Introduction
Thyroid nodules are quite common in the general
population. The cases mentioned in the medical publications report
a prevalence of 50% of thyroid nodules detected during autopsies in
unknown subjects with thyroid pathology. Malignant nodules are
found in approximately 5% of the population (1-5).
Thyroid cancer accounts for 1% of all malignancies,
but is increasingly becoming more common worldwide. Thyroid
carcinoma affects women more often than men, in most cases
affecting patients aged between 25 and 65 years. The annual
incidence of thyroid tumors varies depending on age, sex, race,
geographic region as well as hormonal and environmental factors
(6,7).
The gold standard in the early diagnosis of thyroid
tumors is considered to be fine-needle aspiration biopsy of thyroid
nodules, ultrasound-guided, followed by cytological evaluation. It
is a simple, safe method, which involves minimal costs and has a
favorable diagnostic accuracy (8-11).
Huang et al presented this method as having a
sensitivity and specificity of approximately 83 and 92% with a
failure rate between 1 and 21%, emphasizing that these failure
rates appear because of the technique and experience of execution
(12).
The Bethesda System for Reporting Thyroid
Cytopathology standardized the results obtained through fine-needle
aspiration procedure and facilitated communication and
collaboration between physicians (13). Gupta et al (14) and Miftari et al (15) concluded that the fine-needle
aspiration technique can provide clear evidence in the diagnosis of
aggressive variants of thyroid papillary carcinoma (14,15).
The surgical treatment comprises primarily of total
thyroidectomy with laryngeal recurrent nerve protection (16). Thus, the aim of the present study
was to determine the correlations of thyroid tumors obtained from
patients and those harvested during autopsies. A complex
histopathological diagnosis is imperative in tailoring to each case
for maximum efficiency.
Materials and methods
Case selection for the study
batch
The cases included in the present study came from
thyroids harvested during autopsies, from patients operated in the
surgery wards and from the patients registered in the oncology
ward, from the Braila Emergency County Hospital.
The study batch was composed of 442 males (78%) and
119 females (22%) (sex ratio=3.71), with age ranging from 10 to 94
years (mean=60.32, SD= ±15.42). The highest incidence was found in
patients aged between 60 and 70 years. The inclusion criteria for
the study were: patients presenting thyroid tumors, treated in the
surgery and oncology departments, as well as thyroid tumors
accidentally detected during autopsies in the Department of
Forensics from the Braila Emergency County Hospital in the selected
period of time. None of the subjects with accidentally detected
tumors had evidence of thyroid disease. Patients that had no
histopathologically confirmed diagnostic were excluded. The
urban/rural distribution encountered in patients with thyroidian
neoplasms was approximately equal.
The following types of interventions were performed
on the thyroid gland: fine-needle aspiration technique and
cytological examination, biopsy (in case of non-removable tumor),
unilateral lobectomy with isthmusectomy, subtotal thyroidectomy,
unilateral lobectomy with isthmusectomy and contralateral subtotal
lobectomy, total thyroidectomy, and extended thyroidectomy in the
pre-thyroid muscles. Thyroid nodules <1 cm in diameter were
generally not punctured, unless they showed suspicious
ultrasound-detected changes such as micro-calcifications.
The study was approved by the Ethics Committee of
the County Emergency Hospital Braila (Braila, Romania), approval
no. 37948/08.10.2020, respecting the ethical standards of the 1975
‘Helsinki Declaration’, revised in 2000, and complying with the
national legislation. Written informed consent was obtained from
the patients.
Tissue sampling and staining
The harvested thyroid specimens were fixed in 10%
formalin, buffered for 24 h and in the case of bulky goiter,
several incisions were performed to facilitate fixation. The
thyroid gland was dissected and carefully separated from the soft
tissues of the peri-thyroid space, fixed in 10% formalin and then
weighed and measured.
Macroscopic changes in the observed structure were
noted, especially the whitish star-shaped scarred areas, suspected
of being carcinomas. Multiple systematic cuts were performed to
cover a wide range of undetected pathologies in the macroscopic
examination.
After weighing the thyroidectomy section, 2- to 3-µm
sections were cut to allow complete visualization of the thyroid
capsule and detection of infra-centrimetric carcinomas.
Over 1,000 sections of thyroid tissue were sampled.
