Introduction
Necrotizing fasciitis of the chest wall is a rare
pathology, but is a life-threatening condition with a high
mortality rate. It has an aggressive character, rapidly extensive
and mutilating by widespread musculocutaneous necrosis, with
increased mortality because it is quickly associated with general
septic phenomena with the establishment of multiple organ failure
(MOF) (1-3).
In the 2000's, a total of 4 cases per million were
reported in the USA and 10 cases per million were reported in
Western Europe, while the incidence on the North American continent
was approximately 1,000 cases per year. There is no predisposition
in regards to sex and it has been observed more often in elderly
patients and rarely in children. The first mention case was made by
Hippocrates in the 5th century BC, as a complication of an
erysipelas. In the 18th century, it was described by English
doctors as ‘gangrenous ulcer’, ‘rotten ulcer’ or ‘hospital
gangrene’ (4-6).
In 1983, Pingleton and Jeter reported extensive
thoracic wall gangrene with Bacteroides melaninogenicus and
Viridans streptococci after minimal pleurotomy for empyema.
Vanecko reported clostridial myodestruction of the large pectoralis
major and of the serratus anterior after minimal pleurotomy in a
patient with Boerhaave's syndrome (7).
Thoracic necrotizing fasciitis has been reported as
primary when there is no lesion of cutaneous or secondary when
there is a discontinuity of skin integrity, neighborhood (cervical,
abdominal) or proper thoracic.
In most cases, it has been shown to be directly
related to the state of immunodeficiency, secondary to an
unbalanced diabetes mellitus, steroid treatment, hematological or
oncological disease (8-11).
This proven link does not exclude the possibility of this pathology
in an apparently immunocompetent patient.
The case presented is a secondary one to thoracic
intervention (minimal pleurotomy for pyopneumothorax) in a patient
with active pulmonary tuberculosis. Its peculiarity results from
the presence of a bronchopleural fistula that favored and
