Introduction
Coronary microvasculature dysfunction (CMD) exists
in patients with ST-segment elevation myocardial infarction (STEMI)
undergoing percutaneous transluminal coronary angioplasty or stent
implantation, with a prevalence of 5-50% (1). Current trials have revealed that CMD
has a strong impact on prognosis and outcomes, including persistent
chest pain following primary percutaneous coronary intervention
(PPCI) and reinfarction and is associated with a lower rate of
ST-segment resolution of <50-70%, a higher rate of late-onset
heart failure and increased mortality (2-5).
Therefore, CMD has important clinical significance in investigating
an optimal treatment strategy for patients with CMD following
STEMI.
Nicorandil, a coronary vasodilator that acts on both
macro- and microvascular systems, has been demonstrated to possess
cardioprotective properties, including anti-arrhythmic effects, and
to facilitate ischemic preconditioning, prevention of reperfusion
injury and treatment of the no-reflow/slow-flow phenomenon during
coronary interventions for acute myocardial infarction (6-9).
Thus, nicorandil has been recognized as one of the most important
drugs for the treatment of CMD following STEMI (10).
Alprostadil is a prostacyclin that has been
investigated for the treatment of pulmonary arterial hypertension,
chronic renal failure and diabetic microangiopathy and may improve
impaired microcirculation (11-13),
but the effectiveness of alprostadil for CMD following STEMI has
not been reported, to the best of the authors' knowledge.
The index of coronary microcirculatory resistance
(IMR) is a pressure-temperature sensor guidewire-based measurement
performed during cardiac catherization (14). The IMR is a specific quantitative
measurement and the standard used to assess coronary
microvasculature function; it shows a high predictive capacity for
the extent and severity of myocardial infarction in patients with
STEMI (15,16).
Due to the difficulty of studying CMD in patients
with STEMI immediately following PPCI, the present study, using
normal saline as the negative control group and nicorandil as the
positive control group, investigated the therapeutic effect of
alprostadil on coronary microcirculation function via the IMR of
the left anterior descending artery (LAD) of pigs.
Materials and methods
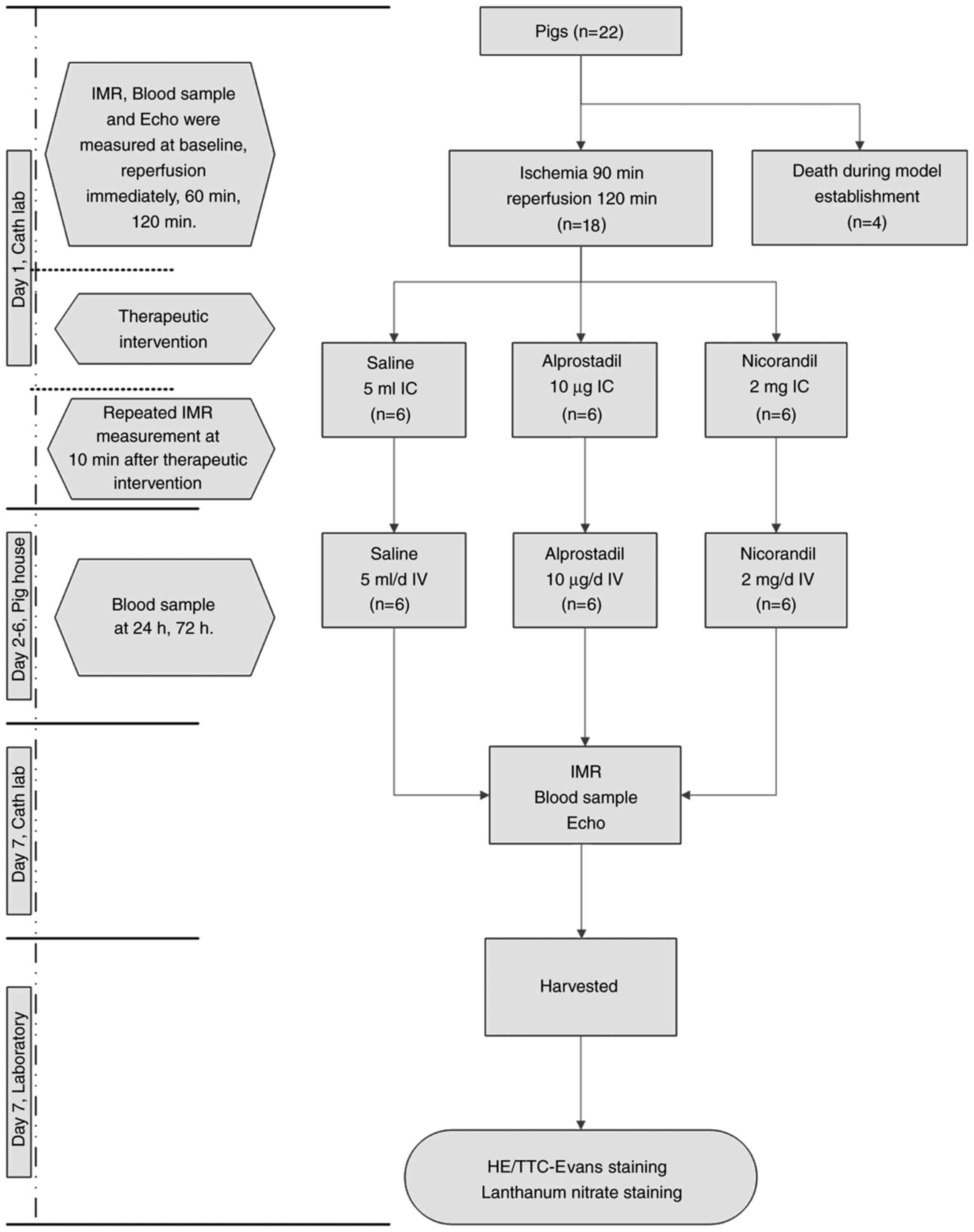
Experimental protocol
The Institutional Animal Care and Use Committee of
General Hospital of Southern Theater Command approved the protocol
of the present study and accounted for the level of mortality
observed during it (ethical approval reference no. K2016012). All
studies were performed in accordance with the ARRIVE (animal
research reporting in vivo experiments) guidelines on the
reporting of animal experiments (17). A total of 22 wuzhishan miniature
pigs (21 males and 1 female; weight, 22.4±2.7 kg; age, 9.6±0.7
months) were provided by Guangzhou Feed Research Institute
[production license no. SCXK (Guangdong) 2015-0036] and were used
for model establishment. The animal experiment center of General
Hospital of Southern Theater Command kept the animals in a clean
environment at a temperature of 16-29˚C, on a 12 h light/dark cycle
and at a humidity of 40-80%. Each miniature pig was fed in a single
house with its own trough and drinking point, feeding was twice per
day (each feed was 3% of body weight) and 1 l water was supplied 5
times per day [use license no. SYXK (Guangdong) 2014-0100; health
and epidemic prevention detection qualified batch no.
44614000000085; quality qualified no. 00131138]. Eventually, a
model of CMD following STEMI was established successfully in 18
pigs, which were then and randomized to three groups of 6 pigs that
received an intracoronary (IC) injection of either normal saline,
nicorandil or alprostadil immediately following IMR measurement and
then an intravenous drip of 5 ml of normal saline, 2 mg of
nicorandil or 10 µg of alprostadil, dependent on the group, once
per day for 6 days. The IMR, cardiac function using ultrasound,
infarct areas and heparanase levels in infarct areas were measured
and compared between the three groups. Details of the experimental
protocol are summarized in Fig.
1.
After establishing the CMD model, 18 pigs survived
and the other 4 pigs died from intractable ventricular fibrillation
(2 pigs), reperfusion arrhythmia (1 pig) and cardiogenic shock (1
pigs). The pig with cardiogenic shock was euthanized after showing
signs of distress.
CMD model establishment
For the 5 days prior to the experiment, the pigs
received aspirin (5 mg/kg, nightly), clopidogrel (5 mg/kg, daily),
perindopril (0.2 mg/kg, daily) and atorvastatin calcium (1 mg/kg,
nightly). General anesthesia was induced via intramuscular
injection of a mixture of ketamine (10 mg/kg), xylazine (0.075
ml/kg, a mixture of haloperidol, xylidinothiazole and
dihydroetorphine) and midazolam (0.5 mg/kg) and then maintained
with a mixture of 0.9% sodium chloride (0.4 ml/kg), ketamine (5
mg/kg) and propofol (2 ml/kg) delivered continuously with a medical
syringe pump through a marginal ear vein (8-18 ml per h) (18,19).
