1. Introduction
Psoriasis is a chronic, systemic, inflammatory,
immune-mediated disease that primarily affects the skin,
characterized by abnormal hyperproliferation of the epidermis
(1-3).
There are many trigger factors involved (stress, infections,
medications, trauma) that can induce the disease in a predisposed
population (3).
To collect reports of psoriasis etiopathogeny, a
literature search was conducted using electronic databases Google
Scholar, PubMed, Key Elsevier, UpToDate and Medscape for the terms
’psoriasis’ in combination with ‘psoriasis in children’,
‘management of psoriasis’, ‘napkin psoriasis’, ‘biologic
therapies’, ‘pathogenesis’, ‘treatment’. Our study includes case
reports, case series, and literature review-type papers. Based on
63 publications found in the literature, we compiled a concised
report, illustrated with images from individual patients. A
parental written informed consent before the publication of
de-identified patient photographs was obtained.
2. Epidemiology
Psoriasis is the second most frequent disorder after
atopic dermatitis in childhood and affects approximately 1% of
children (4,5). The incidence of childhood psoriasis
has increased dramatically over time from 29.6 per 100,000
individuals in 1970-1974 to 62.7 per 100,000 in 1995-1999(6). The disease can appear at any age, more
commonly at 9-10 years, and has a durable course with exacerbation
periods that can last between a few weeks and 1.5 years (7). The incidence of pediatric psoriasis
increases with age. In the UK, the prevalence was found to increase
from 0.55% in children between 0-9 years of age to 1.37% in
children between 10-19 years (8,9). In
Italy, the prevalence of psoriasis in children is estimated at 2.1%
(10). The exacerbations are more
likely to appear in the autumn and winter seasons (7). Psoriasis appears earlier and more
frequently in girls. Earlier onset is also associated with severe
forms of psoriasis (11,12).
3. Pathogenesis
Psoriasis is the result of polygenic predisposition
and environmental triggers. Genetic predisposition is easier to
detect in children than in adults. Family history is present in 35
to 90% of patients with psoriasis, of which 30% are first-degree
relatives and the pathology is more common in monozygotic twins
compared to dizygotic twins (3,13).
Family history has been associated with earlier onset of psoriasis
and the presence of enthesitis (14). There are over 1,300 susceptibility
genes identified in psoriatic skin which are differently expressed
compared to normal skin (15). The
