Introduction
Tea polyphenols (TPs) are a group of polyphenol
compounds that are mainly extracted from green tea, which contain
four substances namely (-)-epigallocatechin-3-gallate (EGCG),
(-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG) and
(-)-epicatechin (EC) (1).
Polyphenols have been reported to exert bioactive effects that are
antioxidant, anti-obesity, anti-inflammatory, cancer preventative,
anti-tumor and regulatory of lipid metabolism (2). As one of the best-studied
antioxidants, it has been demonstrated that TPs exerts definitive
protective effects against oxidative stress, which is associated
with various clinical symptoms and diseases, including age-related,
neurological, cardiovascular and cerebrovascular diseases,
occurring through the elimination of free radicals and the
subsequent regulation of anti-oxidases activity (3,4).
With a basic structure of α-phenyl-benzopyran, the molecular
structure of TPs and polyphenolic compounds, particularly the
position and number of hydroxyl groups, serve an important role in
antioxidant activity (5). Studies
have reported that in laboratory experiments and clinical studies,
TPs can provide a more robust protective effect against oxidative
damage than polyphenolic compounds individually (6,7).
Reactive oxygen species (ROS) are byproducts of
normal cellular metabolism. ROS levels are associated with basic
cellular activities and cause oxidative stress when ROS generation
exceeds the antioxidant capacity of the cell (8). Physiologically, a system of oxidant
and antioxidant enzymes delicately balance intracellular ROS levels
for cellular homeostasis (9,10).
The overproduction of ROS can break cell defenses, leading to
oxidative stress that induces irreversible damage to the
mitochondria, destroying cellular structure and function and
causing increased risk for cardiovascular disease, diabetes
mellitus, cancer and other diseases (10). Previous studies have shown that TPs
exhibit antioxidant effects in the following processes (11,12):
Elimination of free radicals via relatively stable phenolic oxygen
radicals formed with ROS; inhibition and inactivity of oxidant
enzymes and increased production of antioxidant enzymes. A number
of epidemiological studies have revealed that the Kelch-like
ECH-associated protein-1 (Keap1)/nuclear factor (erythroid-derived
2)-like 2 (Nrf2) signaling pathway serves an important role in
antioxidant function and reduces oxidative stress (12,13).
TPs and certain polyphenolic compounds, such as EGCG, stimulate the
activity of the heme oxygenase 1 (HO-1) gene by activating the
Nrf2/antioxidant response element pathway (13,14).
H2O2-induced oxidative damage
is one of the most widely used cellular models of oxidative stress
as the antioxidant effect of potent antioxidants can be evaluated
(15). Macrophages are reportedly
vulnerable to ROS and their functions are affected by oxidative
stress in a direct and indirect manner (16). Macrophages directly kill pathogens
through phagocytosis, secreting large quantities of certain
bioactive molecules, including ROS and nitric oxide (NO). ROS
production is a major defense mechanism against pathogenic
infiltration (17,18). However, the sensitivity of
macrophages to ROS can lead to cell injury and even cell death
(19).
H2O2-induced oxidative damage in macrophages
can provide a drug screening platform for identifying potential
antioxidants from natural products (20).
A study by Wang et al (21) reveals that the cellular
self-protective mechanism of macrophages against oxidative stress
involves the mammalian STE20-like protein kinase (Mst)/Nrf2 axis.
In the same study, Mst1 and Mst2 maintain cellular redox balance by
acting as an ROS sensor and modulating the stability of the
antioxidant transcription factor Nrf2. However, the role of the
Mst/Nrf2 axis in the protective cellular mechanism against
antioxidant damage exerted by TPs remains to be elucidated.
Although a growing number of epidemiological studies
(11,12,22)
have identified the molecular mechanisms associated with the
antioxidant effects exerted by TP, there are a lack of studies
assessing the antioxidant effect of TPs in
H2O2-induced oxidative damaged macrophages.
The aim of the current study was to evaluate the antioxidant
properties and underlying mechanisms of TPs in
H2O2-induced oxidative macrophage injury
using RAW264.7 cells.
Materials and methods
Cell culture
Murine macrophage RAW264.7 cells were purchased from
the Cell Bank of the Chinese Academy of Sciences and cultured in
DMEM (Biological Industries; Sartorius AG) containing 10% fetal
bovine serum (FBS; Biological Industries; Sartorius AG), 100 U/ml
of penicillin and 100 µg/ml of streptomycin (HyClone; Cytiva), in a
humidified atmosphere containing 5% CO2 at 37˚C. For all
experiments, cells were incubated with various concentrations (0.1,
0.5 and 1.0 µg/ml) of TPs (purity ≥98.0%; cat. no. CAS84650-60-2;
Beijing Solarbio Science & Technology Co., Ltd.; containing
46.8% EGCG, 17.3% EGC, 5.2% EC and 1.5% ECG; Fig. S1) for 12 h prior to the addition
of H2O2 for 12 h. EGCG, EGC, ECG and EC were
bought from Aladdin. Methanol of HPLC-grade was purchased from
Sigma-Aldrich (Merck KGaA).
HPLC analysis
The appropriate amounts of four standard substances
(EGCG, EGC, EC and ECG), were accurately weighed and dissolved in
20% methanol (v/v). The tea polyphenol samples were prepared with
20% methanol and injected into the HPLC system for analysis after
filtering through 0.22 µm syringe filters.
The analysis was carried out using an LC 15
(Shimadzu Corporation) for the chromatographic determination. The
sample was injected into a C18 analytical column (4.6 mm I.D x 150
mm, 3.0 µm particle size; Waters Corporation). The mobile phase was
0.1% formic acid solution (solvent A) and methanol (solvent B). The
Diode-Array Detection acquisition wavelength was set at 275 nm. The
gradient elution programmer was as follows: ~0-2 min, 2% B; ~2-13
min, 2-90% B; ~13-16 min, 100%. The flow rate was of 0.3 ml/min,
and the column temperature was set at 40˚C. The injection volume
was 5 µl.
H2O2-induced
oxidative stress model
RAW264.7 cells were inoculated into 96-well plates
at a density of 4x103 cells/well at 37˚C in a humidified
5% CO2 incubator for 12 h. Cells were treated with different final
concentrations of H2O2 (0, 50, 100, 200, 300,
400, 500, 600, 700 and 800 µM) diluted with complete medium for 0,
4, 8, 12 and 24 h. Cells were subsequently treated with Cell
Counting Kit-8 (CCK-8; Shanghai Yeasen Biotech Co., Ltd.) for 3 h.
Absorbance was detected at a wavelength of 450 nm and the results
were expressed as a percentage.
Cell viability assay
TPs cytotoxicity in RAW264.7 cells was analyzed
using a CCK-8 assay in accordance with the manufacturer's protocol.
RAW264.7 cells were seeded into 96-well plates at a density of
4x103 cells/well and left to adhere. Cells were then
treated with 0.0, 0.1, 0.5, 1.0, 5.0 and 10.0 µg/ml TPs for 24 h.
Subsequently, 10 µl CCK-8 reagent was added to each well and
incubated at 37˚C for 3 h. The absorbance value at a wavelength of
450 nm was measured using the Varioskan Flash microplate reader
(Thermo Fisher Scientific, Inc.). Each evaluation of each
concentration was repeated three times.
Effect of TPs on the proliferation of
H2O2-injured cells
Cells were seeded into 96-well plates at a density
of 4x103 cells/well and pre-incubated at 37˚C for 24 h.
The culture medium supernatant was replaced with an equal volume of
solution containing different concentrations of TPs (0.1, 0.5 and
1.0 µg/ml) and incubated at 37˚C for 12 h. Subsequently, samples
were treated with 400 µM H2O2 at 37˚C for a
further 12 h. Proliferations was evaluated using the aforementioned
CCK-8 method.
ROS assay
ROS production was estimated using an ROS Assay kit
(Beyotime Institute of Biotechnology) as previously described
(23). RAW264.7 cells
(2x105 cells/ml) were cultured in a 24-well plates and
incubated at 37˚C overnight. Cells were pretreated with different
concentrations (0.1, 0.5 and 1.0 µg/ml) of TPs at 37˚C for 12 h,
after which each well plate was exposed to 400 µM
H2O2 at 37˚C for a further 12 h.
