Introduction
Axillary bromhidrosis is a common dermatological
condition among the general population, and its overall worldwide
prevalence is reported to be 1-3% (1,2).
Bromhidrosis has a negative effect on a person's quality of life
(3). The treatments for
bromhidrosis include surgery, physical therapy and botulinum toxin
A injection (4,5).
Bromhidrosis results from hyperactivity of axillary
sweat gland (SG)s, and most treatment methods focus on removing the
axillary SG and decreasing axillary sweating (3,6).
However, there is no radical cure for bromhidrosis and the
development of novel drugs or therapies for managing bromhidrosis
remains important. The etiology of bromhidrosis is multifactorial
(7) and is associated with
infection (8) as well as metabolic
and neurological dysfunction (9).
However, the exact etiology of bromhidrosis is unclear.
Paeoniflorin (PF), an agent isolated from
Paeoniae alba, is the primary active component in Shaobei
injection (10). PF has been shown
to promote production of intracellular Ca2+ in salivary
gland cells (11) and exert a
neuroprotective effect (12) by
inducing autophagy-associated pathways (13). In addition, PF regulates the
metabolism of amino acids, cholesterol and fat (14-16)
and inhibits proliferation of fibroblast-like synoviocytes
(17) and pulmonary artery smooth
muscle cells (SMCs) (18). To the
best of our knowledge, however, the effects of PF on SG cells
(SGCs) and its mechanism in treating bromhidrosis have not been
reported.
In clinical practice, patients with axillary
bromhidrosis who had been injected with Shaobei under their armpits
reported that axillary bromhidrosis was notably decreased.
Therefore, the present study aimed to determine the effect of PF on
bromhidrosis both in vivo and in vitro. The effect of
PF on SG morphology, as well as proliferation, apoptosis and
autophagy of SGCs was determined.
Materials and methods
Animals and treatment
The protocols for all animal experiments were
approved by the Institutional Animal Care and Use Committee of the
First Affiliated Hospital of Guangdong Pharmaceutical University.
Male Sprague-Dawley rats (n=25; age, 3-4 weeks; weight, 210±15 g)
were purchased from the Experimental Animal Center of Sun Yat-sen
University and housed at 27˚C, relative humidity 45%, light/dark
cycle of 12 h. Rat chow and water were available ad libitum. PF
(2.5 mg/kg body weight) was injected into the upper right claw via
foot injection. At 6, 24, 48 and 72 h post-treatment (n=5/group),
animals were sacrificed by overdose of pentobarbital sodium (135
mg/kg; R&D Systems, Inc.). The foot skin was dissected and
prepared for histological examination. All experiments were
performed in accordance with the Chinese regulations on the use and
breeding of experimental animals (19).
Histological examination
The foot skin samples were cut into pieces, fixed
with 4% paraformaldehyde at room temperature for 48 h (cat. no.
p1110; Beijing Solarbio Science & Technology Co., Ltd.), then
dehydrated, made transparent, and embedded in paraffin. Next, the
embedded tissues were cut into serial sections (5 µm thickness)
using a microtome. After baking the sections of tissue at 60˚C for
2 h, they were immersed in xylene, ethanol (100, 95, 80 and 70%),
and pure water, counterstained with hematoxylin-eosin at 37˚C for
10 min, dehydrated with gradient ethanol and mounted with neutral
resin as previously reported (20). The photos were captures at the
magnifications of x20 and x400 by an Olympus light microscope (cat.
no. BX51; Olympus Corporation) and the data were analyzed using
Olympus Stream software (Olympus Corporation).
Cell isolation and culture
conditions
Primary human SGCs (hSGCs) were isolated from the
alar skin of five patients with bromhidrosis (three men and two
women; age, 25-45 years) recruited between June and September 2020
at The First Affiliated Hospital of Guangdong Pharmaceutical
University (Guangzhou, China). Bromhidrosis was diagnosed according
to previously reported criteria (21). The protocols for all experiments
involving humans were approved by the Institutional Review Board
and Ethics Committee of The First Affiliated Hospital of Guangdong
Pharmaceutical University Written (approval no. 202182). Written
informed consent was obtained from each of the five patients prior
to enrollment in the study. Primary hSGCs were isolated as
previously reported (22). The
pieces of foot skin were cleaned of subcutaneous fat, washed with
precooled PBS, then incubated with dispase (0.8 U/ml; Roche
Diagnostic Ltd.) at 37˚C for 16-20 h. The dermis was obtained and
subsequently digested with collagenase type IV (2.5 mg/ml; Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C for 1 h. The hSGCs were
then carefully collected from the lysate using a Transferpettor
under a microscope. Next, the hSGCs were cultured in DMEM-F12
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with FBS, 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology), 10X
Insulin Transferrin Selenium, 2 mM l-glutamine (Gibco; Thermo
Fisher Scientific, Inc.), 2 nmol/ml triiodothyronine
(Sigma-Aldrich; Merck KGaA), 10 ng/ml recombinant human epidermal
growth factor (Invitrogen; Thermo Fisher Scientific, Inc.) and 0.4
mg/ml hemisuccinate hydrocortisone (Sigma-Aldrich; Merck KGaA) at
37˚C and 5% CO2. Cells at passage 3-4 were used for
further experiments.
Immunofluorescence assay
Isolated hSGCs were identified using cytokeratin 8
(CK8) and α-smooth muscle actin (SMA) immunofluorescence assays.
Primary cells were harvested, resuspended in DMEM-F12 and then
placed 2x105 cells into 24-well plates with slides (14
mm in diameter; Costar; Corning, Inc.) at 37˚C for 24 h. Next,
cells attached to slides were fixed with 4% Paraformaldehyde at
room temperature for 30 min, treated with 0.5% Triton X-100
(Beyotime Institute of Biotechnology) at room temperature for 5
min, then incubated with antibodies against CK8 (1:100; cat. no.
ab53280; Abcam) and α-SMA (1:200; cat. no. ab32535; Abcam) at 4˚C
overnight. This was followed by incubation with secondary goat
anti-mouse/rabbit IgG antibody labeled with Alexa Fluor 594/488
(both 1:200; cat. no. ab150113; Abcam) at 37˚C for 1 h. Cell nuclei
were stained with DAPI (10 µM) at 37˚C for 10 min. Images of the
stained cells were captured using a fluorescence microscope
(magnification, x20 and x400; Lionheart LX; BioTek Instruments
Inc.).
hSGC treatment
Following identification and passaging, hSGCs at
passage 3-4 were treated with 0, 2, 4, 8, 16, 32 and 64 µM PF
(Sigma-Aldrich; Merck KGaA) at 37˚C in a 5% CO2
atmosphere for 24 h. For inhibition of autophagy, PF and/or 5 mM
3-methyladenine (3-MA; Sigma-Aldrich; Merck KGaA) were added to
hSGCs at 37˚C for 24 h. Each condition was replicated three
times.
