Introduction
Pirarubicin (THP) is a doxorubicin analog that has
gradually replaced doxorubicin in clinical practice (1). The antitumor effects of THP are more
favorable than those of doxorubicin, with lower toxicity and fewer
side effects (2). However,
cardiotoxicity remains an issue (3). Different levels of cardiac damage
have been reported in early THP treatment stages, and its long-term
use may lead to irreversible cardiac damage and increased
cardiovascular end events, thereby limiting its clinical
applications (4,5). Patients with tumors are often
required to reduce or even stop THP therapy due to cardiac
intolerance (6). Previous studies
have shown that oxidative stress injury was implicated in
THP-induced cardiac injury and was the initial step in
cardiomyocyte apoptosis and necrosis, with effects including a
reduced Bcl-2/Bax ratio and activation of the caspase family
(7,8). Other studies reported that reducing
or even reversing oxidative stress injury helped prevent and treat
THP-induced cardiac injury (8,9).
Schisandrin B (SchB), which is derived from
Schisandra chinensis, has very high biphenylcyclooctene
lignin levels and has been clinically shown to improve antioxidant
capacity levels and promote cell mitochondrial functions (10,11).
As a raw material, it may be added to functional foods, herbal
dietary supplements, antiaging health care products and skin care
products to protect the body from free radicals (12-14).
Recent studies have also reported that long-term administration of
low-dose SchB (Below normal treatment or modeling concentrations)
increased the function and antioxidant capacity of mitochondria in
the brain, heart, liver and skeletal muscle of young and old
experimental rats (12,15,16).
Additionally, SchB administered to rats (a myocardial infarction
model) protected cardiomyocytes from ischemia/reperfusion injury
(17).
Therefore, we hypothesized that SchB could protect
the heart from THP damage via antioxidant mechanisms. To the best
of our knowledge, this hypothesis has not yet been previously
confirmed in vivo or in vitro. Therefore, the current
study investigated the antioxidant effects of SchB during
THP-induced cardiac damage and preliminarily evaluated the key
antioxidant mechanisms.
Materials and methods
Materials
SchB and THP, purity ≥98%, were purchased from
Shanghai Aladdin Reagent Co., Ltd. Malondialdehyde (MDA; cat. no.
A003-1-2), superoxide dismutase (SOD; cat. no. A001-3-2), catalase
(CAT; cat. no. A007-1-1), total antioxidant capacity (T-AOC; cat.
no. A015-1-2) and lactate dehydrogenase (LDH; cat. no. A020-2-2)
test kits were obtained from Nanjing Jiancheng Biological
Engineering Research Institute. Brain natriuretic peptide (BNP;
cat. no. MB-1608A), creatine kinase MB (CK-MB; cat. no. MB-6930A)
and cardiac troponin T (cTnT; cat. no. MB-7278A) test kits were
purchased from Shanghai Meixuan Biological Science and Technology,
Ltd. A reactive oxygen species (ROS) detection kit and Cell
Counting Kit (CCK)-8 cell viability and toxicity detection kits
were acquired from Biosharp Life Sciences. Antibodies against SOD2
(cat. no. 13141S), pro/cleaved caspase-3 (cat. nos. 14220S/9664S)
and Bax (cat. no. 14796) were obtained from Cell Signaling
Technology, Inc., and antibodies against NADPH oxidase 2 (NOX2;
cat. no. 19013-1-AP) and Bcl-2 (cat. no. 26593-1-AP) were obtained
from ProteinTech Group, Inc. All chemicals and reagents were of
analytical grade.
Animal experiments Animal model
This study was approved by the Animal Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University (CMU; approval no. 20195101). A total of 20 male Sprague
Dawley (SD) rats (weight, 180-200 g; age, 6 weeks) were obtained
from the CMU Experimental Animal Center. Rats were kept at a
standard room temperature of 22±3˚C with 45±10% humidity
under a 12 h light/dark cycle. The animals were supplied with ad
libitum standard laboratory food and tap water prior to
experimentation. Rats were randomly distributed equally into four
groups (n=5 in each group): Control (CON) group (normal diet for 7
weeks), SchB group (SchB-supplemented diet, 50 mg/kg for 7 weeks),
THP group (3 mg/kg THP was injected via the caudal vein once a week
with a normal diet for 7 weeks) and SchB + THP group (3 mg/kg THP
was injected via the caudal vein once a week with an
SchB-supplemented diet, 50 mg/kg for 7 weeks). The doses of THP and
SchB were converted from the doses taken by patients clinically and
according to our previous research (18-20).
Rats in the CON and THP groups were fed an AIN-76A diet. The
AIN-76A diet contained ~5.2% fat (% by weight, approx. all from
corn oil). The SchB diet in the SchB group and SchB + THP group
contained ~0.5‰ SchB in AIN-76A feed. After conversion, 0.5‰ SchB
in the diet=50 mg/kg in rats. Similar feed processing and feeding
schemes can be found in our previous studies (21,22).
AIN-76A feed and SchB feed processing were completed by Jiangsu
Synergy Pharmaceutical Bioengineering Co., Ltd. Food consumption
and body weight were measured twice a week.
Electrocardiogram (ECG) and Doppler
echocardiography
At week 8, SD rats were anesthetized with inhaled
isoflurane (2%, the maintenance dose was also 2%). Three lead on
ECG was recorded by a BL-420F biological function measurement
system (Chengdu Taimeng Software Co., Ltd.). Doppler
echocardiography was measured by using a Vivid E95 ultrasonic
diagnostic apparatus (General Electric Company).
Sample collection and processing
Rats were sacrificed via cervical dislocation under
anesthesia (inhalation of 2% isoflurane). Blood samples were
collected from the abdominal aorta and centrifuged at 314 x g for
30 min at 4˚C. The supernatant was stored in a refrigerator at
-80˚C. A cardiac tissue sample was then removed and stored at
-80˚C. The levels of LDH, BNP, CK-MB, cTnT, SOD and MDA in serum
were determined according to the instructions of the kits. A total
of ~100 mg heart tissue was homogenized in normal saline at a ratio
of 1:10 and then centrifuged in a low-temperature centrifuge at
1,250 x g for 15 min at 4˚C. The supernatant was obtained to
determine the contents of SOD, MDA and CAT and the T-AOC in
accordance with the manufacturer's protocol.
