Introduction
The typical treatment option for the irreversible
inflammation and necrosis of the dental pulp tissue is root canal
treatment (1). However, the
development of regenerative medicine has enabled the potential
application of regenerative endodontics as an alternative approach,
which aims to regenerate dental pulp-like tissues (2,3). This
method is increasingly attracting the attention of dentists and
researchers due to the reported beneficial effects on both tooth
function and preservation (2,3).
However, identification of the conditions for optimal pulp tissue
regeneration has not been fully realized (4). Stem cells and their microenvironment
serve an important role in pulp regeneration (5). This microenvironment may include
growth factors, scaffold materials and the removal of infection
which can create optimal conditions for the tissue-induced
induction of stem cells (6). During
pulp regeneration, the microenvironment can regulate the
directional differentiation of stem cells, which in turn affects
downstream processes, including the generation of new blood
vessels, neurons and dentin, in the regenerated pulp-like tissues
(6). In the majority of studies
that previously investigated dental pulp-like tissue regeneration,
the regulation of transplanted human dental pulp stem cell (hDPSC)
differentiation serves an important role (7,8).
Regulation of the stem cell microenvironment and its influence of
the direct differentiation of stem cells has been a subject of
intense research. It has been previously reported that the
osteogenic and odontogenic differentiation, vascularization and
neurogenesis of stem cells can be regulated by drug-loaded
scaffolding materials or small-molecular weight agents (2,9). These
findings may provide useful clinical strategies for dental
pulp-like tissue regeneration.
Significant progress has been made in the
exploration of the therapeutic use of naturally-occurring products
for the treatment of several diseases (10,11).
In particular, new applications have emerged with the development
of molecules, such as artemisinin, used in traditional Chinese
medicine (10,11). Salidroside (SAL) is a compound that
can be extracted from the root of Rhodiola rosea and is considered
to be an important Chinese herbal medicine (12). It has been previously reported that
SAL can exert anti-aging, neuroprotective, hepatoprotective,
cardioprotective and anticancer effects (13-16).
However, few studies have performed a therapeutic evaluation of SAL
on hDPSCs. Therefore, the effects of SAL on the regulation of
hDPSCs osteogenic and odontogenic (osteo/odontogenic)
differentiation remain unclear. The aim of the present study was to
explore the potential effects of SAL, a small-molecular weight
compound derived from traditional Chinese medicine, on the
osteo/odontogenic differentiation of hDPSCs in vitro. The
effects of SAL on the viability and osteo/odontogenic
differentiation of hDPSCs and whether these effects were mediated
via the regulation of osteo/odontogenic differentiation-associated
gene expression were examined. The role of the bone morphogenetic
protein (BMP) pathway in osteo/odontogenic differentiation was also
investigated.
Materials and methods
Reagents and antibodies
SAL was purchased from Chengdu Must Bio-Technology
Co., Ltd. (cat. no. MUST-19021504) and was dissolved in dimethyl
sulfoxide (stock concentration of 200 mM). α-modification of
Eagle's minimum essential medium (α-MEM) and
penicillin/streptomycin were purchased from Gibco; Thermo Fisher
Scientific, Inc. Alizarin Red S (ARS), Triton X-100 and DAPI were
obtained from Sigma-Aldrich; Merck KGaA. Primary antibodies against
GAPDH (Cell Signaling Technology, Inc., cat. no. 5174T), Smad 1
(Cell Signaling Technology, Inc., cat. no. 6944T), phosphorylated
(p-)-Smad1/5/8 (Cell Signaling Technology, Inc., cat. no. 13820T),
runt-related transcription factor 2 (RUNX2) (Cell Signaling
Technology, Inc., cat. no. 12556S), bone sialoprotein (BSP) (Cell
Signaling Technology, Inc., cat. no. 5468S), and osteocalcin (OCN)
(Cell Signaling Technology, Inc., cat. no. 12888) were purchased
from Cell Signaling Technology, Inc. Osterix (OSX) (Abcam, cat. no.
ab209484) were purchased from Abcam. Alkaline Phosphatase (ALP)
assay kit (cat. no. P0321S) was obtained from Beyotime Institute of
Biotechnology.
Isolation and culture of hDPSCs
The present study was approved by The Shanghai
Stomatological Hospital Ethics Association [approval no.
SSDC-(2020)0006] and all patients or their parents provided written
informed consent. The hDPSCs were isolated from completely healthy
premolars, without caries or periodontitis, which were extracted
during the course of orthodontic treatment. The donors were aged
between 14 and 18 years (n=10, five male, five female, aged
15.6±1.26 years) and they had their teeth extracted in Shanghai
Stomatological Hospital between August and September 2019. The pulp
was gently separated from the tooth and cultured with a modified
tissue piece method in maintenance medium containing Dulbecco
Modified Eagle Medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 20% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 units ml-1 penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C and 5%
CO2 in an incubator. The medium was replaced every 3
days after cells grew out from the tissue pieces until cells
reached 80-90% confluence. hDPSCs were digested by 0.05%
trypsin-EDTA and then seeded into a new dish at 1:1. Flow
cytometric analysis was used to determine the cell surface markers
present on hDPSCs. The hDPSCs of each donor were isolated and
cultured alone.
Cell viability assay
The effects of SAL on hDPSC proliferation were
measured using a Cell Counting Kit-8 kit (CCK-8; Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocols
(17). In brief, hDPSCs were plated
into 96-well plates at a density of 5x103 cells/well and
were incubated for 48 or 96 h at 37˚C with serial dilutions of SAL
(0, 10, 20, 50 and 100 µM). Subsequently, 10 µl CCK-8 solution was
mixed with 90 µl complete α-MEM and added to each well. Following
incubation for 2 h at 37˚C, the optical density was measured using
an ELX800 absorbance microplate reader (Bio-Tek Instruments, Inc.)
at 450 nm (650 nm reference).
ARS staining assay
hDPSCs were first cultured in 12-well plates
(4x104 cells per well). The wells were then divided into
eight groups as follows at 37˚C and 5% CO2 in an
incubator for 21 days: i) Growth medium group (GM); ii)
osteo/odontogenic induction medium (OM) group; iii) OM + 1 µM SAL
group (1 µM); iv) OM + 5 µM SAL group (5 µM); v) OM + 10 µM SAL
group (10 µM); vi) OM + 20 µM SAL group (20 µM); vii) OM + 50 µM
SAL group (50 µM); viii) and OM + 100 µM SAL group (100 µM).
