Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide for 36 cancers (18.0% of the
total cancer deaths) in 185 countries in year 2020(1), and non-small cell lung cancer (NSCLC)
accounts for up to 80% of total pulmonary malignancies (2). Radiotherapy is the most common
treatment method used for localized lung cancer; it is non-invasive
and well-tolerated (3,4). In patients with NSCLC, radiotherapy
plays a key role in local treatment by inducing DNA damage,
triggering cell cycle arrest and apoptosis of tumor cells (5,6).
However, radioresistance remains an obstacle in achieving
successful treatment. Thus, novel therapeutic strategies are
required to improve the effectiveness of radiotherapy for patients
with NSCLC.
A previous study reported that the wingless-type
(Wnt) pathway is associated with radioresistance in NSCLC (7,8). It
has been reported that Wnt5a expression is upregulated in different
types of cancer, including gastric, pancreatic and prostate cancer
(9-11).
A previous study demonstrated that silencing Wnt5a expression
decreases migration, invasiveness and epithelial-to-mesenchymal
transition (EMT) of NSCLC cells; these effects are reversed
following overexpression of Wnt5a (12). Furthermore, preclinical and
clinical studies have reported that the combination of gene therapy
and conventional anticancer therapy can improve the therapeutic
benefits (13-16).
Although Wnt5a expression is upregulated in radioresistant NSCLC
cells (17), whether Wnt5a
promotes radioresistance in NSCLC cells remains unclear.
The present study aimed to investigate the efficacy
of overexpression or knockdown of Wnt5a combined with radiotherapy
in NSCLC cells. In addition, it has been reported that Wnt5a
overexpression promotes the EMT and metastasis of pancreatic cancer
cells through the β-catenin-dependent canonical signaling (9). Thus, the study also investigated
whether the Wnt/β-catenin pathway was relevant in mediating
radioresistance in NSCLC cells.
Materials and methods
Cell culture
The human NSCLC cell lines, H1650 (cat. no.
CRL-5883) and A549 (cat. no. CCL-185) were purchased from the
American Type Culture Collection. Cells were maintained in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% (w/v) fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.)
and 1% (w/v) penicillin/streptomycin in culture dishes, at 37˚C
with 5% CO2. Cells were seeded into six-well culture
plates at a density of 5x105 cells/well.
Cell transfection
For the knockdown of endogenous Wnt5a expression in
NSCLC cells, small interfering (si)RNAs were used. For
transfection, 1x105 A549 and H1650 parental cells were
seeded into six-well plates and cultured overnight at 37˚C with 5%
CO2 until they reached 80% confluence. The Wnt5a siRNA
expression cassette was subcloned into the pcDNA6 expression vector
(Invitrogen; Thermo Fisher Scientific, Inc.). The target sequence
was 5'-GTTTTGGCCACTGACTGA-3'. For overexpression of Wnt5a,
sequences were amplified by PCR and inserted into pcDNA6.2 vector
to generate fusion plasmids, namely Wnt5a and pcDNA empty vector as
the control. The ratio of the plasmid to the transfection reagent
was 1 µg:3 µl. Transfection was performed at room temperature using
EzWay™ Transfection Reagent according to the manufacturer's
instructions (Invitrogen; Thermo Fisher Scientific, Inc.). To
assess the role of β-catenin, H1650 and A549 cells were transfected
for 24 h with si-β-catenin (20 nM; Shanghai GenePharma Co., Ltd.;
forward, 5'-CATGUGUTGGUAAGCUCUA-3' and reverse,
5'-GCAACAGTTGCAGAGAGGU-3'). A non-specific scramble siRNA was used
as a negative control (20 nM; Shanghai GenePharma Co., Ltd.;
forward, 5'-AUGCUGATCAGUGUCGATU-3' and reverse,
5'-CAGAGAGCTCGUGAGAGTA-3'). Transfection efficiency was determined
via western blotting and reverse transcription-quantitative PCR
(RT-qPCR). Subsequent experiments were performed 48 h
post-transfection.
Radiation treatment
Cell irradiation was performed using a Varian 21EX
(Varian Medical Systems) linear accelerator with a coverage field
of 10x10 cm. H1650 and A549 cells were cultured in 12-well culture
plates (1x104 cells/well) and were treated for 24 h with
0, 2, 4, 6 or 8 Gy of irradiation at a dosage rate of 100 MU/min
and a source-to-surface distance of 100 cm.
Cell proliferation assay
Cell proliferation was assessed via MTT assay
(Sigma-Aldrich; Merck KGaA). At 48 h post-transfection, H1650 and
A549 cells were irradiated (0, 2, 4, 6 or 8 Gy in a single
fraction), cultured in 96-well culture plates (5x103
cells/well) and incubated for 5 days at 37˚C with 5%
CO2. An aliquot of 10 µl MTT solvent (5 mg/ml in PBS)
was added to each well. Following incubation for 2 h at 37˚C, and
then 100 µl isopropanol with 40 mM HCl was added to each well to
dissolve formazan crystals. Optical density (OD) was measured at
wavelengths of 560 and 620 nm, using a measurement parameter editor
(Tecan Group, Ltd.). Cell viability was expressed as OD value of
the transfected cell/OD value of background control (untransfected
cells).
Colony formation assay
H1650 and A549 cells were irradiated (0, 2, 4, 6 or
8 Gy in a single fraction) 48 h post-transfection and subsequently
seeded into 6-well plates at a density of 1x103
cells/well. The RMPI-1640 medium (Thermo Fisher Scientific, Inc.)
was replaced every day and cells were incubated for 14 days at 37˚C
with 5% CO2. After 14 days, cells were fixed with 4%
paraformaldehyde in PBS for 30 min at room temperature and stained
with crystal violet (0.4 g/l; Sigma-Aldrich; Merck KGaA) at room
temperature for 30 min. The number of colonies (determined as
containing >50 cells) was counted manually under a light
microscope (magnification, x10). The surviving fraction (%) was
calculated as follows: Colony forming efficiency = number of
colonies formed following irradiation treatment/number of cells
seeded x100. All experiments were performed in triplicates and
repeated three times.
Cell apoptosis assay
Cell apoptosis was determined via Annexin V-FITC and
PI staining. Following 24 h irradiation, H1650 and A549 cells were
seeded into 24-well plates at a density of 5x104
cells/well and resuspended in 100 µl binding buffer (10.0 HEPES,
140.0 NaCl and 2.5 mM CaCl2; pH 7.4). The cells were
subsequently stained with 5 µl Annexin V-FITC and 5 µl PI using a
FITC Annexin V Detection kit (BD Biosciences) in the dark at room
temperature for 15 min, according to the manufacturer's protocol.
