Introduction
Cancer remains an important global public health
problem and lung cancer is a very common cause of malignant tumor
development (1). With the gradually
deteriorating natural environment due to continuous development and
industrialization, the global morbidity and mortality of lung
cancer in the past decade has increased, with serious implications
for the quality of human life (2).
A number of factors are involved in lung cancer progression, such
as mutation in tumor suppressor genes, cell proliferation,
angiogenesis and chronic inflammation (3). The increased understanding of disease
mechanism has led to the development of a number of new antitumor
agents and associated therapies, the majority of which have been
clinically applied. Aspirin has been a cornerstone in
cardiovascular disease prevention since the late 1980s (4) and the antitumor effect of aspirin has
received increasing attention. Experimental, epidemiological and
clinical data from the last two decades have supported the
hypothesis that aspirin possesses anti-cancer properties and its
use may also reduce the lifetime probability of developing or dying
from a number of cancers (5,6). The
mechanism of action of aspirin as an antineoplastic agent is
multifold through its anti-platelet effect, inhibition of the
enzyme cyclooxygenase (COX), as well as through COX-independent
mechanisms and its impact on multiple metabolic signaling pathways
are considered critical to its anticancer effects (7-9).
Aspirin exerts a proliferation-inhibiting and apoptosis-inducing
potential on a number of cancer cell lines, such as hepatocellular
carcinoma cells (9). However, it
remains to be elucidated whether aspirin has similar inhibitory
effect on lung cancer cells.
Mitogen-activated protein kinase (MAPK) signaling
pathways are involved in mediation of processes of cell growth,
survival and death (10-12).
There are three members in the MAPK family; JNK, p38MAPK and ERK.
They have diverse cellular functions and are controlled separately
by relatively independent upstream signaling molecules (13). These factors are serine/threonine
protein kinases that regulate various cellular activities,
including proliferation, differentiation, apoptosis, survival,
inflammation and innate immunity (14,15).
The abnormal activation of MAPK signaling pathways contributes to
the pathogenesis of diverse human diseases including cancer
(14,16,17).
All the MAPK family members can be activated in response to various
endogenous and exogenous stresses. Upon stimulation, the
phosphorylated/activated ERK can change gene expression to promote
cell growth, differentiation or mitosis (18). In some cases, ERK uses JNK as its
final mediator to stimulate cell proliferation. p38MAPK appears to
play a major role in apoptosis, differentiation, survival,
proliferation, development, inflammation and other stress responses
(19).
In the current study, it was found that although a
high-dose of aspirin showed remarkable tumor suppression in PC-9
lung cancer cells, a relatively low dose of this agent exhibited a
marked growth-promoting effect on PC-9 cells. As for the
growth-promoting mechanism of low-dose aspirin, it was shown that
all the MAPK signaling was involved.
Materials and methods
Materials
Aspirin was freshly dissolved in DMSO (<0.25%
final concentration) before assays. DMEM medium and fetal bovine
serum (FBS) were obtained from Thermo Fisher Scientific, Inc.
Dimethyl sulfoxide (DMSO), MTT and crystal violet were purchased
from Sigma-Aldrich (Merck KGaA). The fluorescein isothiocyanate
(FITC) Annexin V and propidium iodide (PI) kit for apoptosis
detection was obtained from Multisciences (Lianke) Biotech Co.,
Ltd., anti-ERK (cat. no. WL01864), anti-phosphorylated (p)-ERK
(Thr202/Tyr204; cat. no. WLP1512), anti-JNK (cat. no. WL01295),
anti-p-JNK (cat. no. WL01813), anti-p38 (cat. no. WL00764),
anti-p-p38 (cat. no. WLP1576), anti-cleaved-caspase3 (cat. no.
WL02117), anti-Bax (cat. no. WL01637),anti-Bcl-2 antibodies (cat.
no. WL01556) and secondary antibodies (cat no. WLA023) were
purchased from Wanleibio Co., Ltd. PD98059 (an ERK inhibitor),
SP600125 (a JNK inhibitor) and SB203580 (a p38 inhibitor) were
purchased from MedChem Express. Penicillin-streptomycin solution,
100X (cat. no. P1400) and anti-GAPDH (cat. no. K106389P) were
purchased from Beijing Solarbio Science and Technology Co.,
Ltd.
Cell culture
Human lung cancer cell lines PC-9 (formerly known as
PC-14: Please see the following website for further details
https://web.expasy.org/cellosaurus/CVCL_1640) and A549
were obtained from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences. Human colon cancer cell line HCT116
was gifted by Professor Zhang Yu of the Institute of Biological
Sciences, Jinzhou Medical University (Jinzhou, China). Cells were
maintained in DMEM with 10% FBS, 1% penicillin-streptomycin
solution at 37˚C in a humidified atmosphere of 5% CO2.
All cells were tested to ensure that they were mycoplasma
contamination-free using a PCR-based assay. The cells in single
drug groups were treated with 0.25% DMSO (control group) and
different concentrations of aspirin (1, 2, 4, 8 and 16 mM). The
inhibitor groups were pre-incubated with PD98059 (10 µM), SP600125
(10 µM) or SB203580 (10 µM) respectively and then treated with
different concentrations of aspirin.
Colony formation experiments
For this experiment, 600 cells were seeded in
six-well plates and treated with various concentrations of aspirin
in the presence or absence of MAPK inhibitors for 7 days in single
drug groups. For inhibitor groups, cells were pre-treated with a
selective MAPK inhibitor (the ERK inhibitor PD98059, the JNK
inhibitor SP600125 and the p38 MAPK inhibitor SB203580) for 30 min
respectively and then treated with aspirin for 7 days. Control
cells were treated with an equal amount of DMSO (0.25% v/v). The
culture was terminated when colonies were visible to the naked eye
in the culture dish. Then the supernatant was discarded by
aspiration and the cells were washed twice with PBS before 4%
paraformaldehyde (1 ml) was slowly poured into in each petri dish
to fix cells for 20 min at room temperature. The fixative was then
discarded and an appropriate amount of crystal violet added (0.1%)
for 5-10 min at room temperature before finally being slowly washed
away with water. Images were obtained with a camera and ImageJ
version 1.8.0.112 (National Institutes of Health) was used to
calculate the colony area. Every image includes a full well to
ensure the same focal length. The software was used to count the
area of blue colony pixels, a common threshold was set. If the gray
level was greater than this threshold the area was counted as a
colony and if it was less than this value, it was counted as a
background.
