Acute myocardial infarction (AMI) is myocardial
necrosis caused by acute and persistent ischemia and hypoxia in the
coronary arteries (1). There is an
urgent need for improved treatment strategies. The traditional
treatment for AMI is mainly surgery or drug therapy, including
thrombolytic therapy, percutaneous coronary intervention (PCI) and
coronary artery bypass graft (CABG) (2). These traditional treatment methods
can save dying cardiomyocytes, decrease the area of infarction
progression and delay myocardial remodeling. However, they do not
promote myocardial cell regeneration, and the contractile ability
of the scar tissue in the infarcted is limited; therefore, the
myocardial contractile force is gradually reduced, causing
myocardial fibrosis, arrhythmia and ventricular diastolic
dysfunction, leading to advanced congestive heart failure (3). Due to the limits of traditional
methods, significant research has been devoted to post-infarction
repair.
Angiogenesis is essential for correct healing
post-infarction. The blood supply of the cells in the infarcted
area gradually decreases, which restricts oxygen transfer, nutrient
absorption and removal of metabolic waste, and the cardiomyocytes
gradually become necrotic; therefore, restoring the blood supply to
the infarcted area is a favorable repair method (4). The present review outlines the
progress of current research on angiogenesis in myocardial
infarction repair, including the main factors affecting
angiogenesis and the therapeutic methods.
Angiogenesis is the formation of new blood vessels
based on previous vasculature. The formation of blood vessels
starts with the sprouting of endothelial cells (ECs), which adhere
to each other and are connected to the extracellular matrix (ECM),
followed by hydrolytic remodeling of ECM in the presence of various
enzymes. Hydrolytic remodeling of ECM refers to the continuous
process of decomposition and synthesis of the ECM under the action
of various enzymes (5). There are
three main types of ECs, namely tip, stalk and phalanx cells
(6). Tip and stalk cells are
located at the sprouting tip of blood vessels and can secrete a
variety of pro-angiogenic factors, such as vascular endothelial
growth factor (VEGF), fibroblast growth factor and platelet-derived
growth factor (PDGF) (7). There
are numerous factors that affect the formation of blood
vessels.
After AMI, the repair process of cardiac injury
begins with the inflammatory phase. This is characterized by the
activation of natural immune pathways and the recruitment of
inflammatory leukocytes to remove dead cells from the wound, which
involves the complement system, reactive oxygen species and the
participation of various chemokines (8). Some other cells are also activated
and involved in repair, such as macrophages, fibroblasts, ECs,
lymphocytes and other immune cells (9).
Fibroblasts are the main cellular component of
myocardial injury. They are derived from embryonic mesenchymal
cells and can produce collagen and other proteins. Fibroblasts have
multiple phenotypes, and some populations express
fibroblast-specific protein (FSP)1 and α-smooth muscle actin
(α-SMA). FSP1-positive fibroblasts contribute to angiogenesis and
repair, and their reparative effect is greater than that of
α-SMA-positive fibroblasts (12).
Moreover, as infarction progresses, fibroblast function transforms
from inflammation to angiogenesis (13).
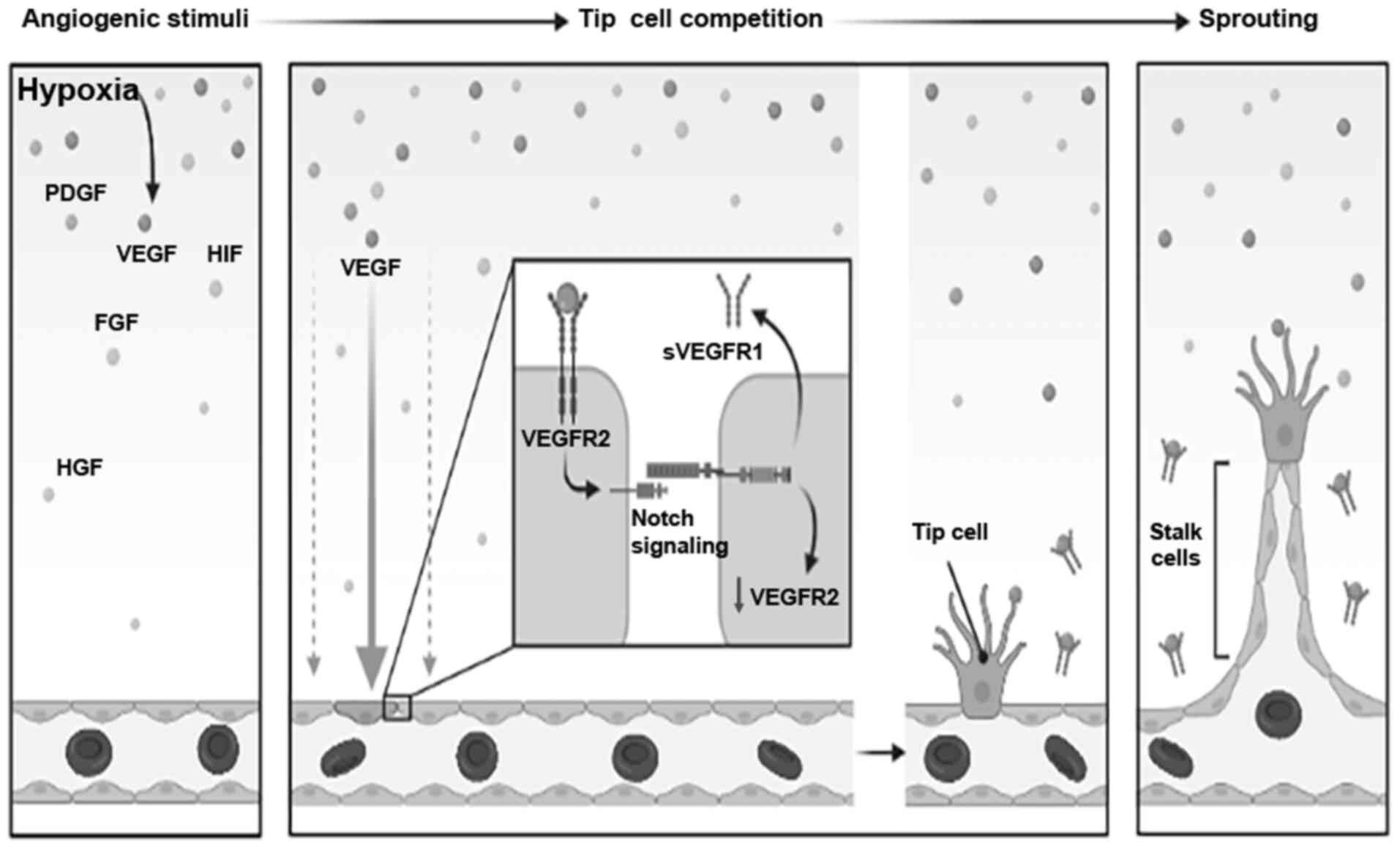
Vascular ECs are simple squamous epithelial cells
that line the inner surface of blood vessels. Hypoxia is a potent
angiogenic stimulus during cardiac repair (Fig. 1). Hypoxia transcriptionally
upregulates angiogenic integrins in microvascular ECs and promotes
the migration and tube formation of HMEC-1 cells (14). Furthermore, hypoxia can induce a
large number of vascular-related signaling pathways in ECs to
upregulate and promote angiogenesis, such as hypoxia-inducible
factor (HIF)-1(15). HIF-1α binds
to the promoter of Twist1 to activate Twist1 transcription and
regulate endothelial-mesenchymal transition (16). It has been reported that
neutrophils and mast cells promote angiogenesis (17,18).
Furthermore, neutrophil extracellular traps produced by dead
neutrophils promote inflammatory angiogenesis in vivo and
in vitro (19). It has also
been shown that mast cells can release some pro-angiogenic
cytokines, such as PDGF and VEGF (20).
