Introduction
Atherosclerosis (AS) is an inflammatory disease
marked by hyperplastic lesions that are formed in the arterial
intima, such as coronary artery, carotid artery and peripheral
artery (1). It is characterized by
the thickening, hardening, elasticity loss and reduction of lumen
size of the arterial wall. AS mainly occurs in large and
medium-sized arteries and internal arterial branches. While it is
presumed that AS is a disease caused by multiple factors, its
etiology remains unknown. Multiple risk factors have been
identified, such as dyslipidemia, hypertension, smoking, diabetes,
abnormal glucose tolerance, age, sex and genetic factors (1). Moreover, AS is the pathophysiological
precursor for the development of ischemic cardio-cerebrovascular
disease (2). With the improvement
in living standards and due to lifestyle-related changes, ischemic
cardio-cerebrovascular disease has become a serious threat to human
health (3). AS treatment includes
surgery and drug therapy. The purpose of the surgical treatments,
such as stenting and bypass surgery, is to restore the blood supply
by correcting the stenosis or occlusion of the arteries, especially
in the coronary and renal arteries, as well as the arteries of the
limbs. Furthermore, drug therapy mainly aims to lower blood lipid
levels to prevent or inhibit the formation and progression of AS
plaques (4). Hence, research on the
pathogenesis of AS and disease prevention strategies has become a
pressing issue. The efforts of numerous researchers are directed at
present towards investigating the prevention and control of the
pathogenesis and progression of AS and its related diseases.
Transient receptor potential channel-1 (TRPC1) is a
Ca2+ channel present on the cell membrane, especially in
vascular smooth muscle cells (VSMCs). As a cell receptor, TRPC1 can
receive signals and stimuli from both inside and outside the cell
(5). The channel is regulated by
intracellular Ca2+ concentration. When Ca2+
ions are depleted from the sarcoplasmic reticulum, TRPC1 opens,
allowing for Ca2+ inflow, resulting in depolarization
and contraction of the cells, which in turn causes contraction of
the blood vessels (6). The large
conductance Ca2+-activated K+ channel (BK) is
widely distributed in the cell membranes of vascular smooth
muscles. Each BK comprises four α subunits, the main function of
which is to regulate the β subunit 1 (BKβ1) (7). When the intracellular Ca2+
concentration is increased, the BK pathway is activated and there
is K+ outflow, resulting in hyperpolarization. This in
turn inhibits the opening of the Ca2+ channel and
reduces the Ca2+ inflow, thereby causing cell
depolarization (7). When the blood
vessels are treated with BK-specific blockers in vitro,
depolarization of the cell membrane of VSMCs occurs, leading to an
increase in vascular tension, which shows that the BKs serve a
critical role in regulating vascular smooth muscle tension.
TRPC1 can interact with transport and scaffolding
proteins, such as β-tublin (8),
ankyrin (9), caveolin (10), Homers (11), MX dynamin like GTPase 1(12), ras homolog family member A (13), synaptosome associated protein
25(13) and vesicle associated
membrane protein 1(14), which are
responsible for vesicle transport, membrane fusion and cytoskeleton
rearrangement process regulation. Moreover, TRPC1 participates in
signal pathways with proteins such as inositol 1,4,5-trisphosphate
receptor type 3(15), calmodulin
(16), Gq/11 (a member of G
proteins) (17), phosphoinositide
phospholipase Cγ (18), plasma
membrane calcium-transporting ATPase (19), sarcoplasmic/endoplasmic reticulum
calcium ATPase (20) and stromal
interaction molecule 1(21) to
regulate the physiological function of cells.
Previous studies have revealed that TRPC1 and BK
form a signal complex on the surface of the VSMC membrane (22,23).
The TRPC1/BK signal complex regulates the changes in intracellular
Ca2+ concentration in a stable physical and chemical
environment to achieve steady vascular tension and regulate the
blood flow (22). The cell membrane
of normal smooth muscle cells possesses a large number of caveolae
that are framed by caveolin 1. A variety of channels, receptors and
regulatory proteins can be found in these caveolae (24,25).
TRPC1 in the caveolae interacts with numerous signal molecules or
membrane proteins to form a complex and mediate signal
transduction. The TRPC1 protein needs to be in the form of a signal
complex to perform its physiological function (22,24,25).
TRPC1 is involved in the depolarization of the aortal VSMCs, and BK
is involved in the repolarization of the arterial smooth muscle
cells (22). The hyperpolarization
of the TRPC1/BK signal complex can reduce the dopant-induced cell
membrane depolarization and prevent excessive contraction of the
arterial smooth muscle cells (22).
Via Ca2+ signal transduction, TRPC1 and BK can regulate
vasoconstriction and diastole. AS leads to a series of
pathophysiological changes in the vessel wall and, therefore, the
ion channels present on the vessel wall are also affected (22). Although there are a few reports on
the involvement of the TRPC1/BK signal complex in the regulation of
tension in the VSMCs, in-depth studies on the functional mechanisms
of TRPC1/BK signaling in vascular disease, particularly
atherosclerotic lesions, are lacking. Further research on the
TRPC1/BK signal complex may provide a novel theoretical basis for
the treatment of atherosclerotic disease and lead to the discovery
of new treatments. Therefore, the present study investigated the
expression of the TRPC1/BK signal complex in arterial tissue at the
molecular level.