most important psoriasis-susceptibility gene (PSORS1) is
human leukocyte antigen HLA Cw6(16). The presence of this gene is
associated with a relative risk of developing psoriasis of 13% for
Caucasians and 25% for the Japanese population. Based on this, some
clinicians consider that there are two types of psoriasis, type I
with early-onset, positive family history, and the presence of HLA
Cw6 and type II with late-onset, without family history and no
expression of HLA Cw6(3). The
corneodesmosin (CDSN) gene has been associated with
psoriasis in the Caucasian population. Other genes involved are the
MHC class I polypeptide-related sequence A (MICA) gene,
according to a study of psoriasis in the Chinese population, and
the angiotensin I converting enzyme (ACE) gene in some
patients (1). Promoter region
polymorphisms in the tumor necrosis factor (TNF)α
gene are associated with psoriasis in the Polish population and
interleukin 12B (IL12B) gene polymorphisms are found in
different patients (1). In
addition, protein tyrosine phosphatase, non-receptor type 22
(PTPN22) region polymorphisms are related to early-onset
psoriasis (1).
The pathogenesis of psoriasis is based on chronic
inflammation, increased keratinocyte proliferation, and
dysfunctional differentiation. Although the main changes in
psoriasis are found in the most superficial layer of the skin,
formed by keratinocytes, the psoriatic plaque is not limited to
inflammation of the epidermis. It is characterized by the
interaction between keratinocytes and many different cells,
extending to the dermal layer of the skin (17-19).
The factors that have a key role in the pathogenesis of psoriasis
are T cells, keratinocytes, Langerhans' cells, macrophages, and
some types of cytokines. Self-antigens activate dendritic cells and
release cytokines including TNF, γ-interferon, interleukin (IL)-12,
and IL-23 that recruit T cells, Th-1, Th-17 and Th-22(2). T cells release cytokines and maintain
the abnormal differentiation of keratinocytes and cell cycle
turnover (16,20). Keratinocytes from psoriatic plaque
express signal transducer and activator of transcription 3 (STAT3),
a transcription factor that was found to produce psoriasis-like
injuries in mice. The fact that STAT3 is activated by cytokines
(IL-6, IL-20, IL-22) could explain the connection between
keratinocyte proliferation and the immune system in the
pathogenesis of psoriasis (3).
There are several vascular changes such as angiogenesis [stimulated
by IL-8 and transforming growth factor (TGF)-α], dilated sinuous
