Introduction
Lung cancer (LC), the main cause of oncological
death in the US and globally, is a significant public health
problem. The incidence of LC is on the rise, much as in
neuroendocrine tumor or gastric carcinoma cases (1,2). In
general, LC is a highly aggressive form of cancer with a rapid rate
of metastasis (3,4).
In recent decades, LC has become the most frequent
form of cancer worldwide, with a possible IL-6 involvement through
its pro-angiogenic properties, which helps in cancer development
and/or progression. The number of new cases was estimated at 1,8
million in 2012 (3,5,6). As
the leading cause of oncological death, with a poor prognosis, LC
has one of the lowest 5-year survival rates at under 15% (7,8).
The aim of the current study was to present the case
of a patient and the cutaneous side effects attributed to
pembrolizumab therapy. The outcome of the current study revealed
that pembrolizumab immune therapy use managed to prolong the
patient's survival with about 4 months, having a good performance
status of 2 (ECOG).
Case report
Subject
The aim of the current study was to present a case
of a patient and the cutaneous side effects attributed to
pembrolizumab therapy. A 63-year-old patient was admitted in March
2017 to the Military Hospital of Galați, Romania, suffering from
right thoracic stabbing pain, dyspnea, dry cough and night sweats.
The patient signed and provided written informed consent for the
