Introduction
Idiopathic membranous nephropathy (IMN) remains one
of the most common causes of nephrotic syndrome (NS) in adults,
accounting for ~20% of all NS cases (1). The proportion of patients with MN
among patients with primary glomerular disease was increased from
10.77% in 2009 to 32.98% in 2018 in mainland China (2). A major breakthrough was the
identification of the podocyte antigen phospholipase A2 receptor
(PLA2R) as the target of circulating antibodies in ~70% of patients
with IMN, which confirmed that IMN is fundamentally an
antibody-mediated autoimmune disease (3). IMN treatment consists of
immunosuppressive therapy (IST) and conservative therapy (4). IST has been proven to be effective in
increasing the probability of the remission of proteinuria and
protecting patients from renal function deterioration (5). Immunosuppressive agents are
recommended in patients at high risk of developing end-stage renal
disease (ESRD) (6). Patients with a
low risk for ESRD are treated with angiotensin-converting enzyme
inhibitors and/or angiotensin II receptor blockers, which are
referred to as ‘conservative therapy’ (7). There are still certain patients who do
not enter remission after taking different types of
immunosuppressive agents for at least 6 months while suffering from
numerous side effects. Therefore, novel useful and predictive
markers to determine the appropriate therapeutic strategy and
predict the prognosis of patients are in high demand.
In recent years, research interests have focused
kidney injury molecule-1 (KIM-1). KIM-1, a sensitive and specific
marker for the presence of tubular damage (8), is not expressed in the normal kidney,
but its expression is induced and markedly increased in proximal
tubular epithelial cells after various types of kidney injury
(9,10). It has been demonstrated that urinary
KIM-1 levels are closely correlated with the severity, therapeutic
response and prognosis of various kidney diseases, including IgA
nephropathy, lupus nephritis and diabetic nephropathy (4,11-14).
In the present retrospective study, KIM-1 levels in
urine and its expression in renal biopsy tissues from adult
patients with IMN and healthy controls were analyzed and the
association between KIM-1 and the therapeutic efficacy of IMN was
determined. Furthermore, KIM-1 expression levels were compared
between patients with different clinical indexes and pathological
parameters.
Materials and methods
Patients
Patients were recruited from the Department of
Nephrology at Qilu Hospital of Shandong University (Jinan, China)
between January 2010 and December 2012. The inclusion criteria were
as follows: i) Typical features of membranous nephropathy detected
by light and electron microscopy; ii) No clinical and/or laboratory
signs of secondary glomerulus nephritis; iii) No previous treatment
with corticosteroids or immunosuppressive drugs; and iv) Renal
tissue samples were available for immunohistochemistry and urine
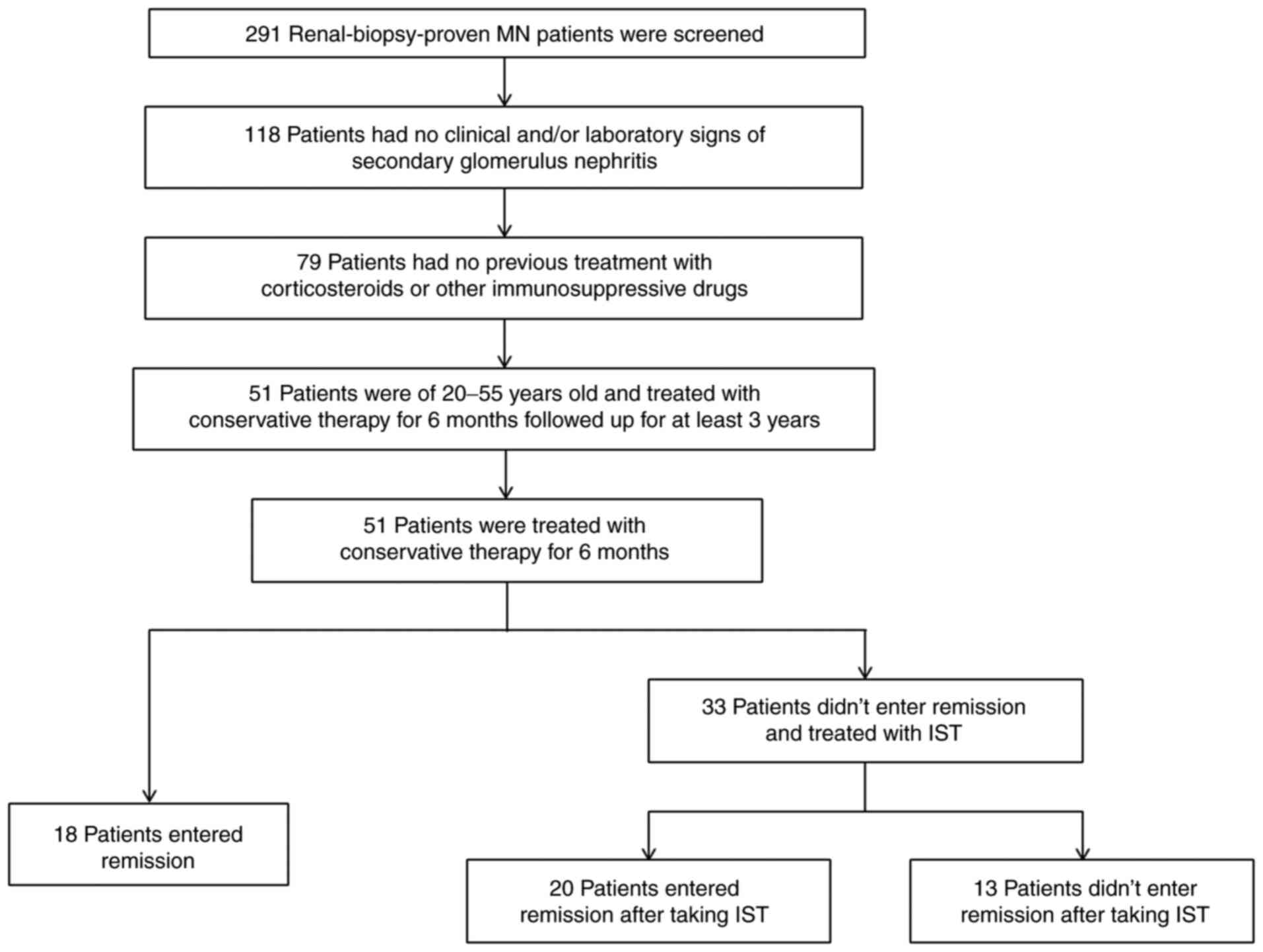
samples for the measurement of urinary KIM-1. A total of 51
patients with IMN aged between 21 and 53 years were included in
this retrospective clinical study. Based on the treatment strategy
(6) and curative effect, patients
were classified into three groups: Spontaneous remission (n=18),
remission with IST (n=20) and nonremission with IST (n=13).
