Introduction
Breast cancer is the most common cancer, accounting
for 11% of all diagnosed cancer sites, and is the leading cause of
cancer-associated mortality worldwide, accounting for 6.6% of all
diagnosed cancer sites (1).
Triple-negative breast cancer (TNBC) is associated with high
metastatic risk and low overall survival (2). Treatment options are particularly
limited for patients with TNBC, which is associated with earlier
time to recurrence, higher risk of distant metastasis and worse
prognosis after recurrence (3).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that are ~22 nucleotides in length, which play an important role in
the post-transcriptional regulation of mRNA (4). miRNAs are vital to several aspects of
cell biology, including apoptosis, proliferation, differentiation
and cell cycle (5). Over the past
decade, increasing evidence suggest that deregulation of miRNAs is
a crucial part of tumor formation, maintenance, metastasis and
immune tolerance, including breast cancer (6). Specific miRNA expression profiles are
associated with distinct breast cancer subtypes (6). For example, miR-218, miR-342,
miR-135b, miR-217, miR-299 and miR-190 are closely associated with
estrogen receptor (ER) positive breast cancer, while the expression
levels of miR-520f-520C, miR-377, miR-527-518a and miR-520g are
closely associated with progesterone receptor (PR) positive breast
cancer (7). It has been reported
that abnormally expressed or mutated miRNAs play an important role
in the pathogenesis of breast cancer (6). Our previous study demonstrated that
miR-3609, which is abnormally expressed in endometrial dysplasia,
oral squamous cell carcinoma and pancreatic cancer (8-10),
was significantly downregulated in breast cancer (11). The results also demonstrated that
restoration of miR-3609 expression sensitized breast cancer to
doxorubicin by blocking the PD-L1 immune checkpoint in vitro
(11). The present study extended
the research in breast cancer based on public databases to perform
a variety of bioinformatics analyses. This study aimed to determine
the potential functions and distinct prognostic values of miR-3609
in breast cancer by analyzing the expression and mutation of the
miR-3609 in patients with breast cancer.
Materials and methods
UALCAN
The UALCAN (http://ualcan.path.uab.edu) is a comprehensive and
interactive web resource for analyzing genomics data from The
Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/), which was used to determine
the expression of miR-3609 in breast cancer. It allows researchers
to analyze the relative expression of potential genes of interest
in tumors and normal samples, and in various tumor subgroups
(12).
cBioPortal
The cBioPortal (https://www.cbioportal.org) is an open-access resource
for interactive exploration of multidimensional cancer genomics
datasets (13). Genetic
alterations of miR-3609 was obtained from cBioPortal, based on TCGA
database.
Kaplan-Meier plotter
The Kaplan Meier Plotter database (https://kmplot.com/analysis) is able to assess the
effect of 54 k genes (mRNAs, miRNAs and proteins) on the survival
of patients with 21 different types of cancer. Sources for the
databases include Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/) and TCGA (14). The overall survival of patients
with breast cancer was analyzed using the Kaplan-Meier Plotter
database.
LinkedOmics
LinkedOmics (http://www.linkedomics.org/login.php) is a publicly
available portal that contains multi-omics and clinical data from
32 types of cancer, and 11,158 patients from TCGA project (15). It also includes mass
spectrometry-based proteomics data generated by the Clinical
Proteomics Tumor Analysis Consortium for TCGA breast, colorectal
and ovarian tumors.
The differentially expressed genes associated with
miR-3609 were screened from TCGA BRCA cohort (n=755), using the
LinkFinder module in the LinkedOmics database. Spearman's
correlation coefficient analysis was performed to assess the
correlation between the miR-3609 expression and genes
differentially expressed in breast cancer.
Gene Ontology (GO; http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; https://www.kegg.jp/) pathway enrichment analyses of
the differentially expressed genes were performed using the
LinkInterpreter module, the results of which were signed and ranked
using the gene set enrichment analysis (GSEA) tool in the
LinkedOmics.
Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING)
STRING (https://string-db.org) is a public database that
predicts protein-protein interactions (PPIs), and aims to achieve a
comprehensive and objective global network and present them with a
unique set of computational predictions (16).
To establish a PPI network, the present study
screened out co-expressed genes with interaction scores >0.4.
Cytoscape software (version 3.8.0; https://cytoscape.org) which is an open source
software platform for visualizing molecular interaction networks
and biological pathways and integrating these networks with
annotations, gene expression profiles and other state data, was
used to visualize the PPI network, and hub genes were identified
using cytoHubba plug-in (version 0.1; https://cytoscape.org) and the degree topological
algorithm.
Cell culture and microRNA mimics
transfection
The TNBC cell line, MDA-MB-231 was purchased from
the American Type Culture Collection and maintained in DMEM
(Hyclone; Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin G and 100 µg/ml streptomycin
(Beijing Solarbio Science & Technology Co., Ltd.), at 37˚C in a
humidified 5% CO2 atmosphere.
miR-3609 mimics (40, 80 and 160 nM; 5'-CAAA
GUGAUGAGUAAUACUGGCUG-3' and 5'-GCCAGUA UUACUCAUCACUUUGUU-3'),
nonspecific miRNA mimics (5'-UUCUCCGAACGUGUCACGUTT-3' and
5'-ACGUGACACGUUCGGAGAATT-3') were synthesized by Shanghai GeneChem
Co., Ltd., and transfected into MDA-MB-231 cells using
Lipofectamine® RNAiMax reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. After transfection at 37˚C for 24 h, miR-3609
expression in MDA-MB-231 cells transfected with 80 nM miR-3609
mimics increased the most (Fig.
S1), MDA-MB-231 cells transfected with 80 nM miR-3609 mimics
were used for subsequent analyses. Non-specific miRNA mimics,
synthesized by Shanghai GeneChem Co., Ltd., were used as the
negative controls. Transfected cells were used for subsequent
experimentation 48 or 72 h post-transfection.