The osteo/odontogenic medium was comprised of 100 nM
dexamethasone, 50 µM ascorbic acid and 10 mM β-glycerophosphate
dissolved in α-MEM (all from Sigma-Aldrich; Merck KGaA) (18). The growth medium was 10% FBS and 1%
Penicillin-Streptomycin in α-MEM. To assess osteogenic
differentiation, the cells were stained with ARS (18). Briefly, hDPSCs were collected on day
21, fixed in 4% paraformaldehyde for 15 min and stained with 40 mM
ARS (pH 4.9; Sigma-Aldrich; Merck KGaA) for 15 min, both at 25˚C.
The plates were observed under an inverted microscope
(magnification, x200; Nikon Corporation). The darker the intensity
of the red dots, the higher the number of calcium nodules and
therefore the higher degree of differentiation.
ALP staining assay
hDPSCs were cultured in 12-well plates
(4x104 cells per well). The wells were divided into
three groups as follows: i) GM; ii) OM; and iii) 10 µM SAL group.
hDPSCs were then treated for 7 days at 37˚C. Subsequently, they
were fixed in 4% paraformaldehyde for 10 min at room temperature.
The cells were stained for ALP using the ALP assay kit (cat. no.
P0321S; Beyotime Institute of Biotechnology) according to the
manufacturer's protocols (19,20).
The cells were subsequently washed three times with distilled
water. ALP staining was observed under an inverted microscope
(magnification, x200; Nikon Corporation). Increases in the
concentration of the insoluble nitroblue tetrazolium formazan was
associated with a change in color from dark blue to blue/purple,
which is in turn associated with higher ALP activity.
RNA extraction and gene expression
analysis using reverse transcription-quantitative PCR
(RT-qPCR)
hDPSCs were cultured in six-well plates
(8x104 cells per well). The wells were divided into
three groups as follows: i) GM; ii) OM; and iii) 10 µM SAL. hDPSCs
were in turn treated for 3 and 7 days at 37˚C. Total mRNA was
extracted using the acid guanidinium thiocyanate-phenol-chloroform
method (21). Complementary DNA
synthesis was performed using a FastQuant RT kit (Tiangen Biotech
Co., Ltd. cat. no. KR106-03). qPCR was performed using the SYBR
Green Premix (Tiangen Biotech Co., Ltd.; cat. no. FP207) according
to the manufacturer's protocols under the following conditions: 15
min of denaturation at 42˚C and 1 cycle of 1 min of denaturation at
95˚C and 40 cycles each of 5 sec of denaturation at 95˚C and 15 sec
of amplification at 60˚C. The following primer sequences were used
as previously described: human actin (22) forward, 5'-CATGTACGTTGCTATCCAGGC-3'
and reverse, 5'-CTCCTTAATGTCACGCACGAT-3'; human ALP (22) forward, 5'-CCTCCTCGGAAGACACTCTG-3'
and reverse, 5'-GCAGTGAAGGGCTTCTTGTC-3'; human RUNX2(22) forward, 5'-CCACTGAACCAAAAAGAAATCCC-3'
and reverse, 5'-GAAAACAACACATAGCCAAACGC-3'; human OCN (23) forward, 5'-GCGCTACCTGTATCAATGGC-3'
and reverse, 5'-AACTCTCACAGTCCGGATT-3'; human dentin
sialophosphoprotein (DSPP) (7)
forward, 5'-CGACATAGGTCACAATGAGGATGTCG-3' and reverse,
5'-TTGCTTCCAGCTACTTGAGGTC-3'; human BSP (7) forward,
5'-CAGGCCACGATATTATCTTTACA-3'and reverse,
5'-CTCCTCTTCTTCCTCCTCCTC-3' and human OSX (24) forward,
5'-CTGTGAAACCTCAAGTCCTATGGA-3' and reverse,
5'-GCTCTGCAGTCAAGGGAGATG-3'. The relative gene expression was
calculated according to the comparative 2-ΔΔCq method
and normalized to that of actin (25).
Immunofluorescence analysis
hDPSCs were cultured in 24-well plates
(4x104 cells per well). The wells were divided into
three groups as follows: i) GM; ii) OM; and iii) 10 µM SAL. hDPSCs
were treated for 3 days at 37˚C. The cell samples were then fixed
in 4% paraformaldehyde in PBS for 20 min at room temperature.
Subsequently, the cells were permeabilized in the presence of PBS
with 0.25% Triton X-100 for 15 min (26) and blocked with 5% non-fat milk
diluted in TBS at room temperature for 1 h. The cells were
incubated overnight at 4˚C with anti-DSPP (1:200; cat. no.
SC-73632; Santa Cruz Biotechnology, Inc.) in the presence of 2.5%
BSA. The cells were subsequently washed three times in PBS with
0.1% Tween-20 (PBST) and stained for 1 h at room temperature with a
goat anti-mouse FITC-conjugated secondary antibody (1:1,000; cat.
no. F-2761; Thermo Fisher Scientific, Inc.) in 2.5% BSA. The
samples were washed five times with PBST and the cells were
counterstained with DAPI (1:10,000) at room temperature for 10 min.
The cells were imaged using a fluorescence microscope
(magnifications, x200; Leica Microsystems, Inc.).
Western blot analysis
hDPSCs were cultured in six-well plates
(8x104 cells per well). The wells were divided into
three groups as follows: i) GM; ii) OM; and iii) 10 µM SAL group.
hDPSCs were treated for 3 days at 37˚C. The cells were collected in
RIPA lysis buffer (Thermo Fisher Scientific, Inc.). The protein
concentration was determined by BCA method (Thermo Fisher
Scientific, Inc., cat. no 23225). The lysate proteins (30 µg) were
separated using 10% SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane. The PVDF membranes were blocked with 5% non-fat
milk diluted in TBS at room temperature for 1 h and were incubated
overnight at 4˚C with a series of primary antibodies targeted at
GAPDH, Smad 1, p-Smad1/5/8, RUNX2, BSP, OSX, and OCN (1:1,000
dilution). The following day, the PVDF membranes were incubated
with HRP-conjugated secondary antibodies (Cell Signaling
Technology, Inc. cat. nos. 7074 and 7076; 1:10,000 dilution) for
1-2 h at room temperature. A Western Chemiluminescent HRP Substrate
system (EMD Millipore; cat. no. WBKLS0100) was used for visualizing
the bands. The signals were captured with an Amersham Imager 600
monitoring system (GE Healthcare). ImageJ 1.8.0 (National
Institutes of Health) was used to quantify the bands.
