Introduction
Bronchiectasis is a chronic pulmonary disease
characterized by a vicious cycle of infection and inflammation of
the airways, leading to permanent structural lung damage (1). The incidence of the disease has risen
since 2000 and there are ≤100,000 individuals in the United States
with bronchiectasis, while the disease is also diagnosed more
frequently worldwide (2).
Bronchiectasis can be detected at all ages and generally presents
with chronic cough, sputum production and recurrent lower
respiratory tract infections (2).
Bronchiectasis is usually post-infection while several lung and
systemic diseases are associated with its development (3).
Bronchiectasis exacerbation is a generally
infectious clinical condition characterized by deterioration of
chronic symptoms with negative consequences on patient prognosis,
caused mostly by bacterial microorganisms. Frequent bronchiectasis
exacerbations lead to lung damage and rapid decline of lung
function (4). Among numerous
microorganisms associated with bronchiectasis, Pseudomonas
aeruginosa is one of the most frequent and important, with its
isolation being associated with frequent exacerbations, poor
quality of life and greater possibility of hospitalization and
mortality (5).
Novel coronavirus SARS-CoV-2, which causes COVID-19
disease, has exhibited a rapid spread worldwide, becoming the
biggest threat ever observed from a pandemic (6). COVID-19 patients have a relatively
low rate of bacterial co-infection or bacterial secondary
infection. These infections are commonly observed in the critically
ill patients (7,8). In addition, it has been reported that
bronchiectasis exacerbation frequency is significantly decreased
during COVID-19 pandemic (9).
Pseudomonas putida is a gram-negative,
aerobic saprophytic bacterium that is a common colonizer of soil,
plants and water. Due to its remarkable metabolism, it is used as a
cell host for synthetic biology and metabolic engineering (10). It has been reported as an
opportunistic pathogen causing sepsis in immunocompromised patients
and infections among hospitalized patients experiencing invasive
procedures due to its ability to contaminate medical solutions
(11,12).
The present study reports a novel case of a
bronchiectasis exacerbation due to Pseudomonas putida
complicating COVID-19 disease.
Case presentation
A 70-year-old female, non-smoker, patient presented
to the Emergency Department of Laiko General Hospital (Athens,
Greece) with complaints of fever, runny nose, cough and progressive
dyspnea at rest over the previous 10 days. She had a medical
history of gastroesophageal reflux, arterial hypertension,
hyperlipidemia, asthma, hypothyroidism, obesity with a body mass
index (BMI) 33 kg/m2, obstructive sleep apnea (OSA),
pulmonary embolism one year ago and appendectomy. Her medications
included pantoprazole, irbesartan/hydrochlorothiazide,
atorvastatin, inhaled fluticazone/salmeterol, montelucast,
levothyroxine sodium and rivaroxaban. She was also using continuous
positive airway pressure therapy for OSA.
Clinical evaluation revealed a febrile patient with
wheezing and crackles on auscultation at the bases of both lungs.
Clinical examination of the other systems was unremarkable. Blood
pressure was 110/60 mmHg, heart rate was 80 beats per min, oxygen
saturation was 90% on room air and body temperature 38.5˚C.
Electrocardiography did not reveal abnormal findings on
admission.