vessels in the papillary dermis, and the formation of high
endothelial venules. Therefore, the size of the microcirculation
expands and facilitates the passage of T lymphocytes (T helper 1
subclass), maintaining psoriatic plaque. Severe psoriasis is
accompanied by microvascular hyperpermeability mediated by vascular
endothelial growth factor (VEGF) (1).
The pathogenesis of psoriasis can be structured in
an initiation phase possibly caused by trauma, infections, or
drugs, and a chronic phase with long-term clinical progression. The
main trigger factors in children include stress and infections,
especially streptococcal infections (3,5,21).
Some possible causes of psoriasis morbidity in children are social
and home problems, emotional issues, pollution, and a decrease in
immune reactivity (5). In addition,
overweight and obesity have been recently discovered as risk
factors for psoriasis in children (2). Paradoxically, TNF inhibitors such as
infliximab and adalimumab may induce psoriasis (4).
4. Clinical features
The history and symptoms of psoriasis can be
different according to the age of children and the type of
psoriasis. Most common psoriasis lesions are found in the
extremities (60%) and the scalp (47%) (6). The most common clinical manifestation
is pruritus (22). In many cases,
infants have persistent diaper rash that remains despite many
treatments. Older children may have an asymptomatic scaly rash or
severe dandruff (2). Adolescents
have the same clinical features as adults. Some children may
present the Koebner phenomenon that consists in the appearance of
new skin lesions on previously unaffected skin secondary to trauma,
lesions that are pronounced and long-lasting (2,5).
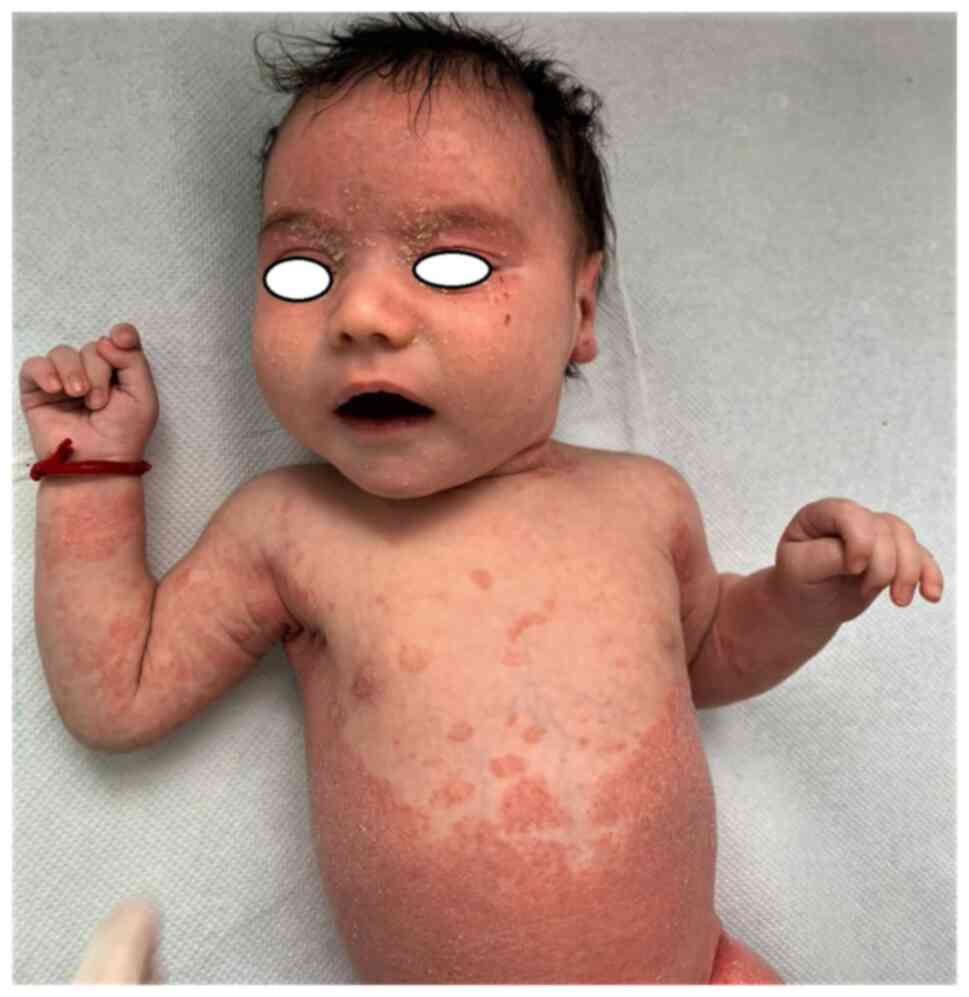
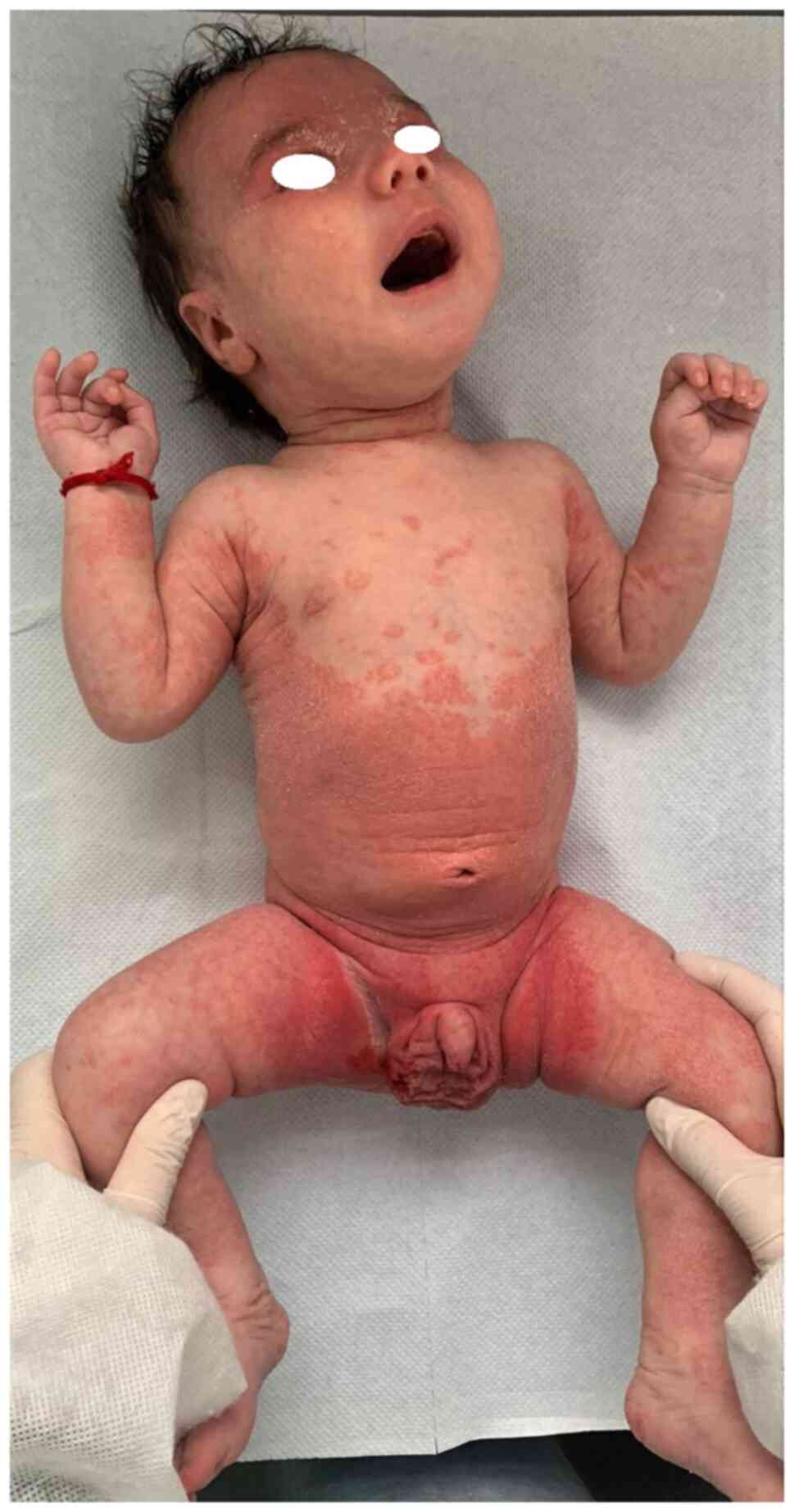
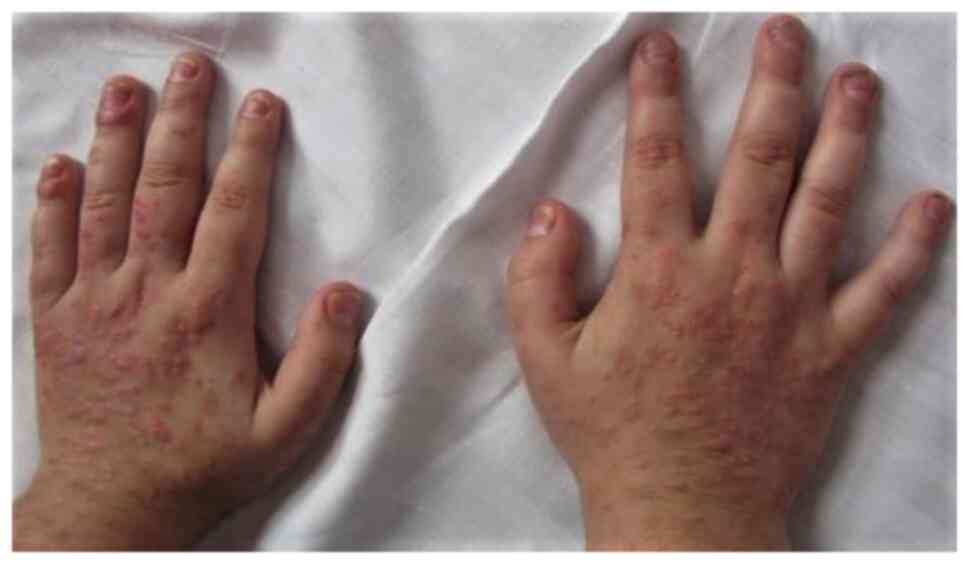
Psoriasis is characterized by erythematous papules
that merge to create well-demarcated plaques with irregular
borders, covered by a silvery-white scale. If the scale is removed,
pinpoint bleeding will result, known as the Auspitz sign. Children
with psoriasis have more facial and flexural lesions and the
plaques are smaller and thinner compared to adults (2,23)
(Figs. 1 and 2). The differential diagnosis is often
difficult to make with seborrheic dermatitis (2,16).
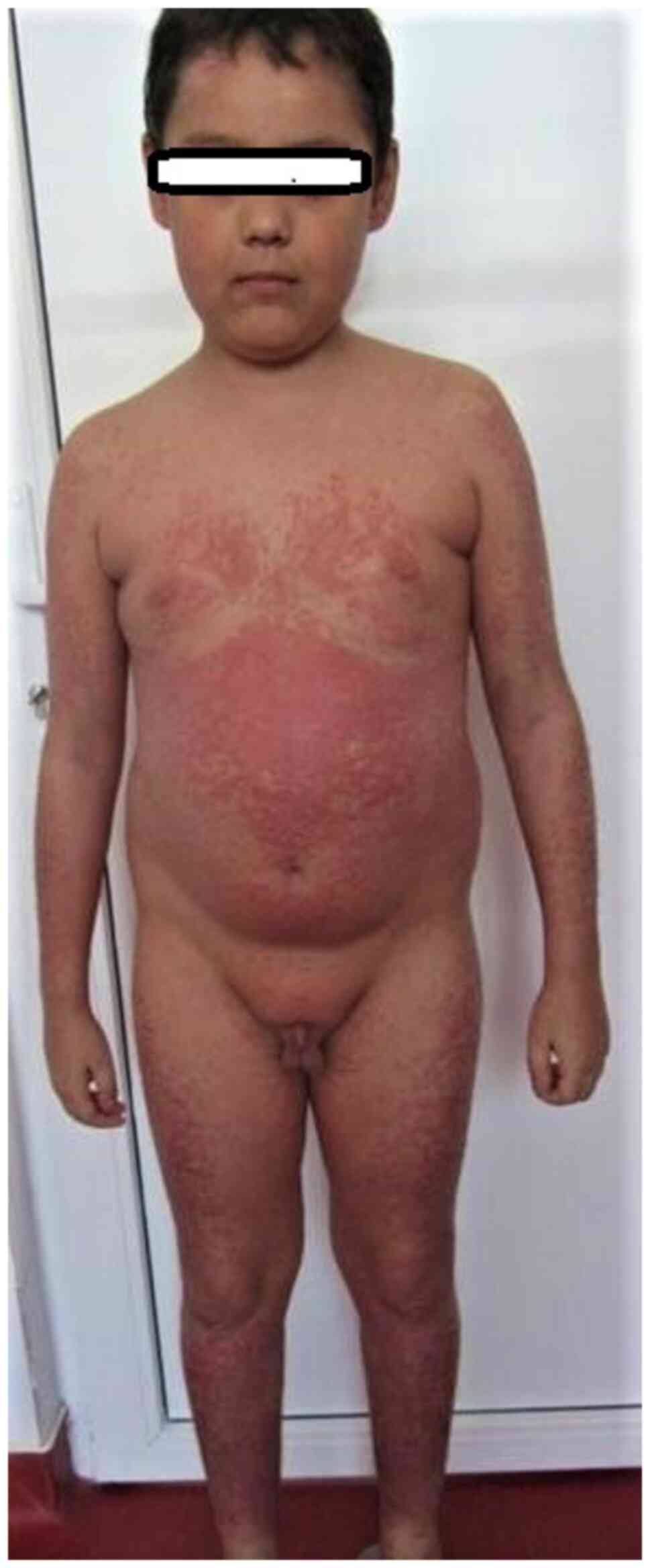
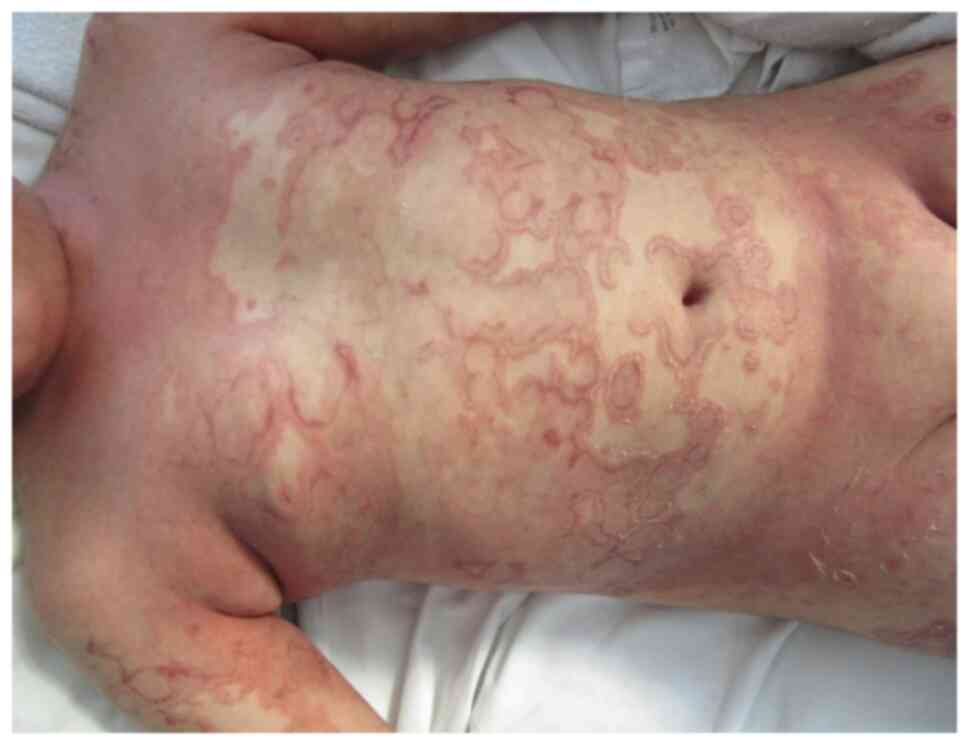
There are several types of psoriasis: vulgaris:
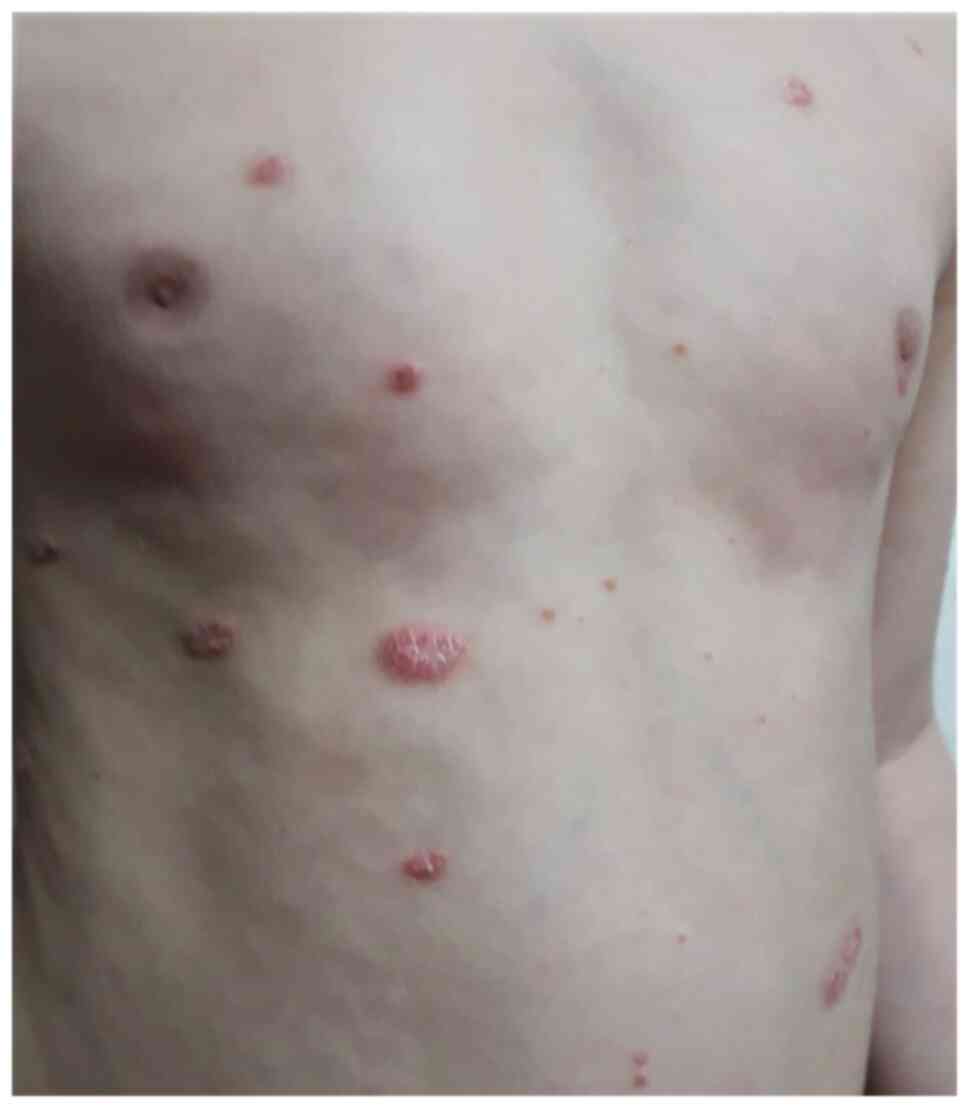
chronic plaque (Fig. 3), guttate
(Fig. 4), and inverse psoriasis
(Fig. 5), pustular and
erythrodermic.
