level of BAG5
and, in 5D, the protein expression levels of BAG5 were
significantly decreased by miR-142-5p mimics compared with
miR-142-5p mimics NC. The immunofluorescence assay demonstrated
that the signal density of BAG5 was markedly reduced by miR-142-5p
mimics transfection compared with miR-142-5p mimics NC transfection
(Fig. 5E).
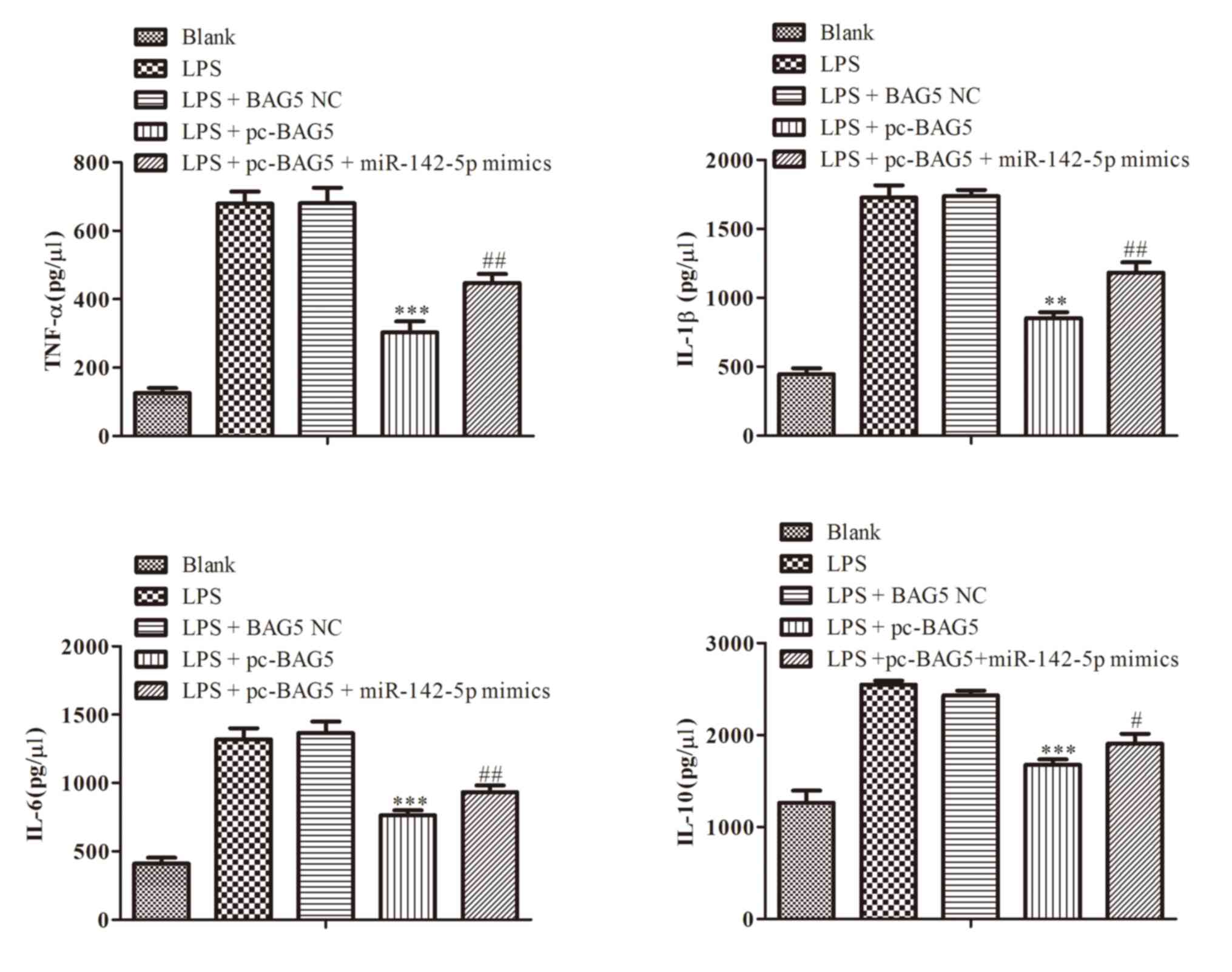
BAG5 regulates the levels of
cytokines
ELISAs were performed to determine the levels of
cytokines in the cell culture medium. The results indicated that
the level of BAG5 was increased by LPS-induced activation of
cytokines, such as TNF-α, IL-1β and IL-6 and IL-8, which was
significantly reversed by transfection with miR-142-5p mimics
(Fig. 6).