Arterial blood gas analysis showed pO2 52
mmHg, pCO2 32 mmHg, pH 7.53 and HCO3 26.7
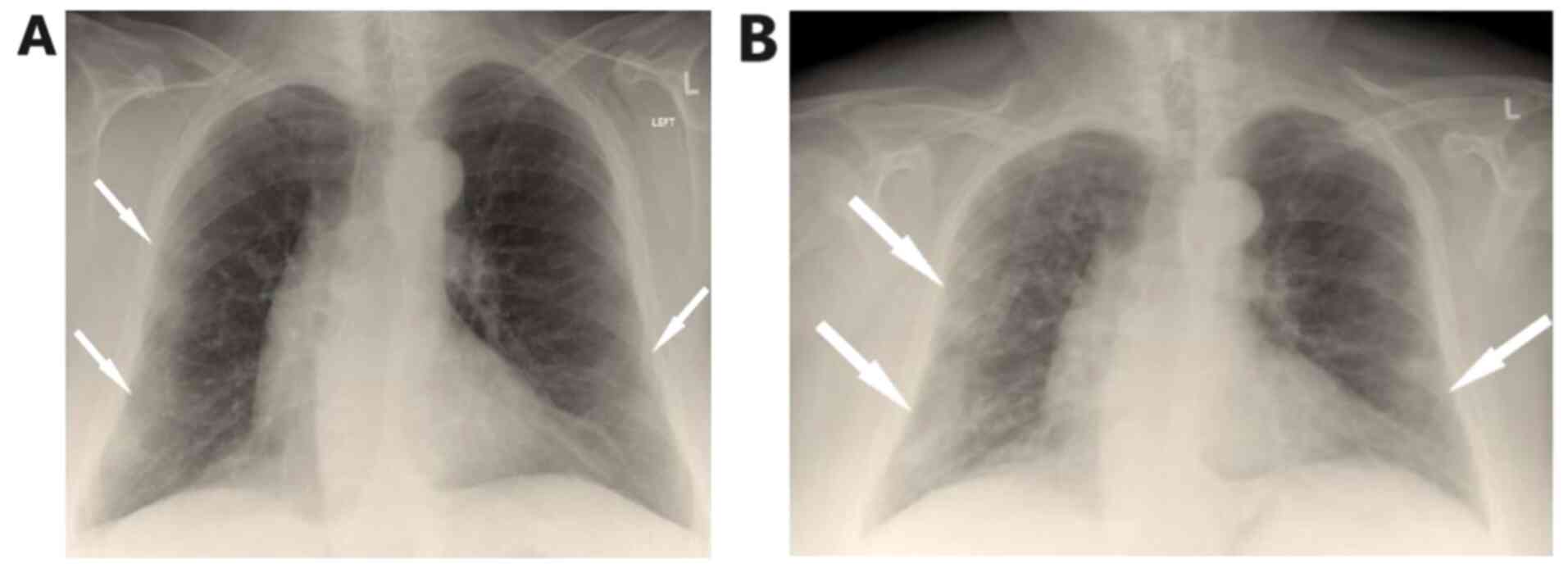
mmol/l on room air. Chest X-Ray showed patchy diffuse infiltrates
in both lungs mostly in the periphery of the left lung (Fig. 1A).
Laboratory investigation included complete blood
cell count, basic biochemistry serum and urine parameters and
coagulation testing. Laboratory findings included hemoglobin 13.1
g/dl (normal 12-16 g/dl), hematocrit 39.5% (normal 38-47%), white
blood cells (WBC) 4.28x103/µl (normal
4-11x103/µl), neutrophils 2.8x103/µl (normal
1.5-6.6x103/µl), lymphocytes 1.24x103/µl
(normal 1.2-3.4x103/µl), platelets 145x103/µl
(normal 140-440x103/µl), serum glucose 110 mg/dl (normal
72-106 mg/dl), blood urea nitrogen 57 mg/dl (normal 15-43 mg/dl),
creatinin 1.36 mg/dl (normal 0.6-1 mg/dl), C-reactive protein 18.78
mg/l (normal <6 mg/l), ferritin 145 ng/ml (normal 15-150 ng/ml),
prothrombin time (PT) 28.5 sec (normal 11-13 sec), activated PTT
52.2 sec (normal 29-40 sec), international normalized ratio (INR)
2.16 (normal 0.9-1.2) and d-dimers 1.21 µg/ml (normal <0.5
µg/ml). The other blood biochemistry parameters were normal.
Urinalysis revealed 1-2 WBCs per high power field (PHF; normal 0-2
PHF), 0-2 RBCs PHF (normal 0-2 PHF), pH 6 (normal 4.5-8), specific
gravity 1.020 (normal 1.005-1.025), nitrites negative and leukocyte
esterase negative.
The patient was tested for coronavirus and had
positive detection of SARS-CoV-2 nucleic acid in examined
nasopharyngeal sample using reverse transcription-PCR.
