Introduction
Bone metabolism, including bone formation and
resorption, is a continuous physiological process that regulates
bone growth and remodeling (1).
Mechanistically, bone formation is initialized by osteoblasts by
synthesizing and secreting the main organic components of bone
matrix, collagen and mucopolysaccharide (2). By contrast, osteoclasts trigger bone
resorption by releasing proteinases to dissolve bone mineral and
degrade bone matrix proteins (3).
In healthy bone remodeling, bone formation and resorption are
maintained in a dynamic balance. However, as the human body ages,
the rate of bone formation decreases, disrupting the aforementioned
balance, thus leading to the development of metabolic bone diseases
such as osteoporosis (4).
Patients with osteoporosis exhibit decreased bone
density and a high risk of fracture (5). In China, osteoporosis is a serious
public health concern due to an increasing aging population
(6). Previous studies have
estimated that there are >60 million individuals in China
suffering with osteoporosis (7,8). A
number of medicines have been used to treat osteoporosis in the
clinic; however, a number of potential side effects have been
associated with these medicines that may impair the health of
patients with osteoporosis during long-term treatment (9). Thus, further investigation into the
development of novel, safe therapeutic strategies for the treatment
of osteoporosis is required.
MicroRNAs (miRNAs/miRs) have been identified as
critical regulators in the development of osteoporosis. Notably,
miR-483-5p was found to be markedly upregulated and promoted
osteoclast differentiation in patients with osteoporosis (10). Another study also investigated the
role of miR-449b-5p in osteogenic differentiation of bone marrow
mesenchymal stem cells (BMSCs) and found that miR-449b-5p could
aggravate osteoporosis by inhibiting osteogenic differentiation
through targeting of Satb2(11).
miR-27b is an intragenic miRNA located in the C9orf3 gene on
chromosome 9(12). miR-27b is
involved in a number of biological processes, such as cell
differentiation, proliferation and apoptosis, by inhibiting the
expression of target genes post-transcriptionally (13-15).
Previous studies revealed that miR-27b expression levels were
abnormally downregulated during the formation of osteoclasts
(16,17). Moreover, miR-27b regulated the
osteogenesis of stem cells from bone marrow and the maxillary sinus
membrane (18,19). Thus, we hypothesize that miR-27b
may be implicated in the pathological process of osteoporosis.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a ligand-activated type II nuclear receptor that is mainly
distributed in adipose tissue (20). As a critical transcription factor,
PPARγ is implicated in a number of metabolic processes, such as
fatty acid and glucose metabolism (21,22).
In bone metabolism, PPARγ inhibits osteoblast formation and
enhances osteoclastogenesis (23,24).
A previous study demonstrated that PPARγ2, an isoform of PPARγ, is
negatively regulated by miR-27b in chondrocytes (25). Thus, miR-27b may play a key role in
the development of osteoporosis by targeting PPARγ2.
An increasing number of studies have suggested that
osteoblast dysfunction disrupts the balance between bone resorption
and bone formation by inhibiting osteoblast differentiation and
proliferation, and enhancing osteoblast apoptosis in
glucocorticoid-induced osteoporosis (26,27).
Exposure to dexamethasone (DEX) induced the apoptosis of
osteoblasts and inhibited MC3T3-E1 cell proliferation (28). In the present study, MC3T3-E1
pre-osteoblasts were treated with DEX to induce osteoporosis in
vitro. The aim of the present study was to determine the
function of the miR-27b/PPARγ axis in DEX-induced proliferation and
osteoblastic differentiation in MC3T3-E1 cells.
Materials and methods
Cell culture and DEX treatment
Mouse MC3T3-E1 pre-osteoblasts obtained from
American Type Culture Collection (cat. no. CRL-2593) were cultured
in DMEM (cat. no. SH30243.01; HyClone; Cytiva) containing 10% FBS
(cat. no. 16000e044; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. P1400; Beijing Solarbio Science
& Technology Co., Ltd.) under 5% CO2 at 37˚C.
Osteogenic differentiation was induced as described in a previous
study (29). MC3T3-E1
pre-osteoblasts were cultured in an osteogenic differentiation
medium consisting of DMEM, 10% FBS, 4 mM glycerophosphate (cat. no.
G9891; Sigma-Aldrich; Merck KGaA) and 25 µg/ml ascorbic acid (cat.
no. A4403; Sigma-Aldrich; Merck KGaA) until cells reached 70%
confluence. Cells were treated with 1 µM DEX (cat. no. D4902;
Sigma-Aldrich; Merck KGaA) to induce osteoporosis in vitro
and DMSO (Beijing Solarbio Science & Technology Co., Ltd.) was
used as a control.
Cell transfection
For overexpression or knockdown of miR-27b-3p, the
miR-27b-3p mimic (5'-UUCACAGUGGCU AAGUUCUGC-3'), miR-27b-3p
inhibitor (5'-GCAGAACUUA GCCACUGUGAA-3') and their corresponding
negative controls (NCs) (NC-mimic, 5'-UGAUACUGUAGACUCGUC AGC-3';
and NC-inhibitor, 5'-CAGUACUUUUGUGUAGUA CAA-3') were synthesized by
Guangzhou RiboBio Co., Ltd. Three PPARγ2 small interfering (si)RNAs
were designed to silence PPARγ2 expression and their sequences were
as follows: siPPARγ2-1, 5'-CGCAUUCCUUUGACAUCAATT-3'; siPPARγ2-2,
5'-CAAUGGUUGCUGAUUACAATT-3'; and siPPARγ2-3,
5'-GGGCGAUCUUGACAGGAAATT-3'. A scrambled siRNA was used as the
corresponding NC (siNC, 5'-UUCUCCGAACGUGUCACGUTT-3'). MC3T3-E1
cells were trypsinized and suspended at a density of
1x106 cells/ml. A total of 2 ml cell suspension was
inoculated into six-well plates overnight at 37˚C in a 5%
CO2 incubator. When MC3T3-E1 cells reached a 60-70%
confluence, cells were transfected with 5 µl mimic, inhibitor or
siRNAs using Lipofectamine® 2000 (cat. no. 11668-019;
Invitrogen; Thermo Fisher Scientific, Inc.) for 4-6 h at 37˚C.
Following 24 h of transfection, serum-free transfer solution was
replaced with complete medium, (DMEM with 10% FBS) and cells were
cultured for a further 48 h.