Cell proliferation assay
MDA-MB-231 cells were transfected with either
miR-3609 mimics or a non-specific miRNA mimic (both at 80 nM),
using Lipofectamine RNAiMax reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Transfected MDA-MB-231 cells were seeded into 96-well plates at a
density of 2x103 cells/well and incubated for 24, 48,
72, 96 or 120 h at 37˚C in DMEM (Hyclone; Cytiva). Cell viability
was assessed via the Cell Counting Kit-8 (CCK-8) assay (Suzhou
Yuheng Biotechnology Co., Ltd.) for 2 h.
Cell cycle assay
Following transfection with either miR-3609 mimics
or a non-specific miRNA mimic for 48 h, MDA-MB-231 cells were
collected after centrifugation with 172.2 x g for 5 min at room
temperature, and washed twice with pre-cooled PBS. Subsequently,
1.0x104 cells were fixed with 500 µl of 70% cold ethanol
overnight at 4˚C, followed by addition of 500 µl of propidium
iodide (PI) solution (11.6 µg/ml PI, 2 mg/ml RNaseA in PBS; Beijing
Solarbio Science & Technology Co., Ltd.), and the mixture was
incubated at room temperature for 30-60 min, in the dark. DNA
content analysis was performed using the FACS Calibur instrument
(BD FACSCantoII™) and CellQuest software (modfit 5.1; BD
Biosciences). The experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). Data are presented as
the mean ± SD of at least three independent experiments. Unpaired
Student's t-test was used to compare differences between two groups
(Figs. 1A, and 4C and D). One-way ANOVA followed by Tukey's post
hoc test was used to compare differences between multiple groups
(Fig. S1). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression and alteration of miR-3609
in breast cancer
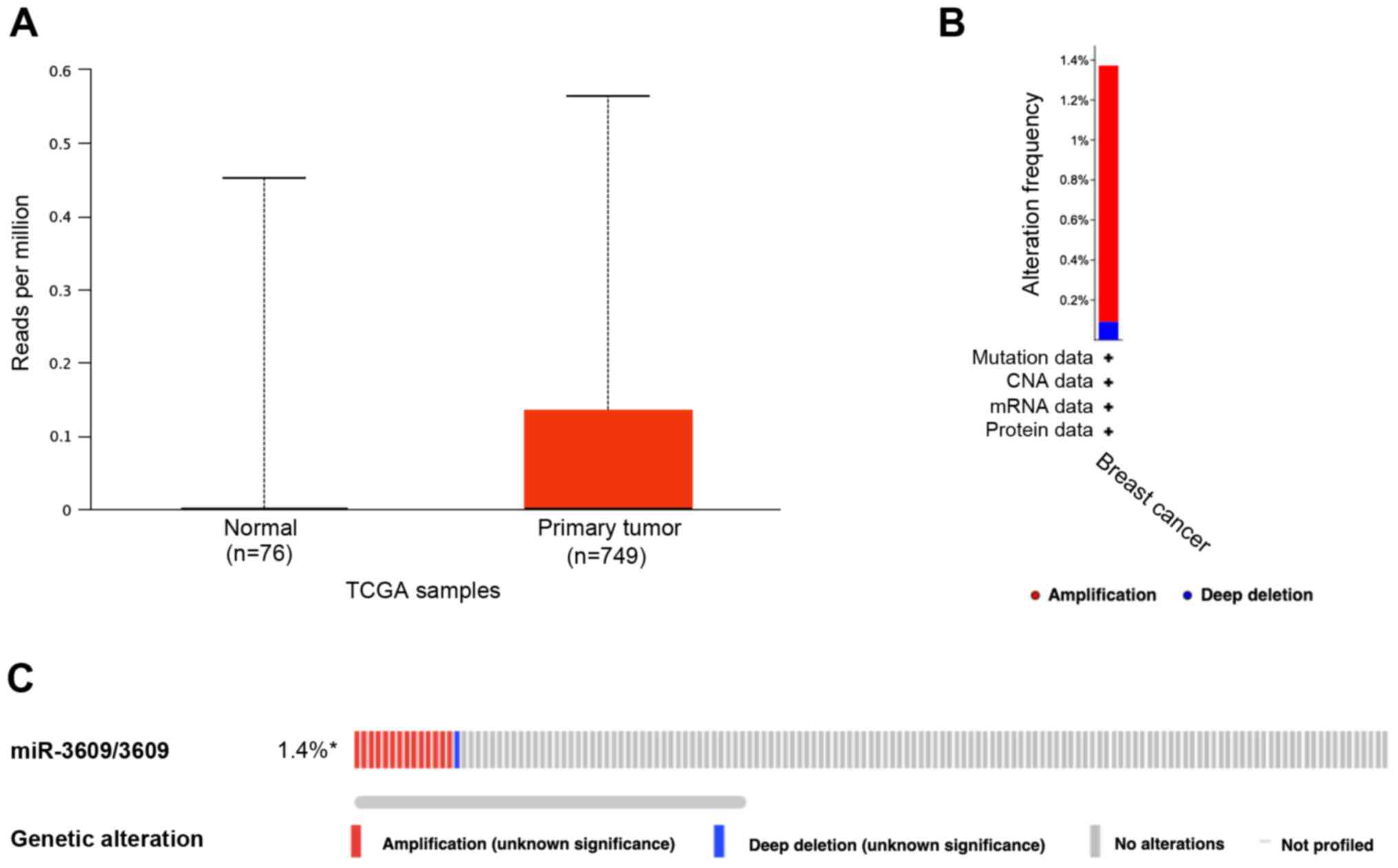
The present study determined the expression of
miR-3609 between normal and primary breast tumor samples in TCGA
database (Fig. 1A). The results
revealed no significant differences between the two groups. The
genetic alterations of miR-3609 in 1,108 breast invasive carcinoma
samples in TCGA database were detected, using cBioPortal. The
results demonstrated that 15 cases had miR-3609 alterations (1.4%)
(Fig. 1B and C).