There are several molecules involved in
angiogenesis. Under the conditions of ischemia and hypoxia,
numerous cells secrete additional pro-angiogenic factors. For
instance, VEGF plays an important role in angiogenesis. VEGF is
upregulated by HIF-1α and regulates angiogenesis by binding to a
specific receptor, VEGFR, and soluble VEGFR1 (sVEGFR1) is secreted
out of the cell to participate in the sprouting process of new
blood vessels (21). HIF-1α and
VEGF are closely associated with Notch signaling (22). Notch and Notch ligand δ-like (Dll)4
signaling is related to angiogenesis (23). VEGFA activates the membrane-bound
ligand Dll4 of tip cells and transmits Dll4 signals to nearby ECs
(24). The angiogenin family and
hepatocyte growth factor (HGF) also contribute to angiogenesis.
HGF/Met induces the proliferation and migration of ECs via
Ras-related C3 botulinum toxin substrate 1 activation. In
fibroblasts, HGF/Met antagonizes the actions of TGF-β1 and
angiotensin II, thereby preventing fibrosis. HGF/Met also
influences the inflammatory response of macrophages and the immune
response of dendritic cells, indicating their protective function
against atherosclerotic and autoimmune diseases (25). In addition, recombinant protein has
been widely used as a molecule to promote angiogenesis. For
instance, the recombinant human PDGF antibody promotes the repair
of cardiac wounds after myocardial infarction by changing the
mechanical mechanism of infarction scarring, thus improving cardiac
function, reducing ventricular arrhythmia and improving survival
rate (26).
There are fewer anti-angiogenic than pro-angiogenic
factors, including cells, secreted factors and recombinant
proteins. M1-like macrophage-derived exosomes suppress angiogenesis
in a myocardial infarction microenvironment, which may be related
to microRNA (miRNA/miR)-155 in exosomes (27). Moreover, 11β-hydroxysteroid-1 in
macrophages can inhibit inflammatory angiogenesis (28). VEGF-A165b is an anti-angiogenic
factor that as has been identified as a regulator of
vascularization (29). In
addition, the anti-angiogenic pigment epithelium-derived factor
suppresses angiogenesis in the human heart by inhibiting
VEGF-induced sprouting (30).
Similarly, Ly6/Plaur domain-containing 1 is a novel antiangiogenic
factor derived from human cardiac fibroblasts, which suppresses EC
network formation (31).
Furthermore, Wnt/β-catenin signaling plays an important role in
angiogenesis. The transcription factor BTB and CNC homology 1
impairs angiogenesis after ischemic injury by suppressing
Wnt/β-catenin signaling (32). It
has also been shown that recombinant human IL-24 can suppress tumor
angiogenesis (33,34). However, to the best of our
knowledge, IL-24 has not been studied in the repair of myocardial
injury. IL-12 is also an anti-angiogenic factor that is mainly
produced by CD11b(+) monocytes in mice after MI. In addition, IL-12
affects the formation of blood vessels in the yolk sac, which can
retard embryonic development (35).
MSCs are stromal cells that have self-renewal
ability and show multilineage differentiation. MSCs can be isolated
from a variety of tissues, such as umbilical cord, endometrial
polyps, menses blood, stem cells, bone marrow and adipose tissue
(36,37). Due to their powerful function and
easy access, MSCs have been widely used by researchers in recent
years, especially in the study of ischemic heart disease (38). For example, placenta-derived MSCs
can be used to promote therapeutic angiogenesis. Such MSCs can
differentiate into vascular-like cells and secrete some provascular
factors to promote angiogenesis. These cytokines include VEGF,
basic fibroblast growth factor, IL-6, IL-8, HGF, insulin-like
growth factor (IGF) binding protein (IGFBP)2, IGFBP3 and IGFBP6.
These factors generate blood vessels by activating key
provascular-related signaling pathways (39).
MSCs from fat and bone marrow promote angiogenesis
via unique cytokines and protease expression mechanisms.
Adipose-derived stem cells promote utilization of the plasminogen
activator/plasmin axis by ECs as the primary means of vessel
invasion and elongation in fibrin (40). MMPs serve a purpose in regulating
capillary diameter and possibly in stabilizing the nascent vessels
(40). MMPs also play an important
role in the differentiation of stromal stem cells (41). MSCs can regulate expression of
MMP9(42). Therefore, MSCs and
MMPs may have important roles in angiogenesis after AMI. In
addition to the role of MSCs, it has been proposed that the
functional benefits observed after MSC transplantation in
experimental models of tissue injury may be associated with the
secretion of soluble factors acting in a paracrine fashion
(43,44). Moreover, stem cells also play an
important role in the repair of other tissues. MSCs derived from
the umbilical cord can relieve limb ischemia via the formation of
blood vessels in mice (45). It
has also been shown that human neural stem cells promote the
proliferation of endogenous neural stem cells and enhance
angiogenesis in the brain of ischemic rats (46).
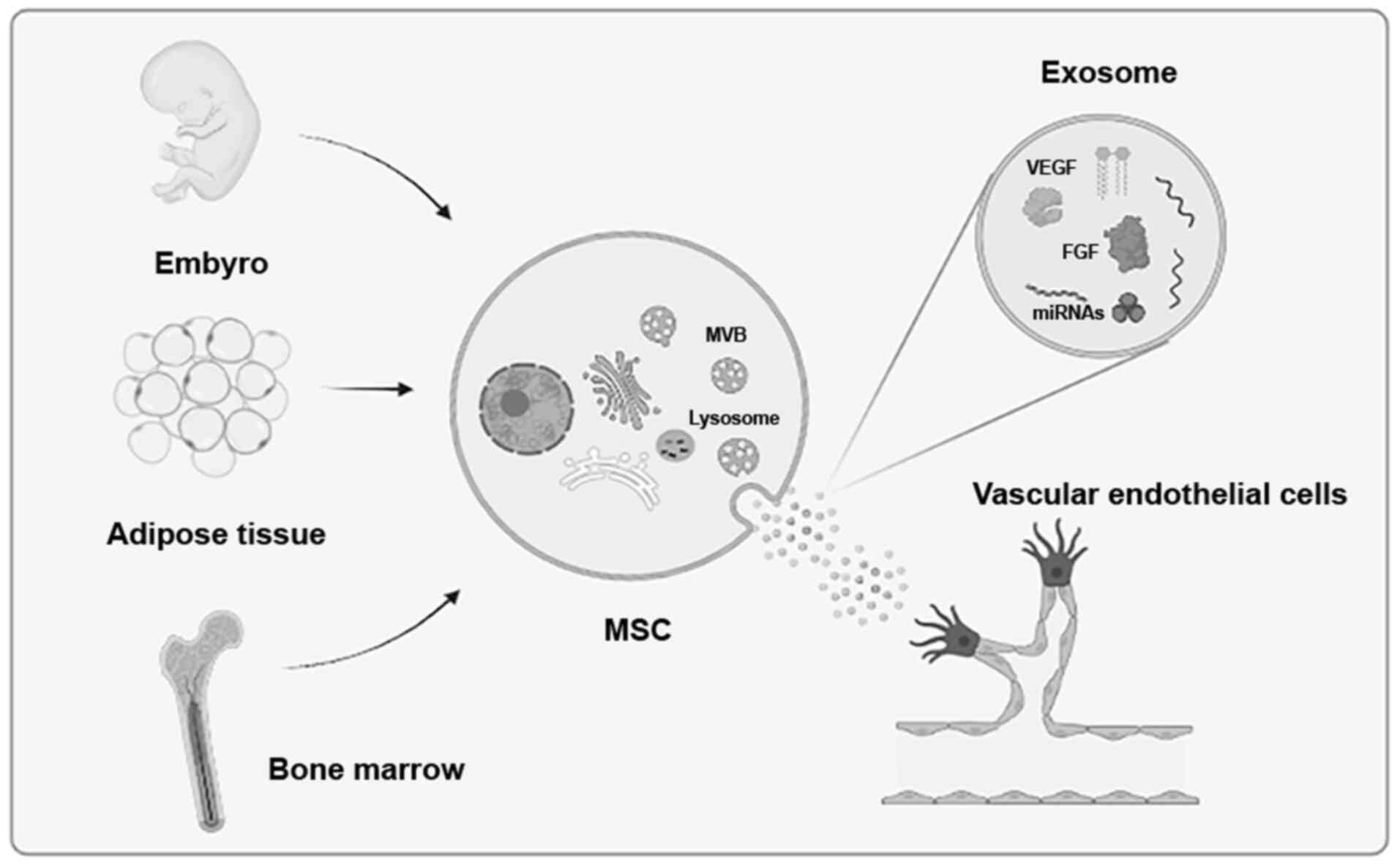
Exosomes are small extracellular vesicles that are
only 50-150 nm in diameter, surrounded by a lipid bilayer membrane
and contain components derived from their original cells (47). Exosomes have a relatively rich
source, existing in various tissues and cells throughout the body,
such as embryos, adipose tissue and bone marrow (Fig. 2).