Cell Counting Kit-8 (CCK-8) assay
MC3T3-E1 cells were re-suspended in PBS at a density
of 2x104 cells/ml for 1 min at 37˚C. A total of 100 µl
suspension was added into a 96-well plate and cultured overnight at
37˚C. Following 0, 12, 24 or 48 h of treatment as aforementioned,
cells were treated with 100 µl CCK-8 solution (Signalway Antibody
LLC) for 1 h. Cell viability was assessed by detecting the OD value
at 460 nm.
Alizarin red S (ARS) staining
Following the induced osteogenic differentiation of
MC3T3-E1 cells, osteogenic differentiation medium was discarded and
the cells were washed three times with PBS. Cells were fixed with
4% paraformaldehyde for 30 min at 37˚C in the dark, and
subsequently stained with 1% ARS (Sigma-Aldrich; Merck KGaA) for
3-5 min at room temperature. Calcification nodules were observed,
and images were captured using an inverted light microscope
(magnification, x100).
Alkaline phosphatase (ALP)
staining
Following the initiation of osteogenic induction,
ALP staining was performed according to the manufacturer's
protocol. Briefly, MC3T3-E1 cells were fixed in 4% formalin for 10
min at 25˚C and washed three times with PBS. ALP staining was
performed using a staining kit (cat. no. G1480; Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
protocol. Images of stained cells were captured using an inverted
light microscope (magnification, x400).
Reverse transcription-quantitative
(RT-q)PCR
RNA samples were extracted from MC3T3-E1 cells using
TRIzol® (Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (cat. no. K1621; Fermentas; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols, and subsequently
amplified using the SYBR Green qPCR Master mix (cat. no. K0223;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The following thermocycling conditions were used for
qPCR: 95˚C for 10 min; followed by 40 cycles at 95˚C for 15 sec and
60˚C for 45 sec; final extension at 95˚C for 15 sec, 60˚C for 1
min, 95˚C for 15 sec and 60˚C for 15 sec. U6 and GAPDH were used as
internal controls, and the relative levels of miR-27b and PPARγ-2
mRNA were calculated using the 2-ΔΔCq method (10). The primer sequences were as
follows: miR-27b-3p forward, 5'-GCGCGTTCACAGTGGC TAAG-3' and
reverse, 5'-AGTGCAGGGTCCGAGGTATT-3'; U6 forward,
5'-GCTTCGGCAGCAC-3' and reverse, 5'-GGAA CGCTTCACG-3; PPARγ2
forward, 5'-TGCGATCAAAGTAG AACC-3' and reverse,
5'-AAGCCTGATGCTTTATCC-3'; and GAPDH forward,
5'-CTGCCCAGAACATCATCC-3' and reverse, 5'-CTCAGATGCCTGCTTCAC-3'.
Western blotting
Target proteins were extracted from MC3T3-E1 cells
using RIPA lysis buffer (Jrdun Biotechnology) and the protein
concentration was determined by a bicinchoninic acid assay kit
(Thermo Fisher Scientific, Inc.). The isolated proteins (25
µg/lane) were separated by electrophoresis in 10%
SDS-polyacrylamide gels, and transferred onto a PVDF membrane.
Membranes were subsequently blocked with 5% non-fat milk overnight
at 4˚C, and incubated with the following primary antibodies:
Anti-PPARγ2 (1:500; cat. no. ab45036; Abcam), anti-bone
morphogenetic protein-2 (BMP2; 1:1,000; cat. no. orb334018;
Biorbyt, Ltd.), anti-runt-related protein 2 (Runx2; 1:1,000; cat.
no. ab23981; Abcam), anti-osteocalcin (OCN; 1:1,000; cat. no.
ab93876; Abcam) and anti-GAPDH (1:2,000; cat. no. 5174; CST
Biological Reagents Co., Ltd.) overnight at 4˚C. Following primary
incubation, membranes were incubated with the horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (Beyotime
Institute of Biotechnology; cat. nos. A0208 and A0216; both
1:1,000) at 37˚C for 1 h. Signal quantification was performed by an
enhanced chemiluminescence system (Bio-Rad Laboratories, Inc.). The
bands were quantified by densitometry with ImageJ software (version
1.51; National Institutes of Health).
Dual-luciferase reporter assay
Bioinformatics software TargetScan 7.2 (targetscan.org/vert_72/) was used to predict
target genes of miR-27b, and the results revealed the binding sites
between miR-27b and PPARγ2. Wild-type (wt) or mutant (mut)
PPARγ2-3'untranslated regions (UTRs) were cloned into a
pGL3-Promoter plasmid containing the firefly luciferase gene
(Promega Corporation). The reconstructed pGL3-Promoter was
introduced into the MC3T3-E1 pre-osteoblasts along with the
pRL-TK-Renilla reporter (Promega Corporation) using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) following manufacturer's protocol for 4-6 h at 37˚C.
Following 6 h of transfection, cells were treated with the miR-27b
mimic. After 24 h, luciferase activity was assessed using a
Dual-Promoter Luciferase Assay kit (cat. no. E1910; Promega
Corporation).
Biochemical detection
Following treatment aforementioned, the supernatant
of MC3T3-E1 pre-osteoblasts was obtained by centrifugation at 800 x
g for 10 min at 4˚C, and ALP activity was determined using an ALP
kit (cat. no. A059-2; Nanjing Jiancheng Bioengineering Institute).
The supernatant and kit solution were mixed and incubated in a
water bath for 15 min at 37˚C, according to the manufacturer's
protocol. The absorbance value was measured at 520 nm.