Cell proliferation analysis
The inhibitory effect of PF on hSGC proliferation
was examined using Cell Counting Kit-8 (CCK-8) assay (5 mg/ml;
Beyotime Institute of Biotechnology) for 2 h according to the
manufacturer's instructions. The absorbance of each well was read
at 450 nm using a microplate reader (BioTek Instruments, Inc.). The
50 and 25 inhibitory concentration (IC) were calculated based on
CCK-8 assay results. Untreated hSGCs were used as the control.
Measurement of intracellular reactive
oxygen species (ROS) levels
Intracellular ROS levels were determined using a
CellROX® Green assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.) Following treatment with PF, hSGCs were incubated
with CellROX® probe at 37˚C and 5% CO2 for 30
min, followed by three washes with DMEM (Gibco; Thermo Fisher
Scientific, Inc.) without FBS. The fluorescence intensity of each
group of cells was measured using a BD FACS Calibur™
flow cytometer (BD Biosciences) and analyzed by FlowJo software
v10.0. N-Acetyl-Cysteine (1 mM) and tert-butylhydroperoxide (200
µM; BD Biosciences) were used as negative and positive controls,
respectively.
Cell apoptosis
Cell apoptosis was determined by flow cytometry and
Hoechst 33258 staining. In brief, hSGCs (2x105 cells/ml)
were placed into 24-well plates (Costar; Corning, Inc.) and
incubated with PF (9.53 µM) for 24 h at 37˚C in a 5%
CO2. The cells were then harvested by Trypsin, fixed
with 70% ethanol at room temperature for 30 min and stained using
Hoechst 33258 DNA intercalating dye (Beyotime Institute of
Biotechnology) or Annexin V-FITC/PI fluorescent double staining
solutions (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. An Olympus fluorescent microscope
(magnification, x400) was used to record images of the Hoechst
33258-stained cells. Cell apoptosis, as indicated by Annexin V/PI
fluorescent double staining, was analyzed using a FACS
Calibur™ flow cytometer (BD Biosciences) and the data
were analyzed using FlowJo 10.07 software (FlowJo LLC).
Cell cycle distribution analysis
The effect of PF on hSGC cell cycle distribution was
analyzed using flow cytometry (BD Biosciences). hSGCs were treated
with PF (9.530 µM) at 37˚C for 24 h, and then the cells were
incubated with Trypsin and 4 mL of blocking reagent DMEM-F12
(Gibco; Thermo Fisher Scientific, Inc.) was added at 37˚C for 10
sec. The cells washed using PBS and fixed with 70% ethanol at 4˚C
for 30 min prior to 50 µg/ml PI/RNase staining solution (Sungene
Biotech Co., Ltd.) at room temperature for 20 min. A FACS
Calibur™ flow cytometer was used for cell cycle analysis
and analyzed by FlowJo software v10.0.
Western blot analysis
The total cellular proteins were extracted from
PF-treated and control hSGCs using lysis buffer (Beyotime Institute
of Biotechnology) and protein determination by BCA Protein Assay
kit (Pierce; Thermo Fisher Scientific, Inc.). Following protein
quantification, an aliquot of total protein (30 µg) from each
extract was separated by 10% SDS-PAGE (Invitrogen; Thermo Fisher
Scientific, Inc.) and the protein bands were electro-transferred
onto PVDF membranes (MilliporeSigma). Then, 5% skimmed milk
(Beyotime Institute of Biotechnology) was used to block the
membrane at room temperature for 30 min, followed by primary
antibody incubation at 4˚C overnight. After washing with TBS- 0.05%
Tween-20 (Sigma-Aldrich; Merck KGaA) and polysorbate buffer
(Invitrogen; Thermo Fisher Scientific, Inc.), the membrane was
incubated with the secondary HRP Goat anti-Rabbit IgG (1: 20000;
BOSTER, cat. no. BA1054) at room temperature for 1 h. Next, the
membranes were incubated with anti-LC3B (1:1,000, cat. no.
ab192890, Abcam), anti-Beclin 1 (1:1,500, cat. no. ab210498,
Abcam), anti-P62 (1:1,000, cat. no. ab109012, Abcam),
phosphorylated (p)-PI3K (1:1,000, cat. no. ab182651, Abcam), PI3K
(1:2,000, cat. no. ab140307, Abcam), Akt (1:500, cat. no. ab8805,
Abcam), p-Akt (1:1,500, cat. no. ab38449, Abcam), and GAPDH
(1:1,000, cat. no. ab8245, Abcam) primary antibodies at 4˚C
overnight. GAPDH served as a control protein. The immunostained
protein bands were visualized using an enhanced chemiluminescence
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The optical density was analyzed by
Image-Pro Plus 6.0 (Easybio Technology Co., Ltd.).