MTT assay
MTT colorimetric assay was used to detect the effect
of aspirin on cell viability. PC-9, A549 and HCT116 cells were
seeded in 96-well plates (2,000 cells/well). After adherence, the
cells were treated with different concentrations of aspirin (0, 1,
2, 4, 8 and 16 mM) for different time points (0, 24, 48 and 72 h).
Subsequently, MTT (5 mg/ml) was added and the plates incubated in
an atmosphere of 5% CO2/95% air at 37˚C for 4 h. The
supernatant was discarded and 150 µl of DMSO was added and agitated
at 37˚C for 10 min until the crystals were sufficiently dissolved.
The absorbance values were measured at 570 nm using a microplate
reader (Bio-Rad Laboratories, Inc.) at different time points (0,
24, 48 and 72 h).
Western blot analysis
Western blot analyses were performed as previously
described (20). The cells were
cultured, harvested and lysed on ice for 30 min in an appropriate
lysis buffer (120 mM NaCl, 40 mM Tris (pH 8.0) and 0.1% NP-40) and
were then centrifuged at 13,000 x g for 15 min at 4˚C. Protein
samples were quantified using a BCA kit (Thermo Fisher Scientific,
Inc.). Lysates from each sample were mixed with 5X sample buffer
(0.375 M Tris-HCl, 5% SDS, 5% β-mercaptoethanol, 50% glycerol and
0.05% bromophenol blue, pH 6.8) and were heated to 95˚C for 5 min.
Equal amounts (30 µg) of protein lysate were separated by 12%
SDS-PAGE and were transferred onto polyvinylidene fluoride
membranes. The membranes were then washed with Tris-buffered saline
(10 mM Tris, 150 mM NaCl) containing 0.05% Tween-20 (TBST) and were
blocked in TBST containing 5% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 h at room temperature. Next, the
membranes were incubated with their respective specific primary
antibodies (ERK, p-ERK, JNK, p-JNK, p38, p-p38, Bcl-2, Bax at a
dilution of 1:500; GAPDH at a dilution of 1:3,000) overnight at
4˚C. After three washes in TBST, the membranes were incubated with
the appropriate secondary antibody conjugated to horseradish
peroxidase (cat no. WLA023; 1:3,000) for 1 h at room temperature.
Then the membranes were washed again and an enhanced
chemiluminescence-based ECL detection kit (Cyanagen Srl) was used.
ImageJ version 1.8.0.112 (National Institutes of Health) was used
for densitometry. Data of specific protein levels are presented
relative to the control. All experiments were repeated at least
three times.
Apoptosis assessment
The Annexin V/PI double staining assay recognizes
the externalization of phosphatidylserine on the cell membrane, a
hallmark of apoptotic cells. In brief, 1x105 cells were