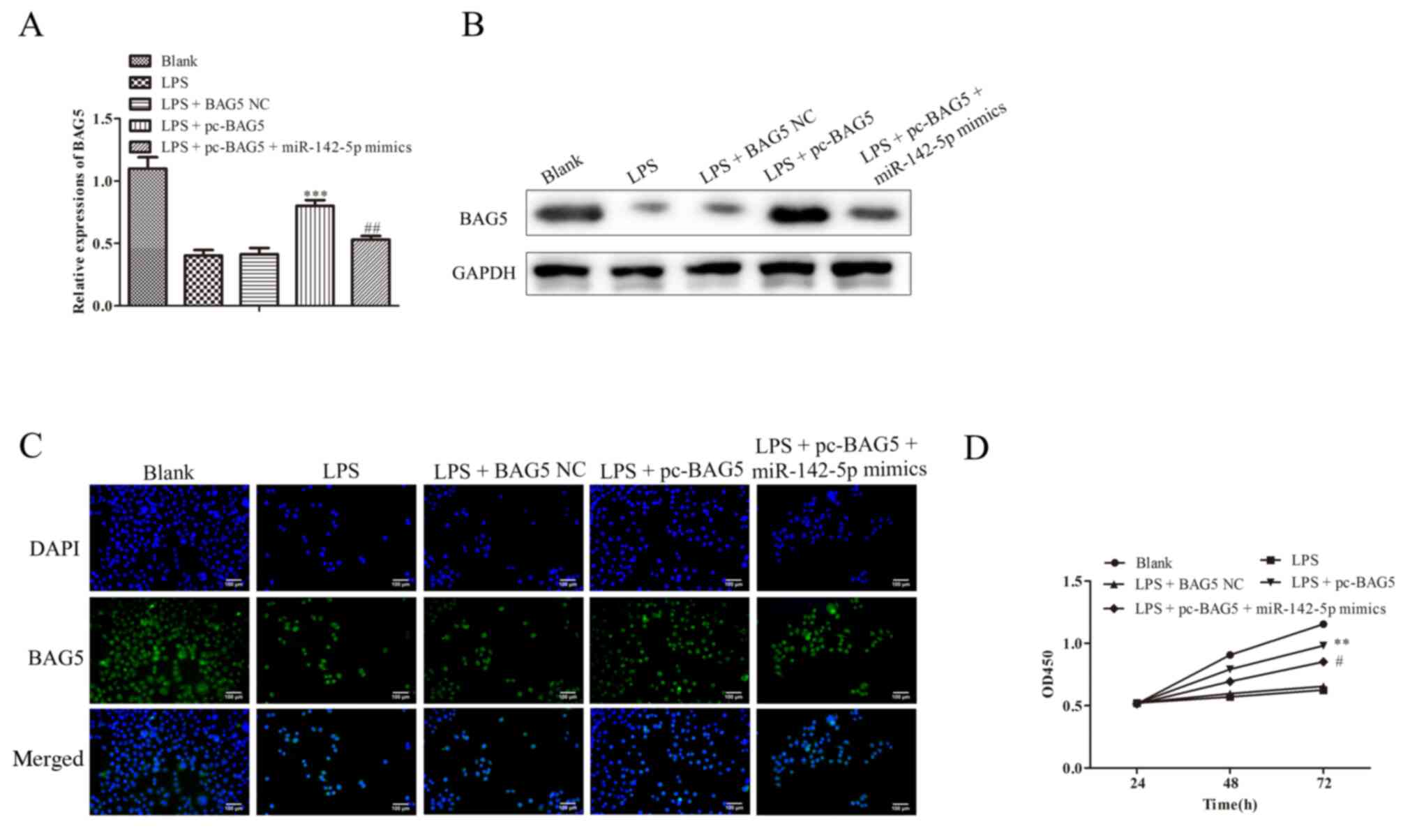
BAG5 regulates the cell viability of
LPS-treated MAC-T cells
As shown in Fig. 7A
and B, the mRNA and protein
expression levels of BAG5 were decreased in cells transfected with
LPS treatment, which was reversed by co-transfection with pc-BAG5.