The patient was unvaccinated for SARS-CoV-2. She was
transferred to the COVID-19 unit and received oxygen therapy with
Venturi mask delivering 35% oxygen and intravenous dexamethasone,
remdesivir and subcutaneous enoxaparin. After three days of
hospitalization she presented with resolution of the fever and
improvement of oxygen level.
On the fifth day of hospitalization, fever
reoccurred and the patient presented with worsening cough with
green sputum, with reduction in partial pressure of oxygen, changes
in findings from lung auscultation with rhonchi sounds found in all
lung fields and worsening infiltrates on chest X-ray (Fig. 1B). The patient received oxygen with
Venturi mask delivering 50% oxygen and intravenous
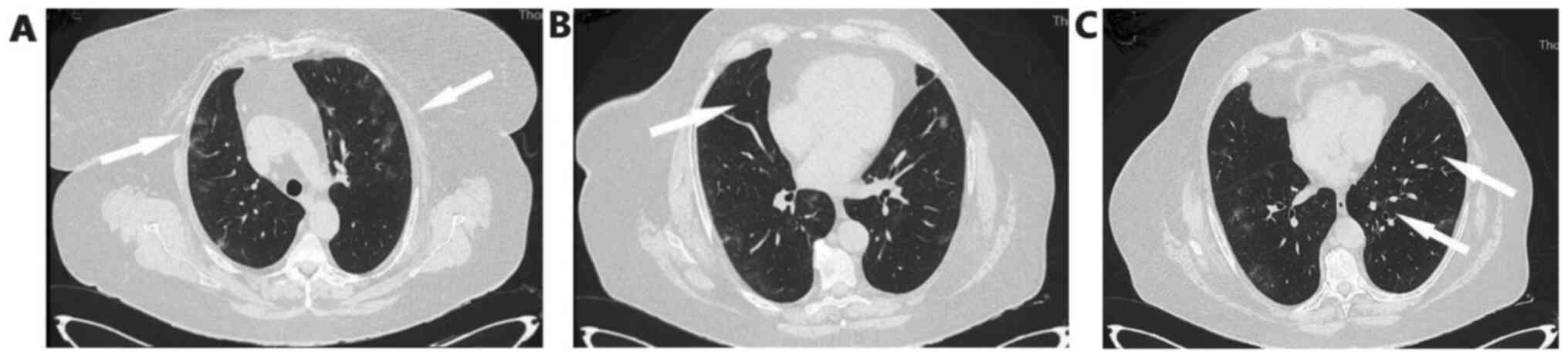
piperacillin-tazobactam empirically. She underwent high resolution
computed tomography of the chest showing bronchiectasis and nodular
ground glass opacifications mostly with peripheral and subpleural
distribution in both lungs (Fig.
2A-C).
Sputum culture was performed which revealed the
presence of Pseudomonas putida. Antimicrobial susceptibility
testing showed that the microorganism is pansusceptible. The
patient presented with improvement after three days of
piperacillin-tazobactam administration. She had gradual recovery
and she was discharged on the 12nd day of hospitalization without
the need of supplemental oxygen and with instructions to receive
oral ciprofloxacin in order to have overall a 14-day antibiotic
course.
Discussion
The case is notable for two reasons. First, it is,
to the best of the authors' knowledge, the second case in
literature mentioning bronchiectasis exacerbation by Pseudomonas
putida. The first case was reported by Fujita et al
(13) in 1998, in a 57-year-old
woman with bronchiectasis and without serious comorbidities. In
that case, the patient was non-immunocompromised, had a known
history of bronchiectasis, presented with increase in the volume
and purulent nature of the sputum and mild hemoptysis and had a
right lower lobe consolidation on imaging. The patient in the
current study was also an immunocompetent host. She did not present
with hemoptysis or consolidation on chest computed tomography scan
and she had no known history of bronchiectasis. Pseudomonas
putida has emerged as an opportunistic pathogen for
non-immunocompetent hosts, causing bacteremia, wound and ocular
infections, urinary tract and lower respiratory tract infections,
central venous catheter and soft tissue infections in this patients
population (14).