publication of data or any images, which is available in the
patient's medical chart. Ethics approval and consent to participate
were obtained from the ‘Sfantul Apostol Andrei’ Emergency Clinical
Hospital's Ethics Committee, with the decision no. 11413 from
03.06.2021.
Computerized tomography scan
After a thoracic computerized tomography (CT) scan
in September 2017, a tumor mass in the right superior pulmonary
lobe with a background of diffuse moderate pulmonary emphysema was
found.
In October 2017, the patient was subjected to a
surgical procedure consisting of right superior lobectomy and
mediastinal lymphadenectomy in the ‘Marius Nasta’ Institute of
Pneumophysiology, in Bucharest, Romania. The pathology laboratory
reported this tumor as a pleomorphic lung cancer with an
adenocarcinoma component, pT2aN0M0, with focal positivity for
thyroid transcription factor 1 (TTF1), without epidermal growth
factor receptor (EGFR) mutations, nor anaplastic lymphoma kinase
(ALK) recombinations, having an initial clinical stage of IB and
programmed death ligand-1 (PD-L1) positivity with a tumor
proportion score of over 70%.
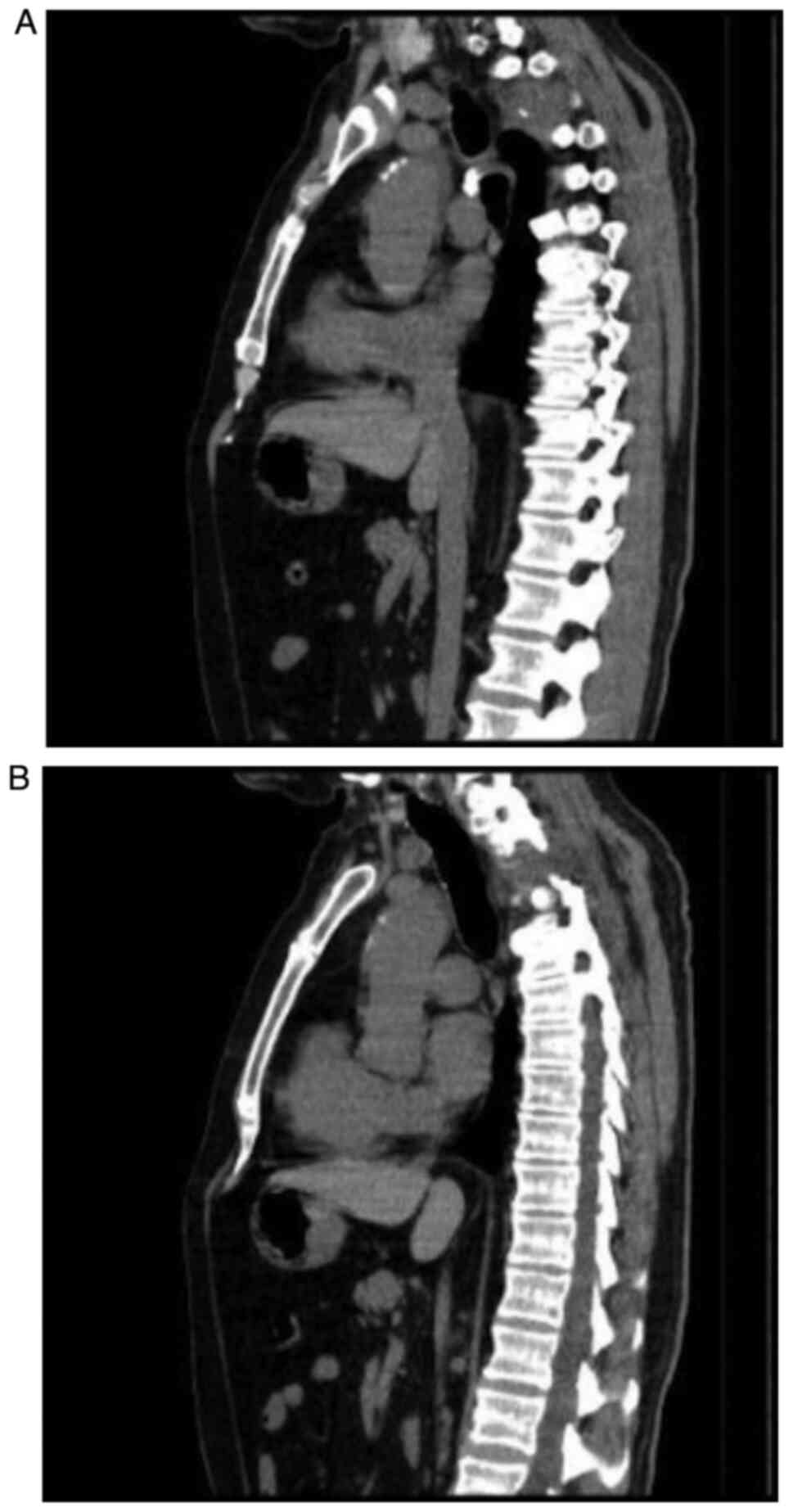
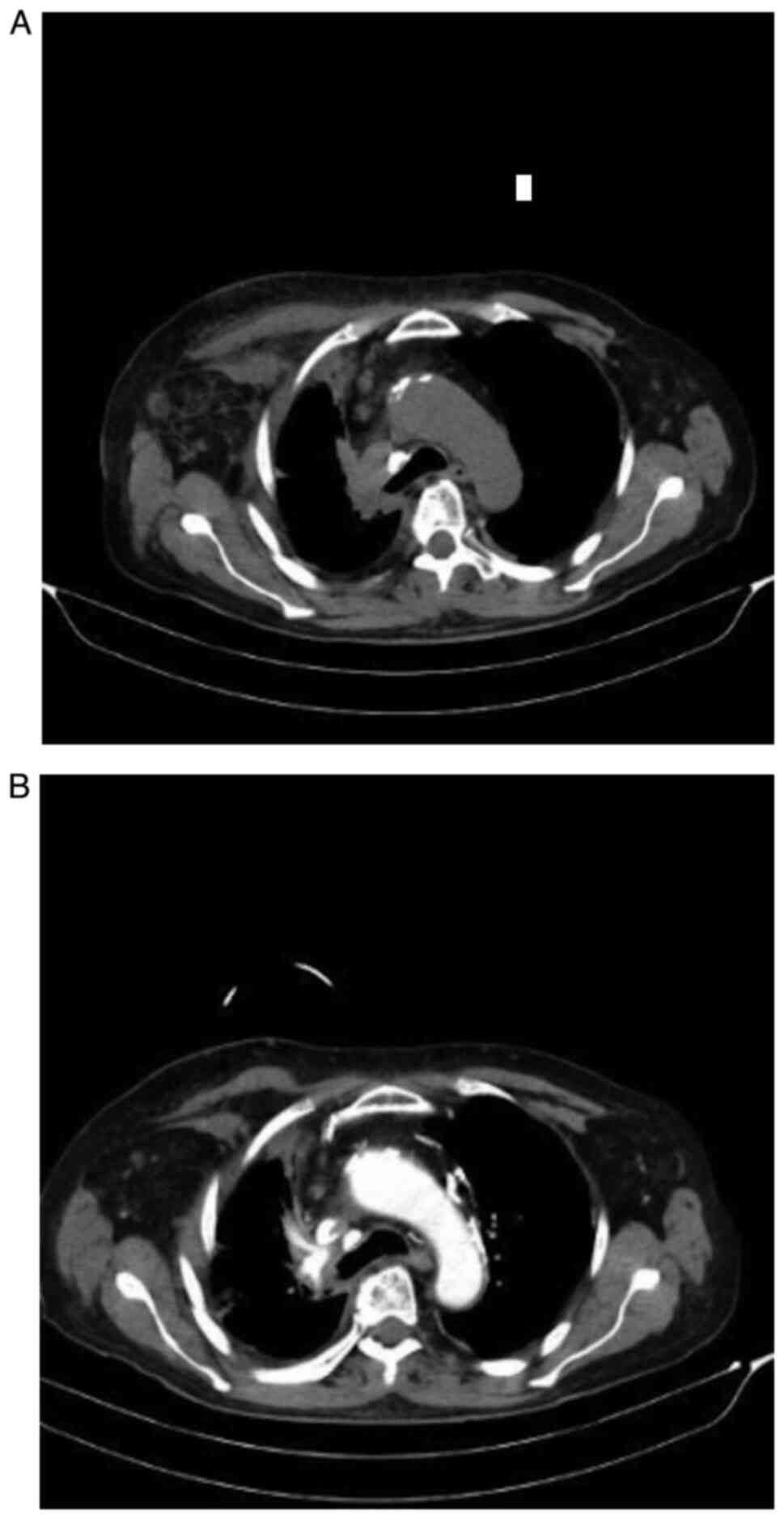
A new thoracic CT scan was performed in March 2018
which revealed a right apex lung tumor with spine involvement and
mediastinal paratracheal ipsilateral adenopathies (Fig. 1A and B).