The extraction methods for exosomes include
ultracentrifugation, immunoprecipitation, size-based isolation
techniques and commercial rapid extraction reagents. The most
widely used methods are ultracentrifugation and rapid extraction
reagents (48). These methods have
both advantages and disadvantages, and thus the appropriate method
to extract exosomes should be selected according to the research
needs. Another important point is the identification of exosomes,
and the quality of exosomes plays a crucial role in research
(49). As early as 2014, the
International Association of Extracellular Vesicles proposed that
the identification of exosomes can be divided into three levels:
Transmission electron microscopy (TEM), nanosight particle size and
protein markers (50). In general
identification, there must be ≥3 vesicle-positive protein makers,
including ≥1 transmembrane or lipid-binding protein and one
cytoplasmic protein, ≥1 vesicle-negative protein maker. The
identification of a single vesicle requires two different but
complementary methods, such as TEM or atomic force microscopy plus
nanoparticle tracking analysis (50).
Circular RNA (circRNA/circ) in exosomes also plays
an important role in tissue repair. Cardiomyocytes subjected to
hypoxia release circ_homeodomain-interacting protein kinase 3
(circHIPK3)-rich exosomes to regulate oxidative stress damage in
cardiac ECs, and circHIPK3 increases the expression of VEGFA by
inhibiting the activity of miR-29a, thereby promoting the
acceleration and proliferation of cardiac ECs (57). It has been confirmed that exosomal
circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates
oxidative damage in cardiac microvascular ECs via the miR-29a/IGF-1
pathway (58). circHIPK3
downregulates miR-421, resulting in increased Forkhead box (FOX)O3a
expression, thereby inhibiting apoptosis and inhibiting release of
IL-1 antioxidant protein and IL-18, ultimately repairing ischemic
injury (59).
Previous research has shown that exosomes from
patients with myocardial ischemia promote angiogenesis via the
miR-939/inducible nitric oxide (NO) synthase/NO pathway (60). There have been similar studies in
skin repair (61-63).
Moreover, it has been shown that exosomes from the serum of
patients with type 2 diabetes can hinder the process of injury
repair, and this effect is related to the miR-20b-5p and
Wnt9b/β-catenin pathways (64).
Some researchers have taken on a new approach and
focused on exosomes themselves. This approach can achieve targeted
repair of injury sites through exosome modification. It was found
that tissue inhibitor of metalloproteinase-2-modified human
umbilical cord MSC-derived exosomes enhanced the repair of
myocardial infarction in a rat model via the Akt/secreted frizzled
related protein 2 pathway (65).
Moreover, overexpression of HIF-1α in MSC-derived exosomes can
enhance angiogenesis after AMI (66). Stromal cell derived factor (SDF)-1
overexpression in MSC-derived exosomes inhibited autophagy of
ischemic myocardial cells and promoted microvascular production of
ECs (67).
At present, the application of engineered exosomes
in injury repair is still the mainstream direction. Exosomes
engineered by ischemic myocardium-targeting peptide (IMTP)
CSTSMLKAC can specifically target ischemic myocardium, and
MSC-derived-IMTP-exosomes exert enhanced therapeutic effects on AMI
(68). CSTSMLKAC is a new peptide
sequence that can preferentially target the ischemic area of the
heart (68). miRNA can also be
used to modify exosomes. miR-322-modified,
cardiac-progenitor-cell-derived exosomes provide protection against
MI via Nox2-dependent angiogenesis (69). Moreover, exosomes derived from
miR-146a-modified adipose-derived stem cells can downregulate early
growth response factor 1 to attenuate AMI-induced myocardial damage
(70).
In addition, with controlled-release properties,
drug-carrying nanoparticle (NP) systems are expected to enhance
cardiac protection in patients with cardiac ischemic events. NPs
can provide sustained and precise exposure of the infarcted area
through direct intramuscular or intravenous injection with drugs
with active targets (71). There
are also exosomes from MSCs modified with mononuclear cell mimics,
which have a high targeting efficiency for injured myocardium and
promote endothelial maturation during angiogenesis (72). In cardiac repair, NPs mainly play a
role by modifying exosomes and acting as nano drug delivery systems
(73).
Some novel ideas and methods have emerged from
research into repair of other organs. By constructing a CD9-HuR
protein, a new exosome was designed, which has a strong ability to
enrich specific RNAs, effectively delivering RNA into cells,
targeting genes in vivo and in vitro, and treating
liver disease in a mouse model (74). Researchers from Switzerland have
reported a series of synthetic biology-inspired control devices
that are known as EXOsomal Transfer Into Cells devices, which serve
to enhance these steps, enabling efficient exosomal mRNA delivery
without the need to concentrate exosomes. This design of exosomes
reduces the neurotoxicity and neuroinflammation of Parkinson's
disease via the delivery of therapeutic catalase mRNA (75).
In recent years, additional research has focused on
repair by using biomaterials (76). Compared with traditional cell
therapy, biomaterials have more advantages, such as being
degradable, easy to obtain and free to regulate the repair process.
Biomaterials are mainly divided into natural and artificial
synthetic materials. Natural materials mainly refer to the various
components of mammalian ECM, such as collagen, fibrinogen, Matrigel
and gelatin. Natural materials also include some ingredients
extracted from plants or animals, such as chitosan and cellulose.
Artificial synthetic materials are easier to obtain and are more
plastic (77).
In addition to using biomaterials to generate new
blood vessels, artificial blood vessels have already been
constructed to treat injuries. The raw materials for manufacturing
artificial blood vessels are polyester, polytetrafluoroethylene,
polyurethane and natural mulberry silk. Artificial blood vessels
supported by spider silk can be constructed in vitro
(87). Moreover, small-diameter
artificial blood vessels can promote in situ
endothelialization (88).
Remarkably, artificial blood vessels are rarely used in cardiac
injury. Therefore, small-diameter artificial blood vessels are
expected to be applied in cardiac repair in the future.