Cell apoptosis was analyzed via flow cytometry (BD FACSCanto™; BD
Biosciences) and expressed as the percentage of cells in each
population (viable, Annexin V-/PI-; early
apoptotic, Annexin V+/PI-; late apoptotic,
Annexin V+/PI+ and necrotic, Annexin
V-/PI+). These data were analyzed by FlowJo
v10.0.7 software (FlowJo LLC).
Western blotting
H1650 and A549 cells were harvested, and cytoplasmic
and nuclear proteins were isolated using the Proteo JET™
Cytoplasmic and Nuclear Protein Extraction kit according to the
manufacturer's instructions (Fermentas; Thermo Fisher Scientific,
Inc.). The Bradford assay was used for protein quantification.
Equal amounts of protein (20 µg/lane) were separated via 8%
SDS-PAGE, transferred onto polyvinylidene difluoride membranes
(Cytiva) and blocked with blocking buffer containing 5% skimmed
milk in TBS-Tween-20 (0.1% Tween-20 in 1X TBS) for 1 h at room
temperature. The membranes were incubated with primary antibodies
against β-catenin (1:1,000; cat. no. 9582s; Cell Signaling
Technology, Inc.), lamin A (1:2,000; cat. no. 86846s; Cell
Signaling Technology, Inc.), Wnt5a (1:800; cat. no. sc-365370;
Santa Cruz Biotechnology, Inc.) and GAPDH (1:2,000; cat. no.
sc-47724; Santa Cruz Biotechnology, Inc.) overnight at 4˚C.
Following primary incubation, membranes were incubated with
HRP-conjugated secondary antibodies [anti-rabbit (1:5,000; cat. no.
211-035-109; Jackson ImmunoResearch Laboratories Inc.) or mouse IgG
(1:5,000; cat. no. 315-035-048; Jackson ImmunoResearch Laboratories
Inc.)] for 1 h at room temperature. Protein bands were detected
using an Enhanced Chemiluminescence System (Pierce: Thermo Fisher
Scientific, Inc.). Immunoreactive bands were quantified with the
TINA v2.10G software (Raytest Isotopenmegerifte GmbH).
RT-qPCR
Total RNA was isolated from H1650 and A549 cells
using TRIzol® according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (2 µg) was
reverse transcribed into complementary DNA (cDNA) using the
First-Strand RT-PCR kit (Promega Corporation). cDNA was
subsequently amplified using PCR specific primers for the target
genes and GAPDH was amplified as the internal control. The
amplification mixture contained 0.5 U of Taq polymerase (Takara
Bio, Inc.). The thermocycling conditions were as follows: 95˚C for
3 min; 30 cycles at 95˚C for 40 sec, 58˚C for 40 sec and 72˚C for
90 sec; final elongation at 72˚C for 10 min. The primer sequences
were as follows: Wnt5a forward, 5'-CGAAGACAGGCATCAAAGAA-3' and
reverse, 5'-GCAAAGCGGTAGCCATAGTC-3'; and GAPDH forward,
5'-ACCACAGTCCATGCCATCAC-3' and reverse, 5'-TCCACCACCCTGTTGCTGTA-3'.
RT-qPCR products were electrophoresed via a 1.5% agarose gel with
ethidium bromide. Signals were quantified by densitometric analysis
using Labworks Image Acquisition 4.0 software (Analytik Jena US
LLC). Statistical analysis was subsequently performed to calculate
the gel intensity using Microsoft Excel software 2010 (Microsoft
Corporation).
Statistical analysis
Statistical analysis was performed using SPSS v21.0
software (IBM Corp.). All experiments were performed in triplicates
and data are presented as the mean ± SD. Statistical differences
were analyzed using one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Wnt5a knockdown enhances
irradiation-induced inhibition of NSCLC cell proliferation and
colony formation
To determine the effects of Wnt5a knockdown on
antitumor radiotherapy in H1650 and A549 cells, MTT assay was
performed to assess cell proliferation following radiation alone or
combined with transfection with si-Wnt5a or empty vector control.
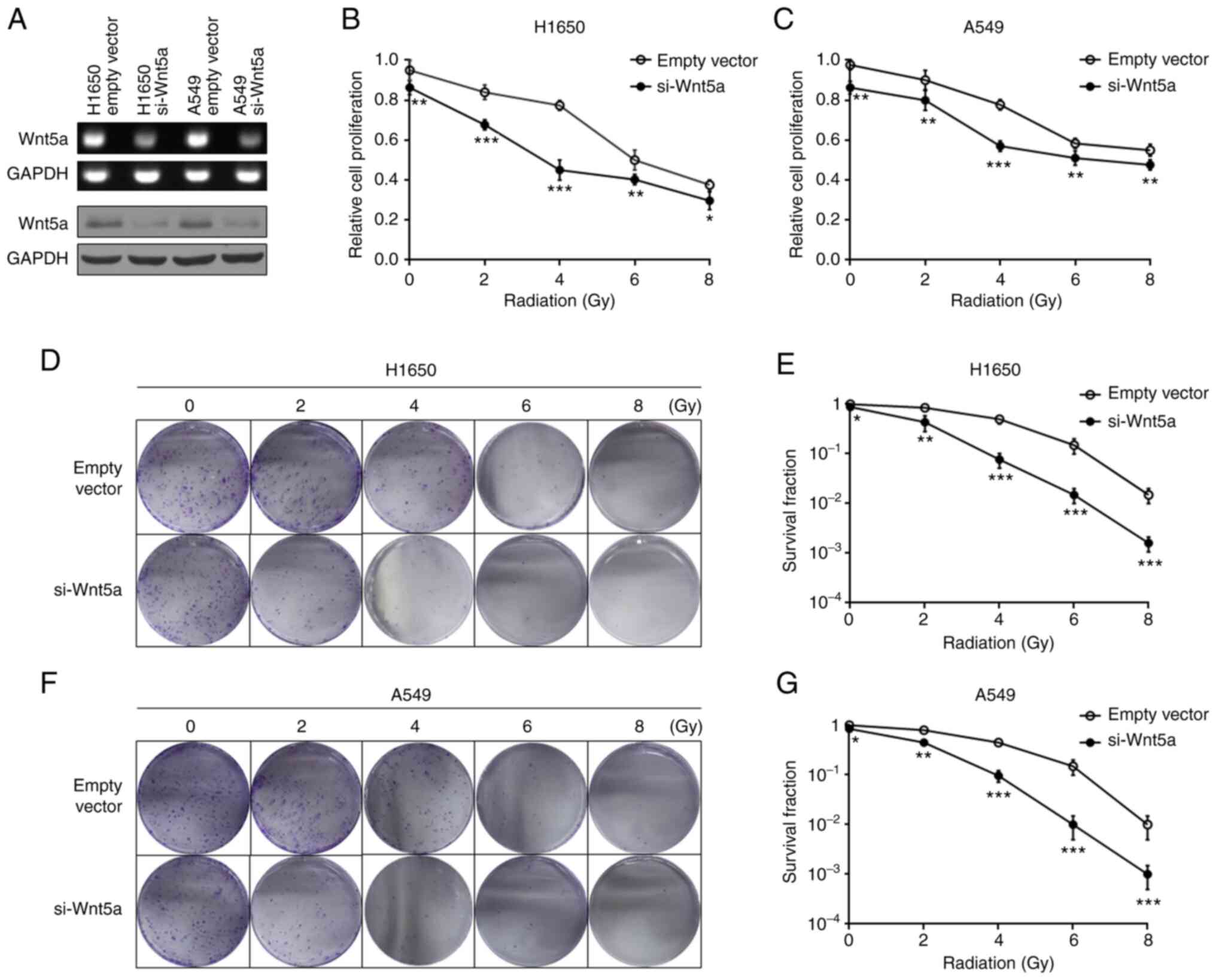
Western blotting and RT-qPCR were performed to detect Wnt5a
expression levels (Fig. 1A).