Statistical analysis
Data analysis was performed using the SPSS software
(ver. 23.0; IBM Corp.). Experiments were repeated independently at
least three times. The results were expressed as mean ± standard
deviation. Statistical analysis of the data was performed with
one-way analysis of variance followed by the Scheffé's post hoc
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
SAL promotes hDPSC viability and
osteo/odontogenic differentiation
To investigate the effects of SAL on the
osteo/odontogenic differentiation of hDPSCs, CCK-8 assay was first
used. Treatment with SAL for 48 or 96 h at concentrations <100
µM did not induce cytotoxicity. SAL (10 or 20 µM) significantly
increased cell viability at 96 h time points of treatment compared
with that in the control group (Fig.
1A and B).
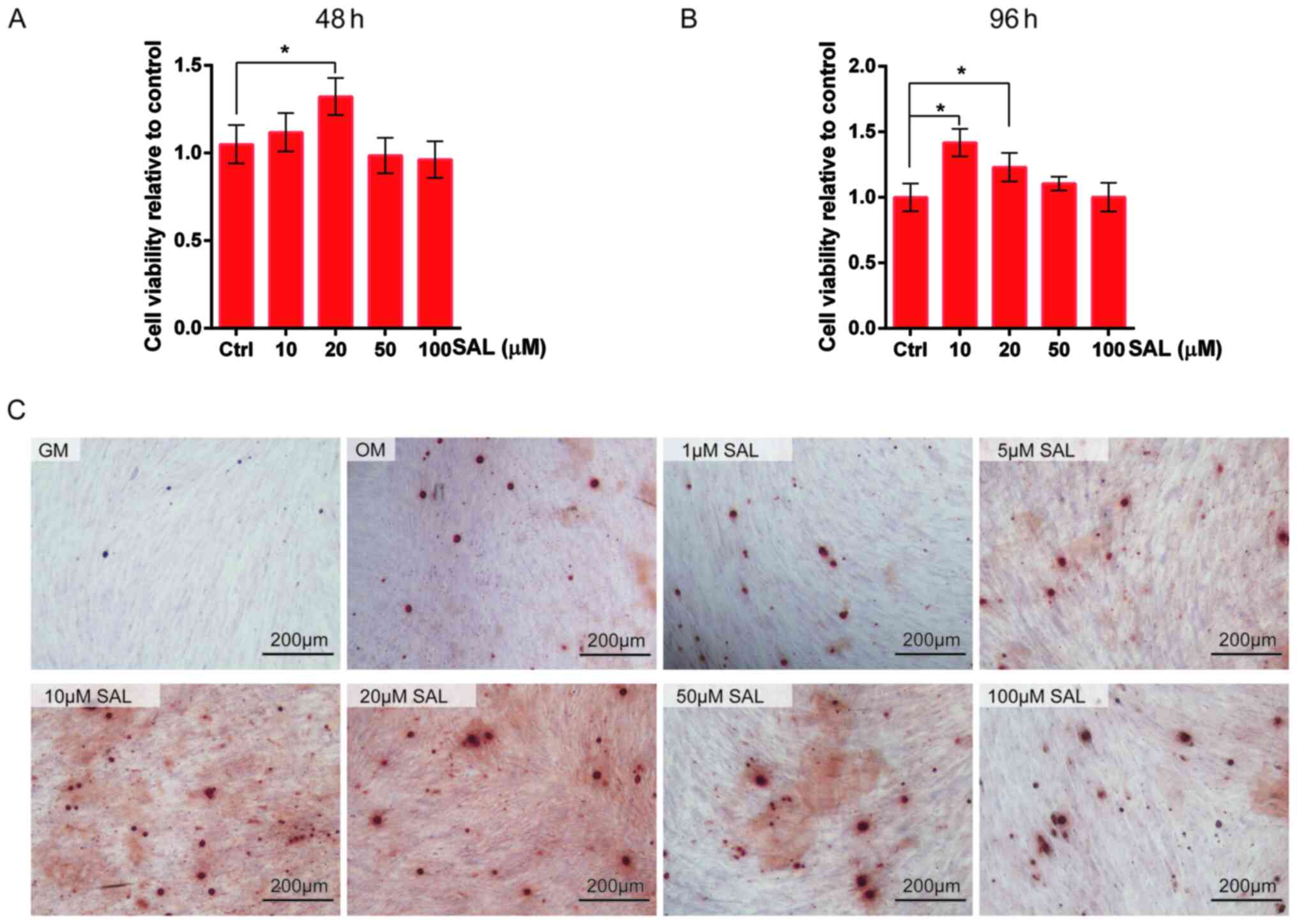 | Figure 1SAL promotes the proliferation and
osteogenesis of hDPSCs. (A) Cell viability of hDPSCs were detected
by Cell Counting Kit-8 following treatment with 10, 20, 50 and 100
µM SAL for (A) 48 and (B) 96 h. (C) Alizarin red staining detected
the osteogenesis of hDPSCs treated with 1, 5, 10, 20, 50 and 100 µM
SAL. Scale bars, 200 µm. *P<0.05. GM, growth medium;
OM, osteogenic medium; SAL, salidroside; hDPSCs, human dental pulp
stem cells. |
ARS was subsequently used to detect the effect of
SAL on the osteogenic differentiation of hDPSCs. SAL promoted the
osteo/odontogenic differentiation of hDPSCs at 100 µM; Fig. 1C showed that differentiation showed
by the calcium nodules was increased in all treatment groups vs.
GM. SAL (10 µM) markedly increased the osteo/odontogenic
differentiation of hDPSCs (Fig.
1C). Based on this observation, 10 µM SAL was selected for the
following experiments.
SAL increases the expression of genes
associated with osteo/odontogenic differentiation
ALP staining indicated that OM increased ALP
activity compared with GM and SAL (10 µM) markedly increased ALP
activity in the hDPSCs compared with OM (Fig. 2A). Additionally, SAL further
promoted the expression levels of ALP mRNA following incubation
with the cells for 3 and 7 days compared with OM (Fig. 2B). The expression levels of OSX were
also significantly increased in hDPSCs following treatment of the
cells with 10 µM SAL for 3 and 7 days compared with those in the OM
group (Fig. 2C). Although SAL
promoted the expression levels of RUNX2, OCN, DSPP and BSP mRNA
following incubation with the cells for 3 and 7 days to some
extent, there was no significant difference (Fig. 2).