Statistical analysis
Quantitative analysis was conducted using GraphPad
Prism 7.0 (GraphPad Software, Inc.) and each experiment was
repeated three independent times. Data are presented as the mean ±
standard deviation. The difference between groups was analyzed
using an unpaired t-test, two-way ANOVA followed by Bonferroni's
multiple comparisons test or one-way ANOVA followed by Tukey's
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
DEX treatment significantly reduces
cell viability and miR-27b expression levels in MC3T3-E1
pre-osteoblasts
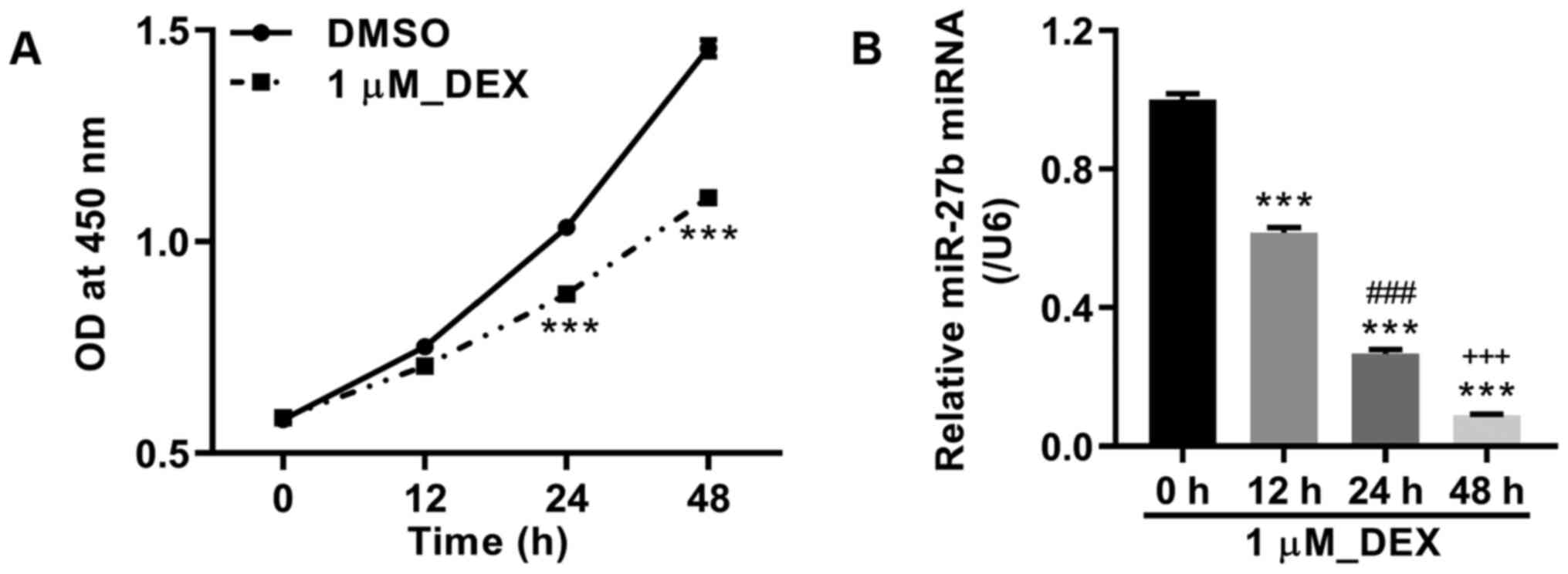
MC3T3-E1 pre-osteoblasts were cultured with 1 µM
DEX, and cell viability was detected at 0, 12, 24 and 48 h after
treatment. The results demonstrated that DEX treatment markedly
inhibited the viability of MC3T3-E1 cells at 24 and 48 h compared
with DMSO (Fig. 1A). In addition,
the miR-27b level was also measured, and DEX treatment
significantly downregulated the expression level of miR-27b at 12,
24 and 48 h compared with 0 h (Fig.
1B).
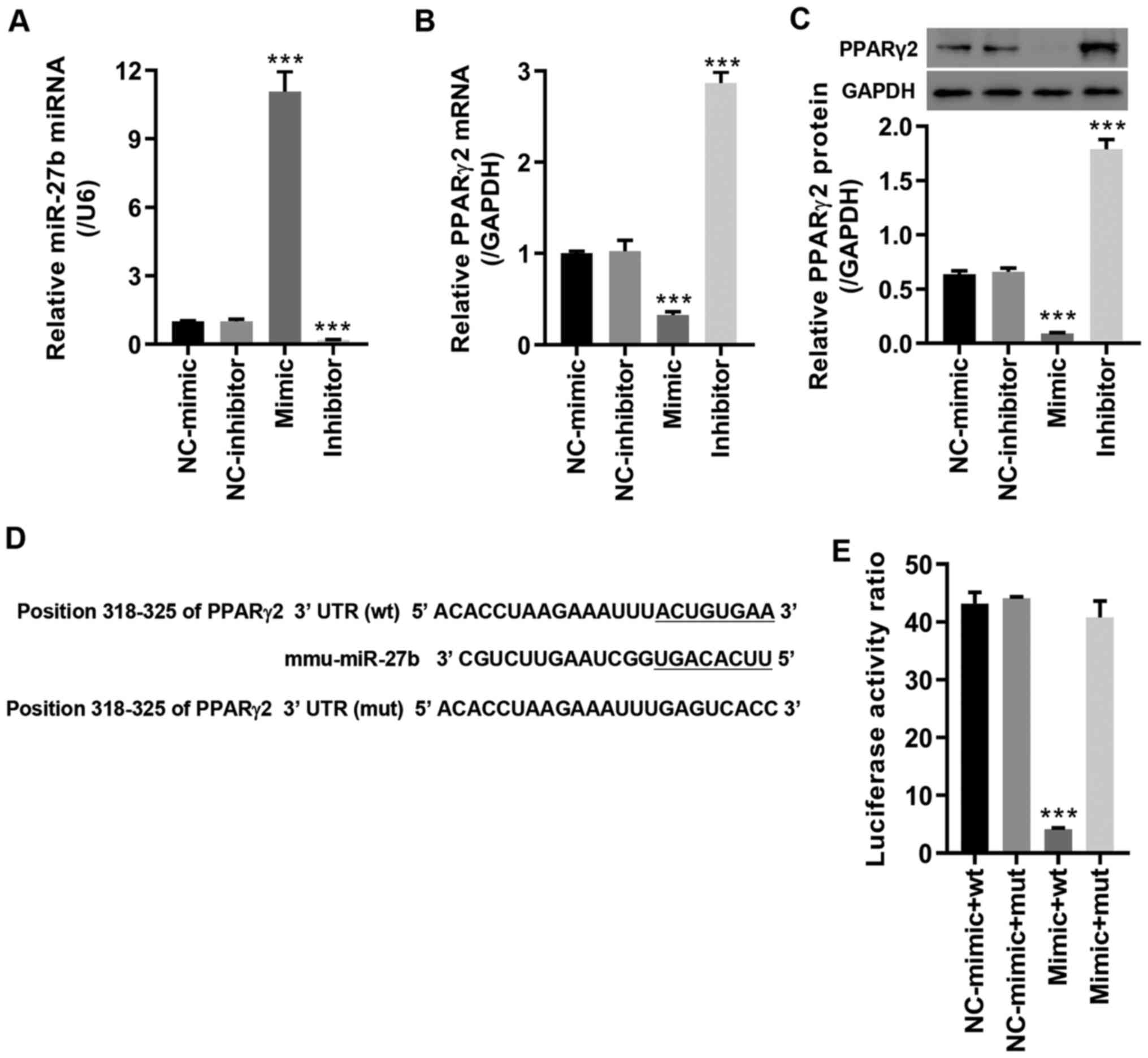
miR-27b directly regulates PPARγ2
The results of the TargetScan bioinformatics
analysis revealed that PPARγ2 was predicted to be a potential
target of miR-27b. To verify this interaction, the miR-27b mimic
and inhibitor were transfected into MC3T3-E1 pre-osteoblasts. The
results indicated that the expression level of miR-27b was markedly
upregulated by the miR-27b mimic and significantly downregulated by
the miR-27b inhibitor compared with its corresponding NC (Fig. 2A). Furthermore, PPARγ2 mRNA and
protein expression was repressed by the miR-27b mimic and
significantly enhanced by the miR-27b inhibitor compared with its
corresponding NC (Fig. 2B and
C). MC3T3-E1 pre-osteoblasts were
co-transfected with the miR-27b mimic and the luciferase vector
containing wt or mut PPARγ2-3'UTR (Fig. 2D). As demonstrated in Fig. 2E, miR-27b overexpression
significantly downregulated the luciferase activity following
transfection with PPARγ2-3'UTR wt compared with NC-mimic; however,
no significant difference was observed in the luciferase activity
following co-transfection with the PPARγ2-3'UTR mut and the miR-27b
mimic. Collectively, these results suggested that miR-27b directly
regulated and repressed the expression level PPARγ2.