Statistical analysis
All data were analyzed using GraphPad Prism 6.0
software (GraphPad Software, Inc.) and results are expressed as the
mean ± standard deviation (n=3). One way analysis of variance
followed by post hoc Tukey's test was performed to compare multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
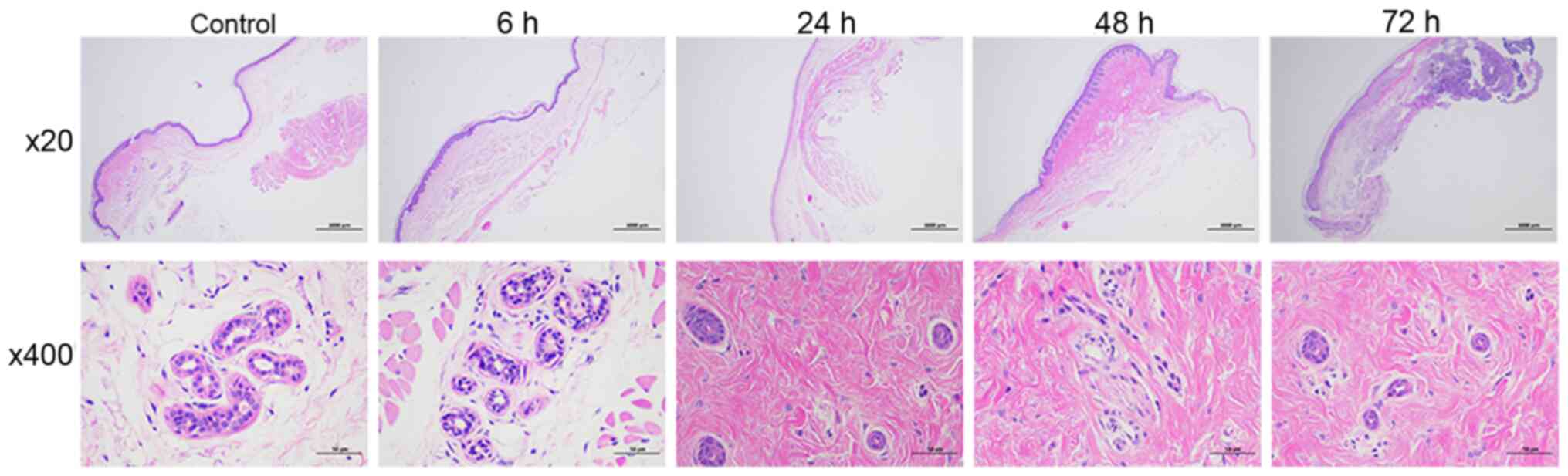
PF alters the histology of skin and
SGs
Treatment with PF for 24, 48 or 72 h significantly
induced nuclear pyknosis in SGs (Fig.
1). At 48 h, SGCs were invisible and small numbers of
inflammatory cells were present. In addition, the cytoplasm of
glandular epithelial cells from rats treated with PF appeared to be
loose compared with cytoplasm in control cells. Treatment with PF
for a short period of time (6 h) did not change the histology of
skin and SGs.
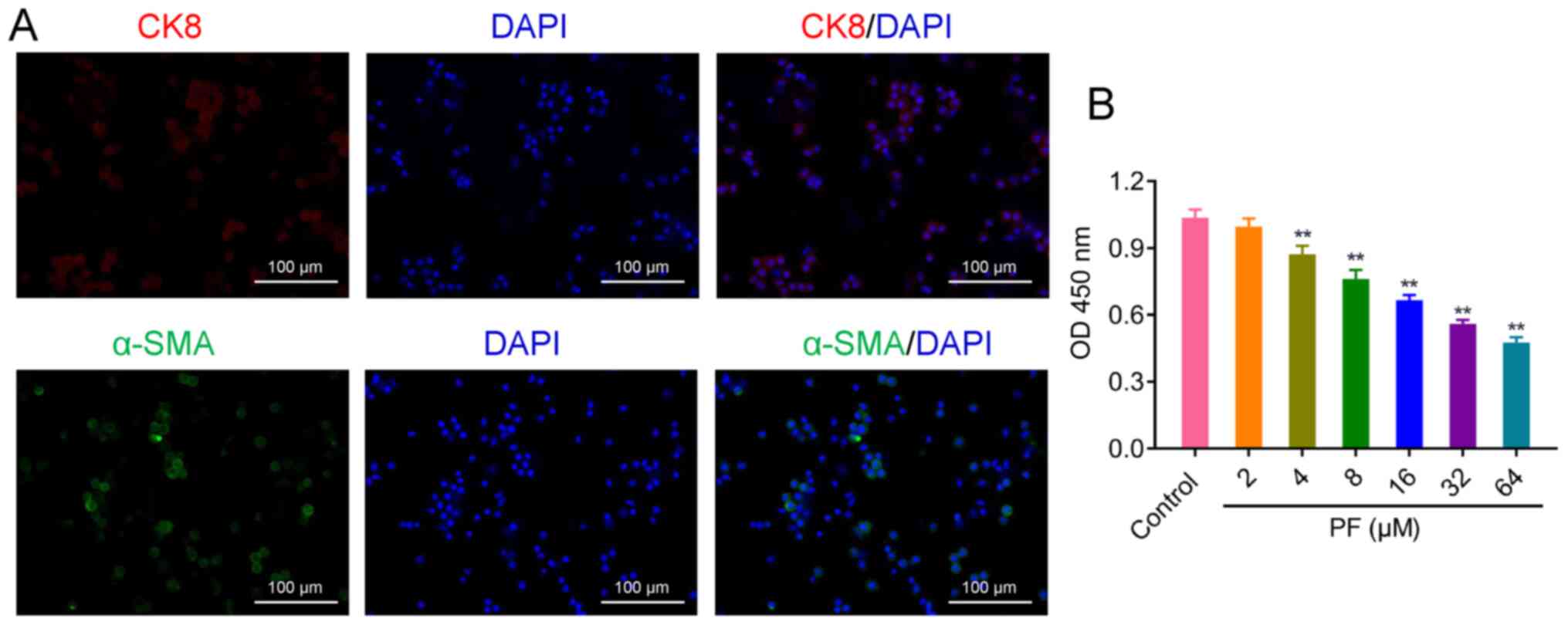
PF inhibits proliferation of
hSGCs
CK8 and α-SMA are specific markers of SGCs (23,24).
To identify SGCs, localization was confirmed by immunohistological
analysis of these maker proteins. Fluorescent images of cells
treated with CK8 and α-SMA showed that hSGCs had been successfully
isolated from human alar skin (Fig.
2A). Primary hSGCs were treated with a series of PF
concentrations; PF inhibited hSGC proliferation in a dose-dependent
manner (Fig. 2B). The
IC50 and IC25 values for PF were 41.013 and
9.530 µM, respectively. After considering the toxicity of PF to
human skin (25), 9.530 µM PF was
subsequently selected for use in treating hSGCs.