seeded on a 24-well plate and treated with aspirin alone or 30 min
pre-treatment of an ERK inhibitor PD98059 or JNK inhibitor SP00125
or P38 inhibitor SB203580 for 24 h. Cells were dissociated with
trypsin, harvested and washed with PBS. The cells were resuspended
in 1X binding buffer (100 µl) and incubated with 1.4 µl of Annexin
V-FITC (200 µg/ml) and 5 µl of PI (30 µg/ml) at room temperature
for 10 min. The cells stained with Annexin V/PI were analyzed by a
flow cytometer (BD Immunocytometry Systems). The amounts of early
apoptosis and late apoptosis were determined as the percentage of
Annexin V+/PI- or Annexin
V+/PI+ cells, respectively. FlowJo version
10.5.3 (FlowJo LLC) was used for analysis.
Cell cycle analysis
For cell cycle analysis, cells were harvested and
fixed in 70% (v/v) ethanol and then washed with PBS. All cells were
stained with PI solution and the DNA content of each cell was
detected by flow cytometry (21).
ModFit LT 4.0 (Verity Software House) was used for analysis.
Statistical analysis
All statistical differences were evaluated using
one-way analysis of variance followed by Dunnet's test. All
experiments were independently performed three times. Data are
presented as the mean ± SD and were analyzed using SPSS 21.0
software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
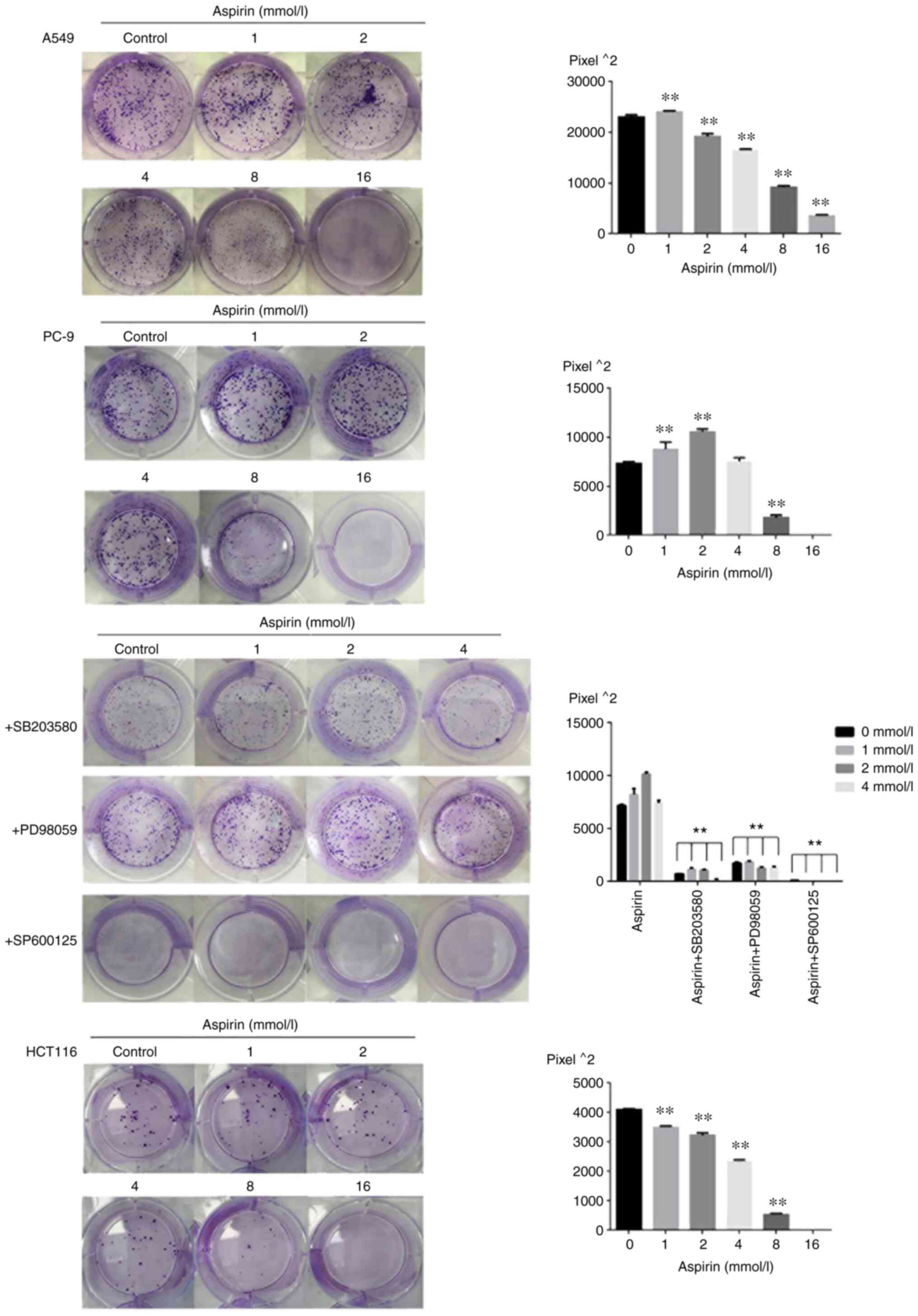
Effects of aspirin on colony formation
area of PC-9 cells
The present study examined the effects of aspirin on
the proliferation of PC-9, A549 and HCT116 cells by colony
formation experiments. Cells were exposed to various concentrations
(0, 1, 2, 4, 8 and 16 mM) of aspirin for 7-12 day. Almost no colony
formation was observed at the concentration of 16 mM. The results
of colony formation are shown as Fig.
1; aspirin significantly promoted the proliferation of PC-9
human lung cancer cells at low concentrations between 1 and 4 mM
and significantly inhibited the proliferation at the concentrations
of 8 and 16 mM. Aspirin-treated colony formation area at 1, 2 and 4
mM were 1.16-, 1.44- and 1.01-fold relative to the control when 600
cells were seeded per well. Aspirin slightly promoted colony growth
of A549 cells at 1 mM concentration and inhibited colony growth of
HCT116 cells at various concentrations.
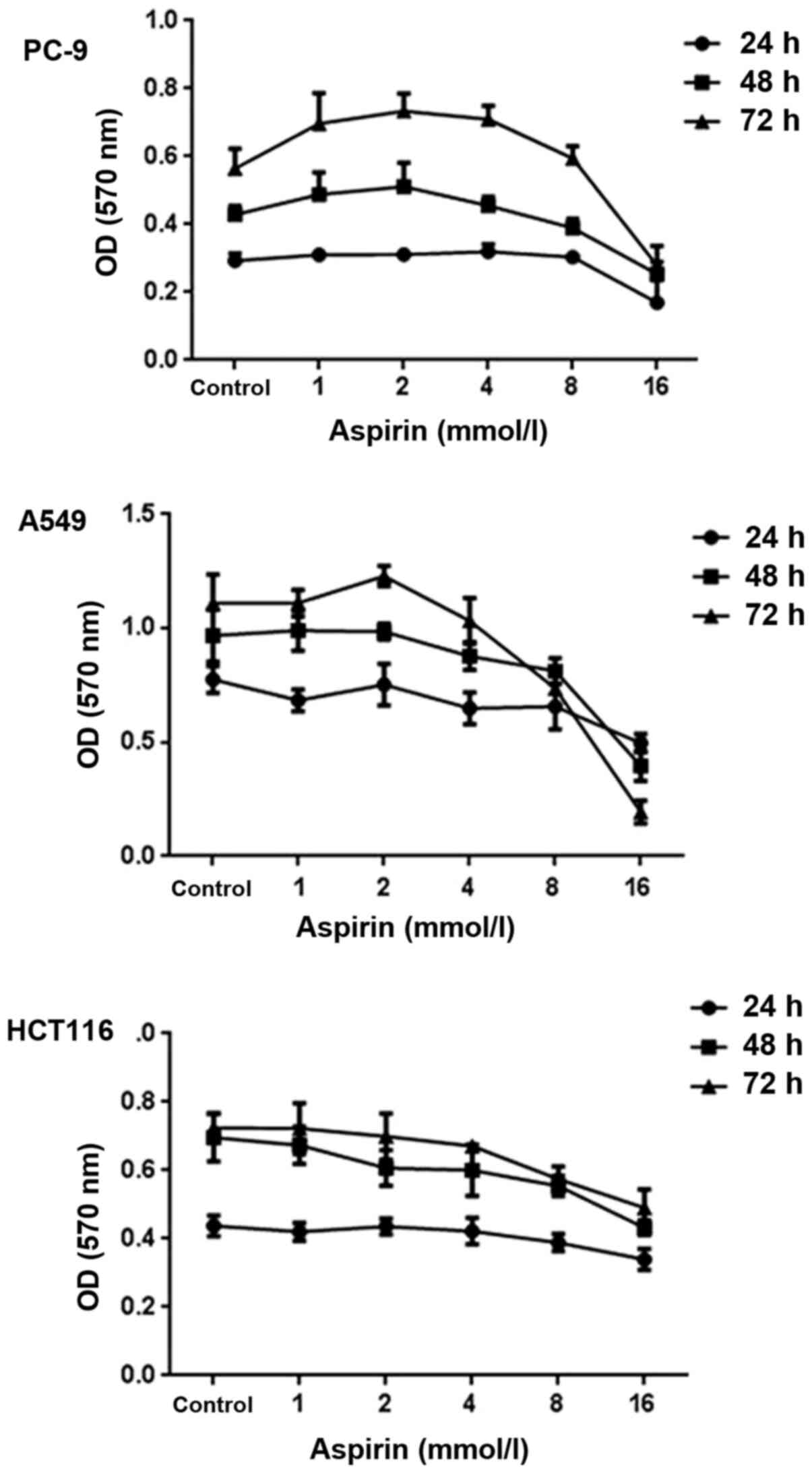
Effects of aspirin on vitality of PC-9
cells
The effects of aspirin on the growth of PC-9 human
lung cancer cells were examined by MTT assay. Cells were exposed to
various concentrations (0, 1, 2, 4, 8 and 16 mM) of aspirin for 0,
24, 48 and 72 h. As shown in Fig.