The immunofluorescence assay results were consistent with the
RT-qPCR and western blotting results (Fig. 7C). The results suggested that Lv
BAG5 markedly increased BAG5 expression levels, which was notably
reversed by co-transfection with miR-142-5p mimics (Fig. 7D).
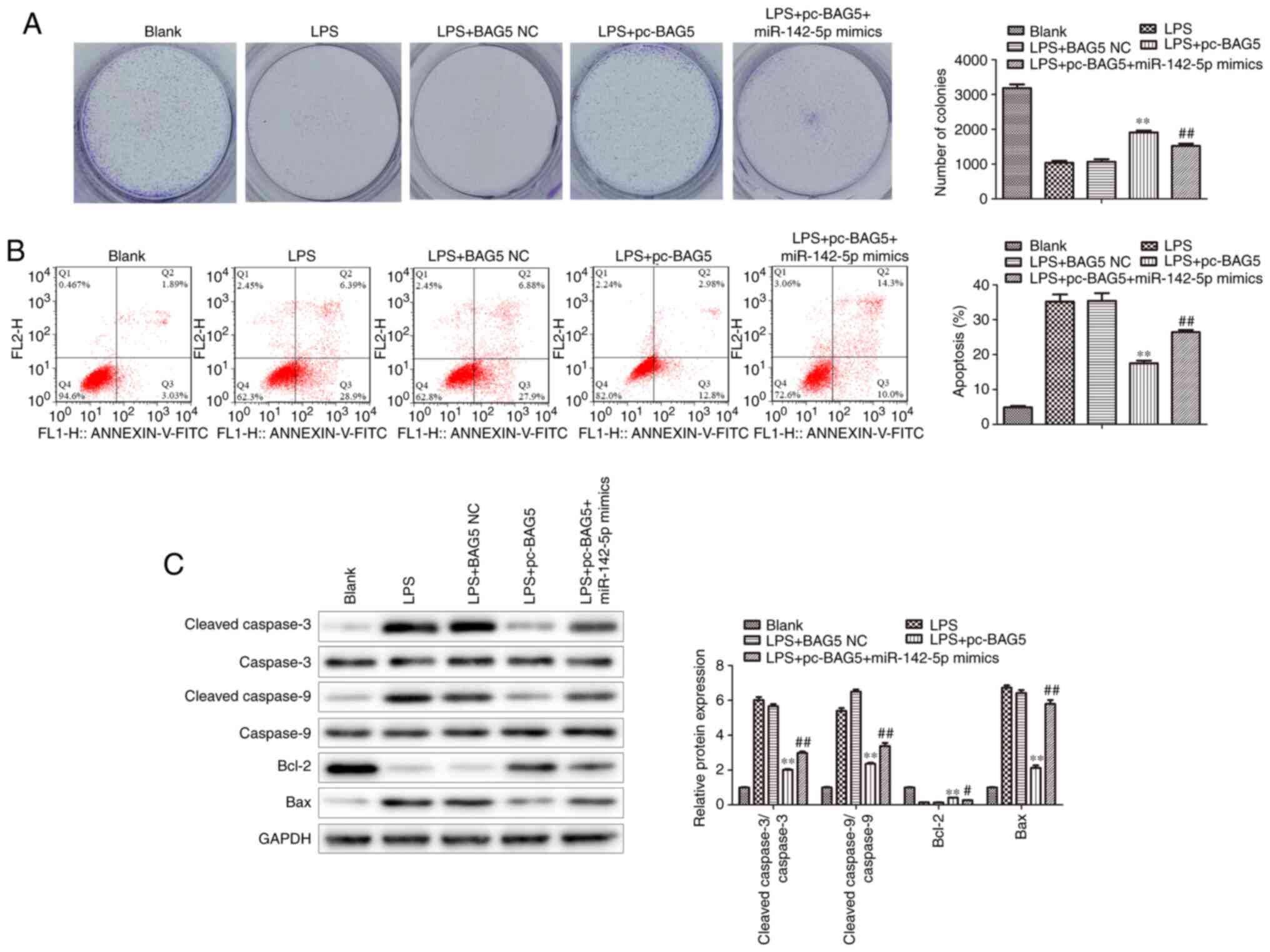
BAG5 regulates the proliferation and
apoptosis of LPS-treated MAC-T cells
BAG5 overexpression increased cell proliferation in
LPS-treated MAC-T cells, which was alleviated by miR-142-5p mimics
transfection (Fig. 8A). As shown in
Fig. 8B, BAG5 overexpression
significantly decreased the apoptotic rate of LPS-treated MAC-T
cells, which was significantly reversed by miR-142-5p mimics
transfection. Moreover, compared with the LPS group, BAG5
overexpression significantly decreased the protein expression
levels of cleaved-Caspase-3/Caspase-3, cleaved-Caspase-9/Caspase-9
and Bax, and significantly increased Bcl-2 protein expression
levels in LPS-treated MAC-T cells, which was significantly reversed
by miR-142-5p mimics transfection (Fig.
8C).
Discussion
Dysregulated miR-142 expression induced by
inflammatory stimuli such as LPS has been revealed in organs, such
as human bones and lungs (19,20).
However, the expression of miR-142 differs under varying conditions
(19,20). For instance, miR-142-3p is
downregulated in human MH7A cells induced by LPS, but upregulated
in LPS-induced acute kidney injury rat mesangial cells (21). As a crucial element of the E.
coli cell membrane, LPS induces an inflammatory response in the
host (4-6).
Increasing evidence has suggested that various miRNAs participate
in inflammatory responses to LPS (11,12).
In the present study, the expression of miR-142-5p was
significantly upregulated in bovine mammary epithelial MAC-T cells
treated with LPS compared with the blank group. In LPS-treated
cells, miR-142-5p mimics transfection significantly increased the
expression of cytokines, which function as prestigious regulators
of inflammation, serve an important role in the pathogenesis of