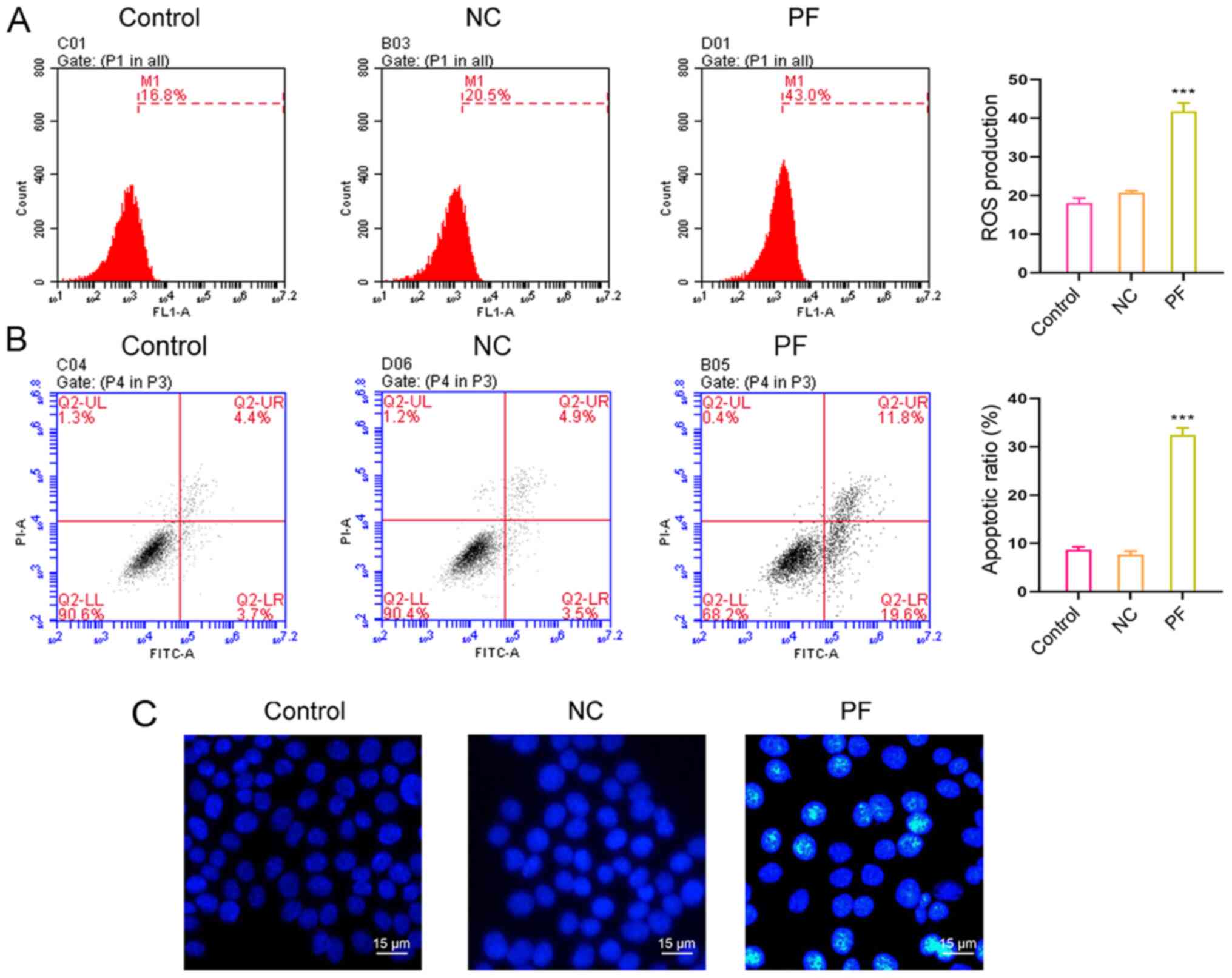
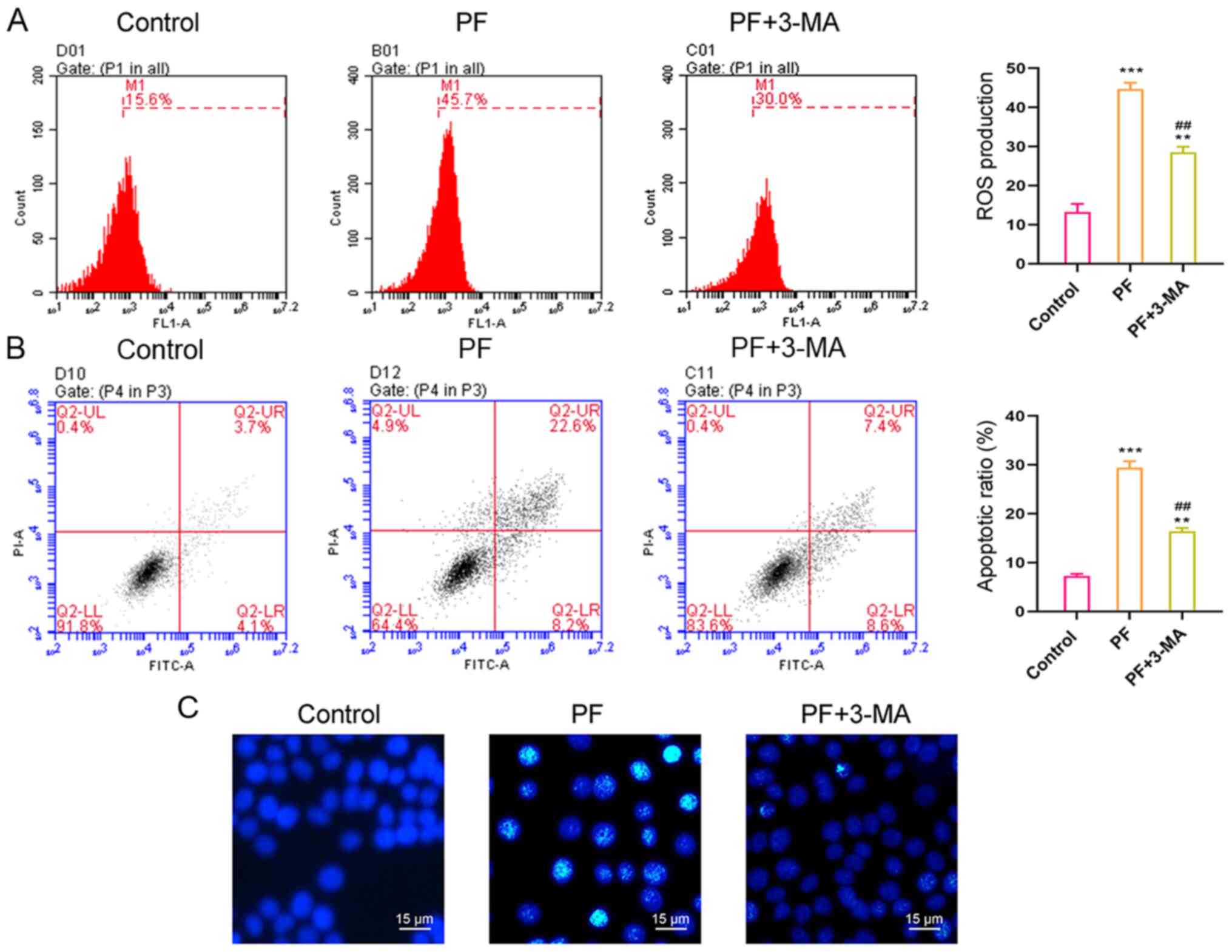
PF promotes intracellular ROS
production and apoptosis
Treatment with 9.530 µM PF for 24 h significantly
increased ROS production (Fig. 3A;
15.62±1.52 vs. 39.44±2.42%) and significantly promoted hSGC
apoptosis (Fig. 3B and C). PF treatment for 24 h increased the
percentage of apoptotic hSGCs from 6.35±0.67 to 32.01±2.78%. These
results suggested PF exerted a cytotoxic effect on hSGCs.
