1. Introduction
Calcium ions (Ca2+) are essential
electrolytes in the body and have a fundamental role in regulating
nerve cell excitation, neurotransmitter release, membrane integrity
and muscle contraction. Thus, Ca2+-triggered signalling
pathways have an important role in neuron survival, plasticity and
nerve transmission.
Over the past decade, transient receptor potential
(TRP) channels have attracted increasing attention. TRP ankyrin 1
(TRPA1), a non-selective cation channel permeable to
Ca2+ ions, is broadly distributed in various parts of
the human body and is associated with various physiological and
pathological states, such as sensations of cold and pain, as well
as itchiness. Numerous studies have explored the role of the
channel in the initiation and development of toxicity. The present
review summarized the progress of research on the structure,
function and distribution of TPRA1, and discussed diseases related
to TPRA1 and Ca2+.
2. TRP family
TRP family members are non-selective cation channels
that were discovered in the visual system of the fruit fly
Drosophila melanogaster (1). There are 28 known TRP cation channels
with different structures and functions (2). All TRP channels have six
transmembrane domains (S1-S6), and both N- and C-termini are
located on the cytoplasmic side of the cell membrane. These
proteins are thought to function as tetramers. TRP channels allow
cations such as Ca2+, Mg2+, Na+
and K+ to pass through, which leads to cell
depolarisation, influx of extracellular Ca2+, release of
Ca2+ from intracellular Ca2+ stores and
binding of Ca2+ to calmodulin (CaM), ultimately
affecting cell proliferation and apoptosis. The functions of the
TRP family are closely related to Ca2+. For instance,
TRP cation channel subfamily V member 5 (TRPV5) and TRPV6 are
Ca2+ uptake channels in epithelial tissues with unique
selectivity for Ca2+ [permeability (P) ratio
PCa/PNa >100] (3).
In mammals, there are six TRP channel subtypes
according to their amino acid sequence homology: The ankyrin TRP
TRPA, the canonical TRPC and TRPV, the melastatin TRP termed TRPM,
the polycystic TRP known as TRPP and the mucolipin TRP TRPML
(4). Although all TRP channels
share certain similarities in sequence and structure, there are
significant differences in physiological functions, including
cation selectivity, ligand binding and sensitivity to temperature
and other environmental conditions among family members (5). The members of TRPV subfamily are
considered to be heat sensors, nociceptive sensors, mechano-sensors
and osmo-sensors (6,7) and the majority of TRPM subfamily
members are implicated in taste, gastric hormone secretion and
insulin release (7,8). Brain development and vaso-motor
regulation have been reported for the TRPC subfamily channels
(7-9),
while the defining characteristic of the TRPP subfamily is their
association with renal development (7-10).
Evidence suggests that the TRPML subfamily is associated with
endocytosis and the regulation of autophagy (7-9).
The TRPA subfamily has been indicated to function as a
thermo-sensor, chemo-sensor and olfactory sensor (6,7). The
major physiological function of TRP family members in mammals are
listed in Table I.
 | Table ITRP family members in mammals. |
Table I
TRP family members in mammals.
| Subfamily | Main physiological
function | (Refs.) |
|---|
| TRPV | Thermo-sensation;
nociception; mechano-sensation; osmo-sensation | (6,7) |
| TRPM | Taste; gastric
hormone secretion; insulin release | (7,8) |
| TRPC | Brain development;
vaso-motor regulation | (7,9) |
| TRPP | Renal
development | (7,10) |
| TRPML | Endocytosis and
endosomal/lysosomal function; regulation of autophagy | (7,9) |
| TRPA | Thermo-sensation;
chemo-sensing; nociception; olfactory responses | (6,7) |
Mutations related to human diseases have been
detected in nearly 30 members of the TRP channel family, which
highlights their importance in human physiology, and they are
likely to continue receiving attention in the future.
3. Structure, distribution and physiological
function of TRPA1
Ankyrins are a group of linker proteins located on
the membrane cytoskeleton that mediate the attachment of intact
membrane proteins to spectrin and actin. TRPA1 was first isolated
from human fetal lung fibroblasts in 1999(11) and was originally called
ankyrin-like with transmembrane domains protein 1(12). It is the only known member of the
TRPA subfamily and consists of 119 amino acid residues with a
molecular weight of 127.4 kDa.
