Heart failure and cancer are two major diseases that
affect human health, and they represent most causes of death and
disability in humans (1,2), The incidence of heart failure and
cancer in oldindividualshas increased quickly in the world
(3,4). In previous studies, epidemiologists
have revealed that cancer treatment makes patients more likely to
suffer from heart failure; this cardio-oncology research focused on
the prevention and treatment of cardiac damage caused by cancer
treatment. Also, the cardiac damage caused by cancer treatment and
treatment of cancer patients with heart disease are discussed in
the present review (Table I)
(3,5-12).
A number of studies have suggested that patients with heart failure
are more likely to have cancer (Table
II) (13-25);
however, the mechanisms and relationships between heart failure and
cancer remain unclear. Certain studies have even confirmed the
presence of a precancerous lesion before carcinogenesis in heart
failure (26,27), and suggested that both heart
failure and cancer are chronic low-level inflammatory diseases
(26). The pathogenesis of heart
failure caused by cancer treatment and the mechanism of cancer
occurrence in patients with heart failure is currently unclear. The
present review outlines the relationship between heart failure and
cancer, and provides clinical strategies towards prevention
according to the pathological mechanism.
Cancer has a high risk of associated cardiac
toxicity, and treatments such as radiation, chemotherapy or
immunosuppressive therapy can also severely affect the heart. At
present, the mechanisms behind cardiac toxicity and
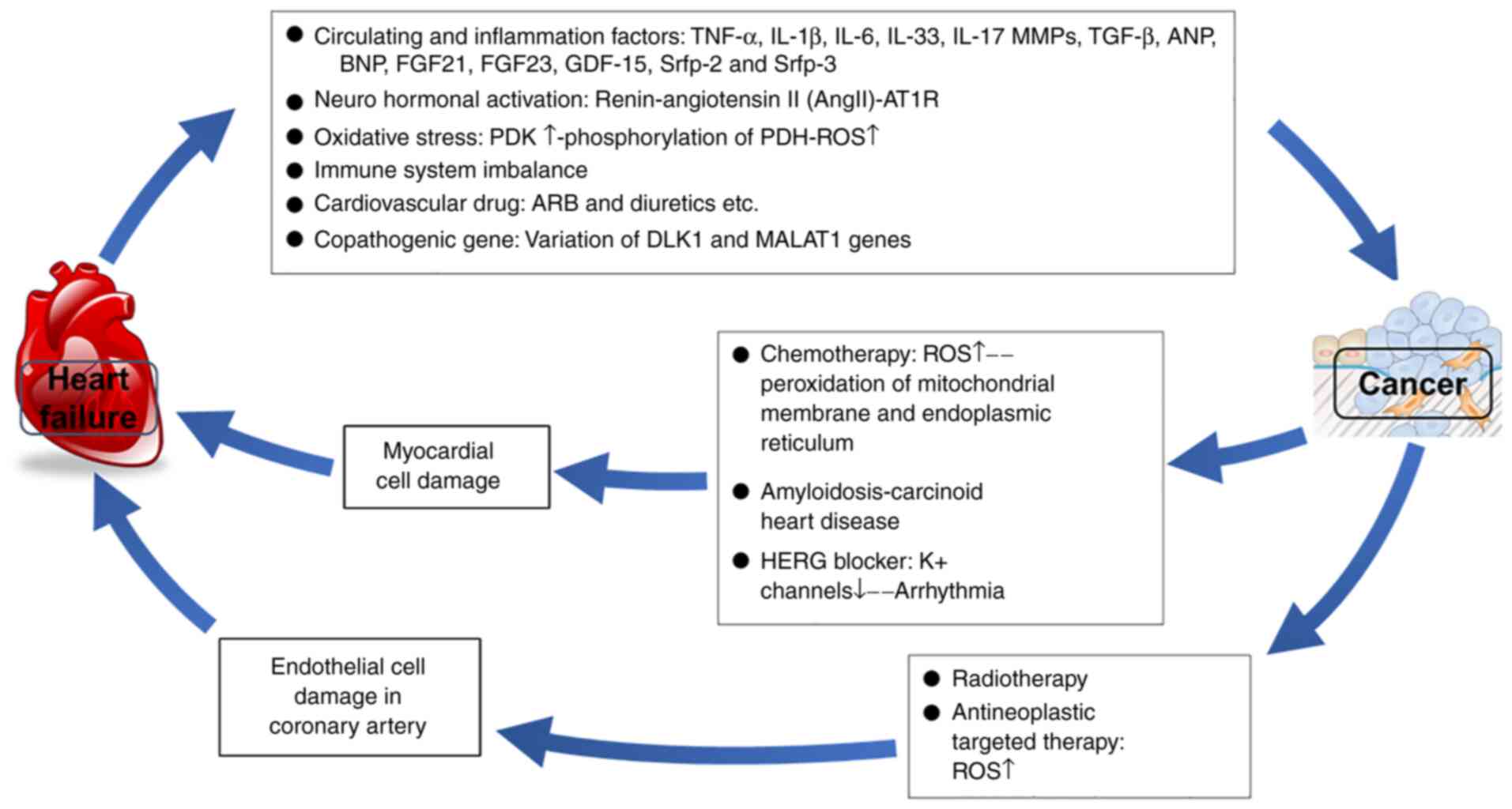
treatment-associated effects are described in Fig. 1 and the following text (28,29).
Anthracyclines are commonly used as chemotherapeutic
drugs for solid and hematological cancer types. Anthracyclines
produce a large number of reactive oxygen free radicals, which
consequently cause myocardial injuries (30). Anthracycline effects induce acute
or chronic cardiotoxicity depending on the dosage of the drug,
ranging from 5% (cumulative dose of 400 mg/m2) to 26%
(cumulative dose of 550 mg/m2) cardiotoxicity (5,9).
However, a study has reported that patients with hematological
diseases treated with low doses of anthracyclines still have
cardiac malfunction (31). This
class of drug has the advantage that after its injection, it is
intercalated into the DNA and blocks the activity of topoisomerase
2, which subsequently inhibits the proliferation of cancerous cells
(32). It has been demonstrated
that cardiac topoisomerase is a key mediator of doxorubicin-induced
cardiotoxicity, which may reduce the efficacy of treatment
(33,34). In fact, doxorubicin induces
apoptosis and DNA damage in a topoisomerase-dependent manner; it
also ultimately affects oxidative phosphorylation and mitochondrial
biogenesis (34). Dexrazoxane, a
topoisomerase inhibitor, is currently used as an effective drug for
preventing and treating heart injury caused by radiotherapy and
chemotherapy (35,36). Other studies previously revealed
that angiotensin-converting enzyme inhibitors (ACEIs) prevented
heart injury, and that, phosphoinositide 3-kinase γ removed damaged
mitochondria in a heart failure model induced by Adriamycin,
suggesting possible treatments to prevent anthracycline-induced
cardiotoxicity (37,38). Radiotherapy is the most common
treatment for breast cancer. Usually, patients who have received
total radiation exposure of >30 Gy, with daily radiation
exposure of >2 Gy, have radiation exposure to the left or front
of the chest; without radiation protection, the heart can easily
manifest symptoms of cardiac damage, including left main coronary
disease and pericarditis (39,40).
One study revealed that radiation therapy can directly cause
myocardial damage through reactive oxygen species (ROS)-induced
activation of Ca2+/calmodulin-dependent protein kinase
II (41). Radiation therapy can
also cause vascular endothelial cell damage, which may contribute
to coronary heart disease (6).
Trastuzumab, a monoclonal antibody against HER2, is
an effective first-line drug for breast cancer (7). By binding to HER2, the trastuzumab
molecule blocks the binding of human epidermal growth factor to
HER2, thereby inhibiting the growth of cancerous cells (7). The cardiotoxicity of trastuzumab
mostly results from symptomatic heart failure or subclinical left
ventricular dysfunction (42).