miR-3609 expression is associated with
overall survival of patients with breast cancer
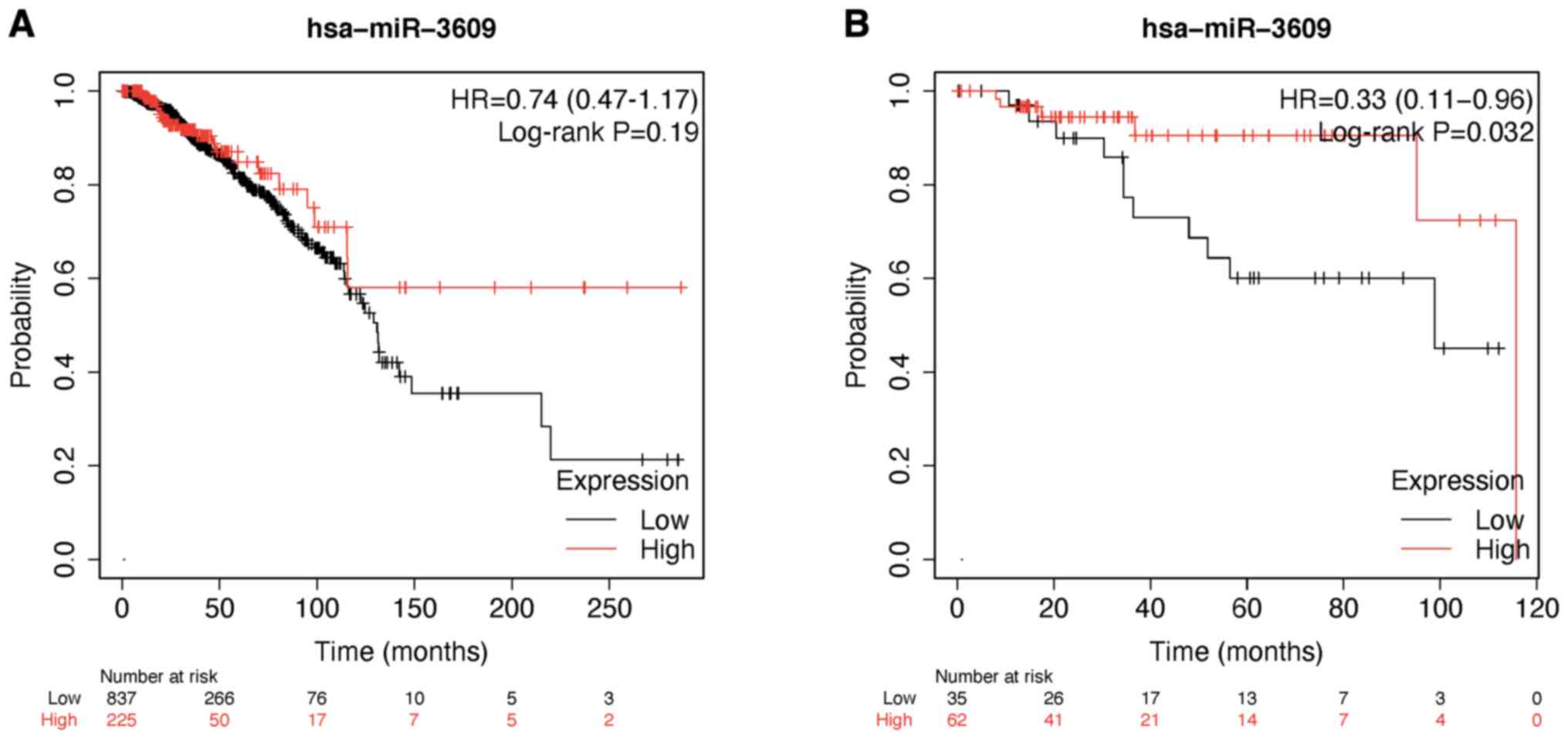
After investigating the overall survival of miR-3609
in breast cancer using the Kaplan-Meier Plotter database, the
results demonstrated no significant differences in overall survival
for miR-3609 among all patients with breast cancer from TCGA
database. However, high miR-3609 expression was associated with
better overall survival in patients with TNBC (Fig. 2A and B).
Co-expression genes correlated with
miR-3609 in breast cancer
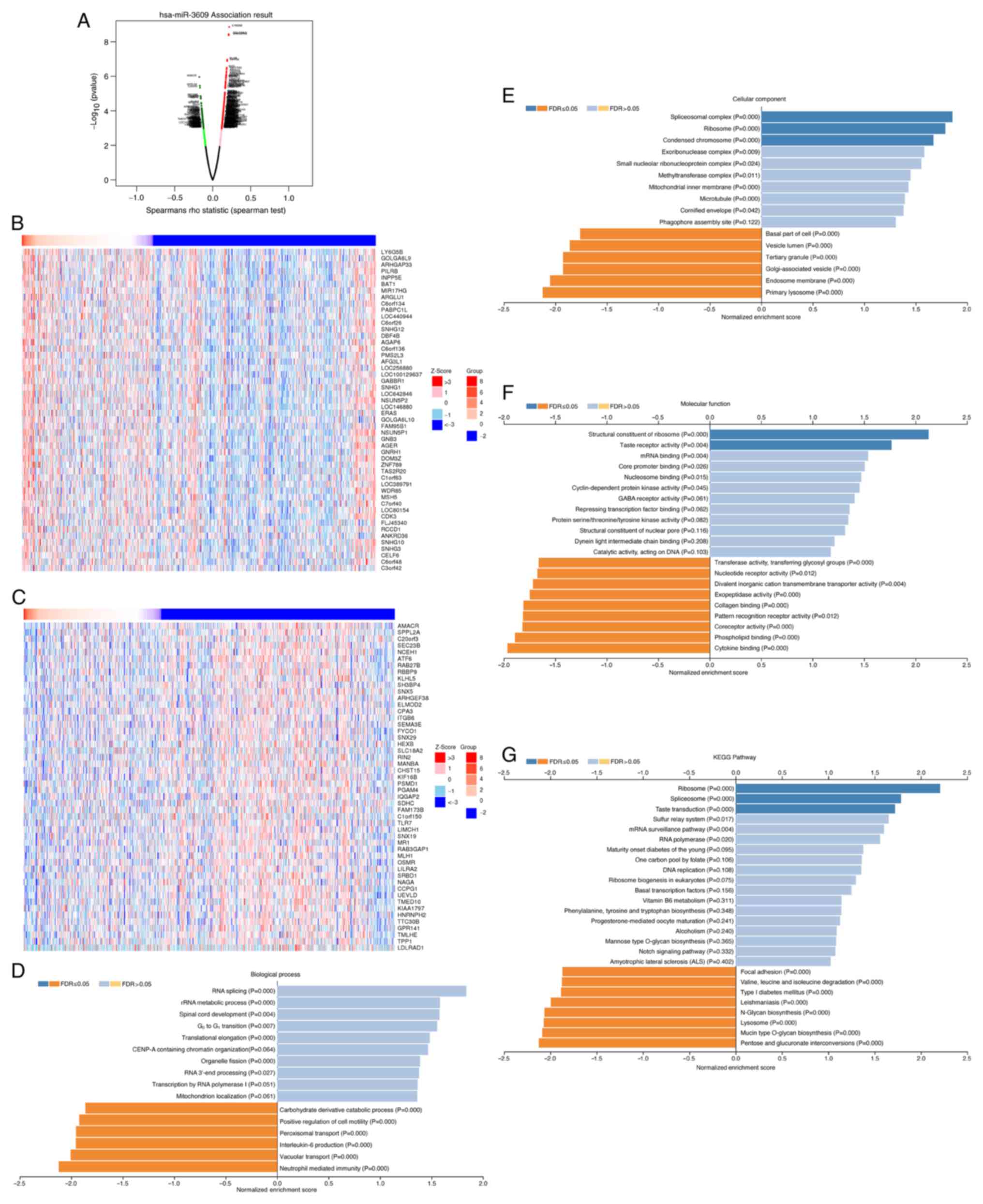
The present study analyzed the co-expressed genes of
miR-3609 in 755 patients with breast cancer, using the LinkedOmics
database. The volcano plot depicts genes positively and negatively
correlated with miR-3609 (Fig.
3A). The top 50 significant gene sets positively or negatively
correlated with miR-3609 are depicted in the heat maps,
respectively (Fig. 3B and C).
GO and KEGG analyses of genes
associated with miR-3609 in breast cancer
