Introduction
Traumatic brain injury (TBI) represents a global
health problem (1). The
pathophysiology of brain injury following craniocerebral trauma is
complex and is characterized by initial and secondary damage for
several days following trauma (2).
Among the numerous causes of morbidity and mortality associated
with trauma, the incidence of TBI is becoming the most important
cause, resulting in severe disability and mortality (3) and functional impairment, which can
affect the quality of life (4,5).
At present, the treatment strategies for brain
trauma mainly include the control of secondary injury and promotion
of neurorehabilitation through training and drug administration
(6). However, this type of
intervention is not enough to fully restore neural system function,
so new strategies should be discovered (1,6). In
the last decade, some studies on stem cell transplantation as a TBI
replacement therapy (7,8), in animal models (9) and in the clinic have produced
promising results (10,11). The benefits of stem cell
transplantation are multifaceted. Firstly, the ability of stem
cells to undergo neural differentiation and long-distance migration
to the injury site allows them to directly replace dead or dying
cells (12,13). Secondly, the presence of stem cells
in lesions indirectly affects the microenvironment. By secreting
growth factors, stem cells can promote the proliferation of nerve
cells and neurotransmitter transmission (14,15).
However, transplanted stem cells have relatively poor survival, and
their limited survival following brain injury and early death of
transplanted cells limits bone marrow mesenchymal stem cell-based
treatment (16,17). In order to fully use the therapeutic
potential of stem cells, it is crucial to determine the cause of
their early death and develop strategies to improve their survival
rate.
Previous studies reported that autophagy has a
protective effect on mesenchymal stem cells (MSCs) under stress
conditions, such as ischemia stroke (18,19).
Autophagy is a process of cellular self-protection that supports
the homeostasis of cells under external and internal environmental
stresses, including undernutrition, infection, presence of cell
debris and protein aggregation (20,21).
Autophagy and apoptosis are the two main pathways involved in cell
protection from external stimuli. These two processes are usually
co-regulated but result in opposite cell outcomes (22). Autophagy is a self-digesting process
during which a double-membrane vesicle, known as the autophagosome,
is produced around the targeted organelle. Autophagosomes degrade
their cargo via a lysosomal mechanism (23). The autophagy process can maintain
cell fitness, nutrition, and energy levels during starvation or
exposure to external stress (24).
Conversely, the programmed cell death of damaged or senescent cells
is known as apoptosis (24).
Previous studies demonstrated that external stress stimuli may
trigger one of these two processes, depending on the cellular
environment (25,26). The inhibition of one of these
pathways can lead to the activation of the other. Triggering
autophagy during programmed cell death can reduce apoptosis and
prolong cell survival, whereas reducing autophagy in normal cells
can increase apoptosis (27,28).
The regulation of autophagy may therefore provide a new mechanism
to prevent cell apoptosis under stress conditions.
Shikonin is a natural naphthoquinone derivative
isolated from the root of plant comfrey, and which possesses some
anti-tumour, anti-inflammatory (29-31),
antibacterial (32) and
antithrombotic (33) activities. A
study has reported that shikonin can inhibit tumour cell
proliferation and metastasis by inhibiting the phosphorylation of
mTOR (34). Shikonin can increase
autophagy in A375 cells with 1,2 µM in 24 h (35). The present investigation illustrates
the protective effect and underlying mechanism of shikonin in human
umbilical MSCs (HUMSCs) in a hypoxic-ischemic state in vitro
and in vivo.
Materials and methods
Materials
Shikonin was purchased from MedChemExpress (cat. no.
HY-N0822) and was dissolved in DMSO to prepare a stock solution(10
mg/ml; stored at -20˚C) and diluted to 1.2, 1.6, 2, 4, and 8 µM for
use. 3-methyladenine (3-MA), an inhibitor of the PI3k/AMPK pathway
used for creating an inhibition model of autophagy, was purchased
from MedChemExpress (cat. no. HY-19312) and dissolved in double
distilled water to prepare stock solutions (5 mM and 1,000 µM
stored at 4˚C). DMEM and fetal bovine serum (FBS) were purchased
from HyClone; GE Healthcare Life Sciences. Trypan blue stain (0.4%;
cat. no. T10282) was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. A One-step TUNEL Apoptosis Detection kit was
purchased from Beyotime Institute of Biotechnology. The purified
antibodies were used as follows: Anti-AMP-activated protein kinase
(AMPK; cat. no. 9158 Cell Signaling Technology, Inc.; 1:1,000);
anti-β-actin (cat. no. 4967 Cell Signaling Technology, Inc.;
1:1,000); anti-GAPDH (cat. no. 5174; Cell Signaling Technology,
Inc.; 1:1,000); anti-sequestosome 1 (SQSTM1)/p62 (cat. no. 5114S;
Cell Signaling Technology, Inc.; 1:1,000); horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (cat. no. 31460; Invitrogen; Thermo Fisher Scientific,
Inc.; 1:5,000); anti-microtubule-associated protein 1A/1B-light
chain 3 (LC3A/B; 1:1,000; cat. no. 12741S; Cell Signaling
Technology, Inc.; 1:1,000); anti-phospho (p)-mTOR (CST; cat. no.
5536S; 1:1,000); anti-mTOR (cat. no. 2983S; Cell Signaling
Technology, Inc.; 1:1,000); anti-p-AMPK (cat. no. 5759; Cell
Signaling Technology, Inc.; 1:1,000); anti-α-tubulin (cat. no.
5335; Cell Signaling Technology, Inc.; 1:1,000); anti-autophagy
protein 5 (ATG5; 1:500; cat. no. ab108327; Abcam; 1:1,000);
anti-Beclin 1 (cat. no. ab207612; Abcam; 1:1,000);
anti-pro-caspase-3 (cat. no. ab32150; Abcam; 1:1000);
anti-cleaved-caspase-3 (cat. no. ab2302; Abcam; 1:500); anti-BCL-2
(cat. no. ab182858; Abcam; 1:1,000) and anti-BAX (cat. no.
ab182733; Abcam; 1:1,000). The Cell Counting Kit-8 (CCK-8), RIPA,
PMSF and BCA kits were purchased from Beyotime Institute of
Biotechnology.
HUMSCs
GFP-labelled HUMSCs were purchased from Cyagen
Biosciences, Inc. (cat. no. HUXUC-01001). A total of
1x106 cells/vial were cryopreserved at passage 2 and
stored in liquid nitrogen. These cells had been collected from the
umbilical cord of healthy pregnant women with normal full-term
delivery by using Wharton's Jelly. The cells were cultured (DMEM +
20% fetal bovine serum + 1% penicillin-streptomycin mixture) and
placed at 37˚C in a humidified incubator containing 5%
CO2. To stimulate hypoxic-ischemic conditions, cells
were subjected to oxygen glucose deprivation (OGD).
OGD
HUMSCs were cultured and passaged in sugar- and
serum-free medium, and the oxygen concentration in the incubator
was adjusted to 0% to prepare a hypoxic environment.
Cell proliferation and treatment
Cells were seeded in a 96-well plate at a density of
105 cells/well in 100 µl culture medium. Cells were
cultured in an incubator containing 5% CO2 at 37˚C for
24 h. Subsequently, different concentrations of shikonin (0.2, 0.4,
0.8, 1.2 and 1.6 µM) were added to the plates. Plates were
incubated for 4, 8, 12 and 24 h under OGD pressure conditions.