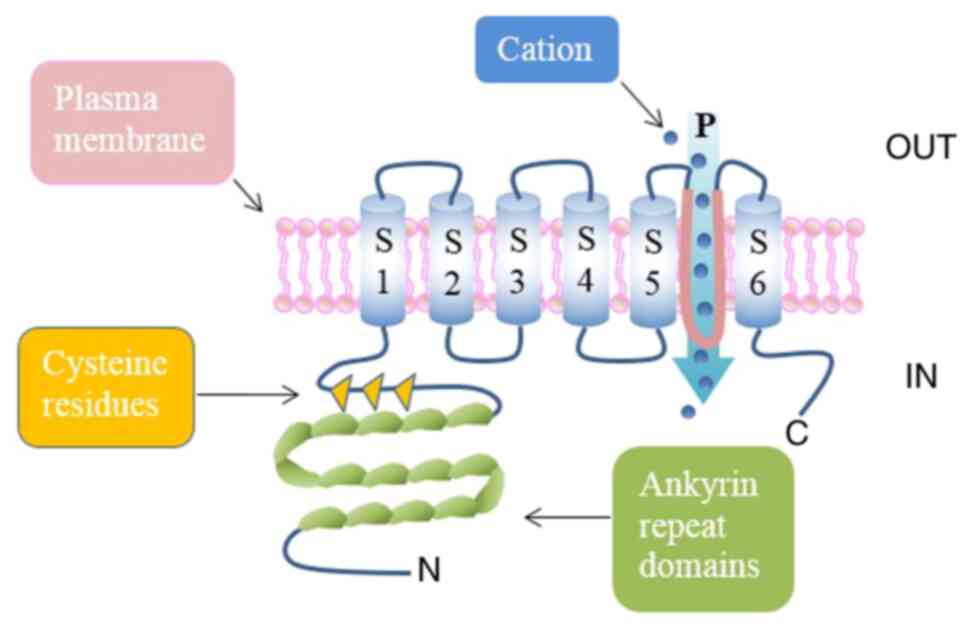
TRPA1 structure
As all TRP channels, TRPA1 is a tetrameric protein
composed of four subunits (119 amino acids) and six transmembrane
α-helices (S1-S6), with the pore loop structure located in the
hydrophilic region between S5 and 6 (13,14).
TRPA1 derives its name from 14-18 ankyrin repeats at the N-terminus
(depending on species) in addition to a large number of active
cysteine residues (15), which is
an unusual structural feature and may be related to its interaction
with intracellular components (16).
TRPA1 may be activated by a series of harmful
external stimuli and endogenous signals related to cell damage. The
latter includes cinnamaldehyde, allicin, allyl isothiocyanate and
reactive oxygen species (17,18).
The major mechanism of activation is the covalent modification of
cysteine and lysine residues at the N-terminus of TRPA1 by highly
electrophilic compounds (19).
This mechanism promotes local conformational changes, leading to
the expansion of the pore loop structure, which increases the
permeability to Ca2+. In addition to being activated by
reactive electrophiles and oxidants, TRPA1 may also be indirectly
activated by the pro-inflammatory factor-mediated phospholipase C
signal, in which cytoplasmic Ca2+ are an important
regulator of channel gating (20).
In addition, as TRPA1 is permeable to both univalent and bivalent
cations (including Ca2+, sodium and potassium), it is
able to depolarise the membrane and activate Ca2+
signals (21). The structure of
TRPA1 is presented in Fig. 1.
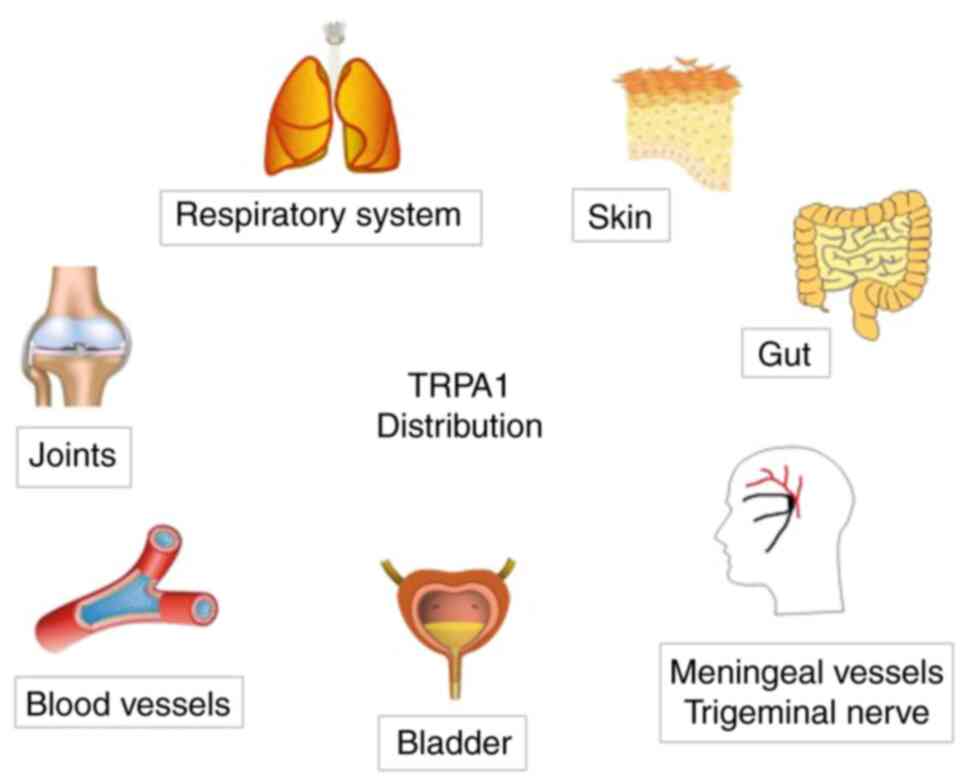
TRPA1 distribution
TRPA1 is a non-selective cation channel present in
various tissues and organs, but it is mainly expressed in sensory
neurons, such as primary sensory neurons in the lung, skin and
brain, and peptidergic neurons (22), particularly those in the mammalian
dorsal root ganglion, trigeminal ganglion, nodular ganglion and
jugular ganglion (23).
In addition, TRPA1 is also present in several
non-nerve cells and tissues, including vascular endothelial cells
and chondrocytes (24,25), but the function of this expression
has remained largely elusive. The major distribution of TRPA1 in
the human body is presented in Fig.
2.