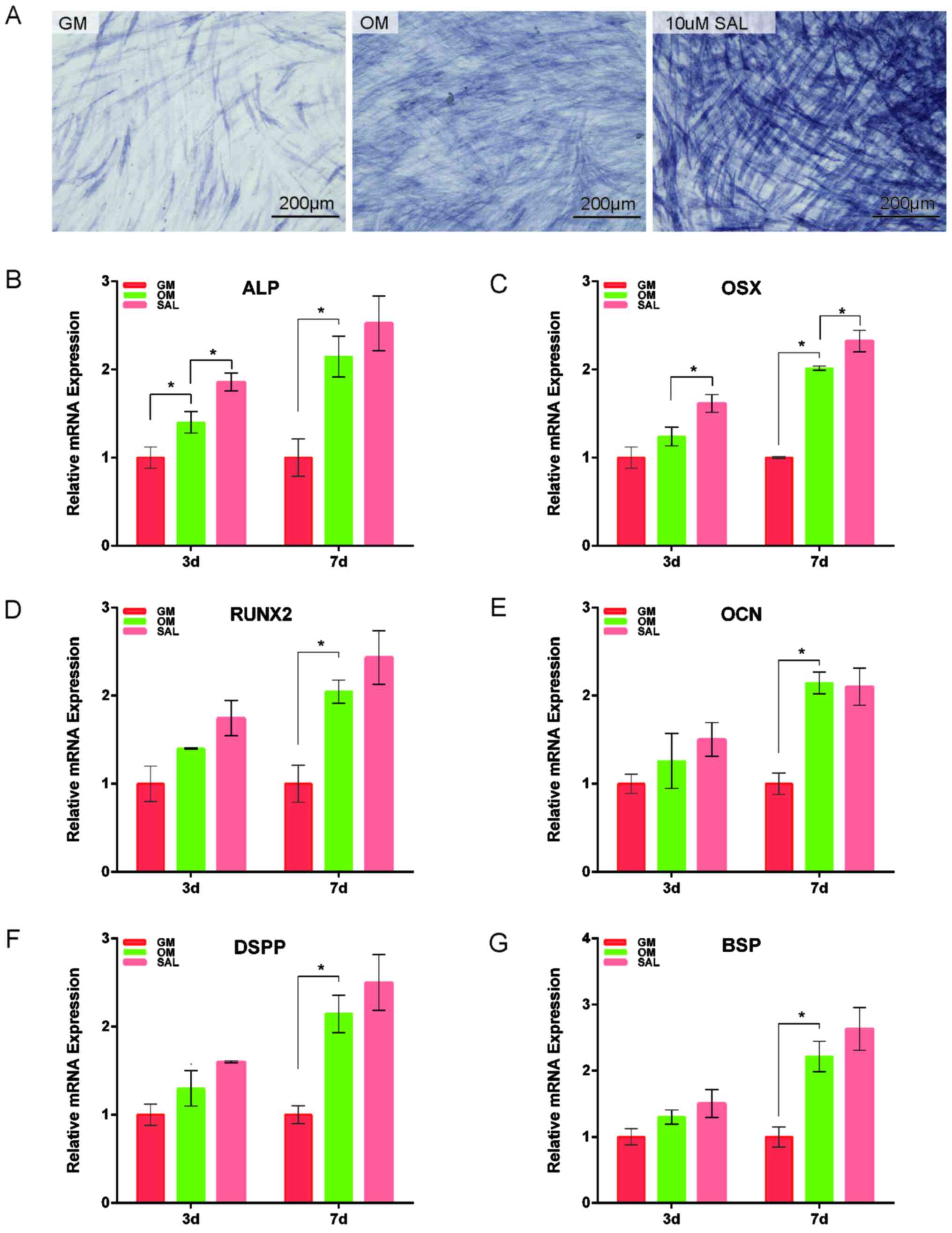 | Figure 2SAL promotes the expression of genes
associated with osteogenesis. (A) Alkaline phosphatase staining was
used to measure the degree of osteogenesis in hDPSCs treated with
10 µM SAL. The mRNA expression of (B) ALP, (C) OSX, (D) RUNX2, (E)
OCN, (F) DSPP and (G) BSP were detected after treatment with 10 µM
SAL for 3 and 7 days. The expression of OSX, RUNX2, OCN, DSPP and
BSP in hDPSCs after treatment for 3 and 7 days with 10 µM SAL was
significantly increased. Scale bars, 200 µm. *P<0.05.
GM, growth medium; OM, osteogenic medium; SAL, salidroside; RUNX2,
runt-related transcription factor 2; BSP, bone sialoprotein; OCN,
osteocalcin; OSX, osterix; ALP, Alkaline Phosphatase; DSPP, dentin
sialophosphoprotein; hDPSCs, human dental pulp stem cells. |
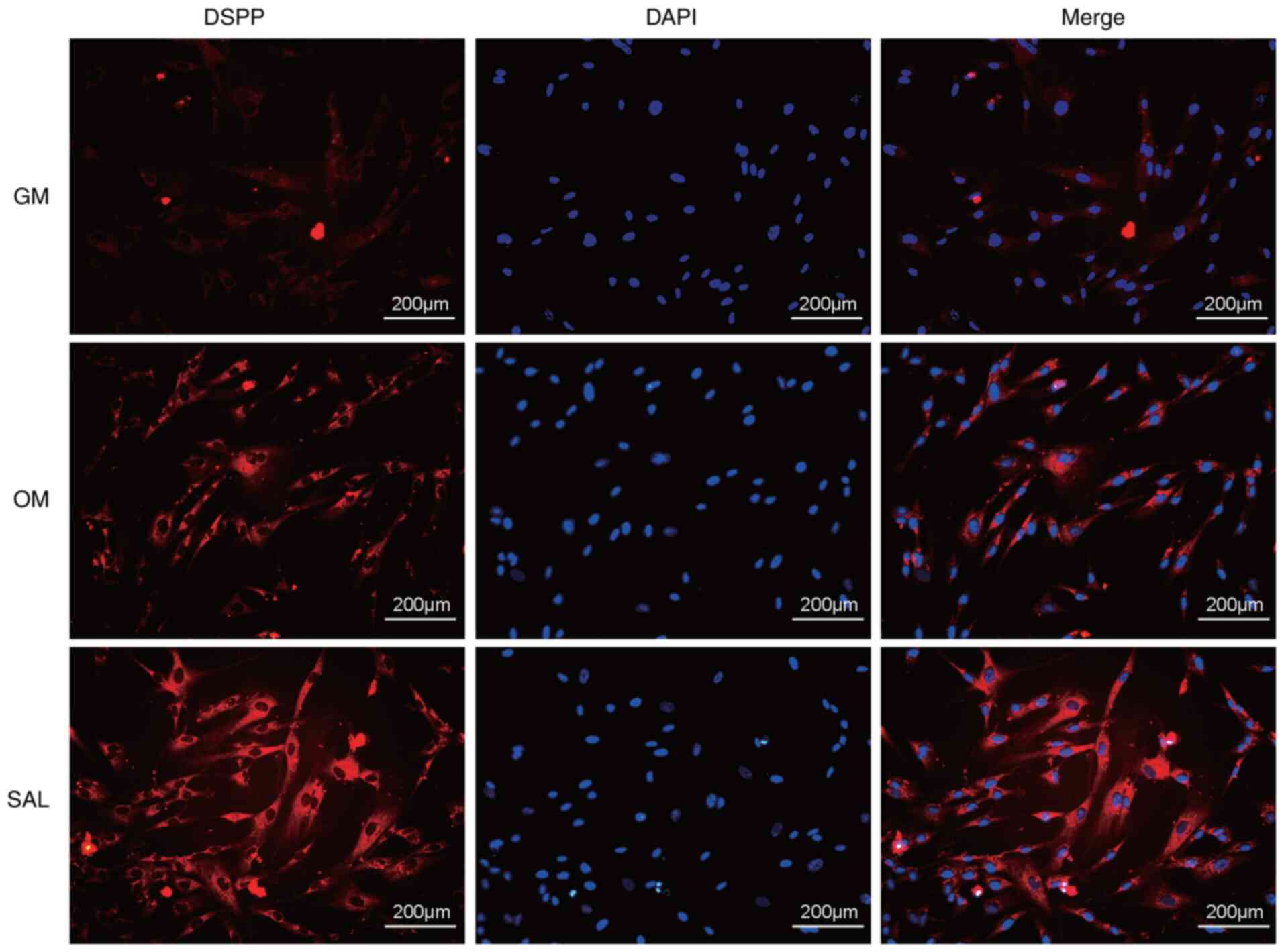
SAL promotes the expression levels of
DSPP in hDPSCs
DSPP is a key protein involved in the process of
odontogenic differentiation (23).
In the OM group, the expression of DSPP was higher compared with
the GM group. SAL (10 µM) effectively promoted the expression of
DSPP in hDPSCs as detected by immunofluorescence analysis compared
with the OM group (Fig. 3).
SAL promotes osteo/odontogenic