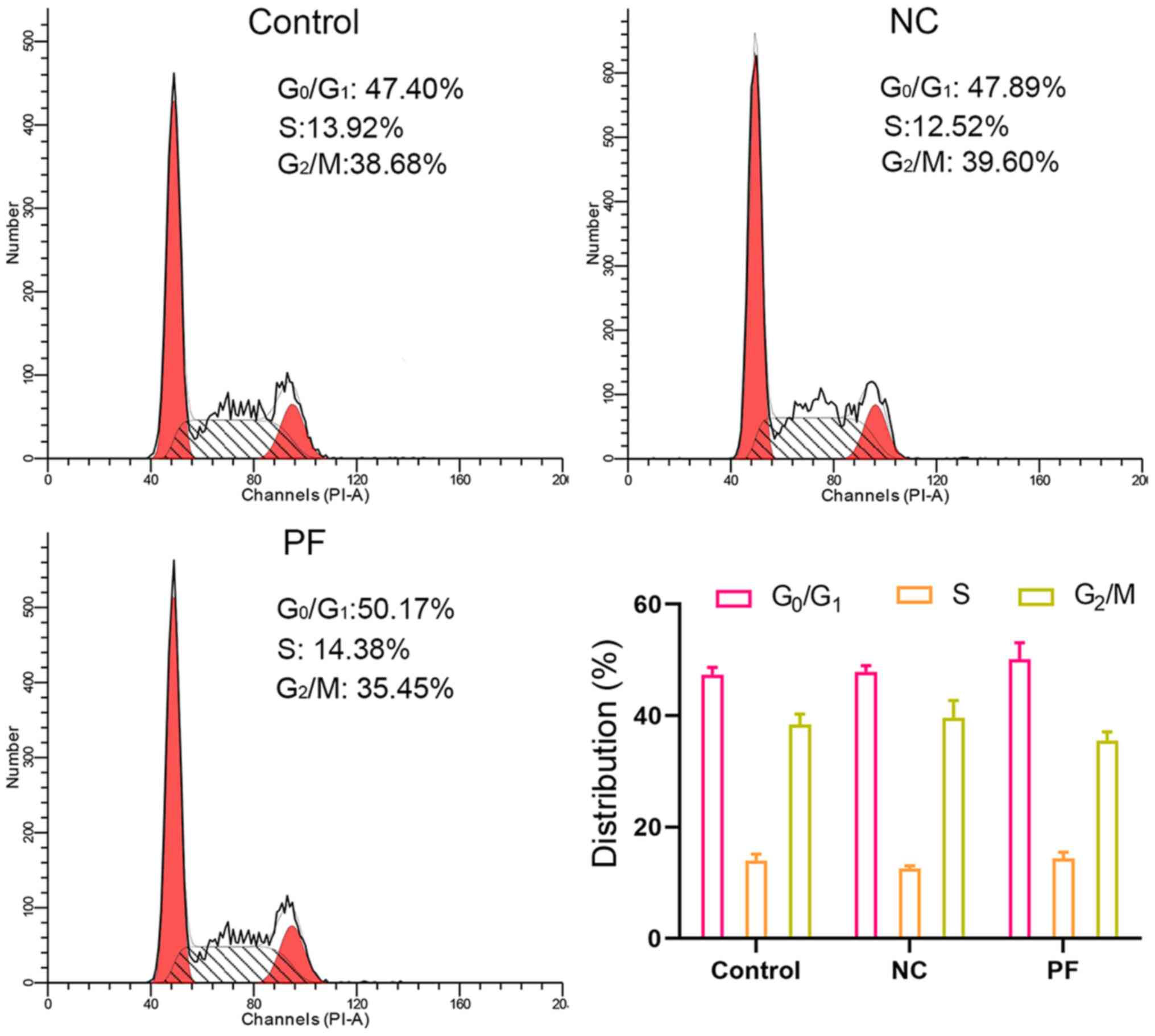
PF alters cell cycle distribution
Flow cytometric analysis of cell cycle distribution
showed that PF treatment for 24 h did not affect the cell cycle
distribution of hSGCs. The percentage of hSGCs at G0/G1 phase
following PF treatment was 59.05±5.91 vs. 56.95±1.54% for control
hSGCs (Fig. 4).
3-MA inhibits PF-induced ROS
production and apoptosis in hSGCs Previous reports have
demonstrated that PF promotes autophagy (26,27)
Here, 3-MA, an autophagy inhibitor, partially
suppressed ROS production and apoptosis in hSGCs that had been
treated with PF (Fig. 5A and
B); these results were confirmed
by Hoechst 33258 staining (Fig.