However, the effect of trastuzumab on the heart is reversible
(43) through the activities of
vascular endothelial growth factor (VEGF), which is an important
regulator of angiogenesis (44).
When cancer metastasizes, VEGF promotes neovascularization to
provide nutrition to the cancer (44). The VEGF gene family consists of
five members, which can activate downstream signaling pathways
after binding to the corresponding VEGF receptor (VEGFR) (44,45);
this phenomenon occurs by blocking the VEGF signaling pathway and
includes the use of anti-VEGF/VEGFR monoclonal antibodies and
VEGFR-tyrosine kinase inhibitor (TKIs). Drugs targeting VEGF
signaling, including humanized anti-VEGF monoclonal antibody,
humanized bevacizumab, TKIs and sorafenib, have certain
cardiovascular side effects such as hypertension, thromboembolism
and cardiomyopathy (44,46). It has been reported that the
administration of bevacizumab combined with anthracyclines
increases the incidence of heart failure from 4 to 14% (47). Meanwhile, VEGF can increase the
release of nitric oxide, facilitate prostacyclin synthesis and
decrease the expression of pro-inflammatory genes such as
cyclooxygenase-2 and E-selectin. This suggests that anti-VEGF
antibodies might cause hypertension and thromboembolic diseases
(48,49). Certain studies have demonstrated
that inhibitors of VEGF can damage endothelial cells and increase
their microparticle production, while the microparticles can
stimulate endothelial cells to generate certain reactions capable
of causing further damage to the endothelial cells; among those
reactions, massive production of endothelin-1, excessive oxidative
stress and inflammatory activation are the most commonly observed
(46,50-52).
In order to improve the safety of TKI drugs, the need for further
studies and an improved understanding of the mechanism of cardiac
injury appears crucial. Finally, cardiotoxicity is also related to
proteasome inhibitors, which are useful for the treatment of
multiple myeloma and other hematological malignancies. According to
a meta-analysis, the second-generation proteasome inhibitor
carfilzomib was associated with higher cardiotoxicity, with an 18%
incidence of cardiovascular adverse events (53,54).
Furthermore, in pigs, inhibition of the ubiquitin-proteasome system
of cardiomyocytes led to decreased cardiac function and the
generation of possible cardiac damage (55).
The human ether-à-go-go-related (HERG) gene belongs
to the voltage-activated outwardly-rectifying EAG family of
K+ channels and is expressed in multiple tissue types,
including cardiac, neural and smooth muscle tissues. HERG loss of
function leads to long QT syndrome (56) and has been demonstrated to
contribute to the occurrence of cancer. Furthermore, transfection
of HERG can induce the malignant transformation of murine
fibroblasts, while HERG blocker (dofetilide) can reverse this
process (57). In previous, HERG
channel antagonists have emerged as new target drugs for cancer
treatment. However, the cardiotoxicity of HERG antagonists remains
the major problem for this method of cancer treatment. HERG
antagonists block the HERG channel, inhibit the proliferation and
migration of cancerous cells, and inhibit the potassium channel of
myocardial cells, resulting in severe arrhythmia (58,59).
The most common arrhythmias are long QT syndrome and ventricular
tachycardia; therefore, designing a drug that can be administered
safely at a reasonable dosage is emerging as the main preventive
strategy for HERG antagonist-related toxicity (60).
Cancer can cause cardiomyopathy, light chain
amyloidosis and carcinoid heart disease. Amyloidosis is a disease
that affects multiple organs, including the myocardium and heart
valves in restrictive cardiomyopathy (61,62).
Heart failure caused by light-chain amyloidosis is severe and might
be related to the direct damage of light-chain amyloidosis in
myocardial cells (8). Studies have
also shown that oxidative stress may cause damage in myocardial
cells (63,64). Currently, the treatment of events
caused by cardiac light-chain amyloidosis is limited to the
treatment of heart failure and related malignant cancer types, and
there is no available treatment for light chain protein deposition
(61). Carcinoid heart disease is
caused by the release of vasoactive mediators, such as serotonin,
bradykinin and histamine. Neuroendocrine tumors (NETs) are a rare
type of cancer found in the gastrointestinal or respiratory tracts.
Mediators released by NETs are inactivated in the liver and
pulmonary blood vessels; therefore, carcinoid heart disease also
occurs in the liver with cancer metastases in the stomach and
intestines, which mainly damages the right ventricle, with
bronchial carcinoids as an outcome (65,66).
Carcinoid heart diseases are characterized by the formation of
fibrotic plaques in the myocardium, which eventually leads to
right-sided heart failure. In addition, fibrotic remodeling of the
tricuspid valve results in regurgitation by the valve, leading to
decompensation of the right ventricle. Medical treatment of
carcinoid syndrome is limited to somatostatin analogs, but this
treatment is not effective for the heart muscle itself or valvular
disease (67). Clinically,
although amyloidosis and carcinoid heart disease are the only forms
of heart failure caused by cancer, it has been demonstrated in
other studies that certain cancer types may affect cardiac function
by releasing cardiac toxic cancer-related metabolites (68,69).
In rats, this mutation stimulates the accumulation and release of
D-2-hydroxyglutarate, which impairs Krebs cycle activity in the
heart and inhibits contractile function (70).
In the last decade, epidemiology studies have
reported a high incidence of cancer in patients with heart failure
(71,72). However, these studies only indicate
the relationship between cancer and heart failure; meanwhile, its
related mechanism is still unclear. Possible mechanisms that
interfere in such relationships (73) are shown in Fig. 1 and described in the following
text.
As an endocrine organ, during heart failure, the
heart can secrete a number of circulating factors, including B-type
natriuretic peptide, which can be used for the diagnosis/risk
stratification and prognosis of heart failure (1,74).
However, various cancer-generated circulating factors influence
surrounding organs (75,76). In heart failure combined with
cancer, increased secretion of important factors, including tumor
necrosis factor, interleukin (IL)-6, IL-1 and VEGF, occurs.
Numerous studies have shown that heart failure stimulates cancer
growth. For example, Meijers et al (27) demonstrated that heart failure
enhanced cancer growth in adenomatous polyposis coli mice. Compared
with that in sham-operated mice, the number and size of the tumors
in mice with heart failure increased by three-fold. The occurrence
and development of cancer is related to cardiac remodeling markers
such as left ventricular ejection fraction (LVEF) and myocardial
fibrosis. In order to further verify these findings, Meijers et
al (27) established a
hemodynamic injury-free model and found that heart failure
accelerated cancer growth. This suggested that heart failure
stimulating cancer growth is not related to the myocardial
infarction model, while some circulating factors secreted by the
heart itself during heart failure may stimulate cancer growth
(27). Certain proteomics studies
discovered that several circulating protein factors were secreted
into the blood during the occurrence of heart failure (77,78);
those protein factors might have various effects on colon tissue
in vitro. Among them, α-1-antichymotrypsin (SerpinA3)
promotes cancer growth by phosphorylating Akt and ribosomal protein
s6 in vitro (27). A
community cohort study with a total of 8,592 subjects showed that
over a follow-up time of 12 years, 1,132 subjects (13.1%) were
diagnosed with cancer, and among these, 132 (11.7%) were diagnosed
with colorectal cancer (27). The
N-terminal pro-B-type natriuretic peptide is an independent risk
factor for colorectal cancer in patients with heart failure, and
the risk of cancer increases with increasing concentration of the
peptide (27,79). Together, these studies indicate
that the secretion of certain biomarkers produced by the heart is
not only a signal of myocardial injury, but also affects the growth
of distant cancer, possibly through cardiac exocrine effects. In
addition, Bertero et al (26) conducted a study focusing on
underlying mechanisms such as inflammation and neurohormones, which
provided some preliminary evidence that heart failure could result
from the adaptation of the body's environment to the onset or
development of cancer.