Subsequently, 10 µl CCK-8 solution was added per well and the plate
was incubated at 37˚C for 1 h. A DeTie microplate reader was used
to measure the absorbance at a wavelength of 450 nm. Following
CCK-8, the optimal processing time and drug concentration was
obtained. All cells were divided into three groups: OGD, OGD +
shikonin and OGD + shikonin + 3-MA. OGD groups were treated only
with OGD. In group OGD + shikonin, shikonin was used to treat cells
with OGD. In OGD + shikonin + 3-MA group, shikonin and 3-MA (5 mM
for 24 h before OGD) were used at same time.
Animal model and tissue
A total of 18 male C57BL/6 mice (15-20 g;
9-12-week-old) were obtained from the Animal Experimental Center of
Wenzhou Medical University. Mice were divided into three groups as
follows: TBI, TBI + shikonin and TBI + shikonin + 3-MA groups (6
mice per group). Treatments were administered for 3 consecutive
days before the TBI model was established. In mice, 50 mg/kg
shikonin (36) was orally
administered with 3-MA (1.5 mg/100 g), as appropriate. TBI was
modelled using an Impact One™ Stereotaxic Impactor (Leica
Microsystems, Inc.) at a depth of 1.0 mm and speed of 3 m/sec
(37) in anesthesized mice. Animal
anesthesia was performed using a Reyward small animal anesthesia
machine and isoflurane inhalation at 3-4%. Once the gas completely
filled the induction box (~1 min), the animals were placed into it.
Once the animals were completely anesthetized (~2-3 min; animals
were turned over and did not attempt resume prone position),
anesthesia was maintained using 1-1.5% isoflurane, and the gas flow
rate was about 300-500 ml/min. GFP-HUMSCs were transplanted 24 h
after TBI. The experimental mice were sacrificed seven days later.
In each group, three mice cortical tissue surrounding the trauma
was obtained and stored at -80˚C immediately. The other three, the
brains were fixed with 4% PFA for 24 h with 4˚C. Then the sections
were dehydrated with 30% sucrose for 48 h with 4˚C, embedded in
optimal cutting temperature compound (OCT) and frozen, then
sectioned at 10 µm. Animal experiments lasted a total of 11 days.
All animal health, behaviour and animal welfare (including regular
cleaning of cages, replacement of food and drinking water and no
more than 5 mice in one cage) were monitored at the Animal
Experiment Center of Wenzhou Medical University. The experimental
mice were sacrificed by decapitation following an overdose of
isoflurane. The study was approved by the Ethics Committee of The
First Affiliated Hospital of Wenzhou Medical University (Wenzhou,
China). All procedures were in compliance with the Animal Care and
Use Committee of Wenzhou Medical University.
Cells transplantation
GFP-HUMSCs were trypsinized with 0.05% trypsin
solution for 3 min at 37˚C before transplantation. Then, HUMSCs
were transferred from the culture dish to the test tube, washed
with PBS for 3 times, and then injected into the TBI modelled area
with a stereotaxic instrument and a micro injection needle
(1x105 cells in 3 µl at a delivery rate of 1
µl/min.).
Trypan blue staining
Trypan blue staining was performed after shikonin
treatment. HUMSCs were detached by trypsin-EDTA, and resuspended in
medium. Subsequently, 200 µl cell suspension was supplemented with
0.4% trypan blue (ratio cell suspension:trypan blue; 9:1) and
incubated for 5 min at room temperature. Under the microscope, dead
cells were stained blue, whereas living cells were transparent and
colourless. ImageJ (v. 1.6.0; National Institutes of Health) was
used to count the number of normal cells and dead cells in the
figure. The rate of living cells was calculated by GraphPad Prism
7.0.
TUNEL immunofluorescence for
HUMSC
To prepare TUNEL detection solution, TdT enzyme was
added with fluorescent labelling solution at a 1:9 dilution. The
HUMSCs were seeded on the glass cover in 24-well plate, and use PBS
to wash redundant medium. The cell culture plate was then washed
twice with PBS or Hank's Balanced Salt Solution for 10 min each
time. Subsequently, 50 µl prepared TUNEL detection solution was
added to each well and incubated at 37˚C for 1 h in a wet box in
the dark. After staining with DAPI, a coverslip was mounted and the
cells observed under a fluorescence microscope (Leica Microsystems,
Inc.). The excitation wavelength range was 450-500 nm (green
fluorescence) and 420-450 nm (blue fluorescence).
Western blotting (WB)
Brain tissues were mashed on the ice, lysed with
RIPA buffer for 30 min on ice and sonicated (30% energy, 2 sec
duration, 2 sec interval, total time 30 sec). Centrifuge at 12,000
rpm for 10 min at 4˚C. Transfer the supernatant to a new tube. PMSF
(1:100) was added, and a BCA kit was used to determine protein
concentration. Cells were washed with PBS, lysed with RIPA buffer
for 30 min on ice and sonicated (30% energy, 2 sec duration, 2 sec
interval, total time 30 sec). PMSF (1:100) was added, and a BCA kit
was used to determine protein concentration. Proteins (60 µg) were
separated by 10% SDS-PAGE and transferred onto PVDF membranes. The
membranes were blocked 3 h (5% milk/PBS at room temperature).
Membranes were incubated with primary antibodies against mTOR,
p-mTOR, AMPK, p-AMPK, GAPDH, SQSTM1/p62, β-actin, LC3A/B, Beclin 1
ATG5 BCL2 Bax pro-caspase-3 and cleaved caspase-3 at 4˚C overnight.
Membranes were washed thrice with TBS-Tween-20 (TBS-T) and
incubated with secondary antibody (dissolved in 1% BSA; 1:5,000) at
room temperature for 1 h. Membranes were washed three times with
TBS-T and exposed to Bio-Rad ChemiDoc XRS (Bio-Rad Laboratories,
Inc.) under West Femto ECL Substrate (Beijing Solarbio Science
& Technology Co., Ltd.) and analysed with Image Lab 3.0
(Bio-Rad Laboratories, Inc.).
Autophagy analysis
After preparing cell protein samples, western
blotting was used to verify the increase in autophagy. Autophagy is
mediated by LC3BI, and increases when LC3BI is converted to LC3BII
(38). Therefore, LC3BI and LC3BII,
as well as the expression of their downstream products in each
experimental group, were used to analyze autophagy.
Statistical analysis
The data were expressed as the means ± standard
deviation. One-way ANOVA with Dunnett's post hoc test were used to
determine the statistical significance of the observed differences.