Cell culture and treatment
The relevant extraction methods for primary
cardiomyocytes have been described in our previous study (18). A total of 20 neonatal male SD rats
(age, 1-3 days; weight, 5-6 g) were kept at a standard room
temperature of 22±3˚C with 45±10% humidity under a 12 h
light/dark cycle at the CMU Experimental Animal Center. Animals
were not fed and were immediately anesthetized and disinfected with
75% ethanol. The ventricular areas were quickly isolated under
aseptic conditions. Blood clots, blood vessels and fat in the heart
were removed, and the remaining tissue was washed clean, cut into
chylous shapes (1 mm3), digested by trypsin + type II
collagenase, filtered, centrifuged (200 x g at 26˚C for
5 min), suspended and seeded. Finally, primary rat cardiomyocytes
were obtained by the differential adhesion method and seeded in
six-well plates at a density of 70-80% (18). The obtained primary rat
cardiomyocytes were further cultured for 24-48 h in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS (PAN-Biotech GmbH) and
2% penicillin/streptomycin at 37.5˚C, pH 7.2-7.4 and 95% air + 5%
CO2. Cardiomyocytes were then treated
(37.5˚C) according to the following methods: Normal
group (CON), SchB group (SchB, 50 µM, 14 h), THP group (THP, 10 µM,
12 h), and THP + SchB coculture group (SchB, 50 µM, 2 h; SchB 50 µM
+ THP 10 µM, 12 h). Approximately every 15 neonatal rat
cardiomyocytes were placed into a standard six-well plate. A total
of ~4 six-well plates were used, with an average of six wells in
each group. Cell viability and oxidative stress in each group were
detected according to the instructions of the CCK-8 and ROS kits
(23).
Western blotting
Heart tissue and primary rat cardiomyocytes were
lysed in RIPA lysis buffer with 1% protease inhibitor (Beyotime
Institute of Biotechnology). A BCA kit was used to determine the
protein concentration in the supernatant (Beyotime Institute of
Biotechnology). In total, ~50 µg heart tissue lysate or 20 µg cell
lysate was used for 12% SDS-PAGE, and proteins were then
transferred to an FL membrane (MilliporeSigma) at 4˚C for 1.5 h.
After blocking with 5% non-fat milk powder (Beyotime Institute of
Biotechnology) at room temperature for 1.5 h, the following primary
antibodies were added and incubated overnight at 4˚C:
SOD2 (1:1,000), pro/cleaved caspase-3 (1:1,000), Bax (1:1,000),
NOX2 (1:2,000) and Bcl-2 (1:1,000) were. Subsequently,
HRP-conjugated goat anti-rabbit IgG (H+L) secondary antibodies
(1:10,000; Thermo Fisher Scientific, Inc.; cat. no. 31460) were
added and incubated at room temperature for 1.5 h. The western
blotting results were analyzed using BeyoECL Plus (Beyotime
Institute of Biotechnology) and Image Lab software (version 5.2.1;
Bio-Rad Laboratories, Inc.). The specific protein expression levels