Activation of the renin-angiotensin-aldosterone
system (RAAS) is one of the central compensatory homeostatic
responses in patients with heart failure. RAAS activation functions
to maintain blood pressure and cardiac output; however, chronic
activation of RAAS can have deleterious effects on the heart,
kidneys and blood vessels (80).
In addition to systemic RAAS, most organ systems such as that of
the heart, blood vessels and kidneys, and even cancerous cells,
have local RAAS. The RAAS has different functions, hormones and
receptors depending on its locality (81); for example, increased expression of
angiotensin II receptor type 1 (AT1R) in cancerous cells suggests
strong cancer aggressiveness and a poor prognosis (82). The regulation of RAAS may also
affect the tumor size, although the results are inconsistent:
Specifically, the angiotensin II (AngII)/AT1R axis is hypothesized
to enhance tumor growth, while the AngII/AT2R signal serves the
opposite role (83,84). RAAS inhibitors such as ACEIs or
angiotensin II receptor blockers (ARBs) represent the cornerstone
of heart failure treatment (85).
The ROS family is the key element for oxidative
stress in eukaryotic cells. The heart inputs and outputs a
consistent amount of energy and mainly relies on oxidative
phosphorylation of mitochondria. ROS serve an important role in
heart failure and cancer (86,87);
however, oxidative phosphorylation of mitochondria also serves an
important role in cancer development (88). Studies have found that dietary
fiber supplementation has positive effects on heart oxidative
stress responses (89,90). In addition, glycolysis increases
the probability of heart failure and glucose oxidation leads to
lactic acid production. Also, in response to rapid cancer growth,
pyruvate dehydrogenase (PDH) and PDH kinase (PDK) play a major role
in mitochondrial oxidative metabolism, which leads to increased
glycolysis. PDH inhibits glucose oxidation and converts pyruvate to
acetyl-CoA (91). PDK can
phosphorylate and inhibit PDH. During heart failure, PDK is
upregulated, but PDH is inhibited (91). Similar mechanisms for PDK
upregulation and PDH inhibition are also present in cancerous cells
(92). Dichloro-acetate, a PDK
inhibitor, enhances PDH activity during heart failure, decreases
ischemic damage and improves cardiac function; these changes
consequently decrease the incidence/development of cancer (92).
Inflammation is closely related to heart failure.
Heart failure increases inflammatory factor secretion, which
supports the premise that inflammation leads to heart failure
(93). Increased secretion of
inflammatory factors during heart failure can cause bone marrow
dysfunction. However, there is no direct evidence that
proinflammatory cytokines released by cardiac cells affect cancer
cells. Furthermore, Meijers et al (27) found that certain inflammatory
factors, such as high-sensitivity C-reactive protein and central
adrenomedullin precursors, are predictors or warning signs of
cancers. The IL-1 inhibitor canakinumab decreased major
cardiovascular events by 25% [hazard ratio (HR), 0.75; 95%
confidence interval (CI), 0.66-0.85] in patients with myocardial
infarction (94). Canakinumab also
significantly decreased the incidence and mortality rate of lung
cancer [highest dose (300 mg): HR, 0.33; 95% CI, 0.18-0.59;
P<0.0001; and HR, 0.23; 95% CI, 0.10-0.54; P=0.0002,
respectively] (95).
Immune system dysfunction is closely related to the
occurrence and development of cancer and heart failure (96,97).
In the early stage of body damage, a large number of immune cells
are beneficial; such cells are able to decrease and repair the area
damaged by injury, but chronic immune activation will generate
severe side effects in the body, such as severe or even fatal
allergic reactions (98). A
complete overview of immune system dysfunction and heart failure
has recently been published by the Working Group on Myocardial
Function of the European Society of Cardiology (99). It is important to note that the
pathogenesis of heart failure is particular. As well as the
differing pathophysiological mechanisms of heart disease, the
immune activation methods of heart disease also vary. For example,
during the first stage of myocardial infarction (a few hours),
neutrophils invade the heart and start the inflammatory response
immediately; furthermore, the infiltration of macrophages breaks
down necrotic tissue and promotes scar formation. In the next stage
of remodeling, the inflammatory response is weakened; the secreted
cytokines will regulate the invasion of inflammatory cells after
myocardial infarction (100-102).
Heart failure with a normal ejection fraction is mostly due to
obesity, hypertension, diabetes and metabolic syndrome (103). Recent studies have revealed that
the immune system may also play a certain role in heart failure
with a normal ejection system, in this case, cardiac hypertrophy
and fibrosis often occur. In heart failure with ejection fraction
retention, macrophages are involved in the process of cardiomyocyte
apoptosis and cardiomyocyte fibrosis, but a decrease in macrophages
can reduce myocardial hypertrophy. Immune system dysfunction is
related to the development of cancer, as cancers can spread to
different organs by weakening the immune system (104).
To date, the impact of cardiovascular drugs on
cancer is still unclear. A number ofmeta-analyses on all types of
antihypertensive drugs showed that the use of ARB, ACEIs,
β-receptor blockers, diuretics and calcium channel blockers has
relatively increased the incidence of cancer and the risk
associated with cancer death by 5.0-10.0% (105,106). However, some meta-analyses have
confirmed that antihypertensive drugs are not associated with
carcinogenesis and development (107,108). Studies on patients with type 2
diabetes showed a negative correlation between losartan and cancer
risk; however, overall, candesartan and telmisartan resulted in an
increased rate of cancer incidence (109). Another drug that affects cancer
is aspirin. A study has found that the use of a low dose of aspirin
results in the acceleration of the progression of cancer in older
individuals (≥70 years old), potentially because aspirin inhibits
antitumor inflammatory or immune responses, which regulate later
stage growth and metastasis (110).
Myocardial fibrosis leads to cardiac remodeling,
promoting heart failure. It has been indicated that the delta like
non-canonical notch ligand 1 (DLK1) gene is a key factor during the
differentiation of fibroblasts into myoblasts (111). The knockdown of the DLK1 gene
leads to the downregulation of microRNA-370 (miR-370), activates
the TGF-β/Smad3 pathway and promotes myocardial cell fibrosis
(112). Excessive deposition of
extracellular matrix infiltrated by myofibroblasts can cause
cardiac dysfunction (112).
However, DLK1 is a type of imprinted gene that participates in the
regulation of the differentiation of a variety of cells; its
expression is increased in a number of cancer types, such as liver,
pancreatic and colorectal cancer. Therefore, this gene plays an
important role in carcinogenesis and cancer development (113). Sialyl-Lewis X (sLex) is the
smallest recognition motif of the P-selectin ligand, which plays an
important role in the adhesion and migration of cancerous cells. A
study has found that miR-370 can specifically inhibit sLex
expression and inhibit cell adhesion in colo-320 cells (114), justifying the fact that,
inhibition of the DLK1 gene and downregulation of miR-370 lead to
myocardial fibrosis/heart failure and cancer metastasis. Thus, some
common targets might exist for heart failure and cancer. The
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)
gene has previously been shown to be involved in the proliferation,
metastasis and function of cancerous cells and the reproduction of
endothelial and smooth muscle cells (115,116). Studies have found that MALAT1 is
the key regulatory factor of mouse atherosclerosis, where its
knockout can significantly increase coronary plaques and affected
area (117). Also, a decrease in
MALAT1 expression in patients with coronary plaques is associated
with a poor prognosis (including heart failure, arrhythmia and
sudden death) (118). At present,
there is no study to determine which genes are directly related to
heart failure and cancer, thus identification and characterization
of genes involved in both cancer and heart failure is required to
improve cancer therapeutic methods in the future.