A skeletal scintigraphy was carried out at the end
of March 2018 which revealed multiple bone metastases localized in
the head of the humerus, the T3 vertebra, right third rib and right
iliac bone, for which the patient received a bone tissue protection
treatment with zolendronic acid until September 2018 and also
antalgic radiotherapy (RTE) with a total dose (TD) of 20 Gy/5 fr
localized at the head of the humerus, right sacroiliac region and
the thoracic spine, levels T1 to T4. No systemic anticancer
treatment was administered from July 2018 until September 2019.
The CT scan of the thorax and abdomen, which was
performed in September 2018, revealed a right apex tumor mass
measuring 65/50/45 mm, which invaded the thoracic wall and
destroyed the 2nd, 3rd and 4th posterior rib arcs. The tumor
protruded from the dorsal medullary canal, as a local, continuous
tumor evolvement. In November 2018, the magnetic resonance imaging
(MRI) of the thoracic spine highlighted the right lung tumor mass
which destroyed the thoracic vertebral bodies of T2 to T4, and
which invaded the intervertebral pedicles on the right side and
also the spinal canal. Consequently, the patient underwent a
surgical procedure for spinal decompression and the pathology
report revealed that the fibro-hyaline fragments of the vertebral
discs had foci of poorly differentiated squamous cell carcinoma
(G3).
Other factors and treatment
At the same time, the laboratory findings revealed
high values for urea and creatinine; the decision for terminating
treatment with zolendronic acid was taken, in favor of a systemic
one, such as chemotherapy or immunotherapy. Due to the high,
oscillating creatinine values, it was possible to administer
chemotherapy based on carboplatin in association with pemetrexed,
paclitaxel or gemcitabine.
The recent inclusion of pembrolizumab in the
national cancer treatment program, the patient's ALK and EGFR
statuses, his PD-L1 level of 70%, and that this drug is excluded
from treatment only in cases with severe renal insufficiency,
supported the initiation of first-line immunotherapy with