aggravated the proper fasciitis and that required a complex
surgical approach, both for infection control and for curative
purposes.
Case report
The case of a 43-year-old man, cachectic, with a
history of left pulmonary tuberculosis treated 10 years ago, is
presented. He presented to the emergency room of the National
Institute of Pneumology, Bucharest, with acute symptoms such as
severe dyspnea, cough with mucopurulent sputum with onset of about
10 days, amid physical and mental asthenia installed for several
months. Biologically, the patient had severe anemia (hemoglobin
level, 6.92 g/dl) and leukocytosis (16,000/µl). The X-ray revealed
a left pyopneumothorax and a minimum left pleurotomy was
performed.
Subsequently, despite the 32 CH tube drainage, major
purulent air and fluid losses were found both at the level of the
underwater sealed drainage and peristomal. These major
periorificial losses were associated with massive local parietal
contamination with rapid extension of the necrotic-suppurative
phenomena, up to the abdominal level, respectively of the left
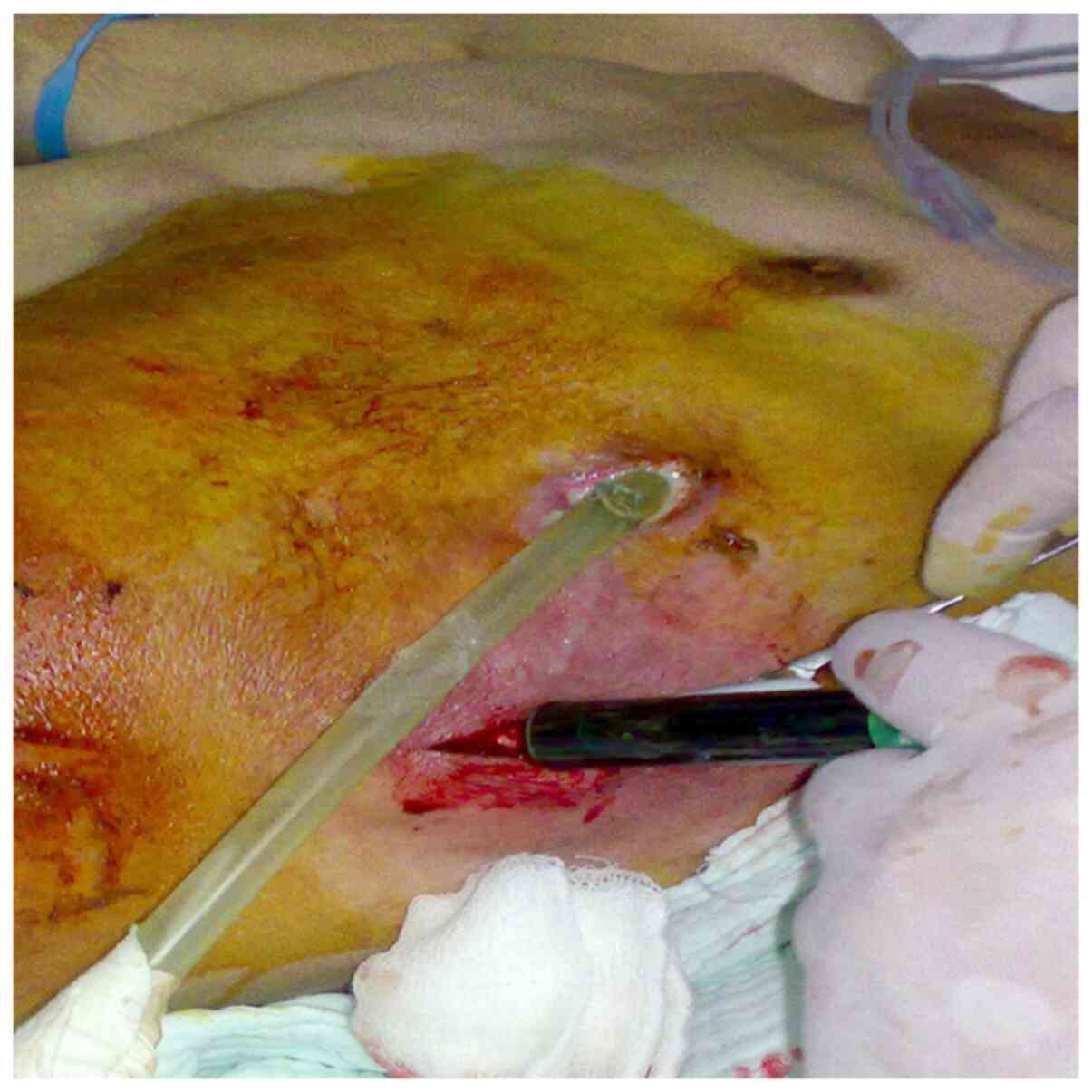
flank (Fig. 1). Thus, at
approximately 48 h, it was necessary to apply drainage incisions
with debridement at the thoracic parietal level, in addition to the
strong antibiotic treatment.
In addition to local betadine lavage and repeated
debridement, negative pressure wound therapy (NPWT) was applied to
areas at a relative distance from the pleurotomy orifice. NPWT
could not be applied extensively due to the lack of tightness
secondary to extensive parietal air loss. Pleural lavage has been
shown to be dysfunctional due to bronchial aspiration. All of these
elements were in favor of a clinically significant bronchopleural
fistula that could not be objectified bronchoscopically.
The sputum examination indicated the presence of
BAAR++, establishing the specific treatment. Streptococcus
pyogenes was identified in the wounds and pleural fluid and
appropriate antibiotic treatment was instituted.
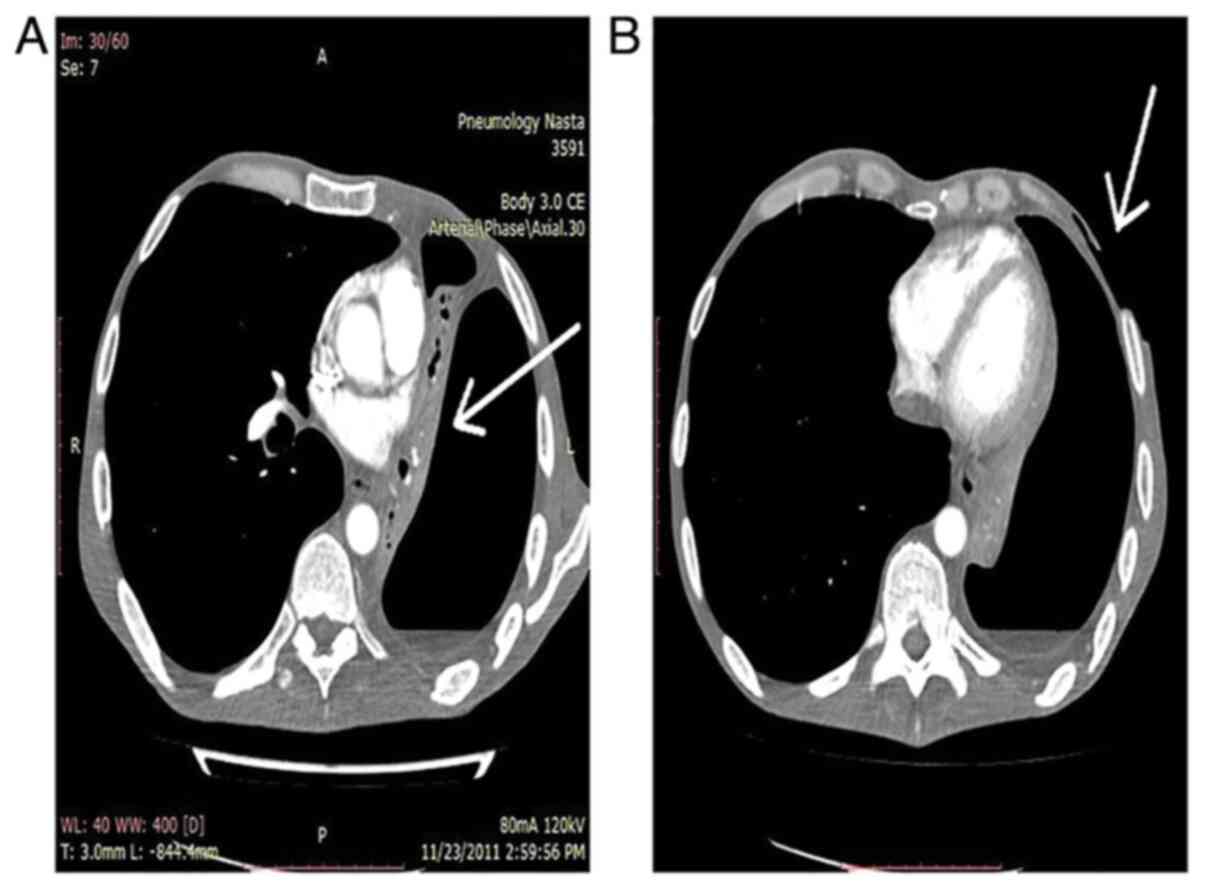
Chest computed tomography (CT) examination indicated