No pig was intubated and oxygen was supplied at 2 l/min. Penicillin
was injected 30 min before the experiment. Following a local
injection of 10 ml of lidocaine in the inguinal area, the right
femoral artery was exposed and isolated after skin incision and the
separation of subcutaneous tissue, and a 6-French (6-F) guiding
catheter sheath (Cordis Corporation) was placed into the artery. A
6-F JR3.5 guiding catheter (Cordis Corporation) was inserted into
the left coronary artery through the arterial sheath. The animals
were heparinized (100 U/kg IC, with another 2,500 units added every
hour). Baseline angiography was performed. Following calibration, a
0.014-inch-diameter coronary pressure wire (St. Jude Medical, Inc.)
was advanced into the distal LAD. The mean aortic pressure (Pa),
mean distal pressure of the LAD (Pd) and mean transit time (Tmn, in
sec) of a 3x3-ml bolus of room temperature saline injected into the
coronary artery were recorded at baseline and at different time
points of reperfusion through the 6-F JR3.5 guide catheter and the
pressure wire. A maximal hyperemic condition was induced by an IC
bolus of papaverine (18 mg) and an IC bolus of nitroglycerine (200
µg) was administered before each IMR measurement. After baseline
measurements, a balloon angioplasty catheter (Maverick, 2.0x20 mm,
Boston Scientific) was advanced to a location between the first and
second diagonal branches of the LAD through the 6-F guiding
catheter. Coronary blood flow was completely interrupted by balloon
inflation and documented via contrast angiography. Balloon
deflation was performed under the same protocol in all animals. In
all cases, a 12-lead real-time electrocardiogram (ECG) monitoring
system (IVT Technology Corporation) recorded the total ECG
waveform, including premature ventricular contractions, ventricular
tachycardia, ventricular fibrillation and ECG ST and T wave
changes.
To reduce the incidence of ventricular
tachycardia/ventricular fibrillation following coronary blood flow
disruption, ischemic preconditioning was performed continuously,
with ischemia for 1, 2, 3 and 5 min before balloon inflation and
1-, 2-, 3- and 5-min intervals between occlusion and reperfusion.
All animals received 0.15 g of amiodarone via intravenous (IV)
administration, followed by continuous infusions of amiodarone at
60 mg/h delivered using a medical syringe pump immediately
following balloon inflation. The pump was not stopped until balloon
deflation and 30 min of reperfusion. If frequent premature
ventricular contractions or brief ventricular tachycardia was
observed, then lidocaine 50 mg IV was administered at once. If
ventricular tachycardia or fibrillation occurred, then electrical
defibrillation at 200 J was performed at once. If heart failure
occurred, then cedilanid (0.2 mg) or furosemide (20 mg) was
administered at once. Dopamine (3 mg) was injected to treat low
blood pressure. To treat unstable vital signs, epinephrine (1 mg)
or atropine (1 mg) were injected.
Interventions during and following the
operation
During the procedure, alprostadil was injected IC
slowly over 2 min. The other solutions were given as an IC bolus
and 10 min later, the IMR of the LAD was measured. After their
wounds were sutured and bandaged, the pigs were returned to the
animal laboratory. Within 3 days after the operation, 4.8 million
units of penicillin was intramuscularly injected. During the next
six days, under anesthesia, the pigs received the three indicated
solutions once a day at the same dose that they originally received
as IC infusions injected into the jugular vein. A 6-F JR3.5 guide
catheter was placed into the right coronary artery through an
arterial sheath to measure the IMR of the LAD on the 7th day.
IMR assessment
The IMR was measured at baseline, balloon occlusion
90 min, after 1, 2 h of reperfusion, 10 min following the
interventions during the operation and on the 7th day after the
operation.
Biochemical assessment
Serum levels of cardiac troponin I (cTnI), brain
natriuretic peptide (BNP) and endothelin-1 (ET-1) were measured
using test kits (cTnI cat. no. N28016833, BNP cat. no. M25016835
and ET-1 cat. no. M25016837, respectively) from the Wuhan Huamei
Biological Engineering Research Center. The serum level of nitric
oxide (NO) was measured using a test kit (cat. no. 20171018) from
the Nanjing Jiancheng Biological Engineering Research Center. All
measurements were performed in duplicate and the values were
averaged.
Ultrasound cardiogram
The left ventricular ejection fraction (EF), left
ventricular anterior wall thickness (LVAWT), left ventricular
end-systolic dimension (LVDs) and left ventricular end-diastolic
dimension (LVDd) were measured at baseline, after 2 h of
reperfusion and on the 7th day following the operation (GE Vivid
E9, GE Healthcare).
Myocardial tissue sample staining and
western blotting
On the 7th day following the operation, the heart
was removed, and the infarct area and non-ischemic area of the left
ventricle were accurately cut (<1 cm3), then fixed in
10% formaldehyde fixative solution for 24 h at 25˚C, dehydrated
with ethanol, embedded in paraffin, and paraffin sections were cut
into 4-µm slices for H&E staining (20) (Beijing Solarbio Science &
Technology Co., Ltd.,), TTC-Evans blue staining (20) (Sigma-Aldrich, Merck KGaA) and
lanthanum nitrate staining (21)
(Electron Microscopy, China), as previously reported.
Heparanase protein levels in infarct areas were
detected by western blotting. Myocardial tissues from infarct areas
of pig hearts were fully lysed with RIPA Lysis buffer (Thermo
Fisher Scientific, Inc.) and the mixture was centrifuged at 300 x g
for 15 min at 4˚C. The supernatant was collected for western
blotting. Protein concentrations were determined using a BCA
quantitative test kit (cat. no. BL8890; Bioworld Technology, Inc.).
A 30 µg protein sample from each group each sample was loaded for
10% SDS-PAGE electrophoresis and transferred on to nitrocellulose
membranes. Membranes were blocked in 5% BSA solution (cat. no.
BS2304; Bioworld Technology, Inc.) for ≥60 min at room temperature
and then incubated overnight at 4˚C with rabbit polyclonal
anti-heparanase antibody (1:2,000; cat. no. bs-1541R; BIOSS) and
rabbit monolonal anti-GAPDH antibody (1:2,000; cat. no. bs-2188R;
BIOSS) with agitation. The next morning, the membranes were washed
and incubated with the appropriate horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:2,000; cat. no.
ab6721; Abcam) for 1 h at room temperature. The membranes were then
washed three times with TBS -0.05% Tween-20 and protein bands were
visualized using the Western Bright ECL kit (cat. no. K-12045-D50;
Advansta). The relative expression of the protein bands was
quantified on images obtained with a digital camera (Nikon D7100;
Nikon Corporation) using ImageJ (version 6; National Institutes of
Health).
Statistical analyses
All statistical analyses were performed using SPSS,
version 22.0 (IBM Corp.). Categorical data are presented as a
frequency or a percentage and differences among groups were
analyzed by χ2 tests. Continuous data are presented as
the median with standard deviation. Differences in data measured at
different time points were analyzed by repeated-measures ANOVA. If
the overall difference was statistically significant, a pairwise
comparison was carried out and a Tukey post hoc test was applied.
P<0.05 was considered to indicate a statistically significant
difference.