various diseases, such as, acute lung injury in human, myocarditis
in human and bovine mastitis, and promote the activation of NF-κB
signaling pathways (22-24).
Previous studies have also demonstrated that miR-142-5p upregulated
the expression of cytokines through activation of NF-κB signaling
pathways (25,26). In the present study, the results
indicated that miR-142-5p activated NF-κB signaling pathways,
further upregulating the expression of cytokines, such as TNF-α,
IL-1β, IL-6 and IL-8, in LPS-treated cells. These results
demonstrated that miR-142-5p may have a proinflammatory role in
bovine mastitis, consistent with Sun et al (15). Furthermore, a 2017 study revealed
that the degradation of epithelial cells induces the secretion of
toxic and proinflammatory endogenous substances, promoting the
progression of bovine mastitis (27). For example, miR-142-5p
overexpression contributes to the proliferation of epithelial cells
in colorectal cancer (28).
However, the potential mechanisms responsible for these outcomes
have not been fully elucidated.
In the present study, to investigate the possible
roles of miR-142-5p in bovine mastitis, MAC-T cells were exposed to
LPS. Subsequently, cell cycle progression and the inflammatory
response, which was assessed by the differential expression of
cytokines, were investigated. An increased rate of apoptosis,
G0/G1 cell cycle phase arrest, and suppressed
cell proliferation and viability following the transfection of
miR-142-5p mimics in LPS-treated cells indicated that miR-142-5p
degraded bovine mammary epithelial cells. Additionally, the results
indicated that miR-142-5p was required for the regulation of cell
cycle-and apoptosis; Caspase-3, Caspase-9, Bcl-2 and Bax protein
expression. These results further revealed that miR-142-5p
negatively regulated the cell cycle progression of MAC-T cells or
induced the degradation of MAC-T cells, which may further
contribute to the progression of bovine mastitis. However, the
underlying molecular mechanisms are still unknown.