2, aspirin-treated cell vitality was significantly increased at
the concentrations of 1, 2 and 4 mM and inhibited at 8 and 16 mM.
After 48 h of exposure, aspirin induced 13.8% growth promotion at
the concentrations of 1 mM, 19.4% at 2 mM and 6.31% at 4 mM,
respectively. After 72 h of exposure, aspirin induced 23.4% growth
promotion at the concentrations of 1 mM, 29.9% at 2 mM and 25.6% at
4 mM, respectively. It was also observed that 2 mM aspirin has a
vitality-promotion at 72 h in A549 cells but no such effect in
HCT116 cells.
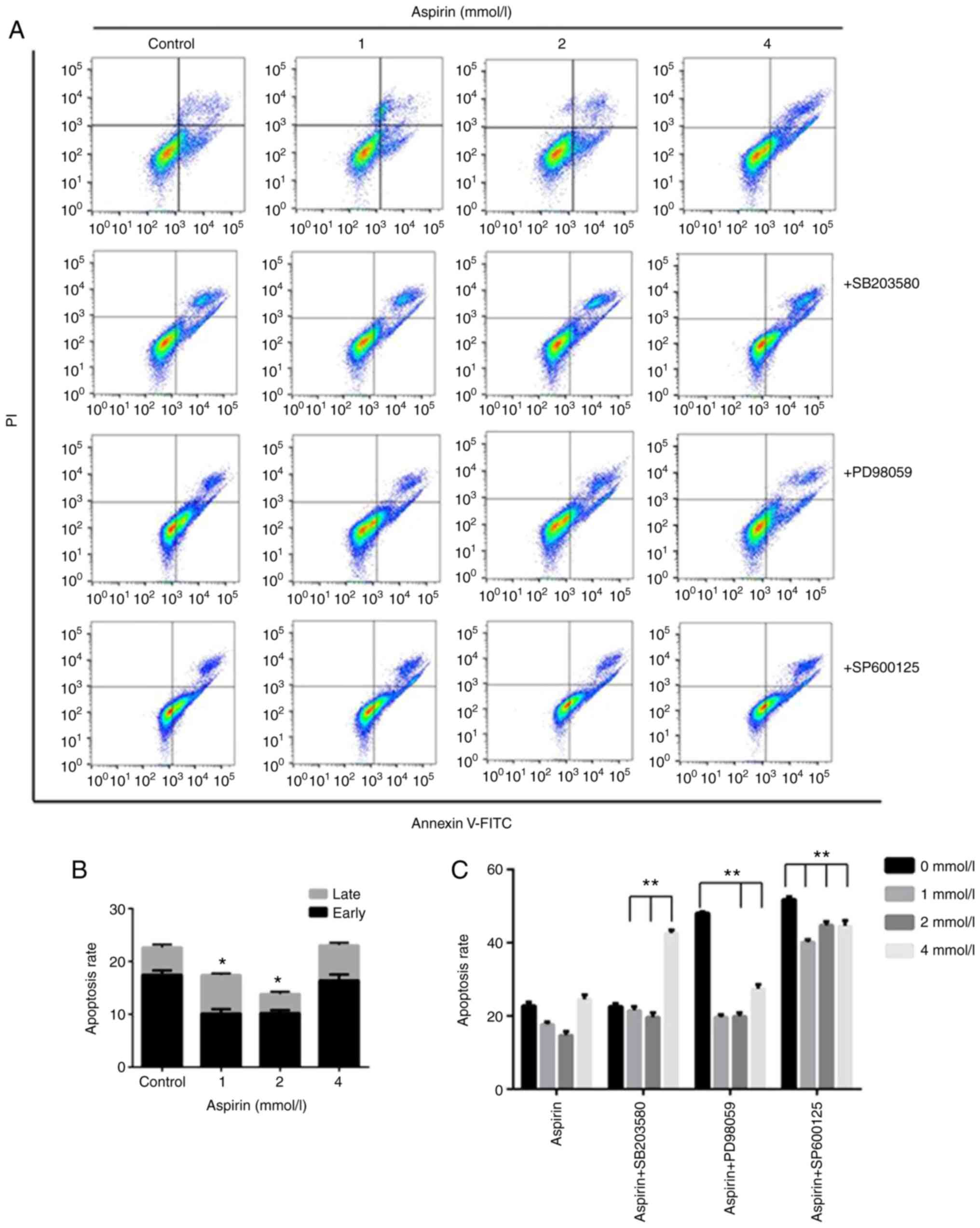
Effects of aspirin on apoptosis in
PC-9 cells
To quantify the extent of apoptotic cells, flow
cytometry analysis was performed using double staining with Annexin
V and PI. The Annexin V-/PI- population was
considered to represent unaffected cells, Annexin
V+/PI- as early apoptosis, Annexin
V+/PI+ as late apoptosis and Annexin
V-/PI+ as necrosis. The results showed that
low-dose aspirin-treated cells significantly decrease the
percentage of apoptotic cells compared with untreated control cells
(Fig. 3A and B). In detail, early apoptotic cell
populations were decreased 7.3% at the concentration of 1 mM, 6.8%
at 2 mM compared with 17.6% of the control. The total apoptotic
cell populations were also decreased at 4.99 and 8.25% at 1 and 2
mM, respectively, compared with 22.78% of the control.