The selected tissue samples were fixed in 10% neutral-buffered
formalin (pH 7.0) for 24-48 hat room temperature and paraffin
embedded. The sections were cut at 5 µm and stained with standard
hematoxylin and eosin at room temperature for 2 h.
The samples harvested from the thyroid gland by
fine-needle aspiration were processed as smears. The slides were
fixed in 95% alcohol and stained with Papanicolaou. Then, the
slides were subjected to complex cyto-histopathological
examination.
All the slides were examined and photographed with a
Nikon Eclipse Ci. Digital images captured using a Digital
Microscope Camera program were processed and analyzed with the
Photos App, running under Windows 10.
Statistical analysis
Variable categories were summarized as counts,
percentages, and continuous variables such as medians. Data are
presented as mean ± SD. Statistical comparisons were made using the
Minitab® program (version 19, Trialware TM), which
showed a mean value of 60.32 for the age (SD, 15.42). The Pearson
Chi-square test was used to compare the type, size of the tumor,
sectioning intervals (0.5 cm for papillary microcarcinomas vs. 1-3
mm as the value for latent papillary carcinomas), multifocality,
lymph node metastases.
The following indicators were calculated in the
statistical analysis: median, mean, standard deviation, asymmetry
and vault indicators for the analyzed immunohistochemical
parameters. P<0.05 was considered statistically significant in
all our tests. There were no repeated experiments.
Results
Following the 526 autopsies performed in a time
interval of 3 years (January 2017-January 2020), 51 thyroid tumors
were identified, 153 cases with thyroid nodules, of which 135 were
multinodular goiters and 18 uninodular goiters.
A further 17 cases were selected from the
histopathological registers between December 2016 and December 2020
from patients operated in the surgery departments and also patients
recorded in the oncology department of the County Emergency
Hospital Braila, who presented thyroid tumors with aggressive
forms.
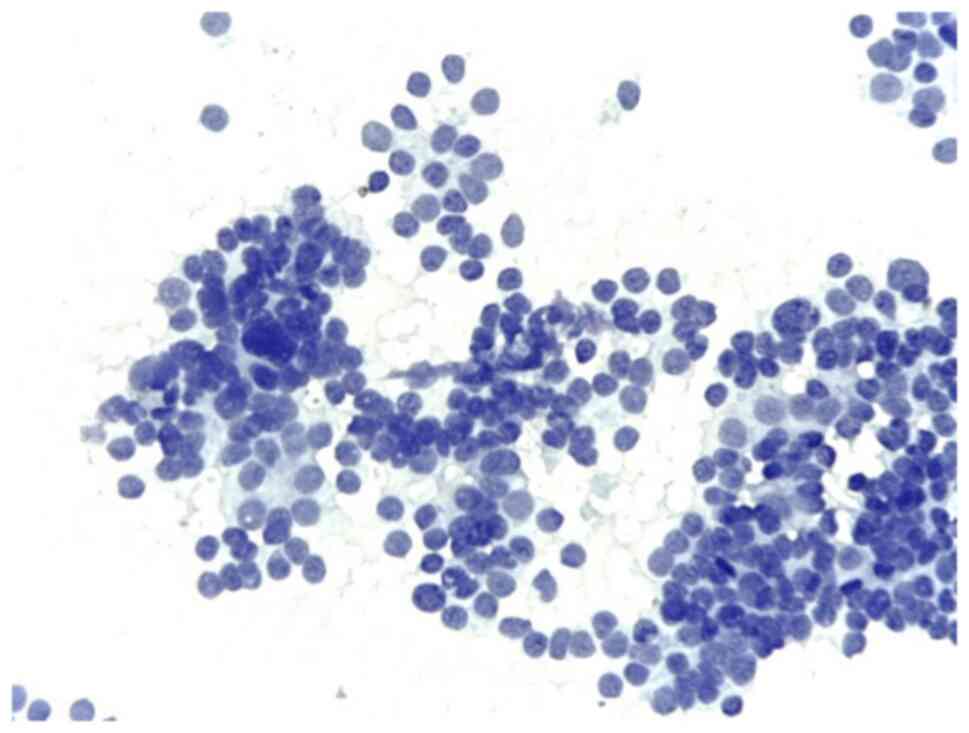
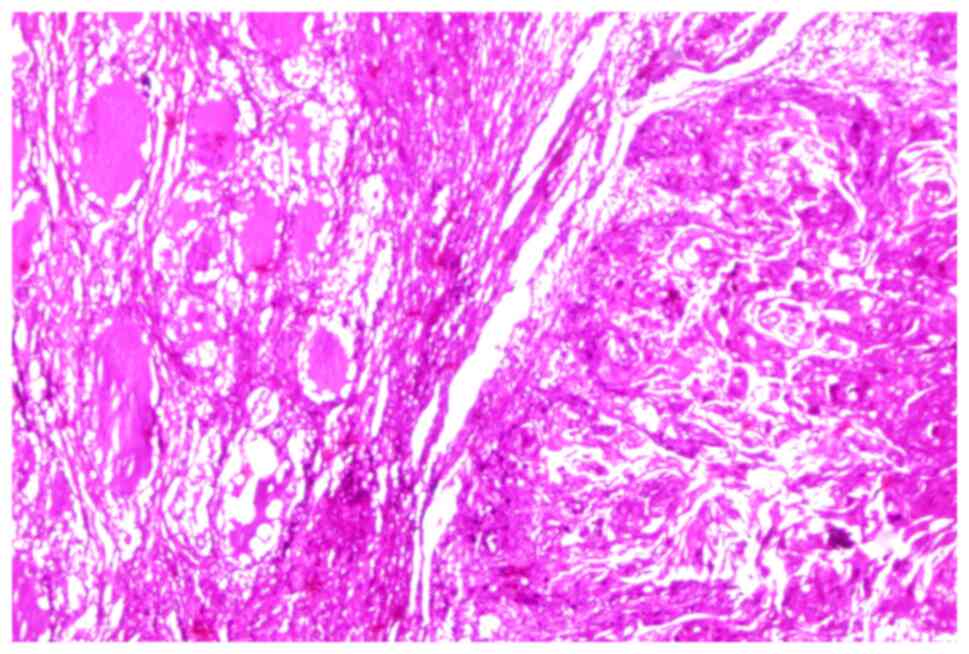