Subsequently, the cells were washed three times with cold PBS and
incubated with 5 µM DCFH2-DA (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C for 30 min. RAW264.7 cells were washed
with DMEM culture medium and determined the fluorescent intensity
was determined using a Bio-Rad ZE5 cell analyzer (Bio-Rad
Laboratories, Inc.).
Antioxidant enzyme activity
assays
RAW264.7 cells were seeded into 6-well plates with a
density of 5x105 cells/cell and cultured at 37˚C
overnight. Cells were treated with 0.1, 0.5 and 1.0 µg/ml of TPs at
37˚C for 12 h, followed by 400 µM H2O2
treatment at 37˚C for an additional 12 h. At the end of incubation,
cells were collected for the detection of NO contents and
superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA)
and glutathione peroxidase (GSH-Px) activity using Griess Regent
and test kits (Nanjing Jiancheng Bioengineering Institute),
respectively.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from 5x106
RAW264.7 cells using TRIzol® reagent according to its
instructions (Invitrogen; Thermo Fisher Scientific, Inc.) and
quantified at 260 nm using a NanoPhotometer (cat. no. P300; Implen
GmbH). cDNA was synthesized from 1 µg total RNA obtained from each
sample using the MonScript RTIII all-in-one Mix according to the
manufacturer's protocols (Monad Biotech Co., Ltd.). Subsequently,
RT-qPCR was performed to quantify mRNA expression levels using the
SYBR Green qPCR Mix (Monad Biotech Co., Ltd.) with the AriaMx
RT-PCR system (Agilent Technologies, Inc.). The primer sequences
used for PCR were as follows: HO-1 forward,
5'-GAAATCATCCCTTGCACGCC-3' and reverse, 5'-CCTGAGAGGTCACCCAGGTA-3';
Keap1 forward, 5'-TGGGTCAAATACGACTGCCC-3' and reverse,
5'-ATCATCCGCCACTCATTCCT-3'; Nrf2 forward,
5'-ACATGGAGCAAGTTTGGCAG-3' and reverse, 5'-TGGAGAGGATGCTGCTGAAA-3';
Mst1 forward, 5'-CAGCCTGCACTCAGACCAAC-3' and reverse, 5'-
TGGCAGTGGAAGAAGCTATGTC-3'; Mst2 forward, 5'-CCAGGCCCTATGTCCAACAG-3'
and reverse, 5'-TGCCTCCTCTTCTTCGCATC-3'; β-actin forward,
5'-AGTGTGACGTTGACATCCGT-3' and reverse,
5'-AGCTCAGTAACAGTCCGCCTA-3'. The amplification program included an
initial denaturation step at 95˚C for 30 sec, followed by 40 cycles
of denaturation at 94˚C for 5 sec, annealing at 58˚C for 30 sec and
extension at 70˚C for 5 sec. The experiments were repeated in
triplicate using independent samples. Relative gene expression was
analyzed using the 2-∆∆Cq method with β-actin as an
internal control (24).
Western blot analysis
Cells were lysed using RIPA lysis buffer (Beyotime
Institute of Biotechnology) containing 1% phenylmethylsulfonyl
fluoride, protease inhibitors and phosphatase inhibitors. The
concentration of protein was subsequently quantified using a BCA
protein estimation kit (Beyotime Institute of Biotechnology). A
total of 40 µg protein was loaded onto 12% SDS-PAGE gels and
transferred onto a nitrocellulose membrane (EDM Millipore). The
membrane was then blocked with 5% nonfat milk in PBST (PBS
containing 0.05% Tween-20) at room temperature for 2 h and
incubated with the following primary antibodies for 14 h at 4˚C:
Keap1 (cat. no. sc-515432; 1:500; Santa Cruz Biotechnology, Inc.),
Mst1 (cat. no. ab51134; 1:1,000; Abcam), Mst2 (cat. no. ab70546;
1:1,000; Abcam), Nrf2 (cat. no. ab92946; 1:1,000; Abcam), HO-1
(cat. no. ab52947; 1:1,000; Abcam) and β-actin (cat. no. ab8226;
1:1,000, Abcam). After washing three times with PBST, the membrane
was incubated for 1 h with HRP-conjugated IgG (cat. nos. ab6721 and
ab6728; 1:10,000; Abcam) at room temperature. The resultant signals
were detected using the LumigenTMA-6 kit (Cytiva). The relative
protein levels were normalized to β-actin, which was used as the
internal control. Quantity One software (Bio-Rad Laboratories,
Inc.) was used for densitometry.
Statistical analysis
All data were presented as the mean ± SD of three
independent experiments and analyzed using GraphPad Prism 8.0.1
software (GraphPad Software, Inc.). Multiple comparisons were
analyzed via one-way ANOVA followed by Tukey's comparison post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
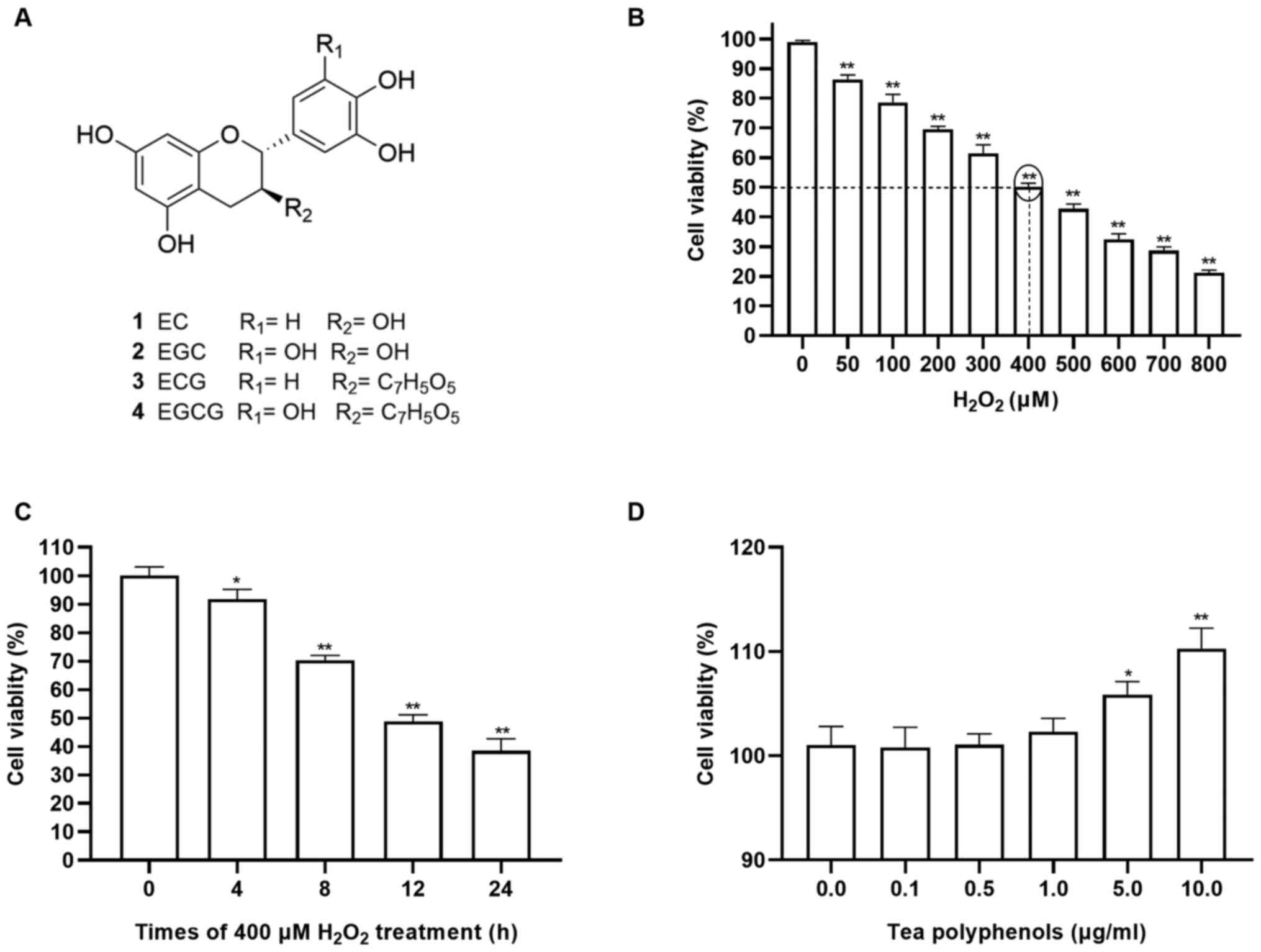
Effects of H2O2
and TPs on RAW264.7 cells
To investigate the cytotoxicity of TPs and
H2O2 in RAW264.7 cells, the viability of
RAW264.7 cells was evaluated following treatment with various
concentrations of TPs or H2O2. The chemical
structures of major catechins in TPs were shown in Fig. 1A. As presented in Fig. 1B, the viability of cells exposed to
H2O2 decreased in a dose-dependent manner
within 12 h. Additionally, treatment with 400 µM
H2O2 exhibited 50% inhibition within 12 h
(Fig. 1B and C) and as such was selected for use in
subsequent experiments. In present study, CCK-8 assays showed that
TPs did not have significant effect on cellular viability after 24
h treatment with 0.1, 0.5 and 1.0 µg/ml (Fig. 1D). Therefore, TPs of 0.1, 0.5 and
1.0 µg/ml were adopted as the suitable concentrations for the
subsequent experiments.