Physiological functions of TRPA1
TRPA1 is a cold-sensitive ion channel that may be
activated to generate a stress response to endogenous and exogenous
chemical stimulation, cold stimulation, mechanical stimulation and
various inflammatory mediators. The activation of TRPA1 is closely
related to the conduction and generation of cold sensation, the
mediation of pain and analgesia, and the regulation of inflammatory
substances.
TRPA1 is not mechanically sensitive under
physiological conditions, but it may be activated at temperatures
<17˚C (26); hence, it serves
as a cold-sensitive receptor that detects changes in temperature in
the internal and external environment. In addition to mediating
temperature sensation and pain (27), TRPA1 functions in mechanical
perception (28) and has a role in
certain inflammatory states (29).
Furthermore, TRPA1 is also associated with hypersensitivity and
overexcitation in certain non-neuronal regions and has an important
role in the pathophysiology of asthma, neuropathic pain, chronic
itching, migraine, gastrointestinal motility disorders, anxiety and
cognitive dysfunction (30-34).
Due to its individual characteristics in different
organs, TRPA1 has been actively studied as a potential target for
treating various diseases.
4. Ca2+ ions
Second messenger is one of the initiating components
of intracellular signal transduction. Ca2+, as an
ubiquitous second messenger in the cytoplasm, is involved in the
regulation of a variety of important physiological processes in
cells, such as the synthesis and release of neurotransmitters,
regulation of germ cell maturation and fertilization, and
regulation of the activities of various enzymes in the body
(35). Ca2+ is also
closely related to hypertension, coronary heart disease,
Alzheimer's disease (AD) and numerous other diseases (36-38).
The intracellular second messenger Ca2+ has been a hot
research topic in recent years.
The cytosolic free Ca2+ concentration in
mammalian cells is generally controlled in the range of 100-200
nmol/l, while the Ca2+ concentration in extracellular
organelles is kept in the order of mmol/l (39). The steep but tightly controlled
concentration gradient of Ca2+ within and outside the
cell membrane, as well as between cytoplasm and organelles, is
maintained and dynamically regulated according to cell needs
through the cooperative work of a variety of ion channels, ion
pumps and transporters (40). The
major channels and transporters for intracellular Ca2+
cycling are presented in Fig.
3.
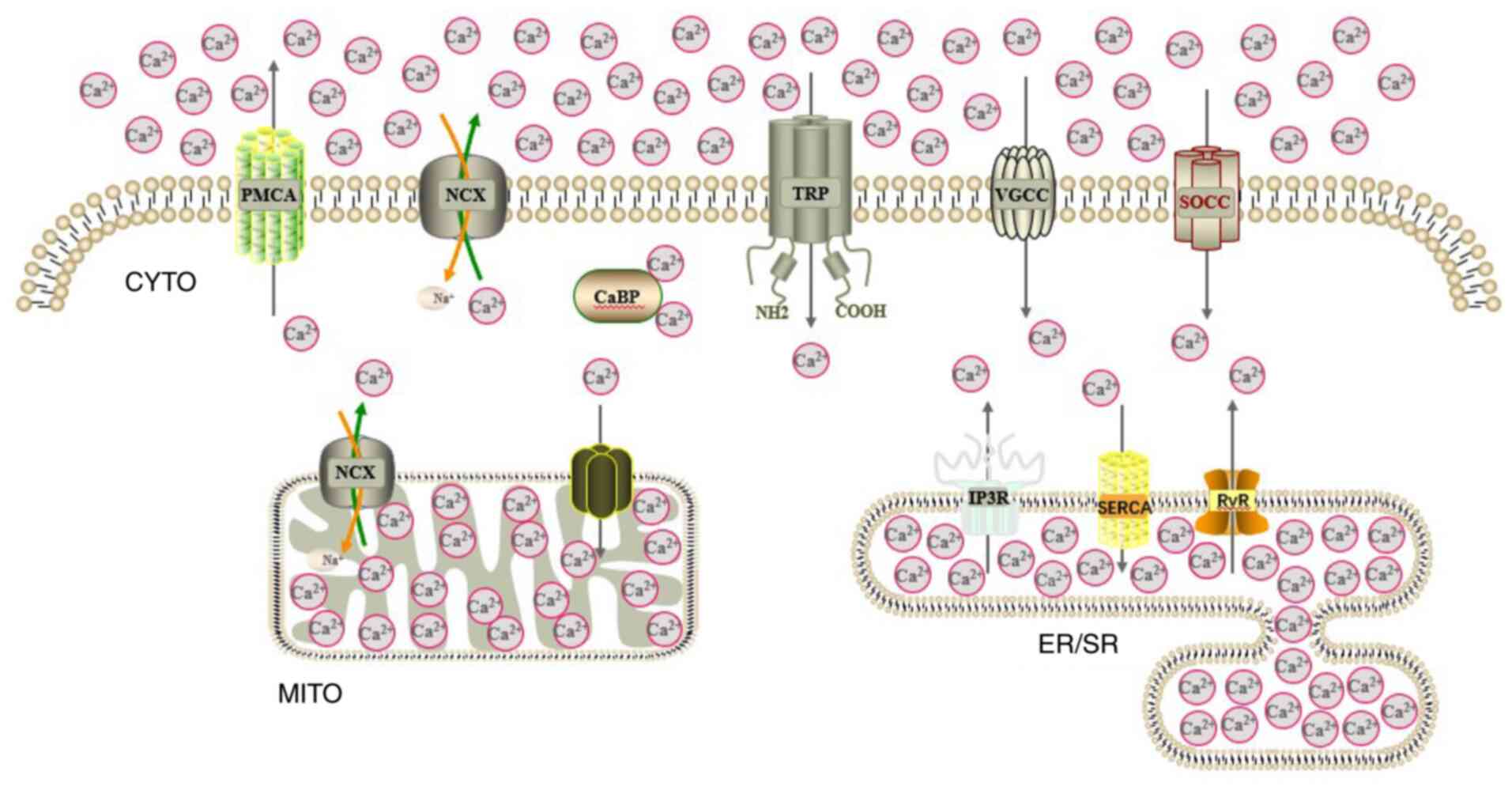 | Figure 3Pattern of mammalian cell
Ca2+ metabolism. The main channels and transporters for
intracellular Ca2+ cycling are displayed.