From the perspective of pathogenesis, the main cause
of heart failure in patients with cancer appears to be cardiac
toxicity caused by cancer-related treatment. At present, the
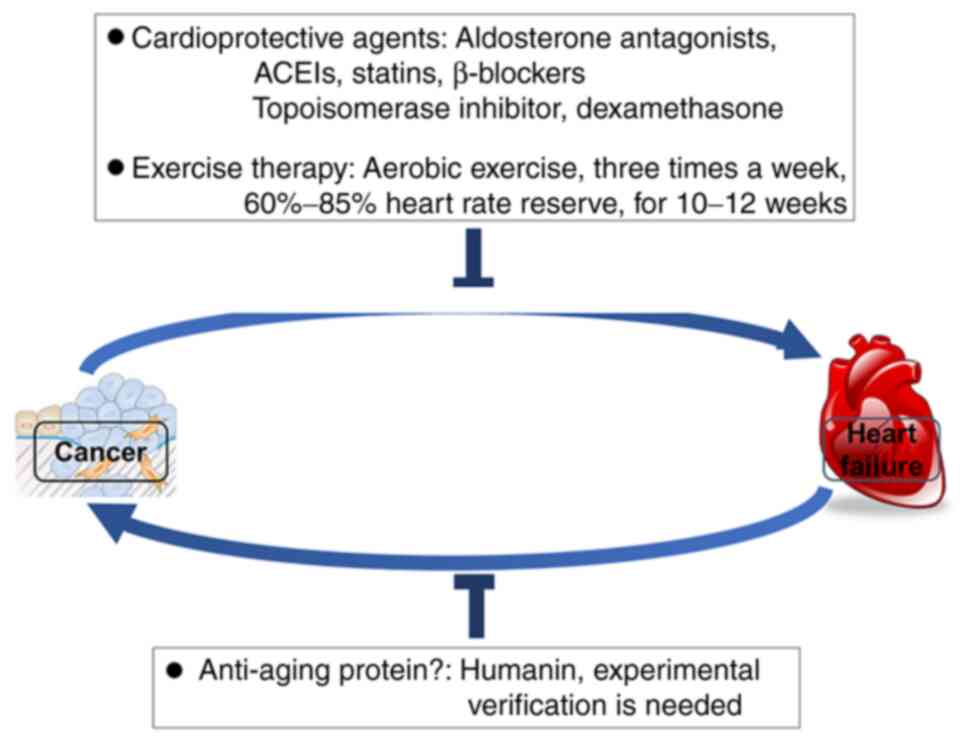
protective measures for such injury mainly include two schemes: The
use of cardioprotective agents and standardized rehabilitation
exercise (Fig. 2). The
cardioprotective agents mainly include the use of traditional
drugs, such as ACEI, to inhibit myocardial remodeling and
topoisomerase inhibitors. In a recent meta-analysis, 15 patients
within randomized controlled trials were selected to analyze the
protective effect of myocardial remodeling drugs on preventing
cardiac toxicity induced by cancer treatment. The study found that
aldosterone antagonists, ACEIs, statins and β-blockers could
substantially improve left ventricular systolic function, while
ARBs displayed no cardioprotective effect and failed to improve the
left ventricular systolic function (measured as LVEF) (119). However, another study proposed
that ARBs are effective in the prevention of heart failure. The
study found that patients administered acetyl-based chemotherapy
had a moderate yet significant benefit in terms of LVEF following
use of β-blockers or ACEIs/ARBs. The β-blocker analysis included
769 patients with cancer, and the ACEI/ARB analysis included a
total of 581 patients with cancer. The mean LVEF difference between
ACEIs and ARBs groups was 4.71% (120). Topoisomerase is a new target to
prevent cancer treatment-related cardiotoxicity, and dexamethasone
and other topoisomerase inhibitors inhibit topoisomerase II
(121). It has been reported that
dapagliflozin protects against doxorubicin-induced cardiotoxicity
in patients with breast cancer and diabetes. Moreover,
dapagliflozin inhibits doxorubicin-induced myocardial fibrosis and
greatly improves cardiac function by inhibiting the apoptosis of
cardiomyocytes and the generation of ROS (122). Therefore, topoisomerase
inhibitors and dapagliflozin can protect the heart from the
toxicity of chemotherapy drugs by inhibiting myocardial
remodeling.
Exercise therapy is a new treatment for
cancer-related heart failure. Cardiorespiratory fitness (CRF) is
closely related to the prognosis of patients with heart failure.
CRF decreases with age, and short-term (12- to 26-week) anticancer
therapy can reduce CRF by 26% (123). The maximum oxygen consumption
rate represents the extent of CRF, which can be improved by
exercise therapy in patients with cancer-related heart failure.
MacVicar et al (124)
formulated an intermittent aerobic exercise prescription for 45
patients with breast cancer who received different chemotherapy
regimens. This treatment recommended exercise three times a week at
60-80% of the normal maximum heart rate, for 10 weeks. The
VO2 peak average of patients receiving this exercise
prescription was increased by 40% compared with that of the
non-exercise group. In another randomized controlled study, 20
patients with advanced breast cancer were randomly divided into two
groups: The chemotherapy group and the chemotherapy + aerobic
exercise group. After 12 weeks, the VO2 peak of the
chemotherapy group decreased by 9%, while the VO2 peak
of the chemotherapy + exercise group increased by 13% (125).
In addition to findings in breast cancer studies,
another study found that exercise therapy was also effective for
prostate cancer and Hodgkin's lymphoma, among others (126). Non-linear aerobic exercise could
maintain the VO2 peak in patients with prostate cancer,
while it could increase the VO2 peak in patients with
Hodgkin's lymphoma from 5 to 17% (126). However, the impact of exercise
therapy on the prognosis of cancer-related heart failure patients
is controversial. In the follow-up period of 35 months, one study
revealed that the all-cause mortality and readmission rate
increased in the exercise group compared with that in the
non-exercise group, and the VO2 peak showed no
significant difference between the two groups (127). However, this result needs to be
further confirmed due to the lack of a long-term exercise therapy
group as a control. Other studies have previously shown that
exercise therapy can improve the VO2 peak and the
short-term prognosis in patients with cancer-related heart failure
(128,129). Therefore, non-linear aerobic
exercise is the recommended exercise for patients with
cancer-related heart failure. It was advised that the patients keep
non-linear aerobic exercise three times a week for 10-12 weeks and
the exercise intensity was 60-85% of the normal maximum heart rate
(128,129).
There are currently no drugs or treatments that can
prevent cancer in patients with heart failure. As the mechanism by
which patients with heart failure are more likely to develop cancer
is known, the inhibition of excessive inflammation during
myocardial remodeling can be a good asset (Fig. 2). Both heart failure and cancer are
aging-related diseases. Rochette et al (130) found that the anti-aging protein
humanin (HN), which is a 24-amino acid, endogenous,
mitochondrial-derived peptide, can inhibit myocardial remodeling
and inflammation. Studies have found that HN can protect
cardiomyocytes through anti-oxidative stress (131,132); furthermore, Qin et al
(133) demonstrated that the
exogenous injection of HN analogs could inhibit age-related
myocardial fibrosis, while HN was able to inhibit cancer
metastasis. In fact, it has been revealed that HN was able to
inhibit the lung metastasis of mouse melanoma cancer cells
(134). However, whether HN can
prevent cancer in patients with chronic heart failure is currently
debated. Large-scale clinical randomized controlled trials and
animal studies are needed to prove its effectiveness in the
future.
With the increase in anticancer drugs discoveries
and prescriptions, the incidence of cancer-related heart disease
has recently increased. Heart failure affects the development of
cancer through a variety of mechanisms. Therefore, cancer and heart
failure are related and interact with each other, by sharing some
usual risks, such as hypertension, diabetes mellitus and obesity,
and even pathogenic genes. The pathophysiological mechanism of
heart failure and cancer remains to be explored in depth.
Currently, preventive strategies are limited to heart failure in
patients with cancer. Further clinical trials are required to
determine how to prevent patients with heart failure from suffering
from cancer.
Not applicable.
No funding was received.