Treatment with ionizing radiation (2-8 Gy) inhibited proliferation
of H1650 and A549 cells in a dose-dependent manner. Furthermore,
Wnt5a knockdown decreased the proliferation of H1650 (Fig. 1B) and A549 (Fig. 1C) cells compared with the control
(empty vector). The present study investigated whether Wnt5a
affects colony formation of H1650 and A549 cells following
radiotherapy. The results demonstrated that cell colony formation
was significantly inhibited by radiotherapy and Wnt5a knockdown
significantly enhanced this inhibitory effect (Fig. 1D-G). Taken together, these results
suggest that combined Wnt5a knockdown and irradiation may improve
the inhibitory effect on NSCLC cell proliferation.
Overexpression of Wnt5a reverses
irradiation-induced inhibition of NSCLC cell proliferation and
colony formation
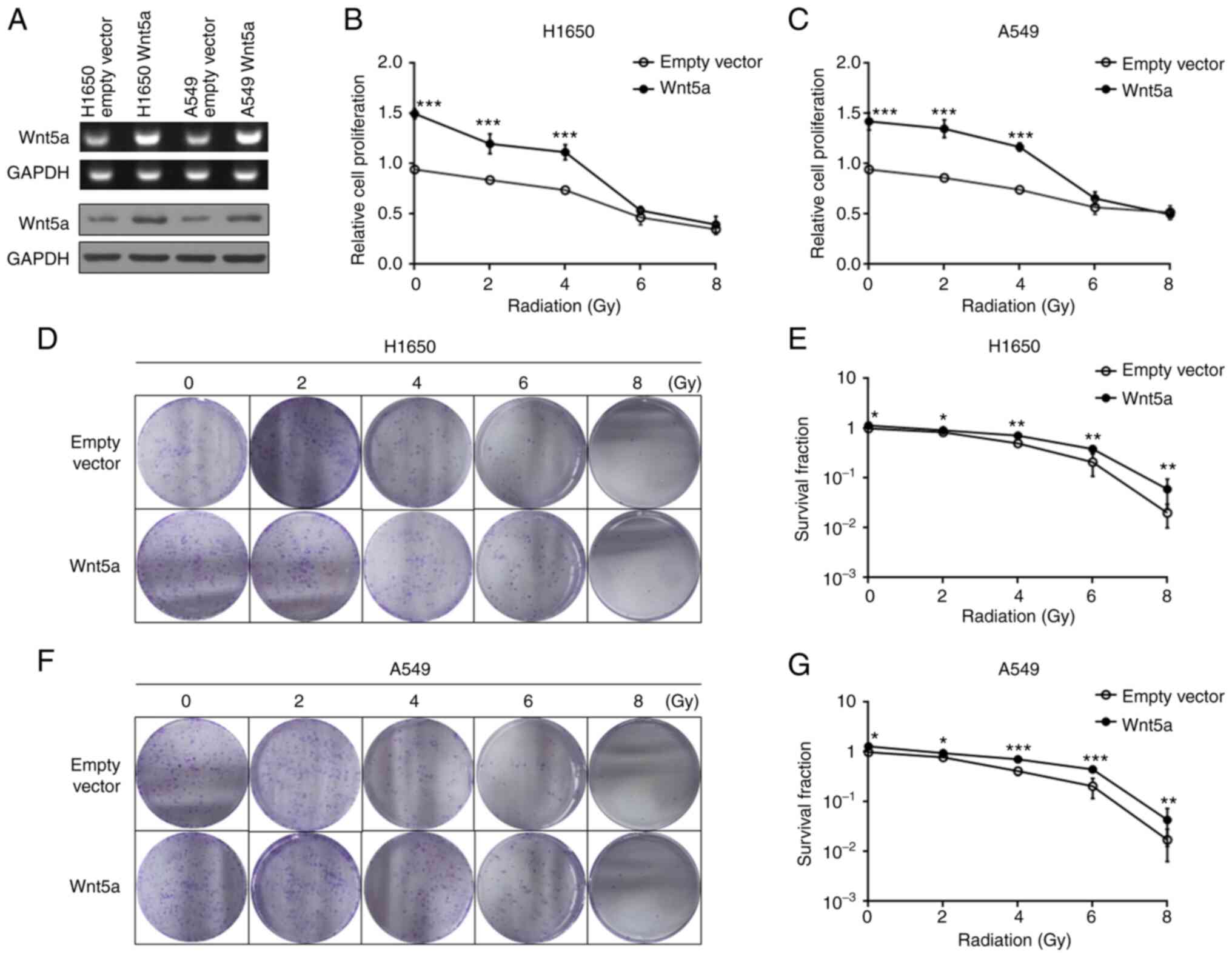
The effect of overexpressing Wnt5a and irradiation
on proliferation of H1650 and A549 cells was investigated. Equal
amounts of H1650 and A549 cells transfected with Wnt5a or empty
vector control were analyzed via western blotting and RT-qPCR
(Fig. 2A). The results
demonstrated that overexpression of Wnt5a increased cell
proliferation following irradiation at 2 or 4 Gy compared with the
empty vector control (Fig. 2B and
C). However, no significant
differences were observed between the Wnt5a overexpression and
empty vector control groups following irradiation at 6 or 8 Gy,
suggesting that 6 and 8 Gy doses may be lethal. H1650 (Fig. 2D and E) and A549 (Fig. 2F and G) cells overexpressing Wnt5a were treated
with radiotherapy; radiation significantly inhibited colony
formation, while overexpression of Wnt5a significantly decreased
this inhibitory effect. Collectively, these results suggest that
overexpression of Wnt5a attenuated the radiotherapeutic effect on
NSCLC cells.