Ca2+, calcium; CYTO, cytoplasm; MITO, mitochondrion;
ER/SR, endoplasmic reticulum/sarcoplasmic reticulum; PMCA, plasma
membrane Ca2+ ATPase; NCX, sodium Ca2+
exchanger; CaBP, Ca2+-binding protein; TRP, transient
receptor potential channel; VGCC, voltage-gated Ca2+
channel; SOCC, store-operated Ca2+ channel; IP3R,
inositol trisphosphate receptor; SERCA, sarcoplasmic reticulum
Ca2+ ATPase; RyR, ryanodine receptor. |
Abnormality of any link may cause instability of
Ca2+ homeostasis. Ca2+ homeostasis is
essential for cell maintenance; under pathological conditions,
Ca2+ homeostasis is altered, with increased cytoplasmic
Ca2+ concentrations. Ca2+ channels are a
basis for revealing the regulatory laws of Ca2+
homeostasis and vital processes. Certain cation channels, including
TRP family members, promote the influx of Ca2+. For
instance, although TRPA1 has high permeability to Ca2+,
Na+ and K+, it has high Ca2+
selectivity. The PCa/PNa is 6 when the channel opens spontaneously
and increases to 9 when the channel is activated by an
electrophilic reagent (41).
5. Coupling TRPA1 with Ca2+
ions
TRPA1 mediates Ca2+
internal flow
Compared with most other TRP channels, TRPA1 has
higher Ca2+ permeability. The N-terminus of TRPA1
contains two helical Ca2+-binding motifs and the
permeation pathway involves two major contraction sites (15), with an aspartate (D918 in mouse,
D915 in human) within the pore loop that is critical for
Ca2+ permeability (13). Total internal reflection
fluorescence and confocal microscopy revealed that the signal
generated by Ca2+ influx from a single TRPA1 channel in
endothelial cells is at least 200 times that of L-type
Ca2+ channels (42).
This is because TRPA1 channels exist in the plasma membrane of
endothelial cells with a tight binary structure. When one of a pair
of channels is opened, afferent Ca2+ is able to bind to
the Ca2+-sensitive EF-Hand protein domain at the
N-terminus, thereby triggering the adjacent channel (43).
There is a highly conserved structural motif in the
TRPA1 channel that is the key site of intracellular Ca2+
elevation caused by Ca2+ storage (44), which explains various
Ca2+-dependent processes, including sensitisation,
desensitisation and coupling with metabolic receptors.
TRPA1-mediated Ca2+ influx is involved in various
biological processes such as factor secretion (45) and gene transcription (46). TRPA1 is able to induce apoptosis in
cardiomyocytes (47),
oligodendrocytes (48) and
hippocampal neurons (49) by
regulating the Ca2+ concentration. Increasing evidence
indicates that TRPA1-mediated Ca2+ influx has a role in
determining the pathophysiological state.
Electrical stimulation of TRPA1 in adult mouse
cardiomyocytes may lead to activation of the calmodulin-dependent
kinase II signalling pathway (45), thereby regulating the availability
and manoeuvrability of intracellular Ca2+. TRPA1 is also
the major contributor to the increase in Ca2+ in
oligodendrocytes caused by ischemia; 70% of the Ca2+
increase may be attributed to TRPA1(50), indicating a key role of the
mediation of Ca2+ influx in myelin injury. The molecular
basis by which TRPA1 regulates nociceptive neurons is also related
to Ca2+; TRPA1 induces local Ca2+ influx at
nerve endings, promotes membrane depolarisation and causes the
release of neuropeptides from large dense core vesicles through
Ca2+-dependent exocytosis, resulting in further
amplification of nociceptive sensation, recruitment of immune
cells, vasodilation and neurogenic inflammation (51).
Ca2+ regulates TRPA1
activity
Ca2+ ions are the most important
endogenous regulators of TRPA1 and they enhance and inhibit the
activity of this channel under chemical stimulation.
Ca2+ binds to the Ca2+ binding domain at the
C-terminus of the molecule, which opens the ion channel. The high
permeability of TRPA1 to Ca2+ not only triggers the
influx of extracellular Ca2+, but also promotes the
release of Ca2+ from intracellular stores, such as the
endoplasmic reticulum (52).
Ca2+ activation may enhance the responses to other
stimuli and desensitise ion channels through a variety of signaling
pathways, which in turn regulate the activity of TRPA1.
The opening kinetics of TRPA1 are strongly affected
by divalent cations. When Ca2+ binds to the channel, the
binding of monovalent cations to the channel is hindered. Under the
spontaneous opening state, ~17% of the TRPA1 current is
Ca2+ current (41).