the presence of a destroyed tuberculous left lung, with
pyopneumothorax, with parietal defects of skin, subcutaneous and
even muscle tissue (Fig. 2).
Considering the evolution of the patient towards the
degradation of the general condition, of extension of the parietal
phenomena, the Azorin procedure of transcervical mediastinoscopic
closure and section of the left primitive bronchus was chosen, in
the hope of diminishing the periorificial air losses and of the
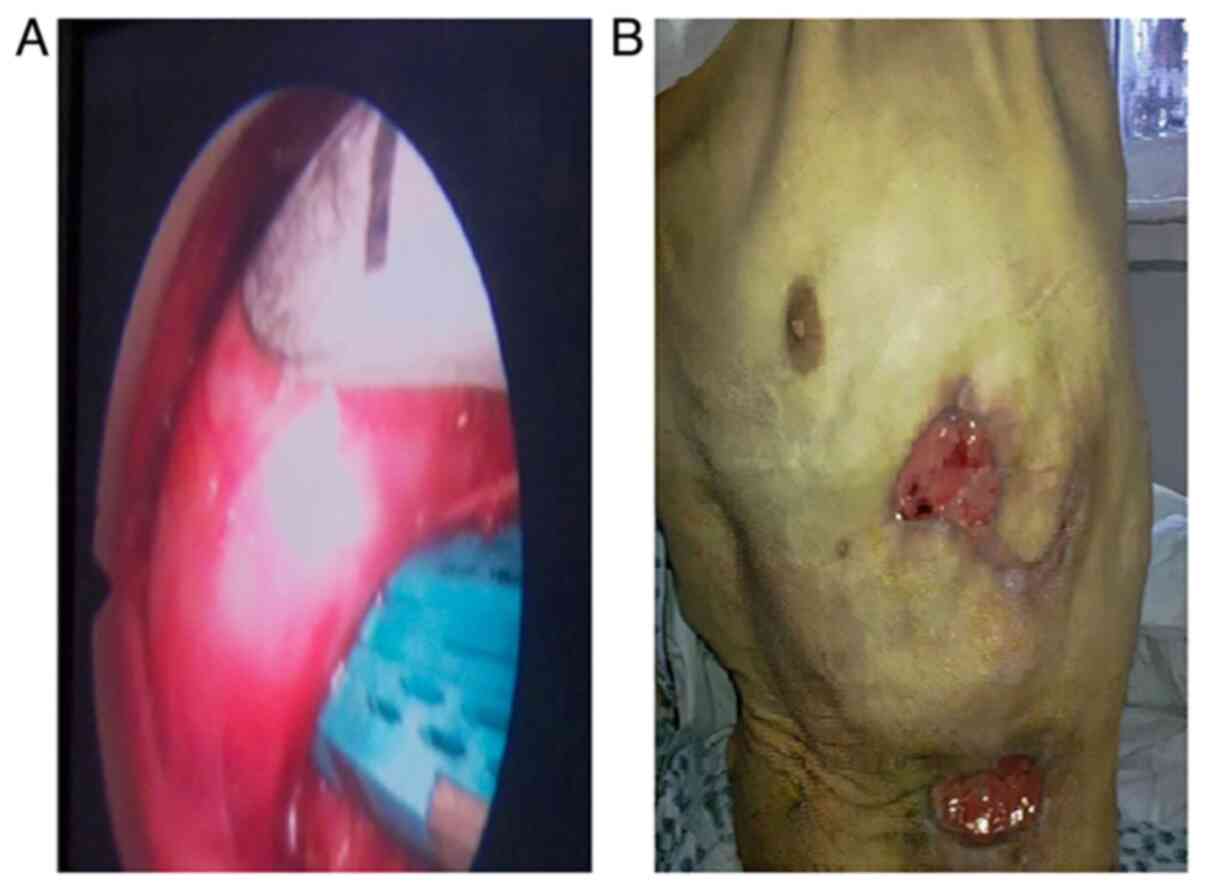
implicit parietal contamination (Fig.
3A).
After 48 h, the patient showed improvement with
diminishing and stoppage of the evolution of acute necrotic
suppurative phenomena at the parietal level. Intrapleural lavage
was introduced and daily debridement and dressings with significant
granulation were continued (Fig.
3B). Subfebrility was recorded for several days, relatively
easily controlled with usual antipyretics. Antituberculosis and
antibiotic treatments were carefully continued. The samples
confirmed the presence of Pseudomonas aeruginosa and a
nosocomial contamination with methicillin-resistant
Staphylococcus, requiring the adaptation of antibiotic
therapy.
At 21 days postoperatively, a left pneumonectomy was
performed, with an abundant intraoperative lavage with betadine
serum. The pneumonectomy technique was a simple one, identifying
relatively easily the distal bronchial stump, mechanically closed
by the previous procedure. The proximal bronchial stump was not
exposed, in order to avoid fistulization. In addition, a flap of
minimal pericardial fat was applied at this level. Moreover,
excision of the pleurocutaneous fistulous tract with suture was
performed and a Clagett-type drain-wash (drainage-circuit lavage
with apical inlet and posterobasal recovery tube) with 1,000 ml of
betadine serum daily for 4 days postoperatively was placed. The
evolution was favorable, the patient being closely monitored
clinically and radiologically for one year.
Discussion
In the literature, the bacteria involved in the
pathogenesis of necrotizing parietal fasciitis is termed ‘flesh