Statistical analysis was performed using and GraphPad Prism 7.0
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell proliferation
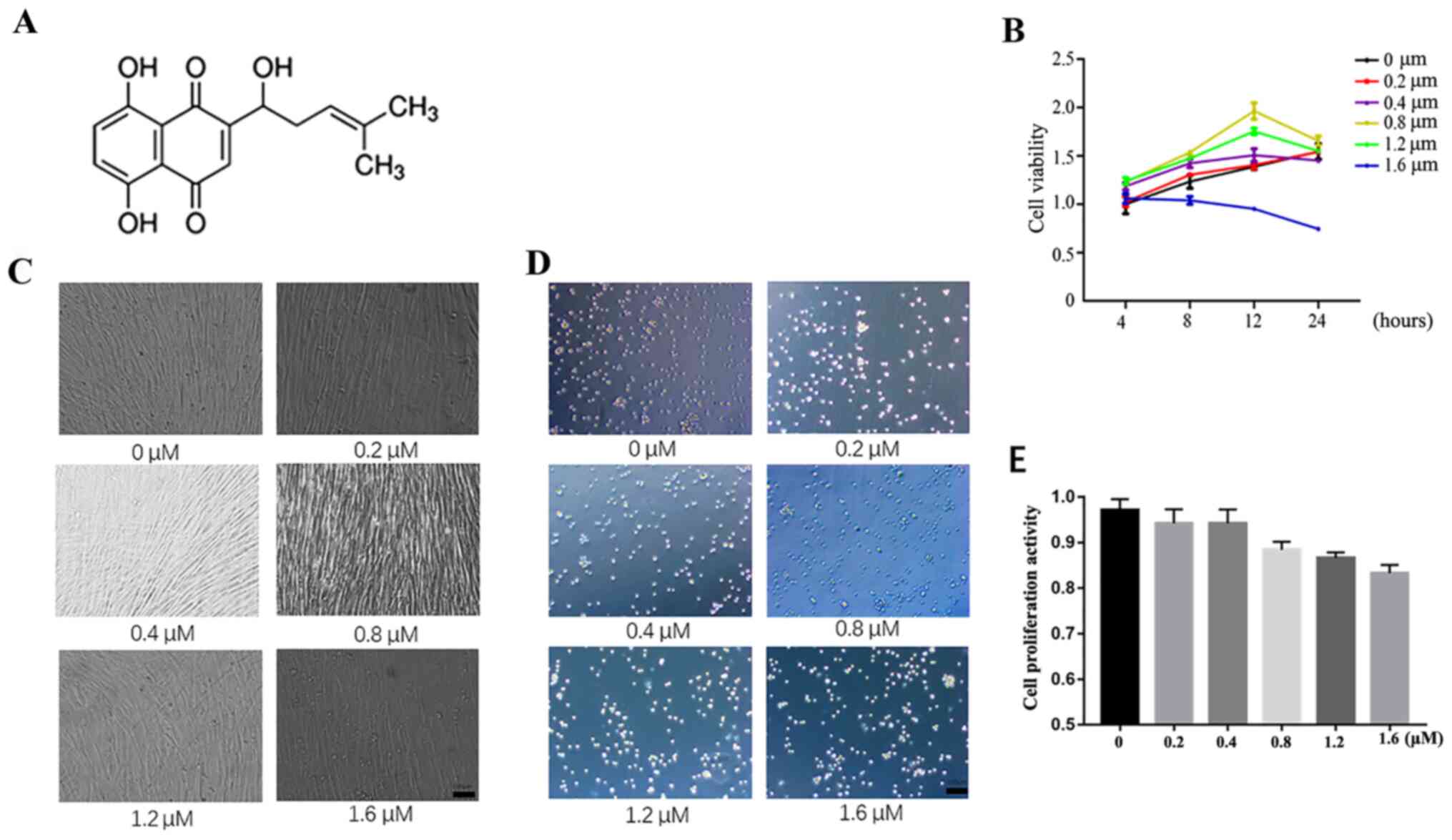
HUMSCs were used to study the effect of shikonin
(Fig. 1A) on cell proliferation.
The results from the CCK-8 assay demonstrated that the optimal
condition under OGD is 0.8 µM shikonin treatment for the 12 h
(Fig. 1B). In normal culture
conditions, no significant cell death was observed after 24-h
treatment with shikonin at different concentrations according to
results from trypan blue staining. (Fig. 1C shows cell density at various
concentrations under light microscope following shikonin treatment,
1D trypan blue staining and 1E quantification of trypan blue
staining).
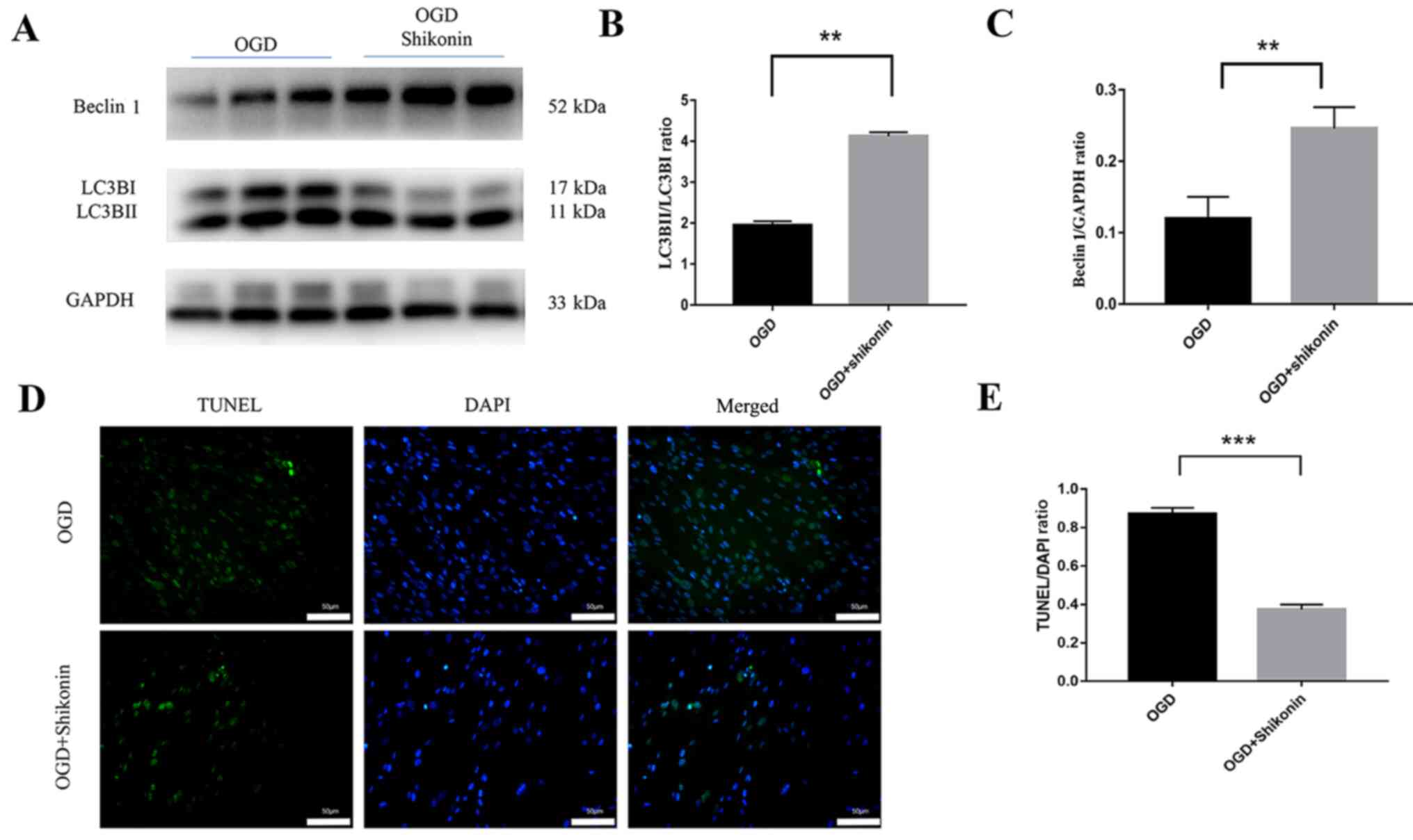
Shikonin protects HUMSCs from
hypoxia-ischemia-induced apoptosis
Previous studies demonstrated that the maximum time
for hypoxia-induced early apoptosis of mesenchymal stem cells
occurs at 24 h (39). The present
study investigated whether shikonin could reverse this process in
HUMSCs. Since 12 h was the optimal time for drug administration,
HUMSCs were exposed to OGD for 12 h together with 0.8 µM shikonin,
and apoptosis was determined using the TUNEL assay (Fig. 2D). The number of cells stained with
TUNEL divided by the number of cells stained with DAPI can reflect
the degree of apoptosis. Shikonin significantly inhibited TUNEL
signal, and the TUNEL/DAPI ratio of the shikonin group was
significantly lower compared with the OGD group (P<0.001;
Fig. 2E). In the present study,
shikonin promoted autophagy at the concentration of 0.8 µM used to
decrease HUMSC apoptosis in vitro. The conversion rate of
the cytosolic form of LC3 [LC3BII; ratio of LC3BII to
LC3-phosphatidylethanolamine conjugate (LC3BI); P<0.01; Fig. 2A and B] and the expression of Beclin-1 (shikonin
+ OGD vs. OGD group; P<0.01; Fig.