Ca2+ is able to directly activate TRPA1, which is not
only important for the basic reaction of TRPA1 at low
Ca2+ concentrations (<1 mM), but may also rapidly
inactivate TRPA1 at high Ca2+ concentrations (>1 mM)
(53). Therefore, Ca2+
has dual and opposite effects on TRPA1. In addition to direct
interaction with TRPA1, indirect interactions occur via cytoplasmic
Ca2+ binding proteins, among which CaM is the most
prominent. TRPA1 binds CaM to form a Ca2+-sensing
channel complex, a key Ca2+ receptor that responds
differently to different Ca2+ signals (54). Extracellular Ca2+
activates and/or enhances TRPA1 at low concentrations but quickly
inactivates TRPA1 following channel activation. This process is
called desensitisation or rapid analgesia (55) and this contrasting effect is the
result of Ca2+ regulating TRPA1 through CaM. Both the N-
and C-terminal regions may be important for these interactions. A
direct effect for Ca2+ on purified human TRPA1 (hTRPA1)
independent of CaM has been demonstrated (56); Ca2+ is able to directly
activate hTRPA1 and cause structural changes by directly
interacting with binding sites other than the N-terminal ankyrin
protein repeat domain of hTRPA1.
Furthermore, thermal activation of TRPA1 in
chameleon, chicken and rat snake depends on extracellular
Ca2+ binding to negatively charged amino acids near the
outer surface of channel pore cells, while other divalent cations
cannot activate the heat-evoked current (57). Although TRPA1 is a hypothermic
receptor, certain researchers assume that harmful hypothermia does
not directly activate TRPA1, but rather that the cold environment
stimulates the release of intracellular Ca2+ stores
(58), activating TRPA1, a
Ca2+-dependent ion channel.
6. Interaction between TRPA1 and
Ca2+ in disease progression
TRPA1 is a neuronal redox-sensitive Ca2+
internal flow channel that is overexpressed in human cancers. It
upregulates Ca2+-dependent anti-apoptosis pathways and
promotes resistance to reactive oxygen species (59). Triclosan, an antibacterial agent,
was indicated to induce activation of TRPA1 and Ca2+
influx in human prostate cancer stromal cells, resulting in the
secretion of VEGF and the growth of prostate cancer epithelial
cells (60).
TRPA1 serves as a physiological medium for
inflammatory signals and appears to perform a functional role in
promoting myoblast migration, fusion and potential activation of
human satellite cells. Thus, it may provide a novel target for
treating muscle injury or muscle-related diseases via the formation
of Ca2+/calmodulin complexes (61).
The inflammatory factor interleukin (IL)-1β
increases the functional expression of TRPA1, which leads to
Ca2+ overload and a significant decrease in
mitochondrial membrane potential. Inhibition of TRPA1 has a
protective effect on mitochondrial dysfunction and even apoptosis
of rat chondrocytes induced by IL-1β (62). Furthermore, the TRPA1 channel may
protect against intestinal fibrosis by mediating Ca2+
mobilisation, in addition to its anti-inflammatory actions
(63).
A considerable amount of research has focused on the
role of TRPA1 in nervous system regions and suggests that TRPA1 has
a promising prospect in both peripheral and central nervous system
regions (64-66).
Current therapies of multiple sclerosis mainly focus
on pathological immune responses, but they cannot prevent the
progression of clinical symptoms (67). TRPA1 regulates the functions of
astrocytes by increasing the intracellular Ca2+
concentration (24).
Organophosphate-induced delayed neurotoxicity refers
to a series of neurological symptoms that occur within 1-3 weeks
after the ingestion of organophosphorus compounds (68). A study from the Shanghai Institute
of Pharmacy, Chinese Academy of Sciences, discovered that TRPA1 is
the major mediator of delayed neuropathy (69). In the same study, verapamil (an
L-type Ca2+ channel blocker that effectively relieves
the symptoms of the disease) was indicated to have a
neuroprotective role by inhibiting TRPA1-mediated Ca2+
influx.
There is evidence to suggest that excessive
Ca2+ in astrocytes may influence synaptic function, and
regulating TRPA1 channel activity in astrocytes may provide a novel
target for blocking early dysfunction in AD (70).
7. Conclusions and perspectives
After years of in-depth research, TRPA1 has
attracted extensive clinical attention due to its function as a
chemical sensor for irritation and cell damage, and its
relationship with various diseases. For instance, hydrogen
sulphide-mediated vasodilation is due to an increase in
Ca2+ concentration in trigeminal ganglion neurons
activated by TRPA1(71). In
addition, TRPA1 is an important subject of toxicology research and
is actively studied by pharmaceutical companies (72). However, the functional regulatory
mechanisms of TRPA1 remain poorly understood and further
investigations may lead to novel protective strategies. In our
opinion, areas worthy of further research include the following: i)
Unveiling the specific mechanism of the interaction between TRPA1
and Ca2+; ii) exploring the therapeutic potential of
TRPA1 in the treatment of pain and airway respiratory diseases
through clinical studies; iii) assessing the potential of TRPA1
antagonist as a therapeutic target; and iv) investigating the
mechanisms by which TRPA1 regulates Ca2+ in different
diseases.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 81673227 and 81172712), the Natural
Science Foundation of Hunan Province (grant no. 2020JJ4080) and the
Key Projects of the Hunan Provincial Department of Education (grant
no. 18A254).