5C). These findings showed that PF-induced ROS production and
apoptosis in hSGCs may be associated with promotion of
autophagy.
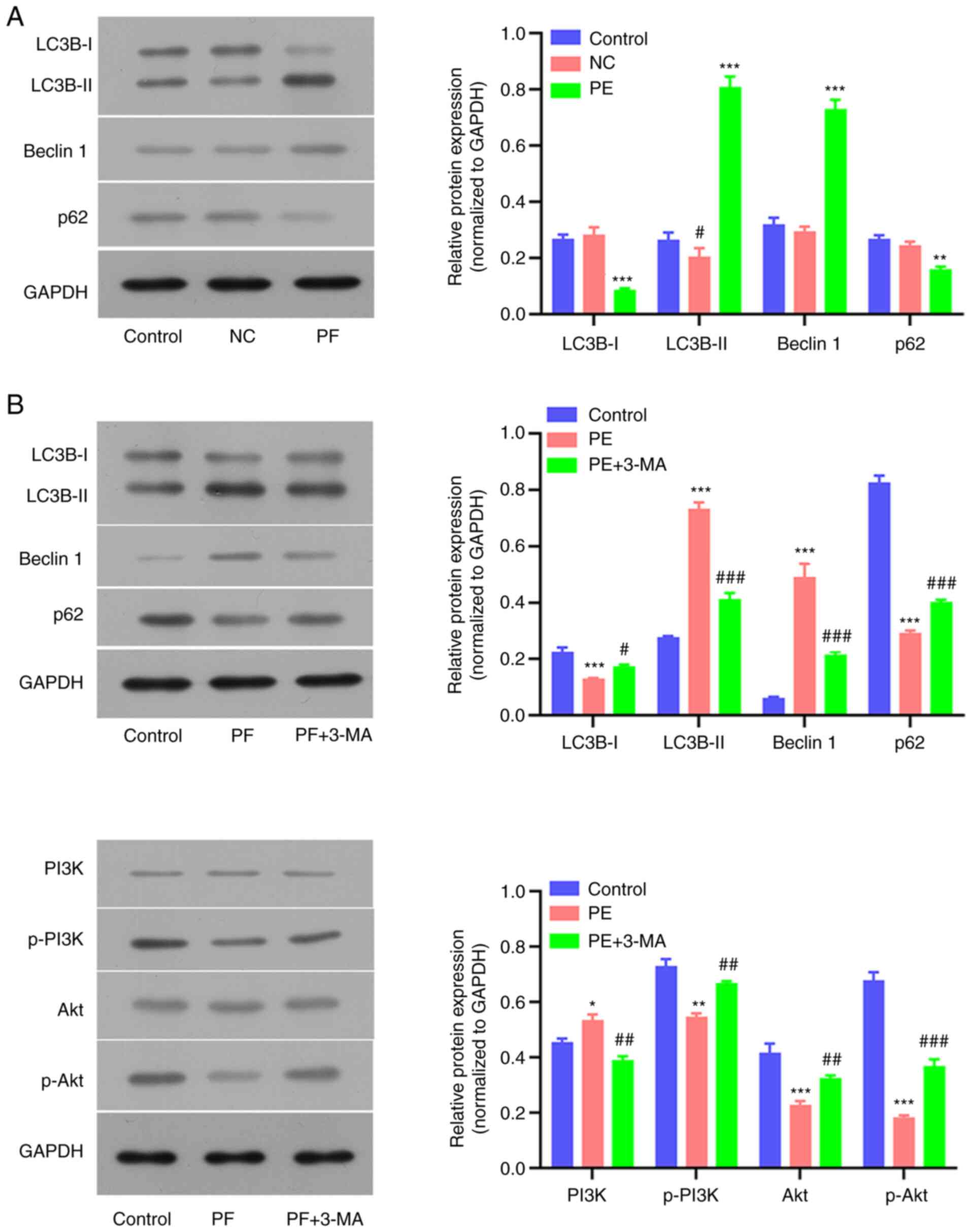
Expression of autophagy-associated
proteins following PF and 3-MA treatment
To verify whether autophagy was associated with the
molecular mechanism underlying PF-induced cellular changes in
hSGCs, expression levels of autophagy-associated proteins were
detected. PF alone significantly upregulated the levels of LC3B and
Beclin 1 expression, but decreased p62 expression in hSGCs
(Fig. 6A and B). By contrast, administration of
autophagy inhibitor (3-MA) partially reversed the PF-induced
changes in LC3B, Beclin 1 and p62 expression (Fig. 6B). In addition, PF-induced
inhibition of p-PI3K and p-Akt expression was also partially
reversed by 3-MA (Fig. 6B). These
protein expression profiles indicated the involvement of autophagy
in PF-induced hSGC cytotoxicity.
Discussion
To the best of our knowledge, the present study is
the first to report the inhibitory effect of PF on SGC
proliferation. The in vivo results showed that PF inhibited
SGC proliferation, while in vitro experiments showed that PF
suppressed SGC proliferation and promoted apoptosis, autophagy and
ROS production.
Cao et al (13) and Sun et al (28) showed that PF administration
attenuates 1-methyl-4-phenylpyridinium-induced production of
cytosolic free Ca2+ in PC12 cells and that levels of
LC3-II protein are upregulated during this process (13,28).
Chen et al (29) reported
that pretreatment with PF restored advanced glycation end product
-modified bovine serum albumin-induced decreases in cell viability
and p62 expression, but enhanced the expression of LC3-II in human
umbilical vein endothelial cells (29). These studies suggested that PF
promotes autophagy in cells.
Additionally, PF is reported to inhibit
proliferation of different types of cell, including fibroblast-like
synoviocytes (17), pulmonary
artery (18) and vascular SMCs
(30) and several types of tumor
cell (31) by regulating various
pathways, including the NF-κB pathway. In accordance with these
findings, the present study showed that PF promoted autophagy and
apoptosis in hSGCs cells by increasing LC3B and Beclin 1 expression
and downregulating p62 expression. The PF-induced inhibition of
hSGC proliferation was consistent with nuclear pyknosis observed in
SGs of rat foot skin in vivo. In addition, PF-induced
inhibition of hSGC proliferation was associated with downregulation
of the pro-proliferative PI3K/Akt pathway. Accordingly, the present
results showed that PF inhibited proliferation of hSGCs,
potentially by inducing autophagy.