pembrolizumab starting from September 2018. Two months after
therapy with pembrolizumab was begun, a CT scan of the head, thorax
and abdomen was carried out and it revealed an evolutionary stable
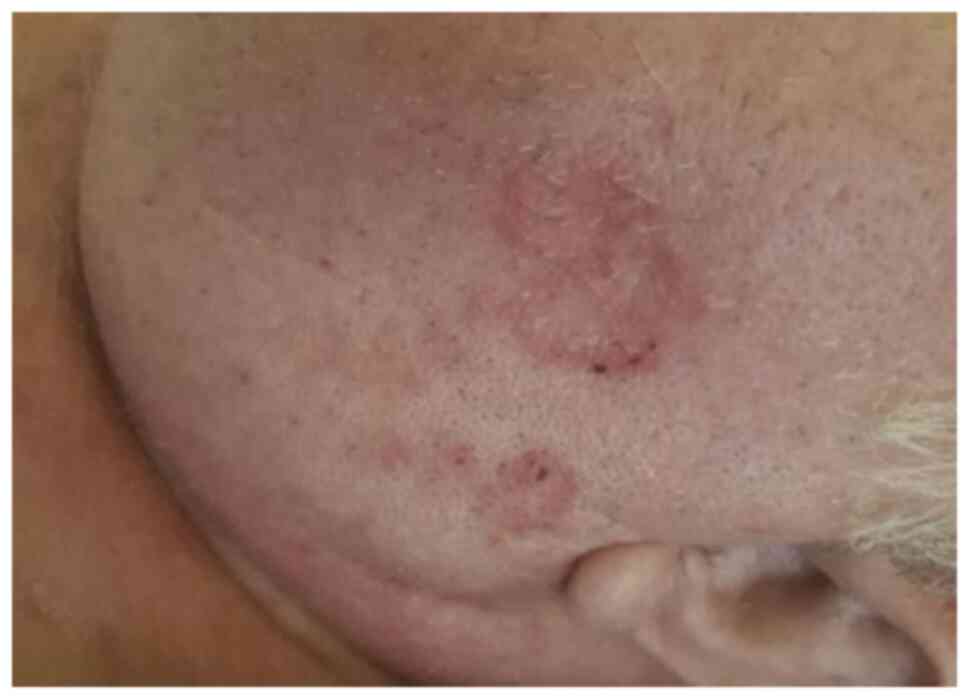
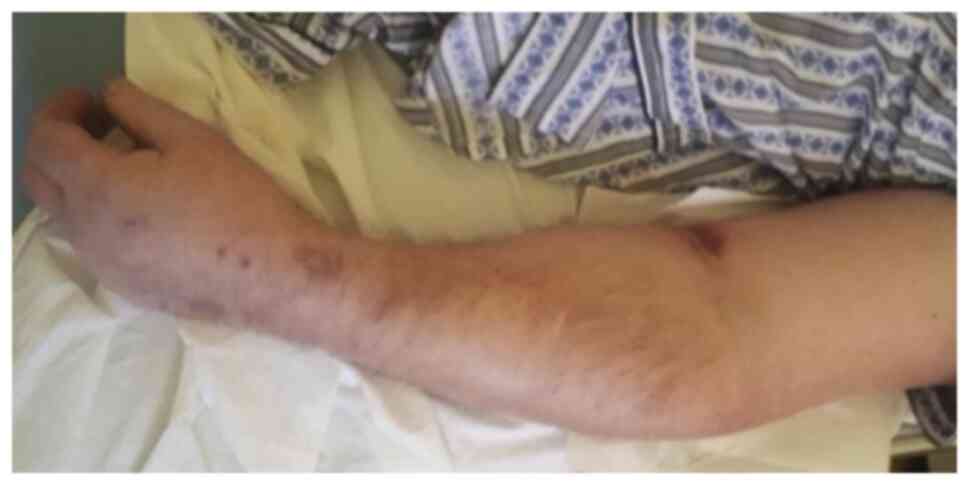
disease. Immunotherapy was continued but some eczematous,
psoriasiform and lichenoid secondary cutaneous side effects
developed, which were localized on the left mandible and left
superior limb and had a partial response to corticoid treatment
with full resolution following pembrolizumab treatment termination
(Figs. 2 and 3) (9-12).
The patient suffered from arterial hypertension for
which he received metoprolol, daily. An important fact that is well
known among physicians is that metoprolol should not be
concomitantly administered with floctafenine, sultopride, bepridil,
diltiazem or verapamil, nor should it be taken during or after a
meal, as food has the property of increasing the bioavailability of
metoprolol. The types of food that are ingested are important, not
only in the case of metoprolol, but also for other medications such
as statins (grapefruit, for example, has the ability to increase
the drug's blood concentration). There are also other factors that
can intervene in certain drug pharmacokinetics, as well as
physiological factors such as decreased fatty tissue or gastric
acidity, or decreased renal excretion. Diuretics
(hydrochlorothiazide), and other anti-hypertensive drugs, can also
have cutaneous adverse reactions including rashes or
photosensitization. The cutaneous adverse reactions of some
ß-blockers have been reported, such as psoriasis precipitation or
exacerbation, but fortunately our patient did not suffer from this
disease (13-19).
Due to the cutaneous adverse reaction development,
dexamethasone was prescribed, as 8 mg injectable doses, 2 vials per
day, for 5 days, which relieved the patient of the cutaneous
lesions, initially attenuating them, followed by complete
extinction. Thus, treatment with pembrolizumab could be reinstated
and continued.