Availability of data and materials
Not applicable.
Authors' contributions
FH: Writing-original draft preparation, review and
editing. XS: Conceptualization. DL: Supervision, funding
acquisition and manuscript revision. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakaguchi R and Mori Y: Transient receptor
potential (TRP) channels: Biosensors for redox environmental
stimuli and cellular status. Free Radic Biol Med. 146:36–44.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alavi MS, Shamsizadeh A, Karimi G and
Roohbakhsh A: Transient receptor potential ankyrin 1
(TRPA1)-mediated toxicity: Friend or foe? Toxicol Mech Methods.
30:1–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saotome K, Singh AK, Yelshanskaya MV and
Sobolevsky AI: Crystal structure of the epithelial calcium channel
TRPV6. Nature. 534:506–511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Skerratt S: Recent progress in the
discovery and development of TRPA1 modulators. Prog Med Chem.
56:81–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li X and Fine M: TRP channel: The
structural era. Cell Calcium. 87(102191)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Venkatachalam K and Montell C: TRP
channels. Annu Rev Biochem. 76:387–417. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nilius B and Flockerzi V: Mammalian
transient receptor potential (TRP) cation channels. Preface. Handb
Exp Pharmacol. 223:5–6. 2014.PubMed/NCBI
|
|
8
|
Dhakal S and Lee Y: Transient receptor
potential channels and metabolism. Mol Cells. 42:569–578.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol.
12(218)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Samanta A, Hughes TET and Moiseenkova-Bell
VY: Transient receptor potential (TRP) channels. Subcell Biochem.
87:141–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Laursen WJ, Anderson EO, Hoffstaetter LJ,
Bagriantsev SN and Gracheva EO: Species-specific temperature
sensitivity of TRPA1. Temperature (Austin). 2:214–226.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andrade EL, Meotti FC and Calixto JB:
TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther.
133:189–204. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Viana F: TRPA1 channels: Molecular
sentinels of cellular stress and tissue damage. J Physiol.
594:4151–4169. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Preti D, Saponaro G and Szallasi A:
Transient receptor potential ankyrin 1 (TRPA1) antagonists. Pharm
Pat Anal. 4:75–94. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paulsen CE, Armache JP, Gao Y, Cheng Y and
Julius D: Structure of the TRPA1 ion channel suggests regulatory
mechanisms. Nature. 520:511–517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Talavera K, Startek JB, Alvarez-Collazo J,
Boonen B, Alpizar YA, Sanchez A, Naert R and Nilius B: Mammalian
transient receptor potential TRPA1 channels: From structure to
disease. Physiol Rev. 100:725–803. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hansted AK, Bhatt DK, Olesen J, Jensen LJ
and Jansen-Olesen I: Effect of TRPA1 activator allyl isothiocyanate
(AITC) on rat dural and pial arteries. Pharmacol Rep. 71:565–572.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tsuchiya Y and Kawamata K: Allicin induces
electrogenic secretion of chloride and bicarbonate ions in rat
colon via the TRPA1 receptor. J Nutr Sci Vitaminol (Tokyo).
65:258–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nilius B and Szallasi A: Transient
receptor potential channels as drug targets: From the science of
basic research to the art of medicine. Pharmacol Rev. 66:676–814.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zygmunt PM and Högestätt ED: TRPA1. Handb
Exp Pharmacol. 222:583–630. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Veldhuis NA, Poole DP, Grace M, McIntyre P
and Bunnett NW: The G protein-coupled receptor-transient receptor
potential channel axis: Molecular insights for targeting disorders
of sensation and inflammation. Pharmacol Rev. 67:36–73.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bátai IZ, Horváth Á, Pintér E, Helyes Z
and Pozsgai G: Role of transient receptor potential ankyrin 1 ion
channel and somatostatin sst4 receptor in the antinociceptive and
anti-inflammatory effects of sodium polysulfide and dimethyl
trisulfide. Front Endocrinol (Lausanne). 9(55)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kurganov E, Saito S, Tanaka Saito C and
Tominaga M: Requirement of extracellular Ca2+ binding to
specific amino acids for heat-evoked activation of TRPA1. J
Physiol. 595:2451–2463. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sághy É, Sipos É, Ács P, Bölcskei K,
Pohóczky K, Kemény Á, Sándor Z, Szőke É, Sétáló G Jr, Komoly S and
Pintér E: TRPA1 deficiency is protective in cuprizone-induced
demyelination-a new target against oligodendrocyte apoptosis. Glia.
64:2166–2180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nummenmaa E, Hämäläinen M, Moilanen LJ,
Paukkeri EL, Nieminen RM, Moilanen T, Vuolteenaho K and Moilanen E:
Transient receptor potential ankyrin 1 (TRPA1) is functionally
expressed in primary human osteoarthritic chondrocytes. Arthritis
Res Ther. 18(185)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miyake T, Nakamura S, Zhao M, So K, Inoue
K, Numata T, Takahashi N, Shirakawa H, Mori Y, Nakagawa T and
Kaneko S: Cold sensitivity of TRPA1 is unveiled by the prolyl
hydroxylation blockade-induced sensitization to ROS. Nat Commun.