Materials and methods
Main reagents and equipment
The following were used in the present study: TRPC1
rabbit anti-mouse primary antibody (cat. no. NB100-98844; Novus
Biologicals, LLC; western blotting dilution, 1:1,000;
immunohistochemistry dilution, 1:1,000), BKα rabbit anti-mouse
primary antibody (cat. no. NBP1-46701; Novus Biologicals, LLC;
western blotting dilution, 1:1,000; immunohistochemistry dilution,
1:500) and BKβ1 rabbit anti-mouse primary antibody (cat.
no. NB300-535; Novus Biologicals, LLC; western blotting dilution,
1:1,000; immunohistochemistry dilution, 1:500); β-actin rabbit
anti-mouse primary antibody (cat. no. 4970S; Cell Signaling
Technology, Inc.; western blotting dilution, 1:1,000;
immunohistochemistry dilution, 1:200); HRP-labeled goat anti-rabbit
secondary antibody (cat. no. ZB-2301; OriGene Technologies, Inc.;
dilution, 1:10,000); ECL kit (Thermo Fisher Scientific, Inc.);
3,3'-diaminobenzidine (DAB) kit (Beijing Golden Bridge
Biotechnology Co., Ltd.); TRIzol® kit (Invitrogen;
Thermo Fisher Scientific, Inc.); PCR two-step kit (Beijing Kangwei
Century Biotechnology Co., Ltd.); electrophoresis instrument
(Shanghai Tianneng Electronics); PCR gene amplification and PCR
electrophoresis (Applied Biosystems; Thermo Fisher Scientific,
Inc.); fluorescence and chemiluminescence imaging analysis system
(Analytik Jena AG); light microscope (Olympus Corporation);
paraffin tissue microtome (Beijing Century Science and Technology
Co., Ltd.); color digital CCD camera (Nikon Corporation); Metamorph
biological imaging software (version 1.12.2; Universal Imaging,
Inc.).
Animal studies
For the animal experiments, 10 apolipoprotein E
(ApoE)-/- mice (purchased from Beijing Huafukang Biotech
Co., Ltd.) were used in the experimental group, and 10 wild-type
C57BL/6J mice (purchased from the Experimental Animal Center of
Anhui Medical University; Hefei, China) were included in the
control group. These mice were 6-8 weeks old, were housed in the
specific pathogen free experimental animal center (temperature,
23˚C; humidity, 67%, light/dark cycle, 12/12 h) and had free access
to autoclaved food and sterilized water. The mice in the
experimental group were kept on a high-fat diet (17.9% crude fat)
to promote the formation of atherosclerotic plaques (26), while the mice in the control group
were kept on normal feed for 10 weeks.
Aortic tissue separation
The mice were anesthetized using an intraperitoneal
injection of 0.5-1 ml 4% pentobarbital (~80 mg/kg). The mice were
fixed on the dissection table, their chest skin was cut along the
median line and was blunt dissected on the left and right sides.
The chest was opened to reveal the heart, and ~0.5 cm section of
the aorta was cut from the heart and carefully separated into the
ascending aorta, aortic arch and thoracic aorta. Immediately after
the artery was separated, the tissue was washed three times with
saline and blotted to remove residual moisture.
Tissue sections
The dissected arterial tissue was immersed in 4%
paraformaldehyde, fixed for 24 h at 4˚C and then embedded with
paraffin. As the AS lesions are mainly found in the ascending aorta
to the aortic arch part during the first occurrence (27), this region was sectioned at 3 µm
layer by layer and made transparent for H&E staining
(hematoxylin staining for 10 min, then eosin staining for 5 min;
room temperature). Sections were observed using a light microscope.
In the film, obvious AS lesions were observed, thereby indicating
the success in modeling AS in the ApoE-/- mice.
Separation of vascular smooth muscle
layer
Using the ApoE-/- mice in which AS were
successfully established, the aorta was isolated using the same
method as aforementioned. To observe the middle vascular smooth
muscle layer, the vascular outer membrane was stripped off with
ophthalmic tweezers and the endovascular layer was scrapped away
using absorbent cotton. Finally, the middle smooth muscle layer was
obtained for the subsequent experimental study, as described
below.
mRNA detection
Reverse transcription PCR (RT-PCR) was performed as
previously reported (28). Total
RNA was extracted using TRIzol reagent (Thermo Fisher Scientific,
Inc.) from VSMC tissue of the middle layer vessels. A universal
RT-PCR detection kit from Beijing ComWin Biotech Co., Ltd. was used
according to the manufacturer’s instructions. Genes coding for
TRPC1, BKα and BKβ1 proteins were denoted as TRPC1,
K+ Ca2+-activated channel subfamily M α 1
(KCNMA1) and K+ Ca2+-activated channel
subfamily M regulatory β subunit 1 (KCNMB1). The following primers
were used: TRPC1 sense, 5’-GCCGTAA GCCCACCTGTAA-3’ and antisense,
5’-TTGTGAGCCA CCACTTTGAG-3’; KCNMA1 sense, 5’-CGGGGTCTTG
CAGGCTAAT-3’ and antisense, 5’-GGTCATCGTCATCGT CTTGGT-3’; KCNMB1
sense, 5’-CGGGGTCTTGCAGGC TAAT-3’ and antisense,
5’-CGCCAAGATGGATAGGGA-3’; and GAPDH sense,
5’-GGAAGCTTGTCATCAACGGG-3’ and antisense,
5-AGTGATGGCATGGACTGTGG-3’. These were designed using the mouse
TRPC1, KCNMA1 and KCNMB1 sequences from PubMed GeneBank. GAPDH was
used as the internal reference. The primers were synthesized by
Sangon Biotech Co., Ltd. RT-PCR was performed using the
Mastercycler personal (Eppendorf). Following RT and cDNA
amplification, PCR was performed with the following thermocycling
conditions: 94˚C for 3 min; 30 cycles of 94˚C for 30 sec, 55˚C for
30 sec, 72˚C for 2 min; and 72˚C for 5 min. The products were
analyzed via electrophoresis on 2% agarose gel, and ethidium
bromide was used for visualization. Next, images were captured
using a radiography gel imaging system (ProteinSimple).