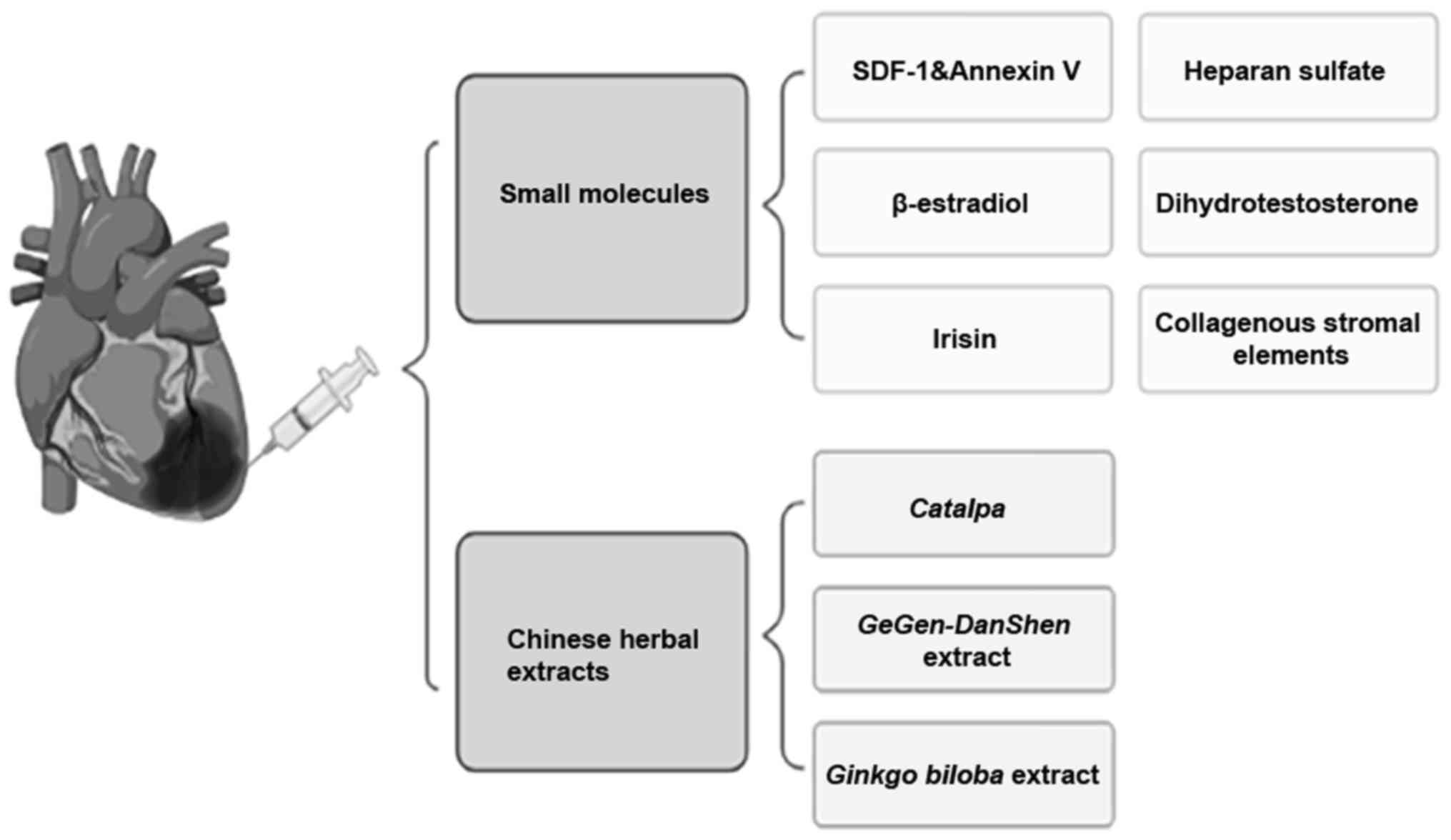
Some biological factors play a role in the repair of
AMI, and an increasing number been applied in recent years
(89). Biological factors are
partly obtained from artificial synthesis. SDF-1 is a distinctive
cytokine that can protect the heart from ischemic injury. Annexin V
can accurately detect dead cells in the body. SDF-1 and Annexin V
in combination can reduce apoptosis, increase angiogenesis, reduce
infarcted area and improve heart function in mice after AMI
(90).
Some small-molecule hormones also play an important
role in injury repair. For instance, β-estradiol promotes recovery
after AMI by enhancing the homing and angiogenesis of
bone-marrow-derived EPCs by enhancing estrogen receptor/SDF-1/C-X-C
motif chemokine receptor 4 crosstalk (91). Hormones also promote myocardial
repair after infarction. Dihydrotestosterone induces angiogenic
factors and helps to nest MSCs into heart tissue (92). Furthermore, irisin plays an anti-MI
role by promoting angiogenesis (93).
A number of other small molecules have restorative
and therapeutic effects. New collagenous stromal elements reduce
left ventricular dilatation after MI by promoting scar formation
and angiogenesis (94). Enhanced
extracellular sulfatase with heparan sulfate enhances the
bioavailability of VEGF during ischemic heart repair, thereby
promoting angiogenesis (95).
circRNAs are also involved in myocardial repair. After AMI,
adeno-associated-virus-9-mediated circ_fibronectin type III domain
containing 3B overexpression in the heart can reduce myocardial
apoptosis, enhance the formation of new blood vessels and improve
left ventricular function (96).
Some Chinese herbal extracts also play an important
role in injury repair. For example, Catalpa extracted from
traditional Chinese medicine has been proven to promote
angiogenesis and VEGF expression in ischemic myocardium (97). Chinese medicine
GeGen-DanShen extract protects against myocardial ischemic
injury by promoting angiogenesis via the upregulation of the
VEGF/VEGFR2 signaling pathway (98). Moreover, the Ginkgo biloba
extract can suppress the inflammation- and apoptosis-regulating p38
MAPKs, NF-κB and Bcl-2 signaling pathways to prevent AMI (Fig. 4) (99).
Gene therapy is an emerging technology. Elabela
(ELA) is a newly discovered hormone peptide containing 32 amino
acids, which is known to regulate endodermal differentiation and
cardiovascular development (100). Jin et al (101) successfully constructed a
p-adeno-associated virus-3 x Flag/ELA-32 fusion expression plasmid
using molecular cloning technology. Their results showed that this
fusion plasmid can promote angiogenesis after MI (101). Gene and stem cell therapies also
hold promise for the treatment of ischemic cardiovascular disease.
The combination of human cord blood CD34(+) cells and Ang1 and VEGF
genes promotes angiogenesis and reduces infarct size (102). Genetic engineering technology is
also widely used to modify cells. FOXO transcription factors can
modulate endothelial gene expression and function (103). Human vascular ECs can be
functionally enhanced by engineering them to express an activated
form of FOXO3(104). Heme
oxygenase (HO)-1 is a cytoprotective, pro-angiogenic and
anti-inflammatory enzyme. Human placental MSCs modified with HO-1
can promote placental angiogenesis by improving the balance of
angiogenic factors (105). In
ischemic skeletal muscle, human adipose-derived stromal cells
expressing VEGF165 can promote angiogenesis (106). Clustered regularly interspaced
short palindromic repeats (CRISPR)/Cas9 has become a powerful
technology to modify cells. Several studies have used CRISPR/Cas9
to edit cells to promote injury of tissues or organs. Deletion of
enhancers and long non-coding RNAs by CRISPR/Cas9 promotes
significant changes in VEGFA and VEGFC expression in ECs (107). Moreover, CRISPR/Cas9 gene therapy
based on TGF-β1 alleviates radiation-induced lung injury (108). Although CRISPR/Cas9 is rarely
used in cardiac repair, it can be expected in the future. The
application of MSCs, exosomes, biomaterials, biological factors and
gene therapy in angiogenesis for cardiac repair was summarized in
Table I.
Promoting angiogenesis in the infarcted area is the
key to the treatment of sequelae of AMI. Restoring the blood supply
to this area can effectively reduce the area of cardiac tissue
necrosis, which in turn can improve cardiac function and quality of
life (109). Although traditional
treatments such as PCI and CABG have numerous advantages, there are
also several complications that are difficult to overcome (110). The common complications of CABG
include postoperative bleeding and acute coronary artery occlusion,
which can lead to severe or even critical conditions (111).
By contrast, the new therapeutic methods mentioned
in this review have greater repair potential. MSCs have superior
therapeutic effects, low antigenicity, wide application and rich
sources. Furthermore, the safety of MSCs has been demonstrated in
clinical trials (112).