7(12840)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Clapham DE: Structural biology:
Pain-sensing TRPA1 channel resolved. Nature. 520:439–441.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moparthi L and Zygmunt PM: Human TRPA1 is
an inherently mechanosensitive bilayer-gated ion channel. Cell
Calcium. 91(102255)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moilanen LJ, Hämäläinen M, Lehtimäki L,
Nieminen RM and Moilanen E: Urate crystal induced inflammation and
joint pain are reduced in transient receptor potential ankyrin 1
deficient mice-potential role for transient receptor potential
ankyrin 1 in gout. PLoS One. 10(e0117770)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang Y, Wang S, Kobayashi K, Hao Y, Kanda
H, Kondo T, Kogure Y, Yamanaka H, Yamamoto S, Li J, et al:
TRPA1-expressing lamina propria mesenchymal cells regulate colonic
motility. JCI Insight. 4(e122402)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gallo V, Dijk FN, Holloway JW, Ring SM,
Koppelman GH, Postma DS, Strachan DP, Granell R, de Jongste JC,
Jaddoe VW, et al: TRPA1 gene polymorphisms and childhood asthma.
Pediatr Allergy Immunol. 28:191–198. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Logashina YA, Korolkova YV, Kozlov SA and
Andreev YA: TRPA1 channel as a regulator of neurogenic inflammation
and pain: Structure, function, role in pathophysiology, and
therapeutic potential of ligands. Biochemistry (Mosc). 84:101–118.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Benemei S and Dussor G: TRP channels and
migraine: Recent developments and new therapeutic opportunities.
Pharmaceuticals (Basel). 12(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee KI, Lin HC, Lee HT, Tsai FC and Lee
TS: Loss of transient receptor potential ankyrin 1 channel
deregulates emotion, learning and memory, cognition, and social
behavior in mice. Mol Neurobiol. 54:3606–3617. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pan S, Ryu SY and Sheu SS: Distinctive
characteristics and functions of multiple mitochondrial Ca2+ influx
mechanisms. Sci China Life Sci. 54:763–769. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen Q, Zhang Y, Ding D, Li D, Yang Y, Li
Q, Chen X, Hu G and Ling W: Associations between serum calcium,
phosphorus and mortality among patients with coronary heart
disease. Eur J Nutr. 57:2457–2467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Popugaeva E, Pchitskaya E and Bezprozvanny
I: Dysregulation of intracellular calcium signaling in Alzheimer's
disease. Antioxid Redox Signal. 29:1176–1188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Touyz RM, Alves-Lopes R, Rios FJ, Camargo
LL, Anagnostopoulou A, Arner A and Montezano AC: Vascular smooth
muscle contraction in hypertension. Cardiovasc Res. 114:529–539.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van der Kant R and Neefjes J: Small
regulators, major consequences-Ca2+ and cholesterol at
the endosome-ER interface. J Cell Sci. 127:929–938. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moccia F, Berra-Romani R and Tanzi F:
Update on vascular endothelial Ca(2+) signalling: A tale of ion
channels, pumps and transporters. World J Biol Chem. 3:127–158.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Karashima Y, Prenen J, Talavera K,
Janssens A, Voets T and Nilius B: Agonist-induced changes in Ca(2+)
permeation through the nociceptor cation channel TRPA1. Biophys J.
98:773–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sullivan MN, Gonzales AL, Pires PW, Bruhl
A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, et al:
Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen
species promote cerebral artery dilation. Sci Signal.
8(ra2)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lin King JV, Emrick JJ, Kelly MJS, Herzig
V, King GF, Medzihradszky KF and Julius D: A cell-penetrating
scorpion toxin enables mode-specific modulation of TRPA1 and pain.
Cell. 178:1362–1374.e16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao J, Lin King JV, Paulsen CE, Cheng Y
and Julius D: Irritant-evoked activation and calcium modulation of
the TRPA1 receptor. Nature. 585:141–145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Andrei SR, Ghosh M, Sinharoy P, Dey S,
Bratz IN and Damron DS: TRPA1 ion channel stimulation enhances
cardiomyocyte contractile function via a CaMKII-dependent pathway.
Channels (Austin). 11:587–603. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yin S, Wang P, Xing R, Zhao L, Li X, Zhang
L and Xiao Y: Transient receptor potential ankyrin 1 (TRPA1)
mediates lipopolysaccharide (LPS)-induced inflammatory responses in
primary human osteoarthritic fibroblast-like synoviocytes.
Inflammation. 41:700–709. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang Z, Wang M, Liu J, Ye J, Jiang H, Xu
Y, Ye D and Wan J: Inhibition of TRPA1 attenuates
doxorubicin-induced acute cardiotoxicity by suppressing oxidative
stress, the inflammatory response, and endoplasmic reticulum
stress. Oxid Med Cell Longev. 2018(5179468)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu M, Zhong S, Kong R, Shao H, Wang C,
Piao H, Lv W, Chu X and Zhao Y: Paeonol alleviates
interleukin-1β-induced inflammatory responses in chondrocytes
during osteoarthritis. Biomed Pharmacother. 95:914–921.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yazğan Y and Nazıroğlu M:
Ovariectomy-induced mitochondrial oxidative stress, apoptosis, and
calcium ion influx through TRPA1, TRPM2, and TRPV1 are prevented by
17β-estradiol, tamoxifen, and raloxifene in the hippocampus and
dorsal root ganglion of rats. Mol Neurobiol. 54:7620–7638.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hamilton NB, Kolodziejczyk K,
Kougioumtzidou E and Attwell D: Proton-gated Ca(2+)-permeable TRP
channels damage myelin in conditions mimicking ischaemia. Nature.