Wnt5a knockdown increases
irradiation-induced apoptosis in NSCLC cells
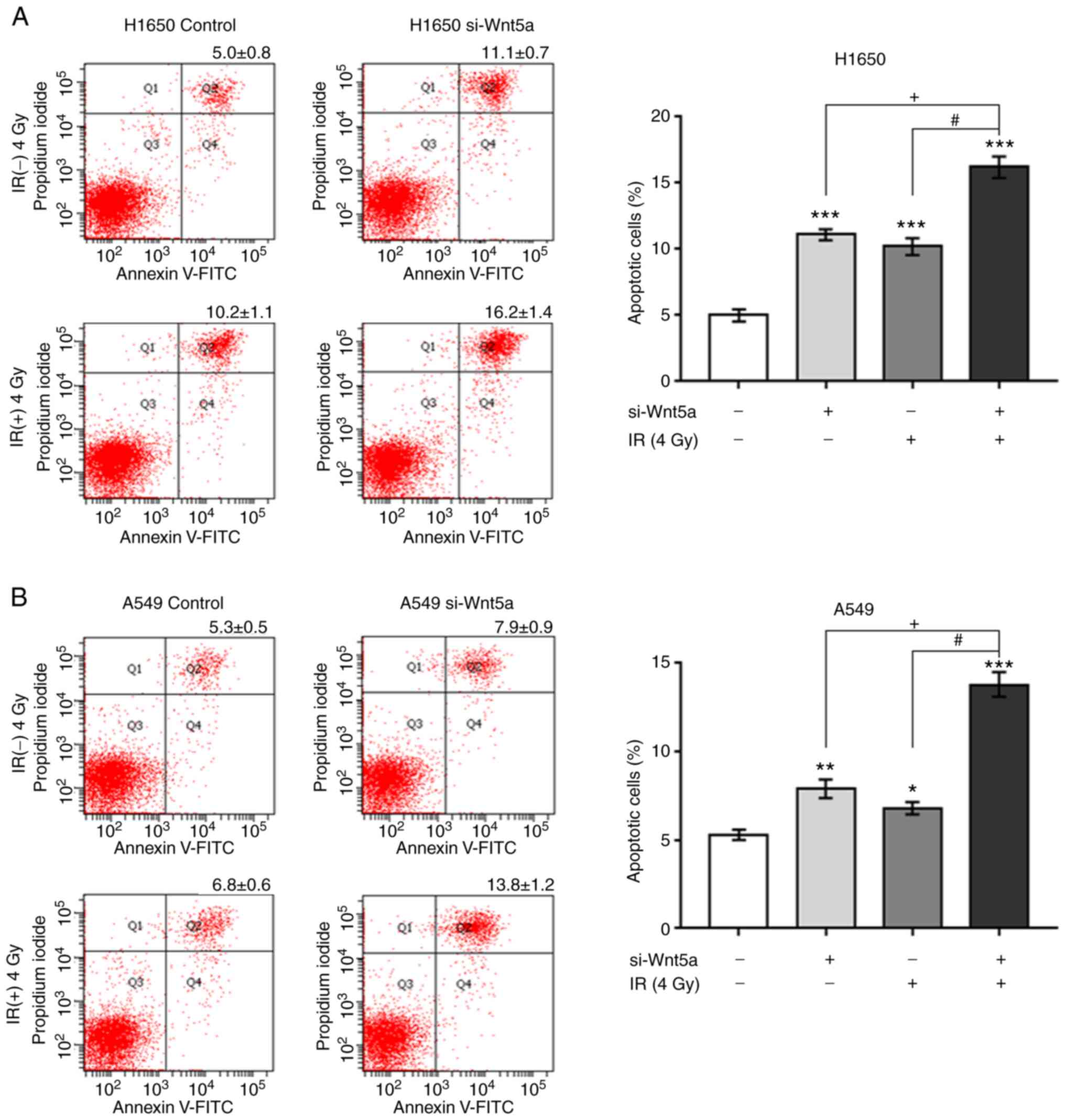
To determine whether Wnt5a knockdown sensitizes
H1650 and A549 cells to irradiation-induced apoptosis, cells were
transfected with si-Wnt5a and subsequently irradiated with either 0
or 4 Gy. After 24 h, the percentage of apoptotic cells was
determined via Annexin V/PI staining (Fig. 3). The apoptosis of H1650 and A549
cells following Wnt5a knockdown or irradiation alone significantly
increased compared with control cells. In addition, combination of
Wnt5a knockdown and irradiation further increased apoptosis. Taken
together, these results suggest that Wnt5a knockdown sensitized
NSCLC cells to irradiation-induced apoptosis.
Overexpression of Wnt5a decreases
irradiation-induced apoptosis in NSCLC cells
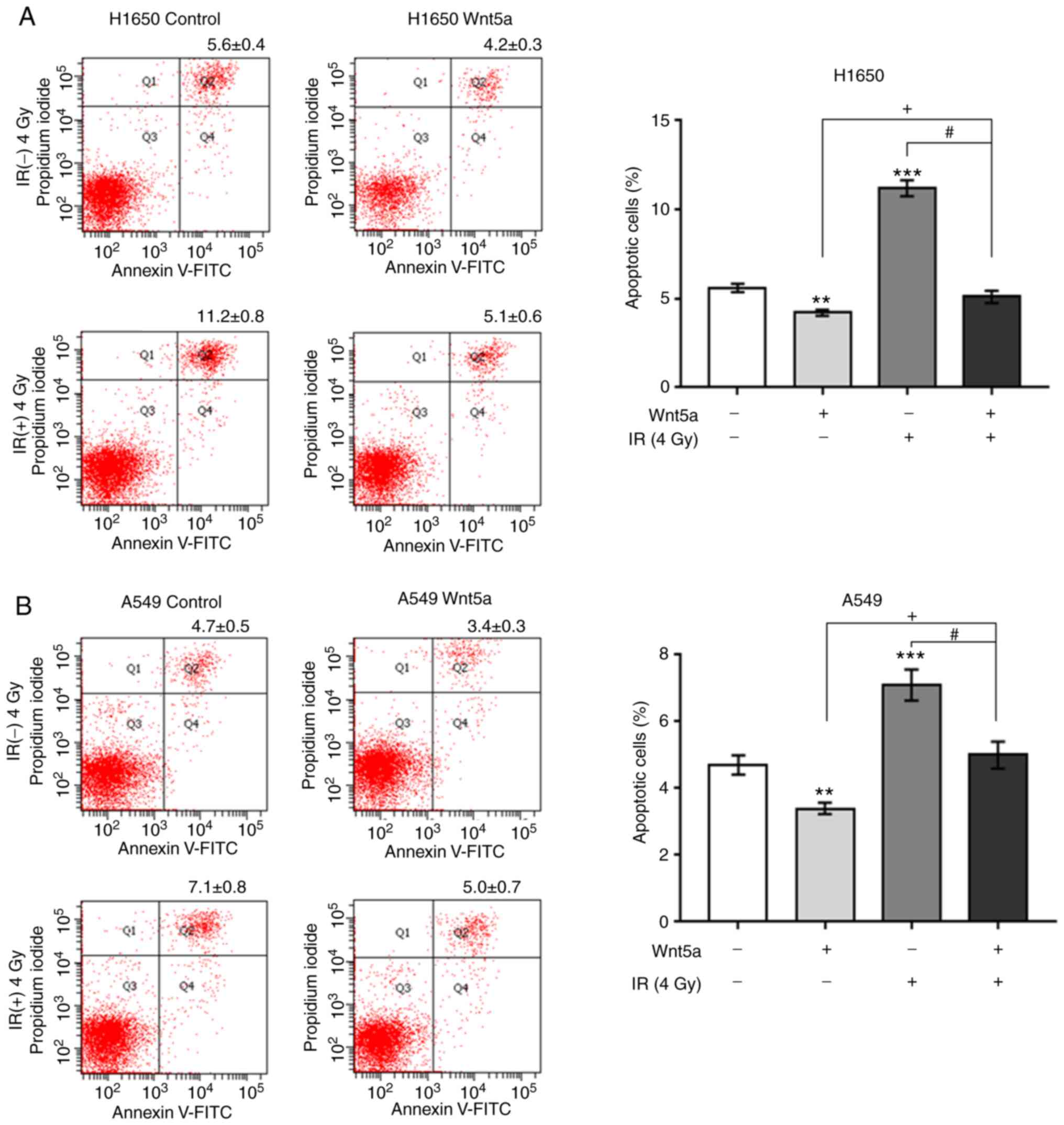
To determine whether overexpression of Wnt5a affects
irradiation-induced apoptosis, H1650 and A549 cells were
transfected with Wnt5a or empty vector control following
irradiation at 0 or 4 Gy (Fig. 4).