2A and C) were also measured.
The results from western blotting demonstrated that
shikonin-treated cells had significantly increased autophagy
compared with OGD-treated cells.
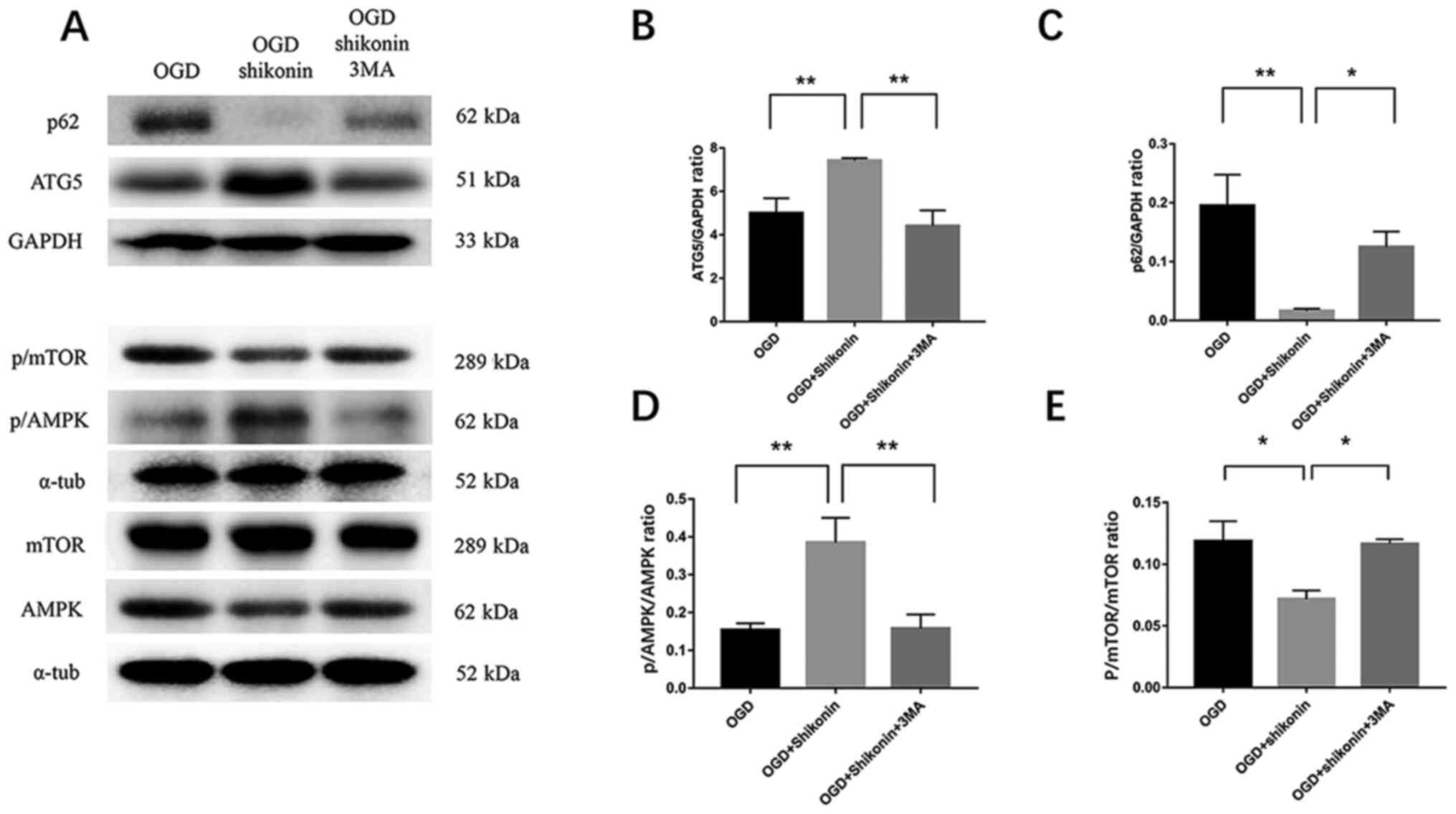
Shikonin treatment increases autophagy
and reduces apoptosis by activating the AMPK/mTOR signalling
pathway
The AMPK/mTOR signalling pathway is an important
regulator of autophagy in numerous cell types (39,40).
The present study investigated whether this signalling pathway
could mediate the anti-apoptotic effect of shikonin in HUMSCs
(Figs. 3 and 4). The results from western blotting
demonstrated that ATG5 and p-AMPK expression was lower in the OGD
group compared with the OGD + shikonin group; however, p62 and
p-mTOR expression was higher in the OGD group compared with the OGD
+ shikonin group. Conversely, shikonin exposure resulted in a
significant increase in the ratio p-AMPK/AMPK (shikonin + OGD vs.