BAG proteins are multifunctional (29). On one hand, the BAG domain
suppresses interactions with the ATPase domain of Hsp70/Hsc70
molecular chaperones; on the other hand, BAG family proteins serve
as regulators or co-chaperone proteins interacting with Hsp70 to
regulate cell behavior, such as proliferation, apoptosis and
inflammatory responses (30). For
example, BAG3 functions as an antiapoptosis gene, suppressing
colorectal cancer cell apoptosis, whereas BAG1 is involved in the
development of chronic rhinosinusitis (31,32).
BAG5 also participates in the progression of breast cancer and
optic nerve head glial activation (33,34).
Interestingly, BAG5 exerts a regulatory role in epithelial cells of
epithelial ovarian cancer (35).
The potential roles of BAG5 in the initiation and progression of
inflammation and breast cancer indicated the potential of BAG5 to
serve a crucial role in bovine mastitis (31,32).
In the present study, BAG5 was shown to be a target of miR-142-5p.
Bioinformatics analysis demonstrated that BAG5 and miR-142-5p
shared a relative high Context++ score, indicating highly
conserved. The present study investigated the potential roles of
BAG5 in bovine mastitis. The results indicated that BAG5
overexpression mediated the upregulation of cytokines and the
activation of NF-κB signaling pathways induced by LPS and/or
miR-142-5p, which suggested that BAG5 served an anti-inflammatory
role in bovine mastitis. Moreover, the present study suggested that
BAG5 served an important role in regulating MAC-T cell viability,
proliferation, cell cycle progression and apoptosis, which was
further reiterated by the regulatory role of BAG5 in cell cycle-
and apoptosis-related protein expression. Additionally, miR-142-5p
mimics transfection abated the effects of BAG5 overexpression on
MAC-T cell viability, proliferation, cell cycle and apoptosis,
indicating that miR-142-5p may promote the degradation of MAC-T
epithelial cells via targeting BAG5.
However, there were some limitations to the present
study. To confirm the results shown here, in vivo
experiments should be conducted It should also be noted that an
miRNA may have several target genes and a gene may be targeted by
several miRNAs. Overall, the present study suggested that
miR-142-5p may serve a proinflammatory role in bovine mastitis via
targeting BAG5, suggesting a novel therapeutic strategy for bovine
mastitis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
Jiangsu Province (China; grant no. BK20151354) and National Natural
Science Foundation of China (grant nos. 31472164 and 31502033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BG and JingL performed the experiments. BG, JinyL,
WL and JiangL collected materials and interpreted the data. JingL
designed and approved the current study. BG and JingL confirm the
authenticity of all the raw data. BG and JiangL reviewed the
results. JinyeL, WL and JingL reviewed the introduction,
methodology and conclusion. All authors read and approved the final
manuscript.
Ethics approval and consent for
participation
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng WN, Jeong CH, Seo HG and Han SG:
Moringa extract attenuates inflammatory responses and increases
gene expression of casein in bovine mammary epithelial cells.
Animals (Basel). 9(391)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fazel F, Jamshidi A and Khoramian B:
Phenotypic and genotypic study on antimicrobial resistance patterns
of E. coli isolates from bovine mastitis. Microb Pathog.
132:355–361. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Locatelli C, Barberio A, Bonamico S,
Casula A, Moroni P and Bronzo V: Identification of
multidrug-resistant escherichia coli from bovine clinical mastitis
using a ceftiofur-supplemented medium. Foodborne Pathog Dis.
16:590–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu
X, Li Y, Jing Y, Yuan X, Fu Y, et al: Evodiamine prevents dextran
sulfate sodium-induced murine experimental colitis via the
regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother.
110:786–795. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang X, Zhao Y, Bai D, Yuan X and Cong S:
Schizandrin protects H9c2 cells against lipopolysaccharide-induced
injury by downregulating Smad3. J Biochem Mol Toxicol.