Nevertheless, some of the problems with treatment with MSCs also
need to be resolved. For example, MSCs are easily differentiated,
heterogeneous and diverse in large-scale production. Furthermore,
MSCs easily die when transplanted into the body and, in cases,
cannot adapt to the microenvironment (113). In response to these problems,
further research and development of new technologies and methods
are required to achieve precise and efficient repairs.
Exosomes have the advantages of being more stable,
capable of mass production, controllable and easy to store. Unlike
MSCs, exosomes can maintain biological activity for a long time
in vitro, thereby overcoming the short-term apoptotic
characteristics of MSCs. In addition, exosomes carry more
diversified information, which can be used for early diagnosis,
relapse monitoring, and drug resistance monitoring (114). Even so, numerous problems still
need to be resolved. For instance, the separation and purification
technology of exosomes is not yet mature, and there is no unified
standard for separation and analysis, to the best of our knowledge.
Otherwise, the identification of exosomes as disease markers still
depends on a large number of clinical trials (115).
Biological factors and gene therapy also play an
important role in cardiac repair. Biological factors are more
effective, but at the same time, they have the problems of short
half-life and easy inactivation. Therefore, new technology is
required to resolve these problems. After >10 years of
development, research on gene therapy has made significant
progress. However, it is still in the early stage of clinical
trials, and cannot guarantee stable efficacy and safety. Despite
the numerous obstacles, the trend towards gene therapy is
encouraging.
In view of all previous studies on angiogenesis in
AMI, it remains necessary to explore the therapeutic mechanisms
mediated by these novel methods, especially when the molecules that
play an effective role in the disease have not yet been identified.
Although MSCs and exosomes face several challenges in
industrialization, their functional research, diagnosis and
treatment applications in some diseases, such as AMI, have shown
potential. Furthermore, there is still much to explore in relation
to drug carriers and regenerative medicine and treatment, with
broad growth potential in the future.
Not applicable.
Funding: This review was supported by the National Natural
Science Foundation of China (grant no. 81800270).
Not applicable.
JL contributed to the design and conception of the
manuscript. YZ and WZ added contributions by revising and editing
the final manuscript. All authors have read and approved the final
version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mehta LS, Beckie TM, DeVon HA, Grines CL,
Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson
KE, et al: American Heart Association Cardiovascular Disease in
Women and Special Populations Committee of the Council on Clinical
Cardiology, Council on Epidemiology and Prevention, Council on
Cardiovascular and Stroke Nursing, and Council on Quality of Care
and Outcomes Research: Acute Myocardial Infarction in Women: A
Scientific Statement From the American Heart Association.
Circulation. 133:916–947. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frangogiannis NG: Cardiac fibrosis: Cell
biological mechanisms, molecular pathways and therapeutic
opportunities. Mol Aspects Med. 65:70–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mitsos S, Katsanos K, Koletsis E, Kagadis
GC, Anastasiou N, Diamantopoulos A, Karnabatidis D and Dougenis D:
Therapeutic angiogenesis for myocardial ischemia revisited: Basic
biological concepts and focus on latest clinical trials.
Angiogenesis. 15:1–22. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lorier G, Touriño C and Kalil RA: Coronary
angiogenesis as an endogenous response to myocardial ischemia in
adults. Arq Bras Cardiol. 97:e140–e148. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vandekeere S, Dewerchin M and Carmeliet P:
Angiogenesis Revisited: An Overlooked Role of Endothelial Cell
Metabolism in Vessel Sprouting. Microcirculation. 22:509–517.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weinstein N, Mendoza L, Gitler I and Klapp
J: A network model to explore the effect of the micro-environment
on endothelial cell behavior during angiogenesis. Front Physiol.
8(960)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Frangogiannis NG: The extracellular matrix
in myocardial injury, repair, and remodeling. J Clin Invest.
127:1600–1612. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Frangogiannis NG: Pathophysiology of
myocardial infarction. Compr Physiol. 5:1841–1875. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ferraro B, Leoni G, Hinkel R, Ormanns S,
Paulin N, Ortega-Gomez A, Viola JR, de Jong R, Bongiovanni D,
Bozoglu T, et al: Pro-angiogenic macrophage phenotype to promote
myocardial repair. J Am Coll Cardiol. 73:2990–3002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang N, Liu C, Wang X, He T, Li L, Liang
X, Wang L, Song L, Wei Y, Wu Q, et al: Hyaluronic acid
oligosaccharides improve myocardial function reconstruction and
angiogenesis against myocardial infarction by regulation of
macrophages. Theranostics. 9:1980–1992. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saraswati S, Marrow SMW, Watch LA and
Young PP: Identification of a pro-angiogenic functional role for
FSP1-positive fibroblast subtype in wound healing. Nat Commun.
10(3027)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mouton AJ, Ma Y, Rivera Gonzalez OJ,
Daseke MJ II, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY
and Lindsey ML: Fibroblast polarization over the myocardial
infarction time continuum shifts roles from inflammation to
angiogenesis. Basic Res Cardiol. 114(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Befani C and Liakos P: Hypoxia upregulates
integrin gene expression in microvascular endothelial cells and
promotes their migration and capillary-like tube formation. Cell
Biol Int. 41:769–778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bartoszewski R, Moszyńska A, Serocki M,
Cabaj A, Polten A, Ochocka R, Dell'Italia L, Bartoszewska S,
Króliczewski J, Dąbrowski M, et al: Primary endothelial
cell-specific regulation of hypoxia-inducible factor (HIF)-1 and
HIF-2 and their target gene expression profiles during hypoxia.
FASEB J. 33:7929–7941. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang B, Niu W, Dong HY, Liu ML, Luo Y and
Li ZC: Hypoxia induces endothelial mesenchymal transition in
pulmonary vascular remodeling. Int J Mol Med. 42:270–278.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ribatti D, Tamma R and Vacca A: Mast cells
and angiogenesis in human plasma cell malignancies. Int J Mol Sci.
20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fetz AE, Radic MZ and Bowlin GL:
Neutrophils in biomaterial-guided tissue regeneration: Matrix
reprogramming for angiogenesis. Tissue Eng Part B Rev. 27:95–106.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aldabbous L, Abdul-Salam V, McKinnon T,
Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C,
Wilkins MR, Toshner M, et al: Neutrophil extracellular traps
promote angiogenesis: Evidence from vascular pathology in pulmonary
hypertension. Arterioscler Thromb Vasc Biol. 36:2078–2087.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mukai K, Tsai M, Saito H and Galli SJ:
Mast cells as sources of cytokines, chemokines, and growth factors.
Immunol Rev. 282:121–150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nishida Y, Yamada Y, Kanemaru H, Ohazama
A, Maeda T and Seo K: Vascularization via activation of VEGF-VEGFR
signaling is essential for peripheral nerve regeneration. Biomed
Res. 39:287–294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Zhao B, Zhu Y, Zhao H and Ma C:
HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint
osteoarthritis. Am J Transl Res. 11:2969–2982. 2019.PubMed/NCBI
|
|
23
|
Pitulescu ME, Schmidt I, Giaimo BD,
Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D,
Rocha SF, et al: Dll4 and Notch signalling couples sprouting
angiogenesis and artery formation. Nat Cell Biol. 19:915–927.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Kume T: Ligand-dependent Notch signaling
in vascular formation. Adv Exp Med Biol. 727:210–222.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gallo S, Sala V, Gatti S and Crepaldi T:
Cellular and molecular mechanisms of HGF/Met in the cardiovascular
system. Clin Sci (Lond). 129:1173–1193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thavapalachandran S, Grieve SM, Hume RD,
Le TY, Raguram K, Hudson JE, Pouliopoulos J, Figtree GA, Dye RP,
Barry AM, et al: Platelet-derived growth factor-AB improves scar
mechanics and vascularity after myocardial infarction. Sci Transl
Med. 12(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang
W, Zhong Y, Chen X, Chen Y, Sabri A, et al: M1-like
macrophage-derived exosomes suppress angiogenesis and exacerbate
cardiac dysfunction in a myocardial infarction microenvironment.