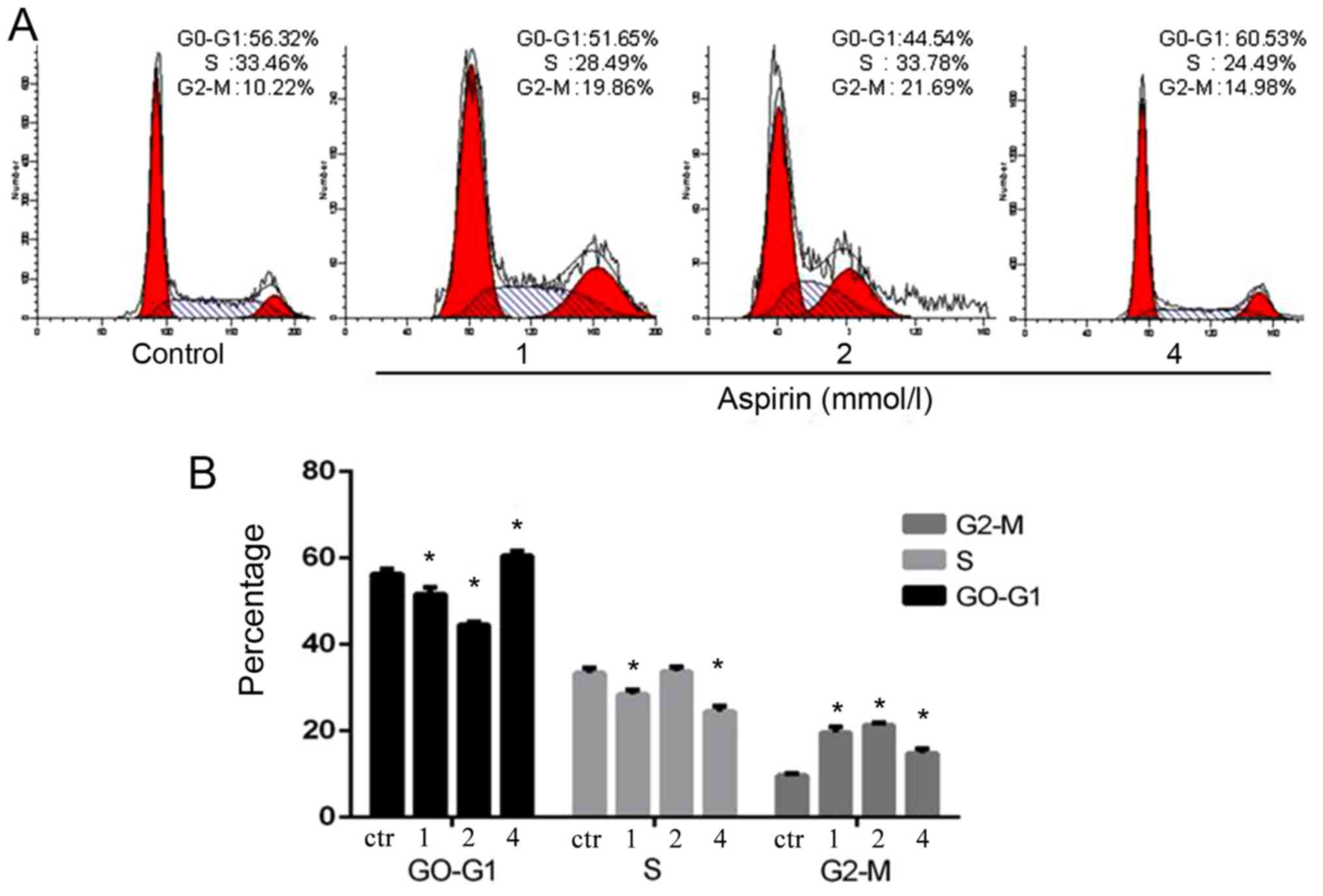
Effects of aspirin on cell cycle phase
distribution in PC-9 cells
The fluorescence-activated cell sorting analysis
clearly indicated that low-dose aspirin could promote G1/S-phase
transition in PC-9 cells. The stimulation of aspirin changed the
cell proliferation cycle, which led to more cells in the 4N phase.
However, it suggested that low-dose aspirin could facilitate cells
into G2-phase of cell cycle (Fig.
4).
Effects of aspirin on the expression
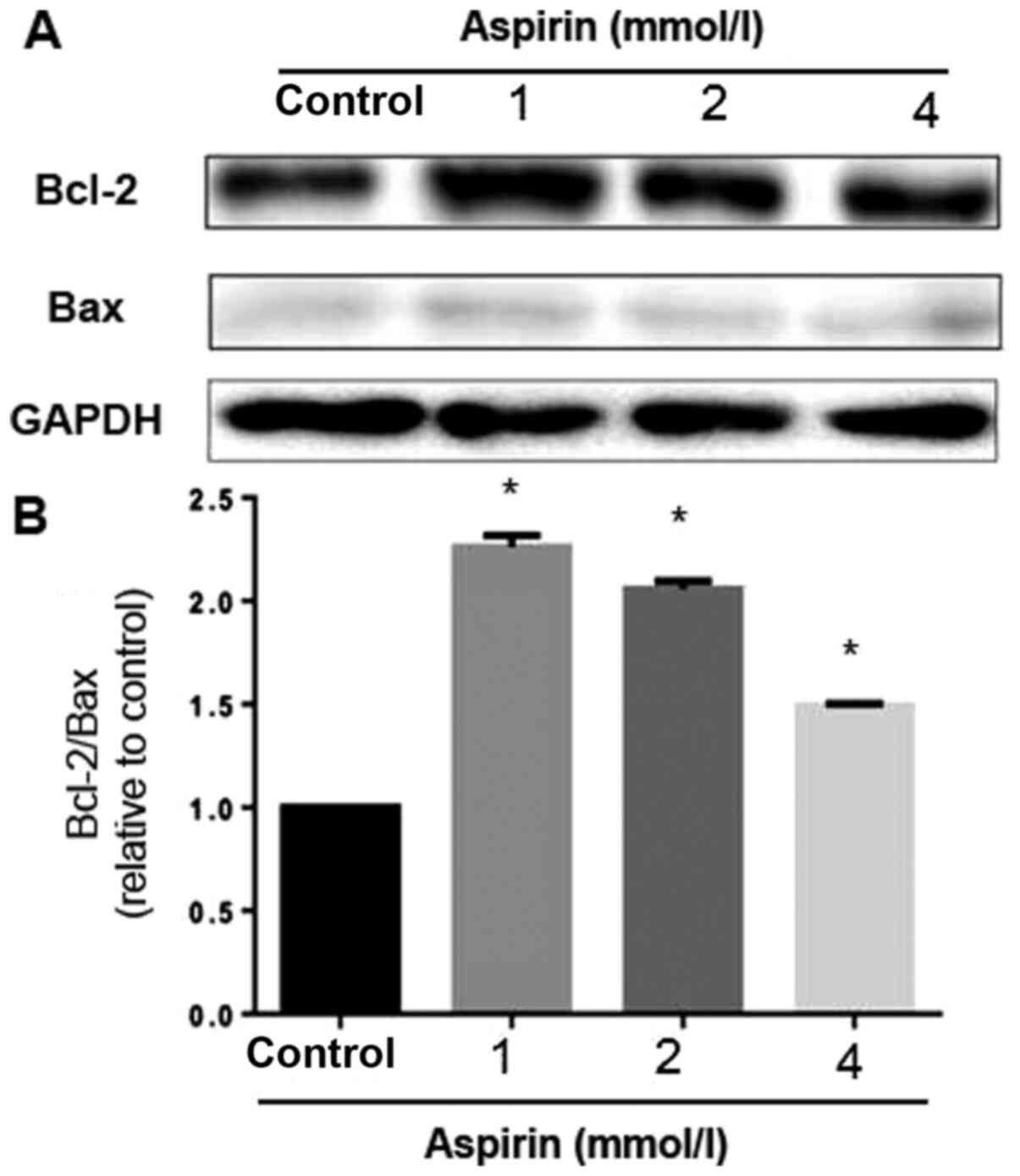
of Bcl-2 family proteins
To study the effects of low-dose aspirin on PC-9
cells, the expression levels of a number of apoptosis regulatory
proteins, including Bcl-2 and Bax, were examined. The mitochondrial
pathway is an important apoptosis pathway as it regulates the
apoptotic cascade via a convergence of signaling at the
mitochondria (22). Bcl-2 interacts
with the mitochondrial plasma membrane and prevents mitochondrial