GO analysis demonstrated that differentially
expressed genes associated with miR-3609 were mainly located in
‘spliceosomal complex’, ‘ribosome’, ‘condensed chromosome’ and
‘exoribonuclease complex’, and these cellular components may have
participated in ‘RNA splicing’, ‘rRNA metabolic process’, ‘spinal
cord development’, ‘G0-G1 transition’ or
other biological processes. They acted as structural constituents
of the ribosome and mRNA binding (Fig.
3D-F). KEGG pathway analysis demonstrated that the functions of
the associated genes were primarily enriched in ribosome and
spliceosome (Fig. 3G).
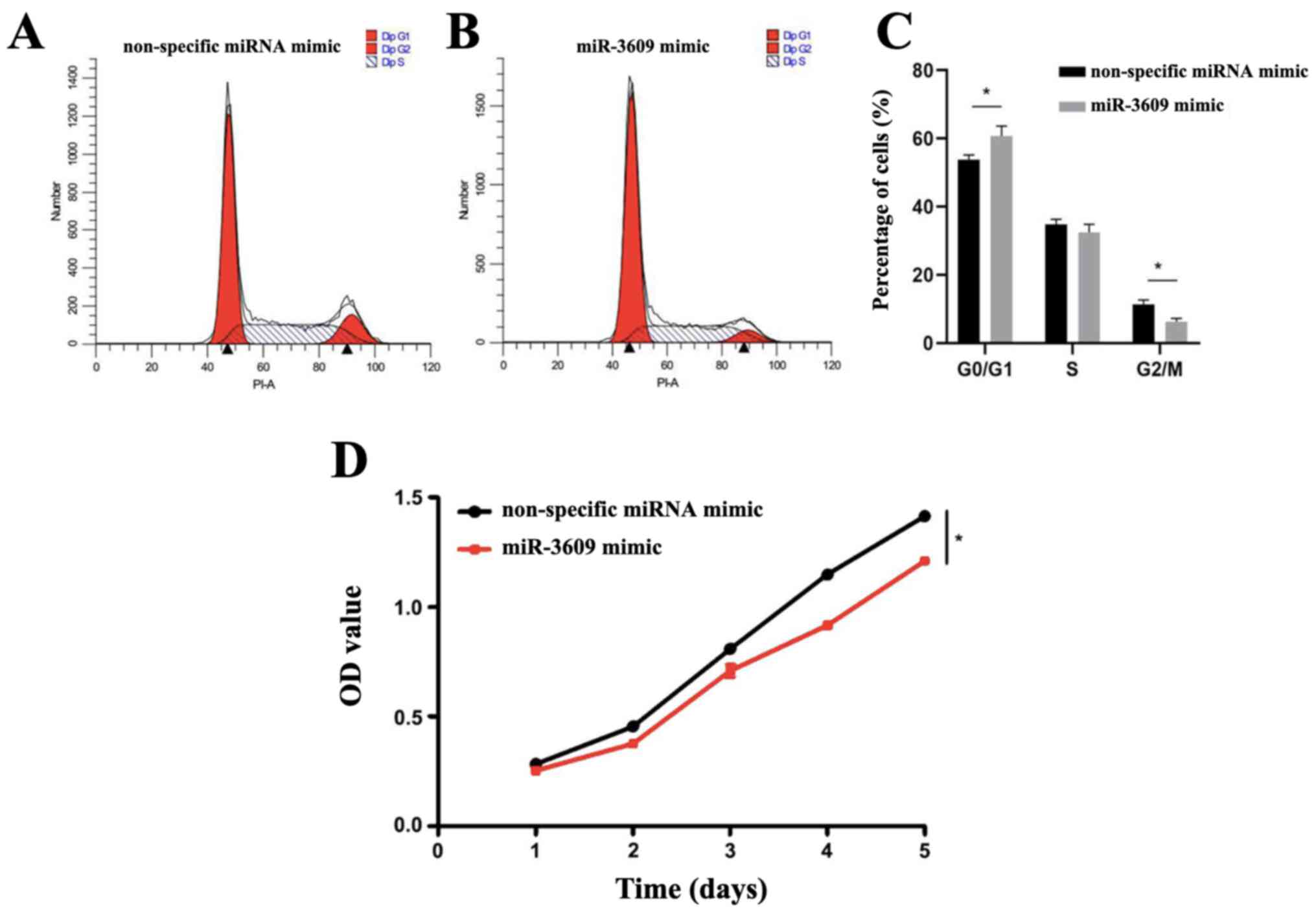
To confirm G0-G1 transition
for biological processes of miR-3609, the cell cycle assay was
performed. The results demonstrated that the proportion of
MDA-MB-231 cells transfected with miR-3609 mimics in the
G0/G1 phase was significantly higher
(P=0.032), and that in the G2/M phase was significantly
lower (P=0.018) compared with the control group (Fig. 4A-C), suggesting that miR-3609
induces cell cycle arrest of TNBC cells in the
G0/G1 phase.