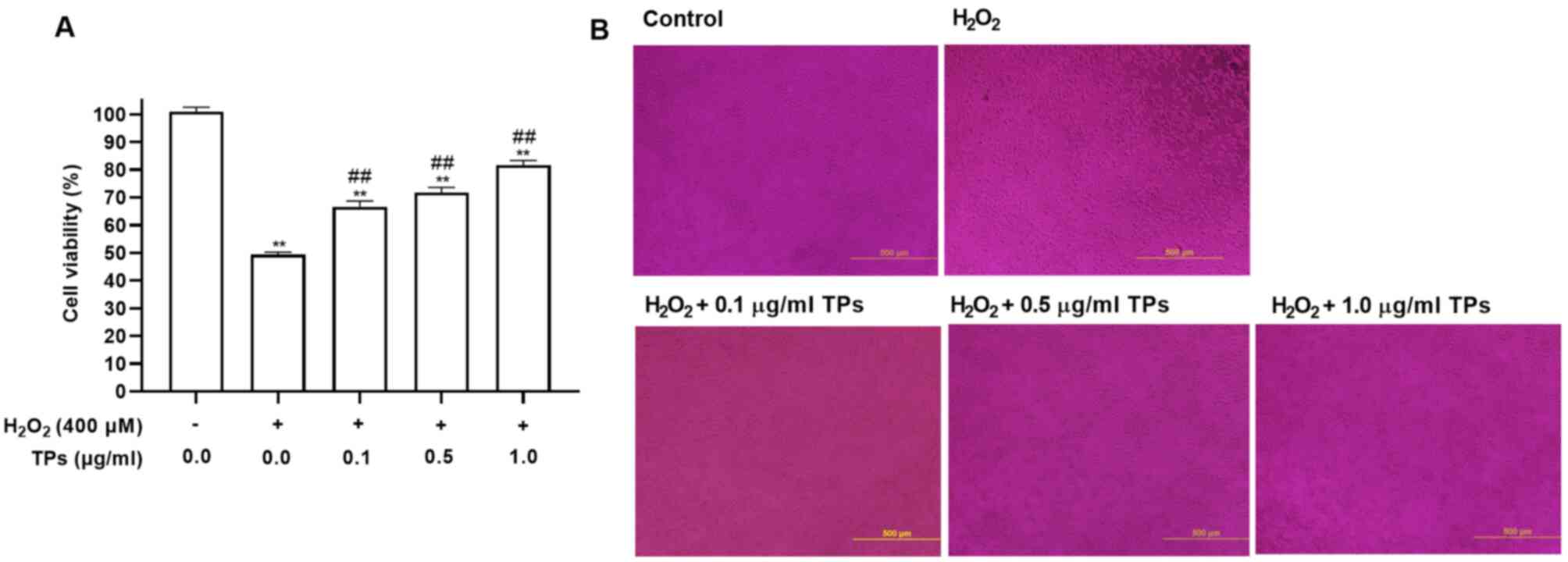
TPs attenuate
H2O2-induced cell injury in an oxidative
stress model
H2O2 can induce mitochondrial
injury and membrane structure disruption due to its excessive
generation (25). CCK-8 analysis
was performed to evaluate the protective effects of TPs on the
viability of cells exposed to H2O2-induced
cytotoxicity. As presented in Fig.
2, TPs reduced H2O2-induced cytotoxic
effects. Additionally, pretreatment with TPs at 0.1, 0.5 and 1.0
µg/ml for 24 h, followed by exposure to 400 µM
H2O2 markedly increased cell viability in a
TPs dose-dependent manner (Fig.
2A). Morphological observations revealed that the number of
RAW264.7 cells in the H2O2 group was
decreased, while pretreatment with TPs could prevent this effect
(Fig. 2B). The results indicated
that TPs could protect cells against
H2O2-induced injury.
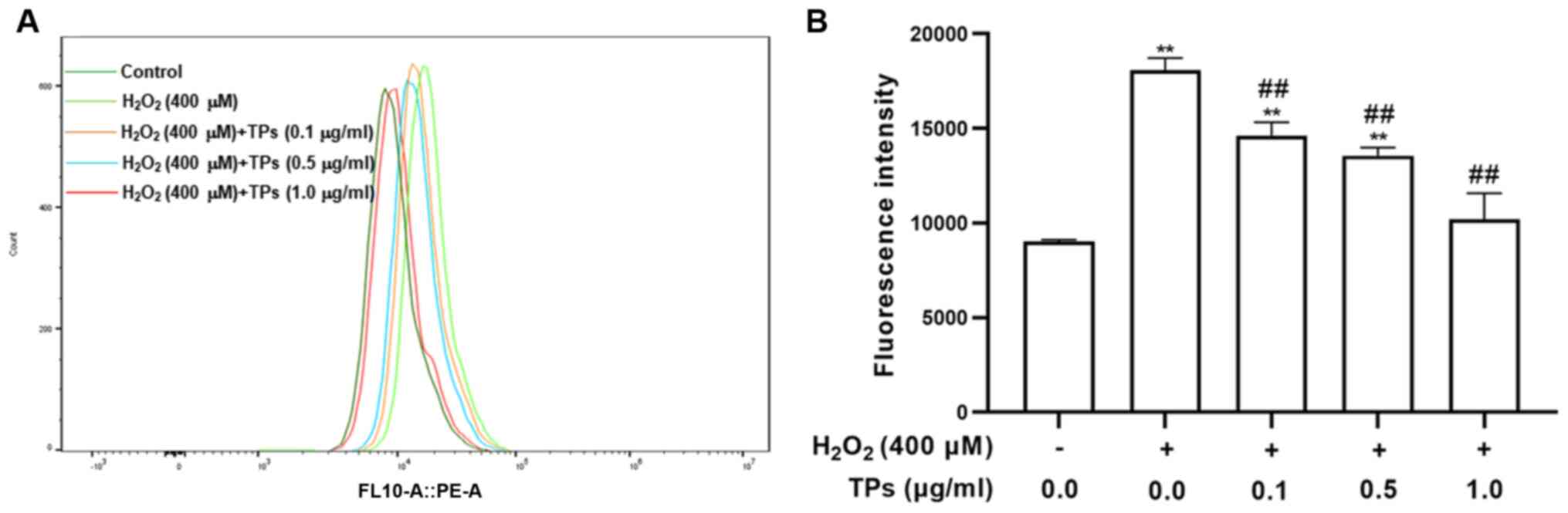
TPs inhibit ROS production in an
oxidative stress model
Given the essential requirement for cellular
homeostasis, ROS are generated in various metabolic processes;
however, external H2O2 can lead to the
excessive production of ROS, which causes damage to cell lipids,
proteins and organelles (26). The
current study measured intracellular ROS content via flow cytometry
using DCFH2-DA. As presented in Fig.
3A and B, following cell
exposure to H2O2 for 12 h, ROS levels were
significantly increased in the H2O2 treated
group compared with the control group (P<0.05). However, ROS
levels were significantly decreased in the three TPs treated groups
compared with the H2O2 group (P<0.05). The
results indicated that TPs enhanced RAW264.7 cell ROS elimination
in a dose-dependent.