Results
No significant differences were observed among the
three groups in the mean body weight, age, diameter of the LAD and
baseline Pa, Pd, HR, IMR, coronary flow reserve (CFR) and
fractional flow reserve (all P>0.05). The baseline
characteristics of the three groups of animals are presented in
Table I and detailed information is
provided in Table SI.
 | Table IBaseline characteristics of the three
groups. |
Table I
Baseline characteristics of the three
groups.
| Characteristic | SHAM (n=6) | NCR (n=6) | ALP (n=6) | P-value |
|---|
| Body weight,
kg | 22.2
(20.0-24.3) | 24.6
(22.2-27.0) | 20.5
(17.9-23.3) | 0.230 |
| Male, % | 100 | 83.3 | 100 | 0.358 |
| Age, months | 9.6 (9.1-9.9) | 9.6 (9.2-10.0) | 9.6 (9.3-9.9) | 0.988 |
| Diameter of LAD,
mm | 2.1 (1.7-2.5) | 2.0 (1.7-2.2) | 2.0 (1.8-2.2) | 0.654 |
| Ventricular
tachycardia or fibrillation, times | 2.5 (0.9-4.1) | 1.7 (0.4-2.9) | 1.5 (0.4-2.6) | 0.370 |
| Electrical
Defibrillation, times | 4.0 (1.6-6.4) | 2.4 (0.6-4.4) | 3.3 (0.5-7.6) | 0.668 |
| Baseline Pa,
mmHg | 114 (107-121) | 104 (94-114) | 108 (97-121) | 0.244 |
| Baseline Pd,
mmHg | 112 (106-118) | 102 (93-109) | 106 (95-118) | 0.126 |
| Baseline HR,
beats/min | 83 (74-92) | 84 (78-91) | 77 (69-85) | 0.211 |
| Baseline IMR | 11.5
(9.6-13.3) | 12.7
(9.4-15.9) | 11.4
(8.7-14.2) | 0.658 |
| Baseline CFR | 4.1 (3.6-4.6) | 3.9 (2.8-4.5) | 3.7 (3.3-4.1) | 0.395 |
| Baseline FFR | 0.94
(0.86-0.99) | 0.92
(0.86-0.97) | 0.94
(0.92-0.98) | 0.571 |
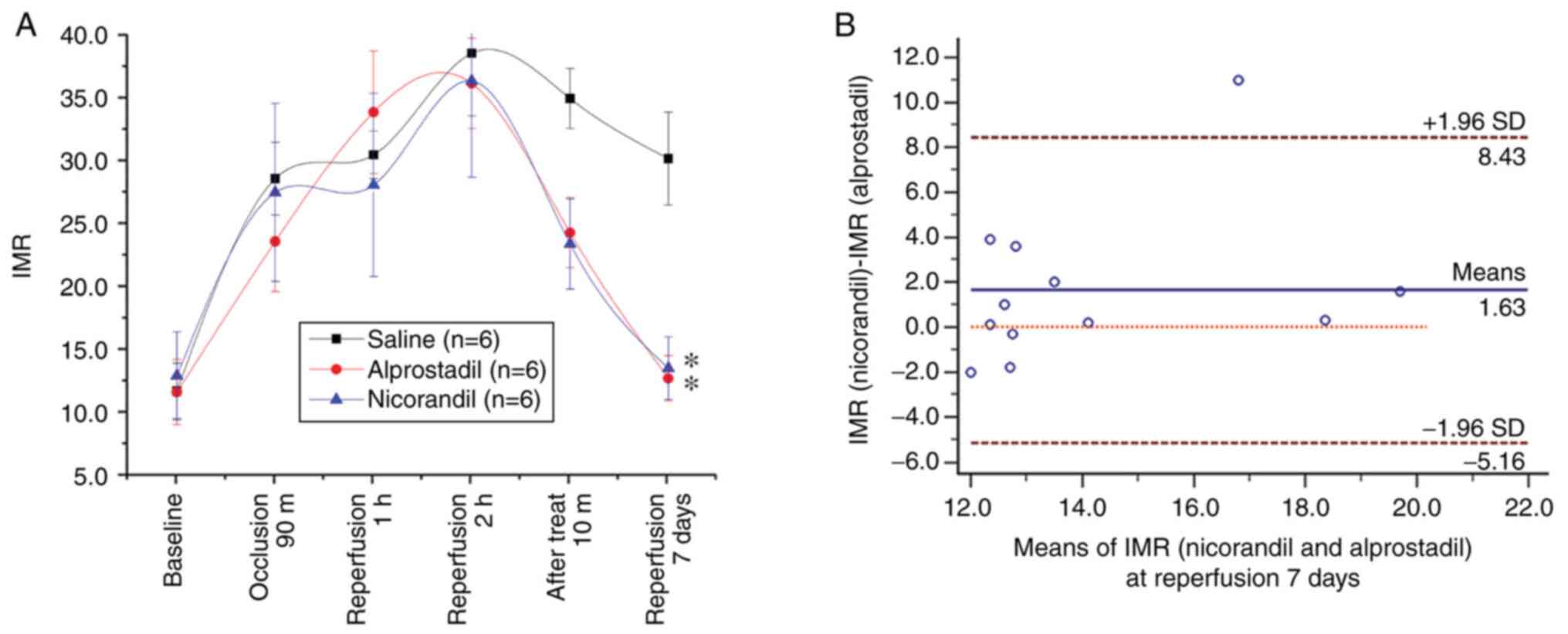
No significant difference in the IMR was observed
after 2 h of reperfusion among the three groups (P>0.05).
Following alprostadil or nicorandil injection, the IMR decreased
markedly 10 min later (both P<0.05), but not after normal saline
injections (P>0.05). After 7 days, the IMRs in the alprostadil
and nicorandil groups were notably lower compared with the normal
saline group (both P<0.05). Moreover, no difference in the IMR
was observed between the alprostadil and nicorandil groups.
Furthermore, an equivalence test was performed between the IMRs of
the two experimental groups and only one IMR value was beyond the
95% conformance scope, with a mean difference of 1.63 and a
standard deviation of 3.47 (Fig.
2).
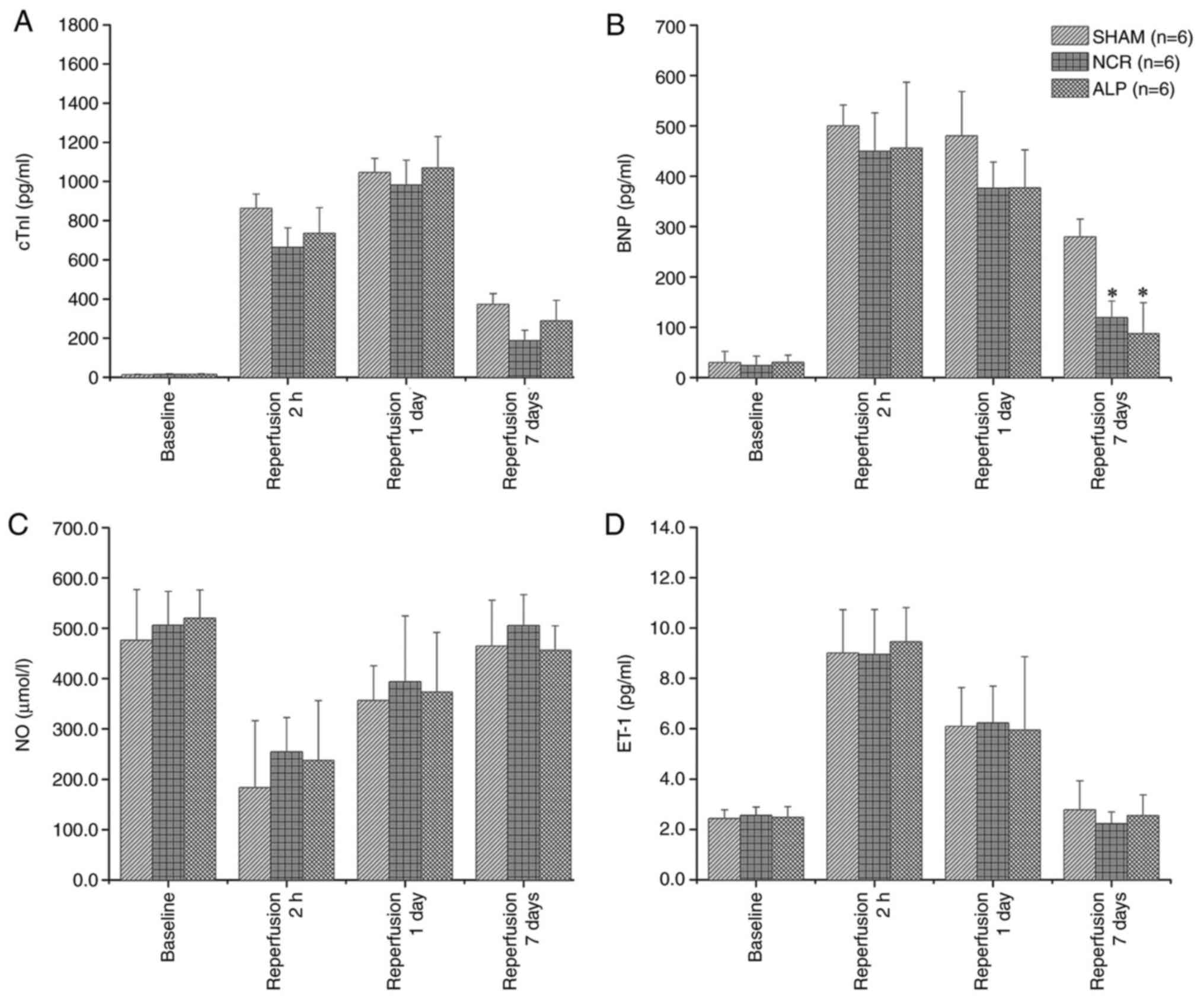
Throughout the course of myocardial infarction, the
serum concentration of cTnI was lowest at baseline, peaked at 24 h
and dropped sharply at 7 days in all three groups. At 7 days, no
significant difference in the serum cTnI was observed among the
three groups (P>0.05). The lowest concentration of serum BNP was
identified at baseline, while the highest level was observed after
2 h of reperfusion. Compared with the concentration in the normal
saline group, a significant difference in the serum BNP
concentration was noted in the alprostadil and nicorandil groups
(both P<0.05). The serum NO concentration decreased gradually
from baseline to the lowest value at 2 h after reperfusion and had
nearly recovered to the baseline level at 7 days. Similar to cTnI,
no significant difference in the serum NO or ET-1 concentration was
identified among the three groups (Fig.