33(e22301)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bielaszewska M, Marejková M, Bauwens A,
Kunsmann-Prokscha L, Mellmann A and Karch H: Enterohemorrhagic
Escherichia coli O157 outer membrane vesicles induce
interleukin 8 production in human intestinal epithelial cells by
signaling via Toll-like receptors TLR4 and TLR5 and activation of
the nuclear factor NF-κB. Int J Med Microbiol. 308:882–889.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gonen E, Nedvetzki S, Naor D and Shpigel
NY: CD44 is highly expressed on milk neutrophils in bovine mastitis
and plays a role in their adhesion to matrix and mammary
epithelium. Vet Res. 39(29)2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Enger BD, Tucker HLM, Nickerson SC,
Parsons CLM and Akers RM: Effects of Staphylococcus aureus
intramammary infection on the expression of estrogen receptor α and
progesterone receptor in mammary glands of nonlactating cows
administered estradiol and progesterone to stimulate mammary
growth. J Dairy Sci. 102:2607–2617. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naveed A, Ur-Rahman S, Abdullah S and
Naveed MA: A concise review of microRNA exploring the insights of
microRNA regulations in bacterial, viral and metabolic diseases.
Mol Biotechnol. 59:518–529. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen X, Tan W, Li W, Li W, Zhu S, Zhong J,
Shang C and Chen Y: miR-1226-3p promotes sorafenib sensitivity of
hepatocellular carcinoma via downregulation of DUSP4 expression. J
Cancer. 10:2745–2753. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lai YC, Fujikawa T, Maemura T, Ando T,
Kitahara G, Endo Y, Yamato O, Koiwa M, Kubota C and Miura N:
Inflammation-related microRNA expression level in the bovine milk
is affected by mastitis. PLoS One. 12(e0177182)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Z, Xu X, Tan T, Chen D, Liang H, Sun
K, Li M, Zhang H, Mao Y and Yang Z: MicroRNA-145 regulates immune
cytokines via targeting FSCN1 in Staphylococcus aureus-induced
mastitis in dairy cows. Reprod Domest Anim. 54:882–891.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang XP, Luoreng ZM, Zan LS, Li F and Li
N: Bovine miR-146a regulates inflammatory cytokines of bovine
mammary epithelial cells via targeting the TRAF6 gene. J Dairy Sci.
100:7648–7658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lai YC, Fujikawa T, Ando T, Kitahara G,
Koiwa M, Kubota C and Miura N: Rapid communication: MiR-92a as a
housekeeping gene for analysis of bovine mastitis-related microRNA
in milk. J Anim Sci. 95:2732–2735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sun J, Aswath K, Schroeder SG, Lippolis
JD, Reinhardt TA and Sonstegard TS: MicroRNA expression profiles of
bovine milk exosomes in response to Staphylococcus aureus
infection. BMC Genomics. 16(806)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun L, Chen L, Wang F, Zheng X, Yuan C,
Niu Q, Li Z, Deng L, Zheng B, Li C and Zhou X: Exogenous hydrogen
sulfide prevents lipopolysaccharide-induced inflammation by
blocking the TLR4/NF-κB pathway in MAC-T cells. Gene. 710:114–121.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang W, Hu X, Shen P, Zhang N and Fu Y:
Sodium houttuyfonate inhibits LPS-induced inflammatory response via
suppressing TLR4/NF-ĸB signaling pathway in bovine mammary
epithelial cells. Microb Pathog. 107:12–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen H, Lan Z, Li Q and Li Y: Abnormal
expression of long noncoding RNA FGD5-AS1 affects the development
of periodontitis through regulating miR-142-3p/SOCS6/NF-κB pathway.
Artif Cells Nanomed Biotechnol. 47:2098–2106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park J, Jeong S, Park K, Yang K and Shin
S: Expression profile of microRNAs following bone marrow-derived
mesenchymal stem cell treatment in lipopolysaccharide-induced acute
lung injury. Exp Ther Med. 15:5495–5502. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu X, Hong C, Wu S, Song S, Yang Z, Cao
L, Song T and Yang Y: Downregulation of lncRNA TUG1 contributes to
the development of sepsis-associated acute kidney injury via
regulating miR-142-3p/sirtuin 1 axis and modulating NF-κB pathway.