Zhao et al (32) showed that PF attenuates α-naphthyl
isothicaynate-induced ROS production in rats. In certain types of
cell, activation of the PI3K/Akt pathway is ROS-dependent (33-35).
However, the overproduction of ROS can damage cellular DNA and
decrease cell membrane stability (36,37).
The interaction between ROS release and autophagy is complex and
multifarious (38). ROS-induced
autophagy and its mechanisms are commonly studied in cancer cells
(38-40).
In salivary gland cells, PF promotes production of intracellular
Ca2+ (11); this effect
can be triggered by ROS production, which trigger short-term
autophagy in cells (41). The
present study showed that ROS production promoted by PF in hSGCs
was associated with apoptosis and autophagy; all these factors were
altered by inhibition of autophagy. These findings suggest a
complex mechanism by which PF inhibits hSGC proliferation. In
summary, PF treatment suppressed SGC proliferation and promoted
apoptosis, autophagy and ROS production, suggesting that PF may be
useful for managing bromhidrosis. However, the exact mechanism by
which PF reduces bromhidrosis was not fully clarifiedand requires
further investigation.
To the best of our knowledge, the present study is
the first to demonstrate that PF inhibits hSGC proliferation by
promoting autophagy, ROS production and apoptosis. The in
vivo anti-proliferative effect of PF on hSGCs was also
confirmed. The present data revealed the effects of autophagy, ROS
production, cell proliferation and apoptosis in the management of
bromhidrosis by PF.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YX, HH and PL conceived, designed and performed the
experiments. HH, PL and HL analyzed and interpreted data. YX and HL
wrote and revised the manuscript. YX and HL confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocols for all animal experiments were
approved by the Institutional Animal Care and Use Committee of the
First Affiliated Hospital of Guangdong Pharmaceutical University
(approval no. 202182). All patients provided written informed
consent. The protocol for the clinical study was approved by the
Research Ethics Committee of the First Affiliated Hospital of
Guangdong Pharmaceutical University (approval no. gyfykydw039). All
procedures were performed in compliance with Ethics Committee
regulations and Animal Research: Reporting of In Vivo
Experiments guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare have no competing interests.
References
|
1
|
Hsu KC and Wang KY: Sparing subcutaneous
septa avoids skin necrosis in the treatment of axillary
bromhidrosis with suction-curettage shaving. J Cosmet Dermatol.
18:892–896. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ardon CB, Molenaar C, van Straalen KR,
Scholtes VC, Prens EP and van der Zee HH: High prevalence of
hidradenitis suppurativa in patients with perianal fistula. Int J
Colorectal Dis. 34:1337–1339. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van TN, Manh TN, Minh PPT, Minh TT, Huu
ND, Cao KP, Huu QN, Cam VT, Huyen ML, Hau KT, et al: The
Effectiveness of Local Surgical Technique in Treatment of Axillary
Bromhidrosis. Open Access Maced J Med Sci. 7:187–191.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He J, Wang T and Dong J: A close positive
correlation between malodor and sweating as a marker for the
treatment of axillary bromhidrosis with Botulinum toxin A. J
Dermatolog Treat. 23:461–464. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kataoka A: Surgical treatment of
bromhidrosis. Rev Bras Cir Plást. 32:377–382. 2001.
|
|
6
|
Coronado MS and Opi JT: Assessment of
axillary hyperhidrosis and bromhidrosis treatment with microwave
technology. J Surg Med. 3:447–451. 2019.
|
|
7
|
Mao GY, Yang SL and Zheng JH: Etiology and
management of axillary bromidrosis: A brief review. Int J Dermatol.
47:1063–1068. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mancini M, Panasiti V, Devirgiliis V,
Pietropaolo V, Fioriti D, Nicosia R, Curzio M, Roberti V, Gobbi S,
Bottoni U, et al: Bromhidrosis induced by sphingomonas
paucimobilis: A case report. Int J Immunopathol Pharmacol.
22:845–848. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hashmonai M, Cameron AEP, Connery CP,
Perin N and Licht PB: The etiology of primary hyperhidrosis: A
systematic review. Clin Auton Res. 27:379–383. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Hu S, Ge S, Wang J and He L:
Paeoniflorin inhibits IgE-mediated allergic reactions by
suppressing the degranulation of mast cells though binding with
FcεRI alpha subunits. Eur J Pharmacol. 886(173415)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qian X, Shi X and Wang H: Effect of
paeoniflorin on the calcium ion concentration in salivary gland
cells using confocal laser scanning microscopy. Am J Transl Res.
8:3678–3688. 2016.PubMed/NCBI
|
|
12
|
Manayi A, Omidpanah S, Barreca D, Ficarra
S, Daglia M, Nabavi SF and Nabavi SM: Neuroprotective effects of
paeoniflorin in neurodegenerative diseases of the central nervous
system. Phytochem Rev. 16:1173–1181. 2017.
|
|
13
|
Cao B-Y, Yang Y-P, Luo W-F, Mao CJ, Han R,
Sun X, Cheng J and Liu CF: Paeoniflorin, a potent natural compound,
protects PC12 cells from MPP+ and acidic damage via autophagic
pathway. J Ethnopharmacol. 131:122–129. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu X, Sun X, Zhao C, Zhang J and Wang X,
Zhang A and Wang X: Exploring the pharmacological effects and
potential targets of paeoniflorin on the endometriosis of cold
coagulation and blood stasis model rats by ultra-performance liquid
chromatography tandem mass spectrometry with a pattern recognition
approach. RSC Advances. 9:20796–20805. 2019.
|
|
15
|
Hu H, Zhu Q, Su J, Wu Y, Zhu Y, Wang Y,
Fang H, Pang M, Li B, Chen S, et al: Effects of an enriched extract
of paeoniflorin, a monoterpene glycoside used in Chinese herbal
medicine, on cholesterol metabolism in a hyperlipidemic rat model.