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
YZ conceived the study. HC and YZ wrote the
manuscript. HM, PC, HC and YZ revised and edited the manuscript.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Christ M, Störk S, Dörr M, Heppner HJ,
Müller C, Wachter R and Riemer U: Trend HF Germany Project. Heart
failure epidemiology 2000-2013: Insights from the German federal
health monitoring system. Eur J Heart Fail. 18:1009–1018.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Armenian SH, Xu L, Ky B, Sun C, Farol LT,
Pal SK, Douglas PS, Bhatia S and Chao C: Cardiovascular disease
among survivors of adult-onset cancer: A community-based
retrospective cohort study. J Clin Oncol. 34:1122–1130.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Choi HM, Park MS and Youn JC: Update on
heart failure management and future directions. Korean J Intern
Med. 34:11–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saiki H, Petersen IA, Scott CG, Bailey KR,
Dunlay SM, Finley RR, Ruddy KJ, Yan E and Redfield MM: Risk of
heart failure with preserved ejection fraction in older women after
contemporary radiotherapy for breast cancer. Circulation.
135:1388–1396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Quarta CC, Solomon SD, Uraizee I, Kruger
J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C and Falk
RH: Left ventricular structure and function in
transthyretin-related versus light-chain cardiac amyloidosis.
Circulation. 129:1840–1849. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van Nimwegen FA, Schaapveld M, Janus CP,
Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM and van
Leeuwen FE: Cardiovascular disease after Hodgkin lymphoma
treatment: 40-year disease risk. JAMA Intern Med. 175:1007–1017.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Laufer-Perl M, Mor L, Milwidsky A,
Derakhshesh M, Amrami N, Moshkovits Y, Arnold J, Topilsky Y, Arbel
Y and Rozenbaum Z: Cancer therapeutics-related cardiac dysfunction
among patients with active breast cancer: A cardio-oncology
registry. Isr Med Assoc J. 22:564–568. 2020.PubMed/NCBI
|
|
11
|
Tian Z, Yang Y, Yang Y, Zhang F, Li P,
Wang J, Yang J, Zhang P, Yao W and Wang X: High cumulative
doxorubicin dose for advanced soft tissue sarcoma. BMC Cancer.
20(1139)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Degens J, De Ruysscher D, Houben R,
Kietselaer B, Bootsma G, Hendriks L, Huijbers E, Schols A and
Dingemans AC: Are patients with stage III non-small cell lung
cancer treated with chemoradiotherapy at risk for cardiac events?
Results from a retrospective cohort study. BMJ Open.
10(e036492)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kjekshus J, Apetrei E, Barrios V, Böhm M,
Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P,
et al: Rosuvastatin in older patients with systolic heart failure.
N Engl J Med. 357:2248–2261. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Linde C, Abraham WT, Gold MR, St John
Sutton M, Ghio S and Daubert C: REVERSE (REsynchronization reVErses
Remodeling in Systolic left vEntricular dysfunction) Study Group.
Randomized trial of cardiac resynchronization in mildly symptomatic
heart failure patients and in asymptomatic patients with left
ventricular dysfunction and previous heart failure symptoms. J Am
Coll Cardiol. 52:1834–1843. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moss AJ, Hall WJ, Cannom DS, Klein H,
Brown MW, Daubert JP, Estes NA III, Foster E, Greenberg H, Higgins
SL, et al: Cardiac-resynchronization therapy for the prevention of
heart-failure events. N Engl J Med. 361:1329–1338. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ruschitzka F, Abraham WT, Singh JP, Bax
JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J III, Gras
D, et al: Cardiac-resynchronization therapy in heart failure with a
narrow QRS complex. N Engl J Med. 369:1395–1405. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Doval HC, Nul DR, Grancelli HO, Perrone
SV, Bortman GR and Curiel R: Randomised trial of low-dose
amiodarone in severe congestive heart failure. Grupo de Estudio de
la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA).
Lancet. 344:493–498. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bigger JT Jr: Prophylactic use of
implanted cardiac defibrillators in patients at high risk for
ventricular arrhythmias after coronary-artery bypass graft surgery.
Coronary artery bypass graft (CABG) patch trial investigators. N
Engl J Med. 337:1569–1575. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kadish A, Dyer A, Daubert JP, Quigg R,
Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A,
et al: Prophylactic defibrillator implantation in patients with
nonischemic dilated cardiomyopathy. N Engl J Med. 350:2151–2158.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Granger CB, McMurray JJ, Yusuf S, Held P,
Michelson EL, Olofsson B, Ostergren J, Pfeffer MA and Swedberg K:
CHARM Investigators and Committees. Effects of candesartan in
patients with chronic heart failure and reduced left-ventricular
systolic function intolerant to angiotensin-converting-enzyme
inhibitors: The CHARM-alternative trial. Lancet. 362:772–776.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McMurray JJ, Ostergren J, Swedberg K,
Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S and Pfeffer
MA: CHARM Investigators and Committees. Effects of candesartan in
patients with chronic heart failure and reduced left-ventricular
systolic function taking angiotensin-converting-enzyme inhibitors:
The CHARM-added trial. Lancet. 362:767–771. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roy D, Talajic M, Nattel S, Wyse DG,
Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, et
al: Rhythm control versus rate control for atrial fibrillation and
heart failure. N Engl J Med. 358:2667–2677. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tavazzi L, Maggioni AP, Marchioli R,
Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M and
Tognoni G: Gissi-HF Investigators. Effect of n-3 polyunsaturated
fatty acids in patients with chronic heart failure (the GISSI-HF
trial): A randomised, double-blind, placebo-controlled trial.
Lancet. 372:1223–1230. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Velazquez EJ, Lee KL, Deja MA, Jain A,
Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et
al: Coronary-artery bypass surgery in patients with left
ventricular dysfunction. N Engl J Med. 364:1607–1616.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bertero E, Canepa M, Maack C and Ameri P:
Linking heart failure to cancer: Background evidence and research
perspectives. Circulation. 138:735–742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meijers WC, Maglione M, Bakker SJL,
Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon
AR, van der Vegt B, et al: Heart failure stimulates tumor growth by
circulating factors. Circulation. 138:678–691. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bertero E, Ameri P and Maack C:
Bidirectional relationship between cancer and heart failure: Old
and new issues in cardio-oncology. Card Fail Rev. 5:106–111.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ausoni S and Azzarello G: Development of
cancer in patients with heart failure: How systemic inflammation
can lay the groundwork. Front Cardiovasc Med.
7(598384)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tan C, Tasaka H, Yu KP, Murphy ML and
Karnofsky DA: Daunomycin, an antitumor antibiotic, in the treatment
of neoplastic disease. Clinical evaluation with special reference
to childhood leukemia. Cancer. 20:333–353. 1967.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vandecruys E, Mondelaers V, De Wolf D,
Benoit Y and Suys B: Late cardiotoxicity after low dose of
anthracycline therapy for acute lymphoblastic leukemia in
childhood. J Cancer Surviv. 6:95–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Frederick CA, Williams LD, Ughetto G, van
der Marel GA, van Boom JH, Rich A and Wang AH: Structural
comparison of anticancer drug-DNA complexes: Adriamycin and
daunomycin. Biochemistry. 29:2538–2549. 1990.PubMed/NCBI
|
|
33
|
Martin E, Thougaard AV, Grauslund M,
Jensen PB, Bjorkling F, Hasinoff BB, Tjørnelund J, Sehested M and
Jensen LH: Evaluation of the topoisomerase II-inactive
bisdioxopiperazine ICRF-161 as a protectant against
doxorubicin-induced cardiomyopathy. Toxicology. 255:72–79.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jones RL: Utility of dexrazoxane for the
reduction of anthracycline-induced cardiotoxicity. Expert Rev
Cardiovasc Ther. 6:1311–1317. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hasinoff BB, Patel D and Wu X: The role of
topoisomerase IIβ in the mechanisms of action of the doxorubicin
cardioprotective agent dexrazoxane. Cardiovasc Toxicol. 20:312–320.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abd El-Aziz MA, Othman AI, Amer M and
El-Missiry MA: Potential protective role of angiotensin-converting
enzyme inhibitors captopril and enalapril against
adriamycin-induced acute cardiac and hepatic toxicity in rats. J
Appl Toxicol. 21:469–473. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li M, Sala V, De Santis MC, Cimino J,
Cappello P, Pianca N, Di Bona A, Margaria JP, Martini M, Lazzarini
E, et al: Phosphoinositide 3-kinase gamma inhibition protects from
anthracycline cardiotoxicity and reduces tumor growth. Circulation.