miR-27b overexpression attenuates
DEX-inhibited proliferation and osteoblastic differentiation in
MC3T3-E1 pre-osteoblasts
The potential functions of miR-27b were observed
following transfection of the miR-27b mimic in DEX-treated MC3T3-E1
cells. The results of the present study demonstrated that miR-27b
overexpression partly reversed DEX-inhibited miR-27b expression and
cell proliferation (Fig. 3A and
B). The ARS and ALP staining of
M3T3-E1 cells revealed that DEX markedly inhibited osteoblastic
differentiation compared with DMSO plus NC-mimic group, while
DEX-mediated effects were abrogated by the miR-27b mimic (Fig. 3C and D). In addition, the ALP activity, and the
expression levels of BMP2, Runx2 and OCN were investigated as
hallmarks of osteoblastic differentiation. The results of the
present study demonstrated that DEX treatment significantly
decreased the ALP activity and protein expression levels of BMP2,
Runx2 and OCN, but increased PPARγ2 protein expression levels
compared with DMSO plus NC-mimic group. Furthermore, DEX-mediated
effects were abrogated by the miR-27b mimic (Fig. 3E-G). These results suggested that
miR-27b overexpression attenuated DEX-inhibited osteoblastic
differentiation.
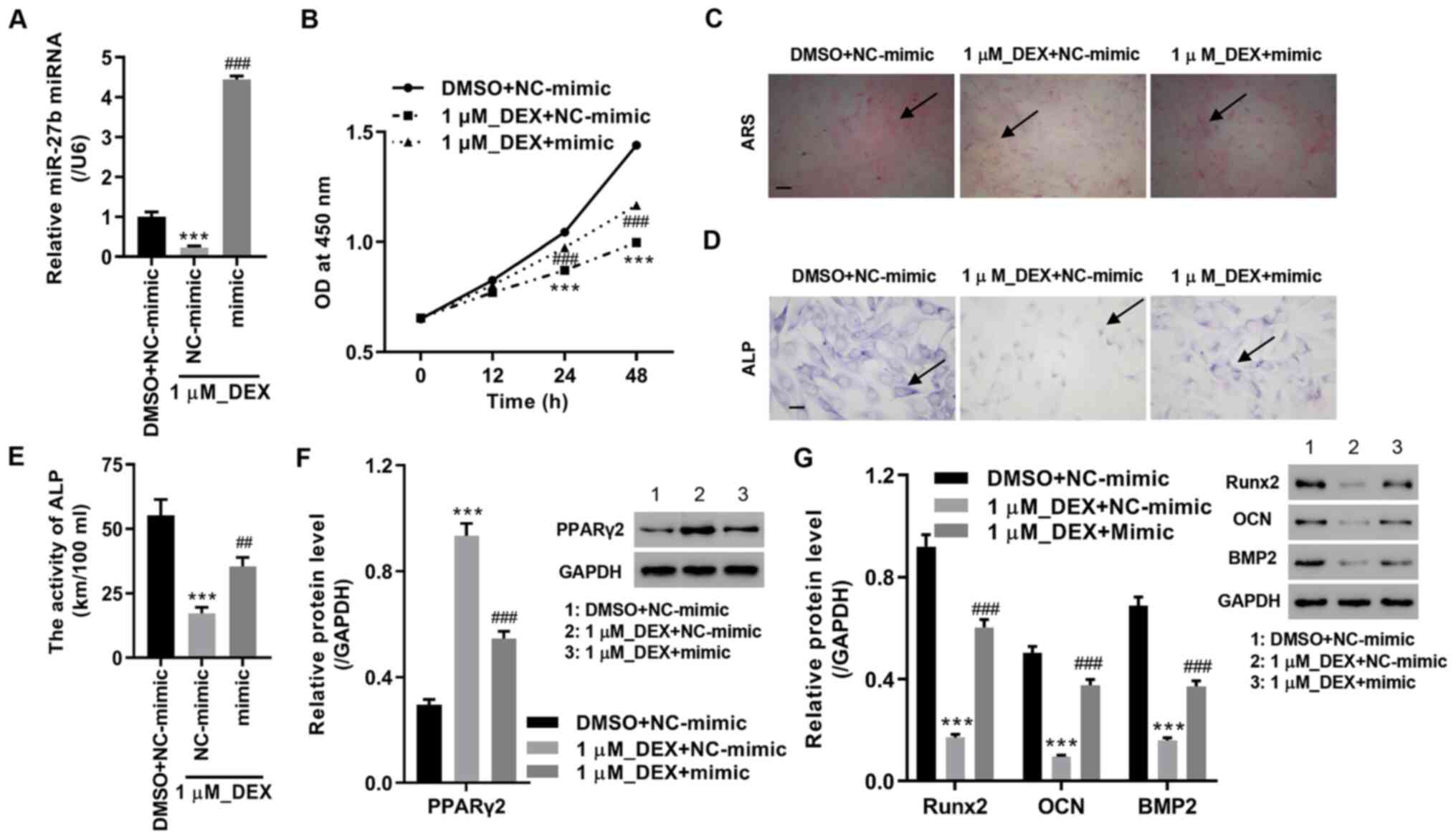 | Figure 3miR-27b mimic attenuates
DEX-inhibited proliferation and osteoblastic differentiation in
MC3T3-E1 pre-osteoblasts. miR-27b mimic and 1µM DEX were used to
treat MC3T3-E1 pre-osteoblasts. (A) miR-27b expression levels were
measured using reverse transcription-quantitative PCR. (B) Cell
viability, (C) ARS staining (scale bar, 100 µm), (D) ALP staining
(scale bar, 25 µm) and (E) ALP activity were measured. Expression
levels of (F) PPARγ2, (G) Runx2, OCN and BMP2 were assessed in
MC3T3-E1 pre-osteoblasts. ***P<0.001 vs. DMSO +
NC-mimic; ##P<0.01 and ###P<0.001 vs. 1
µM_DEX + NC-mimic. miR, microRNA; NC, negative control; DEX,