The results demonstrated that overexpression of Wnt5a significantly
decreased the apoptosis of H1650 and A549 cells compared with the
control group. In addition, irradiation (4 Gy) increased the
apoptosis of both H1650 and A549 cells; this effect was reversed
following overexpression of Wnt5a. Collectively, these results
suggest that overexpression of Wnt5a attenuated irradiation-induced
apoptosis in NSCLC cells.
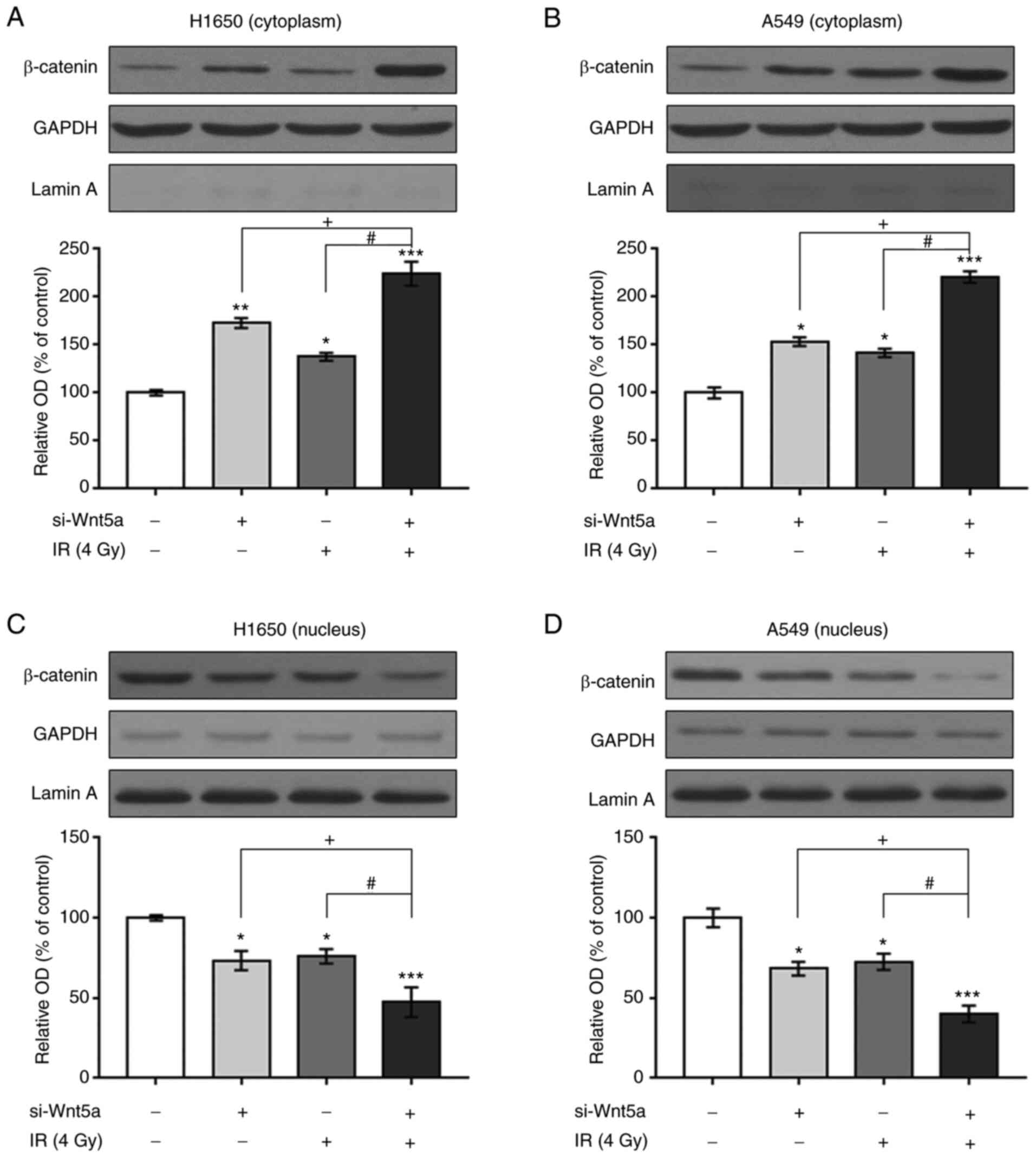
β-catenin expression following Wnt5a
knockdown and/or irradiation in NSCLC cells
To determine whether Wnt5a knockdown and irradiation
inhibit proliferation and induce apoptosis of NSCLC cells via the
β-catenin pathways, the cytoplasm and nucleus were separated and
β-catenin expression was detected via western blotting (Figs. 5 and S1). Cytoplasmic β-catenin expression was
higher following Wnt5a knockdown or irradiation in H1650 and A549
cells compared with the control cells. Combined Wnt5a knockdown and
irradiation was further enhanced the expression of β-catenin.
Conversely, nuclear β-catenin expression was reduced by the
combination of Wnt5a knockdown and irradiation in H1650 and A549
cells.