138:696–711. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Paris F, Fuks Z, Kang A, Capodieci P, Juan
G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C and Kolesnick
R: Endothelial apoptosis as the primary lesion initiating
intestinal radiation damage in mice. Science. 293:293–297.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Orzan F, Brusca A, Conte MR, Presbitero P
and Figliomeni MC: Severe coronary artery disease after radiation
therapy of the chest and mediastinum: Clinical presentation and
treatment. Br Heart J. 69:496–500. 1993.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sag CM, Wolff HA, Neumann K, Opiela MK,
Zhang J, Steuer F, Sowa T, Gupta S, Schirmer M, Hünlich M, et al:
Ionizing radiation regulates cardiac Ca handling via increased ROS
and activated CaMKII. Basic Res Cardiol. 108(385)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
De Keulenaer GW, Doggen K and Lemmens K:
The vulnerability of the heart as a pluricellular paracrine organ:
Lessons from unexpected triggers of heart failure in targeted ErbB2
anticancer therapy. Circ Res. 106:35–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Suter TM, Procter M, van Veldhuisen DJ,
Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin
C, Klijn JG, et al: Trastuzumab-associated cardiac adverse effects
in the herceptin adjuvant trial. J Clin Oncol. 25:3859–3865.
2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410.
2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Carlsson L, Ronquist G, Elisasson R,
Dubois L, Ronquist KG and Larsson A: High concentrations of the
angiogenic peptide VEGF-A in seminal fluid and its association to
prostasomes. Clin Lab. 62:1515–1520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Elice F, Jacoub J, Rickles FR, Falanga A
and Rodeghiero F: Hemostatic complications of angiogenesis
inhibitors in cancer patients. Am J Hematol. 83:862–270.
2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cobleigh MA, Langmuir VK, Sledge GW,
Miller KD, Haney L, Novotny WF, Reimann JD and Vassel A: A phase
I/II dose-escalation trial of bevacizumab in previously treated
metastatic breast cancer. Semin Oncol. 30 (5 Suppl 16):S117–S124.
2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kappers MH, van Esch JH, Sluiter W,
Sleijfer S, Danser AH and van den Meiracker AH: Hypertension
induced by the tyrosine kinase inhibitor sunitinib is associated
with increased circulating endothelin-1 levels. Hypertension.
56:675–681. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li W, Croce K, Steensma DP, McDermott DF,
Ben-Yehuda O and Moslehi J: Vascular and metabolic implications of
novel targeted cancer therapies: Focus on kinase inhibitors. J Am
Coll Cardiol. 66:1160–1178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Neves KB, Rios FJ, Jones R, Evans T,
Montezano AC and Touyz RM: Microparticles from vascular endothelial
growth factor pathway inhibitor-treated cancer patients mediate
endothelial cell injury. Cardiovasc Res. 115:978–988.
2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maurea N, Coppola C, Piscopo G, Galletta
F, Riccio G, Esposito E, De Lorenzo C, De Laurentiis M, Spallarossa
P and Mercuro G: Pathophysiology of cardiotoxicity from target
therapy and angiogenesis inhibitors. J Cardiovasc Med (Hagerstown).
17 (Suppl 1):S19–S26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Schmidinger M, Zielinski CC, Vogl UM,
Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M and Schmidinger H:
Cardiac toxicity of sunitinib and sorafenib in patients with
metastatic renal cell carcinoma. J Clin Oncol. 26:5204–5212.
2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Richardson PG, Sonneveld P, Schuster MW,
Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D,
Lonial S, Goldschmidt H, et al: Bortezomib or high-dose
dexamethasone for relapsed multiple myeloma. N Engl J Med.
352:2487–2498. 2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Waxman AJ, Clasen S, Hwang WT, Garfall A,
Vogl DT, Carver J, O'Quinn R, Cohen AD, Stadtmauer EA, Ky B and
Weiss BM: Carfilzomib-associated cardiovascular adverse events: A
systematic review and meta-analysis. JAMA Oncol.
4(e174519)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Herrmann J, Wohlert C, Saguner AM, Flores
A, Nesbitt LL, Chade A, Lerman LO and Lerman A: Primary proteasome
inhibition results in cardiac dysfunction. Eur J Heart Fail.
15:614–623. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Vandenberg JI, Perry MD, Perrin MJ, Mann
SA, Ke Y and Hill AP: hERG K(+) channels: Structure, function, and
clinical significance. Physiol Rev. 92:1393–1478. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pier DM, Shehatou GS, Giblett S, Pullar
CE, Trezise DJ, Pritchard CA, Challiss RA and Mitcheson JS:
Long-term channel block is required to inhibit cellular
transformation by human ether-à-go-go-related gene (hERG1)
potassium channels. Mol Pharmacol. 86:211–221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Raschi E, Vasina V, Poluzzi E and De Ponti
F: The hERG K+ channel: Target and antitarget strategies in drug
development. Pharmacol Res. 57:181–195. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Stansfeld PJ, Sutcliffe MJ and Mitcheson
JS: Molecular mechanisms for drug interactions with hERG that cause
long QT syndrome. Expert Opin Drug Metab Toxicol. 2:81–94.
2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
He S, Moutaoufik MT, Islam S, Persad A, Wu
A, Aly KA, Fonge H, Babu M and Cayabyab FS: HERG channel and
cancer: A mechanistic review of carcinogenic processes and
therapeutic potential. Biochim Biophys Acta Rev Cancer.
1873(188355)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Falk RH, Alexander KM, Liao R and Dorbala
S: AL (Light-Chain) cardiac amyloidosis: A review of diagnosis and
therapy. J Am Coll Cardiol. 68:1323–1341. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gertz MA, Benson MD, Dyck PJ, Grogan M,
Coelho T, Cruz M, Berk JL, Plante-Bordeneuve V, Schmidt HHJ and
Merlini G: Diagnosis, prognosis, and therapy of transthyretin
amyloidosis. J Am Coll Cardiol. 66:2451–2466. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Brenner DA, Jain M, Pimentel DR, Wang B,
Connors LH, Skinner M, Apstein CS and Liao R: Human amyloidogenic
light chains directly impair cardiomyocyte function through an
increase in cellular oxidant stress. Circ Res. 94:1008–1010.
2004.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sawyer DB, Siwik DA, Xiao L, Pimentel DR,
Singh K and Colucci WS: Role of oxidative stress in myocardial
hypertrophy and failure. J Mol Cell Cardiol. 34:379–388.
2002.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lundin L, Norheim I, Landelius J, Oberg K
and Theodorsson-Norheim E: Carcinoid heart disease: Relationship of
circulating vasoactive substances to ultrasound-detectable cardiac
abnormalities. Circulation. 77:264–269. 1988.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Modlin IM and Sandor A: An analysis of
8305 cases of carcinoid tumors. Cancer. 79:813–829. 1997.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hassan SA, Banchs J, Iliescu C, Dasari A,
Lopez-Mattei J and Yusuf SW: Carcinoid heart disease. Heart.
103:1488–1495. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yeh ET, Tong AT, Lenihan DJ, Yusuf SW,
Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA and Ewer
MS: Cardiovascular complications of cancer therapy: Diagnosis,
pathogenesis, and management. Circulation. 109:3122–3131.