were normalized to that of GAPDH.
Statistical analysis
Data are presented as the mean ± standard deviation
(n=3) and statistical analyses were performed using SPSS Statistics
26 (IBM Corp.). The significance of differences between groups was
analyzed statistically using one or two-way ANOVA, followed by
Tukey's multiple-comparison post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
SchB improves THP-induced body weight,
food intake and echocardiographic changes in rats
The body weights (Fig.
1A) and food intake (Fig. 1B)
of THP-administered rats began to decrease in the third and fourth
weeks. However, significant improvements in the aforementioned
changes were observed after treatment with SchB. Similarly, THP
caused echocardiographic damage in rats, such as a decreased left
ventricular ejection fraction (Fig.
1C), decreased left ventricular fractional shortening (Fig. 1D), increased left ventricular
internal diameter (LVID) at end-diastole (Fig. 1E) and an increased LVID at
end-systole (Fig. 1F). After
treatment with SchB, the aforementioned changes were effectively
alleviated.
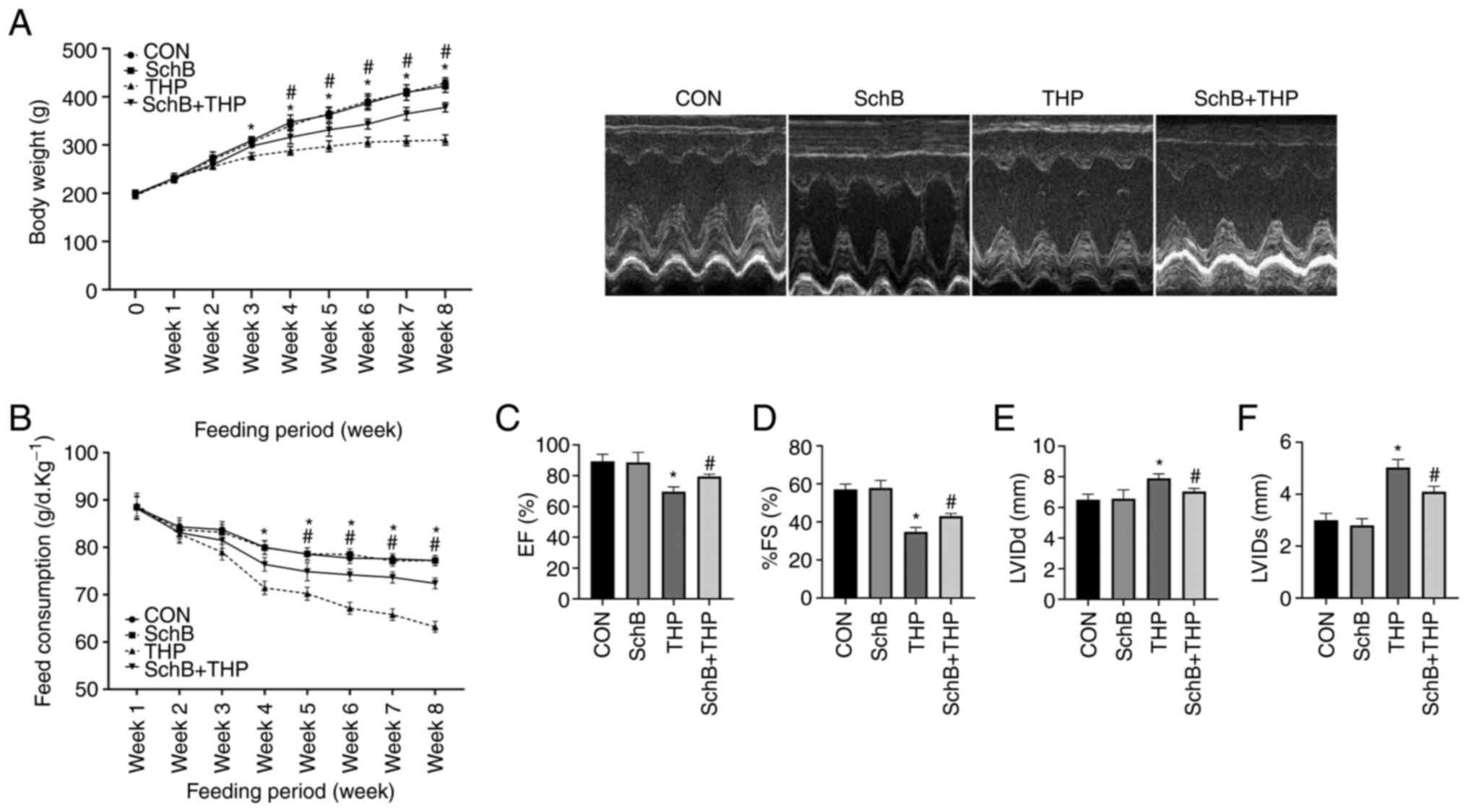 | Figure 1SchB improves THP-induced body
weight, food intake and echocardiographic changes in rats. (A) Body
weights and (B) food intake of THP-treated rats decreased in the
third and fourth weeks. (C) EF and (D) %FS decreased, and (E) LVIDd
and (F) LVIDs increased in THP-treated rats. After treatment with
SchB, the aforementioned changes were effectively alleviated. All
values are presented as the mean ± SD. *P<0.05 vs.
CON; #P<0.05 vs. THP. EF, left ventricular ejection
fraction; FS, left ventricular shortening fraction; LVIDd, left
ventricular internal diameter at end-diastole; LVIDs, left
ventricular internal diameter at end-systole; CON, control; SchB,
schisandrin B; THP, pirarubicin. |
SchB effectively improves THP-induced
myocardial injury in rats
THP also caused myocardial injury in rats, including
increased R waves (Fig. 2A) and T
waves (Fig. 2B), decreased S waves
(Fig. 2C) and prolonged QT
intervals (Fig. 2D). Similarly,
the serum markers of myocardial injury in rats were also abnormal,
including increased LDH (Fig. 2E),
BNP (Fig. 2F), CK-MB (Fig. 2G) and cTnT (Fig. 2H). After SchB treatment, the
aforementioned changes were significantly improved (Fig. 2A-H).
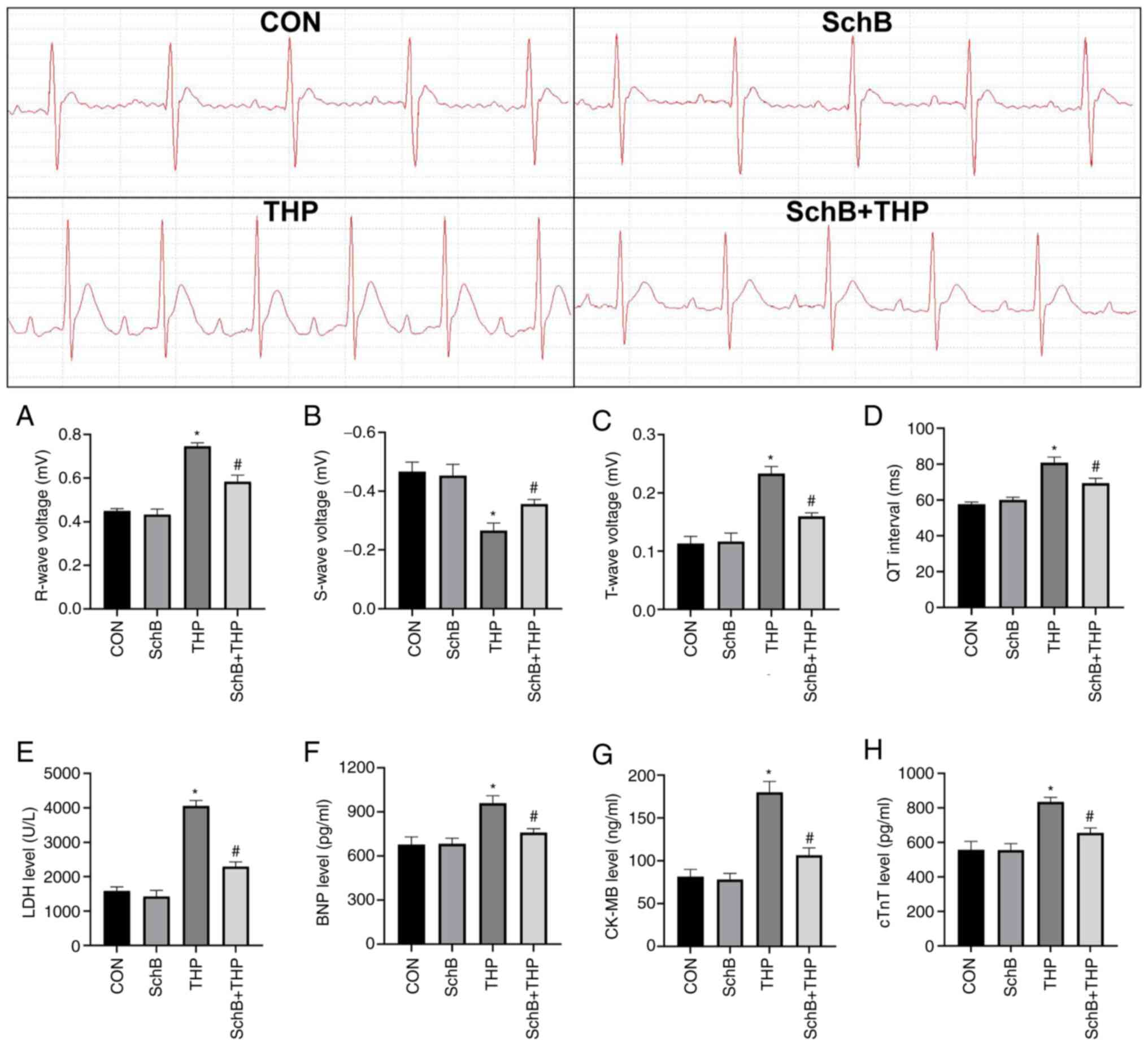 | Figure 2SchB effectively improves THP-induced
myocardial injury in rats. The (A) R wave and (B) S wave increased;
the (C) T wave decreased; and the (D) QT interval was prolonged in
THP-treated rats. Similarly, (E) LDH, (F) BNP, (G) CK-MB and (H)
cTnT increased in the serum of THP-treated rats. After SchB
treatment, the aforementioned changes were significantly improved.