A total of 68 cases with thyroid tumors were
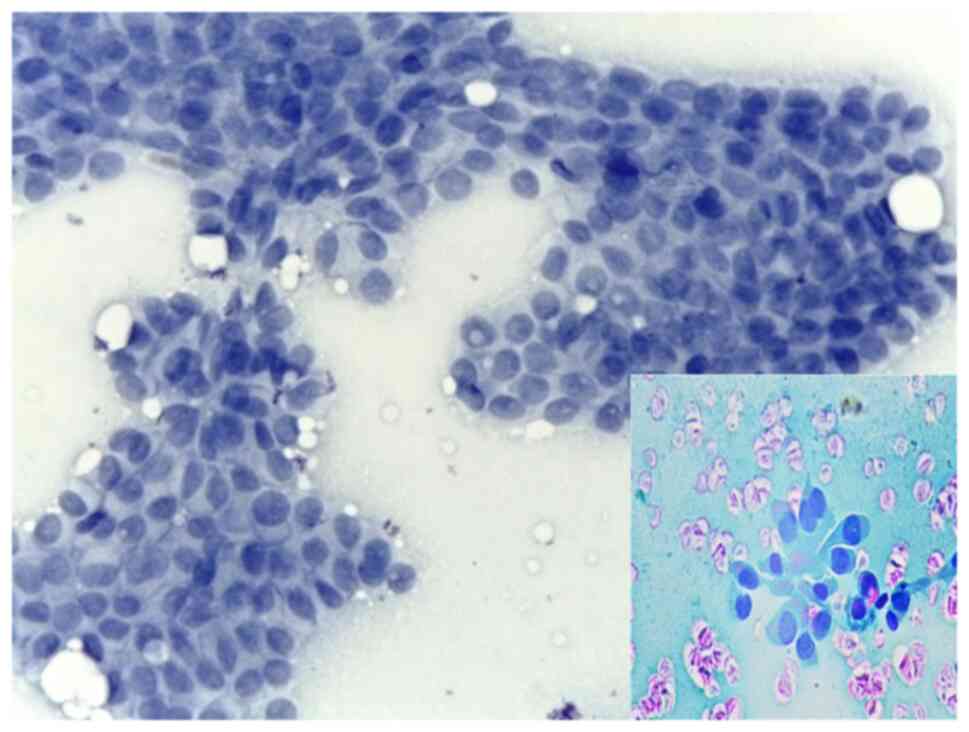
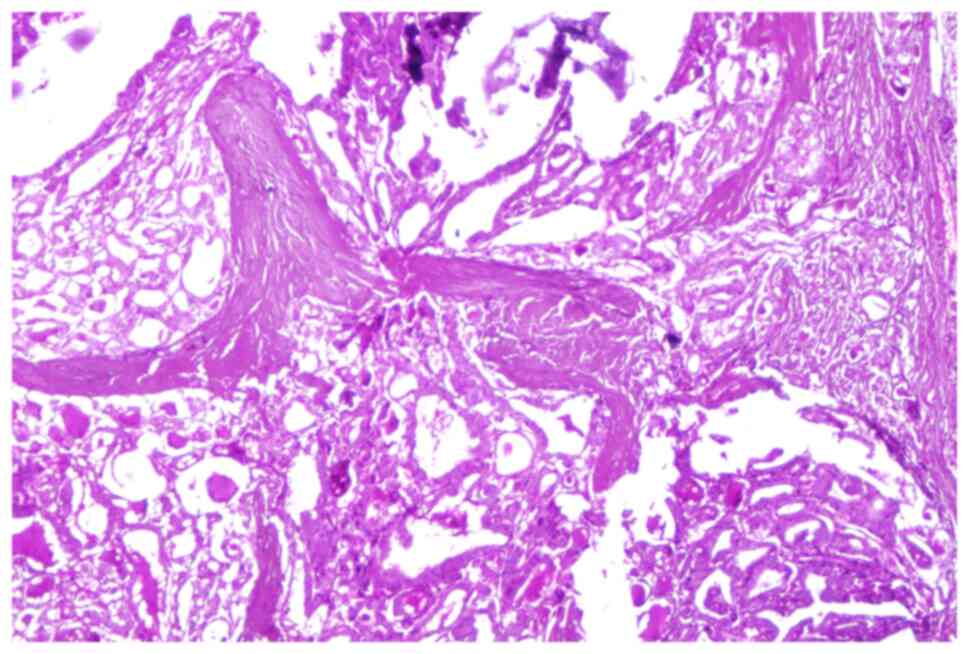
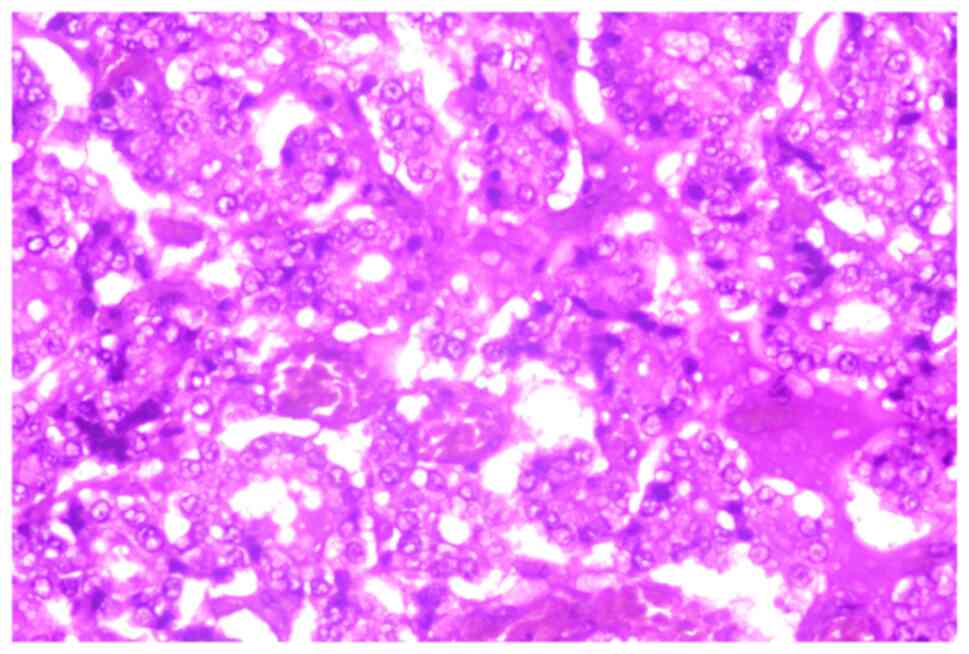
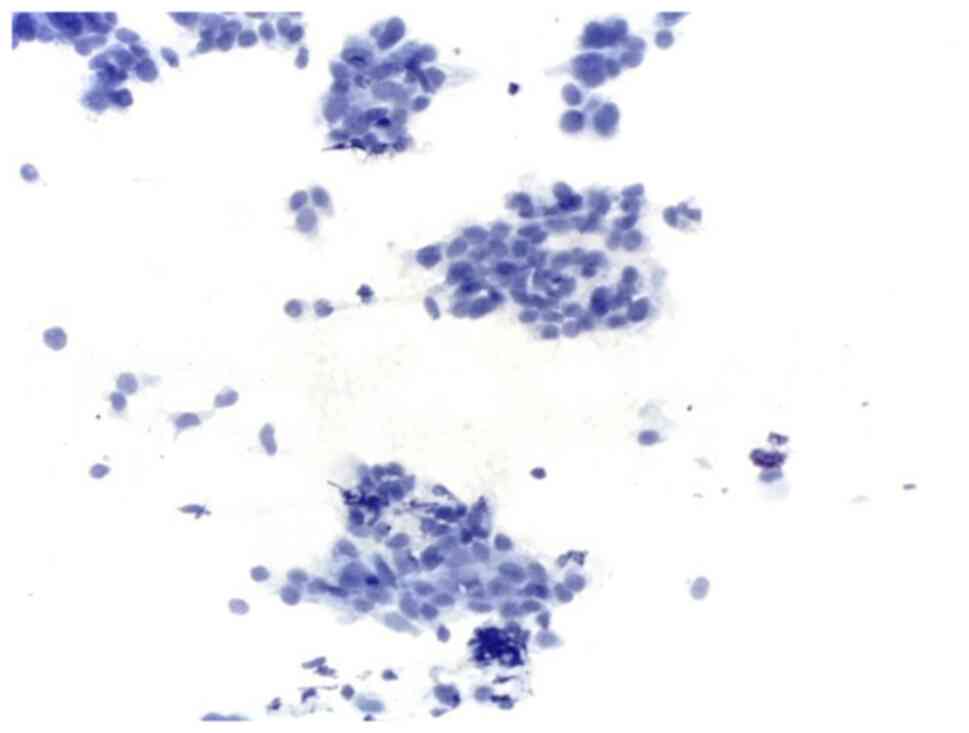
included in the study. From these, 60 were papillary carcinomas
(Fig. 1, Fig. 2 and Fig.
3), 4 follicular carcinomas, two poorly differentiated
(Fig. 4), one medullary (Fig. 5) and one squamous (Fig. 6). Patients included in surgical
treatments also benefited from cytological diagnosis by fine-needle
aspiration.
Papillary carcinomas were carefully reanalyzed to
determine the histological subtype. Microcarcinomas were identified
in the vast majority of cases (55 cases), but there were also 5
cases with variants of papillary carcinoma with aggressive
potential, of which included: tall cell variant (2 cases), one case
with diffuse sclerosis, one hobnail variant and one diffuse
follicular variant.
We used the criteria of the World Health
Organization (WHO) to diagnose and classify the histological
subtypes of the thyroid tumors (17).
Carcinomas that were <1 cm in size were
classified, according to the definition, as papillary
microcarcinomas. These were detected incidentally during the
autopsies. The patients had a long evolution, without symptoms,
their detection being established because of the thorough and
complex analysis of the thyroid gland.
Discussion
By definition, aggressive variants of papillary
thyroid carcinoma have the same characteristic nuclear features
(nuclear inclusions, nuclear notches) with papillary carcinoma but
have different architectural arrangements and distinct cellular
features.
The tall-cell variant of papillary carcinoma was
diagnosed in two male patients, aged 78 and 76 years, respectively,
where the variant showed a characteristic appearance since the
diagnostic cytopuncture, subsequently confirmed by histological
examination.
Leung et al (18) suggested that this variant of
tall-cell papillary carcinoma could not be diagnosed cytologically
by fine-needle aspiration technique. However, Guan et al
(19) and Das et al
(20) showed that the tall-cell
variant was correctly recognized in cytological specimens in
30-100% of cases.