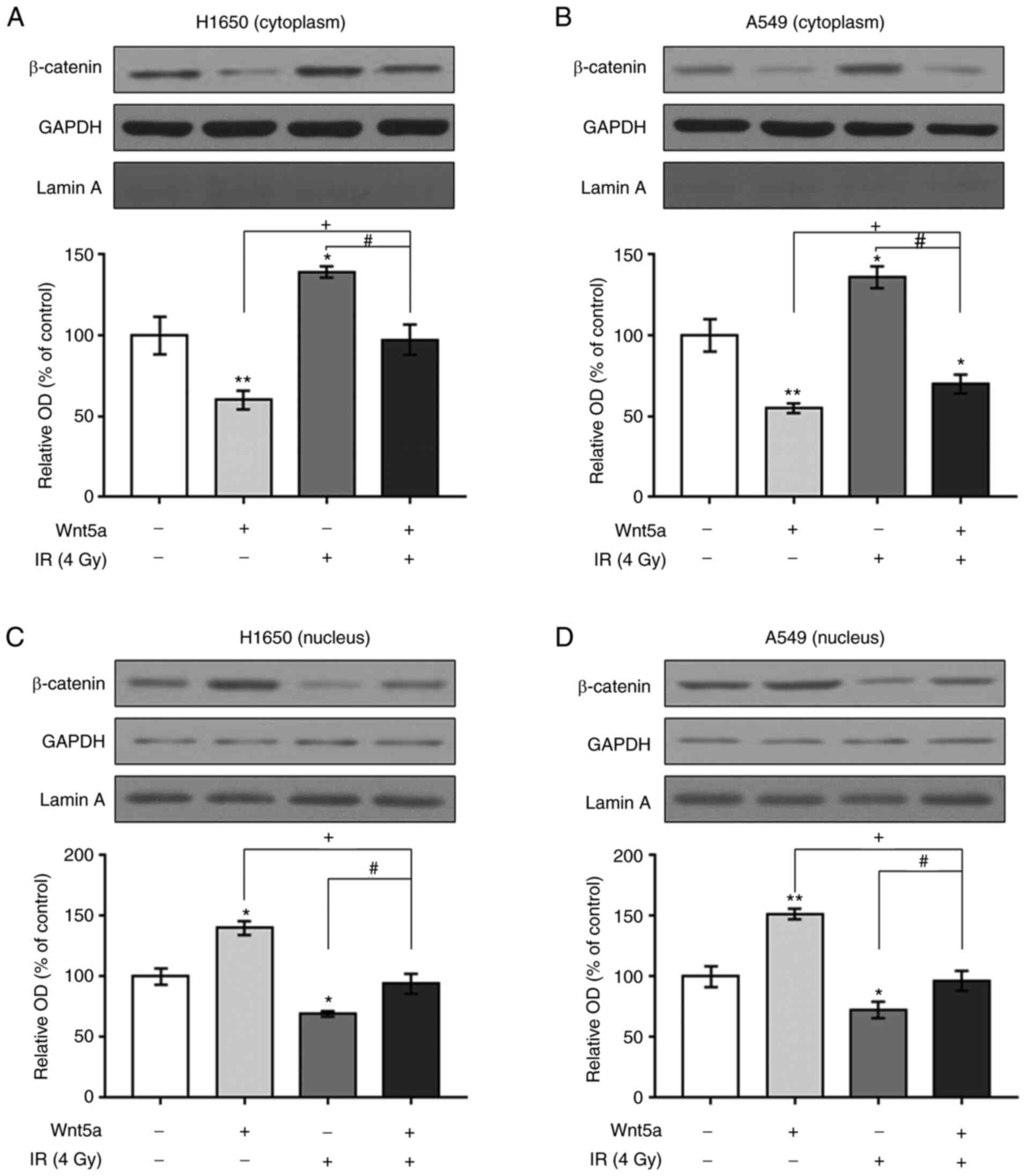
β-catenin expression following
overexpression of Wnt5a and/or irradiation in NSCLC cells
The effects of overexpressing Wnt5a and irradiation
on cytoplasmic and nuclear expression of β-catenin in NSCLC cells
were investigated (Figs. 6 and
S2). Western blot analysis
revealed that overexpression of Wnt5a decreased cytoplasmic but
increased nuclear β-catenin expression in H1650 and A549 cells. In
addition, irradiation treatment increased cytoplasmic and decreased
nuclear β-catenin expression in both H1650 and A549 cells. Notably,
overexpression of Wnt5a reversed the irradiation-induced
alterations in cytoplasmic and nuclear β-catenin expression in
H1650 and A549 cells. Taken together, these results suggested that
overexpression of Wnt5a may cause translocation of β-catenin from
the cytoplasm to the nucleus in NSCLC cells.
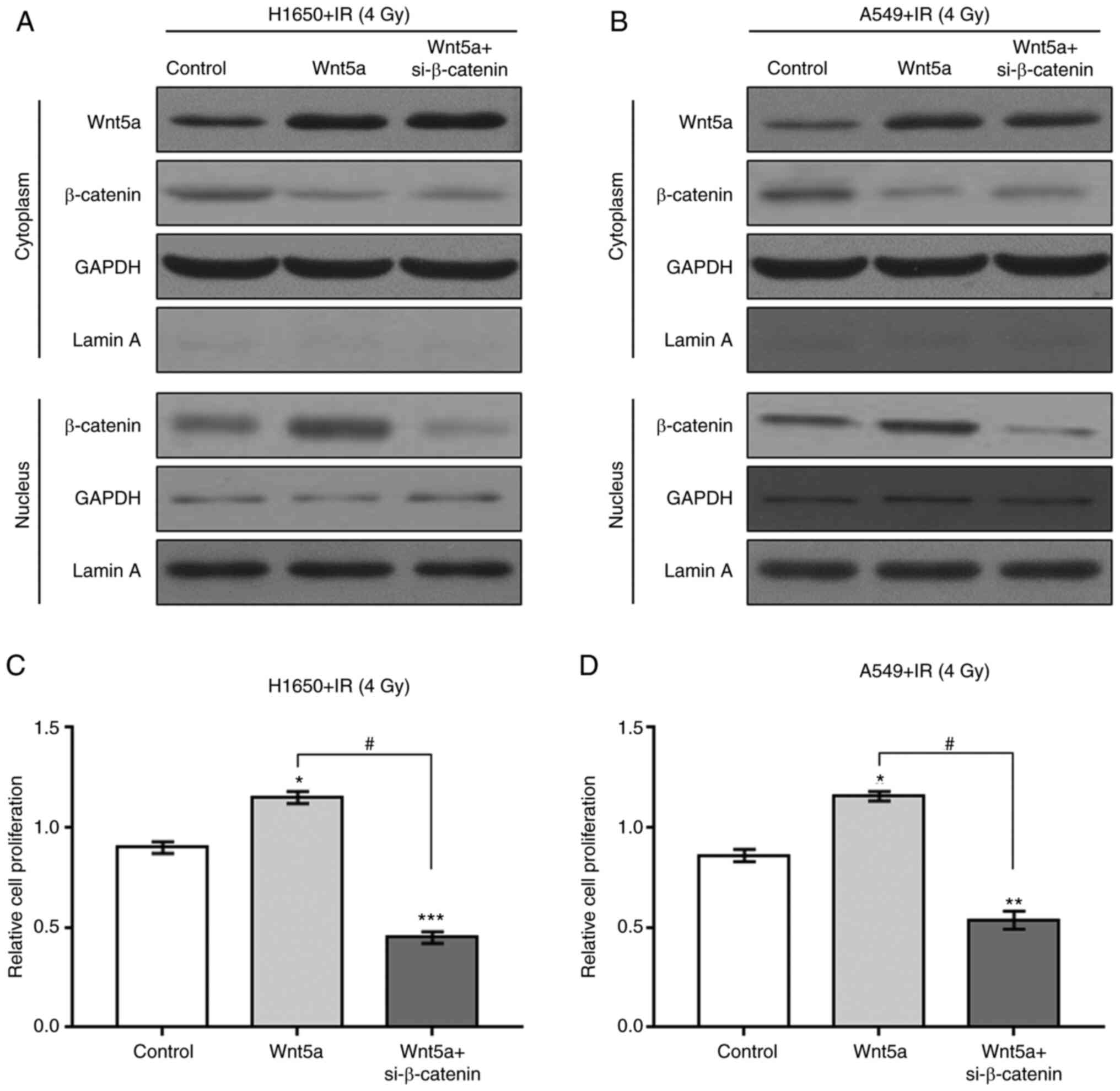
si-β-catenin reverses activation of
NSCLC cell proliferation caused by overexpression of Wnt5a
To determine the role of the β-catenin pathway in
NSCLC cell proliferation induced by overexpression of Wnt5a and
irradiation, H1650 and A549 cells were treated with siRNA for
β-catenin knockdown. Knockdown of β-catenin was confirmed by
western blotting and RT-qPCR (Fig.