differentiation by activating the BMP pathway
The effects of SAL on the expression of the
osteo/odontogenic differentiation-associated proteins were detected
in hDPSCs by western blot analysis. Western blot analysis indicated
that SAL markedly increased the expression levels of the RUNX2 and
BSP proteins compared with the OM group (Fig. 4A). To explore the specific mechanism
underlying the involvement of SAL in the osteo/odontogenic
differentiation of hDPSCs, the effects of SAL on the p38, JNK, ERK
and Smad1/5/8 signaling pathways were examined. The results
indicated that whilst SAL could significantly activate Smad1/5/8
signaling compared with that in the OM group, it had no effect on
the p38, ERK or the JNK MAPK signaling pathways (Fig. 4B-C).
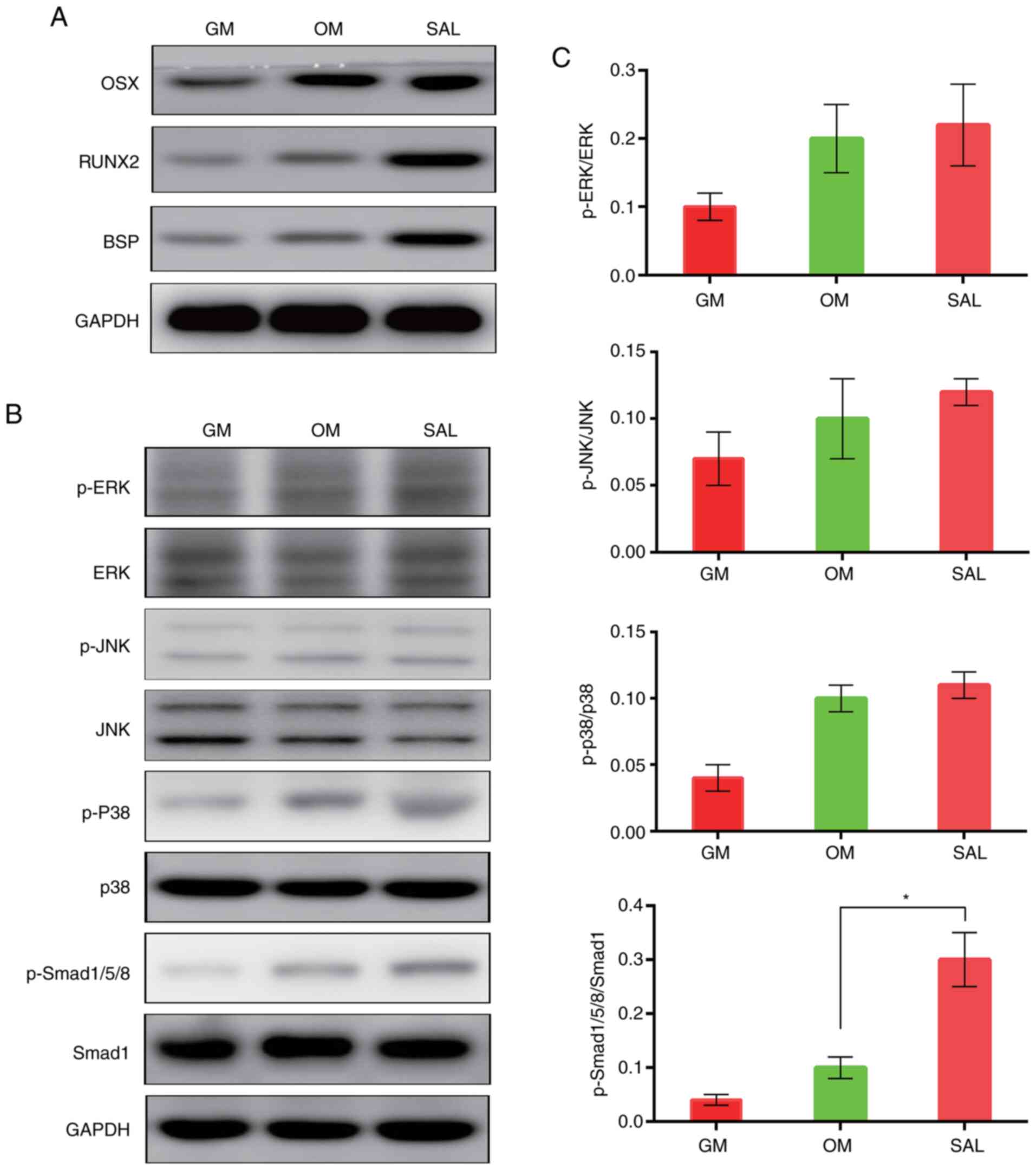 | Figure 4SAL promotes osteogenesis by
activating the BMP signaling pathway. (A) The protein expression of
OSX, RUNX2 and BSP in hDPSCs after treatment with 10 µM SAL were
detected by western blotting. (B) Western blotting was used to
measure the phosphorylation of ERK, JNK, p38 MAPK and Smad1/5/8 in
hDPSCs after treatment with 10 µM SAL, (C) which was quantified
against their corresponding total protein (*P=0.003).