3).
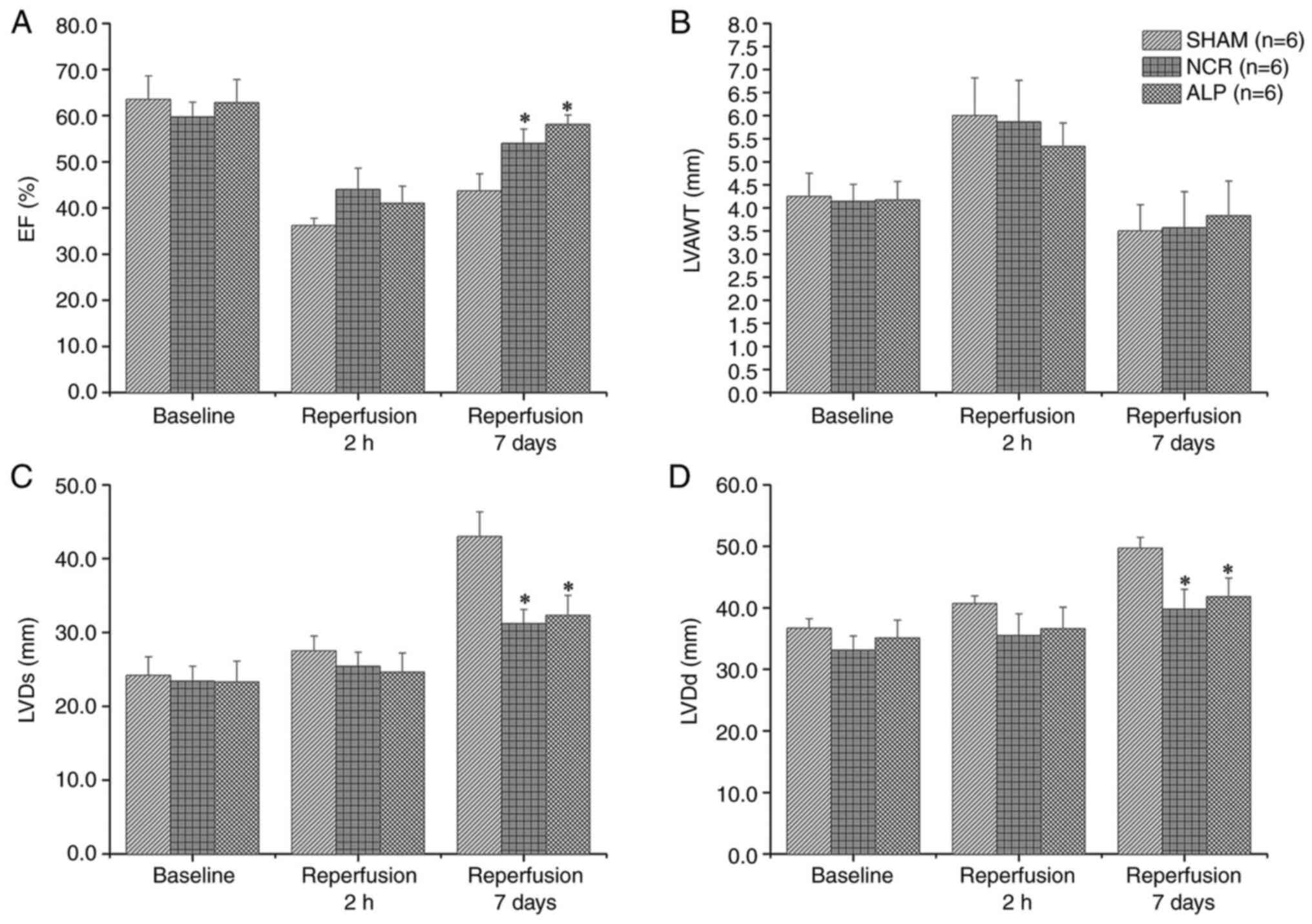
No significant difference was identified among the
three groups for the EF at baseline or after 2 h of reperfusion
(P>0.05 for both time points). However, after 7 days, the EF in
the alprostadil and nicorandil groups was substantially higher
compared with the normal saline group (both P<0.05). Regional
anterior wall motion abnormalities were observed in the left
ventricle after 2 h of reperfusion in all three groups and the
LVAWT was increased. After 7 days, anterior wall edema was reduced
and the LVAWT decreased. The differences in the LVDd and LVDs among
all three groups were similar to those for the EF (Fig. 4).
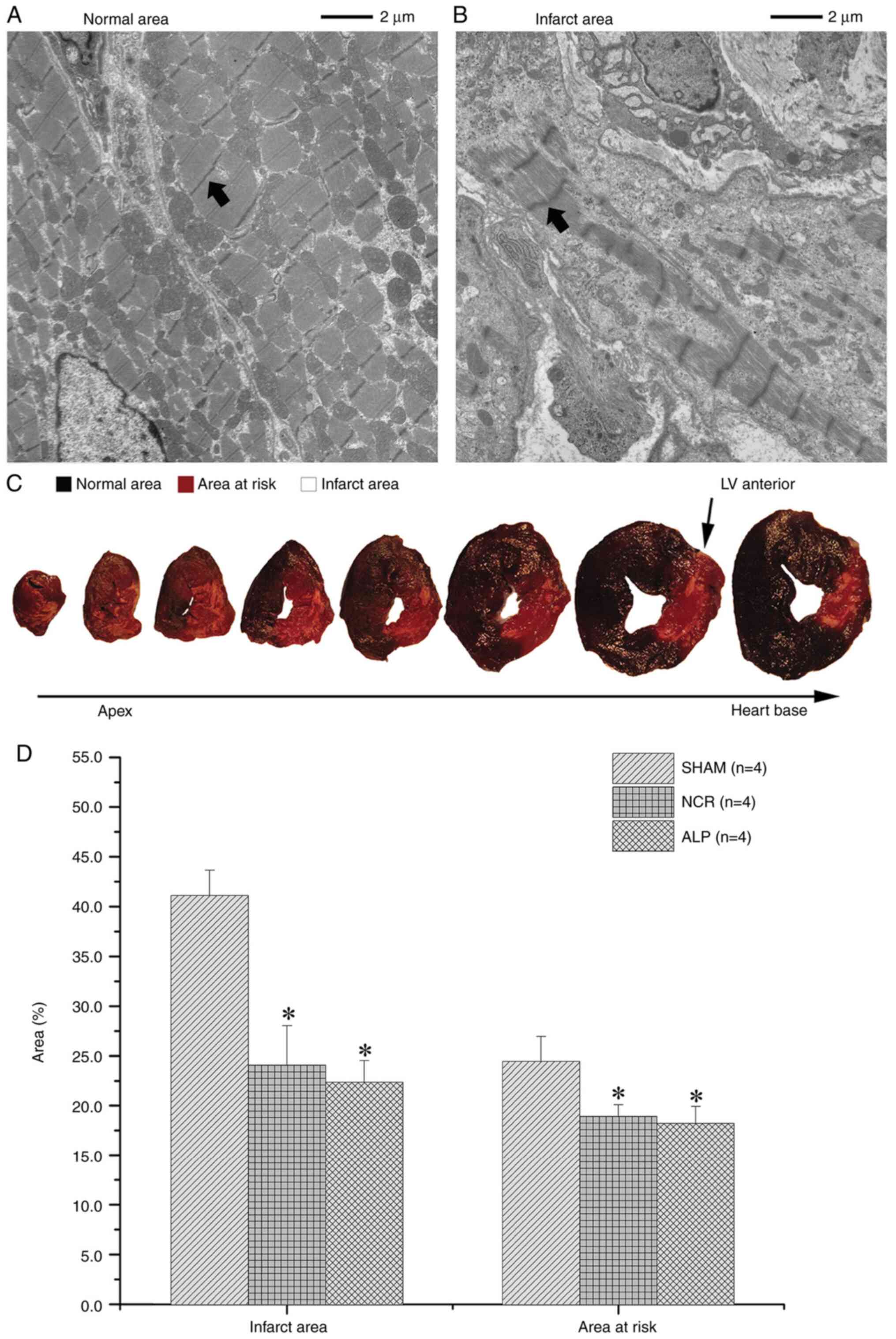
Post-mortem analyses confirmed the presence of
pathological infarction by H&E staining. The TTC-Evans blue
staining results showed that the infarct areas in the alprostadil
and nicorandil groups were evidently smaller compared with those in
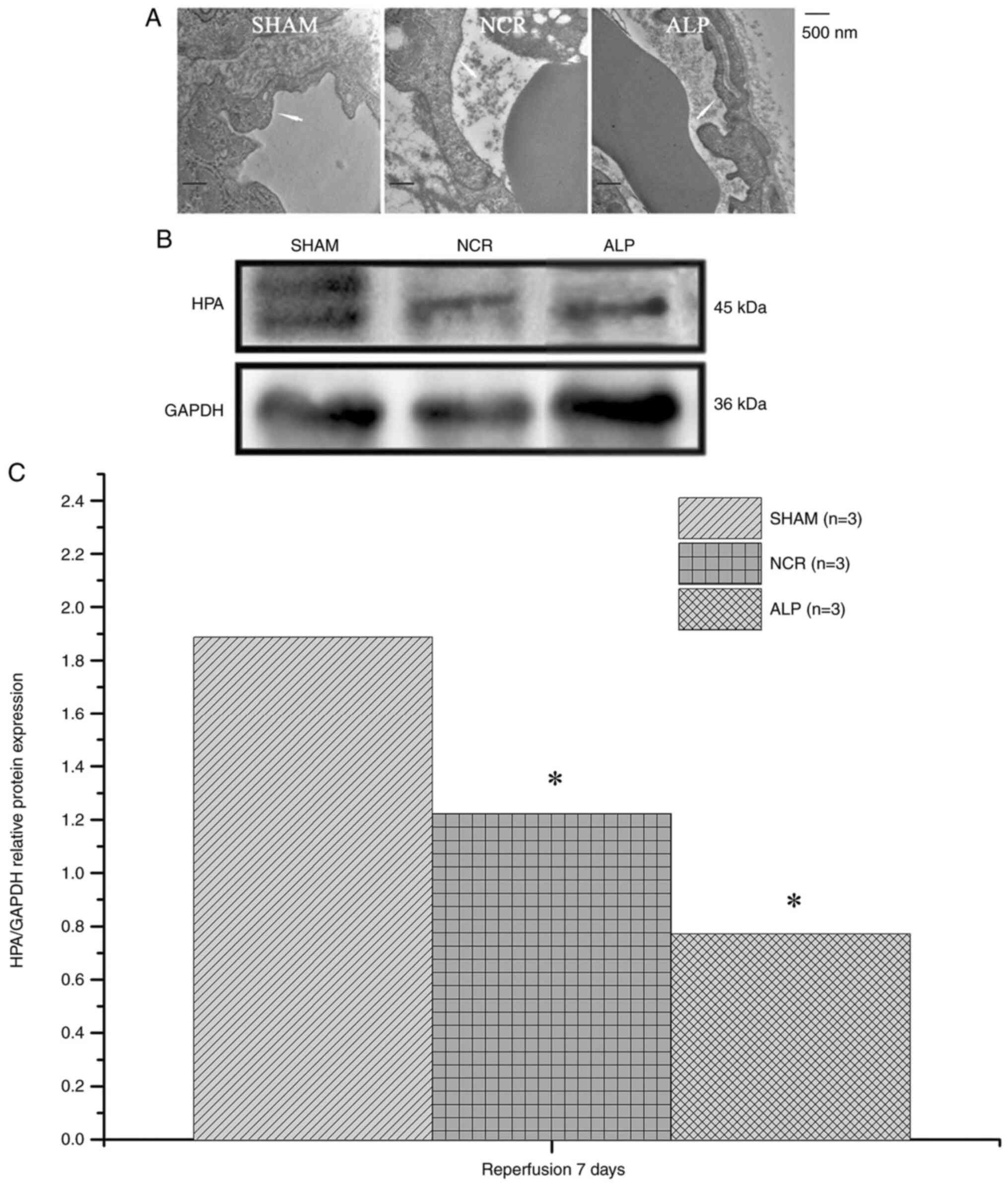
the normal saline group (both P<0.05; Fig. 5). After 7 days, the vascular
endothelial glycocalyx was observed to be degraded and reduced or
had even disappeared in some cases. Myocyte necrosis and heparanase
protein levels in the alprostadil group and nicorandil groups were
much lower than those in the normal saline group (P<0.05;
Fig. 6).
Discussion
The goal of timely reperfusion therapy with
fibrinolytic drugs or PPCI in patients with STEMI is to restore
blood flow to ischemic areas and reduce mortality (22). With improvements in PPCI technology
and progress in the standardized treatment process for STEMI, a
considerably higher proportion of patients can achieve successful
restoration of epicardial coronary artery patency following
prolonged occlusion. However, many of these patients do not
experience adequate reperfusion at the microvascular level,
resulting in high rates of in-hospital mortality and heart failure
(23). Therefore, treatment
strategies for STEMI should shift from opening the epicardial
artery only downstream of the occlusion to opening the
microcirculatory system. The phenomenon of CMD is observed not only
in animal models but also in clinical practice (24,25).
In clinical practice, the mechanism of CMD is not fully understood;