S3). Overexpression of Wnt5a blocked the irradiation-induced
decrease in NSCLC cell proliferation (Figs. 7 and S4). In addition, si-β-catenin reversed
the promotion of NSCLC cell proliferation due to combined Wnt5a
overexpression and irradiation. Collectively, these results suggest
that the β-catenin pathway may be a mediator of Wnt5a
overexpression- and irradiation-induced increases in proliferation
and decreases in apoptosis of NSCLC cells.
Discussion
Wnt5a expression is upregulated in NSCLC cells
(18) and regulates several
biological events associated with tumor growth, EMT and metastasis
of NSCLC cells (9). It has also
been reported that overexpression of Wnt5a increases colony
formation, migration and invasion (12,19).
Thus, to assess whether alterations of Wnt5a expression affected
the response of NSCLC cell lines to radiotherapy, the present study
knocked down or overexpressed Wnt5a in H1650 and A549 cells. The
results demonstrated that Wnt5a knockdown combined with irradiation
decreased proliferation and induced apoptosis of NSCLC cells more
than irradiation or Wnt5a knockdown alone. Conversely,
overexpression of Wnt5a blocked irradiation-induced apoptosis.
These findings suggest that Wnt5a expression served a valuable role
in the radiotherapeutic treatment of NSCLC.
Wnt5a signaling comprises non-canonical
(β-catenin-independent) and canonical (β-catenin-dependent)
pathways (20). β-catenin
signaling serves an important role in regulating the transcription
of several oncogenes, such as cyclin D1 and c-Myc (21,22);
thus, different types of cancer exhibit aberrant activation of this
signaling pathway (23,24). Activated Wnt/β-catenin signaling
pathway has been shown to induce translocation of β-catenin from
the cytoplasm to the nucleus (25,26),
thus promoting the initiation of EMT, tumor invasion, and
metastasis (14,27-29).
The present study demonstrated that the combination of Wnt5a
knockdown and irradiation decreased nuclear but increased
cytoplasmic β-catenin expression in NSCLC cells. These findings
support the hypothesis that the combination of Wnt5a knockdown and
irradiation decreases translocation of β-catenin from the cytoplasm
to the nucleus, thus inhibiting NSCLC cell proliferation and
enhancing apoptosis.
A previous study reported that Wnt5a plays a key
role in regulating NSCLC cell migration and invasion by activating
β-catenin-dependent canonical Wnt signaling (12). Consistent with this finding, the
results of the present study demonstrated that Wnt5a knockdown
increased NSCLC cell apoptosis. In addition, overexpression of
Wnt5a attenuated the killing effect of radiation therapy on NSCLC
cells, whereas si-β-catenin antagonized Wnt5a
overexpression-induced proliferation of NSCLC cells. Increasing
evidence suggest that the Wnt/β-catenin pathway is associated with
radioresistance of cancer cells (7,30).
The enhanced nuclear translocation of β-catenin was more evident in
radioresistance cells (31-33).
Consistent with these findings, the present study demonstrated that
Wnt5a knockdown decreased nuclear β-catenin expression, whereas
overexpression of Wnt5a enhanced nuclear β-catenin expression.
Taken together, these results suggest that the Wnt5a/β-catenin
pathway may exert a radiosensitizing effect in NSCLC.
In conclusion, the present study demonstrated that
Wnt5a knockdown in combination with irradiation inhibited
proliferation and induced apoptosis of NSCLC cells; these effects
were reversed following overexpression of Wnt5a. In addition, Wnt5a
influenced the susceptibility of NSCLC cells to radiotherapy via
activation of β-catenin-dependent canonical Wnt signaling. Thus,
Wnt5a gene therapy may enhance the therapeutic effect of radiation
for the treatment of NSCLC.
Supplementary Material
Effect of Wnt5a knockdown and/or IR on
expression of β-catenin in H1650 and A549 NSCLC cell lines. Wnt5a,
wingless-type protein 5a; si, small interfering; IR,
irradiation.
Effect of Wnt5a overexpression and/or
IR on the expression of β-catenin in H1650 and A549 NSCLC cell
lines. Wnt5a, wingless-type protein 5a; IR, irradiation.
Establishment of β-catenin silencing
by siRNA transfection in H1650 and A549 cells. (A) Knockdown of
β-catenin expression by siRNA in transfected H1650 and A549 cells
was verified by immunoblotting and (B) reverse-transcription
quan-titative PCR with GAPDH as a loading control. A non-targeting
siRNA (scramble) was used as a negative control. Data are presented
as the mean ± SD of three independent repeats.
***P<0.001 vs. control. si, small interfering; OD,
optical density.
Expression of β-catenin in H1650 and
A549 cells with Wnt5a overexpression and/or β-catenin knockdown was
determined using western blot analysis. Wnt5a, wingless-type
protein 5a; si, small interfering.
Acknowledgements
Not applicable.