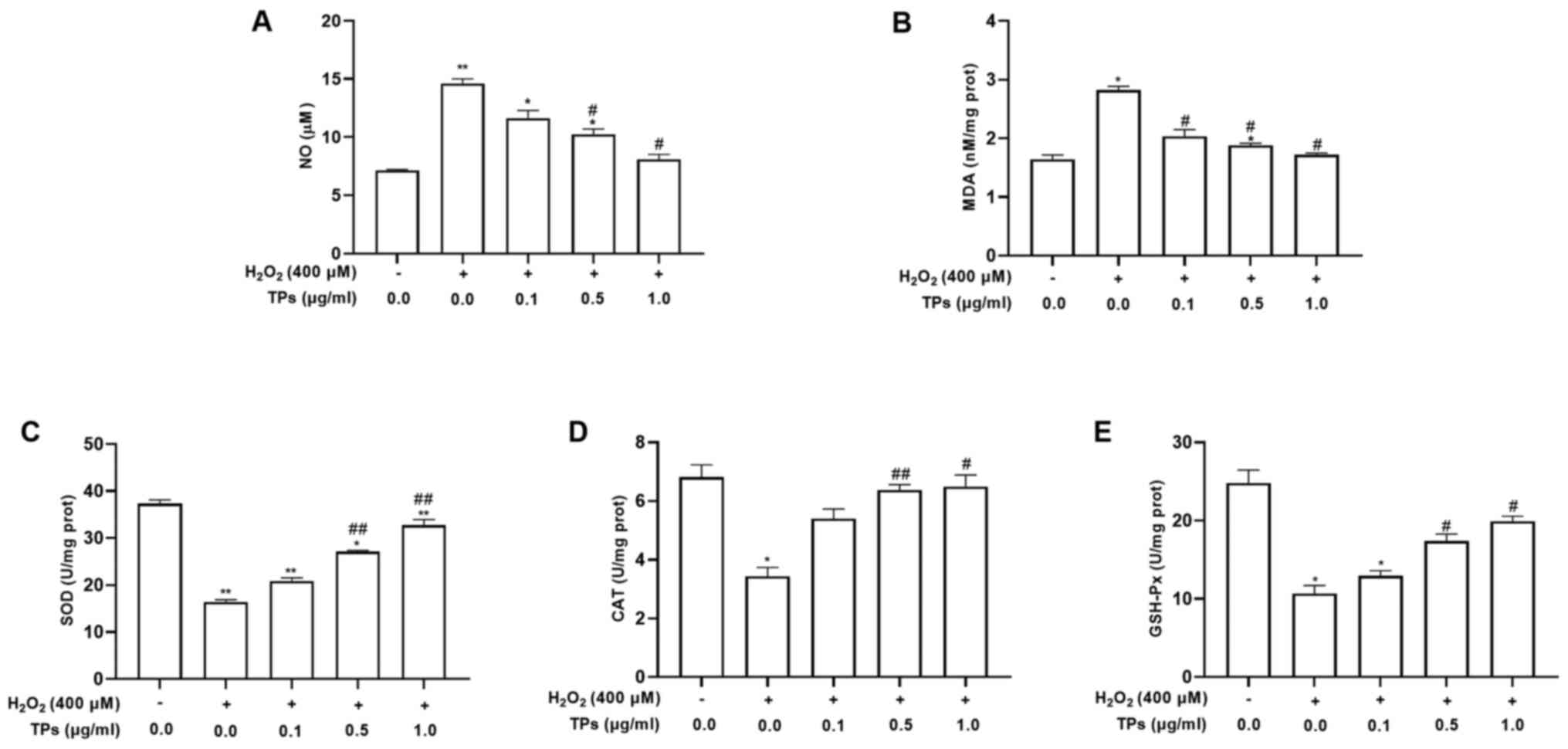
TPs alter the
H2O2-induced expression of antioxidant enzyme
activity in RAW264.7 cells
According to previous studies, TPs, or the main
contents, can reduce levels of NO and MDA, simultaneously
increasing the production of SOD, GSH-Px and CAT (27,28).
To determine the antioxidant effect of TPs under oxidative stress,
the activities of NO and antioxidant enzymes (SOD, CAT, MDA and
GSH-Px) were assessed in TPs-pretreated cells. The results revealed
that increased NO and MDA levels induced by
H2O2 were significantly reduced in TPs
pretreated groups (P<0.05; Fig.
4A and B). In addition, the
three groups treated with TPs demonstrated significantly declined
NO and MDA activities compared with the oxidative injure group
(P<0.05). As presented in Fig.
4C-E, the H2O2 treated group demonstrated
significantly decreased activities of SOD, CAT and GSH-Px compared
with the control group (P<0.05). In addition, significant
increased activities of SOD, CAT and GSH-Px were detected in all
TPs-pretreated groups compared with the H2O2
group (P<0.05).
Mst/Nrf2 axis and Keap1/Nrf2/HO-1
pathway-related gene and protein expressions
Recent studies have demonstrated that ROS activates
the Mst/Nrf2 axis and Keap1/Nrf2/HO-1 pathway to initiate cellular
self-protective mechanisms against oxidative damage (19,21).
To clarify the underlying antioxidant mechanisms of TP, the mRNA
and total protein levels of the Mst/Nrf2 axis and
Keap1/Nrf2/HO-1pathway were measured using RT-qPCR and western
blotting, respectively.
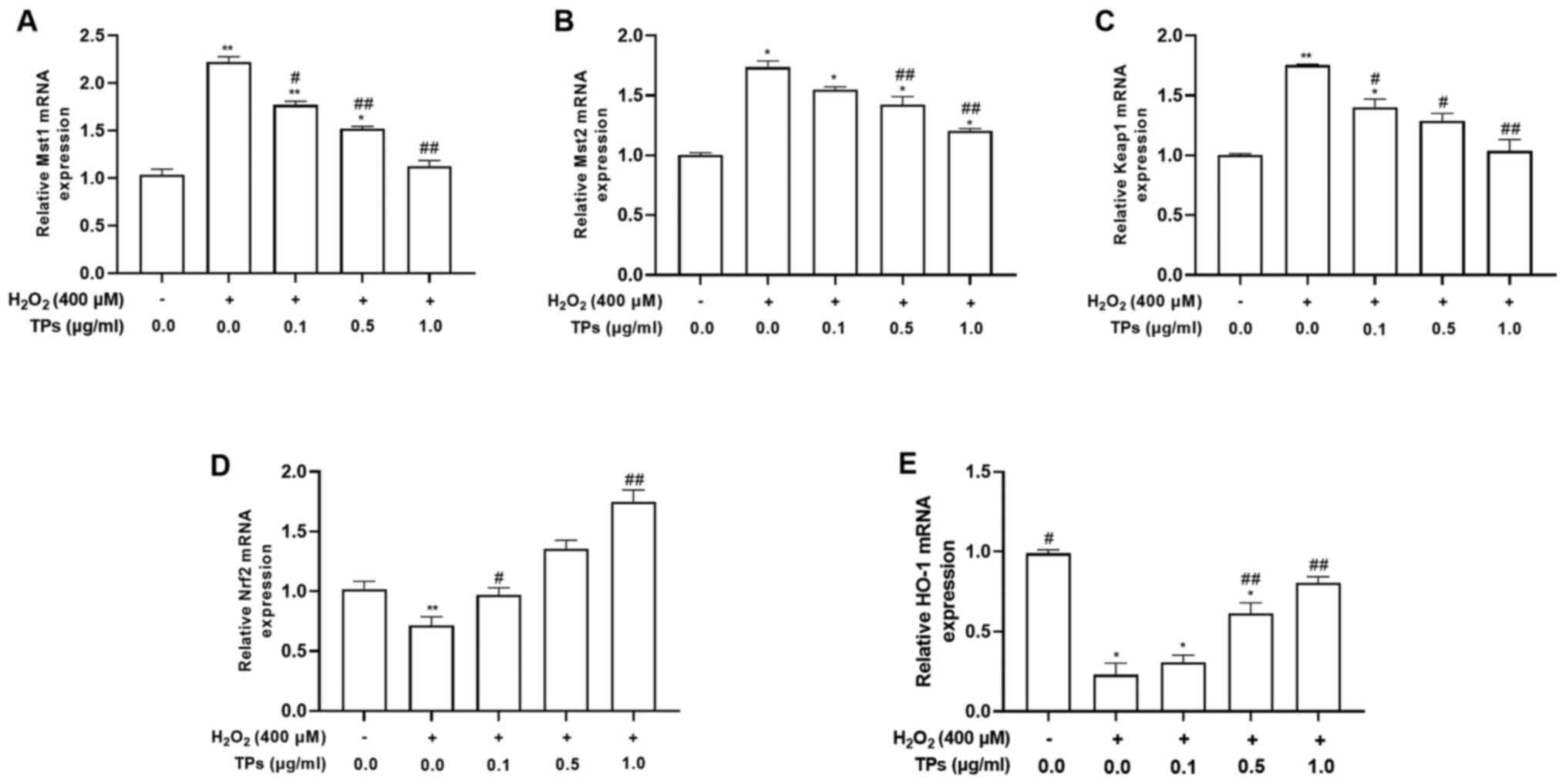
After 12 h of H2O2 and/or 24 h
of TPs pretreatment, the mRNA expression levels of Mst1, Mst2,
Keap1, Nrf2 and HO-1 were evaluated (Fig. 5). The results demonstrated that
Mst1, Mst2 and Keap1 mRNA levels were significantly increased
(P<0.05), while Nrf2 and HO-1 mRNA levels were significantly
decreased (P<0.05) in the H2O2 group
compared with the control group. TPs treatment reduced the mRNA
expression levels of Mst1 and Mst2 in a concentration-dependent
manner, but could not fully restore levels to those prior to
H2O2 oxidative stress induction (Fig. 5A and B). In addition, the three TPs groups
resulted in decreased Keap1 mRNA expression levels compared with
the H2O2-treated group (P<0.01; Fig. 5C). TPs-pretreated groups also
demonstrated significantly increased Nrf2 and HO-1 mRNA expression
levels compared with the H2O2 group
(P<0.01; Fig. 5D and E).