eating bacteria’, although there is no proper mechanism of ‘tissue
devouring’. The mechanisms of the bacteria involved are multiple
and include bacterial necrotoxins (Clostridium perfringens,
Streptococcus pyogenes), thrombosis and microvascular
coagulation with secondary necrosis by heparinase release,
anaerobic environment, release of beta lactamases that interfere
with antibiotic activity (2,4,11).
There is a general specificity of germs depending on
the type of fasciitis, but it is not absolute. Three classes of
fasciitis are described based on the bacteriological criterion. The
first class is a polymicrobial one, with combinations of
gram-positive cocci, gram-negative bacilli and anaerobes of the
Clostridium type. They are located especially in the chest
and perineum. It especially affects the elderly and the
immunocompromised. Often, no obvious trauma or cause is identified,
but the presence of skin discontinuity from old abscesses,
perforations or bacterial translocations is assumed. A special
mention is made of the clostridial subtype that produces gangrene
with specific gas, this being more and more rarely described with
the improvement of sanitary conditions and hygiene. Its mortality
exceeds 50% (11,12). From a practical point of view, the
recommendation is that in the case of a rapid installation of local
phenomena (of the order of hours) to take into account the presence
of Clostridium and Streptococcus species.
Type II involves fasciitis with group A beta
hemolytic Streptococcus alone or in combination with
Staphylococcal species. In addition, it shows a rapid
evolution with high toxicity, observing an impairment of younger
and healthier patients than in type I. Type III is specifically
described on the West coast of the USA with gram-negative marine
Vibrio vulnificus type.
Starting from the criterion of mechanism and
topography, Urschel et al (2) and Moustaide et al (10) reported primary forms of fasciitis
with the involvement mainly of Streptococcus pyogenes, but
also Vibrio vulnificus, Clostridium perfringens or
Bacteroides fragilis, most cases being polymicrobial.