possible mechanisms include existing microvasculature dysfunction,
ischemic injury, reperfusion injury, distal atherothrombotic
embolization and injury-susceptible coronary microcirculation
(26,27). As a result, many treatment
strategies to prevent or overcome the above mechanisms have been
adopted to reduce the occurrence of CMD or to improve the prognosis
of patients with STEMI. Several drugs and methods have been
reported to be effective for the prevention or treatment of CMD
patients and to reduce the incidence of major adverse clinical
events, such as metoprolol, adenosine, nitroprusside, verapamil,
nicorandil and deferred stenting (22,28-34).
Nicorandil has a relaxing effect on coronary
arteries of different diameters, especially small coronary
arteries, and has little influence on blood pressure and heart rate
(35). These effects result from
the nitrate moiety effect and the ability of nicorandil to open
ATP-sensitive potassium channels in coronary arterioles with high
resistance, which is associated with increased cellular potassium
efflux and decreased extracellular influx (36). In a study testing 12 mg of
nicorandil injected into the culprit vessel of patients with STEMI
immediately following PPCI, Kostic et al (34) reported that IC nicorandil
significantly decreases the IMR and improves the CFR and left
ventricular EF in these patients. In a study of 368 patients with
STEMI randomly assigned to receive 12 mg of nicorandil or a placebo
intravenously immediately before PPCI, Ishii (37) reported that the rate of ST-segment
resolution >50% accompanied by lower corrected TIMI frame counts
was higher in a nicorandil group compared with the placebo group.
After a mean follow-up of 2.4 years, patients treated with
nicorandil had a lower incidence of cardiovascular death or
rehospitalization for congestive heart failure following PCI
(37). Therefore, nicorandil has
cardiovascular protective effects and improves coronary
microcirculation function. Based on the above evidence, nicorandil
was employed as a positive control in this study.
Alprostadil, as a coronary vasodilator that acts on
the microvascular system, is reported to be safe and useful in
patients with pulmonary hypertension or chronic heart failure and
diabetic microangiopathy (11-13).