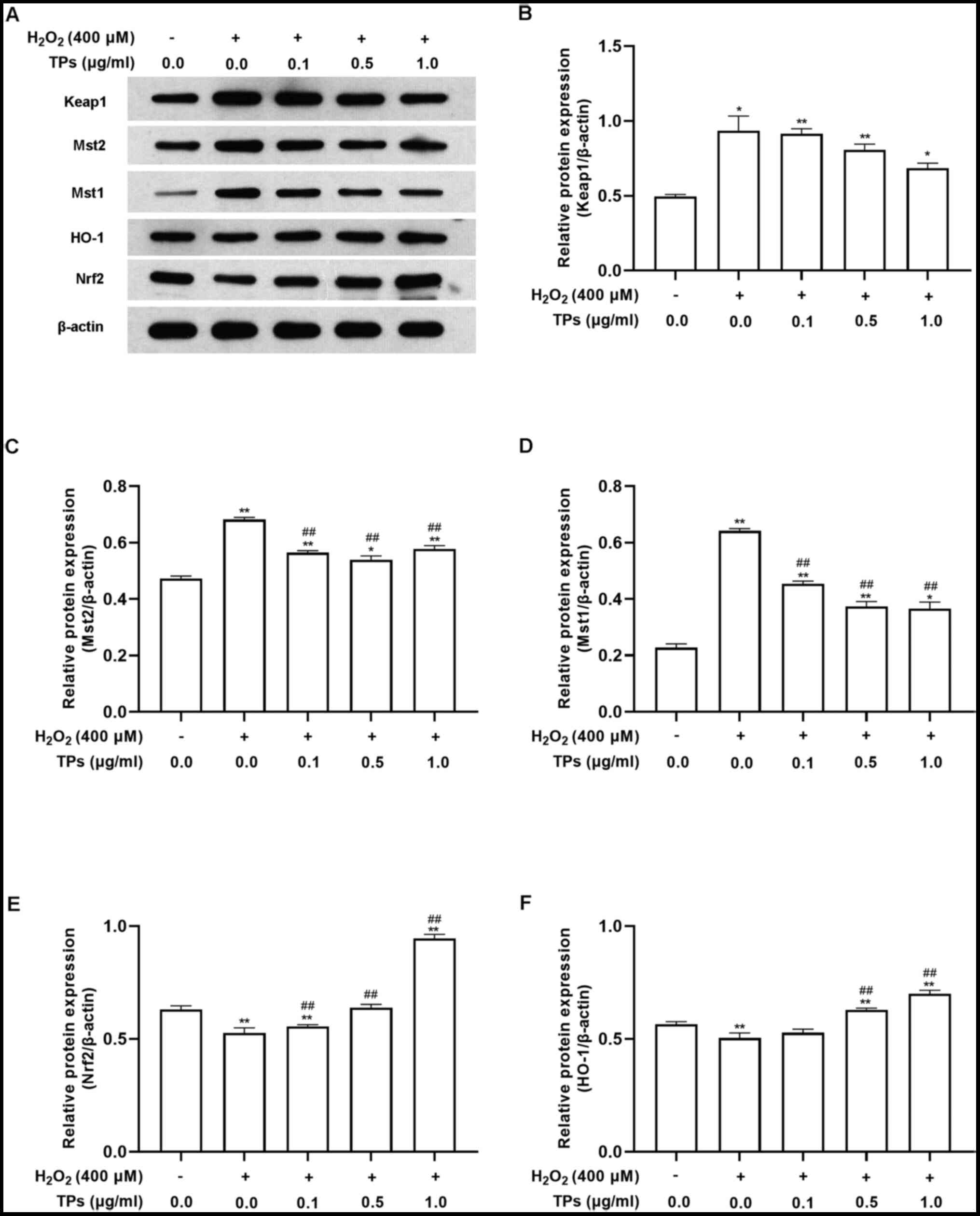
In support of the results of RT-qPCR, western blot
analysis also revealed that H2O2 treatment
significantly affected the Mst/Nrf2 axis and Keap1/Nrf2/HO-1
pathway. As presented in Fig. 6,
when compared with the control group, H2O2
treatment resulted in increased expression of Mst1, Mst2 and Keap1
(Fig. 6A-D) and decreased
expression of Nrf2 and HO-1 (Fig.
6E and F). These effects were
all reversed by preincubation with TP.
Discussion
Accumulating studies indicate that plant bioactive
polyphenols exhibit higher antioxidant activity and lower toxicity
than synthetic compounds (29-31).
Research in our laboratory (Tsinghua University; Beijing) has
focused on the bioactive effects of natural low molecular weight
polyphenols, including lychee, tea, blueberry and other polyphenols
derived from edible plants (32-34).
As recognized natural antioxidants, TPs and their main four
substances have been used in the prevention and treatment of
clinical diseases involving oxidative damage. The TPs content was
~1.8-3.6 mg per gram of dry tea leaves; in addition, 75-147 µg/ml
of polyphenols was dissolved in 100 ml tea liquor from 1 g leaf
(35,36). Frei and Higdon (37) reported that one cup of tea (2 g of
tea leaves infused in hot water for ~1-3 min) will provide 0.15~0.2
g of flavonoids. As few as 2-3 cups/day of tea will therefore
supply a significant contribution to the total flavonoid intake in
most individuals, which is estimated to average 1 g per day. Note
that the concentrations of TPs by drinking tea are much higher than
the experimental concentrations.
High doses of polyphenols are reported to exert
positive effects as well as some negative effects (12). In the pre-experiments of the
present study, the effect of ~0-100 µg/ml TPs on the cell viability
of RAW264.7 cells was assessed. The experiment results indicated
that the non-cytotoxic concentrations were ≤1.0 µg/ml for TPs; 5.0
µg/ml and 10.0 µg/ml enhanced cell viability of RAW264.7 cells;
50.0 µg/ml and 100.0 µg/ml inhibited the viability of RAW264.7
cells. Similar experimental results are reported by Lagha and
Grenier (38); 62.5 µg/ml green
tea extract induces cell viability, and ≥62.5 µg/ml inhibits cell
proliferation of U937 macrophage-like cells. This finding differs
from the fact that TPs inhibits the growth of tumor cells in a
concentration-dependent manner. This phenomenon is puzzling and the
underlying mechanisms remain unclear. The regulation of TPs on the
cell viability in macrophages will become the focus of future
research. Experiments performed on animals have shown that high
concentrations of green tea extract (~500-2000 mg/kg) and of single
tea phenolics, such as EGCG (150 mg/kg/day) produce toxicity in the
liver, intestine and kidneys (39,40).