Second, the case in the current study indicated that
SARS-CoV-2 infection precipitated bronchiectasis exacerbation.
Mask-wearing and social distancing during the COVID-19 pandemic
were associated with a marked decrease in bronchiectasis
exacerbation frequency (15). This
decrease was probably noticed due to a reduction in circulating
viruses and in traffic-related air pollution, documented during the
pandemic (9). However, SARS-CoV-2
infection seems to result in co-infections and exacerbations in
patients with bronchiectasis. Lopinto et al (16) reported the first case of acute
exacerbation of bronchiectasis, precipitated by COVID-19 disease
and complicated by severe hemoptysis. In addition, Faqihi et
al (17) describe a case of
co-infection of Bordetella bronchiseptica and novel
coronavirus in a young patient with idiopathic bronchiectasis
leading to critical illness and requirement of mechanical
ventilation. One may hypothesize that the novel virus triggers
acute bronchial inflammation and exacerbation of the chronic
disease of airways.
It has been reported that ~2% of patients with
COVID-19 have underlying lung disease, which is related to severe
clinical manifestations and higher mortality compared with those
without chronic lung diseases (18). Bronchiectasis is an uncommon
comorbidity observed in COVID-19 patients and thus research about
manifestations and clinical outcome of COVID-19 disease in patients
with bronchiectasis is limite (19,20).
Choi et al (20), in which
8,070 patients with COVID-19 were enrolled, report that the
patients with bronchiectasis are significantly older (P<0.001)
and more frequently exhibit pulmonary comorbidities, including
asthma. The researchers also reported that the rate of SARS-CoV-2
infection is relatively higher in patients with bronchiectasis
compared with those without bronchiectasis and that COVID-19
patients with bronchiectasis present with a higher rate of
supplemental oxygen and extracorporeal membrane oxygenation
requirement and higher mortality than those without bronchiectasis,
indicating that impaired mucociliary clearance and bronchial
inflammation possibly increase their vulnerability to, and severity
of, COVID-19(20). Larger
prospective studies may be needed to address issues concerning
patients with bronchiectasis experiencing SARS-CoV-2 infection.
In conclusion, the present study detailed a rare
case of exacerbation of bronchiectasis by Pseudomonas putida
complicating COVID-19 disease. Pseudomonas putida can cause
pulmonary infection and bronchiectasis exacerbation even in
immunocompetent hosts (13).
Moreover, SARS-CoV-2 infection should be considered a precipitating
factor of bronchiectasis exacerbation, while the presence of
bronchiectasis make individuals susceptible to development and
greater severity of SARS-CoV-2 infection.
Acknowledgements
Not applicable
Funding
No funding was received
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PA, AB and PP conceptualized the case. VEG, KM, NG,
CD, AGk and SC wrote and prepared the draft of the manuscript. NT
and DAS provided critical revisions. PS and AGa prepared the
figures. VEG and AGa confirm the authenticity of all the data. All
authors contributed to manuscript revision and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Written informed was obtained from the patient for
publication of this case report and accompanying images. A copy of
the written consent is available for review by the Editor-in-Chief
of this journal on request.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Cole PJ: Inflammation: A two-edged sword -
the model of bronchiectasis. Eur J Respir Dis Suppl. 147:6–15.
1986.PubMed/NCBI
|
|
2
|
O'Donnell AE: Bronchiectasis update. Curr
Opin Infect Dis. 31:194–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lonni S, Chalmers JD, Goeminne PC,
McDonnell MJ, Dimakou K, De Soyza A, Polverino E, Van de Kerkhove
C, Rutherford R, Davison J, et al: Etiology of non-cystic fibrosis
bronchiectasis in adults and its correlation to disease severity.
Ann Am Thorac Soc. 12:1764–1770. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Georgakopoulou VE, Trakas N, Damaskos C,
Garmpis N, Karakou E, Chatzikyriakou R, Lambrou P and Tsiafaki X:
Neutrophils to lymphocyte ratio as a biomarker in bronchiectasis
exacerbation: A retrospective study. Cureus.