The CCK-8 assay was performed to assess the
viability of MDA-MB-231 cells. The results demonstrated that the
viability of MDA-MB-231 cells transfected with miR-3609 mimics was
significantly lower compared with MDA-MB-231 cells transfected with
non-specific miRNA mimic (P=0.0285), suggesting that high miR-3609
expression decreases the viability of MDA-MB-231 cells (Fig. 4D).
Construction of co-expression gene PPI
network
The 422 significantly co-expressed genes were
selected to construct aPPI network using the STRING database
(P<0.001), and Cytoscape (cytoHubba plug-in) was used to
identify the hub genes, along with the degree topological
algorithm. Based on the degree score, the genes with the highest
scores [interleukin enhancer binding factor 3 (ILF3), ELAV like RNA
binding protein 1 (ELAVL1), heterogeneous nuclear ribonucleoprotein
(HNRNP)L, HNRNPK, HNRNPH1 and polypyrimidine tract binding protein
1 (PTBP1)] were identified as potential hub genes (Fig. 5A and B). The gene of ILF3 encodes a
double-stranded RNA (dsRNA)-binding protein that complexes with
other proteins, dsRNAs, small noncoding RNAs and mRNAs to regulate
gene expression and stabilize mRNAs (17). The protein encoded by ELAVL1 is a
member of the ELAVL family of RNA-binding proteins that contain
several RNA recognition motifs, and selectively bind AU-rich
elements found in the 3' untranslated regions of mRNAs (18). Heterogeneous nuclear RNAs which
include mRNA precursors and mature mRNAs are associated with
specific proteins to form heterogenous ribonucleoprotein (hnRNP)
complexes (19). HNRNPL is stably
associated with hnRNP complexes, and is likely to play a major role
in the formation, packaging, processing, and function of mRNA,
along with other hnRNP proteins (20). HNRNPH1 encodes a member of a
subfamily of ubiquitously expressed hnRNPs (19). Moreover, both HNRNPK and PTBP1
belong to the subfamily of ubiquitously expressed hnRNPs (21,22).
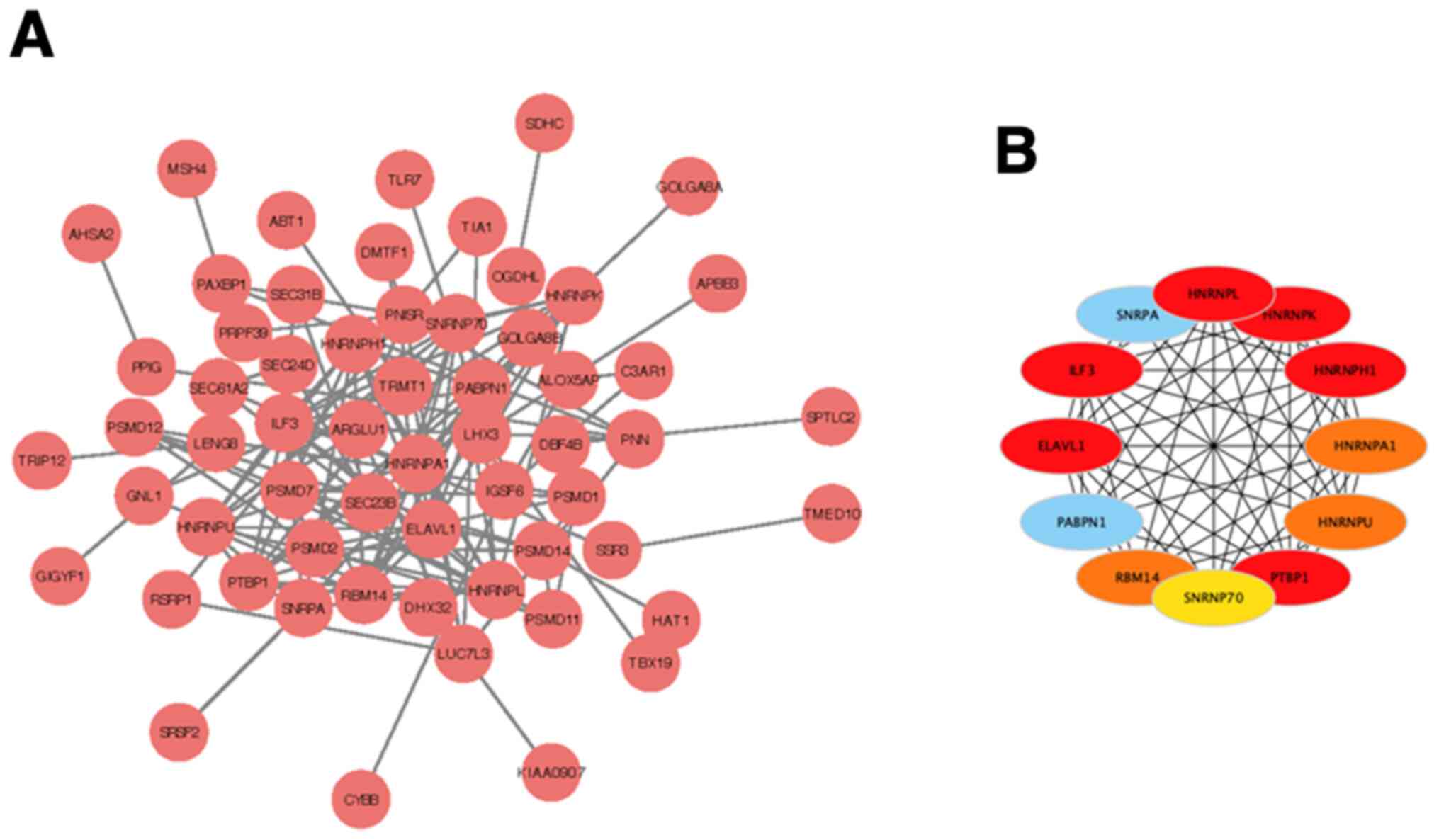 | Figure 5PPI network of co-expression genes.