A previous study reported that alprostadil can improve myocardial
perfusion in dogs with STEMI. Using a conditioned open-chest dog
infarction model, Feld et al (38) reported the effect of alprostadil on
coronary blood flow. Alprostadil administered intravenously
following copper coil-induced coronary thrombosis in the dog model
results in significant acceleration of the thrombolysis time, a
significant improvement in coronary blood flow during reperfusion
and reduction of the infarct area. After four weeks of IV
alprostadil infusion, myocardial perfusion in patients with
ischemic heart disease is improved significantly as assessed by
positron emission tomography (PET) (39).
The following is an explanation for the dose
selection of the three drugs used in the three groups in the
present study. The animals in the nicorandil group were given 2 mg
of nicorandil via IC injection followed by a 2-mg IV injection
based on a previous study (8). That
is, 2 mg of nicorandil was injected before coronary angiography and
2 mg of nicorandil was injected following stent implantation. Some
studies have reported high similarities between pigs and humans for
the coronary arterial system with respect to morphology and size
and even capillary diameter (40,41).
The heart weight of the miniature pigs used in the present study
was ~100 g, which is 40-50% of the heart weight of an adult human;
therefore, 2 mg of nicorandil was selected as the positive control.
Nicorandil (2 mg) was diluted with normal saline to 5 ml for the IC
injection before the IV injection. Therefore, 5 ml of normal saline
IC was used for the negative control group. No study has reported
IC injection of alprostadil in animal experiments or clinical
research, to the best of the authors' knowledge. Hülsmann et
al (11) reported that patients
with dilated cardiomyopathy and end-stage right ventricular
function may be able to tolerate different doses of alprostadil and
the continuous IV injection doses were 2.5, 5, 10, 15, 20, 25, 30,
35 and 40 ng/kg/min until side effects presented, such as headache,
nausea and hypotension. Therefore, in the animal experiment, a
large IC injection dose was selected and 10 µg of alprostadil
diluted in 5 ml of normal saline. The 5-ml mixture was injected
over 2 min. Meanwhile, the heart rate and blood pressure
fluctuations in the miniature pigs was closely monitored.
The present study demonstrated the effect of
ischemia and reperfusion on coronary microvasculature function in a
closed-chest animal model of pigs with STEMI induced by balloon
occlusion of the LAD. Coronary microvasculature function before and
following normal saline, nicorandil or alprostadil injections was
measured using the gold standard angiographic and IMR criteria for
the diagnosis of CMD in clinical practice. The main findings of
this study can be summarized as follows: i) In an animal CMD model
with STEMI, the IMR decreased 10 min after IC infusions of
alprostadil or nicorandil; ii) After 7 days, compared with the
negative control group, the IMRs were lower in the alprostadil and
nicorandil groups, which also showed a higher EF, smaller LVDs and
LVDd values, lower heparanase protein levels and smaller infarct
areas. Meanwhile, no significant difference was observed between
the nicorandil group and the alprostadil group; and iii)
Alprostadil can improve coronary microcirculation function, reduce
the infarct area and limit left ventricular dilatation in a pig
model of CMD following STEMI.
The IMR is a reliable measurement to assess coronary
microvascular dysfunction and changes in the IMR reflect
fluctuations of the microcirculation at the different time points
in the same vessel (42).
Therefore, the changes in the IMR in the present study suggested
that IC administration of alprostadil can effectively improve
microcirculation conditions, including immediate and short-term
effects. The IMR of the miniature pigs in the alprostadil group
after 1 h of reperfusion was higher compared with the normal saline
group and nicorandil group, but the difference between the three
groups was not statistically significant. It was hypothesized that
two reasons may account for this phenomenon. First, a balloon
angioplasty catheter was advanced to a location between the first
and second diagonal branches of the LAD through a 6-F guiding
catheter for model establishment. Coronary blood flow was
completely interrupted by balloon inflation as documented by
contrast angiography. However, differences were noted in the
coronary branches and patterns of each miniature pig. The first
diagonal branch of the miniature pigs in the alprostadil group was
close to the proximal LAD, which resulted in a relatively large
range of myocardial infarction and the IMR of the anterior
descending branch was higher compared with the other two groups
following reperfusion. Second, the average weight of the miniature
pigs in the alprostadil group was approximately 1.7-4.1 kg lower
compared with the other two groups. Therefore, it was hypothesized
that the slight difference in the weights of the miniature pigs may
have affected the IMR of the LAD following acute myocardial
infarction. After 7 days, no significant difference was observed in
the IMR between the nicorandil group and the alprostadil group,
indicating that the effect of alprostadil was equal to that of
nicorandil. The change in the IMR following alprostadil injection
can be attributed to its pharmacological effect. The known effects
of alprostadil include inhibition of vasoconstriction and increased
vasodilatation through several regulatory mechanisms, such as
inhibition of noradrenaline release from nerve terminals,
activation of cAMP, reduction of the concentration of ET-1 and
regulation of the NO and ET-1 ratio (43). During balloon inflation, myocardial
cell necrosis, endothelial cell debris, microthrombi and some
markers of endothelial injury caused distal coronary microvascular
spasm or microcirculation obstruction. Following alprostadil
injection, microvascular spasm was improved and the
microcirculation system was inflated. However, the vasodilation and
increase in blood flow following alprostadil administration were
small and the vasodilating effect appears to be short term
(44). Thus, no significant
difference was observed in the changes in serum NO, ET-1 and BNP
concentrations among the three groups. The IMR is a real-time
indicator of the condition of the coronary microcirculation system
(42,45) and the IMR was significantly reduced
10 min following alprostadil administration.
According to previous studies, the main benefit of
alprostadil is an endothelial protective effect, including
inhibition of the release of thromboxane A2, which changes the
ratio of prostacyclin 2 and thromboxane A2, the interaction between
leucocytes and platelets and the inhibition of platelet
aggregation, although NO bioavailability is increased (44,46).
The endothelial protective effect may develop over a period of at
least several days. Thus, 7 days following the procedure, compared
with the negative control group, a significant difference in the
IMR was identified in the alprostadil group and similar results
were observed for the EF, LVDs, LVDd, heparanase protein levels and
infarct areas.
In addition, 7 days following surgery, the vascular
endothelial glycocalyx was identified to be degraded and reduced or
had even disappeared in some cases and myocyte necrosis and
heparanase protein levels among the three groups also demonstrated
varying degrees of change. Heparan sulfate is a major component of
the glycocalyx, which is a coating composed of linear proteoglycan
compounds that lines the luminal surface of vascular endothelial
cells (47). Heparanase serves a
key role in regulating the arterial structure, vascular
permeability and the vessel barrier system. Heparanase is the only
β-endo-glucuronidase that specifically cleaves heparan sulfate in
mammals (48,49). When the level of heparanase
increases, more heparan sulfate is cleaved and the barrier function
of endothelial cells is impaired (50). Furthermore, loss of function in
heparan sulfate elongation genes EXT1 and EXT 2 results in improved
NO bioavailability and endothelial function (51). Kawamura et al (52) reported that alprostadil suppresses
the production of IL-6 and IL-8 and the change in the balance
between pro-and anti-inflammatory cytokines may be one of the most
important cytoprotective mechanisms of alprostadil during cardiac
surgery. Notably, heparanase activates macrophages, resulting in
the marked induction of cytokine expression associated with
endothelial function (53). In the
present study, heparanase protein levels in the infarct areas of
pigs treated with alprostadil or nicorandil were much lower
compared with the pigs that received normal saline. Therefore, it
is hypothesized that heparanase may serve a vital role in the
pathogenesis of CMD following STEMI in pigs.
As it acts on both the macro- and microvascular
systems, nicorandil is one of the most useful drugs for improving
coronary microcirculation function. In the present study, compared
with the positive control group, no significant difference was
identified in the IMR 10 min after IC alprostadil infusion and the
IMR 7 days later, indicating that alprostadil can improve immediate
and short-term myocardial blood flow in pigs. Meanwhile, similar
changes in the EF, LVDs, LVDd, heparanase protein levels and
infarct areas showed that alprostadil improved the immediate and
short-term coronary microcirculation and has protective effects on
the myocardium of pigs with STEMI.
Several limitations existed in the present study.