12(e9728)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wilson R, Aksamit T, Aliberti S, De Soyza
A, Elborn JS, Goeminne P, Hill AT, Menendez R and Polverino E:
Challenges in managing Pseudomonas aeruginosa in non-cystic
fibrosis bronchiectasis. Respir Med. 117:179–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li X, Yin D, Yang Y, Bi C, Wang Z, Ma G,
Fu X, Ji S, Jiang F and Yu T: Eosinophil: A nonnegligible predictor
in COVID-19 re-positive patients. Front Immunol.
12(690653)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lansbury L, Lim B, Baskaran V and Lim WS:
Co-infections in people with COVID-19: A systematic review and
meta-analysis. J Infect. 81:266–275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Langford BJ, So M, Raybardhan S, Leung V,
Westwood D, MacFadden DR, Soucy JR and Daneman N: Bacterial
co-infection and secondary infection in patients with COVID-19: A
living rapid review and meta-analysis. Clin Microbiol Infect.
26:1622–1629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Crichton ML, Shoemark A and Chalmers JD:
The impact of the COVID-19 pandemic on exacerbations and symptoms
in bronchiectasis: A prospective study. Am J Respir Crit Care Med.
204:857–859. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Volke DC, Calero P and Nikel PI:
Pseudomonas putida . Trends Microbiol. 28:512–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang CH, Young T, Peng MY and Weng MC:
Clinical spectrum of Pseudomonas putida infection. J Formos
Med Assoc. 95:754–761. 1996.PubMed/NCBI
|
|
12
|
Neulier C, Breton N, Pangon B, Le Monnier
A, Henry-Lagarrigue M, Dujon C and Merrer J: Pseudo-outbreak of
Pseudomonas putida respiratory infection caused by
laboratory contamination. Infect Control Hosp Epidemiol.
32:523–525. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Fujita J, Negayama K, Ohara M, Hojo S,
Obayashi Y, Miyawaki H, Yamaji Y and Takahara J: Pneumonia caused
by Pseudomonas putida with a mucoid phenotype. Respir Med.
92:693–695. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peter S, Oberhettinger P, Schuele L,
Dinkelacker A, Vogel W, Dörfel D, Bezdan D, Ossowski S, Marschal M,
Liese J, et al: Genomic characterisation of clinical and
environmental Pseudomonas putida group strains and
determination of their role in the transfer of antimicrobial
resistance genes to Pseudomonas aeruginosa. BMC Genomics.
18(859)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Metersky ML: Fewer bronchiectasis
exacerbations during the ‘Lockdown’ for COVID-19: Can we convert
knowledge into action? Am J Respir Crit Care Med. 204:759–760.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lopinto J, Teulier M, Milon A, Voiriot G
and Fartoukh M: Severe hemoptysis in post-tuberculosis
bronchiectasis precipitated by SARS-CoV-2 infection. BMC Pulm Med.
20(244)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Faqihi F, Alharthy A, Pirompanich P, Noor
A, Shahzad A, Nasim N, Balhamar A, Memish ZA and Karakitsos D:
Co-infection of SARS-CoV-2 and Bordetella bronchiseptica in
a young man with idiopathic non-cystic bronchiectasis and vitamin
D3 deficiency. Respir Med Case Rep. 31(101203)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo
Q, Ji R, Wang H, Wang Y and Zhou Y: Prevalence of comorbidities and
its effects in patients infected with SARS-CoV-2: A systematic
review and meta-analysis. Int J Infect Dis. 94:91–95.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the Coronavirus Disease 2019 (COVID-19)
outbreak in China: Summary of a report of 72,314 cases from the
Chinese Center for Disease Control and Prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Choi H, Lee H, Lee SK, Yang B, Chung SJ,
Yeo Y, Park TS, Park DW, Moon JY, Kim TH, et al: Impact of
bronchiectasis on susceptibility to and severity of COVID-19: A
nationwide cohort study. Ther Adv Respir Dis: Feb 14, 2021 (Epub
ahead of print). doi: 10.1177/1753466621995043.
|