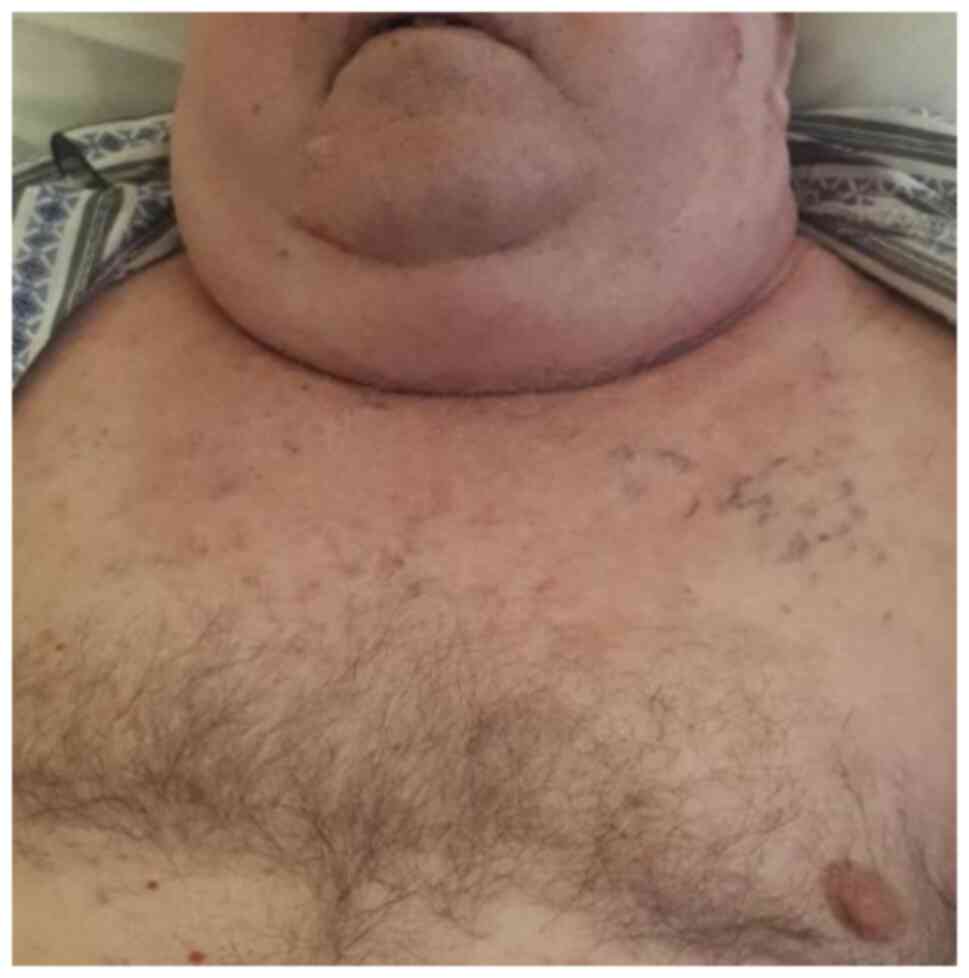
The patient developed a mediastinal compression
syndrome (Fig. 4) for which
mediastinal radiotherapy was administered with a TD of 30 Gy.
Immunotherapy was continued in the subsequent months and was
terminated by March 2019, after a thoracic CT scan was carried out
which revealed progressive disease (Fig. 5A and B).
The patient received 4 doses of pembrolizumab,
having a current unsatisfactory general health status with a
prognosis index of 3 on the Eastern Cooperative Oncology Group
performance status (ECOG). Consequently, immunotherapy was
stopped.
Discussion
Adenocarcinoma is the most frequent pathology
subtype, registering an increased incidence rate among female
patients in industrialized countries over the last decade, a
tendency justified by some authors through a change in toxic
habits, with a higher use of filtered cigarettes, leading to deeper
inhalation with a more peripheral distribution (20,21).
According to the new diagnostic and treatment guides, surgical
intervention, radiotherapy, adjuvant chemotherapy, targeted therapy
and immunotherapy are employed as therapeutic options in non-small
cell lung cancer (NSCLC) with the possibility of being used as
monotherapy or in combination, in accordance with the stage of
disease. Although early diagnosis enables a complete surgical
resection (the therapeutic option with the highest potential for
cure), approximately 40% of patients relapse 5 years after the
procedure (4,22,23).
As part of the NSCLC treatment, new compounds have
been introduced, targeting immune control points, such as
programmed cell death protein-1 (PD-1) or its ligand (PD-L1).
Pembrolizumab is one such immunotherapeutic anti-PD-1 recommended
for treating advanced-stage patients, without EGFR and ALK
mutations, and with high PD-L1 (4,24).
Tumor protein p53 (TP53) and EGFR mutations are strong parameters
that can predict the response to anti-PD-1 treatment in NSCLC
(25,26).
Pembrolizumab was approved by the Food and Drug
Administration (FDA) in the USA for many advanced stage or
metastatic cancers as this therapeutic agent acts by blocking the
protein found on the surface of cancer cells, and the protein known
as PD-L1, thus allowing immune cells to destroy the tumor. Recent
findings suggest that treatment with pembrolizumab can help some
NSCLC patients benefit from a higher survival rate with fewer
adverse effects. The phase I clinical study known as KEYNOTE-001
proved that some advanced stage NSCLC patients who received
pembrolizumab lived 3-4 times longer than expected. The most
frequent cutaneous adverse effects identified were brief cutaneous
eruptions which our patient developed since the second month of
treatment with this medication (27-29).