The most common type of psoriasis in children is
chronic plaque psoriasis (75% of children with psoriasis) followed
by guttate psoriasis (15-30% of children with psoriasis) (2,24). In
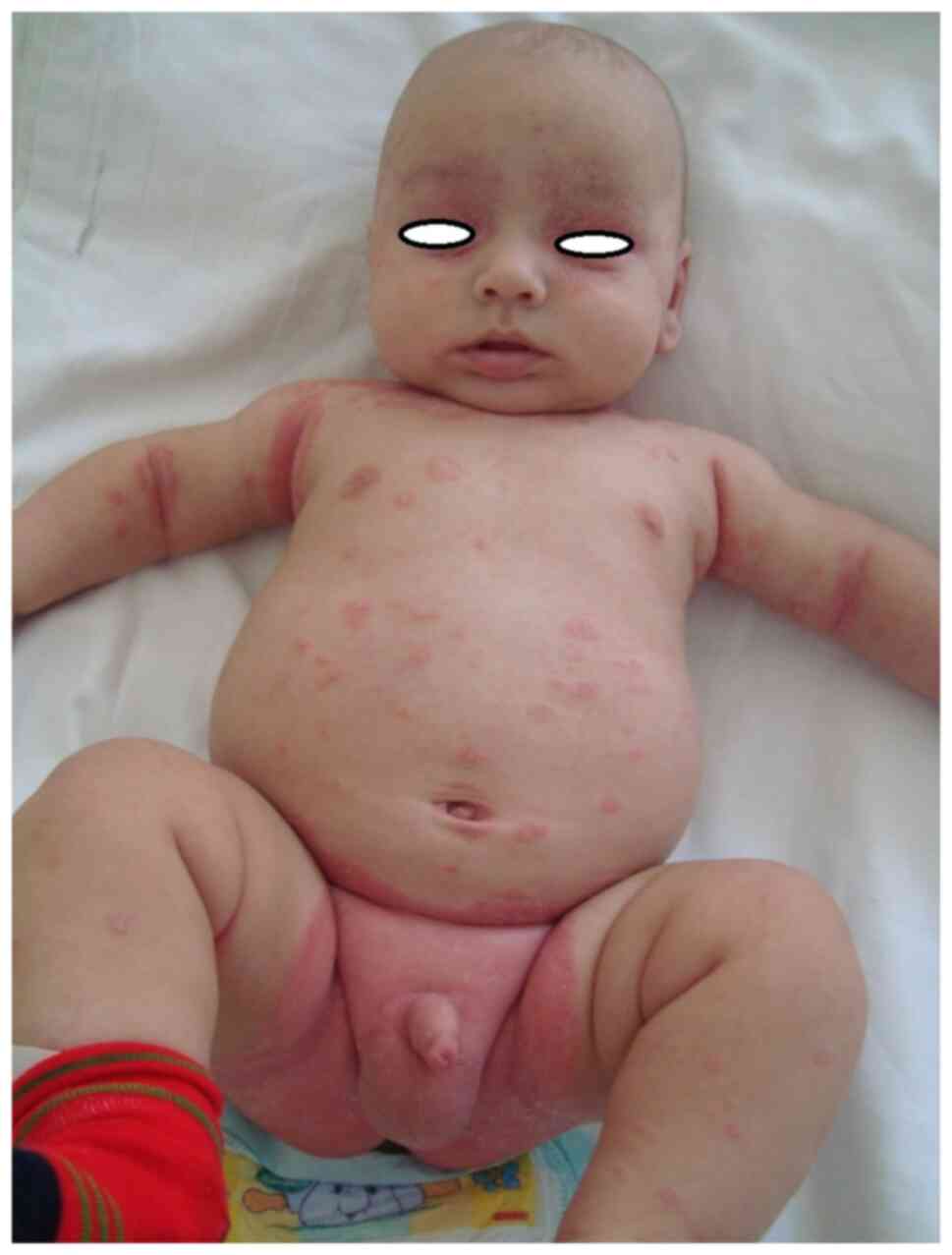
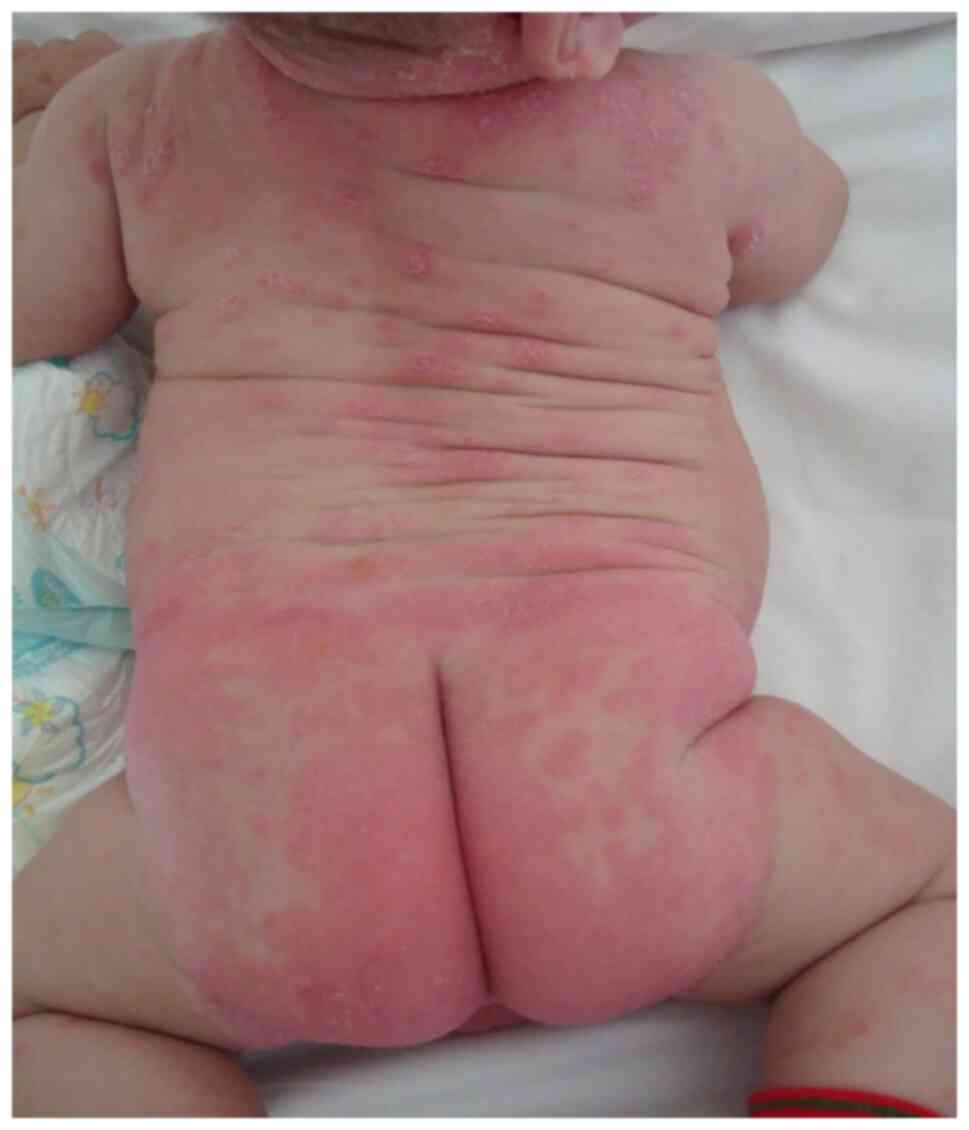
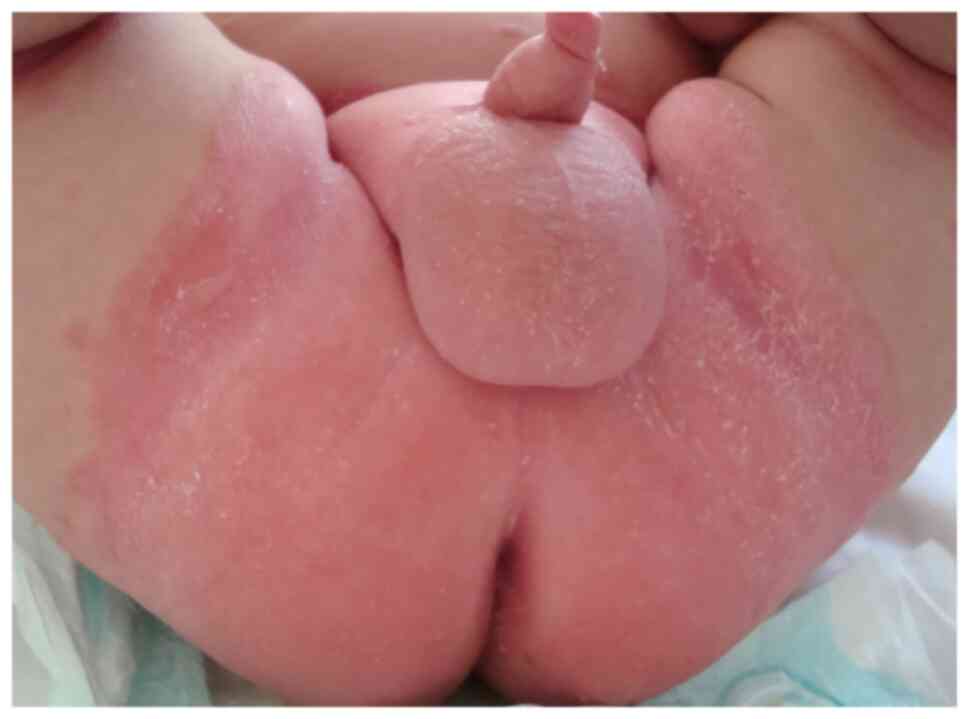
addition, diaper psoriasis is a common variant exclusively seen in
infants (Fig. 6, Fig. 7 and Fig.