The hobnail variant of papillary carcinoma was
detected in a 69-year-old man who showed symptoms of local pain in
the cervical region, dyspnea, dysphonia and dysphagia. The
fine-needle aspiration method showed typical morpho-cytological
features: the nuclei were located at the top of the cell or in the
middle of the cytoplasm, giving the cell the typical hobnail
appearance (21,22).
The diffuse sclerosing variant of the papillary
carcinoma was identified in a 43-year-old patient. In the present
study, aspiration puncture used in the diffuse sclerosing variant
showed a smear with moderate-high cellularity, with the presence of
colloid dispersed in the background. The characteristic cytological
features highlighted the arrangement of the cells in
three-dimensional layers, ball-like groups and cohesive groups of
cells mixed with inflammatory cells. These characteristics are
similar to those mentioned in the literature.
The diffuse follicular variant is defined as having
a characteristic diffuse spreading in the thyroid gland with
pseudo-areas of follicular patterns and nuclear features that are
characteristic for classical thyroid papillary carcinoma. The
diffuse follicular variant was identified in a 46-year-old woman
who came with dyspnea, dysphagia, dysphonia and vocal cord paresis.
Diagnostic cytopuncture and histological examination revealed a
pattern of follicular growth and nuclear features characteristic of
classical papillary thyroid carcinoma (23).
Thyroid follicular carcinoma occurred in 4 cases in
the study group. Of these, three were women and one was a man, all
4 cases, aged between 50 and 60 years. Follicular thyroid carcinoma
occurs in 10-15% of cases with thyroid malignancies and can also
have an invasive behavior (24). In
the present study, aspiration puncture showed a morphology that was
close to normal, with a round nucleus, increased in volume,
chromatin arranged marginally, without clarifications, but with an
increased number of mitoses. One case consisted mainly of Hurthle
cells with small cell microfollicular architecture, intensely
eosinophilic, vesicular cytoplasm, with small, uniform, round
nuclei centered by prominent nucleoli. Poorly differentiated
carcinoma was found in 2 cases of women aged 76 and 83 years,
respectively.
In the present study, fine-needle aspiration
detected high cellularity with the presence of groups of
agglomerated cells showing an increased nucleus/cytoplasmic ratio
with variable nuclear atypia, mitotic figures, chromatin, fine
granular, with distribution in salt and pepper. Poorly
differentiated carcinoma are frequently widely invasive, with
infiltration of perithyroid tissues observed in 60-70% of the cases
presented.
The cytopathological diagnosis presented in
extensive studies could be specified only in 5-14% of cases. The
remaining cases were diagnosed as ‘suspicion of follicular
neoplasm’ or carcinomas (other than papillary carcinoma, follicular
variant of papillary carcinoma or without other specifications)
(25,26).
Medullary carcinoma can exist in two variants:
sporadic and familial. The familial variant has an autosomal
dominant inheritance, involving the mutation of the proto-oncogene
RET (24). Thyroid medullary
carcinoma was detected in a 52-year-old woman who clinically
presented with a hard, painful nodule at the cervical level
accompanied by flush-like symptoms and diarrhea and paraclinically
high levels of plasma serum calcitonin.
Diagnostic cytopuncture revealed a solid
proliferation composed of round and polygonal cells with
amphophilic cytoplasm and medium-sized nuclei, separated by high
vascular stroma, hyalinized with amyloid deposition, with
carcinoid-like growth pattern and the presence of amyloid
deposits.
Squamous cell carcinoma of the thyroid gland is a
very rare entity, the WHO falling under the subchapter ‘other
carcinomas’ in the category of malignant epithelial tumors
(27-29).
Squamous thyroid carcinoma was diagnosed in a 72-year-old woman who
presented with a hoarse voice, dysphagia and cachexia. External
examination of the neck region revealed a tumor mass plunging
retrosternally. Fine-needle aspiration revealed the presence of
colloid and malignant cells.
Cytomorphological characteristics correlated with
clinical data, immunohistochemical markers and the molecular
profile can quickly elucidate complex and difficult cases. In our
study batch, the patients with aggressive variants, who were
preoperatively diagnosed by fine-needle aspiration, received
surgery with extensive dissection in the neck region. They
benefited from imaging scans to rule out the presence of metastases
and were included more rapidlyin national oncology programs.