Densitometry of each band was performed using TotalLab Quant
(version 12; TotalLab Ltd.).
Western blot analysis
The smooth muscle cell layers obtained by peeling
off the adventitial layers with forceps were homogenized and after
a round of high speed (12,000 x g; 4˚C; 5 min) centrifugation, the
supernatant was obtained and the total protein concentration was
measured by detecting the A562 absorbance value. RIPA buffer (cat.
no. P0013B; Beyotime Institute of Biotechnology) was used for
protein extraction. The BCA method was used for protein
determination. The samples (75 µg protein/lane) were subjected to
10% SDS-PAGE and the proteins were transferred onto a PVDF
membrane, which was blocked using 5% skimmed milk at room
temperature for 2 h. After incubating with primary antibodies
(TRPC1, BKα, BKβ1 or β-actin rabbit anti-mouse primary
antibodies) on a shaker at 4˚C overnight, followed by incubation
with secondary antibody (HRP-labeled goat anti-rabbit secondary
antibody) for 1 h at room temperature, the proteins were detected
on an X-ray film using an ECL kit (Thermo Fisher Scientific, Inc.).
Densitometry was performed via ImageJ software (v1.8.0.112;
National Institutes of Health).
Immunohistochemistry
Samples obtained from the operation were fixed at
4˚C in 4% paraformaldehyde for 24 h and then embedded in paraffin.
Paraffin-embedded samples were sectioned into 5-mm-thick sections
and the slides were incubated at 56˚C for 4 h. The sections from
paraffin-embedded aortic smooth muscle layers were de-paraffinized
and hydrated. Antigen retrieval was carried out by adding 10 mmol/l
sodium citrate solution (pH 6.0±0.1) and heating in the microwave
for 10 min. Next, 3% aqueous hydrogen peroxide solution added to
the sections for 10 min at room temperature for reducing the
activity of endogenous peroxidase. The sections were incubated
overnight at 4˚C with the following primary monoclonal antibodies:
TRPC1 (Alomone Labs), BKα (Alomone Labs) and BKβ1
(Alomone Labs). Next, the sections were incubated with appropriate
HRP-conjugated secondary antibodies (OriGene Technologies, Inc.)
for 30 min at 37˚C. Then, DAB was added to the sections for
coloration for 3 min at room temperature and terminated by adding
double distilled water. Finally, hematoxylin counterstaining was
performed for 4 min at room temperature. For negative controls, the
primary antibody was omitted. Subsequently, under light microscope
(magnification, x400), a Nikon color digital CCD camera was used to
capture the images of each group of immunohistochemical staining
sections.
Statistical analysis
Data are presented as the mean ± SEM. Statistical
differences were examined using one-way ANOVA followed by the
Newman-Keuls post hoc test and SigmaStat 4.0 software (Systat
Software, Inc.). All experiments, including RT-PCR, western
blotting and animal tissue staining, were repeated independently on
samples from at least 3 mice, yielding similar results. P<0.05
was considered to indicate a statistically significant difference.
Graphs were plotted using GraphPad Prism 6.01 (GraphPad Software,
Inc.).
Results
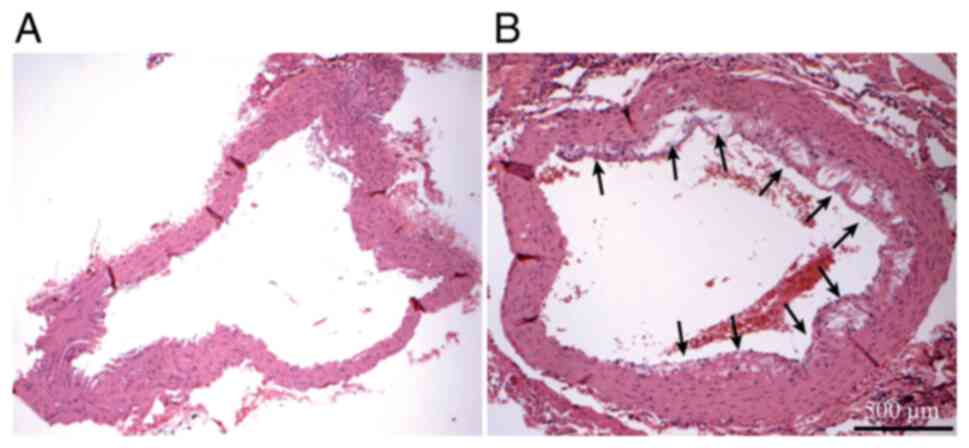
Atherosclerotic lesions
After H&E staining, the aortic tissue sections
from the ApoE-/- mice were observed under an Olympus
microscope (BX 53) (Fig. 1) and
atherosclerotic lesions (shown by arrows in Fig. 1B) were detected, suggesting that the
AS model was successfully established. The aortic sections
collected from the mice in the control group did not have any
atherosclerotic lesions.