OGD group; P<0.01; Fig. 3A and
D); however, the ratio p-mTOR/mTOR
was decreased (shikonin + OGD vs. OGD group; P<0.05; Fig. 3A and E). Compared with the OGD group, there was
a decreased expression of p62 in the shikonin group (shikonin + OGD
vs. OGD group; P<0.01; Fig. 3A
and C), and the expression of ATG5
was higher compared with the OGD group (shikonin + OGD vs. OGD
group; P<0.01; Fig. 3A and
B). Furthermore, treatment with
3-MA lead to a decrease in p-AMPK (shikonin + OGD vs. shikonin +
OGD + 3-MA group, P<0.01 Fig. 3A
and D) and p62 expression (shikonin
+ OGD vs. shikonin + OGD + 3-MA group; P<0.01; Fig. 3A and C), and an increase in ATG5 (shikonin + OGD
vs. shikonin + OGD + 3-MA group; P<0.01; Fig. 3A and B) and p-mTOR expression (shikonin + OGD
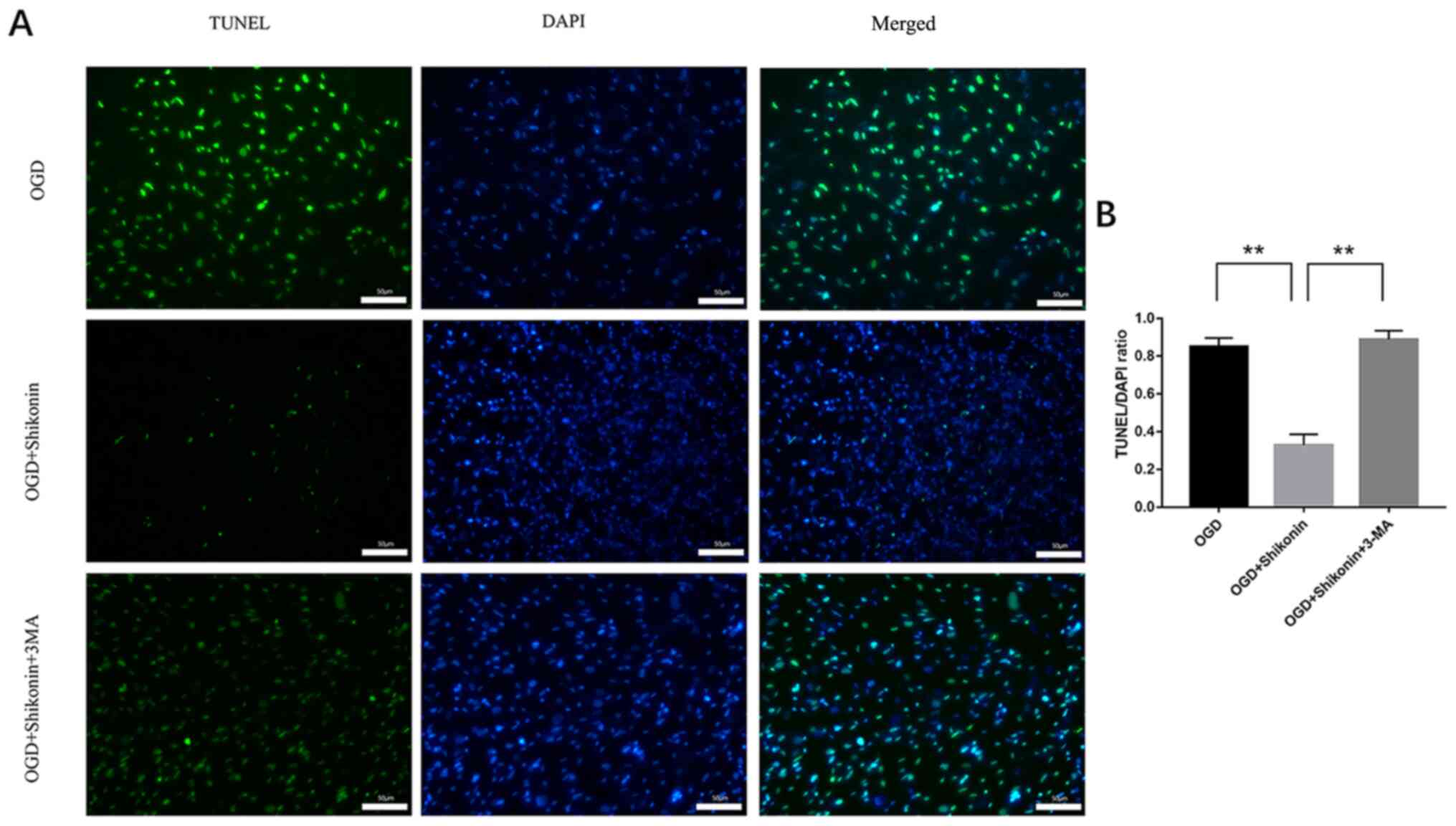
vs. shikonin + OGD + 3-MA group; P<0.05; Fig. 3A and E). In addition, the results from TUNEL
assay demonstrated that the number of TUNEL-positive cells
following treatment with 3-MA and shikonin was significantly higher
compared with shikonin-treated cells (shikonin + OGD vs. OGD group,
P<0.01; shikonin + OGD vs. shikonin + OGD + 3-MA group,
P<0.01; Fig. 4).
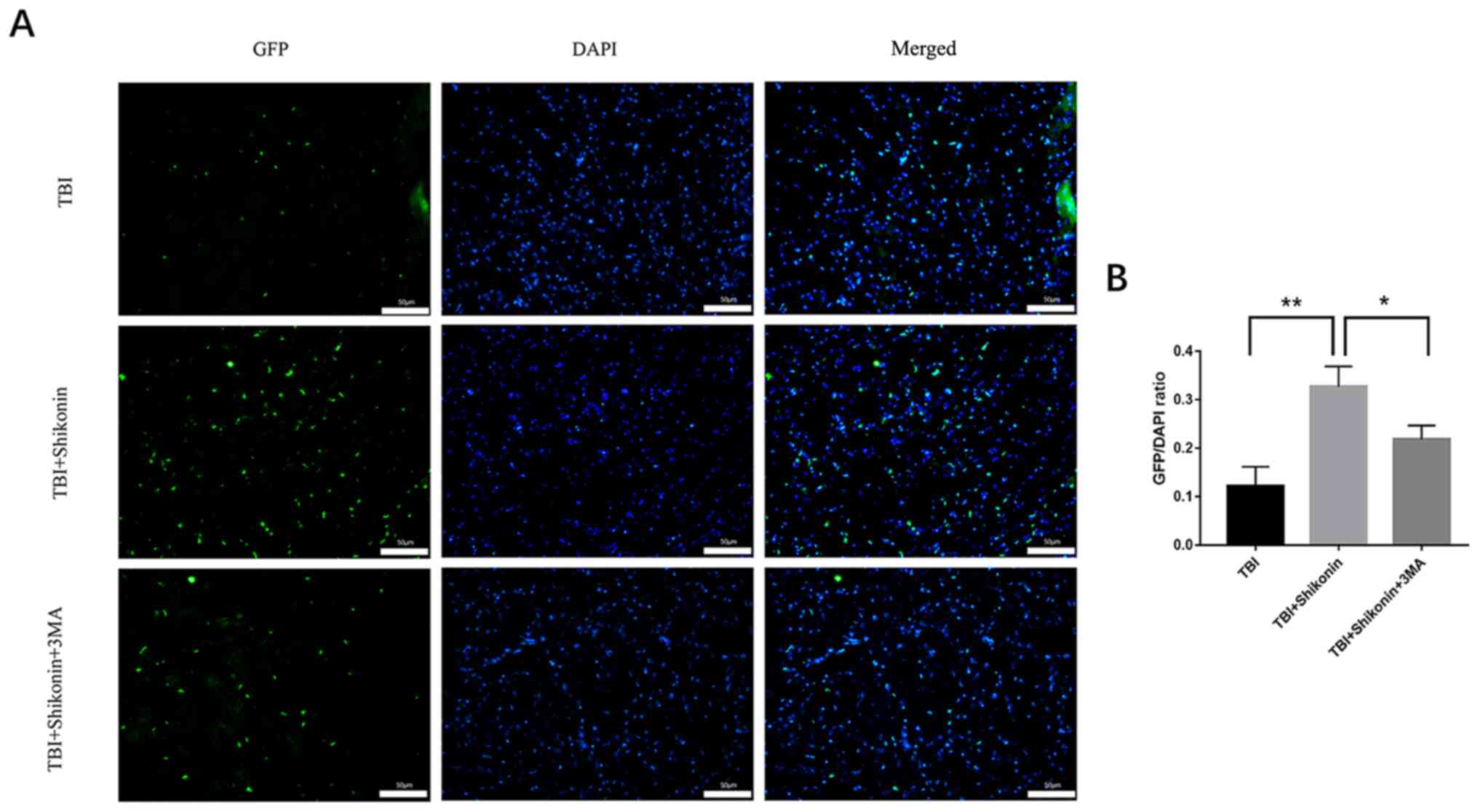
Shikonin increases the survival rate
of transplanted cells in the tissues surrounding the brain
contusion
HUMSCs with GFP protein gene emitting green
fluorescence were found in brain tissue surrounding the TBI,
suggesting that transplanted HUMSCs were able to survive and
migrate to the site of injury (41). In addition, the GFP from HUMSCs was
significantly increased 7 days after transplantation in the
shikonin-pretreated group compared with HUMSCs alone (shikonin +
TBI vs. TBI group, P<0.01; shikonin + TBI vs. shikonin + TBI +
3-MA group, P<0.05; Fig. 5A).