Remission included complete remission and partial remission.
Complete remission was defined as urinary protein excretion of 0.3
g/day [urine protein creatinine ratio (uPCR) 300 mg/g] based on two
values obtained at least 1 week apart accompanied by a normal serum
albumin concentration and normal serum creatinine (sCr) levels.
Partial remission was defined as urinary protein excretion <3.5
g/day (uPCR, 3,500 mg/g) with a 50% or greater reduction based on
the peak values, as indicated by two values obtained at least 1
week apart, accompanied by improvement or normalization of the
serum albumin concentration and a stable sCr (6). The movement of the patients in the
present study is depicted in Fig.
1.
In addition, during the same time window as the
patients, 20 age- and sex-matched healthy adults from the medical
examination center of Qilu Hospital of Shandong University (Jinan,
China) were recruited as controls. The present study was approved
by the Ethics Committee of Qilu Hospital of Shandong University
(Jinan, China).
Clinical examination
Demographic, clinical and biochemical variables were
recorded at the time-point of diagnosis. The blood pressure of all
subjects was measured in a sitting position using a mercury
sphygmomanometer after a 10-min rest. The biochemical parameters
were measured in the clinical laboratory department of Qilu
Hospital of Shandong University (Jinan, China). sCr, blood urea
nitrogen (BUN), serum albumin (sALB), serum cystatin-C (sCys-C),
serum complement C3 (C3), serum C4, routine urine values, urinary
albumin/creatinine ratio (ACR) and urinary β2-microglobulin (β2-MG)
were determined. The estimated glomerular filtration rate (eGFR)
was determined according to the Chronic Kidney Disease Epidemiology
Collaboration formula (15). Plasma
and urine samples were collected. After centrifugation at 867 x g
for 10 min, the supernatants separated from the samples were frozen
at -80˚C until further analysis.
Measurement of anti-PLA2R antibody
(PLA2R-Ab) levels
The levels of PLA2R-Ab in plasma were measured using
an ELISA kit (cat. no. EA 1254-9601 G; Euroimmun).
Measurement of KIM-1 levels in
urine
The KIM-1 concentration in urine was measured by a
commercial ELISA kit (cat. no. DKM100; R&D Systems, Inc.) in
accordance with the manufacturer's protocol. The lower limit of the
detection of urinary KIM-1 was 0.046 ng/ml. The interassay
coefficient of variation (CV) was 6.6% and the intra-assay CV was
4.1%. Urinary KIM-1 levels were normalized to those of urinary
creatinine for each sample. The normalized data are expressed as
the urinary KIM-1 concentration/creatinine concentration
(ng/mg).
Histological parameters
Renal biopsy specimens of all patients were reviewed
by a pathologist at Qilu Hospital of Shandong University (Jinan,
China). The pathologic stage was determined according to Ehrenreich
and Churg and samples were classified as disease stages I-IV
(16). The percentage of glomerular
sclerosis was calculated for all renal biopsies. The extent of
tubular atrophy/interstitial fibrosis was classified according to
the proportion of tubular atrophy and interstitial fibrosis as
follows: Absent (T0), mild (<25%, T1), moderate (25-50%, T2) and
severe (>50%, T3) (17).
Immunohistochemical analysis of KIM-1
in the kidney
Immunohistochemical staining for KIM-1 was performed
on 4-µm paraffin sections of formaldehyde-fixed renal biopsy
tissues. Normal renal tissues adjacent to neoplastic areas
(paraneoplastic) within the nephrectomy specimens used to test for
malignancy were taken as controls. In brief, sections were
incubated in 3% H2O2 in methanol for 15 min
at 37˚C to ablate endogenous peroxidase activity after dewaxing and
rehydration at room temperature. The sections were directly
incubated with mouse anti-human monoclonal antibody against KIM-1
(cat. no. MAB1750; R&D Systems, Inc.; 1:2,000) overnight at
4˚C. Sections were then washed and incubated with polymer helper
(cat. no. D02-18; OriGene Technologies, Inc.) and horseradish
peroxidase-labeled anti-mouse IgG polymer (cat. no. D02-18; OriGene
Technologies, Inc; 1:300) for 20 min at room temperature. The
colorimetric reaction of peroxidase was performed in
3,3-diaminobenzidine solution according to the manufacturer's
protocol (cat. no. D02-18; OriGene Technologies, Inc.) after
washing with PBS three times. The level of KIM-1 expressed in renal
specimens was semiquantified by an image analysis system
(Image-Pro® Plus version 6; Media Cybernetics, Inc).
Statistical analysis
Values are expressed as the median (25 and 75th
percentile) or mean ± standard deviation where appropriate.
Differences in quantitative parameters with a normal distribution
among groups were assessed by one-way ANOVA. Tukey's
multiple-comparisons test was used as post-hoc test after ANOVA.
Differences in quantitative parameters with an abnormal
distribution among groups were assessed by the Kruskal-Wallis
H-test. Dunn's test was used for further comparison after the
Kruskal-Wallis analysis. Categorical variables were described as n
or n (%) and comparisons among groups were performed using
Mantel-Haenszel χ2 tests for dichotomized variables.
Spearman's correlation was used to analyze the correlation between
two parameters. The sensitivity and specificity of urinary KIM-1/Cr
and plasma PLA2R-Ab as indicators of the therapeutic effect in
patients with IMN were compared using receiver operating
characteristic (ROC) curves. An AUC value between 0.85 and 0.95 was
considered to represent a high predictive value. A two-sided
P<0.05 was considered to indicate statistical significance.
Analysis was performed with the SPSS statistical software package
(version 22; IBM Corp.).
Results
Clinical characteristics of patients
with IMN and healthy subjects
The clinical characteristics of patients (n=51) and
healthy controls (n=20) are provided in Table I. There was no difference in the sex
distribution, age, BMI, diastolic or systolic blood pressure,
sCys-C, eGFR, and serum C3 and C4 among the groups. There were more
subjects in the IMN patient group and significantly more patients
in the nonremission with IST group who smoked and drank than in the
control group (P<0.05). sCr in the nonremission with IST group
was significantly higher than that in the control group
(P<0.05). Serum BUN was significantly higher in the nonremission
with IST group than in the control group (P<0.05). The values of
sCr and BUN in the patient groups were still within the normal
range. Furthermore, the sALB level in all IMN groups was
significantly lower than that in the control group (P<0.01).