2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
McKenney AS and Levine RL: Isocitrate
dehydrogenase mutations in leukemia. J Clin Invest. 123:3672–3677.
2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Karlstaedt A, Zhang X, Vitrac H, Harmancey
R, Vasquez H, Wang JH, Goodell MA and Taegtmeyer H: Oncometabolite
d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and
contractile function in rodent heart. Proc Natl Acad Sci USA.
113:10436–10441. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rinde LB, Småbrekke B, Hald EM, Brodin EE,
Njølstad I, Mathiesen EB, Løchen ML, Wilsgaard T, Brækkan SK, Vik A
and Hansen JB: Myocardial infarction and future risk of cancer in
the general population-the Tromsø study. Eur J Epidemiol.
32:193–201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Berton G, Cordiano R, Cavuto F, Bagato F,
Segafredo B and Pasquinucci M: Neoplastic disease after acute
coronary syndrome: Incidence, duration, and features: The
ABC-4* study on heart disease. J Cardiovasc Med
(Hagerstown). 19:546–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
de Boer RA, Meijers WC, van der Meer P and
van Veldhuisen DJ: Cancer and heart disease: Associations and
relations. Eur J Heart Fail. 21:1515–1525. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Arab S, Gramolini AO, Ping P, Kislinger T,
Stanley B, van Eyk J, Ouzounian M, MacLennan DH, Emili A and Liu
PP: Cardiovascular proteomics: Tools to develop novel biomarkers
and potential applications. J Am Coll Cardiol. 48:1733–1741.
2006.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Isaac DL: Biomarkers in heart failure
management. Curr Opin Cardiol. 23:127–133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Dewey CM, Spitler KM, Ponce JM, Hall DD
and Grueter CE: Cardiac-secreted factors as peripheral metabolic
regulators and potential disease biomarkers. J Am Heart Assoc.
5(e003101)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pavo N, Raderer M, Hülsmann M, Scheithauer
W, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG,
Hejna M, et al: Cardiovascular biomarkers in patients with cancer
and their association with all-cause mortality. Heart.
101:1874–1880. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Tuñón J, Higueras J, Tarín N, Cristóbal C,
Lorenzo Ó, Blanco-Colio L, Martín-Ventura JL, Huelmos A, Alonso J,
Aceña Á, et al: N-terminal pro-brain natriuretic peptide is
associated with a future diagnosis of cancer in patients with
coronary artery disease. PLoS One. 10(e0126741)2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
de Boer RA, Daniels LB, Maisel AS and
Januzzi JL Jr: State of the Art: Newer biomarkers in heart failure.
Eur J Heart Fail. 17:559–569. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hartupee J and Mann DL: Neurohormonal
activation in heart failure with reduced ejection fraction. Nat Rev
Cardiol. 14:30–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
George AJ, Thomas WG and Hannan RD: The
renin-angiotensin system and cancer: Old dog, new tricks. Nat Rev
Cancer. 10:745–759. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ino K, Shibata K, Kajiyama H, Yamamoto E,
Nagasaka T, Nawa A, Nomura S and Kikkawa F: Angiotensin II type 1
receptor expression in ovarian cancer and its correlation with
tumour angiogenesis and patient survival. Br J Cancer. 94:552–560.
2006.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Catarata MJ, Ribeiro R, Oliveira MJ,
Robalo Cordeiro C and Medeiros R: Renin-angiotensin system in lung
tumor and microenvironment interactions. Cancers (Basel).
12(1457)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lever AF, Hole DJ, Gillis CR, McCallum IR,
McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL and
Robertson JW: Do inhibitors of angiotensin-I-converting enzyme
protect against risk of cancer? Lancet. 352:179–184.
1998.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Werner CM and Böhm M: The therapeutic role
of RAS blockade in chronic heart failure. Ther Adv Cardiovasc Dis.
2:167–177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Maiuri MC and Kroemer G: Essential role
for oxidative phosphorylation in cancer progression. Cell Metab.
21:11–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Diniz YS, Cicogna AC, Padovani CR, Silva
MD, Faine LA, Galhardi CM, Rodrigues HG and Novelli EL: Dietary
restriction and fibre supplementation: Oxidative stress and
metabolic shifting for cardiac health. Can J Physiol Pharmacol.
81:1042–1048. 2003.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Aune D, Chan DS, Greenwood DC, Vieira AR,
Rosenblatt DA, Vieira R and Norat T: Dietary fiber and breast
cancer risk: A systematic review and meta-analysis of prospective
studies. Ann Oncol. 23:1394–1402. 2012.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal
JS and Stanley WC: Myocardial fatty acid metabolism in health and
disease. Physiol Rev. 90:207–258. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Bonnet S, Archer SL, Allalunis-Turner J,
Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta
L, Bonnet S, et al: A mitochondria-K+ channel axis is suppressed in
cancer and its normalization promotes apoptosis and inhibits cancer
growth. Cancer Cell. 11:37–51. 2007.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Mann DL: Innate immunity and the failing
heart: The cytokine hypothesis revisited. Circ Res. 116:1254–1268.
2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ridker PM, MacFadyen JG, Everett BM, Libby
P, Thuren T and Glynn RJ: CANTOS Trial Group. Relationship of
C-reactive protein reduction to cardiovascular event reduction
following treatment with canakinumab: A secondary analysis from the
CANTOS randomised controlled trial. Lancet. 391:319–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ridker PM, MacFadyen JG, Thuren T, Everett
BM, Libby P and Glynn RJ: CANTOS Trial Group. Effect of
interleukin-1β inhibition with canakinumab on incident lung cancer
in patients with atherosclerosis: Exploratory results from a
randomised, double-blind, placebo-controlled trial. Lancet.
390:1833–1842. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang Y, Bauersachs J and Langer HF:
Immune mechanisms in heart failure. Eur J Heart Fail. 19:1379–1389.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Epelman S, Liu PP and Mann DL: Role of
innate and adaptive immune mechanisms in cardiac injury and repair.
Nat Rev Immunol. 15:117–129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Frantz S, Falcao-Pires I, Balligand JL,
Bauersachs J, Brutsaert D, Ciccarelli M, Dawson D, de Windt LJ,
Giacca M, Hamdani N, et al: The innate immune system in chronic
cardiomyopathy: A European society of cardiology (ESC) scientific
statement from the working group on myocardial function of the ESC.
Eur J Heart Fail. 20:445–459. 2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Stakos DA, Kambas K, Konstantinidis T,
Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki
A, Skendros P, Konstantinides S and Ritis K: Expression of
functional tissue factor by neutrophil extracellular traps in
culprit artery of acute myocardial infarction. Eur Heart J.
36:1405–1414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Sun K, Li YY and Jin J: A double-edged
sword of immuno-microenvironment in cardiac homeostasis and injury
repair. Signal Transduct Target Ther. 6(79)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Oktay AA, Rich JD and Shah SJ: The
emerging epidemic of heart failure with preserved ejection
fraction. Curr Heart Fail Rep. 10:401–410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Pandya PH, Murray ME, Pollok KE and
Renbarger JL: The immune system in cancer pathogenesis: Potential
therapeutic approaches. J Immunol Res. 2016(4273943)2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Wei J, Galaviz KI, Kowalski AJ, Magee MJ,
Haw JS, Narayan KMV and Ali MK: Comparison of cardiovascular events
among users of different classes of antihypertension medications: A
systematic review and network meta-analysis. JAMA Netw Open.
3(e1921618)2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sipahi I, Debanne SM, Rowland DY, Simon DI
and Fang JC: Angiotensin-receptor blockade and risk of cancer:
Meta-analysis of randomised controlled trials. Lancet Oncol.