Med Sci Monit. 23:3412–3427. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang L, Yang B and Yu B: Paeoniflorin
protects against nonalcoholic fatty liver disease induced by a
high-fat diet in mice. Biol Pharm Bull. 38:1005–1011.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen J-Y, Wu H-X, Chen Y, Zhang LL, Wang
QT, Sun WY and Wei W: Paeoniflorin inhibits proliferation of
fibroblast-like synoviocytes through suppressing G-protein-coupled
receptor kinase 2. Planta Med. 78:665–671. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qian G, Cao J, Chen C, Wang L, Huang X,
Ding C, Cai X, Yin F, Chu J, Li G, et al: Paeoniflorin inhibits
pulmonary artery smooth muscle cells proliferation via upregulating
A2B adenosine receptor in rat. PLoS One. 8(e69141)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferry B, Gervasoni D and Vogt C:
Regulatory and Ethical Considerations. In: Stereotaxic Neurosurgery
in Laboratory Rodent. Springer, Paris, pp1-18, 2014.
|
|
20
|
Turkki R, Linder N, Kovanen PE, Pellinen T
and Lundin J: Identification of immune cell infiltration in
hematoxylin-eosin stained breast cancer samples: texture-based
classification of tissue morphologies. In: Proceedings of SPIE -
The International Society for Optical Engineering 9791. Medical
Imaging 2016: Digital Pathology, San Diego, p9791, 2016.
|
|
21
|
Park Y-J and Shin M-S: What is the best
method for treating osmidrosis? Ann Plast Surg. 47:303–309.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang Z, Zhen Y, Yin W, Ma Z and Zhang L:
Shh promotes sweat gland cell maturation in three-dimensional
culture. Cell Tissue Bank. 17:317–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kolakshyapati P, Li X, Chen C, Zhang M,
Tan W, Ma L and Gao C: Gene-activated matrix/bone marrow-derived
mesenchymal stem cells constructs regenerate sweat glands-like
structure in vivo. Sci Rep. 7(17630)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kurata R, Futaki S, Nakano I, Fujita F,
Tanemura A, Murota H, Katayama I, Okada F and Sekiguchi K:
Three-dimensional cell shapes and arrangements in human sweat
glands as revealed by whole-mount immunostaining. PLoS One.
12(e0178709)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang J, Qiu L, Ding L, Wang S, Wang J,
Zhu Q, Song F and Hu J: Ginsenoside Rb1 and paeoniflorin inhibit
transient receptor potential vanilloid-1-activated IL-8 and
PGE2 production in a human keratinocyte cell line HaCaT.
Int Immunopharmacol. 10:1279–1283. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wen J, Xu B, Sun Y, Lian M, Li Y, Lin Y,
Chen D, Diao Y, Almoiliqy M and Wang L: Paeoniflorin protects
against intestinal ischemia/reperfusion by activating LKB1/AMPK and
promoting autophagy. Pharmacol Res. 146(104308)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Y, Che J, Zhao H, Tang J and Shi G:
Paeoniflorin attenuates oxidized low-density lipoprotein-induced
apoptosis and adhesion molecule expression by autophagy enhancement
in human umbilical vein endothelial cells. J Cell Biochem.
120:9291–9299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun X, Cao Y-B, Hu L-F, Yang YP, Li J,
Wang F and Liu CF: ASICs mediate the modulatory effect by
paeoniflorin on α-synuclein autophagic degradation. Brain Res.
1396:77–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen Y, Du X, Zhou Y, Zhang Y, Yang Y, Liu
Z, Liu C and Xie Y: Paeoniflorin protects HUVECs from
AGE-BSA-induced injury via an autophagic pathway by acting on the
RAGE. Int J Clin Exp Pathol. 8:53–62. 2015.PubMed/NCBI
|
|
30
|
Li W, Zhi W, Liu F, Zhao J, Yao Q and Niu
X: Paeoniflorin inhibits VSMCs proliferation and migration by
arresting cell cycle and activating HO-1 through MAPKs and NF-κB
pathway. Int Immunopharmacol. 54:103–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng Y-B, Xiao G-C, Tong S-L, Ding Y,
Wang QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric
carcinoma cell proliferation through up-regulation of microRNA-124
and suppression of PI3K/Akt and STAT3 signaling. World J
Gastroenterol. 21:7197–7207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao Y, Zhou G, Wang J, Jia L, Zhang P, Li
R, Shan L, Liu B, Song X, Liu S, et al: Paeoniflorin protects
against ANIT-induced cholestasis by ameliorating oxidative stress
in rats. Food Chem Toxicol. 58:242–248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wagner G, Lindroos-Christensen J,
Einwallner E, Husa J, Zapf TC, Lipp K, Rauscher S, Gröger M,
Spittler A, Loewe R, et al: HO-1 inhibits preadipocyte
proliferation and differentiation at the onset of obesity via ROS
dependent activation of Akt2. Sci Rep. 7(40881)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mittler R: ROS are good. Trends Plant Sci.
22:11–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen
HL, Bai H and Hai CX: ROS-activated p38 MAPK/ERK-Akt cascade plays
a central role in palmitic acid-stimulated hepatocyte
proliferation. Free Radic Biol Med. 51:539–551. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez
AD, De Vizcaya-Ruíz A and Cebrian ME: Sodium arsenite induces ROS
generation, DNA oxidative damage, HO-1 and c-Myc proteins,
NF-kappaB activation and cell proliferation in human breast cancer
MCF-7 cells. Mutat Res. 674:109–115. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kadowaki H, Nishitoh H, Urano F, Sadamitsu
C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T,
et al: Amyloid β induces neuronal cell death through ROS-mediated
ASK1 activation. Cell Death Differ. 12:19–24. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Poillet-Perez L, Despouy G,
Delage-Mourroux R and Boyer-Guittaut M: Interplay between ROS and
autophagy in cancer cells, from tumor initiation to cancer therapy.
Redox Biol. 4:184–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6(e1604)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu D, Lin J, Su J, Chen X, Jiang P and
Huang K: Glutamine deficiency promotes PCV2 infection through
induction of autophagy via activation of ROS-mediated JAK2/STAT3
signaling pathway. J Agric Food Chem. 66:11757–11766.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Borodkina AV, Shatrova AN, Deryabin PI,
Griukova AA, Abushik PA, Antonov SM, Nikolsky NN and Burova EB:
Calcium alterations signal either to senescence or to autophagy
induction in stem cells upon oxidative stress. Aging (Albany NY).
8:3400–3418. 2016.PubMed/NCBI View Article : Google Scholar
|