The latest literature states that cases with mild
cutaneous adverse reactions (such as lichenoid reactions,
granulomatous skin reactions), as in our patient's case, can
receive treatment with topical corticosteroids at low doses,
moisturizing ointments or oral antihistamines (27,28,30,31).
Some patients who developed cutaneous adverse
reactions such as vitiligo registered skin repigmentation, not as
proof for skin lesion treatment response, but as proof for cancer
(melanoma) progression. The skin lesions were treated with
classical vitiligo treatment, i.e., sun protection, phototherapy,
calcineurin inhibitors, and topical corticosteroids. Psoriasis is a
frequent adverse reaction to pembrolizumab and can develop as de
novo lesions or as an exacerbation of the pre-existing lesions.
Therapeutic management in these cases included topical steroids
and/or vitamin D analogues, systemic retinoids, or even
methotrexate. Cases of pityriasis rubra pilaris have also been
reported after pembrolizumab therapy (some also as a paraneoplastic
syndrome), these patients being successfully treated with acitretin
and topical steroids (27-31).
More severe cutaneous adverse reactions (extensive
bullous pemphigoid lesions) were treated with topical and systemic
corticosteroids and treatment with pembrolizumab was withheld (as a
temporary therapeutic management approach, or even as a permanent
one; as in some patients with lupus erythematosus, toxic epidermal
necrolysis, Stevens-Johnson syndrome or erythema multiforme). These
adverse effects can evolve in various directions: they can resolve
completely, can be ongoing, or they can become exacerbated. Some of
the patients suffering from such therapeutic events, even after
withholding pembrolizumab treatment, had a partial therapeutic
response regarding their skin lesions (27,28,32).
An important issue to further research concerns the
group of pembrolizumab drug interactions. For instance, one case
report presented the case of a patient treated both with
pembrolizumab and rivaroxaban, who developed an intra-cerebellar
hemorrhage; as pembrolizumab seems to have an effect on the liver's
CYP3/A4 system, it also seems to influence the metabolism of such
new anticoagulant medication (33).
Notably, the cutaneous adverse effects treatment
must not interfere with the therapeutic effects of pembrolizumab
(anti-PD1) and patients need to adhere to treatment; such patients
can opt for a dose decrease or can even stop treatment. Skin
lesions being highly visible, the patients suffer from a low
quality of life, with psychological distress and social refrain,
similar to those with systemic sclerosis, for example. Topical
steroids have been used as treatment for such adverse effects,
having beneficial results, mostly due to their multiple biologic
activities, which include anti-inflammatory, immune suppressive, or
anti-proliferative activities. Complete pembrolizumab treatment
withdrawal was found in rare cases in the literature, more often
clinicians opting for continuation of treatment or only for a
temporary withdrawal (for 1 or even 7 weeks) (27,28,33-36).
During clinical trials, patients who develop severe
adverse reactions to immunotherapy (such as pembrolizumab) are not
allowed to resume it, due to the high risk of reoccurrence. This is
also an in-practice issue, as the choice to reinitiate immune
therapy is challenging. One possible approach would involve a class
switch, opting for an anti-CTLA-4, instead of an anti-PD(L)1
(pembrolizumab), these two having different mechanisms of action
(the first one increases the diversity of the host's immune
response, while the second reactivates a suppressed host immune
response). Another approach to patient treatment is the
re-challenge itself, as many patients experience no recurrence of
the side effects. Reintroducing the same anti-PD1 treatment can
also result in some of the patients experiencing the same cutaneous
adverse reactions, or some even new ones. Recurrence of the side
effects may occur with decreased frequency (37-39).
In summary, even with targeted therapy or
immunotherapy, lung cancer remains the main cause of death
worldwide, having an increasing incidence in the last decade with a
low general survival rate. It is mandatory to find a more favorable
approach to identify patients at risk in order to establish more
efficient personalized treatment and part of this is identifying
the adverse effects of new therapies, as an objective for future
studies.
Although immunotherapy is one of the newly reached
frontiers in cancer therapy, clinicians need to have a watchful eye
for (cutaneous) adverse reactions, their early diagnosis and
management having a major impact on patient treatment concerning
adherence, therapeutic result and overall survival. Treatment must
be adapted to each patient, as they can react differently to
pembrolizumab therapy, with various side effects.
Acknowledgements
The current work was academically supported by the
'Dunarea de Jos' University of Galati, Romania, through the
research center - Multidisciplinary Integrated Center of
Dermatological Interface Research (MIC-DIR) [Centrul Integrat
Multidisciplinar de Cercetare de Interfata Dermatologica
(CIM-CID)].
Funding
No funding was received.