All values are presented as the mean ± SD. *P<0.05
vs. CON; #P<0.05 vs. THP. LDH, lactate dehydrogenase;
BNP, brain natriuretic peptide; CK-MB, creatine kinase MB; cTnT,
and cardiac troponin T; CON, control; SchB, schisandrin B; THP,
pirarubicin. |
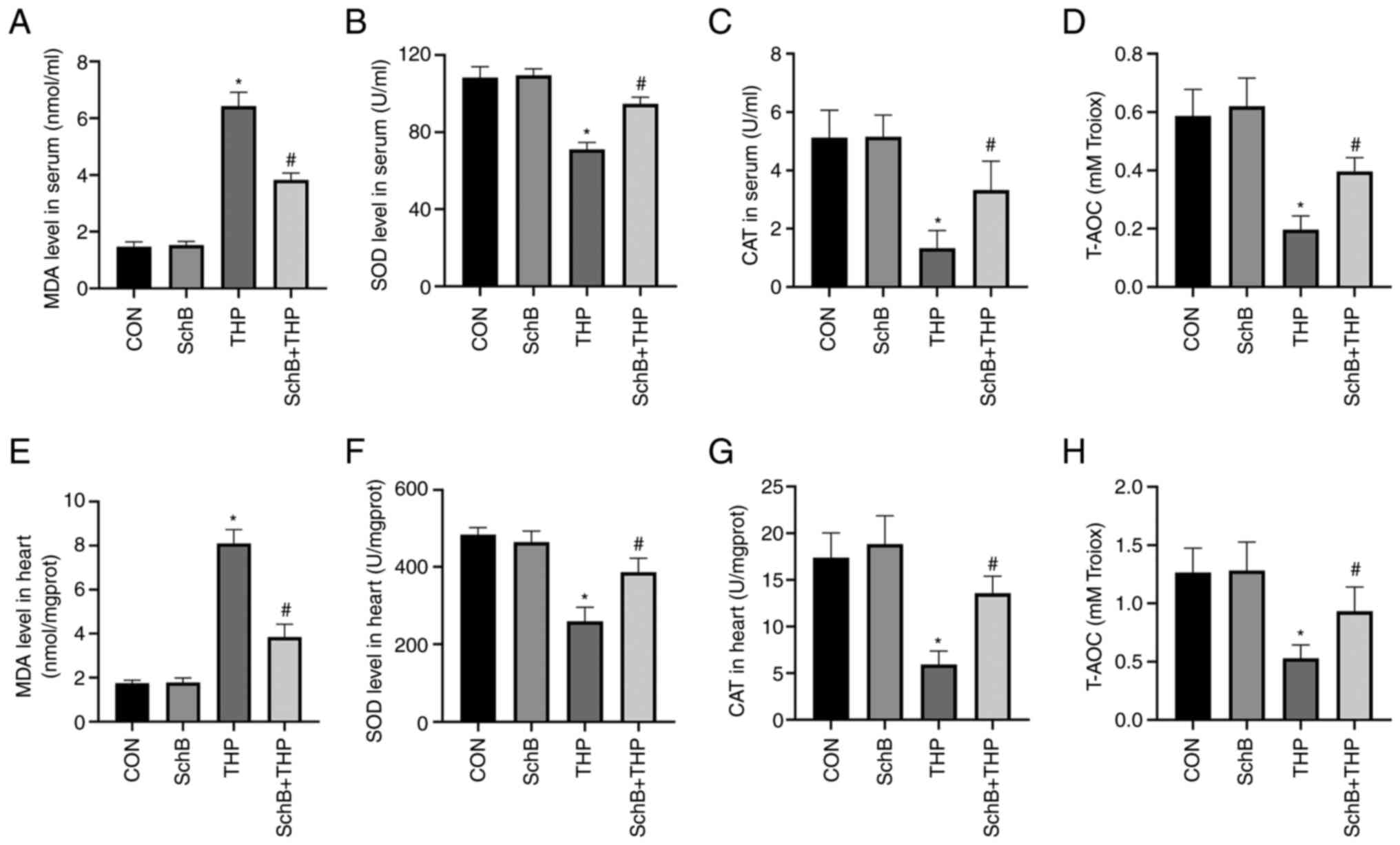
SchB attenuates THP-induced oxidative
stress in rats
The MDA content (Fig.
3A and E) was increased and
the SOD content (Fig. 3B and
F) was decreased in both serum and
heart tissue of the THP group. Similarly, reductions were also
detected with regards to CAT and T-AOC in the serum and heart.
Moreover, SchB improved the THP-induced increase in MDA and the
decrease in SOD, CAT (Fig. 3C and
G) and T-AOC (Fig. 3D and H), indicating that SchB improved the
antioxidant capacity of rats.
Subsequently, the expression of oxidative stress
markers and related downstream proteins in heart tissue were
evaluated. As shown in Fig. 4B,
the expression levels of SOD2, pro-caspase-3 and Bcl-2/Bax were
decreased, while the expression levels of NOX2 and
cleaved-caspase-3 were increased.