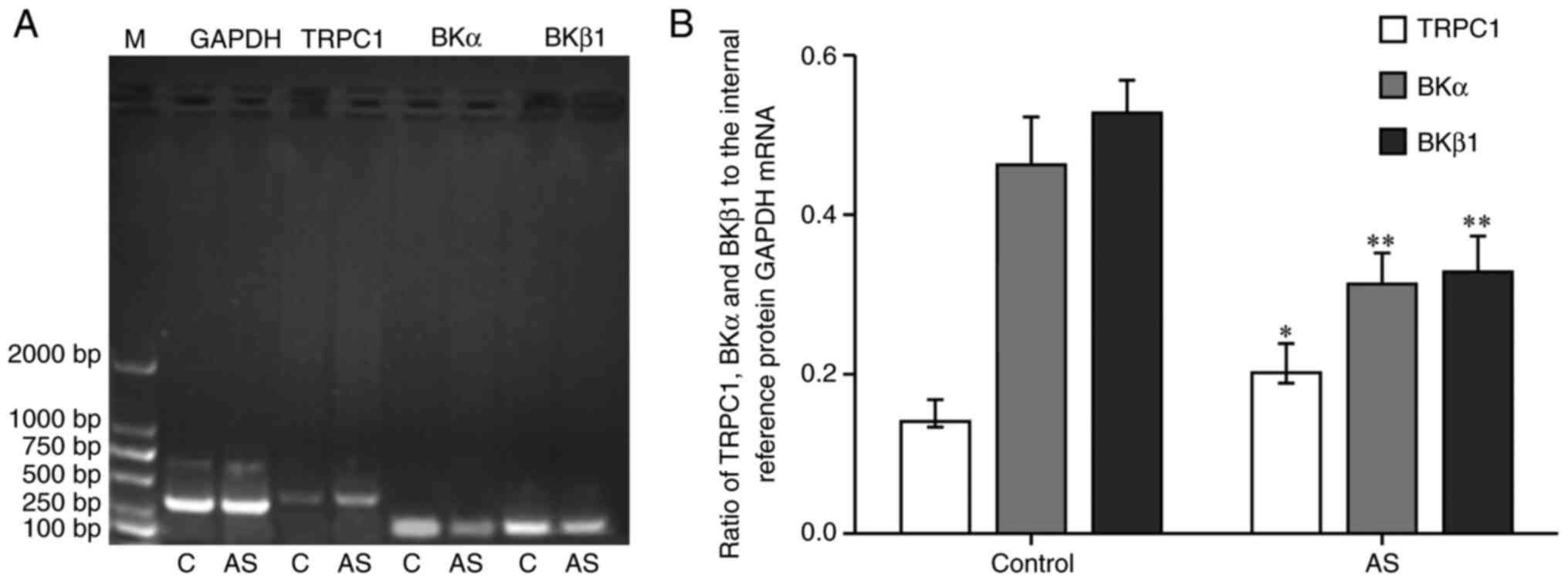
TRPC1, BKα and BKβ1 mRNA
expression
Compared with the control group, the mRNA expression
level of TRPC1 in aortic vascular smooth muscle of AS group was
increased significantly (P<0.05; Fig. 2), whereas the mRNA expression levels
of BKα and BKβ1 were decreased (P<0.01).
TRPC1, BKα and BKβ1 protein
expression
In Fig. 3, the
grayish-yellow regions show the proteins of vascular smooth muscle
tissue stained by the immunohistochemical reaction, and the blue
parts show the nucleus. Compared with the control group, the
protein expression level of TRPC1 in the mice in the AS group was
increased (P<0.01), whereas the expression level of BKα protein
was decreased (P<0.01), as seen in the slides under the same
background light intensity. Moreover, BKβ1 protein
expression was also decreased (P<0.05). The increase and
decrease of these protein expression levels were consistent with
the results of the western blotting (Fig. 4).