Basic Res Cardiol. 115(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Z, Coutinho AE, Man TY, Kipari TM,
Hadoke PW, Salter DM, Seckl JR and Chapman KE: Macrophage 11β-HSD-1
deficiency promotes inflammatory angiogenesis. J Endocrinol.
234:291–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hueso L, Rios-Navarro C, Ruiz-Sauri A,
Chorro FJ, Nunez J, Sanz MJ, Bodi V and Piqueras L: Dynamics and
implications of circulating anti-angiogenic VEGF-A165b isoform in
patients with ST-elevation myocardial infarction. Sci Rep.
7(9962)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rychli K, Kaun C, Hohensinner PJ, Dorfner
AJ, Pfaffenberger S, Niessner A, Bauer M, Dietl W, Podesser BK,
Maurer G, et al: The anti-angiogenic factor PEDF is present in the
human heart and is regulated by anoxia in cardiac myocytes and
fibroblasts. J Cell Mol Med. 14:198–205. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sakamoto S, Matsuura K, Masuda S, Hagiwara
N and Shimizu T: Heart-derived fibroblasts express LYPD-1 and
negatively regulate angiogenesis in rat. Regen Ther. 15:27–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang L, Jia M, Wei X, Guo J, Hao S, Mei
A, Zhi X, Wang X, Li Q, Jin J, et al: Bach1-induced suppression of
angiogenesis is dependent on the BTB domain. EBioMedicine.
51(102617)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie Y, Sheng W, Xiang J, Ye Z, Zhu Y, Chen
X and Yang J: Recombinant human IL-24 suppresses lung carcinoma
cell growth via induction of cell apoptosis and inhibition of tumor
angiogenesis. Cancer Biother Radiopharm. 23:310–320.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Z, Lv J and Zhang T: Combination of
IL-24 and cisplatin inhibits angiogenesis and lymphangiogenesis of
cervical cancer xenografts in a nude mouse model by inhibiting
VEGF, VEGF-C and PDGF-B. Oncol Rep. 33:2468–2476. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nisari M, Ulger H, Unur E, Karaca O and
Ertekin T: Effect of interleukin 12 (IL-12) on embryonic
development and yolk sac vascularisation. Bratisl Lek Listy.
115:532–537. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Konoplyannikov M, Kotova S, Baklaushev V,
Konoplyannikov A, Kalsin V, Timashev P and Troitskiy A: Mesenchymal
stem cell therapy for ischemic heart disease: Advances and
challenges. Curr Pharm Des. 24:3132–3142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mathew SA, Naik C, Cahill PA and Bhonde
RR: Placental mesenchymal stromal cells as an alternative tool for
therapeutic angiogenesis. Cell Mol Life Sci. 77:253–265.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kachgal S and Putnam AJ: Mesenchymal stem
cells from adipose and bone marrow promote angiogenesis via
distinct cytokine and protease expression mechanisms. Angiogenesis.
14:47–59. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Assis-Ribas T, Forni MF, Winnischofer SM,
Sogayar MC and Trombetta-Lima M: Extracellular matrix dynamics
during mesenchymal stem cells differentiation. Dev Biol. 437:63–74.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang W, Wang T, Zhang D, Zhao T, Dai B,
Ashraf A, Wang X, Xu M, Millard RW, Fan GC, et al: Mesenchymal stem
cells overexpressing CX7CR4 attenuate remodeling of postmyocardial
infarction by releasing matrix metalloproteinase-9. Stem Cells Dev.
21:778–789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gnecchi M, Danieli P, Malpasso G and
Ciuffreda MC: Paracrine mechanisms of mesenchymal stem cells in
tissue repair. Methods Mol Biol. 1416:123–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gunawardena TNA, Rahman MT, Abdullah BJJ
and Abu Kasim NH: Conditioned media derived from mesenchymal stem
cell cultures: The next generation for regenerative medicine. J
Tissue Eng Regen Med. 13:569–586. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Z, Zheng L, Lian C, Qi Y, Li W and
Wang S: Human umbilical cord-derived mesenchymal stem cells relieve
hind limb ischemia by promoting angiogenesis in mice. Stem Cells
Dev. 28:1384–1397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ryu S, Lee SH, Kim SU and Yoon BW: Human
neural stem cells promote proliferation of endogenous neural stem
cells and enhance angiogenesis in ischemic rat brain. Neural Regen
Res. 11:298–304. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Davidson SM and Yellon DM: Exosomes and
cardioprotection - A critical analysis. Mol Aspects Med.
60:104–114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and
Li P: Exosome: A review of its classification, isolation
techniques, storage, diagnostic and targeted Therapy applications.
Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Koritzinsky EH, Street JM, Star RA and
Yuen PS: Quantification of exosomes. J Cell Physiol. 232:1587–1590.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Witwer KW, Soekmadji C, Hill AF, Wauben
MH, Buzás EI, Di Vizio D, Falcon-Perez JM, Gardiner C, Hochberg F,
Kurochkin IV, et al: Updating the MISEV minimal requirements for
extracellular vesicle studies: Building bridges to reproducibility.
J Extracell Vesicles. 6(1396823)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kawamoto A and Losordo DW: Endothelial
progenitor cells for cardiovascular regeneration. Trends Cardiovasc
Med. 18:33–37. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zeng CY, Xu J, Liu X and Lu YQ:
Cardioprotective roles of endothelial progenitor cell-derived
exosomes. Front Cardiovasc Med. 8(717536)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pan MC, Lin XY, Wang H, Chen YF and Leng
M: Research advances on the roles of exosomes derived from vascular
endothelial progenitor cells in wound repair. Zhonghua Shao Shang
Za Zhi Zhonghua Shao Shang Za Zhi. 36:883–886. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
54
|
Xing Z, Zhao C, Liu H and Fan Y:
Endothelial progenitor cell-derived extracellular vesicles: A novel
candidate for regenerative medicine and disease treatment. Adv
Healthc Mater. 9(e2000255)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ke X, Yang D, Liang J, Wang X, Wu S, Wang
X and Hu C: Human endothelial progenitor cell-derived exosomes
increase proliferation and angiogenesis in cardiac fibroblasts by
promoting the mesenchymal-endothelial transition and reducing high
mobility group box 1 protein B1 expression. DNA Cell Biol.
36:1018–1028. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang J, Liu H, Chen S, Zhang W, Chen Y and
Yang Y: Moderate exercise has beneficial effects on mouse ischemic
stroke by enhancing the functions of circulating endothelial
progenitor cell-derived exosomes. Exp Neurol.
330(113325)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang Y, Zhao R, Shen C, Liu W, Yuan J, Li
C, Deng W, Wang Z, Zhang W, Ge J, et al: Exosomal CircHIPK3
released from hypoxia-induced cardiomyocytes regulates cardiac
angiogenesis after myocardial infarction. Oxid Med Cell Longev.
2020(8418407)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang Y, Zhao R, Liu W, Wang Z, Rong J,
Long X, Liu Z, Ge J and Shi B: Exosomal circHIPK3 released from
hypoxia-pretreated cardiomyocytes regulates oxidative damage in
cardiac microvascular endothelial cells via the miR-29a/IGF-1
pathway. Oxid Med Cell Longev. 2019(7954657)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yan B, Zhang Y, Liang C, Liu B, Ding F,
Wang Y, Zhu B, Zhao R, Yu XY and Li Y: Stem cell-derived exosomes
prevent pyroptosis and repair ischemic muscle injury through a
novel exosome/circHIPK3/ FOXO3a pathway. Theranostics.