(A) PPI network. (B) The hub genes (red nodes), including ILF3,
ELAVL1, HNRNPL, HNRNPK, HNRNPH1 and PTBP1. PPI, protein-protein
interaction; ILF3, interleukin enhancer binding factor 3; ELAVL1,
ELAV like RNA binding protein 1; HNRNP, heterogeneous nuclear
ribonucleoprotein; PTBP1, polypyrimidine tract binding protein
1. |
Prognostic analysis of hub genes in
breast cancer
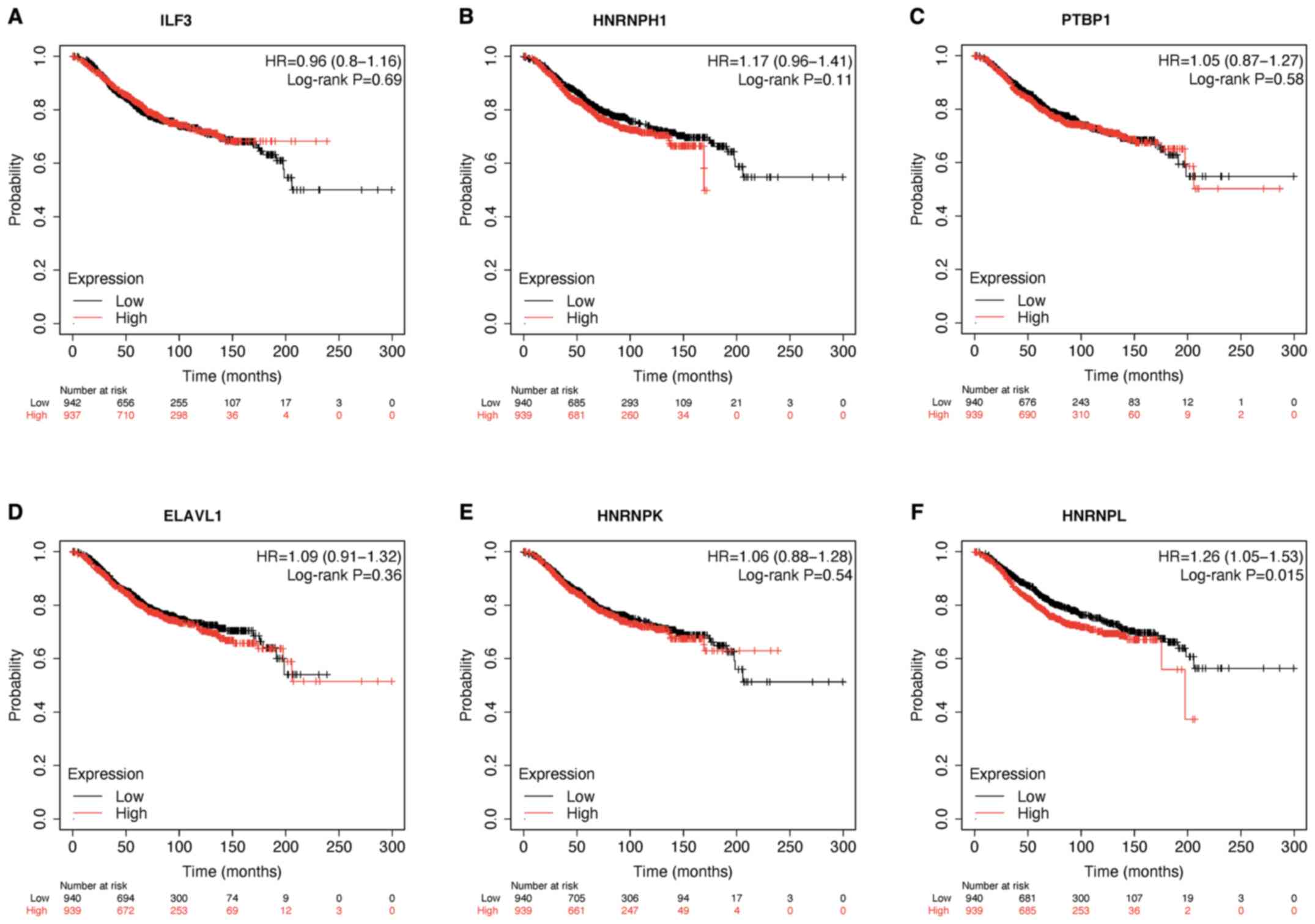
The overall survival of the hub genes in breast
cancer was assessed using the Kaplan-Meier Plotter database. The
results demonstrated that low HNRNPL expression was associated with
a longer overall survival time (P<0.05; Fig. 6).