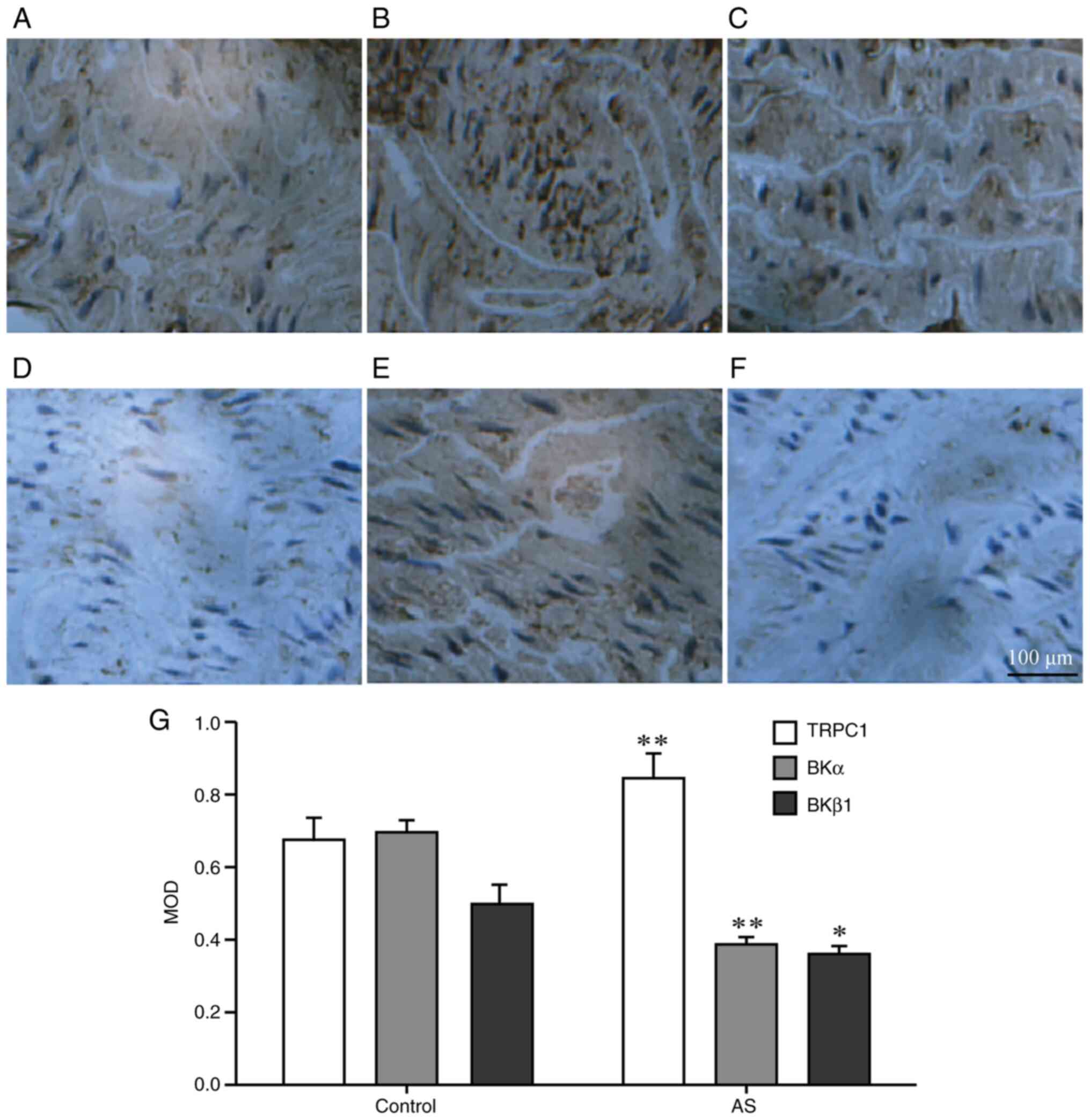 | Figure 3Immunohistochemistry for the
detection of the protein expression levels in the aortic smooth
muscle of the two groups. Expression levels of (A,B) TRPC1, (C,D)
BKα and (E,F) BKβ1 in the aortic vascular smooth muscle
of mice in each group were detected via immunohistochemistry
(n=10). Panels A, C, E show results for the control group, while
panels B, D and F are for the AS group. The protein expression was
observed under an optical microscope (magnification, x400). The
brown-yellow shows the protein localization, while the blue area
shows the nuclei. (G) The MOD of the brown-yellow area was detected
via Metamorph imaging software. *P<0.05,
**P<0.01 vs. respective control group. TRPC1,
transient receptor potential channel-1; BKα, large conductance
calcium-activated potassium channel α subunit; BKβ1,
large conductance calcium-activated potassium channel β1 subunit;
AS, atherosclerosis; MOD, mean optical density. |
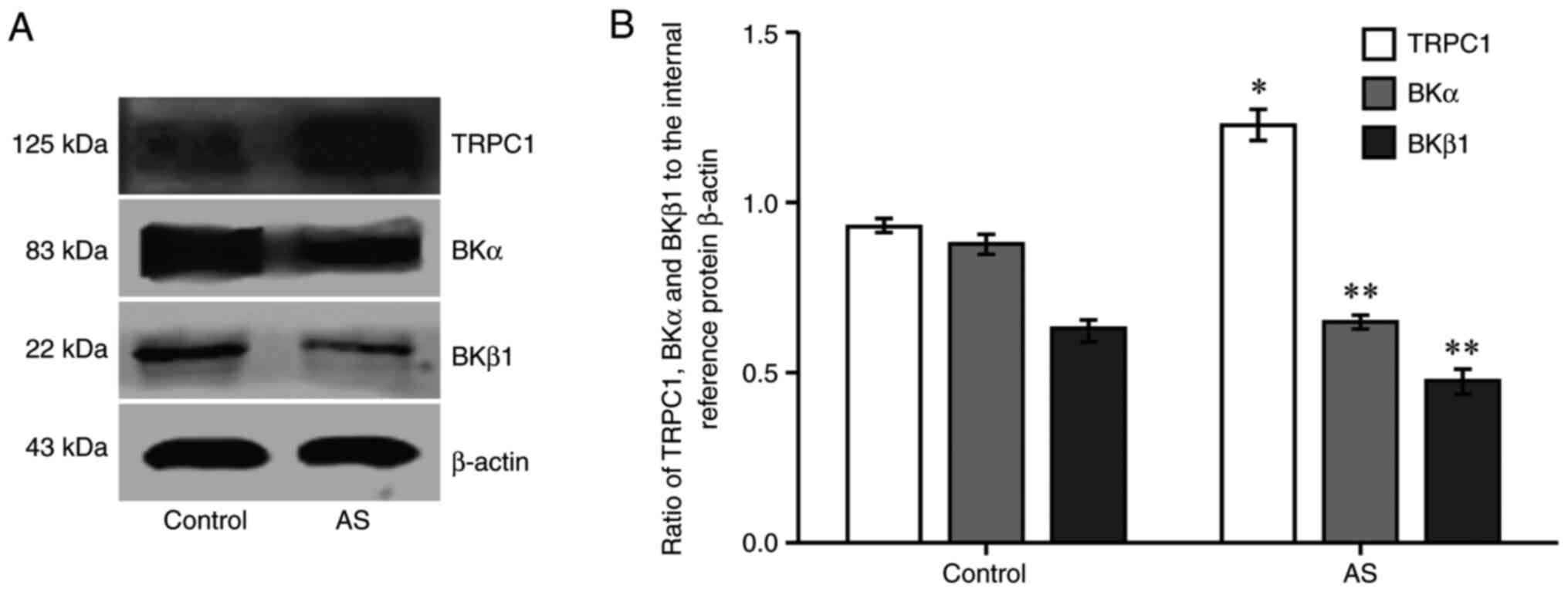
TRPC1, BKα and BKβ1 protein
expression as detected via western blotting
Compared with the control group, the expression
level of TRPC1 protein in the AS group was increased (P<0.05;
Fig. 4), whereas BKα and
BKβ1 protein expression was decreased (P<0.01;
Fig. 4).
Discussion
AS is a form of vascular stenosis disease.
Inflammatory lesions are the first signs of pathogenesis, which are
characterized by the formation of lipid plaques on the arterial
wall that can gradually thicken, resulting in vascular stenosis or
vascular embolism caused by plaque rupture (29). Since hyperlipidemia is the most
important risk factor leading to AS, simulating it in vivo
is an ideal method to establish an AS model. Moreover, ApoE is a
key regulator of plasma lipid levels, as shown by the fact that
mice and humans lacking ApoE have markedly increased plasma
cholesterol levels, as well as AS development (30).
When the ApoE gene is knocked out, ApoE cannot be
synthesized in the body and the resultant lack of it the in blood
can cause significant changes in the content of various
lipoproteins. This leads to accumulation of lipids in the blood