Availability of data and materials
The information generated and analyzed during the
current study is available from the corresponding author on
reasonable request.
Authors' contributions
CB, GM, ALT, SF, EN, MC, LA, LR, MD and AN were
major contributors in writing the manuscript; they were involved in
all the stages of the study, contributed to the conception and
design of the work, as well as revising it; they helped analyze the
data for the work, revised it for important intellectual content
and approved the final version to be published. All authors agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work. All authors have had equal participation, contribution and
equal rights to this article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval and consent to participate were
obtained from the ‘Sfantul Apostol Andrei’ Emergency Clinical
Hospital's Ethics Committee, with the decision no. 11413 from
03.06.2021.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying image.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Craescu M, Rebegea L, Ivan I, Dumitru M,
Serban C and Firescu D: Therapeutic challenges in a case of trachea
neuroendocrine tumor. Acta Med Mediterr. 35:1493–1496. 2019.
|
|
2
|
Fekete GL, Cotoi OS and Fekete JE:
Multiple nodular cutaneous metastases as the first clinical sign of
signet ring cell gastric carcinoma: Case report. Acta
Dermatovenerol Croat. 20:34–37. 2012.PubMed/NCBI
|
|
3
|
Riihimäki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
National Comprehensive Cancer Network
(NCCN): NCCN Clinical Practice Guidelines in Oncology (NCCN
guidelines): Non-small cell lung cancer. Version 5.2019 - June 7,
2019. NCCN, Plymouth Meeting, PA, 2019. https://www.nccn.org/guidelines/category_1#si.
Accessed July 30, 2019.
|
|
5
|
Nomori H, Watanabe K, Ohtusuka T, Naruke
T, Suemasu K and Uno K: The size of metastatic foci and lymph nodes
yielding false-negative and false-positive lymph node staging with
positron emission tomography in patients with lung cancer. J Thorac
Cardiovasc Surg. 127:1087–1092. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Niculet E, Chioncel V, Elisei AM, Miulescu
M, Buzia OD, Nwabudike LC, Craescu M, Draganescu M, Bujoreanu F,
Marinescu E, et al: Multifactorial expression of IL-6 with update
on COVID-19 and the therapeutic strategies of its blockade
(Review). Exp Ther Med. 21(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology and prevention. Clin Chest Med.
32:605–644. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou H, Wu A, Fu W, Lv Z and Zhang Z:
Significance of semaphorin-3A and MMP-14 protein expression in
non-small cell lung cancer. Oncol Lett. 7:1395–1400.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gheorghe I, Tatu AL, Lupu I, Thamer O,
Cotar AI, Pircalabioru GG, Popa M, Cristea VC, Lazar V and
Chifiriuc MC: Molecular characterization of virulence and
resistance features in Staphylococcus aureus clinical strains
isolated from cutaneous lesions in patients with drug adverse
reactions. Rom Biotech Lett. 22:12321–12327. 2017.
|
|
10
|
Hwang SJE and Fernández-Peñas P: Adverse
reactions to biologics: Melanoma (Ipilimumab, Nivolumab,
Pembrolizumab). Curr Probl Dermatol. 53:82–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee M and Seetharamu N: An atypical
presentation of lichen planus-like reaction from pembrolizumab.
Case Rep Dermatol Med. 2019(4065437)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nwabudike LC and Tatu AL: Reply to
Gambichler T et al: Altered epigenetic pathways and cell
cycle dysregulation in healthy appearing skin of patients with
koebnerized squamous cell carcinomas following skin surgery. J Eur
Acad Dermatol Venereol. 33:e3–e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Flaten HK and Monte AA: The
pharmacogenomic and metabolomic predictors of ACE inhibitor and
angiotensin II receptor blocker effectiveness and safety.
Cardiovasc Drugs Ther. 31:471–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Johnson JA: Advancing management of
hypertension through pharmacogenomics. Ann Med. 44 (Suppl
1):S17–S22. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tatu AL, Elisei AM, Chioncel V, Miulescu M
and Nwabudike LC: Immunologic adverse reactions of β-blockers and
the skin (Review). Exp Ther Med. 18:955–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nwabudike LC, Elisei AM, Buzia OD,
Miulescu M and Tatu AL: Statins. A review on structural
perspectives, adverse reactions and relations with non-melanoma
skin cancer. Rev Chim (Bucharest). 69:2557–2562. 2018.
|
|
17
|
Tatu AL, Ciobotaru OR, Miulescu M, Buzia
OD, Elisei AM, Mardare N, Diaconu C, Robu S and Nwabudike LC:
Hydrochlorothiazide: Chemical structure, therapeutic, phototoxic
and carcinogenetic effects in dermatology. Rev Chim (Bucharest).