The secondary ones involve a specific flora and a
specific behavior. The more frequent deep cervical infectious
processes are associated with mediastinitis due to the cervical
fascial anatomy and their connections to the mediastinal fasciae
(11). The superficial ones also
expand gravitationally, but superficially developing fasciitis of
the chest wall, with the aggravation of the initial prognosis
(11,13-20).
The most common causes include dental abscesses, but have also been
cited as secondary to Bezold abscesses or to puncture site for
central catheterization. In terms of flora, Klebsiella
pneumoniae has been identified as the most common and most
aggressive pathogen, associated with increased mortality
(approximately 60%) (15).
Klebsiella pneumoniae has also been identified in nosocomial
forms, associated with multidrug resistance and implicitly with
high mortality (15).
Abdominal extension has been reported following a
retroperitoneal abscess associated with emphysematous
pyelonephritis. This pathology is reported secondary to high
urinary tract infections with Escherichia coli, K.
pneumoniae, Enterobacter aerogenes, Proteus
mirabilis, Pseudomonas species, with anaerobes,
Streptococci and Candida (21,22).
Rebai et al (23) presented
a case of perforating appendicitis resulting in secondary
necrotizing fasciitis of the anterior abdominal and thoracic wall,
identifying gram-negative opportunistic pathogens (Escherichia
coli, Pseudomonas aeroginosus,
Acinetobacter).
Secondary necrotizing thoracic parietal fasciitis is
most often reported due to pleurotomies or surgery for empyema, as
in the present case. In fact, they are described secondary to any
thoracic intervention, but more often secondary to extensive or
esophageal resection, but also secondary to thoracocentesis or
thoracic drainage in Boerhaave syndrome (1-3,24,25).
Thus, globally from a bacteriological point of view,
anaerobes have been identified which include: Bacteroid
species, very often; Peptostreptococcus species,
Clostridium perfringens, Fusobacterium lentum,
Microaerophilic streptococci, Eubacterium, but also
the aerobes, Streptococcus pyogenes, Enteroccus, and
species of Streptococcus. Most associated other anaerobic
and aerobic flora, rarely being unimicrobial. Thus, in 76% of cases
a polymicrobial flora was identified (2,25-27).
In the present case, Streptococcus pyogenes
with general antibiotic sensitivity was initially identified in the
wounds. Subsequently, repeated sampling identified the presence of
Aeruginosa pseudomonas and methicillin-resistant
Staphylococcus, revealing nosocomial contamination and
requiring specific treatment.
Sometimes, bacteriological samples obtained during
surgical debridement prove sterile, but only because of the
introduction of aggressive antibiotic treatments initiated in other
clinics (16). Nosocomial
superinfection adds a negative prognostic factor, being quite
common due to the large areas needed to be managed and the long
period of treatment.
The processes of fasciitis with induration,
collection, erythema, pain, crackles have been obviously
identified, usually at 3-4 days and only where aggressive and early
intervention could be mastered (2).
A characteristic frequently present in this pathology is
represented by the discrepancy between the apparently minimal
visible skin damage and the noisy systemic symptoms (28).
For any suspicion of necrotizing fasciitis, the
finger test is recommended. Thus, after local anesthesia, an
incision 2-3 cm wide enough to insert the index finger to the deep
fascia is made. The lack of bleeding or the appearance of a gray
necrosis fluid are very suggestive for necrotizing fasciitis. The
easy digital dissection of the planes with the minimum tissue
resistance is a golden standard for diagnosis (Fig. 1) (4,29-31).
In the case of obvious clinical forms, imaging
confirmation is required to assess the extent. The presence of air
bubbles is easily made based on the standard radiological
examination and, more recently, POCUS (point of care ultrasound).
The routine paraclinical imaging examination for diagnosis and for
the evaluation of extension is the CT examination (Fig. 2) (22,27,32).
Thus, a CT examination can show specific signs of parietal thoracic
necrotizing fasciitis: signs of parietal collections, edema in the
subcutaneous and fatty tissue, gas collections locally or with
locoregional extension. POCUS examination is becoming more
widespread due to its ease of use even when we have minimal
suspicion. Local pain may limit the examination, but by intensity
disproportionate to the pressure may suggest the diagnosis
(22,29,32,33).
Surgical treatment is based on debridement through
wide drainage incisions. The use of peroxidase, hyperbaric oxygen
therapy, antibody therapy has been reported (2,4,34,35).