J Cell Biochem: Mar 4, 2019 (Epub ahead of print).
|
|
22
|
Ohashi E, Kohno K, Arai N, Harashima A,
Ariyasu T and Ushio S: Adenosine N1-oxide exerts anti-inflammatory
effects through the PI3K/Akt/GSK-3β signaling pathway and promotes
osteogenic and adipocyte differentiation. Biol Pharm Bull.
42:968–976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Q, Zhang KX, Li TY, Piao XM, Lian ML,
An RB and Jiang J: Cardamine komarovii flower extract reduces
lipopolysaccharide-induced acute lung injury by inhibiting
MyD88/TRIF signaling pathways. Chin J Nat Med. 17:461–468.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han FL, Liang F, Jiang TC and Liu M:
Increased expression of CXCR5 and CXCL13 in mice with experimental
autoimmune myocarditis. Eur Rev Med Pharmacol Sci. 21:1860–1867.
2017.PubMed/NCBI
|
|
25
|
Su S, Zhao Q, He C, Huang D, Liu J, Chen
F, Chen J, Liao JY, Cui X, Zeng Y, et al: miR-142-5p and
miR-130a-3p are regulated by IL-4 and IL-13 and control
profibrogenic macrophage program. Nat Commu. 6(8523)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lou Z, Peng Z, Wang B, Li X, Li X and
Zhang X: miR-142-5p promotes the osteoclast differentiation of bone
marrow-derived macrophages via PTEN/PI3K/AKT/FoxO1 pathway. J Bone
Miner Metab. 37:815–824. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Chen W, Ali T, Alkasir R, Yin J,
Liu G and Han B: Staphylococcal enterotoxin H induced apoptosis of
bovine mammary epithelial cells in vitro. Toxins (Basel).
6:3552–3567. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu S, Xiao Z, Ai F, Liu F, Chen X, Cao K,
Ren W, Zhang X, Shu P and Zhang D: miR-142-5p promotes development
of colorectal cancer through targeting SDHB and facilitating
generation of aerobic glycolysis. Biomed Pharmacother.
92:1119–1127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim JA, Kim Y, Kwon BM and Han DC: The
natural compound cantharidin induces cancer cell death through
inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated
athanogene domain 3 (BAG3) expression by blocking heat shock factor
1 (HSF1) binding to promoters. J Biol Chem. 288:28713–28726.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C, Jiang JY, Wang JM, Sun J, An MX, Li
S, Yan J and Wang HQ: BAG3 regulates stability of IL-8 mRNA via
interplay between HuR and miR-4312 in PDACs. Cell Death Dis.
9(863)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li N, Chen M, Cao Y, Li H, Zhao J, Zhai Z,
Ren F and Li K: Bcl-2-associated athanogene 3(BAG3) is associated
with tumor cell proliferation, migration, invasion and
chemoresistance in colorectal cancer. BMC Cancer.
18(793)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin D, Lin H and Xiong X: Expression and
role of BAG-1 in eosinophilic and non-eosinophilic chronic
rhinosinusitis with nasal polyps. Inflammation. 37:1912–1918.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ying Z, Haiyan G and Haidong G: BAG5
regulates PTEN stability in MCF-7 cell line. BMB Rep. 46:490–494.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rogers R, Dharsee M, Ackloo S and Flanagan
JG: Proteomics analyses of activated human optic nerve head lamina
cribrosa cells following biomechanical strain. Invest Ophthalmol
Vis Sci. 53:3806–3016. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bi L, Yang Q, Yuan J, Miao Q, Duan L, Li F
and Wang S: MicroRNA-127-3p acts as a tumor suppressor in
epithelial ovarian cancer by regulating the BAG5 gene. Oncol Rep.
36:2563–2570. 2016.PubMed/NCBI View Article : Google Scholar
|