Among the IMN groups, the spontaneous remission group had the
highest sALB levels (30.9±5.4), while the nonremission with IST
group had the lowest sALB levels (21.3±4.0) and the differences
were statistically significant between any two groups (P<0.05).
The urinary ACR in all IMN groups was significantly higher than
that in healthy subjects, as expected (P<0.01).
 | Table IClinical characteristics of patients
with IMN and healthy subjects. |
Table I
Clinical characteristics of patients
with IMN and healthy subjects.
| | Patients with IMN
(n=51) |
|---|
| Characteristic | Healthy subjects
(n=20) | Spontaneous remission
(n=18) | Remission with IST
(n=20) | Non-remission with
IST (n=13) |
|---|
| Demographic/baseline
data | | | | |
|
Male
sex | 11(55) | 9(50) | 11(55) | 12 (92.3) |
|
Age
(years) | 38.1±10.0 | 36.7±10.9 | 36.0±9.7 | 39.3±8.9 |
|
BMI
(kg/m2) | 23.4±2.6 | 23.8±3.3 | 25.8±4.1 | 26.2±3.2 |
|
SBP
(mmHg) | 121.8±8.5 | 121.6±17.0 | 130.8±26.7 | 139.2±18.8 |
|
DBP
(mmHg) | 76.7±7.8 | 77.6±10.5 | 80.8±12.6 | 83.4±15.3 |
|
Smoking | 0 | 4 (22.2) | 5 (25.0) | 8 (61.5)a |
|
Drinking | 0 | 1 (5.6) | 3(15) | 6 (46.2)a |
|
sCr
(µmol/l) | 51.5±11.7 | 54.7±12.4 | 56.5±13.9 |
65.8±7.5b |
|
BUN
(mmol/l) | 4.14±0.97 | 4.32±1.27 | 5.00±2.00 |
5.61±1.77a |
|
sALB
(g/l) | 44.3±3.3 |
30.9±5.4b |
26.3±6.0b,c |
21.3±4.0b,d,e |
|
Serum Cys-C
(mg/l) | 0.83±0.14 | 0.84±0.16 | 1.01±0.63 | 1.06±0.28 |
|
eGFR
(ml/min/1.73 m2) | 124.5±12.9 | 122.1±11.9 | 123.0±10.9 | 114.6±12.9 |
|
Serum C3
(mg/dl) | (-) | 1.26±0.19 | 1.31±0.34 | 1.20±0.16 |
|
Serum C4
(mg/dl) | (-) | 0.30±0.09 | 0.28±0.09 | 0.30±0.07 |
|
Urinary ACR
(g/g) | 0.01
(0.01-0.03) | 2.09 (1.84,
2.79)a | 2.36 (0.87,
3.91)a | 4.57 (1.54,
6.51)a,e |
|
Urinary
β2-MG (mg/l) | NA | 0.21 (0.20,
0.22) | 0.21 (0.20,
0.22) | 0.22 (0.27,
1.48)d,f |
|
PLA2R-Ab-positive
patients (ELISA) | (-) | 12 (66.7) | 14 (70.0) | 9 (69.2) |
|
PLA2R-Ab
titer (ELISA) (RU/ml)g | (-) | 5.1 (2.4,
28.2) | 57.6 (31.7,
131.4)c | 191.5 (122.8,
406.0)d |
| Histological
scores | | | | |
|
Glomerular
sclerosis (%) | NA | 0 (0,0) | 0 (0,5.8) | 0 (0,10.5) |
|
Interstitial
fibrosis (%) | | | | |
|
Absent | NA | 10 (55.6) | 7 (35.0) | 5 (38.5) |
|
Grade
I | NA | 8 (44.4) | 13 (65.0) | 5 (38.5) |
|
Grade
II | NA | 0 | 0 | 3 (23.0) |
|
Grade
III | NA | 0 | 0 | 0 |
| Therapy | | | | |
|
Renin-angiotensin
system inhibitors | 0 | 11 (61.1) | 12 (60.0) | 8 (61.5) |
|
ACEI
alone | 0 | 2 | 9 | 3 |
|
ARB
alone | 0 | 7 | 3 | 5 |
|
Both | 0 | 2 | 0 | 0 |
|
IST and
duration | | | | |
|
Steroids+CYC,
6 months A | 0 | 0 | 16(80) | 0 |
|
A+Tacrolimus,
6 months B | 0 | 0 | 3(15) | 0 |
|
A+B+MMF,
6 months | 0 | 0 | 1(5) | 13(100) |
| Follow-up data | | | | |
|
Follow-up
duration (months) | | 37.1±6.35 | 37.7±5.37 | 38.2±6.17 |
|
6
months-averaged urinary ACR (g/g) | | 0.9 (0.3, 2.0) | 1 (0.3, 2.3) | 4.3 (2.8,
5.1)c,e |
|
eGFR
declined rate (ml/(min x1.73 m2) per year | | 1.86 (-0.96,
4.20) | 2.66 (1.6,
4.43) | 4.66 (3.33,
6.00) |
Furthermore, the urinary ACR in the nonremission
with IST group was significantly higher than that in the
spontaneous remission group (P<0.05). Urinary β2-MG in the
nonremission with IST group was significantly higher than that in
the spontaneous remission group and remission with IST group
(P<0.01). PLA2R-Ab was detected in 12 (66.7%), 14 (70%) and 9
(69.2%) of patients at baseline in the spontaneous remission,
remission with IST and nonremission with IST groups, respectively
(Table I). The plasma PLA2R-Ab
levels were significantly higher in the remission with IST group
(P<0.05) and nonremission with IST group (P<0.01) than in the
spontaneous remission group. There was no significant difference in
terms of the histological score or renin-angiotensin system
inhibitor therapy among the three patient groups.
All patients were followed up for ~36 months. The
6-month average urinary ACR in the nonremission with IST group was
significantly higher than that in the spontaneous remission group
and remission with IST group (P<0.05). There was no significant
decrease in the rate of eGFR among the three groups (Table I).