Debridement gestures will be applied repeatedly with daily dressing
changes (19,36,37).
Negative pressure wound therapy (NPWT) has greatly
reduced the mortality even in extensive forms, doubled by measures
of muscle plastics, split skin grafts or secondary closure. The
essential condition is the early recognition of suppurative
phenomena (36-40).
In the present case, it was not possible to practice
extensive NPWT, due to air loss near the pleurotomy orifice. Wound
cleansing was achieved by stopping active contamination, applying
bronchial stump closure and by dressing and irrigating with
betadine solution, sometimes repeated daily.
Major damage can cause chest wall stability problems
when extensive costal resections are performed (1,41),
sometimes requiring even prolonged ventilatory support to
facilitate pleural symphysis and the appearance of stabilizing
thickening of pleura. Major damage and extensive lung damage may
require the practice of open drainage (open window-Eloesser
thoracomyoplasty) and Clagett-type lavage (19,36,37).
In the associated forms of mediastinal damage with
parietal fasciitis, various techniques can be used in addition to
superficial debridement incisions, such as mediastinoscopy and
thoracoscopy for mediastinal debridement and drainage (42,43).
The mortality rate was found to be 70%, if delayed
for more than 24 h after clinical diagnosis for drainage and less
than 30% when intervened in less than 24 h. The mortality rate is
71% if limited debridement is performed and 43% if extensive and
repeated debridement is performed (12).
Rapid recognition, prompt and combined application
of aggressive surgical gestures with large debridement and NPW
therapy associated with specific antibiotic therapy were essential
to reduce sepsis and multisystem organ failure phenomena.
The case presented here was a challenge both in
terms of thoracic parietal inflammatory phenomena, and in terms of
the aggravating mechanism of bronchopleural fistula. In addition,
performing a left pneumonectomy in an infected environment,
especially in an immunocompromised, cachectic patient involved an
increased risk of fistulization, with increased morbidity and
mortality.
In fact, the Azorin procedure was initially
described for the treatment of postpneumonectomy bronchopleural
fistulas on long bronchial stump, but can also be practiced as a
preexeresis, preventive method of choice in patients at risk of
postpneumonectomy fistulization (Fig.
3A, bronchial left main into stapler). The principle is to do
bronchial sutures in a clean, uncontaminated environment at the
mediastinal level.
The procedure was found to be the ideal solution for
a destabilized patient with a severe infectious status, in whom the
parietal necrotic suppurative phenomena could not be controlled. In
addition, it allowed a faster recovery than a classic open window
Eloesser thoracoplasty procedure.
In addition, for the preventive purpose of
bronchopleural fistula secondary to pulmonary resection, a patch of
pericardial fat was applied over the bronchial stump and a
Clagett-type lavage was established for 4 days with 1,000 ml of
betadine solution daily.
In conclusion, necrotizing fasciitis is a pathology
with significant mortality, which must always be taken into account
when there is interruption of skin continuity, traumatic or
surgical or when there are minimal elements of suspicion of damage
to subcutaneous cellulite-type tissues. Early recognition and
prompt application of complex medical-surgical treatment ensures
good results. Antibiotic therapy after initiation will be adapted
according to antibiograms, especially in aggressive forms of
nosocomial infections. The application of incisions and wide
debridement ensures the control and limitation of the destructive
phenomena. The entire surgical arsenal will be applied to limit the
spread of infection as fast as possible, in order to achieve a
successful outcome.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Irina Radu, Individual Service
Provider. The figures and case have been previously presented as a
poster at an abstract meeting for the European Respiratory Journal
40: P2414, 2012. The European Respiratory Society Programme Team
has granted the approval to submit a full article based on the
abstract to any other journal.
Funding
Funding: No funding was received.
Availability of data and materials
Data available on request from the authors.
Authors' contributions
CAP wrote the manuscript in light of the literature
data with support from AG and AZ. CAP and RI conceived of the
presented idea. CAP and TC carried out the main intervention. AC
treated and followed the patient. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This case report was approved by the Ethics
Commission of the National Institute of Pneumology, Bucharest,
Romania (no. 23515 from 13.10.2021).
Patient consent for publication
Written informed consent was obtained from the
patient on 12.10.2021.
Competing interest
The authors declare they have no competing
interests.
References
|
1
|
Safran DB and Sullivan WG: Necrotizing
fasciitis of the chest wall. Ann Thorac Surg. 72:1362–1364.
2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Urschel JD, Takita H and Antkowiak JG:
Necrotizing soft tissue infections of the chest wall. Ann Thorac
Surg. 64:276–279. 1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pingleton SK and Jeter J: Necrotizing
fasciitis as a complication of tube thoracostomy. Chest.