Discussion
Breast cancer remains the most common malignancy and
the main cause of cancer-associated mortality in women worldwide,
with incidence and mortality rates of 24.2% and 15.0%, respectively
(23-25).
TNBC is a heterogeneous breast cancer subtype, where the ER, PR and
human epidermal growth factor receptor 2 are negatively expressed
(26-28).
TNBC represents 12-17% of all breast cancer cases (29), and has a more aggressive clinical
course, with greater metastatic potential and poorer prognosis as
demonstrated by the higher relapse (33.9% vs. 20.4%) and lower
survival rates (42.2% vs. 28%) compared with patients with other
breast cancers (30,31). Thus, it is important to identify
novel biomarkers to predict the prognosis of patients with breast
cancer, particularly for TNBC.
Increasing evidence suggest that non-coding RNAs are
active participants in multiple stages of tumor immunity (6,32-35).
Non-coding RNAs, including miRNAs, long non-coding RNAs and
circular RNAs, differentially regulate multiple cellular processes
in development and diseases via a variety of gene-regulation
mechanisms (36). miRNAs are small
non-coding RNA molecules that are ~22 nucleotides in length, which
play important regulatory roles in several aspects of cell
activities, such as cell differentiation, apoptosis and metabolism
(37). Previous studies have
reported that miRNAs, such as miR-1296 and miR-133a, are closely
associated with the occurrence, malignant metastasis and poor
prognosis of breast cancer, and overexpression of these miRNAs can
inhibit the proliferation of breast cancer cells (38-40).
It has also been reported that transfection with miR-3609 mimics
can decrease cyclin-dependent kinase 1 expression in MDA-MB-231
cells (41). Our previous study
demonstrated that miR-3609 can target the 3'-untranslated region of
programmed death-ligand 1 (PD-L1) and effectively inhibit PD-L1
expression in breast cancer cells (11). The present study confirmed that
high miR-3609 expression is associated with better overall survival
in patients with TNBC. Thus, miR-3609 may be used as a potential
prognostic biomarker for TNBC. The results of the present study
demonstrated no significant difference in overall survival for
miR-3609 expression among all patients with breast cancer. This may
have been due to varied miR-3609 expression among diverse subgroups
and subtypes (42). The present
study also investigated the alterations of miR-3609 in breast
cancer using the cBioPortal database. A total of 15 cases of
miR-3609 alterations were found in the cBioPortal database. The
co-expression genes correlated with miR-3609 expression in breast
cancer was also assessed, using the LinkedOmics database. GSEA
suggested that co-expression genes participated in RNA splicing,
rRNA metabolic process and G0-G1 transition
via GO and KEGG pathway analyses. The results of the CCK-8 and cell
cycle assays demonstrated that high miR-3609 expression inhibited
the proliferation of TNBC cells and induced cell cycle arrest of
TNBC cells in the G0/G1 phase. Bioinformatics
analysis was performed to identify hub genes by gene analysis of
tumor data from public databases. ILF3, ELAVL1, HNRNPL, HNRNPK,
HNRNPH1 and PTBP1 were identified as potential hub genes.
Subsequently, the prognostic values of these hub genes were
determined using the Kaplan-Meier Plotter database. Taken together,
the results of the present study suggest that miR-3609 may be used
as a potential prognostic marker and a therapeutic target for
patients with TNBC.
The present study is not without limitations. First,
the effects of miR-3609 on the protein expression levels of ILF3,
ELAVL1, HNRNPL, HNRNPK, HNRNPH1 and PTBP1 were not investigated due
to the lack of antibodies at the time of the study. Secondly, only
one TNBC cell line was used to assess the effect of miR-3609 on
cell cycle arrest. Thus, prospective studies will use other TNBC
cell lines and in vivo models to verify the results
presented here. In addition, further studies are required to
determine the specific mechanisms of miR-3609 involved in the cell
cycle of TNBC cells.
In conclusion, the present study integrated public
sequencing data to guide the research of miR-3609 in breast cancer.
The results presented here confirm that high miR-3609 expression is
associated with a longer overall survival time in patients with
TNBC. In addition, high miR-3609 expression inhibited the
proliferation of TNBC cells and induced cell cycle arrest. Thus,
miR-3609 may be used as a potential biomarker for TNBC diagnosis,
and miRNAs-based therapeutic approaches may be an attractive
alternative option for patients with TNBC.
Supplementary Material
miR-3609 expression in MDA-MB-231
cells transfected with 40, 80 and 160 nM miR-3609 mimics or the
corresponding control. ****P<0.0001 vs. the NC group.
miR, microRNA; NC, negative control.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Research
Projects of Henan Colleges (grant no. 16A350003) and the Henan
Scientific and Technological Research Projects (grant no.
182102410007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
This study was designed by QK and DL. DL, RD and MY
performed the experiments. RD, ZD, MY and XW analyzed the data. DL
and RD confirm the authenticity of all the raw data. RD, DL, QK and
ZD drafted the initial manuscript and critically revised it for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Ruijter TC, Veeck J, de Hoon JP, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:183–192.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Ali Syeda Z, Langden SSS, Munkhzul C, Lee
M and Song SJ: Regulatory mechanism of MicroRNA expression in
cancer. Int J Mol Sci. 21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ediriweera MK and Cho SK: Targeting miRNAs
by histone deacetylase inhibitors (HDACi): Rationalizing
epigenetics-based therapies for breast cancer. Pharmacol Ther.
206(107437)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lowery AJ, Miller N, Devaney A, McNeill
RE, Davoren PA, Lemetre C, Benes V, Schmidt S, Blake J, Ball G, et
al: MicroRNA signatures predict oestrogen receptor, progesterone
receptor and HER2/neu receptor status in breast cancer. Breast
Cancer Res. 11(R27)2009.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Li S, Liu X, Zhou Y, Acharya A, Savkovic
V, Xu C, Wu N, Deng Y, Hu X, Li H, et al: Shared genetic and
epigenetic mechanisms between chronic periodontitis and oral
squamous cell carcinoma. Oral Oncol. 86:216–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang S and Dai Y: RNA sequencing reveals
significant miRNAs in Atypical endometrial hyperplasia. Eur J
Obstet Gynecol Reprod Biol. 225:129–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mao Y, Shen J, Lu Y, Lin K, Wang H, Li Y,
Chang P, Walker MG and Li D: RNA sequencing analyses reveal novel
differentially expressed genes and pathways in pancreatic cancer.