Urinary KIM-1 levels in patients with
IMN
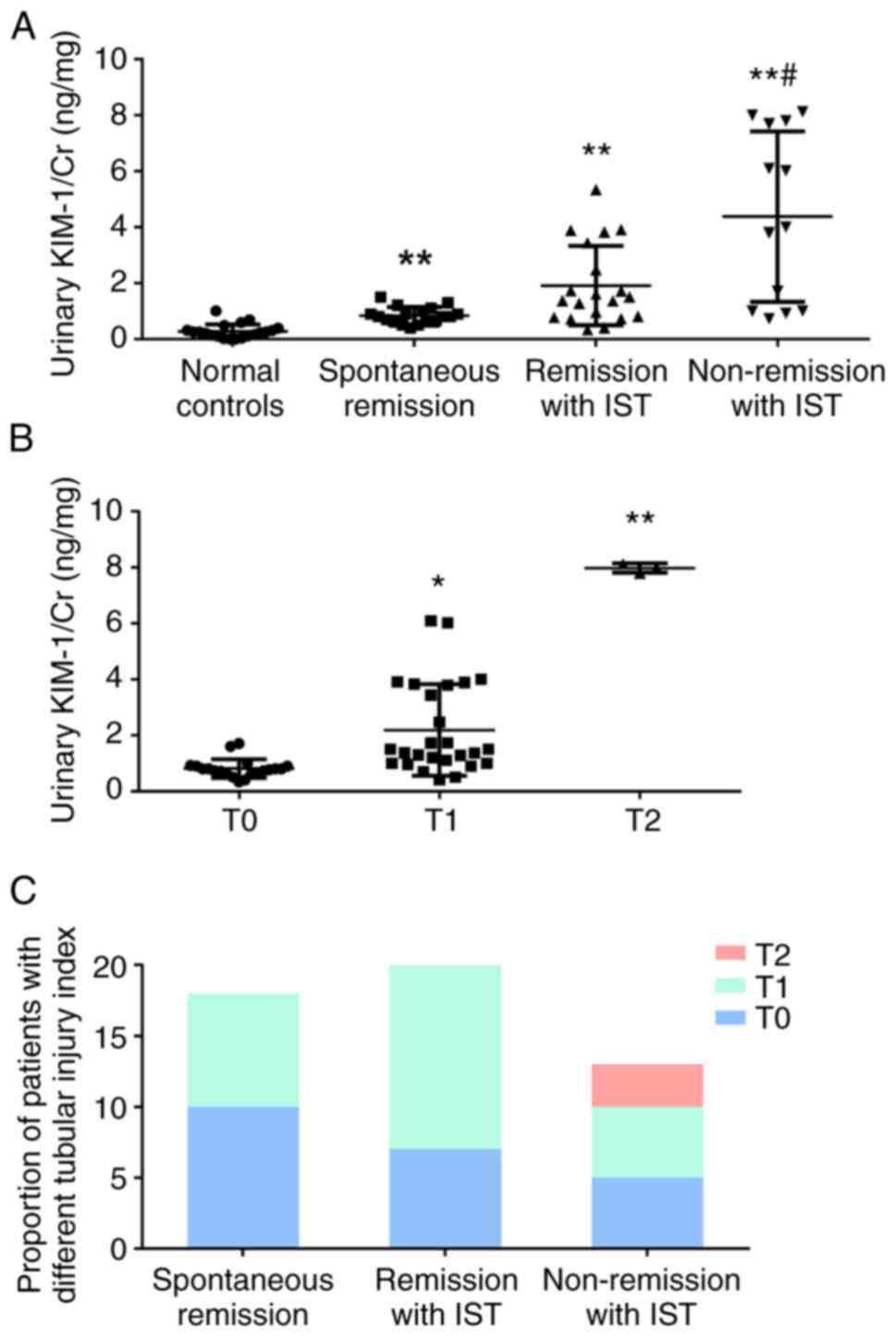
The levels of urinary KIM-1/Cr were significantly
higher in patients with IMN than in healthy subjects (P<0.05,
Fig. 2A). Of note, urinary KIM-1/Cr
levels were significantly increased in the nonremission with IST
group compared with those in the spontaneous remission group
(P<0.05, Fig. 2A).
Urinary KIM-1 levels were also analyzed according to
the tubular atrophy and interstitial fibrosis index based on renal
biopsy (T0, T1 and T2). The urinary KIM-1/Cr levels gradually
increased as tubular atrophy and interstitial fibrosis became more
severe. Patients with both T1 and T2 changes had significantly
higher urinary KIM-1/Cr levels than those with T0 (P<0.05,
Fig. 2B). However, there was no
significant difference between patients with T1 and T2 changes
(P>0.05, Fig. 2B). Consistently,
there were no patients with T2 in any groups other than the
nonremission with IST group (Fig.
2C).
Renal KIM-1 expression in patients
with IMN
KIM-1-positive staining was present in proximal
tubule epithelial cells of patients with IMN, while no
KIM-1-positive cells were observed in normal renal tissue (Fig. 3A). Furthermore, KIM-1 expression was
only detected at a very low level in the tissues from the
spontaneous remission group. KIM-1 expression was significantly
increased in the other two groups of patients compared with that in
the spontaneous remission group (P<0.01), but there was no
significant difference in KIM-1 expression between the two IST
groups (P>0.05, Fig. 3B).