83:925–926. 1983.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hakkarainen TW, Kopari NM, Pham TN and
Evans HL: Necrotizing soft tissue infections: Review and current
concepts in treatment, systems of care, and outcomes. Curr Probl
Surg. 51:344–362. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Loudon I: Necrotizing fasciitis, hospital
gangrene, and phagedena. Lancet. 344:1416–1419. 1994.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Descamps V, Aitken J and Lee M:
Hippocrates on necrotizing fasciitis. Lancet.
344(556)1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
LoCicero J III and Shields T: Infections
of the chest wall. Chapter 45. In: Thoracic surgery. Shields TW,
Locicero J III, Ponn RB and Rusch VW (eds). 6th edition. Lippincott
Williams & Wilkins, Philadelphia, PA, 2009.
|
|
8
|
Rabiou S, Lakranbi M, Issoufou I, Ammor
FZ, Belliraj L, Ouadnouni Y and Smahi M: About two cases of
primitive necrotizing fasciitis of the chest wall. Rev Mal Respir.
33:401–404. 2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
9
|
Belliraj L, Sani R, Issoufou I, Lakranbi
M, Ouadnouni Y and Smahi M: Primitive necrotizing fasciitis of the
thoracic wall: Fatal complication of diabetic patient. Tunis Med.
96:520–523. 2018.PubMed/NCBI
|
|
10
|
Moustaide K, Nassiri A, Aqil N, Baybay H,
Gallouj S and Mernissi FZ: Primary necrotizing fasciitis of the
chest wall. J Stem Cell Res Ther. 4:95–96. 2018.
|
|
11
|
Abbasi Z, Inam H, Das S, Neel S and Fatimi
SH: Fungal cervical abscess complicated by necrotizing fasciitis
leading to descending necrotizing mediastinitis: A case report.
Cureus. 11(e5369)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Freischlag JA, Ajalat G and Busuttil RW:
Treatment of necrotizing soft tissue infections. The need for a new
approach. Am J Surg. 149:751–755. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Medeiros Júnior R, Melo Ada R, Oliveira
HF, Cardoso SM and Lago CA: Cervical-thoracic facial necrotizing
fasciitis of odontogenic origin. Braz J Otorhinolaryngol.
77(805)2011.PubMed/NCBI(In English, Portuguese).
|
|
14
|
Gore MR: Odontogenic necrotizing
fasciitis: A systematic review of the literature. BMC Ear Nose
Throat Disord. 18(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rahim GR, Gupta N, Maheshwari P and Singh
MP: Monomicrobial Klebsiella pneumoniae necrotizing
fasciitis: An emerging life-threatening entity. Clin Microbiol
Infect. 25:316–323. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bakshi J, Virk RS, Jain A and Verma M:
Cervical necrotizing fasciitis: Our experience with 11 cases and
our technique for surgical debridement. Ear Nose Throat J.
89:84–86. 2010.PubMed/NCBI
|
|
17
|
Mao JC, Carron MA, Fountain KR, Stachler
RJ, Yoo GH, Mathog RH and Coticchia JM: Craniocervical necrotizing
fasciitis with and without thoracic extension: Management
strategies and outcome. Am J Otolaryngol. 30:17–23. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Silva VA, Almeida AS, Lavinsky J, Pauna
HF, Castilho AM, Chone CT and Crespo AN: Thorax necrotizing
fasciitis following Bezold's abscess. Clin Case Rep. 8:2848–2851.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lalwani AK and Kaplan MJ: Mediastinal and
thoracic complications of necrotizing fasciitis of the head and
neck. Head Neck. 13:531–539. 1991.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pirvu A, Angelescu D and Savu C: Localized
fibrous tumor of the pleura an unusual cause of severe
hypoglycaemia. Case report. Rev Med Chir Soc Med Nat Iasi.
120:628–630. 2016.PubMed/NCBI
|
|
21
|
Khaladkar SM, Jain KM, Kuber R and Gandage
S: Necrotizing fasciitis of thoracic and abdominal wall with
emphysematous pyelonephritis and retroperitoneal abscess. J Clin
Imaging Sci. 8(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tsitouridis I, Michaelides M, Sidiropoulos
D and Arvanity M: Renal emphysema in diabetic patients: CT
evaluation. Diagn Interv Radiol. 16:221–226. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rebai L, Daghmouri A and Boussaidi I:
Necrotizing fasciitis of chest and right abdominal wall caused by
acute perforated appendicitis: Case report. Int J Surg Case Rep.