dexamethasone; ARS, Alizarin red S; ALP, alkaline phosphatase;
PPARγ2, peroxisome proliferator-activated receptor γ2; BMP2, bone
morphogenetic protein-2; Runx2, runt-related protein 2; OCN,
osteocalcin. |
Inhibition of miR-27b suppresses
proliferation and osteoblastic differentiation in MC3T3-E1
pre-osteoblasts by upregulation of PPARγ2
The transfection efficiency of siRNAs targeting
PPARγ2 in MC3T3-E1 cells was demonstrated by RT-qPCR and western
blot analysis, with the lowest mRNA and protein expression detected
in cells transfected with siPPARγ2-2. siPPARγ2-2 was therefore
selected for subsequent analyses (Fig.
4A and B). To investigate the
potential regulation of PPARγ2 by miR-27b, MC3T3-E1 pre-osteoblasts
were co-transfected with the miR-27b inhibitor and PPARγ2 siRNA.
The results of the present study indicated that miR-27b knockdown
significantly increased PPARγ2 expression, and decreased cell
viability, osteoblastic differentiation, ALP activity and the
expression level of BMP2, Runx2 and OCN. However, these effects
were abrogated by siPPARγ2-2 transfection (Fig. 4C-H). Thus, miR-27b knockdown
inhibited proliferation and osteoblastic differentiation in
MC3T3-E1 pre-osteoblasts by the upregulation of PPARγ2.
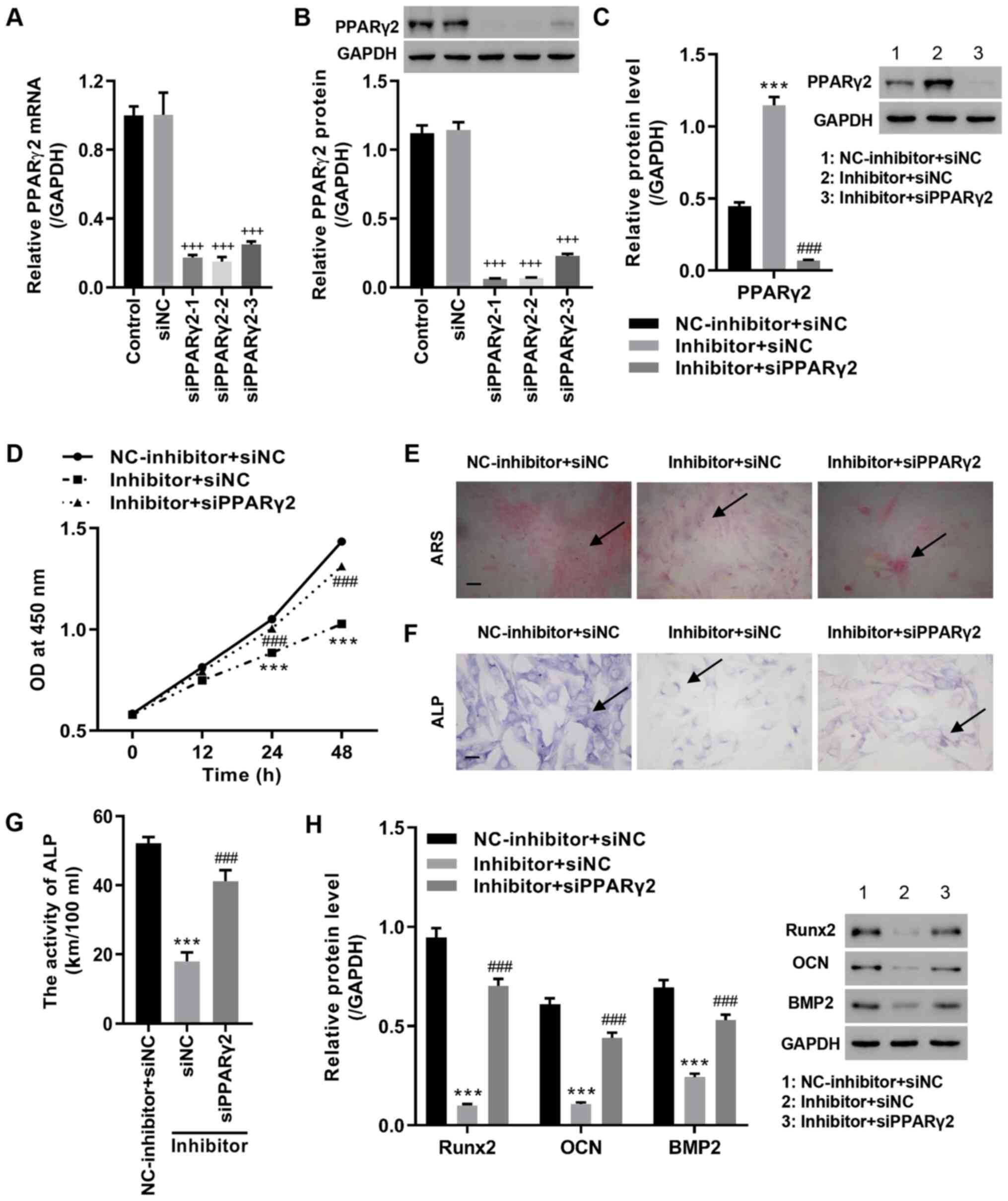 | Figure 4miR-27b inhibitor represses
proliferation and osteoblastic differentiation in MC3T3-E1
pre-osteoblasts by regulating PPARγ2. Cells were transfected with
siPPARγ2, and after 48 h, PPARγ2 (A) mRNA and (B) protein levels
were measured. MC3T3-E1 pre-osteoblasts were also co-transfected
with miR-27b inhibitor and siPPARγ2. (C) PPARγ2 expression was
measured by western blot analysis. (D) Cell viability, (E) ARS
staining (scale bar, 100 µm), (F) ALP staining (scale bar, 25 µm)
and (G) ALP activity were measured. (H) Expression levels of Runx2,
OCN and BMP2 were measured in MC3T3-E1 pre-osteoblasts.