GM, growth medium; OM, osteogenic medium; SAL, salidroside; RUNX2,
runt-related transcription factor 2; BSP, bone sialoprotein; OSX,
osterix; hDPSCs, human dental pulp stem cells. |
Discussion
Dental pulp regeneration, compared with root canal
therapy for the young permanent teeth with pulpitis, exhibits a
number of methodological advantages as it restores the nutrition
supply of tooth tissue and make the tooth harder to break (27,28).
It would be of considerable benefit for the young permanent teeth
with pulpitis to be able to regulate the directed differentiation
of transplanted hDPSCs using different methods such as releasing
cytokines or drugs by biomaterials (29). Small molecules derived from
traditional Chinese medicine have been reported to confer specific
biological activities that may resemble those of natural products
(30). Previous studies have
successively used molecules such as corylin and artemisinin for the
regulation of stem cell differentiation (31,32).
The aim of the present study was to explore whether SAL has the
ability to affect the osteo/odontogenic differentiation of hDPSCs
in vitro. The data demonstrated that SAL promoted the
viability and osteo/odontogenic differentiation of hDPSCs.
In the present study, SAL exerted no cytotoxicity in
the concentration range of 0-100 µM, but could instead
significantly increase the viability of hDPSCs at 10 and 20 µM,
which is consistent previous observations by Chen et al
(13). However, further increases
in SAL concentration to 50 and 100 µM did not promote the
proliferation of hDPSCs. This may be because with this increase,
the toxicity of SAL increased correspondingly, counteracting its
effect on cell viability. The possible regulatory effect of SAL on
the osteo/odontogenic differentiation of hDPSCs was then detected
by ARS. The results of the ARS assay demonstrated that SAL exerted
a promoting effect on the osteo/odontogenic differentiation of
hDPSCs, which was the most prominent at 10 µM. The biological
effects of SAL on promoting osteogenesis have also been reported in
other cells. Zhang et al (33) previously demonstrated that SAL
treatment counteracted H2O2-induced
osteogenic inhibition by increasing ALP activity and mineralization
in the MC3T3-E1 osteoblastic cell line. It has also been shown that
SAL promotes osteoblastic differentiation in the murine pluripotent
mesenchymal cell line C3H10T1/2(13). A previous study reported that SAL
promoted human periodontal ligament cell proliferation and
osteocalcin secretion via the ERK1/2 and PI3K/AKT signaling
pathways (16). These previous
studies aforementioned at least in part support the findings of the
present study.
ALP is a key marker of osteoblastic cells and serves
a central role in the regulation of early osteogenesis, where it
promotes cell maturation and calcification (24,34).
To evaluate the effects of SAL, the expression levels of ALP were
assessed by ALP staining and RT-qPCR. The results of ALP staining
and gene expression analysis demonstrated that 10 µM SAL exerted a
significant promoting effect on early hDPSC osteogenesis compared
with that in the OM groups. OSX is a zinc finger-containing
transcription factor that is expressed in osteoblasts and may serve
an important role in the osteoblast differentiation pathway by
controlling the expression of specific genes such as OCN and
collagen (35). A number of
transcription factors are specific to regulating osteoblast
differentiation and bone formation. RUNX2 is a multifunctional
transcription factor that can regulate the differentiation of
osteoblasts into mesenchymal stem cells and induces the
differentiation of mesenchymal stem cells into osteoblasts
(36). Furthermore, RUNX2 is
necessary for tooth formation and is intimately involved in the
development of calcified tooth tissues (35). Miyazaki et al (37) reported that RUNX2 is expressed in
preodontoblasts, where its expression level is decreased during
odontoblast differentiation. In the present study, the results
suggested that RUNX2 was highly expressed during the early stages
of hDPSC osteoblast/odontoblastic differentiation following SAL
treatment compared with the OM group. RUNX2 and OCN are two
extensively studied osteoblast-specific transcription factors that
are also key markers of osteoblastic cells (38). RUNX2 is typically expressed during
early osteoblast differentiation and is indispensable for the
differentiation and appropriate functioning of osteoblasts
(38,39). OCN is considered to be a marker of
mature osteocytes that is also expressed in odontoblasts (40). OCN has been reported to regulate the
growth rate and direction of hydroxyapatite crystals during bone
development (39).
During dentin formation, DSPP is considered the
marker of dentin differentiation (41). DSPP is only expressed in
odontoblasts and is regulated by RUNX2 to promote differentiation
into odontoblasts (42). By
contrast, to these observations, BSP comprises the majority of
non-collagenous extracellular matrix in mineralized tissues and is
one of the osteoblast-specific markers regulated by RUNX2(43). In the present study, the expression
levels of DSPP and BSP were significantly increased following SAL
treatment. This indicates that SAL may promote
osteoblastic/odontoblastic cell differentiation and mineralization
by regulating DSPP and BSP gene expression in hDPSCs. BSP functions
as a hydroxyapatite nucleator and it is possible that the bony
spicules observed in the frontal and parietal bones may be formed
as a result of BSP upregulation (44). In the present study, SAL (10 µM)
caused a significant upregulation in the mRNA expression levels of
OSX, RUNX2, DSPP and BSP on either day 3 or day 7. These findings
in the present study suggest that SAL may serve an important role
in promoting the osteogenic and odontoblast differentiation of
hDPSCs.