Oncotarget. 8:42537–42547. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li D, Wang X, Yang M, Kan Q and Duan Z:
miR3609 sensitizes breast cancer cells to adriamycin by blocking
the programmed death-ligand 1 immune checkpoint. Exp Cell Res.
380:20–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nagy Á, Munkácsy G and Győrffy B:
Pancancer survival analysis of cancer hallmark genes. Sci Rep.
11(6047)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Castella S, Bernard R, Corno M, Fradin A
and Larcher JC: Ilf3 and NF90 functions in RNA biology. Wiley
Interdiscip Rev RNA. 6:243–256. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Upadhyay R, Sanduja S, Kaza V and Dixon
DA: Genetic polymorphisms in RNA binding proteins contribute to
breast cancer survival. Int J Cancer. 132:E128–E138.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han SP, Tang YH and Smith R: Functional
diversity of the hnRNPs: Past, present and perspectives. Biochem J.
430:379–392. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gu J, Chen Z, Chen X and Wang Z:
Heterogeneous nuclear ribonucleoprotein (hnRNPL) in cancer. Clin
Chim Acta. 507:286–294. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu Y, Wu W, Han Q, Wang Y, Li C, Zhang P
and Xu H: Post-translational modification control of RNA-binding
protein hnRNPK function. Open Biol. 9(180239)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Han F, Liu W and Shi X: PTBP1
promotes tumorigenesis by regulating apoptosis and cell cycle in
colon cancer. Bull Cancer. 105:1193–1201. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kalimutho M, Nones K, Srihari S, Duijf
PHG, Waddell N and Khanna KK: Patterns of genomic instability in
breast cancer. Trends Pharmacol Sci. 40:198–211. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang R, Zhu Q, Yin D, Yang Z, Guo J,
Zhang J, Zhou Y and Yu JJ: Identification and validation of an
autophagy-related lncRNA signature for patients with breast cancer.
Front Oncol. 10(597569)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gong Y, Ji P, Yang YS, Xie S, Yu TJ, Xiao
Y, Jin ML, Ma D, Guo LW, Pei YC, et al: Metabolic-pathway-based
subtyping of triple-negative breast cancer reveals potential
therapeutic targets. Cell Metab. 33:51–64.e9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu H, Paddock MN, Wang H, Murphy CJ, Geck
RC, Navarro AJ, Wulf GM, Elemento O, Haucke V, Cantley LC, et al:
The INPP4B tumor suppressor modulates EGFR trafficking and promotes
triple-negative breast cancer. Cancer Discov. 10:1226–1239.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brown M, Tsodikov A, Bauer KR, Parise CA
and Caggiano V: The role of human epidermal growth factor receptor
2 in the survival of women with estrogen and progesterone
receptor-negative, invasive breast cancer: The California Cancer
Registry, 1999-2004. Cancer. 112:737–747. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cortez MA, Anfossi S, Ramapriyan R, Menon
H, Atalar SC, Aliru M, Welsh J and Calin GA: Role of miRNAs in
immune responses and immunotherapy in cancer. Genes Chromosomes
Cancer. 58:244–253. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang Q, Cao W, Wang Z, Zhang B and Liu J:
Regulation of cancer immune escape: The roles of miRNAs in immune
checkpoint proteins. Cancer Lett. 431:73–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xie M, Ma L, Xu T, Pan Y, Wang Q, Wei Y
and Shu Y: Potential regulatory roles of microRNAs and long
noncoding RNAs in anticancer therapies. Mol Ther Nucleic Acids.
13:233–243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Denaro N, Merlano MC and Lo Nigro C: Long
noncoding RNAs as regulators of cancer immunity. Mol Oncol.
13:61–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin C-P and He L: Noncoding RNAs in cancer
development. Annu Rev Cancer Biol. 1:163–184. 2017.https://doi.org/10.1146/annurev-cancerbio-050216-034443.
|
|
37
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen LL, Zhang ZJ, Yi ZB and Li JJ:
MicroRNA-211-5p suppresses tumour cell proliferation, invasion,
migration and metastasis in triple-negative breast cancer by
directly targeting SETBP1. Br J Cancer. 117:78–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Breunig C, Erdem N, Bott A, Greiwe JF,
Reinz E, Bernhardt S, Giacomelli C, Wachter A, Kanthelhardt EJ,
Beißbarth T, et al: TGFβ1 regulates HGF-induced cell migration and
hepatocyte growth factor receptor MET expression via C-ets-1 and
miR-128-3p in basal-like breast cancer. Mol Oncol. 12:1447–1463.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang DY, Gendoo DMA, Ben-David Y, Woodgett
JR and Zacksenhaus E: A subgroup of microRNAs defines
PTEN-deficient, triple-negative breast cancer patients with poorest
prognosis and alterations in RB1, MYC, and Wnt signaling. Breast
Cancer Res. 21(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fitzpatrick C, Bendek MF, Briones M,
Farfán N, Silva VA, Nardocci G, Montecino M, Boland A, Deleuze JF,
Villegas J, et al: Mitochondrial ncRNA targeting induces cell cycle
arrest and tumor growth inhibition of MDA-MB-231 breast cancer
cells through reduction of key cell cycle progression factors. Cell
Death Dis. 10(423)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiao B, Zhang W, Chen L, Hang J, Wang L,
Zhang R, Liao Y, Chen J, Ma Q, Sun Z, et al: Analysis of the
miRNA-mRNA-lncRNA network in human estrogen receptor-positive and
estrogen receptor-negative breast cancer based on TCGA data. Gene.
658:28–35. 2018.PubMed/NCBI View Article : Google Scholar
|