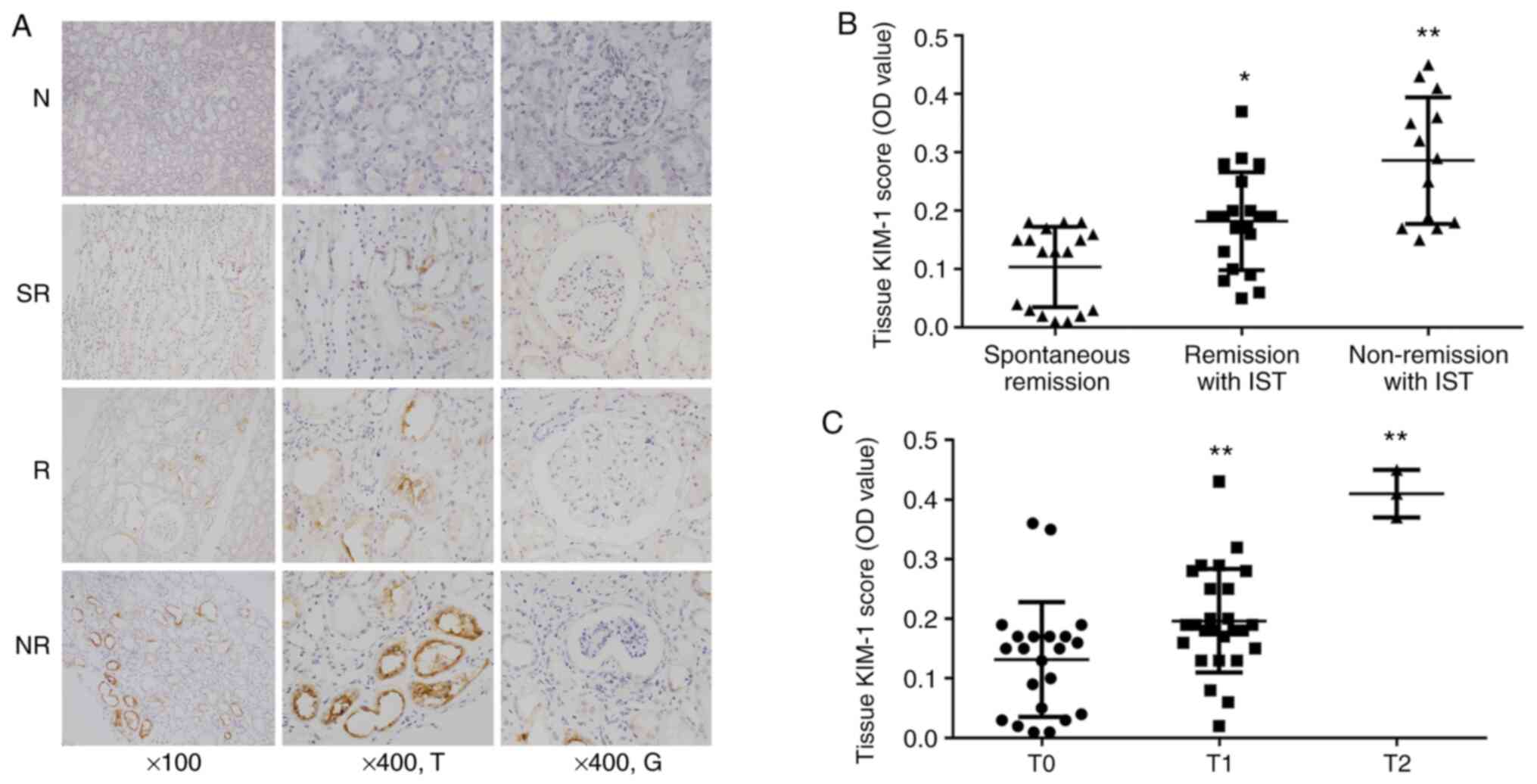 | Figure 3(A) Representative histology images of
renal KIM-1 expression in the control and IMN groups (magnification
x100 or x400; T and G indicate tubules and glomeruli, respectively.
Samples were counterstained with hematoxylin with brown areas
indicating positive staining). Groups: N, normal control group; SR,
spontaneous remission group; R, remission with IST group; NR,
nonremission with IST group. (B) Semiquantitative analysis of
tissue KIM-1 expression levels in different groups of patients with
IMN. *P<0.05, **P<0.01 vs spontaneous
remission group. (C) Semiquantitative analysis of tissue KIM-1
expression levels in patients with IMN with different tubular
injury indexes. **P<0.01 vs T0. KIM-1, kidney injury
molecule-1; IMN, idiopathic membranous nephrology; OD, optical
density; IST, immunosuppressive therapy. |
Renal KIM-1 levels were analyzed again according to
the tubular atrophy and interstitial index. Renal tissues with T2
had significantly higher KIM-1 expression than those with T0
(P<0.01, Fig. 3C). Although
KIM-1-positive staining appeared to be deeper in renal tissues with
T2 than those with T1 (Fig. S1),
there was no significant difference in quantitative analysis
between these tissues (P>0.05; Fig.
3C). This may be due to there being a greater proportion of
patients with T2 in the nonremission with IST group than in the
remission with IST and spontaneous remission groups.
Correlation of KIM-1 and plasma
PLA2R-Ab levels with clinical parameters
As presented in Table
II, urinary KIM-1/Cr was positively correlated with BUN
(r=0.282, P=0.045), sCr (r=0.311, P=0.026), sCys-C (r=0.433,
P=0.001), the urinary ACR (r=0.306, P=0.029), urinary β2-MG
(r=0.410, P=0.003) and the tubular atrophy and interstitial
fibrosis index (r=0.572, P<0.001). Furthermore, it was
negatively correlated with sALB (r=-0.343, P=0.014) at the time of
renal biopsy. However, it was not correlated with the eGFR,
Ehrenreich-Churg stage or glomerular sclerosis index in renal
tissues.
 | Table IICorrelation of urinary or renal KIM-1
expression with serum PLA2R-Ab and clinical or histological indexes
in patients with idiopathic membranous nephrology. |
Table II
Correlation of urinary or renal KIM-1
expression with serum PLA2R-Ab and clinical or histological indexes
in patients with idiopathic membranous nephrology.
| | Urinary
KIM-1/Cr | Renal KIM-1
expression | Plasma
PLA2R-Ab |
|---|
| Item | r-Value | P-value | r-Value | P-value | r-Value | P-value |
|---|
| Clinical data | | | | | | |
|
BUN
(mmol/l) | 0.282 | 0.045 | 0.361 | 0.009 | 0.227 | 0.190 |
|
sCr
(umol/l) | 0.311 | 0.026 | 0.315 | 0.024 | 0.403 | 0.016 |
|
sCys-C
(mg/l) | 0.433 | 0.001 | 0.321 | 0.022 | 0.143 | 0.412 |
|
eGFR (ml/min
x 1.73 m2) | -0.146 | 0.306 | -0.185 | 0.194 | -0.369 | 0.029 |
|
sAlb
(g/l) | -0.343 | 0.014 | -0.433 | 0.002 | -0.454 | 0.006 |
|
Urinary ACR
(g/g) | 0.306 | 0.029 | 0.396 | 0.004 | 0.390 | 0.020 |
|
Urinary
β2-MG (mg/l) | 0.410 | 0.003 | 0.497 | <0.001 | 0.378 | 0.025 |
| Histology
parameters | | | | | | |
|
Ehrenreich-Churg
stage | 0.274 | 0.052 | 0.175 | 0.220 | 0.161 | 0.356 |
|
Glomerular
sclerosis (%) | 0.149 | 0.296 | 0.199 | 0.161 | 0.419 | 0.012 |
|
Tubular
atrophy and interstitial fibrosis index | 0.572 | <0.001 | 0.498 | <0.001 | 0.055 | 0.753 |
Renal tissue KIM-1 expression levels were positively
correlated with BUN (r=0.361, P=0.009), sCr (r=0.315, P=0.024),
sCys-C (r=0.321, P=0.022), the urinary ACR (r=0.396, P=0.004),
tubular atrophy and interstitial fibrosis index (r=0.498,
P<0.001) and urinary β2-MG (r=0.497, P<0.001) and negatively
correlated with sALB (r=-0.433, P=0.002).