membrane pores from opening during apoptosis, blocking the signals
of apoptotic factors, such as Bax (23). Low-dose aspirin decreased Bax
expression but increased the expression of Bcl-2. A densitometric
analysis of the bands revealed that low-dose aspirin caused a
decreased Bax/Bcl-2 ratio (Fig. 5).
These results suggested that aspirin can reduce apoptosis through
the regulation of apoptosis-related protein expression in PC-9
cells at low concentration.
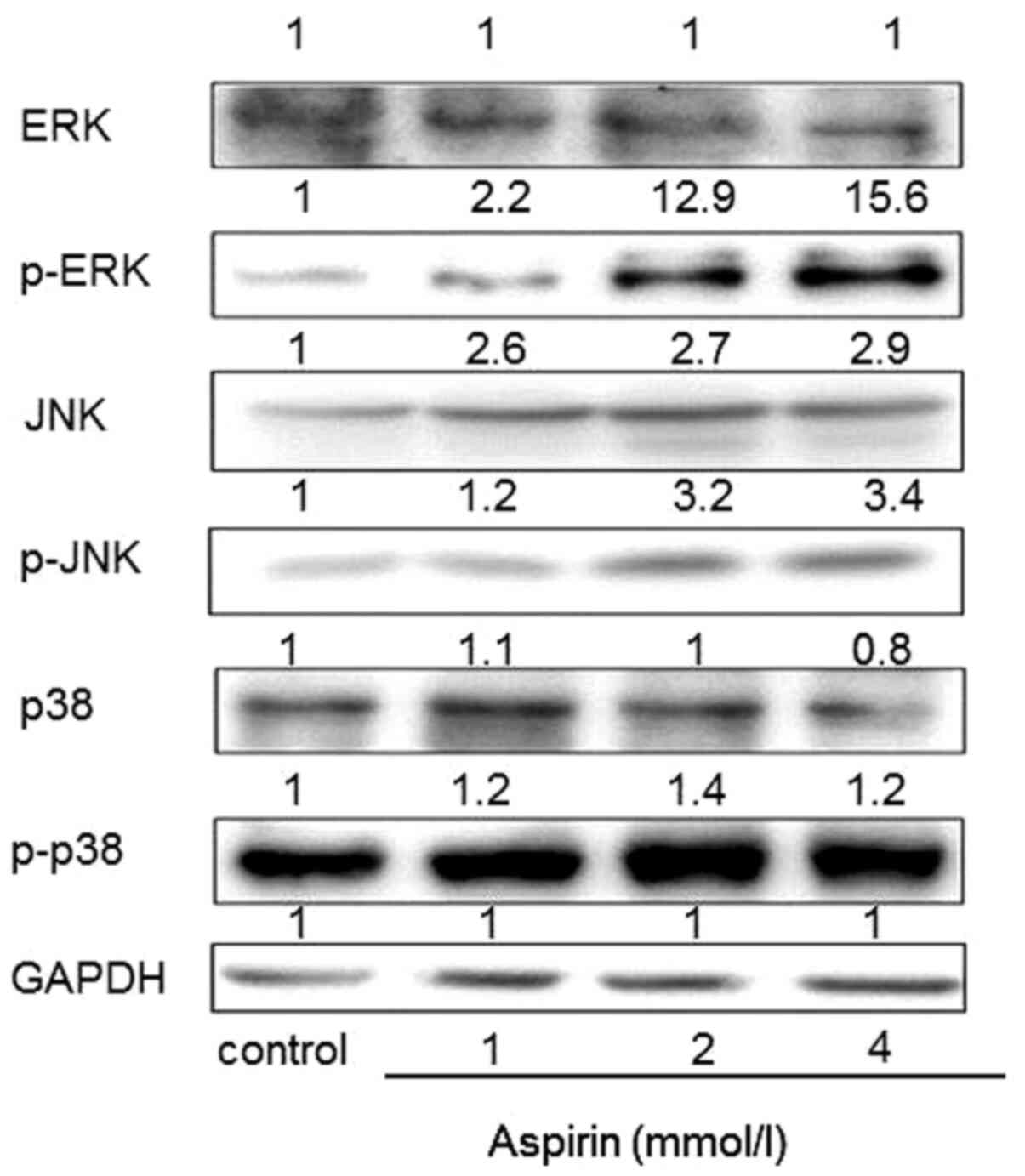
Effects of aspirin on the expression
of MAPK in PC-9 cells
MAPKs signaling cascades, including JNK, ERK and p38
kinase, are found in all eukaryotes and have been demonstrated to
play a central role in regulating cell proliferation,
differentiation and apoptosis (24). To further determine whether MAPKs
are involved in the low-dose aspirin-induced PC-9 cells
proliferation promotion, the phosphorylation expression levels of
MAPKs were examined. As shown in Fig.