69:2110–2114. 2018.
|
|
18
|
Dobre M, Georgescu C, Stefanescu V,
Cuciureanu M, Nechita A and Arbune M: Homeostatic changes during
anticonvulsant medication in children. Farmacia. 63:402–406.
2015.
|
|
19
|
Jáuregui-Garrido B and Jáuregui-Lobera I:
Interactions between antihypertensive drugs and food. Nutr Hosp.
27:1866–1875. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fidler-Benaoudia MM, Torre LA, Bray F,
Ferlay J and Jemal A: Lung cancer incidence in young women vs.
young men: A systematic analysis in 40 countries. Int J Cancer.
147:811–819. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Özmen S and Ceylan O: Trends in lung
cancer incidence within the last 10 years: An Eastern Anatolian
single center experience. J Surg Med. 4:112–115. 2020.
|
|
22
|
Blandino G and Di Agostino S: New
therapeutic strategies to treat human cancers expressing mutant p53
proteins. J Exp Clin Cancer Res. 37(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
NSCLC Meta-analyses Collaborative Group.
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y and
Yang J: Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer:
Toward personalized medicine and combination strategies. J Immunol
Res. 2018(6984948)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Biton J, Mansuet-Lupo A, Pécuchet N,
Alifano M, Ouakrim H, Arrondeau J, Boudou-Rouquette P, Goldwasser
F, Leroy K, Goc J, et al: TP53, STK11, and EGFR mutations predict
tumor immune profile and the response to anti-PD-1 in lung
adenocarcinoma. Clin Cancer Res. 24:5710–5723. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu CH and Hwang MJ: Risk stratification
for lung adenocarcinoma on EGFR and TP53 mutation status,
chemotherapy, and PD-L1 immunotherapy. Cancer Med. 8:5850–5861.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sanlorenzo M, Vujic I, Daud A, Algazi A,
Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K and
Ortiz-Urda S: Pembrolizumab cutaneous adverse events and their
association with disease progression. JAMA Dermatol. 151:1206–1212.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simonsen AB, Kaae J, Ellebaek E, Svane IM
and Zachariae C: Cutaneous adverse reactions to anti-PD-1
treatment-a systematic review. J Am Acad Dermatol. 83:1415–1424.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fekete GL, Boda D, Căruntu C and Fekete L:
Paraneoplastic pityriasis rubra pilaris in association with
prostate carcinoma: A case report and literature review. Exp Ther
Med. 18:5052–5055. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Coleman E, Panse G, Haldas J, Gettinger SN
and Leventhal JS: Pityriasis rubra pilaris-like erythroderma in the
setting of pembrolizumab therapy responsive to acitretin. JAAD Case
Rep. 4:669–671. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Creţu A, Dimitriu A, Brănişteanu D and
Brinişteanu DE: Erythema multiforme-etiopathogenic, clinical and
therapeutic aspects. Rev Med Chir Soc Med Nat Iasi. 119:55–61.
2015.PubMed/NCBI
|
|
33
|
Joshi N (ed): Pembrolizumab/rivaroxaban
interaction. In: Reactions Weekly. Vol 1704. Springer Nature
Switzerland AG, Cham, p304, 2018.
|
|
34
|
Bobeică C, Tatu AL, Crăescu M and
Solovăstru L: Dynamics of digital ulcers in systemic sclerosis. Exp
Ther Med. 20:61–67. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bobeica C, Niculet E, Craescu M, Onisor C,
Bujoreanu F, Draganescu ML, Halip IA and Gheuca-Solovastru L:
Epidemiological profile of systemic sclerosis in the southeast
region of Romania. Exp Ther Med. 21(77)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Niculet E, Bobeica C and Tatu AL:
Glucocorticoid-induced skin atrophy: The old and the new. Clin
Cosmet Investig Dermatol. 13:1041–1050. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Haanen J, Ernstoff M, Wang Y, Menzies A,
Puzanov I, Grivas P, Larkin J, Peters S, Thompson J and Obeid M:
Rechallenge patients with immune checkpoint inhibitors following
severe immune-related adverse events: Review of the literature and
suggested prophylactic strategy. J Immunother Cancer.
8(e000604)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Winer A, Bodor JN and Borghaei H:
Identifying and managing the adverse effects of immune checkpoint
blockade. J Thorac Dis. 10 (Suppl 3):S480–S489. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bhatlapenumarthi V, Patwari A and Harb AJ:
Immune-related adverse events and immune checkpoint inhibitor
tolerance on rechallenge in patients with irAEs: A single-center
experience. J Cancer Res Clin Oncol. 147:2789–2800. 2021.PubMed/NCBI View Article : Google Scholar
|