In addition, plasma PLA2R-Ab levels were positively
correlated with sCr (r=0.403, P=0.016), the urinary ACR (r=0.390,
P=0.020), urinary β2-MG (r=0.378, P=0.025) and the glomerular
sclerosis index (r=0.419, P=0.012). It was negatively correlated
with sALB (r=-0.454, P=0.006) and the eGFR (r=-0.369, P=0.029).
However, it was not correlated with BUN, sCys-C, the
Ehrenreich-Churg stage or the interstitial fibrosis index in renal
tissues.
Correlation of urinary KIM-1 and renal
KIM-1
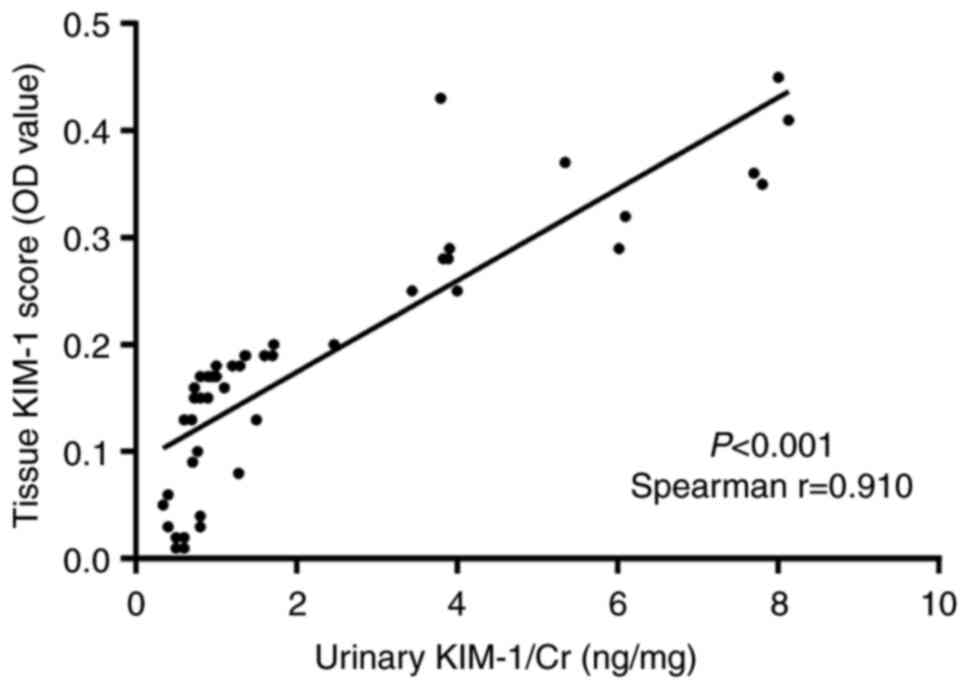
Next, it was investigated whether there was a
correlation between the urinary and renal KIM-1 levels. As
presented in Fig. 4, there was a
significant correlation between the urinary KIM-1/Cr and renal
KIM-1 levels (Spearman r=0.910, P<0.001).
Predictive power of urinary KIM-1 and
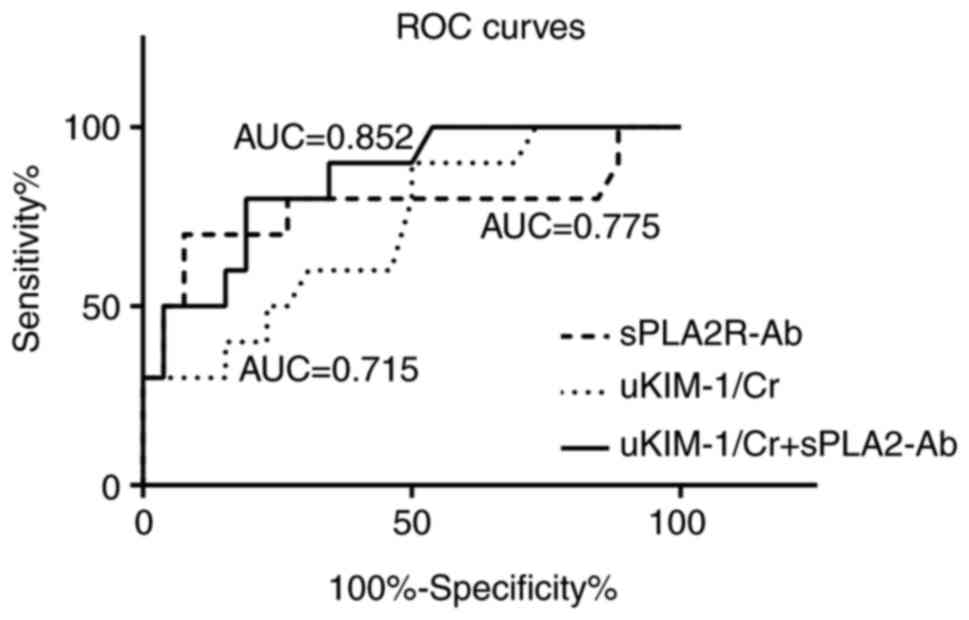
plasma PLA2R-Ab for the therapeutic effect
ROC curve analysis was performed in
PLA2R-Ab-positive individuals to compare the predictive power of
urinary KIM-1 and plasma PLA2R-Ab as biomarkers of the therapeutic
effect in patients with IMN. The area under the ROC curve (AUC) was
measured to evaluate the sensitivity and specificity of urinary
KIM-1/Cr, PLA2R-Ab and their combination. As presented in Fig. 5, the AUC for urinary KIM-1/Cr in
predicting the therapeutic effect was 0.715 (95% CI, 0.533-0.898)
and the AUC of PLA2R-Ab was 0.775 (95% CI, 0.560-0.990). The
predictive power of their combination was the highest, with an AUC
of 0.852 (95% CI, 0.721-0.983).
Discussion
The presence and severity of membranous nephropathy
are generally indicated by the degree of podocyte injury. High
urinary concentrations of proteins that reflect tubular or
glomerular damage precede a decline in the eGFR in patients with
IMN (18,19). Urinary KIM-1 is a novel biomarker of
tubular damage. There is increasing evidence of the prognostic
value of KIM-1 in patients with glomerular disease, including
diabetic kidney disease and IMN (20,21).