6, low-dose aspirin treatment for 24 h significantly increased
phosphorylation of JNK, ERK and p38 MAPK. Total protein levels of
ERK and p38 remained constant. Notably, total protein levels of JNK
were markedly increased.
Effects of MAPK inhibitor on growth
promotion of PC-9 cells induced by low-dose aspirin
In order to investigate the significance of MAPK
activation in the low-dose aspirin treatment, PC-9 cells were
treated with low-dose aspirin in the presence or absence of
SP600125, PD98059 or SB203580. As shown in Fig. 3A and C the treatment of MAPK inhibitor SP600125,
PD98059 and SB203580 effectively prevented the aspirin-induced
growth promotion. The results of double staining with Annexin V and
PI showed that treatment with low-dose aspirin in PC-9 cells in
presence of MAPK inhibitor significantly increased the percentage
of apoptotic cells compared with that in absence of MAPK inhibitor.
The effects of MAPK inhibitors on low-dose aspirin-induced
proliferation promotion in PC-9 cells was also examined by the
colony formation as shown in Fig.
1. The results showed that the colony formation area of the
inhibitor group was smaller than that of the aspirin group.
Discussion
Aspirin can be regarded as one of the most commonly
used synthetic drugs in human history. It was originally developed
and sold at the end of the 19th century, mainly for the treatment
of inflammatory diseases. However, its mechanism of action remains
to be determined. Low doses of aspirin are also used for primary
and secondary prevention of cardiovascular disease to prevent
antithrombotic formation (25).
Previous findings have shown that aspirin has a role in reducing
cancer risk, but whether it can be used as an anticancer drug for
cancer patients was unknown until recently (26). Based on the safety of aspirin for
clinical use over the years and its anti-tumor activity (25), it was investigated whether aspirin
can also play a role in lung cancer treatment and the effect of
aspirin on lung cancer PC-9 cells observed. However, the results of
the present study indicated that aspirin stimulated the
proliferation of PC-9 cells at low concentrations and significantly
inhibited PC-9 cells growth at 8 mM or higher concentrations.
Apoptosis is an important phenomenon due to its
maintenance of cellular homeostasis by regulating cell division and
cell death. There is increasing evidence that the processes of
neoplastic transformation, progression and metastasis involve
alterations in the normal apoptotic pathways (27). Furthermore, the majority of
chemotherapeutic agents as well as radiation utilize the apoptotic
pathway to induce cancer cell death (28). The present study investigated
apoptosis by flow cytometry in PC-9 lung cancer cells which were
treated with various concentrations of aspirin in the presence or
absence of SP600125, PD98059 and SB203580. The results showed that
treatment of PC-9 cells with low-dose aspirin significantly
decreased the percentage of apoptotic cells compared with untreated
control cells and the treatment of MAPK inhibitor (SP600125,
PD98059 and SB203580) effectively prevented the aspirin-induced
growth promotion. Low-dose aspirin treatment for 24 h significantly
increased the phosphorylation of JNK, ERK and p38 MAPK in PC-9
cells. These results indicated that low-dose aspirin-induced
proliferation promotion in PC-9 may be involved in the activation
of MAPK.
At present, apoptotic signals have two main
pathways: the extrinsic or death receptor pathway and the intrinsic
or mitochondrial-mediated pathway (29). The mitochondrial-related pathway is
regulated by anti-apoptotic (Bcl-2, Bcl-x and Bcl-XL) and
pro-apoptotic members (Bax, Bcl-2 homologous antagonist/killer, BH3
interacting-domain death agonist, Bad and Bcl-2-like protein 11) of
the Bcl-2 family (30). The
anti-apoptotic proteins on the outer membranes of mitochondria
maintain the integrity of the mitochondria by inhibiting apoptosis
in the presence of various apoptotic stimuli (31). Therefore, the balance between the
expression levels of Bax and Bcl-2 is important to cell survival as
well as death. Data from the present study showed that
aspirin-induced PC-9 growth promotion was associated with the
downregulation of Bax protein as well as the upregulation of Bcl-2
expression. Previous findings have also suggested that the
Bax/Bcl-2 ratio is a determining factor in regulating the apoptotic
process (32). An analysis of the
present data indicated that low-dose aspirin may decrease the
Bax/Bcl-2 ratio in PC-9 cells, which suggested that low-dose
aspirin has anti-apoptotic function in PC-9 cells.
The results of apoptosis were consistent with the
results of cell cycle analysis. Low concentrations of aspirin
promoted G1/S phase to G2 phase of the cell cycle process and
promoted cell proliferation, which was consistent with the MTT and
colony experiment. This once again validated the hypothesis of the
present study. The comparative results of inhibitor group and the
single drug group also showed that MAPK inhibitors can prevent the
low-dose aspirin-caused proliferation and promotion of PC-9 cells
in the MTT and colony formation experiments.
The present study identified that aspirin promoted
the growth of human PC-9 lung cancer cells at low concentrate,
particularly <4 mM. Low-dose aspirin treatment for 24 h
significantly increased phosphorylation of JNK, ERK and p38 MAPK in
PC-9 cells. All of the above results indicated that low-dose
aspirin could stimulate the proliferation of lung cancer PC-9 cells
through activation of MAPK signaling.