529:523–527. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kádková A, Synytsya V, Krusek J, Zímová L
and Vlachová V: Molecular basis of TRPA1 regulation in nociceptive
neurons. A review. Physiol Res. 66:425–439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Stueber T, Eberhardt MJ, Caspi Y, Lev S,
Binshtok A and Leffler A: Differential cytotoxicity and
intracellular calcium-signalling following activation of the
calcium-permeable ion channels TRPV1 and TRPA1. Cell Calcium.
68:34–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hasan R and Zhang X: Ca2+
regulation of TRP ion channels. Int J Mol Sci.
19(1256)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hasan R, Leeson-Payne AT, Jaggar JH and
Zhang X: Calmodulin is responsible for Ca2+-dependent
regulation of TRPA1 channels. Sci Rep. 7(45098)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cordero-Morales JF, Gracheva EO and Julius
D: Cytoplasmic ankyrin repeats of transient receptor potential A1
(TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc
Natl Acad Sci USA. 108:E1184–E1191. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Moparthi L, Moparthi SB, Wenger J and
Zygmunt PM: Calcium activates purified human TRPA1 with and without
its N-terminal ankyrin repeat domain in the absence of calmodulin.
Cell Calcium. 90(102228)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kurganov E and Tominaga M: Dependence of
heat-evoked TRPA1 activation on extracellular Ca2.
Channels (Austin). 11:271–272. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zurborg S, Yurgionas B, Jira JA, Caspani O
and Heppenstall PA: Direct activation of the ion channel TRPA1 by
Ca2+. Nat Neurosci. 10:277–279. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
59
|
Takahashi N, Chen HY, Harris IS, Stover
DG, Selfors LM, Bronson RT, Deraedt T, Cichowski K, Welm AL, Mori
Y, et al: Cancer cells co-opt the neuronal redox-sensing channel
TRPA1 to promote oxidative-stress tolerance. Cancer Cell.
33:985–1003.e7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Derouiche S, Mariot P, Warnier M,
Vancauwenberghe E, Bidaux G, Gosset P, Mauroy B, Bonnal JL,
Slomianny C, Delcourt P, et al: Activation of TRPA1 channel by
antibacterial agent triclosan induces VEGF secretion in human
prostate cancer stromal cells. Cancer Prev Res (Phila). 10:177–187.
2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Osterloh M, Böhm M, Kalbe B, Osterloh S
and Hatt H: Identification and functional characterization of TRPA1
in human myoblasts. Pflugers Arch. 468:321–333. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yin S, Zhang L, Ding L, Huang Z, Xu B, Li
X, Wang P and Mao J: Transient receptor potential ankyrin 1 (trpa1)
mediates il-1β-induced apoptosis in rat chondrocytes via calcium
overload and mitochondrial dysfunction. J Inflamm (Lond).
15(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kurahara LH, Hiraishi K, Hu Y, Koga K,
Onitsuka M, Doi M, Aoyagi K, Takedatsu H, Kojima D, Fujihara Y, et
al: Activation of myofibroblast TRPA1 by steroids and pirfenidone
ameliorates fibrosis in experimental crohn's disease. Cell Mol
Gastroenterol Hepatol. 5:299–318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kheradpezhouh E, Choy JMC, Daria VR and
Arabzadeh E: TRPA1 expression and its functional activation in
rodent cortex. Open Biol. 7(160314)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kittaka H and Tominaga M: The molecular
and cellular mechanisms of itch and the involvement of TRP channels
in the peripheral sensory nervous system and skin. Allergol Int.
66:22–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bölcskei K, Kriszta G, Sághy É, Payrits M,
Sipos É, Vranesics A, Berente Z, Ábrahám H, Ács P, Komoly S and
Pintér E: Behavioural alterations and morphological changes are
attenuated by the lack of TRPA1 receptors in the cuprizone-induced
demyelination model in mice. J Neuroimmunol. 320:1–10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Habek M: Immune and autonomic nervous
system interactions in multiple sclerosis: Clinical implications.
Clin Auton Res. 29:267–275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Richardson RJ, Fink JK, Glynn P, Hufnagel
RB, Makhaeva GF and Wijeyesakere SJ: Neuropathy target esterase
(NTE/PNPLA6) and organophosphorus compound-induced delayed
neurotoxicity (OPIDN). Adv Neurotoxicol. 4:1–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ding Q, Fang S, Chen X, Wang Y, Li J, Tian
F, Xu X, Attali B, Xie X and Gao Z: TRPA1 channel mediates
organophosphate-induced delayed neuropathy. Cell Discov.
3(17024)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Bosson A, Paumier A, Boisseau S,
Jacquier-Sarlin M, Buisson A and Albrieux M: TRPA1 channels promote
astrocytic Ca2+ hyperactivity and synaptic dysfunction
mediated by oligomeric forms of amyloid-β peptide. Mol
Neurodegener. 12(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hajna Z, Sághy É, Payrits M, Aubdool AA,
Szőke É, Pozsgai G, Bátai IZ, Nagy L, Filotás D, Helyes Z, et al:
Capsaicin-sensitive sensory nerves mediate the cellular and
microvascular effects of H2S via TRPA1 receptor activation and
neuropeptide release. J Mol Neurosci. 60:157–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kaneko Y and Szallasi A: Transient
receptor potential (TRP) channels: A clinical perspective. Br J
Pharmacol. 171:2474–2507. 2014.PubMed/NCBI View Article : Google Scholar
|