First, the number of pigs used to investigate the effects of the
interventions on CMD was small, which may bias the results. Second,
due to shortages of research funding and equipment, TTC-Evans blue
staining was used to assess differences in infarct areas and
ischemic areas in pigs receiving various treatments rather than
PET, which is the gold standard for assessing infarct areas.
Lastly, the effect of only one dose of alprostadil was studied and
whether this dose is optimal for treating microcirculation
dysfunction and whether the cardiac protective effect of
alprostadil is dose-dependent remains to be elucidated. Additional
studies are needed to determine the effects of other treatment
doses on improving microcirculation function. In addition, the
present study did not identify a clear signaling pathway for the
cardioprotective effects of alprostadil, thus rendering it less
innovative. Further experiments in pigs are impractical to perform
at present, but the signaling pathways affected by alprostadil will
be investigated in subsequent studies.
In conclusion, alprostadil infusion improved
coronary circulation function, reduced infarct areas and limited
left ventricular dilatation in a pig model of CMD following
STEMI.
Supplementary Material
Baseline and hyperemia characteristics
of the three groups of animals.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Institute of Health fund ‘Establishment and improvement of
emergency medical treatment systems for acute myocardial
infarction’ (grant no. 2016YFC1301201) and ‘Research on artificial
intelligence diagnosis and treatment model and key technology of
acute chest pain disease based on big data’ (grant no.
202002020036). The funders had no role in study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TD prepared the experiments, acquired the data and
drafted the manuscript; JZ measured the IMR at different time
points; RS anaesthetized the pigs; RK prepared blood samples during
measurements; WH performed cardiac ultrasound testing and collected
experimental data; and DX designed the work, edited the article and
approved the final version for submission. TD and DX confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
conducted in accordance with the ARRIVE (animal research reporting
in vivo experiments) guidelines on the reporting of animal
experiments. The Institutional Animal Care and Use Committee of
General Hospital of Southern Theater Command approved the protocol
of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niccoli G, Burzotta F, Galiuto L and Crea
F: Myocardial No-Reflow in humans. J Am Coll Cardiol. 54:281–292.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fearon W, Shah M, Ng M, Brinton T, Wilson
A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, et
al: Predictive value of the index of microcirculatory resistance in
patients with ST-segment elevation myocardial infarction. J Am Coll
Cardiol. 51:560–565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ndrepepa G, Tiroch K, Fusaro M, Keta D,
Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A and
Kastrati A: 5-year prognostic value of no-reflow phenomenon after
percutaneous coronary intervention in patients with acute
myocardial infarction. J Am Coll Cardiol. 55:2383–2389.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamirani YS, Wong A, Kramer CM and Salerno
M: Effect of microvascular obstruction and intramyocardial
hemorrhage by CMR on LV remodeling and outcomes after myocardial
infarction: A systematic review and meta-analysis. JACC Cardiovasc
Imaging. 7:940–952. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi
FA, Spertus JA, Krumholz HM and Jiang L: China PEACE Collaborative
Group. ST-segment elevation myocardial infarction in China from
2001 to 2011 (the China PEACE-Retrospective Acute Myocardial
Infarction Study): A retrospective analysis of hospital data.
Lancet. 385:441–451. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyazawa A, Ikari Y, Tanabe K, Nakajima H,
Aoki J, Iijima R, Nakayama T, Hatori M, Nakazawa G, Tanimoto S, et
al: Intracoronary nicorandil prior to reperfusion in acute
myocardial infarction. EuroIntervention. 2:211–217. 2006.PubMed/NCBI
|
|
7
|
Matsuo H, Watanabe S, Watanabe T, Warita
S, Kojima T, Hirose T, Iwama M, Ono K, Takahashi H, Segawa T, et
al: Prevention of no-reflow/slow-flow phenomenon during rotational
atherectomy-A prospective randomized study comparing intracoronary
continuous infusion of verapamil and nicorandil. Am Heart J.
154:994.e1–6. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee HC, An SG, Choi JH, Lee TK, Kim J, Kim
JH, Chun KJ, Hong TJ, Shin YW and Lee SK: Effect of intra-coronary
nicorandil administration prior to reperfusion in acute ST segment
elevation myocardial infarction. Circ J. 72:1425–1429.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kobatake R, Sato T, Fujiwara Y, Sunami H,
Yoshioka R, Ikeda T, Saito H and Ujihira T: Comparison of the
effects of nitroprusside versus nicorandil on the slow/no-reflow
phenomenon during coronary interventions for acute myocardial
infarction. Heart Vessels. 26:379–384. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Galasso G, Schiekofer S, D'Anna C, Gioia
GD, Piccolo R, Niglio T, Rosa RD, Strisciuglio T, Cirillo P,
Piscione F and Trimarco B: No-reflow phenomenon: Pathophysiology,
diagnosis, prevention, and treatment. A review of the current
literature and future perspectives. Angiology. 65:180–189.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hülsmann M, Stefenelli T, Berger R, Sturm
B, Parkner A, Zuckermann A, Woloszczuk W and Pacher R: Response of
right ventricular function to prostaglandin E1 infusion predicts
outcome for severe chronic heart failure patients awaiting urgent
transplantation. J Heart Lung Transplant. 19:939–945.
2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Friesenecker B, Tsai AG, Dunser MW,
Martini J, Hasibeder W and Intaglietta M: Lowered microvascular
vessel wall oxygen consumption augments tissue pO2 during
PgE1-induced vasodilation. Eur J Appl Physiol. 99:405–414.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gupta V, Rawat A and Ahsan F: Feasibility
study of aerosolized prostaglandin E1 microspheres as a noninvasive
therapy for pulmonary arterial hypertension. J Pharm Sci.
99:1774–1789. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fearon WF, Balsam LB, Farouque HM,
Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG and Yeung AC:
Novel index for invasively assessing the coronary microcirculation.
Circulation. 107:3129–3132. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McGeoch R, Watkins S, Berry C, Steedman T,
Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H and Oldroyd
K: The index of microcirculatory resistance measured acutely
predicts the extent and severity of myocardial infarction in
patients with ST-segment elevation myocardial infarction. JACC
Cardiovasc Interv. 3:715–722. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cuculi F, De Maria GL, Meier P,
Dall'Armellina E, de Caterina AR, Channon KM, Prendergast BD,
Choudhury RP, Forfar JC, Kharbanda RK and Banning AP: Impact of
microvascular obstruction on the assessment of coronary flow
reserve, index of microcirculatory resistance, and fractional flow
reserve after ST-segment elevation myocardial infarction. J Am Coll
Cardiol. 64:1894–1904. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8(e1000412)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lervik A, Raszplewicz J, Ranheim B, Solbak
S, Toverud SF and Haga HA: Dexmedetomidine or fentanyl?
Cardiovascular stability and analgesia during propofol-ketamine
total intravenous anaesthesia in experimental pigs. Vet Anaesth
Analg. 45:295–308. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duan T, Zhang J, Xiang D, Song R and Kong
R: Effect of combined anesthesia on Wuzhishan miniature pigs in
surgery lasting up to 8 hours. Chin J Comp Med. 28:80–85. 2018.(In
Chinese).
|
|
20
|
Liu X, Xie W, Liu P, Duan M, Jia Z, Li W
and Xu J: Mechanism of the cardioprotection of rhEPO pretreatment
on suppressing the inflammatory response in ischemia-reperfusion.
Life Sci. 78:2255–2264. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tachi M, Okada H, Matsuhashi N, Takemura
G, Suzuki K, Fukuda H, Niwa A, Tanaka T, Mori H, Hara A, et al:
Human colorectal cancer infrastructure constructed by the
glycocalyx. J Clin Med. 8(1270)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stone GW, Webb J, Cox DA, Brodie BR,
Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford
BD, et al: Distal microcirculatory protection during percutaneous
coronary intervention in acute ST-segment elevation myocardial
infarction: A randomized controlled trial. JAMA. 293:1063–1072.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Menees DS, Peterson ED, Wang Y, Curtis JP,
Messenger JC, Rumsfeld JS and Gurm HS: Door-to-balloon time and
mortality among patients undergoing primary PCI. N Engl J Med.
369:901–909. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kloner RA, Ganote CE and Jennings RB: The
‘No-Reflow’ phenomenon after temporary coronary occlusion in the
dog. J Clin Invest. 54:1496–1508. 1974.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bekkers SC, Yazdani SK, Virmani R and
Waltenberger J: Microvascular obstruction: Underlying
pathophysiology and clinical diagnosis. J Am Coll Cardiol.