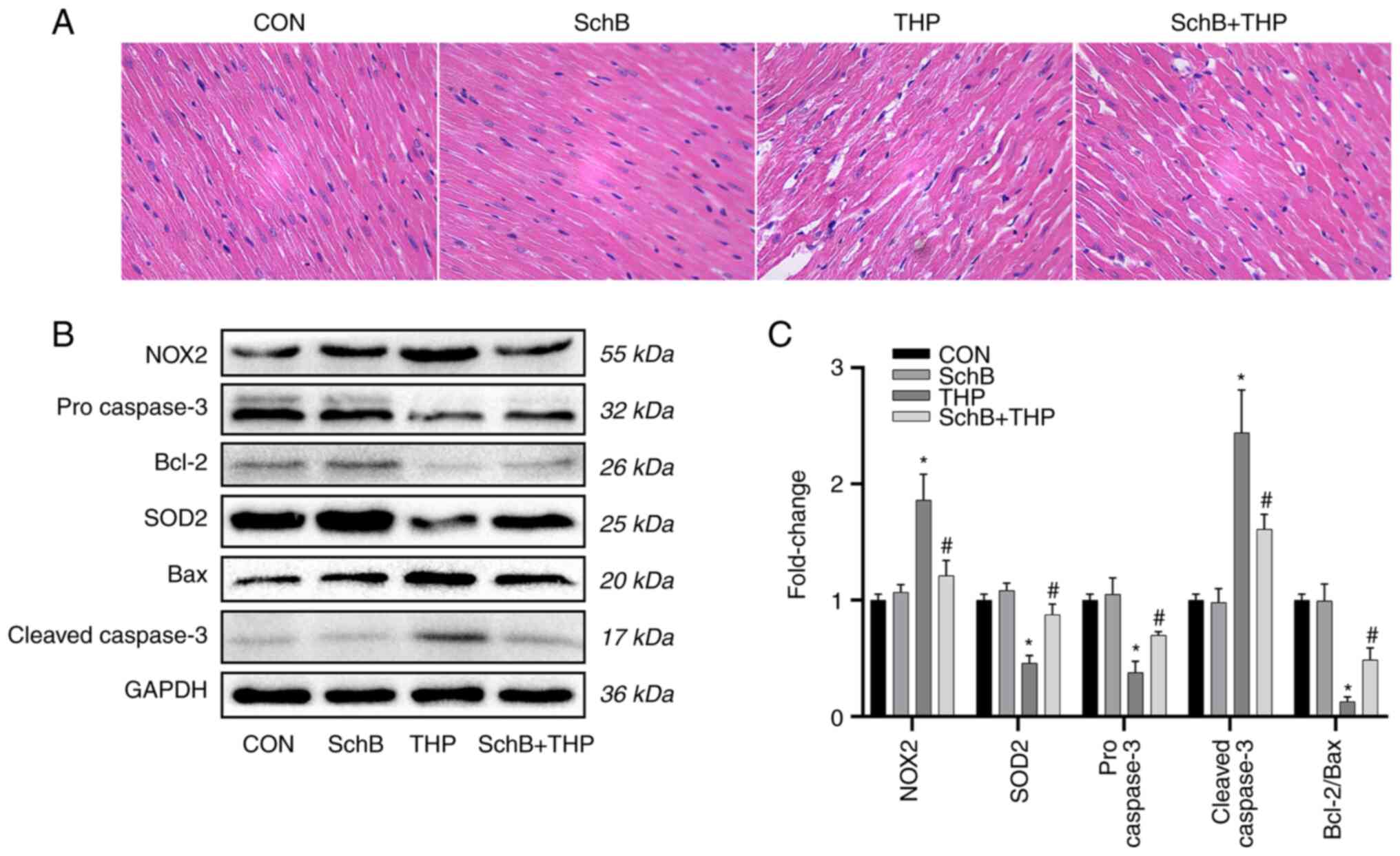 | Figure 4SchB improves myocardial tissue
changes induced by THP in rats. (A) The myocardial cells in the THP
group were disordered, the intercellular space was increased and
the myocardial tissue was calcified or denatured. However, these
changes in the heart were alleviated after SchB treatment
(magnification, x200). (B) The expression levels of SOD2,
pro-caspase-3 and Bcl-2/Bax decreased, while the expression levels
of NOX2 and cleaved-caspase-3 increased. However, after treatment
with SchB, the aforementioned changes were significantly improved.
(C) Semi-quantitative analysis of western blotting results.
Magnification, x200. All values are presented as the mean ± SD.
*P<0.05 vs. CON; #P<0.05 vs. THP. SOD,
superoxide dismutase; NOX, NADPH oxidase; CON, control; SchB,
schisandrin B; THP, pirarubicin. |
However, after treatment with SchB, the
aforementioned changes were significantly improved, as shown by the
semi-quantitative analyses (Fig.
4C).
SchB improves myocardial tissue
changes induced by THP in rats
As shown in Fig.
4A, the myocardial cells in the THP group were disordered, the
intercellular space was increased and the myocardial tissue was
calcified or denatured. However, these changes in the heart were
alleviated after SchB treatment.
SchB attenuates THP-induced oxidative
stress in primary cardiomyocytes
As shown in Fig. 5A
and B, THP reduced primary
cardiomyocyte viability and increased ROS levels. Similarly, the
expression levels of oxidative stress markers and related
downstream proteins in primary cardiomyocytes were detected. The
results suggested that the expression levels of SOD2, pro-caspase-3
and Bcl-2/Bax were decreased, while the expression levels of NOX2
and cleaved-caspase-3 were increased in the THP group (Fig. 5C).