Moreover, these patients required close supervision and monitoring,
and in advanced cases of the disease they benefited from complex
palliative care.
On some cytological smears it was not possible to
specifically diagnose an aggressive histological subtype of thyroid
carcinoma. However, even in these cases, the visualized
morphological characteristics were reported, signaling the
possibility of the existence of an aggressive variant of thyroid
carcinoma. Despite the increasing incidence, thyroid cancer
mortality has been decreasing in recent decades due to an improved
diagnostic process and thyroid treatment strategy expected with the
development of knowledge in the field.
In conclusion, recognition of aggressive variants of
thyroid carcinoma requires the application of more aggressive
surgical treatment from the beginning with the optimization and
minimization of post-therapeutic sequelae and the avoidance of
further surgeries. It is also important for a cytopathologist to
have experience and be familiarized with the cytomorphological
features encountered in poorly differentiated carcinomas and
aggressive variants of thyroid carcinomas to avoid misdiagnosis
with other types of primary or secondary thyroid tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IM and DT performed the histological and cytological
examinations, and had major contributions in writing the
manuscript. IM and DM analyzed and interpreted the patient data.
BS, DS and MC searched the literature for similar work and articles
and contributed to writing the manuscript. All authors read and
approved the final manuscript. All authors confirm the authenticity
of the data and the paper.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki. The protocol was
approved (no. 37948/08.10.2020) by the local Bioethics Committee
from the Brăila Emergency County Hospital (Braila, Romania). All
patients previously signed a written informed consent about
hospitalization, treatment and a possible future publication of
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramirez-Gonzalez LR, Sevilla-Vizcaino R,
Monge-Reyes P, Aldaz-Dorantes JE, Marquez-Valdez AR,
Garcia-Martinez D, Gonzalez-Ojeda A and Fuentes-Orozco C: Findings
of thyroid nodules in autopsies in Western Mexico. Rev Med Inst Mex
Seguro Soc. 55:594–598. 2017.PubMed/NCBI(In Spanish).
|
|
2
|
Vaideeswar P, Singaravel S and Gupte P:
The thyroid in ischemic heart disease: An autopsy study. Indian
Heart J. 70 (Suppl 3):S489–S491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Davies L, Morris LG, Haymart M, Chen AY,
Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong
RJ, et al: American association of clinical endocrinologists and
American college of endocrinology disease state clinical review:
The increasing incidence of thyroid cancer. Endocr Pract.
21:686–696. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Milas Z, Shin J and Milas M: New
guidelines for the management of thyroid nodules and differentiated
thyroid cancer. Minerva Endocrinol. 36:53–70. 2011.PubMed/NCBI
|
|
5
|
Mohorea IS, Socea B, Şerban D, Ceausu Z,
Tulin A, Melinte V and Ceausu M: Incidence of thyroid carcinomas in
an extended retrospective study of 526 autopsies. Exp Ther Med.
21(607)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaliszewski K, Zubkiewicz-Kucharska A,
Kiełb P, Maksymowicz J, Krawczyk A and Krawiec O: Comparison of the
prevalence of incidental and non-incidental papillary thyroid
microcarcinoma during 2008-2016: A single-center experience. World
J Surg Oncol. 16(202)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slijepcevic N, Zivaljevic V, Marinkovic J,
Sipetic S, Diklic A and Paunovic I: Retrospective evaluation of the
incidental finding of 403 papillary thyroid microcarcinomas in 2466
patients undergoing thyroid surgery for presumed benign thyroid
disease. BMC Cancer. 15(330)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park SY, Jung YS, Ryu CH, Lee CY, Lee YJ,
Lee EK, Kim SK, Kim TS, Kim TH, Jang J, et al: Identification of
occult tumors by whole-specimen mapping in solitary papillary
thyroid carcinoma. Endocr Relat Cancer. 22:679–686. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee YS, Lim H, Chang HS and Park CS:
Papillary thyroid microcarcinomas are different from latent
papillary thyroid carcinomas at autopsy. J Korean Med Sci.
29:676–679. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Janovsky CCPS, Bittencourt MS, Novais MAP,
Maciel RMB, Biscolla RPM and Zucchi P: Thyroid cancer burden and
economic impact on the Brazilian public health system. Arch
Endocrinol Metab. 62:537–544. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Miyauchi A, Ito Y and Oda H: Insights into
the management of papillary microcarcinoma of the thyroid. Thyroid.