Similarly, a previous study revealed that the continuous
administration of high-dose plant bioactive polyphenols does not
achieve the expected continuous prophylactic and therapeutic
effects in the treatment of RAW264.7 cells and oxidative-damaged
RAW264.7 cells. The data from the present study suggested that low
doses of TPs exerted effective preventive biological effects. The
doses of TPs (0.1, 0.5, 1.0 µg/ml) that protected RAW264.7 cells
against H2O2-induced injury were much lower
compared with those used in other studies (~10-100 µg/ml TPs;
~10-50 µM EGCG) (41-43).
Pharmacokinetics studies in humans or mice show that the peak serum
concentrations of EGCG, EGC or ECG are affected by an individual's
metabolism and the number of catechins in the ingested type of
green tea ranged from 34.7 ng/ml-3.39 µg/ml (44-46).
Accordingly, the concentrations of TPs and main substances used in
the present study were comparable to their serum
concentrations.
H2O2 can diffuse freely
through the cell due to its membrane permeability (47). When an organism is stimulated by
exogeneous H2O2, excessive ROS interferes
with the balance of intricate defense mechanisms, triggering
oxidative damage (48). Therefore,
the H2O2-induced cell injury model is ideal
for in vitro antioxidant research (15). Forman et al (49) reveal that
H2O2 concentration in blood plasma is ~1-5
µM, which would be >100-fold higher than that estimated to occur
within cells. An estimated 100-fold concentration gradient from
extracellular to intracellular is given for rough orientation and
ensures to maintain homeostasis adjacent to normal cells (15). This also suggests that 400 µM
H2O2 was a reasonable concentration in an
injury cell model. Based on the results of flow cytometry analysis
in the current study, all three doses of TPs alleviated the
excessive production of ROS induced by
H2O2.
The oxidoreductase system, which involves CAT, SOD,
GSH-Px and MDA, serves an important regulatory role in
H2O2-induced injury. A number of reports have
revealed that natural antioxidants can increase CAT, SOD and GSH-Px
levels and inhibit the production of MDA both in vivo and
in vitro. Similarly, treatment with TPs or its four
components can reduce MDA production and increase levels of
antioxidant enzymes (3,5,11).
In the present study, H2O2-treated RAW264.7
cells that were exposed to TPs increased the levels of CAT, SOD and
GSH-Px and decreased levels of MDA, as well as No.
H2O2 can also increase NO levels in RAW264.7
cells, which in turn further promotes the production of
H2O2, inhibiting catalase activity and
creating a cycle of aggravated oxidative damage to cells (25,50,51).
The current data demonstrated that TPs efficiently reduced NO
release, alleviating oxidative damage to RAW264.7 cells.
To elucidate the underlying protective mechanism of
TPs in H2O2-injured RAW264.7 cells, the
expression of the Mst/Nrf2 axis and Keap1/Nrf2/HO-1 pathway was
evaluated. The Nrf2 transcription factor is an essential regulator
of cellular responses against oxidative stress (52), which mainly activates the
antioxidant response and promotes the production of antioxidant
proteins and enzymes (53). In the
cytoplasm of normal cells, Nrf2 is maintained at low levels by
polymerizing into dimers and binding to Keap1, which facilitates
polyubiquitination and enables proteasomal degradation of
Nrf2(54). When cells are exposed
to excessive endogenous or exogenous ROS under conditions of
stress, Nrf2 dissociates from Keap1, migrates to the nucleus and
exerts its function (55),
ultimately stimulating HO-1 activity (54). In addition, Nrf2 can mediate the
expression of certain antioxidant factors including SOD, CAT and
NAD(P)H quinone oxidoreductase 1(54). In both animal and cell line models,
the preventive and therapeutic effects of TPs are alleviated via
the Nrf2/Keap1/HO-1 pathway (56,57).
In the present study, the results of RT-qPCR and western blotting
revealed that TPs upregulated Nrf2 and HO-1 expressions,
downregulated Keap1 expression and reversed oxidative damage in
RAW264.7 cells induced by H2O2. The results
suggested that the possible mechanisms in TPs-alleviated cellular
damage may be through the modulation of Nrf2/Keap1/HO-1
pathway.
Recent research revealed that the Mst/Nrf2 axis
maintains cellular redox homeostasis when sensing the production of
ROS in macrophages (21). ROS
recruits and activates the protective kinases Mst1 and Mst2, which
induce Keap1 phosphorylation, prevent Keap1 polymerization and
block Nrf2 ubiquitination and degradation. The stability of Nrf2 is
a major factor for the protection of cells against oxidative damage
(54). Consistent with previous
studies, the results of the current study also supported the view
that Mst1 and Mst2 are markedly activated by
H2O2-induced ROS release, achieving
self-protection in RAW264.7 cells. In addition, the production of
ROS was downregulated following TPs treatment, with Mst1 and Mst2
exhibiting lower expressions compared with the
H2O2-treated group; however, the levels were
still higher compared with the control group. The results suggested
that the Mst/Nrf2 axis may be a specific regulatory system for the
defense against oxidative damage induced by
H2O2. However, the present study did not
conduct verification experiments by intervention strategies, such
as inhibitors, activators, some genes and epigenetic modifications,
targeting the relative pathways. The promising findings are not
conclusive due to some limitations of the present study. The
underlying mechanism needs further investigation and will be
addressed in future studies.
A number of epidemiological studies have been
conducted to investigate the effects of tea consumption on human
benefits with special reference to antioxidant, cancer and
cardiovascular diseases. Tea drinking is shown to be associated
with a lower risk for several types of cancer (58). During an 11-year follow-up, a
prospective cohort study containing 285 males and 203 female cancer
patients was conducted with green tea. Individuals who consumed
>10 cups of green tea per day showed marked reductions of
relative risk for lung, colon and liver cancers than other groups
(<3 cups; 4-9 cups) (59). In
addition, increasing frequency, duration and quantity of green tea
consumed weakened the risk of prostate cancer (60). Therefore, a cell line model
consuming TPs continuously will need to be established to simulate
regular tea consumption in a follow-up study. In addition, two
commonly used human cell lines, THP-1 and U937, will be used in
future to identify the most effective dosage and frequency to
achieve the maximum human health benefits.
In conclusion, TPs treatment was applied in the
current study to induce antioxidant activity, reducing the effects
of H2O2-induced cellular injury. The
protective effects of TPs partly involved the reduction of
intracellular ROS generation, NO release and MDA levels, along with
the restoration of SOD and GSH-Px activity. The underlying
mechanism may possibly be associated with the Mst/Nrf2 axis and
Keap1/Nrf2/HO-1 signaling pathway for the sensing and regulation of
antioxidant defense (Fig. 7).
Since the oxidative injury model serves a key role in screening
antioxidants and protective substances, the current study provides
further evidence for the application of TPs in pro-oxidant
ROS-mediated oxidative stress.