8). Chronic plaque psoriasis is most common on the extensor
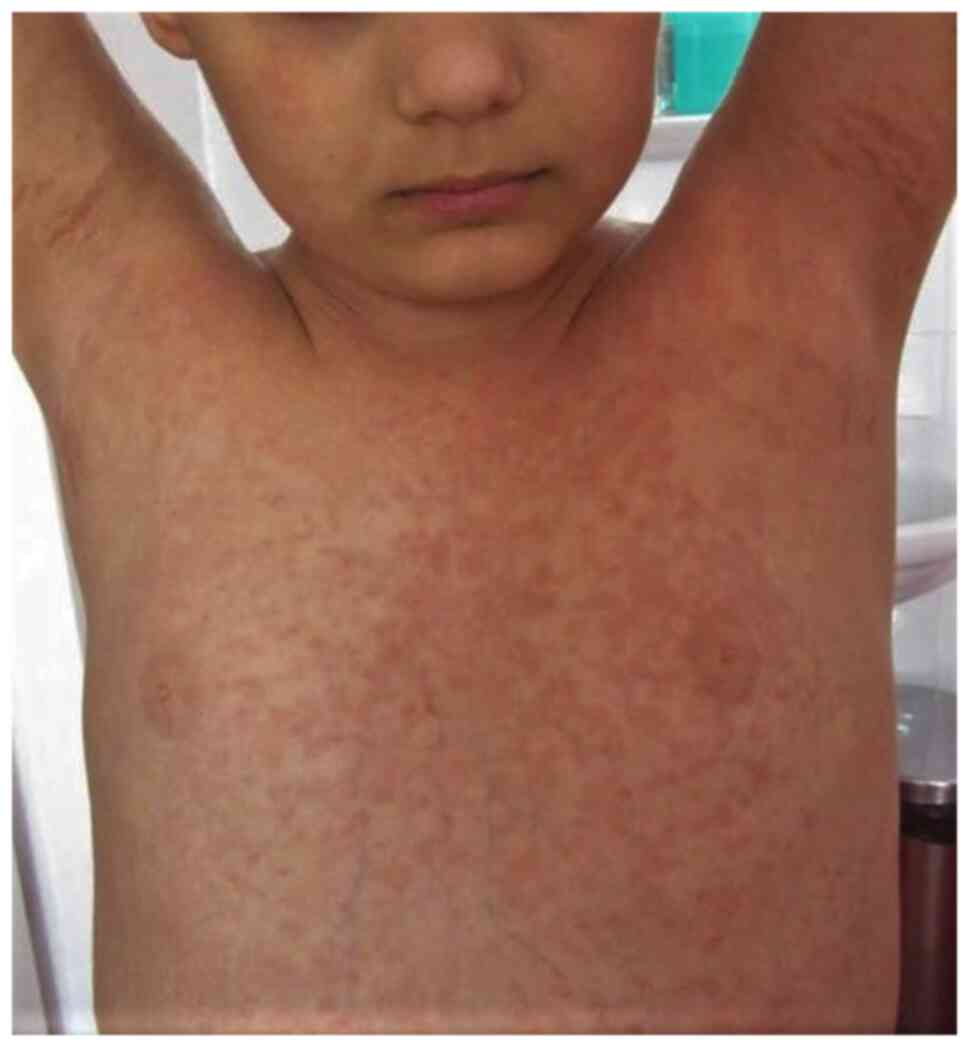
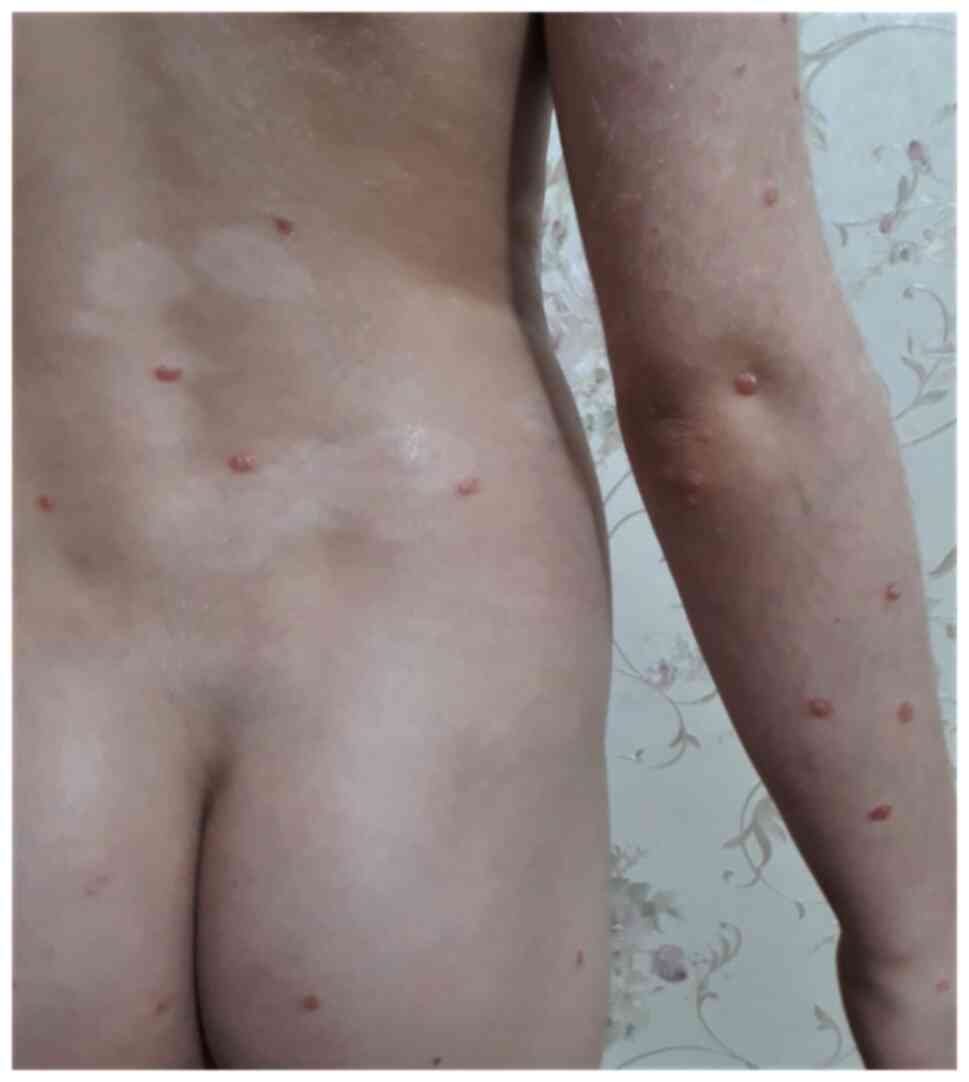
extremities, trunk, flexures, and face. Guttate psoriasis is
characterized by the sudden onset of droplet papules with
symmetrical disposal over the trunk, limbs, and face (Fig. 9). These lesions may be preceded by
streptococcal infection and resorb within 3 to 4 weeks or they can
persist and transform into severe psoriasis (2). The development of lesions in flexural
areas and the face is more common in children and is known as
inverse psoriasis. The psoriasis of the scalp has well-defined,
scaly, erythematous, and pruritic lesions. In children with
alopecia, the hair grows back when the disease is under control
(2). Nail psoriasis is less
frequent in children compared to adults but can be observed in
approximately 40% of the children. The most common changes are
pitting, leukonychia, and longitudinal ridges that are very
difficult to treat (2,25,26)
(Fig. 10). Pustular psoriasis is
rare and consists of sterile superficial pustules with annular
disposition (Fig. 11). If pustules
are generalized, children may develop a fever and they may be often
mistaken for an infection. Another variant of this disease is
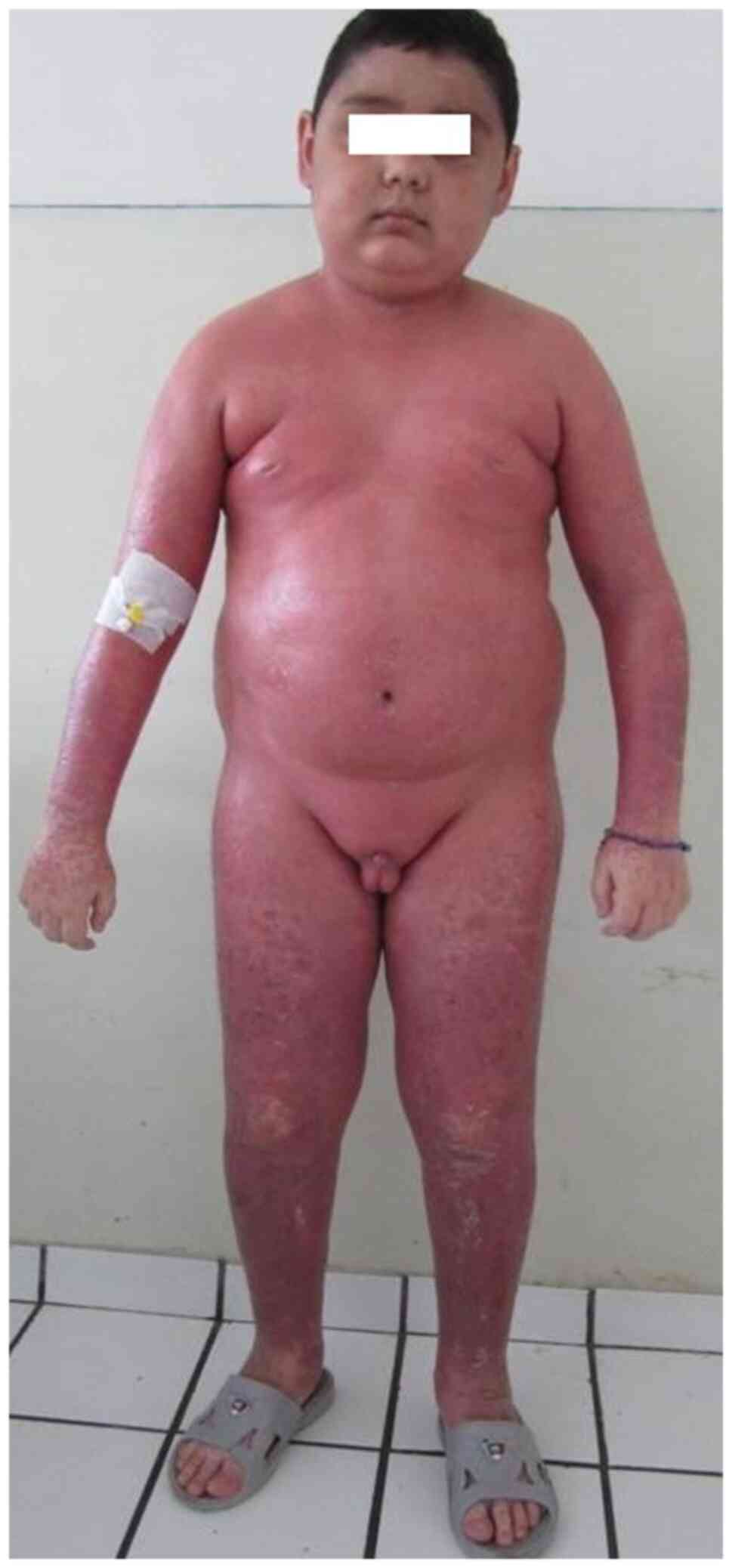
erythrodermic psoriasis (Fig. 12)
that affects more than 90% of the body surface and is extremely
rare (2,16).