+++P vs. siNC; ***P<0.001 vs. NC-inhibitor
+ siNC; ###P<0.001 vs. Inhibitor + siNC. PPARγ2,
peroxisome proliferator-activated receptor γ2; si, small
interfering; NC, negative control; ARS, Alizarin red S; ALP,
alkaline phosphatase; BMP2, bone morphogenetic protein-2; Runx2,
runt-related protein 2; OCN, osteocalcin; miR, microRNA. |
Discussion
miRNAs are a type of non-coding RNA that function by
inhibiting the expression of downstream target genes (30). A previous study has demonstrated
that miRNAs are critical regulators during the formation, viability
and death of osteoblasts and osteoclasts (31). A number of miRNAs, such as
miR-7b-5p and miR-19a-3p, alleviate the progression of osteoporosis
(32,33). Thus, a number of miRNAs may act as
novel targets for the development of safe and effective
osteoporosis treatment options.
Individuals develop osteoporosis due to decreased
viability and function of osteoblasts caused by glucocorticoid
treatment (26). In the present
study, DEX treatment significantly decreased cell viability, ALP
activity and osteoblastic differentiation of mouse MC3T3-E1
pre-osteoblasts, indicating the successful establishment of an
osteoporosis model induced by DEX. miR-27b is an intragenic miRNA
involved in a number of diseases. For example, miR-27b suppresses
cancer cell proliferation and enhances apoptosis in neuroblastoma,
bladder and gastric cancer (15,34,35).
In cardiac disease, adenoviral vector encoding sense miR-27b
overexpression causes cardiac hypertrophy and fibrosis (36,37).
In osteoarthritis, miR-27b decreases the degradation of the
extracellular matrix in chondrocytes (38). However, the exact pathological
mechanisms underlying miR-27b in osteoporosis remain to be
elucidated. The results of previous studies demonstrated that
miR-27b inhibited osteogenesis in maxillary sinus membrane stem
cells, and promoted osteoblastic differentiation in BMSCs (18,19).
These results suggested that the effect of miR-27b on osteogenesis
depends on the cell type. The results of the present study revealed
that miR-27b knockdown repressed proliferation and osteoblastic
differentiation in MC3T3-E1 pre-osteoblasts, which is consistent
with the findings by Seenprachawong et al (18) that miR-27b promotes osteogenesis in
human MSCs. Moreover, miR-27b overexpression attenuated
DEX-inhibited proliferation and osteoblastic differentiation,
highlighting the potential protective role of miR-27b in
osteoporosis. However, the decreased cell viability and
osteoblastic differentiation in MC3T3-E1 pre-osteoblasts induced by
DEX were not reversed by miR-27b. Previous studies have reported
that a number of other miRNAs, such as miR-365(39), miR-199a (40), let-7f-5p (41) and miR-216a (42), play roles in the function of DEX in
osteoporosis.
PPARγ, a member of the nuclear receptor family,
regulates the transcription of target genes by binding to the
specific PPAR response element (43). Previous studies have reported that
PPARγ is directly regulated by miR-27b in a number of cell lines,
including adipocytes, neuroblastoma cells and BMSCs (18,34,44).
An isoform of PPARγ, PPARγ2, is the target of miR-27b in
chondrocytes (25). Consistent
with the findings of previous studies, the results of the present
study revealed the regulatory effect of miR-27b on PPARγ2 in
MC3T3-E1 cells. A previous study has revealed that PPARγ2 inhibits
osteoblastogenesis and enhances adipogenesis (45). The results of the present study
revealed that miR-27b enhanced proliferation and osteoblastic
differentiation in MC3T3-E1 cells by targeting PPARγ2, highlighting
the importance of PPARγ2 in the formation of osteoblasts.
Previous studies indicated that the expression level
of miR-27b was significantly downregulated during the
differentiation of Raw264.7 pre-osteoclasts into osteoclasts
(16,17), implying the potential involvement
of miR-27b in osteoclastic formation. In addition, PPARγ enhanced
osteoclastic differentiation and activity (46,47).
The results of previous studies have revealed that a number of
miRNAs, such as miR-20a and miR-27a, regulate osteoclastic
formation by targeting PPARγ (46,48).
Thus, we hypothesize that miR-27b may also function in
osteoclastogenesis by regulating PPARγ. The results of a previous
study indicated that miR-27b enhanced osteogenesis in human BMSCs
by the specific downregulation of PPARγ (18). Furthermore, inhibition of PPARγ
ameliorated DEX-induced osteoporosis in a mouse model (49), highlighting the role of miR-27b and
PPARγ in osteoporosis. However, the molecular mechanism underlying
the increased expression of PPARγ induced by DEX remains to be
elucidated. To the best of our knowledge, the present study is the
first to demonstrate the increased level of miR-27b induced by DEX,
and the function of the miR-27b/PPARγ axis in DEX-induced
proliferation and osteoblastic differentiation in MC3T3-E1
cells.
In conclusion, the results of the present study
demonstrated that miR-27b alleviated DEX-inhibited proliferation
and differentiation in MC3T3-E1 pre-osteoblasts. Therefore, miR-27b
may act as a potential target for the treatment of osteoporosis.