Subsequently, the present study explored the
signaling pathways that were affected by SAL treatment with regards
to the osteogenic differentiation of hDPSCs. It was found that SAL
exerted its effects on the hDPSCs mainly by activating Smad1/5/8,
instead of by activating the MAPK signaling pathway. The BMP
pathway is one of the main signaling cascades that stimulate the
osteogenic and odontoblastic differentiation of hDPSCs (45). The phosphorylation of Smad1/5/8 is
an important starting point for the activation of the BMP signaling
pathway (46). Phosphorylation of
Smad1/5/8 promotes the translocation of the downstream target Smad4
to the nucleus, where it activates intranuclear signaling
transduction (47). SAL caused a
significant increase in the phosphorylation levels of Smad1/5/8,
which largely indicated that it may regulate the differentiation of
hDPSCs through the BMP signaling pathway. These findings may
provide novel preliminary evidence of the involvement of BMP
signaling in the SAL-induced osteo/odontogenic differentiation of
hDPSCs. However, they also demonstrated that SAL did not affect the
osteo/odontogenic differentiation of hDPSCs via the MAPK signaling
pathway.
It should be noted here that other pathways may also
be involved in the regulation of hDPSCs by SAL. In addition, the
present study lacks in vivo experiments and the mechanism of
SAL action requires further investigation. Animal models will be
constructed in subsequent studies to assess the in vivo
effects of SAL and to provide potential clinical applications of
this compound for the osteo/odontogenic differentiation of
hDPSCs.
In conclusion, the present study demonstrated that
SAL promoted the viability and osteo/odontogenic differentiation of
hDPSCs, where it also increased the expression of osteo/odontogenic
differentiation-associated genes. This process was mediated by
activation of the BMP pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
Project of the Shanghai Municipal Commission of Health and Family
Planning (grant no. 201740051).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JL, HL and CN performed experiments. JL, CN and
DC wrote and revised the manuscript. CN and DC conceived and
designed the study. DC and CN confirm the authenticity of all raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Shanghai
Stomatological Hospital Ethics Association [approval no.
SSDC-(2020)0006] and all patients or their parents provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peters OA: The Guidebook to Molar
Endodontics. Springer, New York, NY, 2017.
|
|
2
|
Moonesi RR, Atila D, Akgun EE, Evis Z,
Keskin D and Tezcaner A: Evaluation of human dental pulp stem cells
behavior on a novel nanobiocomposite scaffold prepared for
regenerative endodontics. Mater Sci Eng C Mater Biol Appl.
100:928–948. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eramo S, Natali A, Pinna R and Milia E:
Dental pulp regeneration via cell homing. Int Endod J. 51:405–419.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jung C, Kim S, Sun T, Cho YB and Song M:
Pulp-dentin regeneration: Current approaches and challenges. J
Tissue Eng. 10(1544418625)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ran R, Yang H, Cao Y, Yan W, Jin L and
Zheng Y: Depletion of EREG enhances the osteo/dentinogenic
differentiation ability of dental pulp stem cells via the p38 MAPK
and Erk pathways in an inflammatory microenvironment. BMC Oral
Health. 21(314)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang X, Li Z, Liu A, Liu X, Guo H, Wu M,
Yang X, Han B and Xuan K: Microenvironment influences odontogenic
mesenchymal stem cells mediated dental pulp regeneration. Front
Physiol. 12(656588)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hao J, Yang H, Cao Y, Zhang C and Fan Z:
IGFBP5 enhances the dentinogenesis potential of dental pulp stem
cells via JNK and ErK signalling pathways. J Oral Rehabil.
47:1557–1565. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsutsui TW: Dental pulp stem cells:
Advances to applications. Stem Cells Cloning. 13:33–42.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen H, Fu H, Wu X, Duan Y, Zhang S, Hu H,
Liao Y, Wang T, Yang Y, Chen G, et al: Regeneration of
pulpo-dentinal-like complex by a group of unique multipotent
CD24a+ stem cells. Sci Adv. 6(eaay1514)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu A, Bao Y, Yu H, Zhou Y and Lu Q:
Berberine accelerates odontoblast differentiation by
Wnt/beta-Catenin Activation. Cell Reprogram. 21:108–114.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li F, Sun X, Ma J, Ma X, Zhao B, Zhang Y,
Tian P, Li Y and Han Z: Naringin prevents ovariectomy-induced
osteoporosis and promotes osteoclasts apoptosis through the
mitochondria-mediated apoptosis pathway. Biochem Biophys Res
Commun. 452:629–635. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Biswal S, Barhwal KK, Das D, Dhingra R,
Dhingra N, Nag TC and Hota SK: Salidroside mediated stabilization
of Bcl-xL prevents mitophagy in CA3 hippocampal neurons during
hypoxia. Neurobiol Dis. 116:39–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen JJ, Zhang NF, Mao GX, He XB, Zhan YC,
Deng HB, Song DQ, Li DD, Li ZR, Si SY, et al: Salidroside
stimulates osteoblast differentiation through BMP signaling
pathway. Food Chem Toxicol. 62:499–505. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo XQ, Qi L, Yang J, Wang Y, Wang C, Li
ZM, Li L, Qu Y, Wang D and Han ZM: Salidroside accelerates fracture
healing through cell-autonomous and non-autonomous effects on
osteoblasts. Cell Tissue Res. 367:197–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sun M, Lu Z, Cai P, Zheng L and Zhao J:
Salidroside enhances proliferation and maintains phenotype of
articular chondrocytes for autologous chondrocyte implantation
(ACI) via TGF-β/Smad3 Signal. Biomed Pharmacother.
122(109388)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ying Y and Luo J: Salidroside promotes
human periodontal ligament cell proliferation and osteocalcin
secretion via ERK1/2 and PI3K/Akt signaling pathways. Exp Ther Med.
15:5041–5045. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Niu C, Xiao F, Yuan K, Hu X, Lin W, Ma R,
Zhang X and Huang Z: Nardosinone suppresses RANKL-induced
osteoclastogenesis and attenuates lipopolysaccharide-induced
alveolar bone resorption. Front Pharmacol. 8(626)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu J, Wang Y, Li J and Zhang X, Geng Y,
Huang Y, Dai K and Zhang X: IL-12p40 impairs mesenchymal stem
cell-mediated bone regeneration via CD4+ T cells. Cell
Death Differ. 23:1941–1951. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jin Q, Yuan K, Lin W, Niu C, Ma R and
Huang Z: Comparative characterization of mesenchymal stem cells
from human dental pulp and adipose tissue for bone regeneration
potential. Artif Cells Nanomed Biotechnol. 47:1577–1584.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T,
Hao J, Guo CL, Ma QW, Wang L, et al: iPS cells produce viable mice
through tetraploid complementation. Nature. 461:86–90.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Zhao Q, Yang X, Yu X, Yu D and
Zhao W: Effects of cobalt chloride on the stem cell marker
expression and osteogenic differentiation of stem cells from human
exfoliated deciduous teeth. Cell Stress Chaperones. 24:527–538.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi R, Yang H, Lin X, Cao Y, Zhang C, Fan
Z and Hou B: Analysis of the characteristics and expression
profiles of coding and noncoding RNAs of human dental pulp stem
cells in hypoxic conditions. Stem Cell Res Ther.