28:23–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang LY, Lee YL, Chou P, Chiu WY and Chu
D: Thyroid fine-needle aspiration biopsy and thyroid cancer
diagnosis: A nationwide population-based study. PLoS One.
10(e0127354)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rossi ED, Faquin WC and Pantanowitz L:
Cytologic features of aggressive variants of follicular-derived
thyroid carcinoma. Cancer Cytopathol. 127:432–446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gupta S, Sodhani P, Jain S and Kumar N:
Morphologic spectrum of papillary carcinoma of the thyroid: Role of
cytology in identifying the variants. Acta Cytol. 48:795–800.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miftari R, Topçiu V, Nura A and
Haxhibeqiri V: Management of the patient with aggressive and
resistant papillary thyroid carcinoma. Med Arch. 70:314–317.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alecu L, Slavu I, Tulin A, Braga V, Socea
B and Nitipir C: A current view on recurrent laryngeal nerve injury
in total thyroidectomy. Mod Med. 28:7–12. 2021.
|
|
17
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J (eds): WHO Classification of Tumours of Endocrine Organs. Vol 10.
4th edition. International Agency for Research on Cancer, Lyon,
2017.
|
|
18
|
Leung AK, Chow SM and Law SC: Clinical
features and outcome of the tall cell variant of papillary thyroid
carcinoma. Laryngoscope. 118:32–38. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guan H, Vandenbussche CJ, Erozan YS,
Rosenthal DL, Tatsas AD, Olson MT, Zheng R, Auger M and Ali SZ: Can
the tall cell variant of papillary thyroid carcinoma be
distinguished from the conventional type in fine needle aspirates?
A cytomorphologic study with assessment of diagnostic accuracy.
Acta Cytol. 57:534–542. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Das DK, Mallik MK, Sharma P, Sheikh ZA,
Mathew PA, Sheikh M, Mirza K, Madda JP, Francis IM and Junaid TA:
Papillary thyroid carcinoma and its variants in fine needle
aspiration smears. A cytomorphologic study with special reference
to tall cell variant. Acta Cytol. 48:325–336. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sheu SY, Schwertheim S, Worm K, Grabellus
F and Schmid KW: Diffuse sclerosing variant of papillary thyroid
carcinoma: Lack of BRAF mutation but occurrence of RET/PTC
rearrangements. Mod Pathol. 20:779–787. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ
and Kang CS: Liquid-based cytology improves preoperative diagnostic
accuracy of the tall cell variant of papillary thyroid carcinoma.
Diagn Cytopathol. 42:11–17. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Gupta S, Ajise O, Dultz L, Wang B, Nonaka
D, Ogilvie J, Heller KS and Patel KN: Follicular variant of
papillary thyroid cancer: Encapsulated, nonencapsulated, and
diffuse: Distinct biologic and clinical entities. Arch Otolaryngol
Head Neck Surg. 138:227–233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Humphrey PA, Dehner LP, Pfeifer JD,
Agarwal A and Al-Kateb H: The Washington Manual of Surgical
Pathology. 2nd edition. Lippincott Williams & Wilkins,
Philadelphia, pp.410-416, 2012.
|
|
25
|
Bongiovanni M, Sadow PM and Faquin WC:
Poorly differentiated thyroid carcinoma: A cytologic-histologic
review. Adv Anat Pathol. 16:283–289. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kane SV and Sharma TP: Cytologic
diagnostic approach to poorly differentiated thyroid carcinoma: A
single-institution study. Cancer Cytopathol. 123:82–91.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lam AK: Squamous cell carcinoma of
thyroid: A unique type of cancer in World Health Organization
classification. Endocr Relat Cancer. 27:R177–R192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hedinger C, Williams ED and Sobin LH
(eds): Other carcinomas. In: Histological Typing of Thyroid Tumours
(World Health Organ. International Histological Classification of
Tumours), 2nd edition, pp. 14-15. Springer, Germany, 1988.
|
|
29
|
Lam AK: Pathology of endocrine tumors
update: World health organization new classification 2017 - other
thyroid tumors. AJSP: Rev Rep. 22:209–216. 2017.
|