The severity of skin disease is classified based on
the affected body surface area (BSA) According to this, the disease
is mild if lesions cover less than 5% of BSA, moderate if it
affects between 5 and 10% of BSA and severe if the BSA involved is
greater than 10% (16).
Fortunately, most children with psoriasis have mild disease
(2,22).
5. Comorbidities
Some recent studies have discovered that children
with psoriasis are twice as likely to have comorbidities as those
without psoriasis (2). Psoriasis
has been associated with type 1 diabetes, rheumatoid arthritis,
Crohn's disease, systemic lupus erythematosus, vitiligo (Fig. 13), alopecia areata, eczema, and
lichen planus (2,27,28).
Psoriasis can be mistaken with seborrheic dermatitis and
avitaminosis, but they can also coexist. Just like in adults, the
association with atopic dermatitis is frequently seen in children.
In terms of association with systemic diseases, children with
psoriasis have a higher rate of obesity, especially boys. Patients
with this comorbidity are more likely to have a severe form of
psoriasis compared to normal-weight psoriatic children (29). The major comorbidity is psoriatic
arthritis. Prevalence in the children population is still unknown.
Psoriatic arthritis can occur at any age, but the onset is more
common between 2 and 3 years and 9 to 12 years of age. This
condition causes joint pain and mostly affects finger and toe
joints (30,31). Younger children with psoriasis,
especially girls, present an onset with an oligoarticular disease
or dactylitis while older children, especially boys, present an
onset with enthesitis and axial joint involvement.
Pediatric patients with concomitant arthritis can
develop uveitis (4). Children and
adolescents with this condition are reported to have an increased
risk of hypertension, hyperlipidemia, and diabetes, probably due to
the high rate of metabolic syndrome. Low levels of HDL cholesterol
and high concentrations of fasting glucose appear in the early
stages of metabolic syndrome in psoriatic children (32).
The presence of skin lesions harms the children.
Many patients are stigmatized by other children and recreational
activity is highly affected. This stigmatization can induce changes
in behavior, depression, and anxiety. More than that, the
association between psoriasis and obesity increases the risk of
social isolation, withdrawal, depression, and anxiety (4,33).
Psoriasis affects the quality of life of children and their parents
and they should receive psychosocial support (34).
As in most systemic, inflammatory, chronic diseases,
ocular manifestations can occur (35). The incidence of ocular involvement
in psoriasis largely varies in the literature between 12 and 58%
(36,37) and even up to 81.4% in more recent
studies (38). The eyelid
manifestations include blepharitis, which is the most frequent one,
together with erythema, psoriatic plaques, and edema leading to
eyelid abnormalities such as ectropion, trichiasis, and madarosis.
In moderate and severe forms of the disease, chronic conjunctivitis
can lead to limbal lesions and keratoconjunctivitis sicca further
responsible for corneal lesions such as punctate keratitis,
recurrent erosions, opacities, and vascularization (39). The anterior uveitis associated with
childhood psoriatic arthritis and onset before the age of 6 years
has been described as bilateral, long-lasting, and severe requiring
biologic therapy (40).
6. Treatment
Psoriasis therapy varies according to patient age,
type of psoriasis, affected sites, and extension of the disease
(16). This condition requires the
involvement of several specialists such as dermatologists,
pediatricians, and rheumatologist. There are 4 types of treatment
as described.
Topical agents
Topical agents such as emollients, vitamin D
analogs, and corticosteroids should be the first choice of
treatment for children with mild to moderate psoriasis (41). Vitamin D analogs, calcipotriene, and
calcitriol inhibit the proliferation of keratinocytes and are safe
to use.
Corticosteroids reduce inflammation and
proliferation of keratinocytes, scaling, and erythema. Side effects
of topical steroids are divided into local and systemic. Local side
effects occur with prolonged treatment and are related to the
corticosteroid potency and site of application (42). The most frequent side effects are
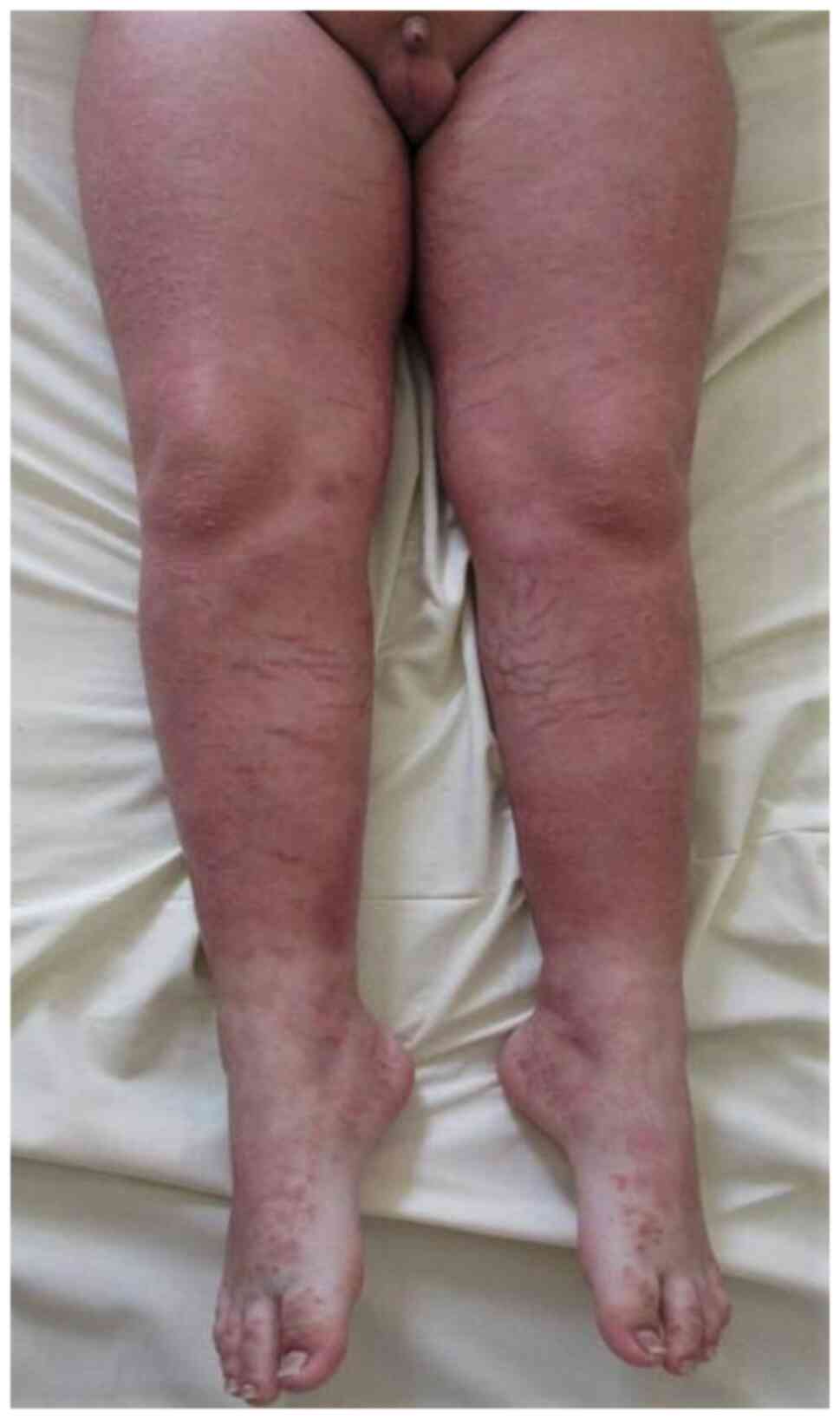
atrophy, striae (Figs. 14 and
15) acne, rosacea, perioral
dermatitis, and purpura. Patients can also experience less common
side effects such as hypertrichosis, delayed wound healing, pigment
alteration, and aggravation of skin infections (42). Systemic side effects are more common
in long-term topical treatment with highly potent corticosteroids
on large areas, thin skin, or inflamed surfaces. Children have a
large body surface area as related to the body volume so they have
a higher risk of developing hypothalamic-pituitary-adrenal axis
suppression due to systemic absorption. Some systemic adverse
effects are iatrogenic Cushing syndrome (moon face, centripetal
obesity, striae) (Fig. 12),
corticosteroid-related Addison crises, growth retardation (reduced
bone mineral density, osteopathy), hyperglycemia and diabetes
mellitus, edema, hypocalcemia, hypertension. During continued
treatment, psoriatic patients may develop tachyphylaxis. Rebound
phenomena may occur upon withdrawal of topic steroids applied on
large areas of psoriasis for a long time. They are characterized by
relapse or a papulopustular flare and may precipitate severe
generalized pustular psoriasis (42).