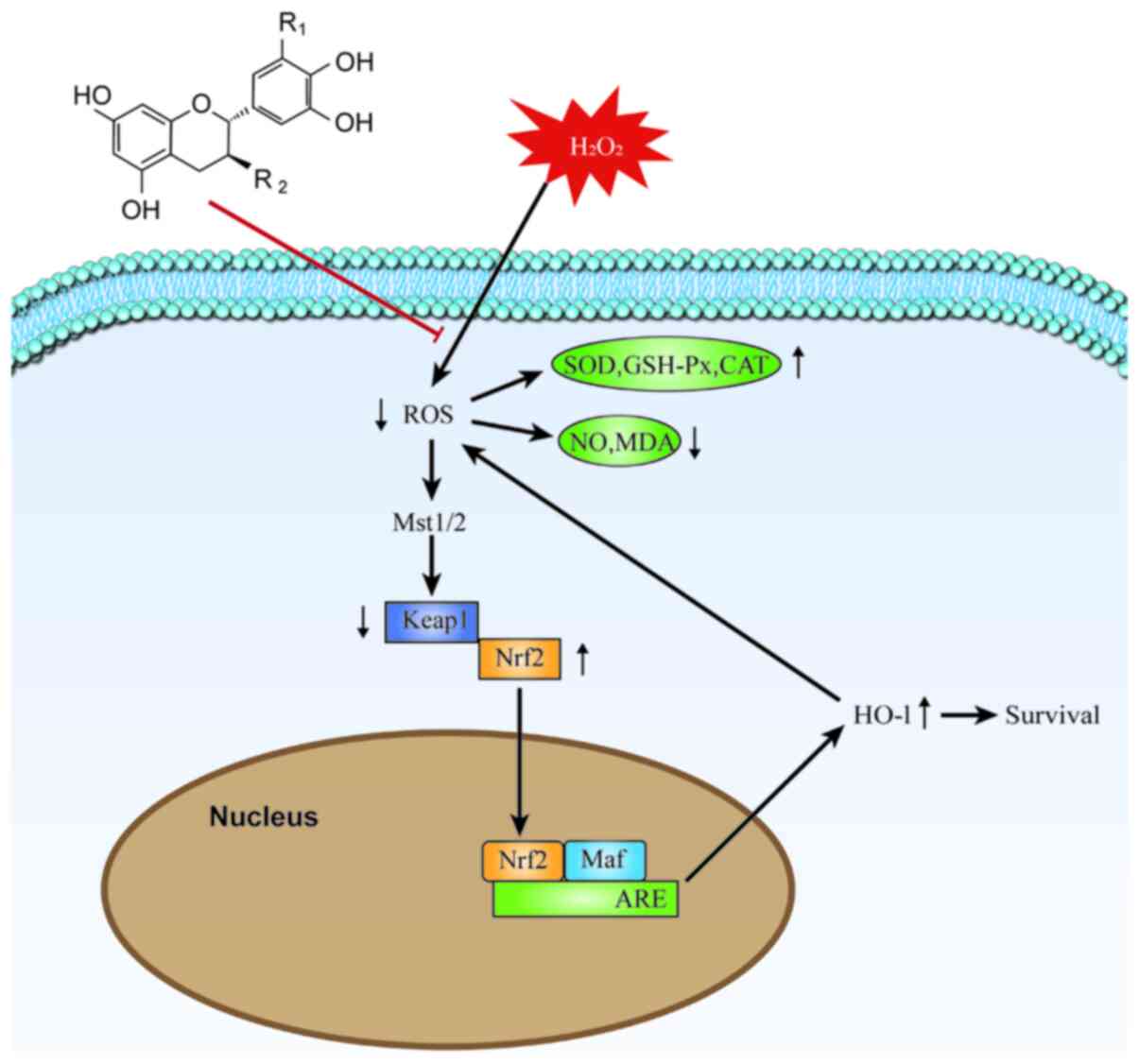 | Figure 7Molecular mechanism of TPs protection
against H2O2-induced RAW264.7 cell injury.
The excessive production of ROS in
H2O2-injured RAW264.7 cells is alleviated and
the balance of the oxidoreductase system is maintained by TPs. The
expression of Nrf2 is directly affected by the change of
intracellular ROS levels, following the Mst/Nrf2 axis maintenance
of cellular redox balance and via Nrf2 binding to the ARE in the
nucleus and thereby activating HO-1 protein expression. Induction
via the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway appears to
represent the antioxidant mechanism of TPs. TPs, tea polyphenols;
ROS, reactive oxygen species; Nrf2, nuclear factor
(erythroid-derived 2)-like 2; Mst, mammalian STE20-like protein
kinase; HO-1, heme oxygenase 1; Keap1, Kelch-like ECH-associated
protein 1; ARE, antioxidant response element; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; NO,
nitric oxide; MDA, malondialdehyde; Maf, musculoaponeurotic
fibrosarcoma; ARE, antioxidant response element. |
Supplementary Material
The representative chromatograms of
tea polyphenols and four standard compounds. 1. EGCG; 2. EC; 3.
EGC; 4. ECG. The content of EGCG (46.8%) was the highest, followed
by EGC (17.3%), EC (5.2%) and ECG (1.5%). EGCG,
-epigallocatechin-3-gallate; EC, -epicatechin; EGC,
-epicatechin-3-gallate; ECG, -epigallocatechin.
Acknowledgements
The authors would like to thank Mrs. Jia-Qi Tan, Dr
Yu-Jin Xu and Dr Ce-Shu Gao (Department of Neurology, Beijing
Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua
University) for their technical support and assistance with data
analysis.
Funding
The current study was supported by the National Natural Science
Foundation of China (grant no. 81670090).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
QL, YW and HX performed the experiments and
analyzed the data; QL and ZQ obtained the data and wrote the
manuscript. XC and YZ analyzed the data. JT, LL and CG
conceptualized and guided the research. QL and HX confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang CS, Kim S, Yang GY, Lee MJ, Liao J,
Chung JY and Ho CT: Inhibition of carcinogenesis by tea:
Bioavailability of tea polyphenols and mechanisms of actions. Proc
Soc Exp Biol Med. 220:213–217. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khan N and Mukhtar H: Tea polyphenols in
promotion of human health. Nutrients. 11(39)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zuo AR, Dong HH, Yu YY, Shu QL, Zheng LX,
Yu XY and Cao SW: The antityrosinase and antioxidant activities of
flavonoids dominated by the number and location of phenolic
hydroxyl groups. Chin Med. 13(51)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ganesan K and Xu B: A critical review on
polyphenols and health benefits of black soybeans. Nutrients.
9(455)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Namal Senanayake SPJ: Green tea extract:
Chemistry, antioxidant properties and food applications - A review.
J Funct Foods. 5:1529–1541. 2013.
|
|
6
|
Gundimeda U, McNeill TH, Fan TK, Deng R,
Rayudu D, Chen Z, Cadenas E and Gopalakrishna R: Green tea
catechins potentiate the neuritogenic action of brain-derived
neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen
peroxide. Biochem Biophys Res Commun. 445:218–224. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ueda-Wakagi M, Nagayasu H, Yamashita Y and
Ashida AH: Green tea ameliorates hyperglycemia by promoting the
translocation of glucose transporter 4 in the skeletal muscle of
diabetic rodents. Int J Mol Sci. 20(2436)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Griendling KK, Sorescu D, Lassègue B and
Ushio-Fukai M: Modulation of protein kinase activity and gene
expression by reactive oxygen species and their role in vascular
physiology and pathophysiology. Arterioscler Thromb Vasc Biol.
20:2175–2183. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yan Z, Zhong Y, Duan Y, Chen Q and Li F:
Antioxidant mechanism of tea polyphenols and its impact on health
benefits. Anim Nutr. 6:115–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xing L, Zhang H, Qi R, Tsao R and Mine Y:
Recent advances in the understanding of the health benefits and
molecular mechanisms associated with green tea polyphenols. J Agric
Food Chem. 67:1029–1043. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang D, Wang Y, Wan X, Yang CS and Zhang
J: Green tea polyphenol (-)-epigallocatechin-3-gallate triggered
hepatotoxicity in mice: Responses of major antioxidant enzymes and
the Nrf2 rescue pathway. Toxicol Appl Pharmacol. 283:65–74.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qi G, Mi Y, Fan R, Zhao B, Ren B and Liu
X: Tea polyphenols ameliorates neural redox imbalance and
mitochondrial dysfunction via mechanisms linking the key circadian
regular Bmal1. Food Chem Toxicol. 110:189–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sies H: Hydrogen peroxide as a central
redox signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biol. 11:613–619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wen ZS, Liu LJ, OuYang XK, Qu YL, Chen Y
and Ding GF: Protective effect of polysaccharides from Sargassum
horneri against oxidative stress in RAW264.7 cells. Int J Biol
Macromol. 68:98–106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
West AP, Brodsky IE, Rahner C, Woo DK,
Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS and
Ghosh S: TLR signalling augments macrophage bactericidal activity
through mitochondrial ROS. Nature. 472:476–480. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin X, Bai D, Wei Z, Zhang Y, Huang Y,
Deng H and Huang X: Curcumin attenuates oxidative stress in
RAW264.7 cells by increasing the activity of antioxidant enzymes
and activating the Nrf2-Keap1 pathway. PLoS One.
14(e0216711)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang XT, Sun XQ, Wu C, Chen JL, Yuan JJ,
Pang QF and Wang ZP: Heme oxygnease-1 induction by methylene blue
protects RAW264.7 cells from hydrogen peroxide-induced injury.
Biochem Pharmacol. 148:265–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang P, Geng J, Gao J, Zhao H, Li J, Shi
Y, Yang B, Xiao C, Linghu Y, Sun X, et al: Macrophage achieves
self-protection against oxidative stress-induced ageing through the
Mst-Nrf2 axis. Nat Commun. 10(755)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang CS, Lambert JD and Sang S:
Antioxidative and anti-carcinogenic activities of tea polyphenols.