In summary, the present study identified that
low-dose aspirin has the potential to promote the proliferation of
PC-9 lung cancer cells and that its mechanism was related to MAPK
activation. Low-dose aspirin (75-300 mg/day) is used as a basic
medication to treat cardiovascular and cerebrovascular disease.
However, the results of the present study suggested that the use of
low-dose aspirin may pose a risk of cancer progression in lung
cancer and a cardiovascular co-morbidity. The conclusions of the
present study need further verification.
The results of the present study suggested that the
activation of MAPK signaling, at least partially, was responsible
for low-dose aspirin-induced proliferation promotion. By contrast,
high-dose aspirin attenuated the proliferation of PC-9 cells. These
results may suggestion caution should be exercised when employing
aspirin in the clinic.
Acknowledgements
The authors thank Ms. Danyang Zhang (The First
Affiliated Hospital of Jinzhou Medical University, Jinzhou, China)
for experimental guidance and Dr Lei Zhou (The First Affiliated
Hospital of Jinzhou Medical University, Jinzhou, China) for
language assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD, YQ and HL conceived and designed the study; YQ
performed the experiments and wrote the paper. HD revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang SW, Zheng RS, Yang ZX, Zeng HM, Sun
KX, Gu XY, Li H, Chen WQ and He J: Trend analysis on incidence and
age at diagnosis for lung cancer in cancer registration areas of
China, 2000-2014. Zhonghua Yu Fang Yi Xue Za Zhi. 52:579–585.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
2
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang X and Guo Z: The role of sulfur in
platinum anticancer chemotherapy. Anticancer Agents Med Chem.
7:19–34. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim J and Becker RC: Aspirin dosing
frequency in the primary and secondary prevention of cardiovascular
events. J Thromb Thrombolysis. 41:493–504. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Okada S, Morimoto T, Ogawa H, Sakuma M,
Matsumoto C, Soejima H, Nakayama M, Doi N, Jinnouchi H, Waki M, et
al: Effect of aspirin on cancer chemoprevention in Japanese
patients with type 2 diabetes: 10-Year observational follow-up of a
randomized controlled trial. Diabetes Care. 41:1757–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hua H, Zhang H, Kong Q, Wang J and Jiang
Y: Complex roles of the old drug aspirin in cancer chemoprevention
and therapy. Med Res Rev. 39:114–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Di Francesco L, López Contreras LA, Sacco
A and Patrignani P: New insights into the mechanism of action of
aspirin in the prevention of colorectal neoplasia. Curr Pharm Des.
21:5116–5126. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Usman MW, Luo F, Cheng H, Zhao JJ and Liu
P: Chemopreventive effects of aspirin at a glance. Biochim Biophys
Acta. 1855:254–263. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang G, Wang Y, Feng J, Liu Y, Wang T,
Zhao M, Ye L and Zhang X: Aspirin suppresses the abnormal lipid
metabolism in liver cancer cells via disrupting an NFκB-ACSL1
signaling. Biochem Biophys Res Commun. 486:827–832. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, Wei X, Wu Y, Wang Y, Qiu Y, Shi J,
Zhou H, Lu Z, Shao M, Yu L, et al: Giganteaside D induces
ROS-mediated apoptosis in human hepatocellular carcinoma cells
through the MAPK pathway. Cell Oncol (Dordr). 39:333–342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liang Z, Chi YJ, Lin GQ, Luo SH, Jiang QY
and Chen YK: MiRNA-26a promotes angiogenesis in a rat model of
cerebral infarction via PI3K/AKT and MAPK/ERK pathway. Eur Rev Med
Pharmacol Sci. 22:3485–3492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geest CR and Coffer PJ: MAPK signaling
pathways in the regulation of hematopoiesis. J Leukoc Biol.
86:237–250. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu ZJ, Xiao M, Balint K, Smalley KS,
Brafford P, Qiu R, Pinnix CC, Li X and Herlyn M: Notch1 signaling
promotes primary melanoma progression by activating
mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt
pathways and up-regulating N-cadherin expression. Cancer Res.
66:4182–4190. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rehman SK, Li SH, Wyszomierski SL, Wang Q,
Li P, Sahin O, Xiao Y, Zhang S, Xiong Y, Yang J, et al: 14-3-3ζ
orchestrates mammary tumor onset and progression via
miR-221-mediated cell proliferation. Cancer Res. 74:363–373.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tibbles LA and Woodgett JR: The
stress-activated protein kinase pathways. Cell Mol Life Sci.
55:1230–1254. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ryu MJ and Chung HS: [10]-Gingerol induces
mitochondrial apoptosis through activation of MAPK pathway in
HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim.
51:92–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu T, Ren MX, Chen GP, Jin ZM and Wang G:
Rrp15 affects cell cycle, proliferation, and apoptosis in NIH3T3
cells. FEBS Open Bio. 6:1085–1092. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ryu MJ, Kim AD, Kang KA, Chung HS, Kim HS,
Suh IS, Chang WY and Hyun JW: The green algae Ulva fasciata Delile
extract induces apoptotic cell death in human colon cancer cells.
In Vitro Cell Dev Biol Anim. 49:74–81. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stadheim TA and Kucera GL: c-Jun
N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is
required for mitoxantrone- and anisomycin-induced apoptosis in
HL-60 cells. Leuk Res. 26:55–65. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sarbacker GB, Lusk KA, Flieller LA and Van
Liew JR: Aspirin use for the primary prevention of cardiovascular
disease in the elderly. Consult Pharm. 31:24–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pasche B, Wang M, Pennison M and Jimenez
H: Prevention and treatment of cancer with aspirin: Where do we
stand? Semin Oncol. 41:397–401. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suvarna V, Singh V and Murahari M: Current
overview on the clinical update of Bcl-2 anti-apoptotic inhibitors
for cancer therapy. Eur J Pharmacol. 862(172655)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
30
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond - mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK, et al: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Leung HW, Yang WH, Lai MY, Lin CJ and Lee
HZ: Inhibition of 12-lipoxygenase during baicalein-induced human
lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol.
45:403–411. 2007.PubMed/NCBI View Article : Google Scholar
|