Second-line topical treatments include retinoids,
tars, anthralin, and keratolytics. Tazarotene decreases
inflammation and helps to restore normal epidermal proliferation
and differentiation (43). It is
effective in the treatment of nail psoriasis. Tars have
antipruritic and antiproliferative effect. Anthralin is especially
used on thick plaques or large involved areas. Phenol and saline
solution can be used for scalp lesions (2,16).
Phototherapy
Phototherapy [narrow-band ultraviolet B (NB-UVB);
psoralen plus ultraviolet A (PUVA)] is an alternative therapy for
children with chronic plaque or guttate psoriasis that have an
unsatisfactory result to topical treatment. Side effects of
phototherapy in children include erythema, burning,
hyperpigmentation, viral reactivation, and risk of cutaneous
carcinogenesis in long-term treatment with PUVA. Psoralen is
avoided in children under 12 years of age. Phototherapy is
contraindicated in children with cutaneous cancer syndromes or
generalized erythroderma (4,16).
Systemic therapy
Systemic therapy is required in moderate to severe
disease. Methotrexate is the most common systemic medication used
for psoriasis in children and it requires folic acid
supplementation. The most serious side effects of methotrexate are
bone marrow suppression, hepatic and pulmonary toxicity. Other
options may include cyclosporine and systemic retinoids.
Cyclosporin is well tolerated in pediatric patients and has a quick
effect on severe, pustular, and erythrodermic psoriasis (4,16,44-46).
Biologic therapy
Biologic therapy is becoming much more commonly used
in pediatric patients. FDA has approved etanercept (TNF inhibitor)
for patients 4 years and older, ustekinumab (IL-12 and IL-23
antagonist) for patients 12 years and older, and adalimumab (TNF
inhibitor) was approved in Europe for patients 4 years and older
(4). Etanercept is the favorite
biologic therapy for pediatric patients and it was proved to be
effective for moderate to severe psoriasis (15). It is a TNF inhibitor, which prevents
activation of the inflammatory cascade. Studies have revealed that
etanercept is more efficient and safe in long-term usage compared
to other systemic treatments such as methotrexate, cyclosporine,
and PUVA. Side effects include mild injection site reaction,
increased risk of infections and cold-like illnesses, hepatitis B
virus reactivation, and the possibility of weight gain (47,48).
Studies have shown that adalimumab is more efficient than
methotrexate and has a similar safety profile (49). The advantage of adalimumab over
other biologics consists in the efficacy in the treatment of
psoriatic arthritis. Just like other TNF-α inhibitors, adalimumab
has absolute contraindications to treatments such as tuberculosis
and other severe infections (50).
The precautions for the use of TNF-α inhibitors are hepatitis B
infection, allergy, history of malignancy, and demyelinating
conditions (51). Ustekinumab
targets the IL-12 and IL-23 axis and it is efficient for psoriasis
and psoriatic arthritis. A statistically significant increased
response to ustekinumab in HLA-Cw6-positive patients has been
noted. The different action mechanism of this drug provides an
alternative treatment to TNF inhibitor failure, but there can be a
certain problem for some patients regarding the slow onset of
action (52). Some biological
therapies are off-label (53).
Infliximab has been approved for children with Crohn's disease, and
several cases of induced psoriasis have been reported after a few
weeks of therapy (54). Infliximab
is used in limited cases such as for children with severe pustular
psoriasis (55,56). Certolizumab pegol is approved for
psoriasis only in adults and it is currently being investigated in
childhood arthritis and Crohn's disease (57,58).
Secukinumab is not FDA-approved for any medical condition in
children, but clinical trials are ongoing to evaluate the efficacy
of this therapy in pediatric psoriasis (59,60).
Other biologics such as ixekizumab, guselkumab, and brodalumab,
currently approved for adults with psoriasis, have been included in
clinical trials for children with psoriasis (61-63).
7. Conclusions
Pediatric psoriasis is an increasing chronic skin
disease, with exacerbations and remissions. The complexity of the
pathology requires the involvement of a multidisciplinary team.
Psychosocial support is an important part of psoriasis treatment
due to the significant impact of the disease on the quality of life
of the patients. Unfortunately, the lack of standardized treatment
guidelines and the limited number of randomized clinical trials
concerning biologic therapy make systemic treatment challenging in
children with severe psoriasis.
Acknowledgements
Not applicable.
Funding
Publishing funds were supported by the Association of
Dermatologists from Moldova.
Availability of data and materials
All information provided in this review is
documented by relevant references.
Authors' contributions
DEB and DCB contributed to the study design,
participated in the entire review process, and prepared the
manuscript. SG, DB, ACN and CIB contributed to collecting the
relevant literature, data analysis, and critical interpretation.
RB, MAM, MG, ACP, AD and ILS conceived the concept of the review
and modified the manuscript. All authors read and approved the
final version of the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
A parental written informed consent before the
publication of de-identified patient photographs was obtained.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Patterson JW: The psoriasiform reaction
pattern. In: Weedon's Skin Pathology. 5th edition. Elsevier,
Philadelphia, PA, pp99-120.e11, 2021.
|
|
2
|
Tollefson MM: Diagnosis and management of
Psoriasis in children. Pediatr Clin North Am. 61:261–277.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kerkhof PCM and van de Nestlé FO:
Psoriasis. In: Dermatology. 4th edtion. Philadelphia, PA,
pp138-160, 2018.
|
|
4
|
Menter A, Cordoro KM, Davis DMR,
Kroshinsky D, Paller AS, Armstrong AW, Connor C, Elewski BE,
Gelfand JM, Gordon KB, et al: Joint American Academy of
Dermatology-National Psoriasis Foundation guidelines of care for
the management and treatment of psoriasis in pediatric patients. J
Am Acad Dermatol. 82:161–201. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tkach VY, Voloshynovych MS, Girnyk GY and
Kozak NV: Clinical features and the course of psoriasis in
children. Przegl Dermatol. 107:476–480. 2020.
|
|
6
|
Tollefson MM, Crowson CS, McEvoy MT and
Maradit Kremers H: Incidence of psoriasis in children: A
population-based study. J Am Acad Dermatol. 62:979–987.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murzina E: Pediatric Psoriasis: Clinical
Features and Course. OAJBS. 1(OAJBS.ID.000147)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gelfand JM, Weinstein R, Porter SB,
Neimann AL, Berlin JA and Margolis DJ: Prevalence and treatment of
psoriasis in the United Kingdom: A population-based study. Arch
Dermatol. 141:1537–1541. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pinson R, Sotoodian B and Fiorillo L:
Psoriasis in children. Psoriasis (Auckl). 6:121–129.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM: Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team. Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Paller AS, Singh R, Cloutier M,
Gauthier-Loiselle M, Emond B, Guérin A and Ganguli A: Prevalence of
psoriasis in children and adolescents in the United States: A
claims-based analysis. J Drugs Dermatol. 17:187–194.
2018.PubMed/NCBI
|
|
12
|
Seyhan M, Coşkun BK, Sağlam H, Ozcan H and
Karincaoğlu Y: Psoriasis in childhood and adolescence: Evaluation
of demographic and clinical features. Pediatr Int. 48:525–530.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwon HH, Na SJ, Jo SJ and Youn JI:
Epidemiology and clinical features of pediatric psoriasis in
tertiary referral psoriasis clinic. J Dermatol. 39:260–264.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ran D, Cai M and Zhang X: Genetics of
psoriasis: A basis for precision medicine. Precis Clin Med.
2:120–130. 2019.
|
|
15
|
Paller AS, Siegfried EC, Pariser DM, Rice
KC, Trivedi M, Iles J, Collier DH, Kricorian G and Langley RG:
Long-term safety and efficacy of etanercept in children and
adolescents with plaque psoriasis. J Am Acad Dermatol.
74:280–287.e1-e3. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kliegman RM, Geme JWS, Blum NJ, Shah SS,
Tasker RC and Wilson KM: Diseases of the epidermis. In: Nelson
Textbook of Pediatrics. Kliegman RM and Geme JWS (eds). Elsevier,
Philadelphia, PA, pp3501-3510.e1, 2020.
|
|
17
|
Rendon A and Schäkel K: Psoriasis
pathogenesis and treatment. Int J Mol Sci. 20(1475)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):S1191–S1196.
2014.PubMed/NCBI
|
|
20
|
Das RP, Jain AK and Ramesh V: Current
concepts in the pathogenesis of psoriasis. Indian J Dermatol.
54:7–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qing M, Liu P, Zhu W, Chen M, Chen M and
Kuang Y: Analysis for 208 children with psoriasis vulgaris. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 45:804–811. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese,
English).
|
|
22
|
Nguyen TT: Papulosquamous eruptions. In:
Conn's Current Therapy 2021. Kellerman RD and Rakel D (eds).