vessel walls, which is a direct consequence of the formation of
lipid oxidation products, such as oxidized low density lipoprotein
cholesterol (30). This can also
promote phagocytosis in the blood vessel wall and lead to the
formation of foamy macrophages, causing a series of inflammatory
responses within the blood vessels involving of a variety of cells.
The phenomenon occurs in a loop and results in the formation of
vascular atherosclerotic plaques (31,32).
Atherosclerotic lesions gradually penetrate the
endothelium and invade the smooth muscle layers and the outer
layers (27). During the
progression of AS, the protein expression of various ion channels
on the vascular wall cells is affected to varying degrees (33). The alterations in the ion channel
proteins of the VSMCs change the internal and external
physiological and biochemical processes which is crucial for the
vascular smooth muscle systolic and diastolic function, thereby
affecting blood flow (33,34). The proteins related to the TRPC1
pathway have been detected on a variety of cell membranes,
particularly on the VSMC membrane (34). BK is also an ion channel that is
present on the cell membrane and is highly dependent on the
intracellular Ca2+ concentration and mediates
intracellular K+ transport (35). In previous years, studies using
immunofluorescence, immunoprecipitation, patch clamp tests and
vascular tension measurements, as well as other research
techniques, have shown that TRPC1 and BK form an ion signal complex
in the thoracic aortic VSMC membrane of C57BL/6J mice (22,31).
The common regulation of intracellular Ca2+
concentration affects the cell surface membrane potential and, in
turn, controls the vascular tension (22). In addition, studies using diabetic
rats have shown significant differences in the expression of the BK
protein in the coronary artery of these rats compared with that in
the normal rat coronary artery (36). The present results demonstrated that
there was a significant difference in the expression levels of
TRPC1 and BK in atherosclerotic and normal mouse aortic smooth
muscle tissue, which was consistent with these previous
studies.