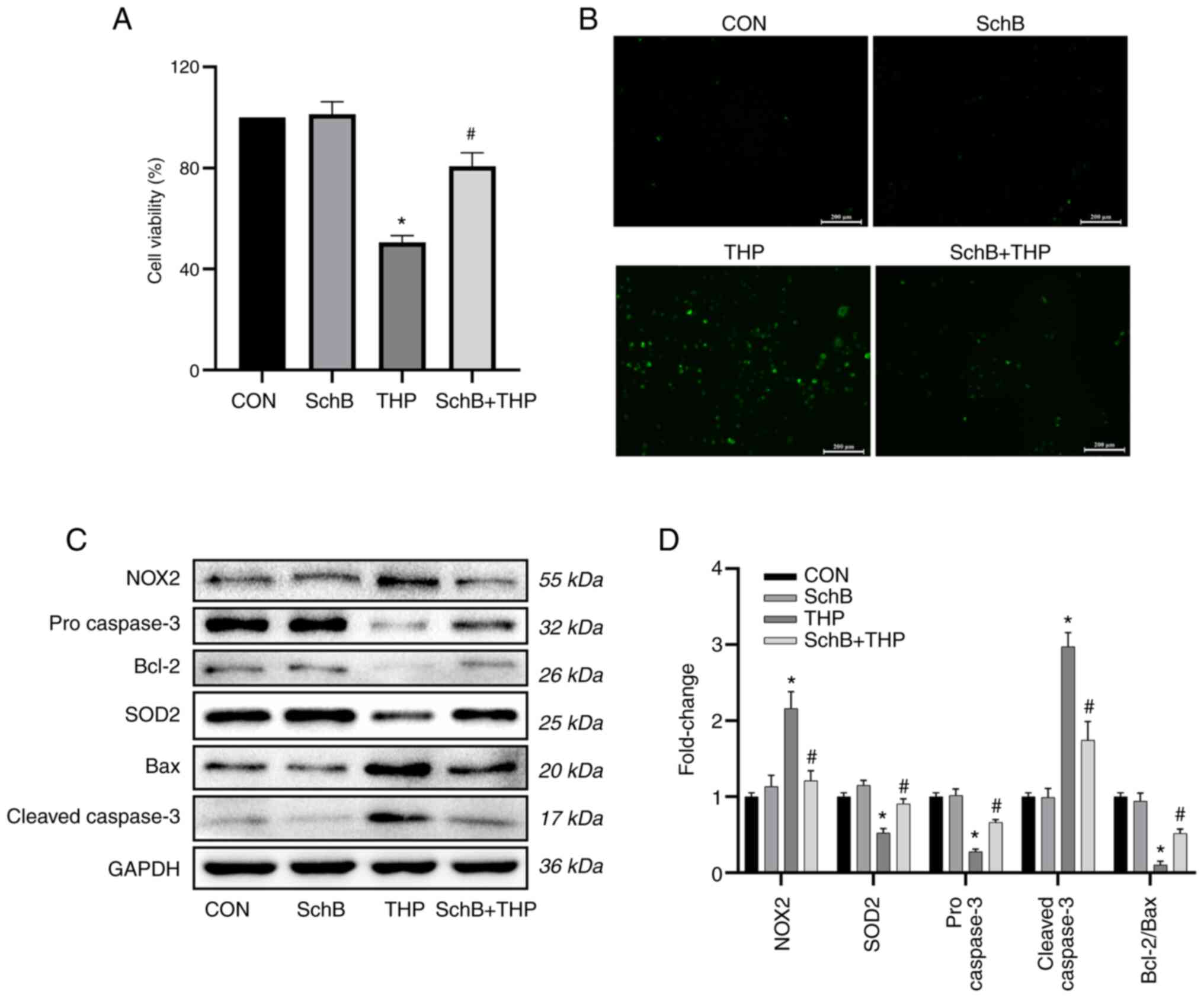 | Figure 5SchB attenuates THP-induced oxidative
stress in primary cardiomyocytes. THP reduced H9C2 (A)
cardiomyocyte viability and (B) increased reactive oxygen species.
Scale bar, 200 µm. (C) Similarly, the expression levels of SOD2,
pro-caspase-3 and Bcl-2/Bax decreased, while the expression levels
of NOX2 and cleaved-caspase-3 increased. However, after treatment
with SchB, the aforementioned changes were significantly improved.
(D) Semi-quantitative analysis of western blotting results. All
values are presented as the mean ± SD. *P<0.05 vs.
CON; #P<0.05 vs. THP. SOD, superoxide dismutase; NOX,
NADPH oxidase; CON, control; SchB, schisandrin B; THP,
pirarubicin. |
However, after treatment with SchB, the
aforementioned changes were significantly improved, as shown by the
semi-quantitative analyses (Fig.
5D).
Discussion
At a 50-mg/kg dose, SchB displayed novel and
promising effects by improving THP-induced cardiotoxicity in rats
and produced considerable improvements in a series of cardiac
injury manifestations. Consistent with our hypothesis, the key to
improving THP-induced cardiac injury by SchB was combatting
increased oxidative stress. Thus, the current study provided an
improved understanding of the pharmacological effects of SchB and
novel insights for the development of effective natural products to
prevent THP cardiotoxicity.
At present, SchB has shown some achievements in the
study of the cardiotoxicity of anthracycline antitumor drugs.
However, the data are relatively old and all focus on adriamycin
(24,25). Adriamycin has been gradually
abandoned in clinical practice, while other corresponding
anthracycline antitumor drugs, such as THP, are more widely used.
THP is an anthracycline antitumor drug; however, cardiotoxicity
issues have limited its clinical application (3). Often, patients with cancer must
reduce doses or even stop therapy due to cardiac intolerance
(6). Echocardiography and ECG are
commonly used as cardiac function test methods to monitor patients
receiving anthracycline drugs (26,27).
Without intervention, patients or animals on long-term
anthracyclines may incur some level of echocardiography and ECG
damage, which is consistent with our present observations (26,27).
In addition, myocardial injury serum markers, such as CK-MB, cTnT
and BNP, also reflect damage to cardiac function and structure
(28,29).
Cardiotoxicity induced by anthracyclines is closely
associated with oxidative stress (30). High ROS levels activate cytotoxic
signals leading to DNA damage, mitochondrial dysfunction, altered
protein synthesis and calcium overload, eventually leading to
irreversible cardiomyocyte damage (30,31).
The end-product of oxidation is MDA, which causes cross-linking and
polymerization of proteins, nucleic acids and other key
macromolecules to induce cytotoxicity (32), thereby affecting the activity of
in vitro mitochondrial respiratory chain complexes and key
enzymes in mitochondria (22). SOD
is also regarded as a major destroyer of oxygen free radicals in
the body, which resists, blocks and recovers the damage caused by
oxygen free radicals and repairs damaged cells over time (33,34).
Similarly, in the current THP model, MDA levels increased, and SOD
activity, CAT levels and the T-AOC decreased in both serum and
heart tissue, suggesting that THP induced abnormal increases in
oxidative stress in the model. NOX is the main ROS source in
cardiovascular systems, and NOX2 is the earliest NOX subtype that
transfers NADPH electrons to molecular oxygen, generating
superoxide anions (O2-) and inducing disease (35-37).