These findings suggested that shikonin may improve the number and
cell viability of HUMSCs in the transplanted area.
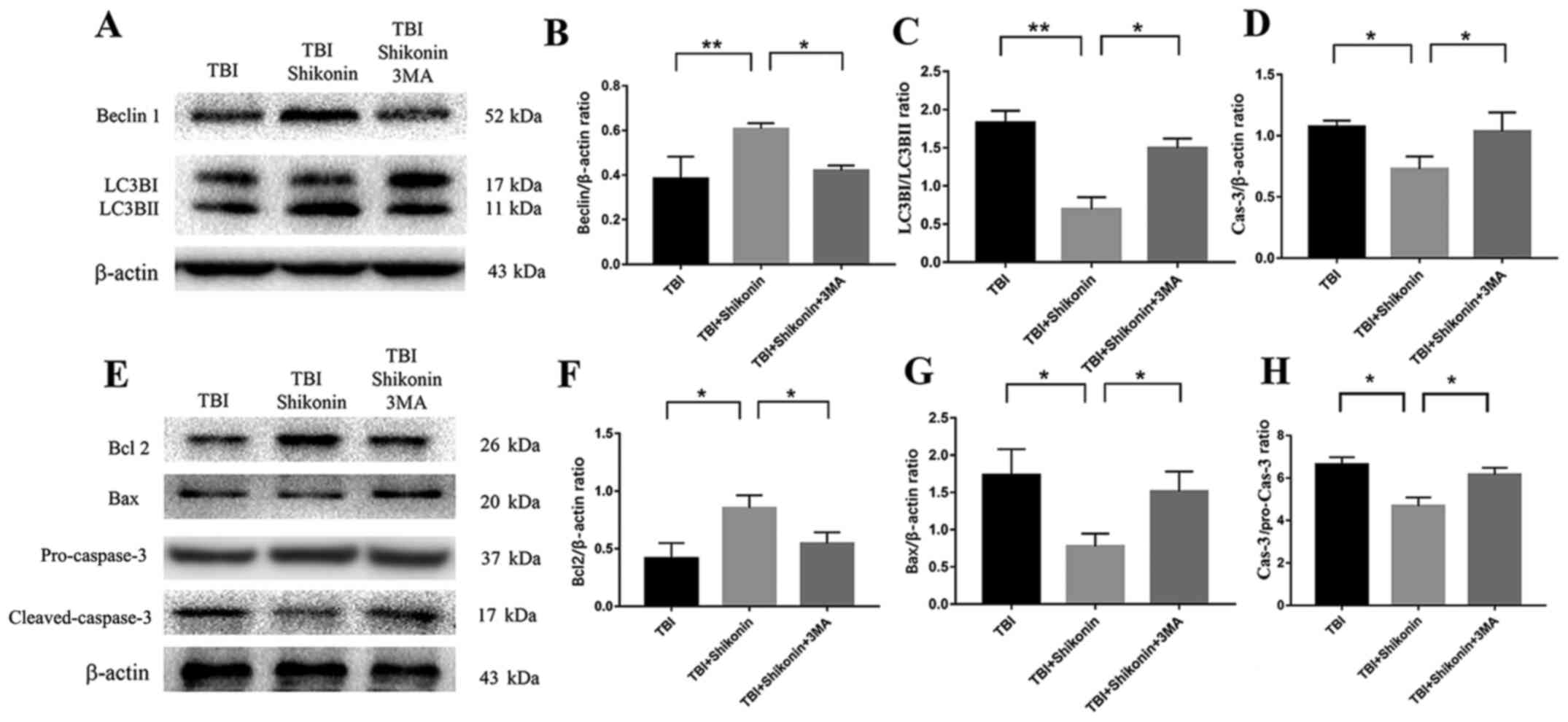
Expression of autophagy- and
apoptosis-related proteins in tissues surrounding trauma
The expression of autophagy- and apoptosis-related
proteins in the tissues surrounding the brain injury 12 h after
HUMSC transplantation was analyzed using western blotting. The
results demonstrated that Beclin-1 (shikonin + TBI vs. TBI group;
P<0.01; Fig. 6A and B), Bcl-2/Bax ratio(shikonin + TBI vs. TBI
group; P<0.01; Fig. 6H) and
Bcl-2 (shikonin + TBI vs. TBI group; P<0.05; Fig. 6E and F) expression, and LC3B (shikonin + TBI vs.
TBI group; P<0.01; Fig. 6A and
C) conversion in the shikonin + TBI
group were higher compared with the TBI group. However, expression
of Bax (shikonin + TBI vs. TBI group; P<0.05; Fig. 6E and G) and caspase-3 (shikonin + TBI vs. TBI
group; P<0.05; Fig. 6E, D and H)
was decreased. In mice transplanted with HUMSCs and co-treated with
3-MA and shikonin, these effects were reversed (shikonin + TBI vs.
shikonin + TBI + 3-MA group; P<0.05; Fig. 6).
Discussion
Acute TBI is associated with severe complications,
including tissue ischemia, excitotoxicity and overproduction of
free radicals, leading to the release of inflammatory molecules,
and to axonal and neuroendothelial cell damage (42,43).
Stem cell transplantation represents a novel treatment that could
have a crucial role in the prognosis of patients with TBI (1). In the acute phase of TBI, there is
severe cerebral ischemia in the region of brain contusion. At this
point, the transplanted stem cells in this region are in a hypoxic
and hypotrophic state, eventually resulting in severe apoptosis,
which impairs the therapeutic benefits of stem cell therapy
(42-44).
Therefore, improving the survival rate of transplanted stem cells
in this environment is crucial. In the present study,
shikonin-induced autophagy served an important role in protecting
mesenchymal stem cells from hypoxia-ischemia-induced apoptosis
through AMPK/mTOR-related signalling pathway, which may allow MSCs
to become resistant to the fluctuation of growth factors and
nutrient deprivation in the ischemic microenvironment, particularly
following TBI injury.
Although human and rodent brains share some
physiological similarities, there are significant differences in
the structure and function of their brain, which may cause rodents
to respond differently to trauma (45). Furthermore, although the Glasgow
coma score is an important indicator in TBI clinical experiments
(45,46), it is not usable in animals.
Therefore, since computerized tomography, Magnetic resonance
imaging and other imaging methods can be used to directly evaluate
the prognosis of TBI, these methods may be able to confirm the
results of the present study. Previous studies demonstrated that
shikonin can improve the survival of chondrocytes, reduce
chondrocyte apoptosis and promote tissue repair after spinal injury
(29). These studies indicated that
shikonin would be a relatively effective inhibitor of apoptosis. It
has been demonstrated that shikonin can increase the expression of
Beclin-1 and reduce the conversion rate of LC3-II, and the lesion
volume (35,36). Previous studies reported an
endogenous protective mechanism of tissue ischemia and an increase
in AMPK during tissue ischemia, indicating that this pathway is
active during tissue ischemia (47-49).