10(89)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Niu C, Yuan K, Ma R, Gao L, Jiang W, Hu X,
Lin W, Zhang X and Huang Z: Gold nanoparticles promote osteogenic
differentiation of human periodontal ligament stem cells via the
p38 MAPK signaling pathway. Mol Med Rep. 16:4879–4886.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang W, Yu L, Han X, Pan J, Deng J, Zhu
L, Lu Y, Huang W, Liu S, Li Q and Liu Y: The secretome of human
dental pulp stem cells protects myoblasts from hypoxiainduced
injury via the Wnt/β-catenin pathway. Int J Mol Med. 45:1501–1513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dissanayaka WL and Zhang C: The role of
vasculature engineering in dental pulp regeneration. J Endod. 43
(Suppl):S102–S106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahmed GM, Abouauf EA, AbuBakr N, Dorfer CE
and El-Sayed KF: Tissue engineering approaches for enamel, dentin,
and pulp regeneration: An update. Stem Cells Int.
2020(5734539)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hashemi-Beni B, Khoroushi M, Foroughi MR,
Karbasi S and Khademi AA: Tissue engineering: Dentin-pulp complex
regeneration approaches (A review). Tissue Cell. 49:552–564.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu K, Wang XJ, Li YN, Li B, Qi JS, Zhang
J and Wang Y: Tongxinluo reverses the Hypoxia-suppressed Claudin-9
in cardiac microvascular endothelial cells. Chin Med J (Engl).
129:442–447. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fang J, Zhao X, Li S, Xing X, Wang H,
Lazarovici P and Zheng W: Protective mechanism of artemisinin on
rat bone marrow-derived mesenchymal stem cells against apoptosis
induced by hydrogen peroxide via activation of
c-Raf-Erk1/2-p90rsk-CREB pathway. Stem Cell Res Ther.
10(312)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu AX, Xu ML, Yao P, Kwan KK, Liu YX, Duan
R, Dong TT, Ko RK and Tsim KW: Corylin, a flavonoid derived from
Psoralea Fructus, induces osteoblastic differentiation via estrogen
and Wnt/β-catenin signaling pathways. FASEB J. 34:4311–4328.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang JK, Yang L, Meng GL, Yuan Z, Fan J,
Li D, Chen JZ, Shi TY, Hu HM, Wei BY, et al: Protection by
salidroside against bone loss via inhibition of oxidative stress
and bone-resorbing mediators. PLoS One. 8(e57251)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang J, Zhang W, Dai J, Wang X and Shen
SG: Overexpression of Dlx2 enhances osteogenic differentiation of
BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and
Alp. Int J Oral Sci. 11(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Camilleri S and McDonald F: Runx2 and
dental development. Eur J Oral Sci. 114:361–373. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gomathi K, Akshaya N, Srinaath N, Moorthi
A and Selvamurugan N: Regulation of Runx2 by post-translational
modifications in osteoblast differentiation. Life Sci.
245(117389)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miyazaki T, Kanatani N, Rokutanda S,
Yoshida C, Toyosawa S, Nakamura R, Takada S and Komori T:
Inhibition of the terminal differentiation of odontoblasts and
their transdifferentiation into osteoblasts in Runx2 transgenic
mice. Arch Histol Cytol. 71:131–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chan W, Tan Z, To M and Chan D: Regulation
and role of transcription factors in osteogenesis. Int J Mol Sci.
22(5445)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Komori T: Functions of osteocalcin in
bone, pancreas, testis, and muscle. Int J Mol Sci.
21(7513)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Komori T: Regulation of bone development
and extracellular matrix protein genes by RUNX2. Cell Tissue Res.
339:189–195. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
D'Souza RN, Cavender A, Sunavala G,
Alvarez J, Ohshima T, Kulkarni AB and MacDougall M: Gene expression
patterns of murine dentin matrix protein 1 (Dmp1) and dentin
sialophosphoprotein (DSPP) suggest distinct developmental functions
in vivo. J Bone Miner Res. 12:2040–2049. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li S, Kong H, Yao N, Yu Q, Wang P, Lin Y,
Wang J, Kuang R, Zhao X, Xu J, et al: The role of runt-related
transcription factor 2 (Runx2) in the late stage of odontoblast
differentiation and dentin formation. Biochem Biophys Res Commun.
410:698–704. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao RT, Zhan LP, Meng C, Zhang N, Chang
SM, Yao R and Li C: Homeobox B7 promotes the osteogenic
differentiation potential of mesenchymal stem cells by activating
RUNX2 and transcript of BSP. Int J Clin Exp Med. 8:10459–10470.
2015.PubMed/NCBI
|
|
44
|
Baht GS, O'Young J, Borovina A, Chen H,
Tye CE, Karttunen M, Lajoie GA, Hunter GK and Goldberg HA:
Phosphorylation of Ser136 is critical for potent bone
sialoprotein-mediated nucleation of hydroxyapatite crystals.
Biochem J. 428:385–395. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hrubi E, Imre L, Robaszkiewicz A, Virág L,
Kerényi F, Nagy K, Varga G, Jenei A and Hegedüs C: Diverse effect
of BMP-2 homodimer on mesenchymal progenitors of different origin.
Hum Cell. 31:139–148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang L, Liu C and Wu F: Low-level laser
irradiation enhances the proliferation and osteogenic
differentiation of PDLSCs via BMP signaling. Lasers Med Sci: Jul
10, 2021 (Epub ahead of print).
|
|
47
|
Kang X, Qian Z, Liu J, Feng D, Li H, Zhang
Z, Jin X, Ma Z, Xu M, Li F, et al: Neuropeptide Y acts directly on
cartilage homeostasis and exacerbates progression of osteoarthritis
through NPY2R. J Bone Miner Res. 35:1375–1384. 2020.PubMed/NCBI View Article : Google Scholar
|