Increased NOX2 expression in the rat heart suggested that THP
induced ROS overproduction by activating NOX2 expression. Similar
results were obtained in vitro.
Another important finding of the present study was
that SchB had promising protective effects on THP-induced cardiac
injury; these injuries were improved, and SchB reduced oxidative
stress levels in rats and primary cardiomyocytes. Consistent with
other studies (12,38), SchB increased SOD levels, inhibited
lipid peroxidation, reduced the release of LDH, MDA and ROS, and
directly scavenged free radicals. These scavenging effects on
oxygen free radicals were a significant feature of SchB function,
and, critically, these effects on hydroxyl free radicals were
greater than those of vitamin C at the same concentration (39). Excessive oxidative stress in the
heart and myocardial cells also induces apoptosis and eventually
leads to myocardial cell death (40). Consistent with the present results,
excessive ROS production led to activation of the caspase protein
family and decreased the Bcl-2/Bax ratio, which eventually led to
increased cardiomyocyte apoptosis and affected normal heart
function (41,42). SchB effectively reversed these
harmful changes both in vivo and in vitro.
The present study confirmed that the cardiovascular
protective effects of SchB were dependent on reducing oxidative
stress levels. The current results provided evidence that SchB, as
a natural molecule, exerted strong cardiovascular protective
effects and highlighted its key potential antioxidant stress
mechanisms. However, at the end of the experiment, representative
images of rats in each group were not captured, and the general
state of rats was not observed. In addition, how SchB regulated
oxidative stress and some specific mechanisms remains unclear.
Therefore, further studies are required to clarify the potential
role of SchB as a new and effective antioxidant drug for
drug-induced cardiovascular disease and other cardiovascular
diseases.
In conclusion, the present study identified that
SchB effectively improved heart injury caused by THP, which was
closely associated with its strong antioxidant capacity. Based on
this evidence, SchB may be a promising new drug for the prevention
and treatment of cardiovascular disease caused by abnormal
increases in oxidative stress mediated by drugs or other causes. A
clinical study by the authors will be conducted soon, but at
present the basic research is ongoing and will explore other
mechanisms and therapeutic targets.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by a research grant from the
National Natural Science Foundation of China (grant no.
31501097).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT and JZ conceived and designed the experiments. PP
and LW implemented experimental improvements. HT and JZ acquired
and analyzed the data. HT, PP, LW and RF analyzed and interpreted
the data. HT, PP and RF drafted manuscript and critically revised
it for important intellectual content. All authors confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University (approval no. 20195101).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Minotti G, Recalcati S, Menna P,
Salvatorelli E, Corna G and Cairo G: Doxorubicin cardiotoxicity and
the control of iron metabolism: Quinone-dependent and independent
mechanisms. Methods Enzymol. 378:340–361. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weiss RB: The anthracyclines: Will we ever
find a better doxorubicin? Semin Oncol. 19:670–686. 1992.PubMed/NCBI
|
|
3
|
Maayah ZH, Abdelhamid G, Elshenawy OH,
El-Sherbeni AA, Althurwi HN, McGinn E, Dawood D, Alammari AH and
El-Kadi AOS: The role of soluble epoxide hydrolase enzyme on
daunorubicin-mediated cardiotoxicity. Cardiovasc Toxicol.
18:268–283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Skrypnyk I, Maslova G, Lymanets T and
Gusachenko I: L-arginine is an effective medication for prevention
of endothelial dysfunction, a predictor of anthracycline
cardiotoxicity in patients with acute leukemia. Exp Oncol.
39:308–311. 2017.PubMed/NCBI
|
|
5
|
Nicolazzi MA, Carnicelli A, Fuorlo M,
Scaldaferri A, Masetti R, Landolfi R and Favuzzi AMR: Anthracycline
and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev
Med Pharmacol Sci. 22:2175–2185. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartlett JJ, Trivedi PC and Pulinilkunnil
T: Autophagic dysregulation in doxorubicin cardiomyopathy. J Mol
Cell Cardiol. 104:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Afsar T, Razak S, Batoo KM and Khan MR:
Acacia hydaspica R. Parker prevents doxorubicin-induced cardiac
injury by attenuation of oxidative stress and structural
Cardiomyocyte alterations in rats. BMC Complement Altern Med.
17(554)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Songbo M, Lang H, Xinyong C, Bin X, Ping Z
and Liang S: Oxidative stress injury in doxorubicin-induced
cardiotoxicity. Toxicol Lett. 307:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotoxicity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu TL, Wu XY, Song Y, Chen H, Xu B, Zhou
Y, Huang ZJ, Sun Y and Mao CQ: Effect of acupuncture on target
tissue distribution of Schisandra lignans. Acupunct Med.
31:207–213. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Y, Li ZC, Yao LQ, Li M and Tang M:
Schisandrin B alleviates acute oxidative stress via modulation of
the Nrf2/Keap1-mediated antioxidant pathway. Appl Physiol Nutr
Metab. 44:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lam PY and Ko KM: Schisandrin B as a
hormetic agent for preventing age-related neurodegenerative
diseases. Oxid Med Cell Longev. 2012(250825)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lam PY, Yan CW, Chiu PY, Leung HY and Ko
KM: Schisandrin B protects against solar irradiation-induced
oxidative stress in rat skin tissue. Fitoterapia. 82:393–400.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nasser MI, Zhu S, Chen C, Zhao M, Huang H
and Zhu P: A comprehensive review on schisandrin B and its
biological properties. Oxid Med Cell Longev.
2020(2172740)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu H, Zhang X, Guan J, Cui B, Zhao L and
Zhao X: Pharmacokinetics and tissue distribution study of
schisandrin B in rats by ultra-fast liquid chromatography with
tandem mass spectrometry. J Pharm Biomed Anal. 78-79:136–140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang EP, Li H, Yu CR, Yu CY, Jing S, Sun
HX, Wang CM, Fan XT, Chen JG and Wang S: Schisandrin B protects
PC12 cells against oxidative stress of neurodegenerative diseases.