The present study demonstrated that shikonin could regulate
autophagy by inhibiting the AMPK/mTOR pathway and reduce apoptosis
and increase the survival rate of HMSCs, providing therefore a
protective effect against contused peripheral ischemic tissue in
stem cell-based therapies.
Decreased cell viability under ischemic stress is a
barrier to stem cell therapy. Autophagy is a cellular response that
is critical for the survival of cells under metabolic stress and
energy starvation (7,20). Autophagy is a catabolic process that
transfers cytoplasmic components to lysosomes for their degradation
(50). Hypoxia and ischemia are
sources of cellular stress (51).
By eliminating cellular energy supplies and destructing organelles
damaged by free radicals, autophagy serves a vital role in cell
survival (52,53). Previous studies on shikonin reported
that it increases autophagy (35,54).
In the present study, shikonin reduced the apoptosis of HUMSCs
under hypoxic-ischemic conditions, which was accompanied by an
increase in autophagy. Furthermore, the protective effect of
shikonin was significantly decreased with the addition of the
autophagy inhibitor 3-MA, which confirmed that shikonin exerted an
anti-apoptotic effect by regulating autophagy in HUMSCs.
AMPK is a stress-signalling kinase that is a key
regulator of energy production and depletion pathways, thus
protecting cells from hypoxia and cell death (18,40).
One of the main mechanisms of AMPK action is the regulation of
autophagy under stress conditions (48). Under hypoxia/SD or other energy
shortages, AMPK may act as a sensor of cellular energy change,
activated by a lower ATP/AMP ratio (18,40).
Furthermore, mTOR is a major downstream target of AMPK and can
stimulate autophagy by inactivating the autophagy pathway inhibitor
mTOR complex 1(55). It was
therefore hypothesized that the AMPK/mTOR signalling pathway could
play a positive regulatory role in hypoxia, starvation and other
energy stress events (55). The
present study demonstrated that under hypoxic-ischemic conditions,
the expression of p-AMPK was increased by shikonin, whereas the
expression of p-mTOR was decreased. The opposite effects on p-AMPK
and p-mTOR were observed when the AMPK inhibitor 3-MA was used
together with shikonin. These results suggested that the regulation
of autophagy via the AMPK/mTOR signalling pathway and, ultimately,
the inhibition of apoptosis, may be considered as a potential
mechanism through which shikonin inhibits apoptosis in HUMSCs.
In conclusion, shikonin promoted the survival of
HUMSCs under TBI conditions. The results from the present study
indicated that shikonin may regulate autophagy via the AMPK/mTOR
signalling pathway and protect HUMSCs from apoptosis induced by
hypoxia/ischemia. These results provided a basis for the clinical
application of stem cell therapy in brain trauma treatment
strategies.
Acknowledgements
Not applicable.
Funding
This work was partially supported by a grant from the Municipal
Science and Technology Bureau of Wenzhou (grant no. Y20190568).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and LR designed the study. XZ and KWu performed
cell culture and TUNEL detection. XZ, LH and ZS performed the
western blot analysis. KWu and JR performed CCK-8 assays and trypan
blue staining. KWa and LH performed animal experiments and
immunofluorescence. XZ, LH, LR and QZ performed data analysis and
statistics. XZ, LH and LR confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wenzhou Medical University (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Feigin VL, Nichols E, Alam T, Bannick MS,
Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG,
et al: GBD 2016 Neurology Collaborators: Global, regional, and
national burden of neurological disorders, 1990-2016: A systematic
analysis for the Global Burden of Disease Study 2016. Lancet
Neurol. 18:459–480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu J, Chen L, Huang X, Wu K, Ding S, Wang
W, Wang B, Smith C, Ren C, Ni H, et al: Calpain inhibitor MDL28170
improves the transplantation-mediated therapeutic effect of bone
marrow-derived mesenchymal stem cells following traumatic brain
injury. Stem Cell Res Ther. 10(96)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Taylor CA, Bell JM, Breiding MJ and Xu L:
Traumatic Brain Injury-Related Emergency Department Visits,
Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR
Surveill Summ. 66:1–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bennett MH, Trytko B and Jonker B:
Hyperbaric oxygen therapy for the adjunctive treatment of traumatic
brain injury. Cochrane Database Syst Rev.
12(CD004609)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang
ZG, Morris DC and Chopp M: Neuroprotective and neurorestorative
effects of thymosin β4 treatment following experimental traumatic
brain injury. Ann N Y Acad Sci. 1270:51–58. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hawryluk GWJ, Rubiano AM, Totten AM,
O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N,
Shutter L, et al: Guidelines for the Management of Severe Traumatic
Brain Injury: 2020 Update of the Decompressive Craniectomy
Recommendations. Neurosurgery. 87:427–434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mahmood A, Lu D, Yi L, Chen JL and Chopp
M: Intracranial bone marrow transplantation after traumatic brain
injury improving functional outcome in adult rats. J Neurosurg.
94:589–595. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fairless R and Barnett SC: Olfactory
ensheathing cells: Their role in central nervous system repair. Int
J Biochem Cell Biol. 37:693–699. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chopp M and Li Y: Treatment of neural
injury with marrow stromal cells. Lancet Neurol. 1:92–100.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cox CS Jr, Baumgartner JE, Harting MT,
Worth LL, Walker PA, Shah SK, Ewing-Cobbs L, Hasan KM, Day MC, Lee
D, et al: Autologous bone marrow mononuclear cell therapy for
severe traumatic brain injury in children. Neurosurgery.
68:588–600. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang ZX, Guan LX, Zhang K, Zhang Q and
Dai LJ: A combined procedure to deliver autologous mesenchymal
stromal cells to patients with traumatic brain injury. Cytotherapy.
10:134–139. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Skardelly M, Gaber K, Burdack S, Scheidt
F, Hilbig H, Boltze J, Förschler A, Schwarz S, Schwarz J,
Meixensberger J, et al: Long-term benefit of human fetal neuronal
progenitor cell transplantation in a clinically adapted model after
traumatic brain injury. J Neurotrauma. 28:401–414. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tate MC, Shear DA, Hoffman SW, Stein DG,
Archer DR and LaPlaca MC: Fibronectin promotes survival and
migration of primary neural stem cells transplanted into the
traumatically injured mouse brain. Cell Transplant. 11:283–295.
2002.PubMed/NCBI
|
|
14
|
Lee JP, Jeyakumar M, Gonzalez R, Takahashi
H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, et al: Stem
cells act through multiple mechanisms to benefit mice with
neurodegenerative metabolic disease. Nat Med. 13:439–447.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Redmond DE Jr, Bjugstad KB, Teng YD,
Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R,
Blanchard BC, Kim SU, et al: Behavioral improvement in a primate
Parkinson's model is associated with multiple homeostatic effects
of human neural stem cells. Proc Natl Acad Sci USA.