11:627–636. 2010.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Bangalore S, Kumar S, Kjeldsen SE, Makani
H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C and
Messerli FH: Antihypertensive drugs and risk of cancer: Network
meta-analyses and trial sequential analyses of 324,168 participants
from randomised trials. Lancet Oncol. 12:65–82. 2011.PubMed/NCBI View Article : Google Scholar
|
|
108
|
ARB Trialists Collaboration. Effects of
telmisartan, irbesartan, valsartan, candesartan, and losartan on
cancers in 15 trials enrolling 138,769 individuals. J Hypertens.
29:623–635. 2011.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Chang CH, Lin JW, Wu LC and Lai MS:
Angiotensin receptor blockade and risk of cancer in type 2 diabetes
mellitus: A nationwide case-control study. J Clin Oncol.
29:3001–3007. 2011.PubMed/NCBI View Article : Google Scholar
|
|
110
|
McNeil JJ, Gibbs P, Orchard SG, Lockery
JE, Bernstein WB, Cao Y, Ford L, Haydon A, Kirpach B, Macrae F, et
al: Effect of aspirin on cancer incidence and mortality in older
adults. J Natl Cancer Inst. 113:258–265. 2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Falix FA, Aronson DC, Lamers WH and
Gaemers IC: Possible roles of DLK1 in the Notch pathway during
development and disease. Biochim Biophys Acta. 1822:988–995.
2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Rodriguez P, Sassi Y, Troncone L, Benard
L, Ishikawa K, Gordon RE, Lamas S, Laborda J, Hajjar RJ and Lebeche
D: Deletion of delta-like 1 homologue accelerates
fibroblast-myofibroblast differentiation and induces myocardial
fibrosis. Eur Heart J. 40:967–978. 2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Akiyama J, Okamoto R, Iwasaki M, Zheng X,
Yui S, Tsuchiya K, Nakamura T and Watanabe M: Delta-like 1
expression promotes goblet cell differentiation in
Notch-inactivated human colonic epithelial cells. Biochem Biophys
Res Commun. 393:662–667. 2010.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Wei Y, Shao J, Wang Y, Shen H, Yu S, Zhang
J and Yin L: Hsa-miR-370 inhibited P-selectin-induced cell adhesion
in human colon adenocarcinoma cells. Mol Cell Biochem. 450:159–166.
2019.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding
Q, Weng H, Shu YJ, Liu TY, Jiang L, et al: MALAT1 promotes the
proliferation and metastasis of gallbladder cancer cells by
activating the ERK/MAPK pathway. Cancer Biol Ther. 15:806–814.
2014.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li
YJ, Yan B and Jiang Q: Pathogenic role of lncRNA-MALAT1 in
endothelial cell dysfunction in diabetes mellitus. Cell Death Dis.
5(e1506)2014.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Gast M, Rauch BH, Nakagawa S, Haghikia A,
Jasina A, Haas J, Nath N, Jensen L, Stroux A, Böhm A, et al: Immune
system-mediated atherosclerosis caused by deficiency of long
non-coding RNA MALAT1 in ApoE-/-mice. Cardiovasc Res. 115:302–314.
2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Cremer S, Michalik KM, Fischer A,
Pfisterer L, Jaé N, Winter C, Boon RA, Muhly-Reinholz M, John D,
Uchida S, et al: Hematopoietic deficiency of the long noncoding RNA
MALAT1 promotes atherosclerosis and plaque inflammation.
Circulation. 139:1320–1334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Li X, Li Y, Zhang T, Xiong X, Liu N, Pang
B, Ruan Y, Gao Y, Shang H and Xing Y: Role of cardioprotective
agents on chemotherapy-induced heart failure: A systematic review
and network meta-analysis of randomized controlled trials.
Pharmacol Res. 151(104577)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Totzeck M, Mincu RI, Heusch G and Rassaf
T: Heart failure from cancer therapy: Can we prevent it? ESC Heart
Fail. 6:856–862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kollárová-Brázdová P, Jirkovská A,
Karabanovich G, Pokorná Z, Bavlovič Piskáčková H, Jirkovský E,
Kubeš J, Lenčová-Popelová O, Mazurová Y, Adamcová M, et al:
Investigation of structure-activity relationships of dexrazoxane
analogs reveals topoisomerase IIβ interaction as a prerequisite for
effective protection against anthracycline cardiotoxicity. J
Pharmacol Exp Ther. 373:402–415. 2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Chang WT, Lin YW, Ho CH, Chen ZC, Liu PY
and Shih JY: Dapagliflozin suppresses ER stress and protects
doxorubicin-induced cardiotoxicity in breast cancer patients. Arch
Toxicol. 95:659–671. 2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Fitzgerald MD, Tanaka H, Tran ZV and Seals
DR: Age-related declines in maximal aerobic capacity in regularly
exercising vs sedentary women: A meta-analysis. J Appl Physiol
(1985). 83:160–165. 1997.PubMed/NCBI View Article : Google Scholar
|
|
124
|
MacVicar MG, Winningham ML and Nickel JL:
Effects of aerobic interval training on cancer patients' functional
capacity. Nurs Res. 38:348–351. 1989.PubMed/NCBI
|
|
125
|
Jones LW, Fels DR, West M, Allen JD,
Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, et
al: Modulation of circulating angiogenic factors and tumor biology
by aerobic training in breast cancer patients receiving neoadjuvant
chemotherapy. Cancer Prev Res (Phila). 6:925–937. 2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Courneya KS, Sellar CM, Stevinson C,
McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N,
Mazurek A and Reiman T: Randomized controlled trial of the effects
of aerobic exercise on physical functioning and quality of life in
lymphoma patients. J Clin Oncol. 27:4605–4612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Jones LW, Douglas PS, Khouri MG, Mackey
JR, Wojdyla D, Kraus WE, Whellan DJ and O'Connor CM: Safety and
efficacy of aerobic training in patients with cancer who have heart
failure: An analysis of the HF-ACTION randomized trial. J Clin
Oncol. 32:2496–2502. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Hughes DC, Lenihan DJ, Harrison CA and
Basen-Engquist KM: Exercise intervention for cancer survivors with
heart failure: Two case reports. J Exerc Sci Fit. 9:65–73.
2011.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Maginador G, Lixandrão ME, Bortolozo HI,
Vechin FC, Sarian LO, Derchain S, Telles GD, Zopf E, Ugrinowitsch C
and Conceição MS: Aerobic exercise-induced changes in
cardiorespiratory fitness in breast cancer patients receiving
chemotherapy: A systematic review and meta-analysis. Cancers
(Basel). 12(2240)2020.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Rochette L, Meloux A, Zeller M, Cottin Y
and Vergely C: Role of humanin, a mitochondrial-derived peptide, in
cardiovascular disorders. Arch Cardiovasc Dis. 113:564–571.
2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Cai H, Liu Y, Men H and Zheng Y:
Protective mechanism of humanin against oxidative stress in
aging-related cardiovascular diseases. Front Endocrinol (Lausanne).
12(683151)2021.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Thummasorn S, Shinlapawittayatorn K,
Khamseekaew J, Jaiwongkam T, Chattipakorn SC and Chattipakorn N:
Humanin directly protects cardiac mitochondria against dysfunction
initiated by oxidative stress by decreasing complex I activity.
Mitochondrion. 38:31–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Qin Q, Mehta H, Yen K, Navarrete G,
Brandhorst S, Wan J, Delrio S, Zhang X, Lerman LO, Cohen P and
Lerman A: Chronic treatment with the mitochondrial peptide humanin
prevents age-related myocardial fibrosis in mice. Am J Physiol
Heart Circ Physiol. 315:H1127–H1136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Lue Y, Swerdloff R, Wan J, Xiao J, French
S, Atienza V, Canela V, Bruhn KW, Stone B, Jia Y, et al: The potent
humanin analogue (HNG) protects germ cells and leucocytes while
enhancing chemotherapy-induced suppression of cancer metastases in
male mice. Endocrinology. 156:4511–4521. 2015.PubMed/NCBI View Article : Google Scholar
|