53:32–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Floether L, Bucher M, Benndorf R and
Burgdorff AM: Necrotizing fasciitis caused by the treatment of
chronic non-specific back pain. BMC Anesthesiol.
20(245)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cai Y, Cai Y, Shi W, Feng Q and Zhu L:
Necrotizing fasciitis of the breast: A review of the literature.
Surg Infect (Larchmt). 22:363–373. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Andreasen TJ, Green SD and Childers BJ:
Massive infectious soft-tissue injury: Diagnosis and management of
necrotizing fasciitis and purpura fulminans. Plast Reconstr Surg.
107:1025–1035. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rogers PJ, Lewis BM, Odak M and Bucher J:
Spontaneous necrotizing fasciitis. Cureus.
12(e11880)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jansen-Winkeln B, Langer S, Hoang Do M and
Gockel I: Necrotizing fasciitis. Chirurg: Jan 9, 2020 (Epub ahead
of print). doi: 10.1007/s00104-019-01108-3.
|
|
29
|
Goh T, Goh LG, Ang CH and Wong CH: Early
diagnosis of necrotizing fasciitis. Br J Surg. 101:e119–e125.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Hösl VM, Kehrer A and Prantl L:
Necrotizing fasciitis-a surgical emergency. Unfallchirurg.
123:807–815. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
31
|
Neeki MM, Dong F, Au C, Toy J, Khoshab N,
Lee C, Kwong E, Yuen HW, Lee J, Ayvazian A, et al: Evaluating the
laboratory risk indicator to differentiate cellulitis from
necrotizing fasciitis in the emergency department. West J Emerg
Med. 18:684–689. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Savu C, Melinte A, Balescu I and Bacalbasa
N: Azygos vein anevrysm mimicking a mediastinal mass. In Vivo.
34:2135–2140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamaoka M, Furusawa K, Uemsatsu T and
Yasuda K: Early evaluation of necrotizing fasciitis with use of CT.
J Craniomaxillofac Surg. 22:268–721. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tata MD, Kwan KC, Abdul-Razak MR,
Paramalingam S and Yeen WC: Adjunctive use of superoxidized
solution in chest wall necrotizing soft tissue infection. Ann
Thorac Surg. 87:1613–1614. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Riseman JA, Zamboni WA, Curtis A, Graham
DR, Konrad HR and Ross DS: Hyperbaric oxygen therapy for
necrotizing fasciitis reduces mortality and the need for
debridements. Surgery. 108:847–850. 1990.PubMed/NCBI
|
|
36
|
Deschamps C, Allen MS, Miller DL, Nichols
FC III and Pairolero PC: Management of postpneumonectomy empyema
and bronchopleural fistula. Semin Thorac Cardiovasc Surg. 13:13–19.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Perentes JY, Abdelnour-Berchtold E,
Blatter J, Lovis A, Ris HB, Krueger T and Gonzalez M:
Vacuum-assisted closure device for the management of infected
postpneumonectomy chest cavities. J Thorac Cardiovasc Surg.
149:745–750. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
O'Connor J, Kells A, Henry S and Scalea T:
Vacuum-assisted closure for the treatment of complex chest wounds.
Ann Thorac Surg. 79:1196–1200. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Birnbaum DJ, D'Journo XB, Casanova D and
Thomas PA: Necrotizing fasciitis of the chest wall. Interact
Cardiovasc Thorac Surg. 10:483–484. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Garcia-Orozco VH, Solar-Aguirre C and
Lopez-Yerena I: Successful treatment of necrotizing fasciitis using
handmade negative pressure system wound therapy. Cir Cir. 88 (Suppl
1):S24–S27. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kovács O, Szántó Z and Krasznai G:
Fulminant isolated necrotizing fasciitis of the chest wall,
complicating thoracic empyema. Magy Seb. 69:27–30. 2016.PubMed/NCBI View Article : Google Scholar : (In
Hungarian).
|
|
42
|
Zhu ZC, Yang X, Zheng F, Zheng L and Xu
TS: Clinical study of cervical necrotizing fasciitis accompanied
with descending necrotizing mediastinitis treated with cervical
double parallel incision combined with mediastinoscope or
thoracoscope. Zhonghua Kou Qiang Yi Xue Za Zhi. 54:309–314.
2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
43
|
Peetermans M, de Prost N, Eckmann C,
Norrby-Teglund A, Skrede S and De Waele JJ: Necrotizing skin and
soft-tissue infections in the intensive care unit. Clin Microbiol
Infect. 26(817)2020.PubMed/NCBI View Article : Google Scholar
|