However, the influence of the levels of KIM-1 in urine and renal
tissue on the therapeutic efficacy for IMN has remained to be
determined. In the present study, KIM-1 expression levels were
analyzed according to the therapeutic outcomes for patients with
IMN. The results suggested that patients with IMN had elevated
urinary and renal KIM-1 levels compared with those in normal
control subjects. Significantly increased urinary and renal KIM-1
levels were observed in the nonremission with IST group compared
with those in the spontaneous remission group. The present study
suggested that higher KIM-1 levels in both urine and tissue at the
time of renal biopsy are associated with poorer treatment outcomes
for IMN. The present study also confirmed the link between urinary
and tubular KIM-1 expression in IMN, since urinary KIM-1 levels are
strongly correlated with tubular KIM-1 expression in experimental
and human renal disease in both acute kidney injury and ESRD
(22). Due to the correlation
between urinary and tubular KIM-1 expression, the levels of KIM-1
may be detected directly from urine without necessitating an
invasive kidney biopsy in the future.
KIM-1, which is located on epithelial cells,
mediates the phagocytosis of apoptotic and necrotic cells by
binding to phosphatidylserine and oxidized lipid epitopes on the
apoptotic cell surface (23).
Various experimental and clinical studies suggested that KIM-1
reflects tubulointerstitial injury and repair (24). In the present study, increased
tubular KIM-1 expression was indicated to be positively associated
with BUN, sCr, sCys-C, the urinary ACR and urinary β2-MG at the
time of renal biopsy, which suggested that KIM-1 provides
additional information on tubular processes involved in progressive
renal failure.
MN is the major cause of nephrotic syndrome with
special pathological features resulting from the formation of
immune complexes in the space between podocytes and the glomerular
basement membrane. PLA2R-Ab production is common and is detected in
70-80% of patients with IMN (25).
Autoantibodies against M-type PLA2R are specific markers of IMN.
The PLA2R-Ab level was reported to be an independent predictor of
the risk of remission in proteinuria and to be of prognostic value
in IMN (26). The strong
correlation between the clinical conditions and PLA2R-Ab levels
allowed the prediction of the distribution of the prevalence in
patients with active disease and partial and complete remission
(27). In the present study, plasma
PLA2R-Ab levels were positively correlated with proteinuria. A
significantly higher plasma PLA2R-Ab level was observed in the
nonremission with IST group than in the spontaneous remission
group. Furthermore, significantly higher urinary KIM-1 levels were
observed in the nonremission with IST group than in the spontaneous
remission group. Therefore, KIM-1 has prognostic value for tubular
injury and may be considered a promising biomarker to evaluate the
prognosis of patients with IMN together with PLA2R-Ab, which is of
prognostic value for glomerular injury.
The patients of the present study were biopsied at
an early stage of the disease and subjected to conservative
treatment or IST. Patients who were treated with IST but who did
not enter remission displayed much more highly elevated urinary
KIM-1 levels than those in the spontaneous remission group. This
may aid the identification of the IST group at the early stage of
the disease. Children with steroid-resistant idiopathic nephrotic
syndrome were likely to present with high urinary KIM-1 excretion
and had a higher risk of tubulointerstitial fibrosis (22). The primary finding of the report is
that chronic KIM-1 expression in renal epithelial cells directly
causes interstitial inflammation followed by progressive fibrotic
renal disease (28). The present
study also indicated that urinary KIM-1 levels exhibited a
gradually increasing trend as tubular atrophy and interstitial
fibrosis became more severe. KIM-1 excretion rates correlated with
the excretion rates of other tubular damage markers, such as
urinary α1 microglobulin and urinary β2-MG, and predicted the
outcome for patients with IMN. In the present study, it was
indicated that urinary KIM-1 levels were positively associated with
the Ehrenreich-Churg stage, but this correlation was not observed
for renal KIM-1 expression. The urinary and renal KIM-1 expression
levels increased as tubular atrophy and interstitial fibrosis
became more severe, which confirmed previous observations.
The major limitation of the present study is that it
is a single-center descriptive study with a small sample size,
which may limit the broader applicability of the results. Trends
regarding differences in KIM-1 expression levels between the two
groups of patients treated with IST may be significantly different
in a study with a more adequate sample size. All patients included
in the present study were at the early stage of IMN with an
Ehrenreich and Churg stage of I or II. However, histology scoring
could have been performed instead of measuring the OD value when
analyzing the expression of KIM-1 in renal tissues. In addition,
long-term follow-up of the patients is required to perform a more
comprehensive analysis. The dynamic change of KIM-1 levels may
provide more values associated with therapeutic treatment and
outcomes. Earlier data for urinary KIM-1 levels were not gathered,
since the present study had a retrospective design, and a
prospective study that dynamically describes the relationship
between KIM-1 levels and outcome for patients with IMN will be
performed in the near future. Furthermore, the present study mainly
focused on the relationship of KIM-1 levels with therapeutic
outcomes for IMN and without considering the impact of smoking and
drinking. It may also be interesting to compare the
non-smoking/drinking subjects to the non-smoking/drinking subjects
in the same group if the group sizes were decent in a future
prospective study.
In conclusion, urinary and renal KIM-1 levels may be
used as biomarkers of tubulointerstitial damage and to evaluate the
therapeutic effects of IMN treatment. Further studies are required
to elucidate the exact role of KIM-1 in IMN and the correlation
between tubulointerstitial injury and the treatment effect.
Supplementary Material
Representative KIM-1
immunohistochemistry images for T0, T1 and T2 stages (magnification
x400; counterstained with hematoxylin; brown areas indicate
positive staining). KIM-1, kidney injury molecule-1.
Acknowledgements
Not applicable.
Funding
This work was funded by the General Financial Grant from the
China Postdoctoral Science Foundation (grant. no. 2015 M572048) and
the International Exchange Project of the China Postdoctoral
Science Foundation (grant. no. 2019GSF108111).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and XYX were responsible for the study design.