Neuroreport. 26:360–366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao X, Xiang Y, Cai C, Zhou A, Zhu N and
Zeng C: Schisandrin B protects against myocardial
ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol
Med Rep. 17:556–561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi H, Zeng Q, Wei Y, Yang H, Tang H, Wang
D, Pu P and Feng R: Canagliflozin is a potential cardioprotective
drug but exerts no significant effects on pirarubicin-induced
cardiotoxicity in rats. Mol Med Rep. 24(703)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen N and Ko M: Schisandrin B-induced
glutathione antioxidant response and cardioprotection are mediated
by reactive oxidant species production in rat hearts. Biol Pharm
Bull. 33:825–829. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Miller AA and Salewski E: Prospects for
pirarubicin. Med Pediatr Oncol. 22:261–688. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang H, Zeng Q, Tang T, Wei Y and Pu P:
Kaempferide improves glycolipid metabolism disorder by activating
PPARγ in high-fat-diet-fed mice. Life Sci.
270(119133)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang H, Zeng Q, Ren N, Wei Y, He Q, Chen M
and Pu P: Kaempferide improves oxidative stress and inflammation by
inhibiting the TLR4/IκBα/NF-κB pathway in obese mice. Iran J Basic
Med Sci. 24:493–498. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li
J and Zhang Q: Resveratrol reverses Doxorubicin resistance by
inhibiting epithelial-mesenchymal transition (EMT) through
modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 36(19)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li L, Pan Q, Han W, Liu Z, Li L and Hu X:
Schisandrin B prevents doxorubicin-induced cardiotoxicity via
enhancing glutathione redox cycling. Clin Cancer Res. 13:6753–6760.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu Y, Liu Z, Sun J, Pan Q, Sun F, Yan Z
and Hu X: Schisandrin B prevents doxorubicin-induced chronic
cardiotoxicity and enhances its anticancer activity in vivo. PLoS
One. 6(e28335)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Anqi Y, Yu Z, Mingjun X, Xiaoli K,
Mengmeng L, Fangfang L and Mei Z: Use of echocardiography to
monitor myocardial damage during anthracycline chemotherapy.
Echocardiography. 36:495–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Saidi A and Alharethi R: Management of
chemotherapy induced cardiomyopathy. Curr Cardiol Rev. 7:245–249.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simões R, Silva LM, Cruz A, Fraga VG, de
Paula Sabino A and Gomes KB: Troponin as a cardiotoxicity marker in
breast cancer patients receiving anthracycline-based chemotherapy:
A narrative review. Biomed Pharmacother. 107:989–996.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cardinale D, Sandri MT, Colombo A, Colombo
N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G,
Fiorentini C and Cipolla CM: Prognostic value of troponin I in
cardiac risk stratification of cancer patients undergoing high-dose
chemotherapy. Circulation. 109:2749–2754. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cappetta D, De Angelis A, Sapio L,
Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S and
Urbanek K: Oxidative stress and cellular response to doxorubicin: A
common factor in the complex milieu of anthracycline
cardiotoxicity. Oxid Med Cell Longev. 2017(1521020)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Farías JG, Molina VM, Carrasco RA, Zepeda
AB, Figueroa E, Letelier P and Castillo RL: Antioxidant therapeutic
strategies for cardiovascular conditions associated with oxidative
stress. Nutrients. 9(966)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu Y and Zheng G: Troxerutin protects
against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat
model of type 2 diabetes. Mol Med Rep. 15:3473–3478.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huang ZW, Liu N, Li D, Zhang HY, Wang Y,
Liu Y, Zhang LL and Ju XL: Angiopoietin-1 modified human umbilical
cord mesenchymal stem cell therapy for endotoxin-induced acute lung
injury in rats. Yonsei Med J. 58:206–216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun HL, Peng ML, Lee SS, Chen CJ, Chen WY,
Yang ML and Kuan YH: Endotoxin-induced acute lung injury in mice is
protected by 5,7-dihydroxy-8-methoxyflavone via inhibition of
oxidative stress and HIF-1α. Environ Toxicol. 31:1700–1709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gray SP and Jandeleit-Dahm KA: The role of
NADPH oxidase in vascular disease - hypertension, atherosclerosis
and stroke. Curr Pharm Des. 21:5933–5944. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Parajuli N, Patel VB, Wang W, Basu R and
Oudit GY: Loss of NOX2 (gp91phox) prevents oxidative stress and
progression to advanced heart failure. Clin Sci (Lond).
127:331–340. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manuneedhi Cholan P, Cartland SP and
Kavurma MM: NADPH oxidases, angiogenesis, and peripheral artery
disease. Antioxidants (Basel). 6(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xin DQ, Hu ZM, Huo HJ, Yang XJ, Han D,
Xing WH, Zhao Y and Qiu QH: Schisandrin B attenuates the
inflammatory response, oxidative stress and apoptosis induced by
traumatic spinal cord injury via inhibition of p53 signaling in
adult rats. Mol Med Rep. 16:533–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim SR, Lee MK, Koo KA, Kim SH, Sung SH,
Lee NG, Markelonis GJ, Oh TH, Yang JH and Kim YC:
Dibenzocyclooctadiene lignans from Schisandra chinensis protect
primary cultures of rat cortical cells from glutamate-induced
toxicity. J Neurosci Res. 76:397–405. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu
SC, Yuan YP, Deng W, Ma ZG and Tang QZ: FNDC5 alleviates oxidative
stress and cardiomyocyte apoptosis in doxorubicin-induced
cardiotoxicity via activating AKT. Cell Death Differ. 27:540–55.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu Y, Wang B, Xu H, Tang L, Li Y, Gong L,
Wang Y and Li W: Probiotic Bacillus attenuates oxidative
stress-induced intestinal injury via p38-mediated autophagy. Front
Microbiol. 10(2185)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang M, Meng XB, Yu YL, Sun GB, Xu XD,
Zhang XP, Dong X, Ye JX, Xu HB, Sun YF and Sun XB: Elatoside C
protects against hypoxia/reoxygenation-induced apoptosis in H9c2
cardiomyocytes through the reduction of endoplasmic reticulum
stress partially depending on STAT3 activation. Apoptosis.
19:1727–1735. 2014.PubMed/NCBI View Article : Google Scholar
|