10:6728–6742. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Li H, Liao Y, Gao L, Zhuang T, Huang Z,
Zhu H and Ge J: Coronary serum exosomes derived from patients with
myocardial ischemia regulate angiogenesis through the
miR-939-mediated nitric oxide signaling pathway. Theranostics.
8:2079–2093. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Xu J, Bai S, Cao Y, Liu L, Fang Y, Du J,
Luo L, Chen M, Shen B and Zhang Q: miRNA-221-3p in endothelial
progenitor cell-derived exosomes accelerates skin wound healing in
diabetic mice. Diabetes Metab Syndr Obes. 13:1259–1270.
2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen K, Yu T and Wang X: Inhibition of
circulating exosomal miRNA-20b-5p accelerates diabetic wound
repair. Int J Nanomedicine. 16:371–381. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ren S, Chen J, Duscher D, Liu Y, Guo G,
Kang Y, Xiong H, Zhan P, Wang Y, Wang C, et al: Microvesicles from
human adipose stem cells promote wound healing by optimizing
cellular functions via AKT and ERK signaling pathways. Stem Cell
Res Ther. 10(47)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xiong Y, Chen L, Yan C, Zhou W, Endo Y,
Liu J, Hu L, Hu Y, Mi B and Liu G: Circulating Exosomal miR-20b-5p
inhibition restores Wnt9b signaling and reverses
diabetes-associated impaired wound healing. Small.
16(e1904044)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ni J, Liu X, Yin Y, Zhang P, Xu YW and Liu
Z: Exosomes derived from TIMP2-modified human umbilical cord
mesenchymal stem cells enhance the repair effect in rat model with
myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med
Cell Longev. 2019(1958941)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu
X, Yang Z and Shen Z: HIF-1α overexpression in mesenchymal stem
cell-derived exosomes mediates cardioprotection in myocardial
infarction by enhanced angiogenesis. Stem Cell Res Ther.
11(373)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gong XH, Liu H, Wang SJ, Liang SW and Wang
GG: Exosomes derived from SDF1-overexpressing mesenchymal stem
cells inhibit ischemic myocardial cell apoptosis and promote
cardiac endothelial microvascular regeneration in mice with
myocardial infarction. J Cell Physiol. 234:13878–13893.
2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun
J, Yang Z, Chen Y, Li J, Ma T, et al: Engineered exosomes with
ischemic myocardium-targeting peptide for targeted therapy in
myocardial infarction. J Am Heart Assoc. 7(e008737)2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Youn SW, Li Y, Kim YM, Sudhahar V,
Abdelsaid K, Kim HW, Liu Y, Fulton DJ, Ashraf M, Tang Y, et al:
Modification of cardiac progenitor cell-derived exosomes by miR-322
provides protection against myocardial infarction through
Nox2-dependent angiogenesis. Antioxidants (Basel).
8(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pan J, Alimujiang M, Chen Q, Shi H and Luo
X: Exosomes derived from miR-146a-modified adipose-derived stem
cells attenuate acute myocardial infarction-induced myocardial
damage via downregulation of early growth response factor 1. J Cell
Biochem. 120:4433–4443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Fan C, Joshi J, Li F, Xu B, Khan M, Yang J
and Zhu W: Nanoparticle-mediated drug delivery for treatment of
ischemic heart disease. Front Bioeng Biotechnol.
8(687)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhang N, Song Y, Huang Z, Chen J, Tan H,
Yang H, Fan M, Li Q, Wang Q, Gao J, et al: Monocyte mimics improve
mesenchymal stem cell-derived extracellular vesicle homing in a
mouse MI/RI model. Biomaterials. 255(120168)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ho YT, Poinard B and Kah JC: Nanoparticle
drug delivery systems and their use in cardiac tissue therapy.
Nanomedicine (Lond). 11:693–714. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R,
Sun W, Duan Y, Yang G and Yuan L: In vitro and in vivo RNA
inhibition by CD9-HuR functionalized exosomes encapsulated with
miRNA or CRISPR/dCas9. Nano Lett. 19:19–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kojima R, Bojar D, Rizzi G, Hamri GC,
El-Baba MD, Saxena P, Ausländer S, Tan KR and Fussenegger M:
Designer exosomes produced by implanted cells intracerebrally
deliver therapeutic cargo for Parkinson's disease treatment. Nat
Commun. 9(1305)2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Xiang Gu G, Su I, Sharma S, Voros JL, Qin
Z and Buehler MJ: Three-dimensional-printing of bio-inspired
composites. J Biomech Eng. 138(021006)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Chattopadhyay S and Raines RT: Review
collagen-based biomaterials for wound healing. Biopolymers.
101:821–833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Smagul S, Kim Y, Smagulova A, Raziyeva K,
Nurkesh A and Saparov A: Biomaterials loaded with growth
factors/cytokines and stem cells for cardiac tissue regeneration.
Int J Mol Sci. 21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Oduk Y, Zhu W, Kannappan R, Zhao M,
Borovjagin AV, Oparil S and Zhang JJ: VEGF nanoparticles repair the
heart after myocardial infarction. Am J Physiol Heart Circ Physiol.
314:H278–H284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Liu Y, Li P, Qiao C, Wu T, Sun X, Wen M
and Zhang W: Chitosan hydrogel enhances the therapeutic efficacy of
bone marrow-derived mesenchymal stem cells for myocardial
infarction by alleviating vascular endothelial cell pyroptosis. J
Cardiovasc Pharmacol. 75:75–83. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Yuan Z, Tsou YH, Zhang XQ, Huang S, Yang
Y, Gao M, Ho W, Zhao Q, Ye X and Xu X: Injectable citrate-based
hydrogel as an angiogenic biomaterial improves cardiac repair after
myocardial infarction. ACS Appl Mater Interfaces. 11:38429–38439.
2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Song C, Zhang X, Wang L, Wen F, Xu K,
Xiong W, Li C, Li B, Wang Q, Xing MM, et al: An injectable
conductive three-dimensional elastic network by tangled
surgical-suture spring for heart repair. ACS Nano. 13:14122–14137.
2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chachques JC, Lila N, Soler-Botija C,
Martinez-Ramos C, Valles A, Autret G, Perier MC, Mirochnik N,
Monleon-Pradas M, Bayes-Genis A, et al: Elastomeric cardiopatch
scaffold for myocardial repair and ventricular support. Eur J
Cardiothorac Surg. 57:545–555. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang X, Wang L, Wu Q, Bao F, Yang H, Qiu X
and Chang J: Chitosan/calcium silicate cardiac patch stimulates
cardiomyocyte activity and myocardial performance after infarction
by synergistic effect of bioactive ions and aligned nanostructure.
ACS Appl Mater Interfaces. 11:1449–1468. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sondermeijer HP, Witkowski P, Seki T, van
der Laarse A, Itescu S and Hardy MA: RGDfK-peptide modified
alginate scaffold for cell transplantation and cardiac
neovascularization. Tissue Eng Part A. 24:740–751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Nasser M, Wu Y, Danaoui Y and Ghosh G:
Engineering microenvironments towards harnessing pro-angiogenic
potential of mesenchymal stem cells. Mater Sci Eng C. 102:75–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Dastagir K, Dastagir N, Limbourg A,
Reimers K, Strauss S and Vogt PM: In vitro construction of
artificial blood vessels using spider silk as a supporting matrix.
J Mech Behav Biomed Mater. 101(103436)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Guo HF, Dai WW, Qian DH, Qin ZX, Lei Y,
Hou XY and Wen C: A simply prepared small-diameter artificial blood
vessel that promotes in situ endothelialization. Acta Biomater.