Further in vitro experiments and clinical practice are
required to explore the potential role of miR-27b in
osteoporosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was approved by the Inner Mongolia
Natural Science Foundation (grant. no. 2017MS08118) and the Inner
Mongolia Medical University ‘Science and Technology Million
Project' (grant no. YKD2016kjbw010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and HL designed this study. TY and MW performed
the experiments. AH, HJ and MM analyzed and interpreted the data.
TY and SL confirm the authenticity of all the raw data. HL, TY and
SL wrote the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gaffney-Stomberg E: The impact of trace
minerals on bone metabolism. Biol Trace Elem Res. 188:26–34.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kini U and Nandeesh B: Physiology of bone
formation, remodeling, and metabolism. In: Radionuclide and Hybrid
Bone Imaging. Fogelman I, Gnanasegaran G and van der Wall H (eds).
Springer, Berlin, Heidelberg, pp29-57, 2012.
|
|
3
|
Strålberg F, Kassem A, Kasprzykowski F,
Abrahamson M, Grubb A, Lindholm C and Lerner UH: Inhibition of
lipopolysaccharide-induced osteoclast formation and bone resorption
in vitro and in vivo by cysteine proteinase inhibitors. J Leukoc
Biol. 101:1233–1243. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Demontiero O, Vidal C and Duque G: Aging
and bone loss: New insights for the clinician. Ther Adv
Musculoskelet Dis. 4:61–76. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang N and Xia W: Assessment of bone
quality in patients with diabetes mellitus. Osteoporos Int.
29:1721–1736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin X, Xiong D, Peng YQ, Sheng ZF, Wu XY,
Wu XP, Wu F, Yuan LQ and Liao EY: Epidemiology and management of
osteoporosis in the People's Republic of China: Current
perspectives. Clin Interv Aging. 10:1017–1033. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang
Q, Huang J, Wen Q, Hu R, Wang L, et al: The prevalence of
osteoporosis in China, a nationwide, multicenter DXA survey. J Bone
Miner Res. 34:1789–1797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cui Z, Meng X, Feng H, Zhuang S, Liu Z,
Zhu T, Ye K, Xing Y, Sun C, Zhou F, et al: Estimation and
projection about the standardized prevalence of osteoporosis in
mainland China. Arch Osteoporos. 15(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skjødt MK, Frost M and Abrahamsen B: Side
effects of drugs for osteoporosis and metastatic bone disease. Br J
Clin Pharmacol. 85:1063–1071. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li K, Chen S, Cai P, Chen K, Li L, Yang X,
Yi J, Luo X, Du Y and Zheng H: miRNA-483-5p is involved in the
pathogenesis of osteoporosis by promoting osteoclast
differentiation. Mol Cell Probes. 49(101479)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li JY, Wei X, Sun Q, Zhao XQ, Zheng CY,
Bai CX, Du J, Zhang Z, Zhu LG and Jia YS: MicroRNA-449b-5p promotes
the progression of osteoporosis by inhibiting osteogenic
differentiation of BMSCs via targeting Satb2. Eur Rev Med Pharmacol
Sci. 23:6394–6403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kida K, Nakajima M, Mohri T, Oda Y, Takagi
S, Fukami T and Yokoi T: PPARα is regulated by miR-21 and miR-27b
in human liver. Pharm Res. 28:2467–2476. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen D, Si W, Shen J, Du C, Lou W, Bao C,
Zheng H, Pan J, Zhong G, Xu L, et al: miR-27b-3p inhibits
proliferation and potentially reverses multi-chemoresistance by
targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis.
9(188)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Henriksen TI, Davidsen PK, Pedersen M,
Schultz HS, Hansen NS, Larsen TJ, Vaag A, Pedersen BK, Nielsen S
and Scheele C: Dysregulation of a novel miR-23b/27b-p53 axis
impairs muscle stem cell differentiation of humans with type 2
diabetes. Mol Metab. 6:770–779. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu X, Yan T, Wang Z, Wu X, Cao G and Zhang
C: lncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and
inhibits apoptosis by regulating miR-27b. Biomed Pharmacother.
96:299–304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han Z, Zhan R, Chen S, Deng J, Shi J and
Wang W: miR-181b/Oncostatin m axis inhibits prostate cancer bone
metastasis via modulating osteoclast differentiation. J Cell
Biochem. 121:1664–1674. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takigawa S, Chen A, Wan Q, Na S, Sudo A,
Yokota H and Hamamura K: Role of miR-222-3p in c-Src-Mediated
Regulation of Osteoclastogenesis. Int J Mol Sci.
17(240)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Seenprachawong K, Tawornsawutruk T,
Nantasenamat C, Nuchnoi P, Hongeng S and Supokawej A: miR-130a and
miR-27b enhance osteogenesis in human bone marrow mesenchymal stem
cells via specific down-regulation of peroxisome
proliferator-activated receptor γ. Front Genet.
9(543)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng W, Zhu S, Li X, Weng J and Chen S:
miR-27b-3p suppressed osteogenic differentiation of maxillary sinus
membrane stem cells by targeting Sp7. Implant Dent. 26:492–499.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guo M, Li C, Lei Y, Xu S, Zhao D and Lu
X-Y: Role of the adipose PPARγ-adiponectin axis in susceptibility
to stress and depression/anxiety-related behaviors. Mol Psychiatry.
22:1056–1068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Calvier L, Chouvarine P, Legchenko E,
Hoffmann N, Geldner J, Borchert P, Jonigk D, Mozes MM and Hansmann
G: PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle
cells, regulating cell proliferation and glucose metabolism. Cell
Metab. 25:1118–1134.e1117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ye G, Gao H, Wang Z, Lin Y, Liao X, Zhang
H, Chi Y, Zhu H and Dong S: PPARα and PPARγ activation attenuates
total free fatty acid and triglyceride accumulation in macrophages
via the inhibition of Fatp1 expression. Cell Death Dis.
10(39)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan Y, Chong L-W and Evans RM: PPAR-γ
regulates osteoclastogenesis in mice. Nat Med. 13:1496–1503.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Zhuang H, Zhang X, Zhu C, Tang X, Yu F,
Shang GW and Cai X: Molecular mechanisms of PPAR-γ governing MSC
osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther.
11:255–264. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu J, Lv S, Hou Y, Xu K, Sun D, Zheng Y,
Zhang Z, Li X, Li Y and Chi G: miR-27b promotes type II collagen
expression by targetting peroxisome proliferator-activated
receptor-γ2 during rat articular chondrocyte differentiation.