The present study identified that in the aortic
vascular smooth muscle tissue of arterial AS mouse models and
normal mice, mRNA and protein expression levels of TRPC1, BKα and
BKβ1 were different, indicating that the development of
AS affects this ion channel complex, at least in the upstream
transcription stage of protein synthesis from DNA to RNA. An
increase in the expression of TRPC1 on the cell membrane was
observed in AS, which leads to increase in the number of open
channels and subsequent increase in the Ca2+ influx
leading to contraction of VSMCs (34). The Ca2+ ions can also
promote intracellular and extracellular matrix proliferation and
increase AS vascular stenosis (37). Conversely, the expression levels of
the BK proteins are decreased on the cell membrane in AS, resulting
in decreased K+ outflow that causes reduction in the
hyperpolarization of smooth muscle cells and maintains the
contraction state, further aggravating vascular stenosis (35). In addition, TRPC1 and BK are
physically associated with each other in VSMCs, and Ca2+
influx via TRPC1 activates BK, leading to membrane
hyperpolarization (22), and thus,
we hypothesize that an interaction of these two channels forms the
TRPC1/BK signal complex. Furthermore, the dysfunction of this
complex can also increase arterial atherosclerotic lesions and
vascular stenosis. The TRPC1/BK signal complex may serve as a new
target for the treatment of AS-related diseases. The mechanism of
TRPC1/BK in the cardiovascular system is complex and has been well
studied (38). However, novel
strategies need to be developed using this signal complex in order
to treat or prevent cardiovascular diseases in the future.
The present study has limitations. Since an animal
model was used in the study, only the protein and mRNA expression
levels were detected. Future studies will clarify the function of
the two target proteins using inhibitors. Furthermore,
immunoprecipitation tests will be conducted to help further
understand the role of this complex. At present, the current
experiment focused the determination of RNA and protein content. In
the future, further in-depth research, such as Ca2+
measurement in VSMCs and the association of other ions with
TRPC1/BK signal complex, will be examined.
In conclusion, the present study demonstrated that
the mRNA and protein expression of the TRPC1/BK complex molecules
is different in vascular AS compared with the control, which may
provide a novel target to study vascular disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LFW designed this study and performed the critical
revision of the manuscript. HL and QXT participated in the design
of the study. DYL performed the majority of experiments and drafted
the manuscript. MHX, LWT and YH performed acquisition of data. MXH,
LWT, YH, HL, QXT and ZC performed data collection. BZZ, HTL, YFW
and XGZ analyzed the data. ZC interpreted the research data. LFW
and DYL confirmed the authenticity of the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol has been reviewed and approved by the Institutional Animal
Care and Use Committee of The 901st Hospital of Joint Logistics
Support Force of PLA (approval no. 20150615).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47 (Suppl 8):C7–12. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peng R, Ji H, Jin L, Lin S, Huang Y, Xu K,
Yang Q, Sun D and Wu W: Macrophage-Based Therapies for
Atherosclerosis Management. J Immunol Res.
2020(8131754)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bai X, Wang WX, Fu RJ, Yue SJ, Gao H, Chen
YY and Tang YP: Therapeutic Potential of Hydroxysafflor Yellow A on
Cardio-Cerebrovascular Diseases. Front Pharmacol.
11(01265)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nicholls SJ, Ballantyne CM, Barter PJ,
Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M,
Wolski K, et al: Effect of two intensive statin regimens on
progression of coronary disease. N Engl J Med. 365:2078–2087.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reyes RC, Verkhratsky A and Parpura V:
TRPC1-mediated Ca2+ and Na+ signalling in
astroglia: Differential filtering of extracellular cations. Cell
Calcium. 54:120–125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Y, Ye P, Chen SL and Zhang DM:
Functional regulation of large conductance
Ca2+-activated K+ channels in vascular
diseases. Metabolism. 83:75–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ferguson SD, Zhou S, Xiu J, Hashimoto Y,
Sanai N, Kim L, Kesari S, de Groot J, Spetzler D and Heimberger AB:
Ependymomas overexpress chemoresistance and DNA repair-related
proteins. Oncotarget. 9:7822–7831. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu P, Verhaar AP and Peppelenbosch MP:
Signaling Size: Ankyrin and SOCS Box-Containing ASB E3 Ligases in
Action. Trends Biochem Sci. 44:64–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kruglikov IL and Scherer PE: Caveolin as a
Universal Target in Dermatology. Int J Mol Sci.
21(80)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Son A, Kang N, Oh SY, Kim KW, Muallem S,
Yang YM and Shin DM: Homer2 and Homer3 modulate RANKL-induced
NFATc1 signaling in osteoclastogenesis and bone metabolism. J
Endocrinol. 242:241–249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ciminski K, Pulvermüller J, Adam J and
Schwemmle M: Human MxA is a potent interspecies barrier for the
novel bat-derived influenza A-like virus H18N11. Emerg Microbes
Infect. 8:556–563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nguyen LK, Kholodenko BN and von
Kriegsheim A: Rac1 and RhoA: Networks, loops and bistability. Small
GTPases. 9:316–321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Redondo PC, Harper AG, Salido GM, Pariente
JA, Sage SO and Rosado JA: A role for SNAP-25 but not VAMPs in
store-mediated Ca2+ entry in human platelets. J Physiol.
558:99–109. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Antigny F, Konig S, Bernheim L and Frieden
M: Inositol 1,4,5 trisphosphate receptor 1 is a key player of human
myoblast differentiation. Cell Calcium. 56:513–521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dhar M, Wayman GA, Zhu M, Lambert TJ,
Davare MA and Appleyard SM: Leptin-induced spine formation requires
TrpC channels and the CaM kinase cascade in the hippocampus. J
Neurosci. 34:10022–10033. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Marom M, Birnbaumer L and Atlas D:
Membrane depolarization combined with Gq-activated
G-protein-coupled receptors induce transient receptor potential
channel 1 (TRPC1)- dependent potentiation of catecholamine release.