54:107–116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yifa O, Weisinger K, Bassat E, Li H, Kain
D, Barr H, Kozer N, Genzelinakh A, Rajchman D, Eigler T, et al: The
small molecule Chicago Sky Blue promotes heart repair following
myocardial infarction in mice. JCI Insight. 4(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Huang FY, Xia TL, Li JL, Li CM, Zhao ZG,
Lei WH, Chen L, Liao YB, Xiao D, Peng Y, et al: The bifunctional
SDF-1-AnxA5 fusion protein protects cardiac function after
myocardial infarction. J Cell Mol Med. 23:7673–7684.
2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Yuan Z, Kang L, Wang Z, Chen A, Zhao Q and
Li H: 17β-estradiol promotes recovery after myocardial infarction
by enhancing homing and angiogenic capacity of bone marrow-derived
endothelial progenitor cells through ERα-SDF-1/CXCR4 crosstalking.
Acta Biochim Biophys Sin (Shanghai). 50:1247–1256. 2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Popa MA, Mihai MC, Constantin A, Şuică V,
Ţucureanu C, Costache R, Antohe F, Dubey RK and Simionescu M:
Dihydrotestosterone induces pro-angiogenic factors and assists
homing of MSC into the cardiac tissue. J Mol Endocrinol. 60:1–15.
2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Liao Q, Qu S, Tang LX, Li LP, He DF, Zeng
CY and Wang WE: Irisin exerts a therapeutic effect against
myocardial infarction via promoting angiogenesis. Acta Pharmacol
Sin. 40:1314–1321. 2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lindsey ML, Iyer RP, Zamilpa R,
Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA,
Bratton D, Flynn ER, et al: A novel collagen matricryptin reduces
left ventricular dilation post-myocardial infarction by promoting
scar formation and angiogenesis. J Am Coll Cardiol. 66:1364–1374.
2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Korf-Klingebiel M, Reboll MR, Grote K,
Schleiner H, Wang Y, Wu X, Klede S, Mikhed Y, Bauersachs J,
Klintschar M, et al: Heparan sulfate-editing extracellular
sulfatases enhance vegf bioavailability for ischemic heart repair.
Circ Res. 125:787–801. 2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun.
10(4317)2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ju X, Xue D, Wang T, Ge B, Zhang Y and Li
Z: Catalpol promotes the survival and VEGF secretion of bone
marrow-derived stem cells and their role in myocardial repair after
myocardial infarction in rats. Cardiovasc Toxicol. 18:471–481.
2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Zhai S, Zhang XF, Lu F, Chen WG, He X,
Zhang CF, Wang CZ and Yuan CS: Chinese medicine GeGen-DanShen
extract protects from myocardial ischemic injury through promoting
angiogenesis via up-regulation of VEGF/VEGFR2 signaling pathway. J
Ethnopharmacol. 267(113475)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Li Y, Zhang Y, Wen M, Zhang J, Zhao X,
Zhao Y and Deng J: Ginkgo biloba extract prevents acute
myocardial infarction and suppresses the inflammation and apoptosis
regulating p38 mitogen activated protein kinases, nuclear factor-κB
and B cell lymphoma 2 signaling pathways. Mol Med Rep.
16:3657–3663. 2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Ho L, van Dijk M, Chye STJ, Messerschmidt
DM, Chng SC, Ong S, Yi LK, Boussata S, Goh GH, Afink GB, et al:
ELABELA deficiency promotes preeclampsia and cardiovascular
malformations in mice. Science. 357:707–713. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Jin L, Pan Y, Li Q, Li J and Wang Z:
Elabela gene therapy promotes angiogenesis after myocardial
infarction. J Cell Mol Med. 25:8537–8545. 2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Chen HK, Hung HF, Shyu KG, Wang BW, Sheu
JR, Liang YJ, Chang CC and Kuan P: Combined cord blood stem cells
and gene therapy enhances angiogenesis and improves cardiac
performance in mouse after acute myocardial infarction. Eur J Clin
Invest. 35:677–686. 2005.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Czymai T, Viemann D, Sticht C, Molema G,
Goebeler M and Schmidt M: FOXO3 modulates endothelial gene
expression and function by classical and alternative mechanisms. J
Biol Chem. 285:10163–10178. 2010.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Yan P, Li Q, Wang L, Lu P, Suzuki K, Liu
Z, Lei J, Li W, He X, Wang S, et al: FOXO3-engineered human
ESC-derived vascular cells promote vascular protection and
regeneration. Cell Stem Cell. 24:447–461.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Wu D, Liu Y, Liu X, Liu W, Shi H, Zhang Y,
Zou L and Zhao Y: Heme oxygenase-1 gene modified human placental
mesenchymal stem cells promote placental angiogenesis and spiral
artery remodeling by improving the balance of angiogenic factors in
vitro. Placenta. 99:70–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Shevchenko EK, Makarevich PI, Tsokolaeva
ZI, Boldyreva MA, Sysoeva VY, Tkachuk VA and Parfyonova YV:
Transplantation of modified human adipose derived stromal cells
expressing VEGF165 results in more efficient angiogenic response in
ischemic skeletal muscle. J Transl Med. 11(138)2013.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Mushimiyimana I, Tomas Bosch V, Niskanen
H, Downes NL, Moreau PR, Hartigan K, Ylä-Herttuala S, Laham-Karam N
and Kaikkonen MU: Genomic landscapes of noncoding RNAs regulating
VEGFA and VEGFC expression in endothelial cells. Mol Cell Biol.
41(e0059420)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Zhen S, Qiang R, Lu J, Tuo X, Yang X and
Li X: TGF-β1-based CRISPR/Cas9 gene therapy attenuate
radiation-induced lung injury. Curr Gene Ther: Dec 29, 2020 (Epub
ahead of print). doi: 10.2174/1566523220666201230100523.
|
|
109
|
van der Laan AM, Piek JJ and van Royen N:
Targeting angiogenesis to restore the microcirculation after
reperfused MI. Nat Rev Cardiol. 6:515–523. 2009.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Tarantini G, Ramondo A, Napodano M,
Favaretto E, Gardin A, Bilato C, Nesseris G, Tarzia V, Cademartiri
F, Gerosa G, et al: PCI versus CABG for multivessel coronary
disease in diabetics. Catheter Cardiovasc Interv. 73:50–58.
2009.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Montrief T, Koyfman A and Long B: Coronary
artery bypass graft surgery complications: A review for emergency
clinicians. Am J Emerg Med. 36:2289–2297. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Wang L, Huang S, Li S, Li M, Shi J, Bai W,
Wang Q, Zheng L and Liu Y: Efficacy and safety of umbilical cord
mesenchymal stem cell therapy for rheumatoid arthritis patients: A
prospective phase I/II study. Drug Des Devel Ther. 13:4331–4340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Watanabe Y, Tsuchiya A and Terai S: The
development of mesenchymal stem cell therapy in the present, and
the perspective of cell-free therapy in the future. Clin Mol
Hepatol. 27:70–80. 2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Yu B, Zhang X and Li X: Exosomes derived
from mesenchymal stem cells. Int J Mol Sci. 15:4142–4157.
2014.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Yamashita T, Takahashi Y and Takakura Y:
Possibility of exosome-based therapeutics and challenges in
production of exosomes eligible for therapeutic application. Biol
Pharm Bull. 41:835–842. 2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
He X, Wang Q, Zhao Y, Zhang H, Wang B, Pan
J, Li J, Yu H, Wang L, Dai J, et al: Effect of intramyocardial
grafting collagen scaffold with mesenchymal stromal cells in
patients with chronic ischemic heart disease: A randomized clinical
trial. JAMA Netw Open. 3(e2016236)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Topaloğlu Demir F, Özkök Akbulut T, Kıvanç
Altunay İ, Aytekin S, Oğuz Topal İ, Kara Polat A, Özkur E and
Karadağ AS: Evaluation of the adverse effects of biological agents
used in the treatment of psoriasis: A multicenter retrospective
cohort study. Dermatol Ther. 33(e14216)2020.PubMed/NCBI View Article : Google Scholar
|