Elsevier, Philadelphia, PA, pp1056-1061, 2021.
|
|
23
|
Kim SK, Kang HY, Kim YC and Lee ES:
Clinical comparison of psoriasis in Korean adults and children:
Correlation with serum anti-streptolysin O titers. Arch Dermatol
Res. 302:295–299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Say M, Boralévi F, Lenormand C, Bursztejn
AC, Estève E, Phan A, Bourrat E, Lacour JP, Richard MA, Acher A, et
al: Clinical and therapeutic aspects of linear psoriasis: A study
of 30 cases. Am J Clin Dermatol. 19:609–615. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thomas L, Azad J and Takwale A: Management
of nail psoriasis. Clin Exp Dermatol. 46:3–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Uber M, Carvalho VO, Abagge KT, Robl Imoto
R and Werner B: Clinical features and nail clippings in 52 children
with psoriasis. Pediatr Dermatol. 35:202–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Narla S and Silverberg JI: 15838
Autoimmune comorbidities of psoriasis in US adults and children. J
Am Acad Dermatol. 83(AB48)2020.
|
|
28
|
Brandon TG, Manos CK, Xiao R, Ogdie A and
Weiss PF: Pediatric psoriatic arthritis: A population-based cohort
study of risk factors for onset and subsequent risk of inflammatory
comorbidities. J Psoriasis Psoriatic Arthritis. 3:131–136.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thomas J and Parimalam K: Treating
pediatric plaque psoriasis: Challenges and solutions. Pediatric
Health Med Ther. 7:25–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomark Med. 9:513–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brihan I, Hălmăjan A, Boda D, Ianoși SL,
Fekete GL and Zdrîncă M: Role of osteodensitometry in osteoporosis
and osteopenia in psoriatic arthritis. Exp Ther Med.
20(188)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Taranu T, Constantin M, Ungureanu D, Esanu
IM and Toader MP: IL6 is correlated with metabolic syndrome
parameters in oral lichen planus. In: 2015 E-Health and
Bioengineering Conference (EHB). pp1-4, 2015. http://toc.proceedings.com/29132webtoc.pdf.
|
|
33
|
Brihan I, Ianoși SL, Boda D, Hălmăjan A,
Zdrîncă M and Fekete LG: Implications of self-esteem in the quality
of life in patients with psoriasis. Exp Ther Med.
20(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gånemo A, Wahlgren CF and Svensson Å:
Quality of life and clinical features in Swedish children with
psoriasis. Pediatr Dermatol. 28:375–379. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Branisteanu DC, Stoleriu G, Branisteanu
DE, Boda D, Branisteanu CI, Maranduca MA, Moraru A, Stanca HT,
Zemba M and Balta F: Ocular cicatricial pemphigoid (Review). Exp
Ther Med. 20:3379–3382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Karabulut AA, Yalvac IS, Vahaboglu H,
Nurozler AB and Duman S: Conjunctival impression cytology and
tear-film changes in patients with psoriasis. Cornea. 18:544–548.
1999.PubMed/NCBI
|
|
37
|
Kilic B, Dogan U, Parlak AH, Goksugur N,
Polat M, Serin D and Ozmen S: Ocular findings in patients with
psoriasis. Int J Dermatol. 52:554–559. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cruz NFSD, Brandão LS, Cruz SFSD, Cruz
SASD, Pires CAA and Carneiro FRO: Ocular manifestations of
psoriasis. Arq Bras Oftalmol. 81:219–225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Erbagci I, Erbagci Z, Gungor K and Bekir
N: Ocular anterior segment pathologies and tear film changes in
patients with psoriasis vulgaris. Acta Med Okayama. 57:299–303.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Salek SS, Pradeep A, Guly C, Ramanan AV
and Rosenbaum JT: Uveitis and juvenile psoriatic arthritis or
psoriasis. Am J Ophthalmol. 185:68–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mahé E: Childhood psoriasis. Eur J
Dermatol. 26:537–548. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Coondoo A, Phiske M, Verma S and Lahiri K:
Side-effects of topical steroids: A long overdue revisit. Indian
Dermatol Online J. 5:416–425. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ogawa E, Sato Y, Minagawa A and Okuyama R:
Pathogenesis of psoriasis and development of treatment. J Dermatol.
45:264–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Negrei C, Ginghina O, Caruntu C,
Burcea-Dragomiroiu GTA, Jinescu G and Boda D: Investigation
relevance of methotrexate polyglutamates in biological systems by
high performance liquid chromatography. REV CHIM (Bucharest).
66:766–768. 2015.
|
|
45
|
Negrei C, Caruntu C, Ginghina O,
Burcea-Dragomiroiu GTA, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. REV CHIM
(Bucharest). 66:607–610. 2015.
|
|
46
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
47
|
Nguyen TU and Koo J: Etanercept in the
treatment of plaque psoriasis. Clin Cosmet Investig Dermatol.
2:77–84. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Branisteanu DE, Voicu CM, Cretu A,
Dimitriu A, Luca MC and Salavastru CM: Adverse reactions of
biological therapy for psoriasis. Rev Med Chir Soc Med Nat Iasi.
119:38–44. 2015.PubMed/NCBI
|
|
49
|
Papp K, Thaçi D, Marcoux D, Weibel L,
Philipp S, Ghislain PD, Landells I, Hoeger P, Kotkin C, Unnebrink
K, et al: Efficacy and safety of adalimumab every other week versus
methotrexate once weekly in children and adolescents with severe
chronic plaque psoriasis: A randomised, double-blind, phase 3
trial. Lancet. 390:40–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Khobzey K, Liskova I, Szegedi A, Pavlovsky
L, Lunder T, Kingo K, Miljković J, Péč J, Bohinc M and Hojnik M:
Effectiveness of adalimumab in the treatment of scalp and nail
affection in patients with moderate to severe plaque psoriasis in
routine clinical practice. Acta Dermatovenerol Alp Pannonica
Adriat. 26:11–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mocanu M, Toader MP, Rezus E and Taranu T:
Aspects concerning patient adherence to anti-TNFα therapy in
psoriasis: A decade of clinical experience. Exp Ther Med.
18:4987–4992. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Savage LJ, Wittmann M, McGonagle D and
Helliwell PS: Ustekinumab in the treatment of psoriasis and
psoriatic arthritis. Rheumatol Ther. 2:1–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cline A, Bartos GJ, Strowd LC and Feldman
SR: Biologic treatment options for pediatric psoriasis and atopic
dermatitis. Children (Basel). 6(103)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Broge T, Nguyen N, Sacks A and Davis M:
Infliximab-associated psoriasis in children with Crohn's disease
may require withdrawal of anti-tumor necrosis factor therapy.
Inflamm Bowel Dis. 19:E75–E77. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Menter MA and Cush JM: Successful
treatment of pediatric psoriasis with infliximab. Pediatr Dermatol.
21:87–88. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Skrabl-Baumgartner A, Weger W, Salmhofer W
and Jahnel J: Childhood generalized pustular psoriasis: Longtime
remission with combined infliximab and methotrexate treatment.
Pediatr Dermatol. 32:e13–e14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
UCB Celltech: (2018). The Use of
Certolizumab Pegol for Treatment of Active Crohn's Disease in
Children and Adolescents (NURTURE). ClinicalTrials.gov Identifier: NCT00899678.
https://clinicaltrials.gov/ct2/show/NCT00899678.
|
|
58
|
UCB BIOSCIENCES GmbH: (2020). Pediatric
Arthritis Study of Certolizumab Pegol (PASCAL). ClinicalTrials.gov Identifier: NCT01550003.
https://clinicaltrials.gov/ct2/show/NCT01550003.
|
|
59
|
Novartis Pharmaceuticals: (2020).
Pediatric Study in Children and Adolescents With Severe Plaque
Psoriasis. ClinicalTrials.gov Identifier:
NCT02471144. https://clinicaltrials.gov/ct2/show/NCT02471144.
|
|
60
|
Novartis Pharmaceuticals: (2020). Study to
Assess the Long-term Safety, Tolerability, Efficacy of Secukinumab
in Pediatric Patients of Age 6 to <18 Years, With Moderate to
Severe Plaque Psoriasis. ClinicalTrials.gov Identifier: NCT03668613.
https://clinicaltrials.gov/ct2/show/NCT03668613.
|
|
61
|
Eli Lilly and Company: (2020). Study of
Ixekizumab (LY2439821) in Children 6 to Less Than 18 Years With
Moderate-to-Severe Plaque Psoriasis (Ixora-peds). ClinicalTrials.gov Identifier: NCT03073200.
https://clinicaltrials.gov/ct2/show/NCT03073200.
|
|
62
|
Janssen Research & Development, LLC:
(2020). A Study to Evaluate the Efficacy, Safety, and
Pharmacokinetics of Subcutaneously Administered Guselkumab for the
Treatment of Chronic Plaque Psoriasis in Pediatric Participants
(PROTOSTAR). ClinicalTrials.gov Identifier:
NCT03451851. https://clinicaltrials.gov/ct2/show/NCT03451851.
|
|
63
|
Bausch Health Americas, Inc. (2021). An
Open-label, Single-dose Study to Evaluate Safety, Tolerability, and
Pharmacokinetics of Brodalumab in Pediatric Subjects. ClinicalTrials.gov Identifier: NCT03240809.
https://clinicaltrials.gov/ct2/show/NCT03240809.
|