55:1649–1660. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Herrmann J, Kaski JC and Lerman A:
Coronary microvascular dysfunction in the clinical setting: From
mystery to reality. Eur Heart J. 33:2771–2781b. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Niccoli G, Scalone G, Lerman A and Crea F:
Coronary microvascular obstruction in acute myocardial infarction.
Eur Heart J. 37:1024–1033. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bestehorn HP, Neumann FJ, Buttner HJ, Betz
P, Stürzenhofecker P, von Hodenberg E, Verdun A, Levai L, Monassier
JP and Roskamm H: Evaluation of the effect of oral verapamil on
clinical outcome and angiographic restenosis after percutaneous
coronary intervention: The randomized, double-blind,
placebo-controlled, multicenter Verapamil slow-release for
prevention of cardiovascular events after angioplasty (VESPA)
trial. J Am Coll Cardiol. 43:2160–2165. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Van't Hof AW, Ten Berg J, Heestermans T,
Dill T, Funck RC, van Werkum W, Dambrink JH, Suryapranata H, van
Houwelingen G, Ottervanger JP, et al: Prehospital initiation of
tirofiban in patients with ST-elevation myocardial infarction
undergoing primary angioplasty (On-TIME 2): A multicentre,
double-blind, randomised controlled trial. Lancet. 372:537–546.
2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Niccoli G, Rigattieri S, De Vita MR,
Valgimigli M, Corvo P, Fabbiocchi F, Romagnoli E, De Caterina AR,
La Torre G, Lo Schiavo P, et al: Open-Label, Randomized,
Placebo-Controlled evaluation of intracoronary adenosine or
nitroprusside after thrombus aspiration during primary percutaneous
coronary intervention for the prevention of microvascular
obstruction in acute myocardial infarction: The REOPEN-AMI study
(Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial
Infarction). JACC Cardiovasc Interv. 6:580–589. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pizarro G, Fernández-Friera L, Fuster V,
Fernández-Jiménez R, García-Ruiz JM, García-Álvarez A, Mateos A,
Barreiro MV, Escalera N, Rodriguez MD, et al: Long-Term benefit of
early Pre-Reperfusion metoprolol administration in patients with
acute myocardial infarction: Results from the METOCARD-CNIC trial
(Effect of Metoprolol in Cardioprotection During an Acute
Myocardial Infarction). J Am Coll Cardiol. 63:2356–2362.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hillegass WB, Dean NA, Liao L, Rhinehart
RG and Myers PR: Treatment of no-reflow and impaired flow with the
nitric oxide donor nitroprusside following percutaneous coronary
interventions: Initial human clinical experience. J Am Coll
Cardiol. 37:1335–1343. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carrick D, Oldroyd KG, McEntegart M, Haig
C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, et
al: A randomized trial of deferred stenting versus immediate
stenting to prevent no- or slow-reflow in acute ST-segment
elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol.
63:2088–2098. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kostic J, Djordjevic-Dikic A, Dobric M,
Milasinovic D, Nedeljkovic M, Stojkovic S, Stepanovic J, Tesic M,
Trifunovic Z, Zamaklar-Tifunovic D, et al: The effects of
nicorandil on microvascular function in patients with ST segment
elevation myocardial infarction undergoing primary PCI. Cardiovasc
Ultrasound. 13(26)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jang HJ, Koo BK, Lee HS, Park JB, Kim JH,
Seo MK, Yang HM, Park KW, Nam CW, Doh JH and Kim HS: Safety and
efficacy of a novel hyperaemic agent, intracoronary nicorandil, for
invasive physiological assessments in the cardiac catheterization
laboratory. Eur Heart J. 34:2055–2062. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Costa AD and Garlid KD: Intramitochondrial
signaling: Interactions among mitoKATP, PKCepsilon, ROS, and MPT.
Am J Physiol Heart Circ Physiol. 295:H874–H882. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ishii H, Ichimiya S, Kanashiro M, Amano T,
Imai K, Murohara T and Matsubara T: Impact of a single intravenous
administration of nicorandil before reperfusion in patients with
ST-Segment-Elevation myocardial infarction. Circulation.
112:1284–1288. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Feld S, Li G, Amirian J, Felli P, Vaughn
WK, Accad M, Tolleson TR, Swenson C, Ostro M and Smalling RW:
Enhanced thrombolysis, reduced coronary reocclusion and limitation
of infarct size with liposomal prostaglandin E1 in a canine
thrombolysis model. J Am Coll Cardiol. 24:1382–1390.
1994.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Huang CL, Wu YW, Wang SS, Tseng CD, Chiang
FT, Hsu KL, Lee CM and Tzen KY: Continuous intravenous infusion of
prostaglandin E1 improves myocardial perfusion reserve in patients
with ischemic heart disease assessed by positron emission
tomography: A pilot study. Ann Nucl Med. 25:462–468.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hildebrand F, Andruszkow H, Huber-Lang M,
Pape H and van Griensven M: Combined hemorrhage/trauma models in
pigs-current state and future perspectives. Shock. 40:247–273.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kumar M, Kasala ER, Bodduluru LN, Dahiya
V, Sharma D, Kumar V and Lahkar M: Animal models of myocardial
infarction: Mainstay in clinical translation. Regul Toxicol
Pharmacol. 76:221–230. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
De Maria GL, Cuculi F, Patel N, Dawkins S,
Fahrni G, Kassimis G, Choudhury RP, Forfar JC, Prendergast BD,
Channon KM, et al: How does coronary stent implantation impact on
the status of the microcirculation during primary percutaneous
coronary intervention in patients with ST-elevation myocardial
infarction? Eur Heart J. 36:3165–3177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gensch C, Clever Y, Werner C, Hanhoun M,
Bohm M and Laufs U: Regulation of endothelial progenitor cells by
prostaglandin E1 via inhibition of apoptosis. J Mol Cell Cardiol.
42:670–677. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Weiss T, Fischer D, Hausmann D and Weiss
C: Endothelial function in patients with peripheral vascular
disease: Influence of prostaglandin E1. Prostaglandins Leukot
Essent Fatty Acids. 67:277–281. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sirol M, Cescau A, Sideris G, Logeart D,
Dillinger JG, Mercadier JJ and Henry P: Index of microcirculatory
resistance as an early predictive factor of Lv remodeling after
reperfused myocardial infarction. J Am Coll Cardiol.
65(A1934)2015.
|
|
46
|
Wu CC, Wu CI, Wang WY and Wu YC: Low
concentrations of resveratrol potentiate the antiplatelet effect of
prostaglandins. Planta Med. 73:439–443. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Baker AB, Groothuis A, Jonas M, Ettenson
DS, Shazly T, Zcharia E, Vlodavsky I, Seifert P and Edelman ER:
Heparanase alters arterial structure, mechanics, and repair
following endovascular stenting in mice. Circ Res. 104:380–387.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li JP and Vlodavsky I: Heparin, heparan
sulfate and heparanase in inflammatory reactions. Thromb Haemost.
102:823–828. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Peterson SB and Liu J: Multi-faceted
substrate specificity of heparanase. Matrix Biol. 32:223–227.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Baker AB, Gibson WJ, Kolachalama VB,
Golomb M, Indolfi L, Spruell C, Zcharia E, Vlodavsky I and Edelman
ER: Heparanase regulates thrombosis in vascular injury and
Stent-Induced flow disturbance. J Am Coll Cardiol. 59:1551–1560.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mooij HL, Cabrales P, Bernelot Moens SJ,
Xu D, Udayappan SD, Tsai AG, van der Sande MA, de Groot E,
Intaglietta M, Kastelein JJ, et al: Loss of function in heparan
sulfate elongation genes EXT1 and EXT 2 results in improved nitric
oxide bioavailability and endothelial function. J Am Heart Assoc.
3(e001274)2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kawamura T, Nara N, Kadosaki M, Inada K
and Endo S: Prostaglandin E1 reduces myocardial reperfusion injury
by inhibiting proinflammatory cytokines production during cardiac
surgery. Crit Care Med. 28:2201–2208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Vlodavsky I, Blich M, Li JP, Sanderson RD
and Ilan N: Involvement of heparanase in atherosclerosis and other
vessel wall pathologies. Matrix Biol. 32:241–251. 2013.PubMed/NCBI View Article : Google Scholar
|