Arch Toxicol. 83:11–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Green LC, Wagner DA, Glogowski J, Skipper
PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite, and
[15N]nitrate in biological fluids. Anal Biochem. 126:131–138.
1982.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-∆∆C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wen ZS, Liu LJ, Qu YL, Ouyang XK, Yang LY
and Xu ZR: Chitosan nanoparticles attenuate hydrogen
peroxide-induced stress injury in mouse macrophage RAW264.7 cells.
Mar Drugs. 11:3582–3600. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rani V, Deep G, Singh RK, Palle K and
Yadav UC: Oxidative stress and metabolic disorders: Pathogenesis
and therapeutic strategies. Life Sci. 148:183–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmed NA, Radwan NM, Aboul Ezz HS and
Salama NA: The antioxidant effect of Green Tea Mega EGCG against
electromagnetic radiation-induced oxidative stress in the
hippocampus and striatum of rats. Electromagn Biol Med. 36:63–73.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen TS, Liou SY, Lin HH, Hung MY, Lin CC,
Lin YM, Lin KH, Padma VV, Yao CH, Kuo WW, et al: Oral
administration of green tea Epigallocatechin-3-gallate reduces
oxidative stress and enhances restoration of cardiac function in
diabetic rats receiving autologous transplantation of
adipose-derived stem cells. Arch Physiol Biochem. 127:82–89.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Valu MV, Soare LC, Sutan NA, Ducu C, Moga
S, Hritcu L, Boiangiu RS and Carradori S: Optimization of
ultrasonic extraction to obtain erinacine A and polyphenols with
antioxidant activity from the fungal biomass of hericium
erinaceus. Foods. 9(1889)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Urbańska B and Kowalska J: Comparison of
the total polyphenol content and antioxidant activity of chocolate
obtained from roasted and unroasted cocoa beans from different
regions of the world. Antioxidants. 8(283)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xia EQ, Deng GF, Guo YJ and Li HB:
Biological activities of polyphenols from grapes. Int J Mol Sci.
11:622–646. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xue HK, Tan JQ, Li Q, Cai X and Tang JT:
Optimization ultrasound-assisted extraction of anthocyanins from
cranberry using response surface methodology coupled with genetic
algorithm and identification anthocyanins with HPLC-MS2. J Food
Process Preserv. 45(e15378)2021.
|
|
33
|
Tan J, Li Q, Xue H and Tang J:
Ultrasound-assisted enzymatic extraction of anthocyanins from grape
skins: Optimization, identification, and antitumor activity. J Food
Sci. 85:3731–3744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xue H, Tan J, Li Q, Tang J and Cai X:
Ultrasound-assisted deep eutectic solvent extraction of
anthocyanins from blueberry wine residues: Optimization,
identification, and HepG2 antitumor activity. Molecules.
25(5456)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mukhtar H and Ahmad N: Cancer
chemoprevention: Future holds in multiple agents. Toxicol Appl
Pharmacol. 158:207–210. 1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Khan N and Mukhtar H: Tea polyphenols for
health promotion. Life Sci. 81:519–533. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Frei B and Higdon JV: Antioxidant activity
of tea polyphenols in vivo: Evidence from animal studies. J Nutr.
133:S3275–S3284. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lagha AB and Grenier D: Tea polyphenols
inhibit the activation of NF-κB and the secretion of cytokines and
matrix metalloproteinases by macrophages stimulated with
Fusobacterium nucleatum. Sci Rep. 6(34520)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Galati G, Lin A, Sultan AM and O'Brien PJ:
Cellular and in vivo hepatotoxicity caused by green tea phenolic
acids and catechins. Free Radic Biol Med. 40:570–580.
2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Isbrucker RA, Edwards JA, Wolz E,
Davidovich A and Bausch J: Safety studies on epigallocatechin
gallate (EGCG) preparations. Part 2: Dermal, acute and short-term
toxicity studies. Food Chem Toxicol. 44:636–650. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Coyle CH, Philips BJ, Morrisroe SN,
Chancellor MB and Yoshimura N: Antioxidant effects of green tea and
its polyphenols on bladder cells. Life Sci. 83:12–18.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang GY, Liao J, Li C, Chung J, Yurkow EJ,
Ho CT and Yang CS: Effect of black and green tea polyphenols on
c-jun phosphorylation and H(2)O(2) production in transformed and
non-transformed human bronchial cell lines: Possible mechanisms of
cell growth inhibition and apoptosis induction. Carcinogenesis.
21:2035–2039. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma YF, Zhao L, Coleman DN, Gao M and Loor
JJ: Tea polyphenols protect bovine mammary epithelial cells from
hydrogen peroxide-induced oxidative damage in vitro by activating
NFE2L2/HMOX1 pathways. J Dairy Sci. 102:1658–1670. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee MJ, Maliakal P, Chen L, Meng X, Bondoc
FY, Prabhu S, Lambert G, Mohr S and Yang CS: Pharmacokinetics of
tea catechins after ingestion of green tea and
(-)-epigallocatechin-3-gallate by humans: Formation of different
metabolites and individual variability. Cancer Epidemiol Biomarkers
Prev. 11:1025–1032. 2002.PubMed/NCBI
|
|
45
|
Nakagawa K, Okuda S and Miyazawa T:
Dose-dependent incorporation of tea catechins,
(-)-epigallocatechin-3-gallate and (-)-epigallocatechin, into human
plasma. Biosci Biotechnol Biochem. 61:1981–1985. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ullmann U, Haller J, Decourt JP, Girault
N, Girault J, Richard-Caudron AS, Pineau B and Weber P: A single
ascending dose study of epigallocatechin gallate in healthy
volunteers. J Int Med Res. 31:88–101. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Avshalumov MV, Bao L, Patel JC and Rice
ME: H2O2 signaling in the nigrostriatal
dopamine pathway via ATP-sensitive potassium channels: Issues and
answers. Antioxid Redox Signal. 9:219–231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mao X, Gu C, Chen D, Yu B and He J:
Oxidative stress-induced diseases and tea polyphenols. Oncotarget.
8:81649–81661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Forman HJ, Bernardo A and Davies KJ: What
is the concentration of hydrogen peroxide in blood and plasma? Arch
Biochem Biophys. 603:48–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Brown GC: Reversible binding and
inhibition of catalase by nitric oxide. Eur J Biochem. 232:188–191.
1995.PubMed/NCBI View Article : Google Scholar
|
|
51
|
McBride AG, Borutaité V and Brown GC:
Superoxide dismutase and hydrogen peroxide cause rapid nitric oxide
breakdown, peroxynitrite production and subsequent cell death.
Biochim Biophys Acta. 1454:275–288. 1999.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jadeja RN, Upadhyay KK, Devkar RV and
Khurana S: Naturally occurring Nrf2 activators: Potential in
treatment of liver injury. Oxid Med Cell Longev.
2016(3453926)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295.
2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shukla K, Pal PB, Sonowal H, Srivastava SK
and Ramana KV: Aldose reductase inhibitor protects against
hyperglycemic stress by activating Nrf2-dependent antioxidant
proteins. J Diabetes Res. 2017(6785852)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Qi GY, Mi YS, Fan R, Li RN, Wang YW, Li
XY, Huang SX and Liu XB: Tea polyphenols ameliorate hydrogen
peroxide- and constant darkness-triggered oxidative stress via
modulating the Keap1/Nrf2 transcriptional signaling pathway in
HepG2 cells and mice liver. RSC Advances. 7:32198–32208. 2017.
|
|
57
|
Wang D, Zhang M, Wang T, Liu T, Guo Y and
Granato D: Green tea polyphenols mitigate the plant lectins-induced
liver inflammation and immunological reaction in C57BL/6 mice via
NLRP3 and Nrf2 signaling pathways. Food Chem Toxicol.
144(111576)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sueoka N, Suganuma M, Sueoka E, Okabe S,
Matsuyama S, Imai K, Nakachi K and Fujiki H: A new function of
green tea: Prevention of lifestyle-related diseases. Ann N Y Acad
Sci. 928:274–280. 2001.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jian L, Xie LP, Lee AH and Binns CW:
Protective effect of green tea against prostate cancer: A
case-control study in southeast China. Int J Cancer. 108:130–135.
2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Miyata Y, Shida Y, Hakariya T and Sakai H:
Anti-cancer effects of green tea polyphenols against prostate
cancer. Molecules. 24(193)2019.PubMed/NCBI View Article : Google Scholar
|