YDZ, CHX, LYG, YYZ and JHZ contributed to acquiring and analyzing
the data. YDZ and CHX were involved in writing the manuscript. All
authors read and approved the final version of the manuscript. All
authors have confirmed the authenticity of the raw data.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee at the institution
in which the study was conducted and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. The present study was approved by the Ethics Committee
of Qilu Hospital of Shandong University [Jinan, China;
institutional review board approval no. KYLL-2018(KS)-234]. Written
informed consent was obtained from all subjects and/or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ronco P and Debiec H: Pathophysiological
advances in membranous nephropathy: Time for a shift in patient's
care. Lancet. 385:1983–1992. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu R, Quan S, Wang Y, Wang Y, Zhou Y,
Zhang Y, Liu L, Zhou XJ and Xing G: Spectrum of biopsy proven renal
diseases in Central China: A 10-year retrospective study based on
34,630 cases. Sci Rep. 10(10994)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Beck LH Jr, Bonegio RG, Lambeau G, Beck
DM, Powell DW, Cummins TD, Klein JB and Salant DJ: M-type
phospholipase A2 receptor as target antigen in idiopathic
membranous nephropathy. N Engl J Med. 361:11–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Timmermans SA, Hamid MA, Tervaert JW,
Damoiseaux JG and van Paassen P: Limburg Renal Registry. Anti-PLA2R
antibodies as a prognostic factor in PLA2R-related membranous
nephropathy. Am J Nephrol. 42:70–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van de Logt AE, Hofstra JM and Wetzels JF:
Pharmacological treatment of primary membranous nephropathy in
2016. Expert Rev Clin Pharmacol. 9:1463–1478. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kidney Disease: Improving global outcomes.
KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney
International Supplements. 2(139)2012.
|
|
7
|
Polanco N, Gutierrez E, Covarsi A, Ariza
F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C,
Pons S, et al: Spontaneous remission of nephrotic syndrome in
idiopathic membranous nephropathy. J Am Society Nephrol.
21:697–704. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Han WK, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bonventre JV: Kidney injury molecule-1
(KIM-1): A urinary biomarker and much more. Nephrol Dial
Transplant. 24:3265–3268. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Timmeren MM, van den Heuvel MC, Bailly
V, Bakker SJ, van Goor H and Stegeman CA: Tubular kidney injury
molecule-1 (KIM-1) in human renal disease. J Pathol. 212:209–217.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Waanders F, Vaidya VS, van Goor H,
Leuvenink H, Damman K, Hamming I, Bonventre JV, Vogt L and Navis G:
Effect of renin-angiotensin-aldosterone system inhibition, dietary
sodium restriction, and/or diuretics on urinary kidney injury
molecule 1 excretion in nondiabetic proteinuric kidney disease: A
post hoc analysis of a randomized controlled trial. Am J Kidney
Dis. 53:16–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peters HP, Waanders F, Meijer E, van den
Brand J, Steenbergen EJ, van Goor H and Wetzels JF: High urinary
excretion of kidney injury molecule-1 is an independent predictor
of end-stage renal disease in patients with IgA nephropathy.
Nephrol Dial Transplant. 26:3581–3588. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vaidya VS, Niewczas MA, Ficociello LH,
Johnson AC, Collings FB, Warram JH, Krolewski AS and Bonventre JV:
Regression of microalbuminuria in type 1 diabetes is associated
with lower levels of urinary tubular injury biomarkers, kidney
injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int.
79:464–470. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Du Y, Hou L, Guo J, Sun T, Wang X and Wu
Y: Renal neutrophil gelatinase-associated lipocalin and kidney
injury molecule-1 expression in children with acute kidney injury
and Henoch-Schonlein purpura nephritis. Exp Ther Med. 7:1130–1134.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levey AS, Stevens LA, Schmid CH, et al: A
new equation to estimate glomerular filtration rate. Ann Intern
Med. 150:604–612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Churg J, Grishman E, Golstein MH, Yunis SL
and Porush JG: Idiopathic nephrotic syndrome in adults. A study and
classification based on renal biopsies. N Engl J Med. 28:165–174.
1965.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Roberts ISD, Burrows C, Shanks JH, Venning
M and McWilliam LJ: Interstitial myofibroblasts: Predictors of
progression in membranous nephropathy. J Clin Pathol. 50:123–127.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bazzi C, Petrini C, Rizza V, et al:
Urinary excretion of IgG and alpha(1)-microglobulin predicts
clinical course better than extent of proteinuria in membranous
nephropathy. Am J Kidney Dis. 38:240–248. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hofstra JM, Deegens JKJ, Willems HL and
Wetzels JF: Beta-2-microglobulin is superior to
N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic
membranous nephropathy. Nephrol Dial Transplant. 23:2546–2551.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Carvalho JAM, Tatsch E, Hausen BS, et
al: Urinary kidney injury molecule-1 and neutrophil
gelatinase-associated lipocalin as indicators of tubular damage in
normoalbuminuric patients with type 2 diabetes. Clin Biochem.
49:232–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maas RJH, van den Brand JA, Waanders F, et
al: Kidney injury molecule-1 and neutrophil gelatinase-associated
lipocalin as prognostic markers in idiopathic membranous
nephropathy. Ann Clin Biochem. 53:51–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bieniaś B, Zajączkowska M, Borzęcka H,
Sikora P, Wieczorkiewicz-Płaza A and Wilczyńska B: Early markers of
tubulointerstitial fibrosis in children with idiopathic nephrotic
syndrome: Preliminary report. Medicine (Baltimore).
94(e1746)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ichimura T, Asseldonk EJPV, Humphreys BD,
Gunaratnam L, Duffield JS and Bonventre JV: Kidney injury
molecule-1 is a phosphatidylserine receptor that confers a
phagocytic phenotype on epithelial cells. J Clin Invest.
118:1657–1668. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Brooks CR and Bonventre JV: KIM-1/TIM-1 in
proximal tubular cell immune response. Oncotarget. 6:44059–44060.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu W, Gao C, Dai H, et al: Immunological
pathogenesis of membranous nephropathy: Focus on PLA2R1 and its
role. Front Immunol. 10(1809)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han WW, Tang LJ, Kong XL, Yang H and Xu
DM: Clinical significance of autoantibodies in the assessment and
treatment of idiopathic membranous nephropathy. Exp Ther Med.
17:1825–1830. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Radice A, Trezzi B, Maggiore U, et al:
Clinical usefulness of autoantibodies to M-type phospholipase A2
receptor (PLA2R) for monitoring disease activity in idiopathic
membranous nephropathy (IMN). Autoimmun Rev. 15:146–154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Humphreys BD, Xu F, Sabbisetti V, et al:
Chronic epithelial kidney injury molecule-1 expression causes
murine kidney fibrosis. J Clin Invest. 123:4023–4035.
2013.PubMed/NCBI View
Article : Google Scholar
|