Biosci Rep. 38(BSR20171109)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu WN, Zheng HL, Yang RZ, Jiang LS and
Jiang SD: HIF-1α Regulates Glucocorticoid-Induced Osteoporosis
Through PDK1/AKT/mTOR Signaling Pathway. Front Endocrinol
(Lausanne). 10(922)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li P, Mao WW, Zhang S, Zhang L, Chen ZR
and Lu ZD: Sodium hydrosulfide alleviates dexamethasone-induced
cell senescence and dysfunction through targeting the miR-22/sirt1
pathway in osteoblastic MC3T3-E1 cells. Exp Ther Med.
21(238)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang L, Liu S, Mu S, Guo R, Zhou L and Fu
Q: Paeoniflorin Attenuates Dexamethasone-Induced Apoptosis of
Osteoblast Cells and Promotes Bone Formation via Regulating
AKT/mTOR/Autophagy Signaling Pathway. Evid Based Complement
Alternat Med. 2021(6623464)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu C, Zheng XF, Yang YH, Li B, Wang YR,
Jiang SD and Jiang LS: LGR4 acts as a key receptor for R-spondin 2
to promote osteogenesis through Wnt signaling pathway. Cell Signal.
28:989–1000. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gulyaeva LF and Kushlinskiy NE: Regulatory
mechanisms of microRNA expression. J Transl Med.
14(143)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ge DW, Wang WW, Chen HT, Yang L and Cao
XJ: Functions of microRNAs in osteoporosis. Eur Rev Med Pharmacol
Sci. 21:4784–4789. 2017.PubMed/NCBI
|
|
32
|
Chen R, Qiu H, Tong Y, Liao F, Hu X, Qiu Y
and Liao Y: miRNA-19a-3p alleviates the progression of osteoporosis
by targeting HDAC4 to promote the osteogenic differentiation of
hMSCs. Biochem Biophys Res Commun. 516:666–672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li QQ, Wei Q, Zhai XC, Qin L, Li HB, Meng
R and Chen SC: miRNA-7b-5p attenuates the progression of
osteoporosis by inhibiting adipose differentiation of hMSCs via
regulating IRS2. Eur Rev Med Pharmacol Sci. 23:9207–9214.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee JJ, Drakaki A, Iliopoulos D and Struhl
K: miR-27b targets PPARγ to inhibit growth, tumor progression and
the inflammatory response in neuroblastoma cells. Oncogene.
31:3818–3825. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tao J, Zhi X, Zhang X, Fu M, Huang H, Fan
Y, Guan W and Zou C: miR-27b-3p suppresses cell proliferation
through targeting receptor tyrosine kinase like orphan receptor 1
in gastric cancer. J Exp Clin Cancer Res. 34(139)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Song Y, Zhang Y, Xiao H, Sun Q,
Hou N, Guo S, Wang Y, Fan K, Zhan D, et al: Cardiomyocyte
overexpression of miR-27b induces cardiac hypertrophy and
dysfunction in mice. Cell Res. 22:516–527. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hou N, Wang J, Li Z, Cao Y, Fan K and Yang
X: Cardiomycyte overexpression of miR-27b resulted in cardiac
fibrosis and mitochondria injury in mice. Yi Chuan. 34:326–334.
2012.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
38
|
Li YF, Li SH, Liu Y and Luo YT: Long
noncoding RNA CIR promotes chondrocyte extracellular matrix
degradation in osteoarthritis by acting as a sponge for Mir-27b.
Cell Physiol Biochem. 43:602–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu D, Gao Y, Hu N, Wu L and Chen Q:
miR-365 Ameliorates Dexamethasone-Induced Suppression of
Osteogenesis in MC3T3-E1 Cells by Targeting HDAC4. Int J Mol Sci.
18(977)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tang J, Yu H, Wang Y, Duan G, Wang B, Li W
and Zhu Z: MicroRNA-199a counteracts glucocorticoid inhibition of
bone marrow mesenchymal stem cell osteogenic differentiation
through regulation of Klotho expression in vitro. Cell Biol Int.
44:2532–2540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shen GY, Ren H, Shang Q, Zhao WH, Zhang
ZD, Yu X, Huang JJ, Tang JJ, Yang ZD, Liang D, et al: Let-7f-5p
regulates TGFBR1 in glucocorticoid-inhibited osteoblast
differentiation and ameliorates glucocorticoid-induced bone loss.
Int J Biol Sci. 15:2182–2197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li H, Li T, Fan J, Li T, Fan L, Wang S,
Weng X, Han Q and Zhao RC: miR-216a rescues dexamethasone
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT
pathway. Cell Death Differ. 22:1935–1945. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Corrales P, Izquierdo-Lahuerta A and
Medina-Gómez G: Maintenance of kidney metabolic homeostasis by PPAR
gamma. Int J Mol Sci. 19(2063)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Karbiener M, Fischer C, Nowitsch S,
Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ and Scheideler M:
MicroRNA miR-27b impairs human adipocyte differentiation and
targets PPARgamma. Biochem Biophys Res Commun. 390:247–251.
2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shockley KR, Lazarenko OP, Czernik PJ,
Rosen CJ, Churchill GA and Lecka-Czernik B: PPARgamma2 nuclear
receptor controls multiple regulatory pathways of osteoblast
differentiation from marrow mesenchymal stem cells. J Cell Biochem.
106:232–246. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Guo L, Chen K, Yuan J, Huang P, Xu X, Li
C, Qian N, Qi J, Shao Z, Deng L, et al: Estrogen inhibits
osteoclasts formation and bone resorption via microRNA-27a
targeting PPARγ and APC. J Cell Physiol. 234:581–594.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Patel JJ, Butters OR and Arnett TR: PPAR
agonists stimulate adipogenesis at the expense of osteoblast
differentiation while inhibiting osteoclast formation and activity.
Cell Biochem Funct. 32:368–377. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang H and Shen Y: MicroRNA 20a negatively
regulates the growth and osteoclastogenesis of THP 1 cells by
downregulating PPARγ. Mol Med Rep. 20:4271–4276. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang Y, Pan Z and Chen F: Inhibition of
PPARγ by bisphenol A diglycidyl ether ameliorates
dexamethasone-induced osteoporosis in a mouse model. J Int Med Res.
47:6268–6277. 2019.PubMed/NCBI View Article : Google Scholar
|