104:12175–12180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arataki S, Tomizawa K, Moriwaki A, Nishida
K, Matsushita M, Ozaki T, Kunisada T, Yoshida A, Inoue H and Matsui
H: Calpain inhibitors prevent neuronal cell death and ameliorate
motor disturbances after compression-induced spinal cord injury in
rats. J Neurotrauma. 22:398–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fillmore N, Huqi A, Jaswal JS, Mori J,
Paulin R, Haromy A, Onay-Besikci A, Ionescu L, Thébaud B,
Michelakis E, et al: Effect of fatty acids on human bone marrow
mesenchymal stem cell energy metabolism and survival. PLoS One.
10(e0120257)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xia W and Hou M: Macrophage migration
inhibitory factor induces autophagy to resist hypoxia/serum
deprivation-induced apoptosis via the AMP-activated protein
kinase/mammalian target of rapamycin signaling pathway. Mol Med
Rep. 13:2619–2626. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Herberg S, Shi X, Johnson MH, Hamrick MW,
Isales CM and Hill WD: Stromal cell-derived factor-1β mediates cell
survival through enhancing autophagy in bone marrow-derived
mesenchymal stem cells. PLoS One. 8(e58207)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ferraro E and Cecconi F: Autophagic and
apoptotic response to stress signals in mammalian cells. Arch
Biochem Biophys. 462:210–219. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: Critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Palikaras K, Lionaki E and Tavernarakis N:
Mitophagy: In sickness and in health. Mol Cell Oncol.
3(e1056332)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bi Y, Zhu Y, Zhang M, Zhang K, Hua X, Fang
Z, Zhou J, Dai W, Cui Y, Li J, et al: Effect of Shikonin on Spinal
Cord Injury in Rats Via Regulation of HMGB1/TLR4/NF-κB Signaling
Pathway. Cell Physiol Biochem. 43:481–491. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao
J, Chen L, Cui L and Qiao H: Protective effect of shikonin in
experimental ischemic stroke: Attenuated TLR4, p-p38MAPK, NF-κB,
TNF-α and MMP-9 expression, up-regulated claudin-5 expression,
ameliorated BBB permeability. Neurochem Res. 39:97–106.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gan L, Wang ZH, Zhang H, Zhou R, Sun C,
Liu Y, Si J, Liu YY and Wang ZG: Protective effects of shikonin on
brain injury induced by carbon ion beam irradiation in mice. Biomed
Environ Sci. 28:148–151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan
Q, Luo J, Zen K and Yang J: Inhibiting aerobic glycolysis
suppresses renal interstitial fibroblast activation and renal
fibrosis. Am J Physiol Renal Physiol. 313:F561–F575.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li F, Zhang K, Liu H, Yang T, Xiao DJ and
Wang YS: The neuroprotective effect of mesenchymal stem cells is
mediated through inhibition of apoptosis in hypoxic ischemic
injury. World J Pediatr. 16:193–200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li W, Liu J and Zhao Y: PKM2 inhibitor
shikonin suppresses TPA-induced mitochondrial malfunction and
proliferation of skin epidermal JB6 cells. Mol Carcinog.
53:403–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Y, Kang X, Niu G, He S, Zhang T, Bai
Y, Li Y, Hao H, Chen C, Shou Z, et al: Shikonin induces apoptosis
and prosurvival autophagy in human melanoma A375 cells via
ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed
Biotechnol. 47:626–635. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Z, Liu T, Gan L, Wang T, Yuan X,
Zhang B, Chen H and Zheng Q: Shikonin protects mouse brain against
cerebral ischemia/reperfusion injury through its antioxidant
activity. Eur J Pharmacol. 643:211–217. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Romine J, Gao X and Chen J: Controlled
cortical impact model for traumatic brain injury. J Vis Exp.
90(e51781)2014.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
New J and Thomas SM: Autophagy-dependent
secretion: Mechanism, factors secreted, and disease implications.
Autophagy. 15:1682–1693. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Q, Yang YJ, Wang H, Dong QT, Wang
TJ, Qian HY and Xu H: Autophagy activation: A novel mechanism of
atorvastatin to protect mesenchymal stem cells from hypoxia and
serum deprivation via AMP-activated protein kinase/mammalian target
of rapamycin pathway. Stem Cells Dev. 21:1321–1332. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tang Z, Gao J, Wu J, Zeng G, Liao Y, Song
Z, Liang X, Hu J, Hu Y, Liu M, et al: Human umbilical cord
mesenchymal stromal cells attenuate pulmonary fibrosis via
regulatory T cell through interaction with macrophage. Stem Cell
Res Ther. 12(397)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Napoli I and Neumann H: Microglial
clearance function in health and disease. Neuroscience.
158:1030–1038. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Carrico KM, Vaishnav R and Hall ED:
Temporal and spatial dynamics of peroxynitrite-induced oxidative
damage after spinal cord contusion injury. J Neurotrauma.
26:1369–1378. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with Akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
McNamara EH, Grillakis AA, Tucker LB and
McCabe JT: The closed-head impact model of engineered rotational
acceleration (CHIMERA) as an application for traumatic brain injury
pre-clinical research: A status report. Exp Neurol.
333(113409)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bolouri H and Zetterberg H: Animal Models
for Concussion: Molecular and Cognitive Assessments - Relevance to
Sport and Military Concussions. Chapter 46. In: Brain Neurotrauma.
Kobeissy FH (ed). CRC Press/Taylor & Francis, Boca Raton, FL,
pp645-658, 2015.
|
|
47
|
Liu B, Jin J, Zhang Z, Zuo L, Jiang M and
Xie C: Shikonin exerts antitumor activity by causing mitochondrial
dysfunction in hepatocellular carcinoma through PKM2-AMPK-PGC1α
signaling pathway. Biochem Cell Biol. 97:397–405. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S,
Yang Y and Gu C: AMPK: Potential Therapeutic Target for Ischemic
Stroke. Theranostics. 8:4535–4551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Qiang L, Wu C, Ming M, Viollet B and He
YY: Autophagy controls p38 activation to promote cell survival
under genotoxic stress. J Biol Chem. 288:1603–1611. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang H, Bosch-Marce M, Shimoda LA, Tan
YS, Baek JH, Wesley JB, Gonzalez FJ and Semenza GL: Mitochondrial
autophagy is an HIF-1-dependent adaptive metabolic response to
hypoxia. J Biol Chem. 283:10892–10903. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bachmann J, Ehlert E, Becker M, Otto C,
Radeloff K, Blunk T and Bauer-Kreisel P: Ischemia-Like Stress
Conditions Stimulate Trophic Activities of Adipose-Derived
Stromal/Stem Cells. Cells. 9:1935–1955. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hamacher-Brady A, Brady NR, Logue SE,
Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA and Gustafsson AB:
Response to myocardial ischemia/reperfusion injury involves Bnip3
and autophagy. Cell Death Differ. 14:146–157. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gwon SY, Ahn J, Jung CH, Moon B and Ha
T-Y: Shikonin Attenuates Hepatic Steatosis by Enhancing Beta
Oxidation and Energy Expenditure via AMPK Activation. Nutrients.
12:1133–1145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang F, Mayca Pozo F, Tian D, Geng X, Yao
X, Zhang Y and Tang J: Shikonin Inhibits Cancer Through P21
Upregulation and Apoptosis Induction. Front Pharmacol. 11:861–873.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kapahi P, Chen D, Rogers AN, Katewa SD, Li
PW, Thomas EL and Kockel L: With TOR, less is more: A key role for
the conserved nutrient-sensing TOR pathway in aging. Cell Metab.
11:453–465. 2010.PubMed/NCBI View Article : Google Scholar
|