Neuroscience. 189:132–145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tu CL, Chang W and Bikle DD: Phospholipase
cgamma1 is required for activation of store-operated channels in
human keratinocytes. J Invest Dermatol. 124:187–197.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Singh BB, Liu X, Tang J, Zhu MX and
Ambudkar IS: Calmodulin regulates Ca(2+)-dependent feedback
inhibition of store-operated Ca(2+) influx by interaction with a
site in the C terminus of TrpC1. Mol Cell. 9:739–750.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Selli C, Erac Y and Tosun M: Simultaneous
measurement of cytosolic and mitochondrial calcium levels:
Observations in TRPC1-silenced hepatocellular carcinoma cells. J
Pharmacol Toxicol Methods. 72:29–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dyrda A, Koenig S and Frieden M: STIM1
long and STIM1 gate differently TRPC1 during store-operated calcium
entry. Cell Calcium. 86(102134)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kwan HY, Shen B, Ma X, Kwok YC, Huang Y,
Man YB, Yu S and Yao X: TRPC1 associates with BK(Ca) channel to
form a signal complex in vascular smooth muscle cells. Circ Res.
104:670–678. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kochukov MY, Balasubramanian A, Noel RC
and Marrelli SP: Role of TRPC1 and TRPC3 channels in contraction
and relaxation of mouse thoracic aorta. J Vasc Res. 50:11–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
ÁvilaMedina J: CalderónSánchez E,
GonzálezRodríguez P, Monje-Quiroga F, Rosado JA, Castellano A,
Ordóñez A and Smani T: Orai1 and TRPC1 colocalize with CaV1.2
channels to form a signal complex in vascular smooth muscle cells.
J Biol Chem. 291:21148–21159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nascimento Da Conceicao V, Sun Y, Zboril
EK, De la Chapa JJ and Singh BB: Loss of Ca2+ entry via
Orai-TRPC1 induces ER stress, initiating immune activation in
macrophages. J Cell Sci. 133(jcs237610)2019.PubMed/NCBI View Article : Google Scholar : doi.org/10.1242/jcs.237610.
|
|
26
|
Packard RR and Libby P: Inflammation in
atherosclerosis: From vascular biology to biomarker discovery and
risk prediction. Clin Chem. 54:24–38. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Andrés-Manzano MJ, Andrés V and Dorado B:
Oil Red O and Hematoxylin and Eosin Staining for Quantification of
Atherosclerosis Burden in Mouse Aorta and Aortic Root. Methods Mol
Biol. 1339:85–99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang LF, Gu L, Huang MX, Zhou WB, Li H and
Zhang BZ: Effects of spironolactone towards rabbit atrial
remodeling with rapid pacing. Pak J Pharm Sci. 29:151–156.
2016.PubMed/NCBI
|
|
29
|
Cochain C and Zernecke A: Macrophages and
immune cells in atherosclerosis: Recent advances and novel
concepts. Basic Res Cardiol. 110(34)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Davignon J, Cohn JS, Mabile L and Bernier
L: Apolipoprotein E and atherosclerosis: Insight from animal and
human studies. Clin Chim Acta. 286:115–143. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Libby P, Lichtman AH and Hansson GK:
Immune effector mechanisms implicated in atherosclerosis: From mice
to humans. Immunity. 38:1092–1104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tousoulis D, Psarros C, Demosthenous M,
Patel R, Antoniades C and Stefanadis C: Innate and adaptive
inflammation as a therapeutic target in vascular disease: The
emerging role of statins. J Am Coll Cardiol. 63:2491–2502.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tangvoraphonkchai K and Davenport A:
Magnesium and Cardiovascular Disease. Adv Chronic Kidney Dis.
25:251–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nesin V and Tsiokas L: TRPC1. In:
Mammalian Transient Receptor Potential (TRP) Cation Channels.
Handbook of Experimental Pharmacology. Nilius B and Flockerzi V
(eds). Vol 222. Springer, Berlin, Heidelberg, pp15-51, 2014.
|
|
35
|
Latorre R, Castillo K, Carrasquel-Ursulaez
W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C and Alvarez O:
Molecular Determinants of BK Channel Functional Diversity and
Functioning. Physiol Rev. 97:39–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang X, Qian LL, Wang RX, Yao Y, Dang SP,
Wu Y, Wang W, Ji Y, Sun MQ, Xia DY, et al: Regulation of coronary
arterial large conductance Ca2+-activated K+
channel protein expression and function by n-3 polyunsaturated
fatty acids in diabetic rats. J Vasc Res. 54:329–343.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tykocki NR, Boerman EM and Jackson WF:
Smooth muscle ion channels and regulation of vascular tone in
resistance arteries and arterioles. Compr Physiol. 7:485–581.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schmidt K, Dubrovska G, Nielsen G, Fesüs
G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, de
Wit C, et al: Amplification of EDHF-type vasodilatations in
TRPC1-deficient mice. Br J Pharmacol. 161:1722–1733.
2010.PubMed/NCBI View Article : Google Scholar
|