Funding
The present work was supported by The General Program (grant
nos. 817368 and 819MS135) and Youth Project (both Department of
Science and Technology; grant no. 819QN359) and The Regional
Science Foundation Program (National Natural Science Foundation;
grant no. 81860414).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL, SX and XX conceptualized and designed the study.
JL, SX, XW, HD and LHW acquired the data and drafted the
manuscript. JL, SX, XW, HD and LHW performed data analysis. SX and
XX wrote the manuscript. JL, SX, XW, HD and XX revised the
manuscript. JL, SX and XX confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harris JP, Murphy JD, Hanlon AL, Le QT,
Loo BW Jr and Diehn M: A population-based comparative effectiveness
study of radiation therapy techniques in stage III non-small cell
lung cancer. Int J Radiat Oncol Biol Phys. 88:872–884.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim E, Song C, Kim MY and Kim JS:
Long-term outcomes after salvage radiotherapy for postoperative
locoregionally recurrent non-small-cell lung cancer. Radiat Oncol
J. 35:55–64. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palayoor ST, Macklis RM, Bump EA and
Coleman CN: Modulation of radiation-induced apoptosis and G2/M
block in murine T-lymphoma cells. Radiat Res. 141:235–243.
1995.PubMed/NCBI
|
|
6
|
Ning S and Knox SJ: Arrest and death by
apoptosis of HL60 cells irradiated with exponentially decreasing
low-dose-rate gamma radiation. Radiat Res. 151:659–669.
1999.PubMed/NCBI
|
|
7
|
Lee SB, Gong YD, Park YI and Dong MS:
2,3,6-Trisubstituted quinoxaline derivative, a small molecule
inhibitor of the Wnt/beta-catenin signaling pathway, suppresses
cell proliferation and enhances radiosensitivity in A549/Wnt2
cells. Biocchem Biophys Res Commun. 431:746–752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst.
106(djt356)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bo H, Zhang S, Gao L, Chen Y, Zhang J,
Chang X and Zhu M: Upregulation of Wnt5a promotes
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells. BMC Cancer. 13(496)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee GT: Prostate cancer bone metastases
acquire resistance to androgen deprivation via WNT5A-mediated BMP-6
induction. Br J Cancer. 110:1634–1644. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kanzawa M, Semba S, Hara S, Itoh T and
Yokozaki H: WNT5A is a key regulator of the epithelial-mesenchymal
transition and cancer stem cell properties in human gastric
carcinoma cells. Pathobiology. 80:235–244. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang B, Tang Z, Gong H, Zhu L and Liu X:
Wnt5a promotes epithelial-to-mesenchymal transition and metastasis
in non-small-cell lung cancer. Biosci Rep.
37(BSR20171092)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mokhtari RB, Homayouni TS, Baluch N,
Morgatskaya E, Kumar S, Das B and Yeger H: Combination therapy in
combating cancer. Oncotarget. 8:38022–38043. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fang B and Roth JA: The role of gene
therapy in combined modality treatment strategies for cancer. Curr
Opin Mol Ther. 5:475–482. 2003.PubMed/NCBI
|
|
15
|
Yap TA, Omlin A and De Bono JS:
Development of therapeutic combinations targeting major cancer
signaling pathways. J Clin Oncol. 31:1592–1605. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Blagosklonny MV: Analysis of FDA approved
anticancer drugs reveals the future of cancer therapy. Cell Cycle.
3:1033–1042. 2004.PubMed/NCBI
|
|
17
|
Ahn SJ, Choi C, Choi YD, Kim YC, Kim KS,
Oh IJ, Ban HJ, Yoon MS, Nam TK, Jeong JU, et al: Microarray
analysis of gene expression in lung cancer cell lines treated by
fractionated irradiation. Anticancer Res. 34:4939–4948.
2014.PubMed/NCBI
|
|
18
|
Whang YM, Jo U, Sung JS, Ju HJ, Kim HK,
Park KH, Lee JW, Koh IS and Kim YH: Wnt5a is associated with
cigarette smoke-related lung carcinogenesis via protein kinase C.
PLoS One. 8(e53012)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang Y, Liu G, Zhang B, Xu G, Xiong W and
Yang H: Wnt-5a regulates proliferation in lung cancer cells. Oncol
Rep. 23:177–181. 2010.PubMed/NCBI
|
|
20
|
Pukrop T and Binder C: The complex
pathways of Wnt5a in cancer progression. J Mol Med (Berl).
86:259–266. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, Da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saegusa M, Hashimura M, Kuwata T, Hamano M
and Okayasu I: β-Catenin simultaneously induces activation of the
p53-p21WAF1 pathway and overexpression of cyclin D1 during squamous
differentiation of endometrial carcinoma cells. Am J Pathol.
164:1739–1749. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anastas JN and Moon RT: WNT signaling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–25. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
de Sousa E, Melo F and Vermeulen L: Wnt
signaling in cancer stem cell biology. Cancers (Basel).
8(60)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li XQ, Yang XL, Zhang G, Wu SP, Deng XB,
Xiao SJ, Liu QZ, Yao KT and Xiao GH: Nuclear β-catenin accumulation
is associated with increased expression of Nanog protein and
predicts poor prognosis of non-small cell lung cancer. J Transl
Med. 11(114)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Eger A, Stockinger A, Schaffhauser B, Beug
H and Foisner R: Epithelial mesenchymal transition by c-Fos
estrogen receptor activation involves nuclear translocation of
β-catenin and upregulation of β-catenin/lymphoid enhancer binding
factor-1 transcriptional activity. J Cell Biol. 148:173–188.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Roy LD, Sahraei M, Subramani DB, Besmer D,
Nath S, Tinder TL, Bajaj E, Shanmugam K, Lee YY, Hwang SI, et al:
MUC1 enhances invasiveness of pancreatic cancer cells by inducing
epithelial to mesenchymal transition. Oncogene. 30:1449–1459.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang L, Zhang XM, Li Z, Liu XJ, Chai J,
Zhang GY and Cheng YF: Overexpression of nuclear β-catenin in
rectal adenocarcinoma is associated with radioresistance. World J
Gastroenterol. 19:6876–6882. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tanaka H, Kawaguchi M, Shoda S, Miyoshi T,
Iwasaki R, Hyodo F, Hyodo F, Mori T, Hara A, Tomita H and Matsuo M:
Nuclear Accumulation of β-catenin in cancer stem cell
radioresistance and stemness in human colon cancer. Anticancer Res.
39:6575–6583. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo Y, Li M, Zuo X, Basourakos SP, Zhang
J, Zhao J, Han Y, Lin Y, Wang Y, Jiang Y, et al: β-catenin nuclear
translocation induced by HIF1α overexpression leads to the
radioresistance of prostate cancer. Int J Oncol. 52:1827–1840.
2018.PubMed/NCBI View Article : Google Scholar
|