Introduction
Bisphosphonates (BPs) are a series of recently
developed drugs targeting musculoskeletal diseases and disorders of
calcium metabolism. BPs act via binding with hydroxyapatite in the
bones to attenuate the activity of osteoclasts, thereby inhibiting
bone resorption (1). Therefore,
BPs have been used to treat osteoporosis, scleromalacia, metastatic
bone cancer-induced hypercalcemia and itai-itai disease (2). Osteonecrosis of the jaw (ONJ) is a
rare condition that occurs in patients with cancer treated with
multimodality therapies, including intravenous or oral
administration of BPs (3). The
incidence of drug-induced ONJ is the highest among patients treated
with third-generation BPs, such as zoledronic acid (ZA) (4,5).
VEGF regulates the proliferation and differentiation
of osteoblasts, as well as attenuating blood vessel growth and bone
regeneration by blocking the VEGF receptors VEGFR1 and
VEGFR2(6). Previous studies have
demonstrated that exogenous VEGF could improve cell mineralization
and osteogenesis in vivo in different bone defect animal
models (7-9).
Additionally, a recent study revealed a positive association
between the occurrence of ONJ and treatment with bone resorption
and VEGFR tyrosine kinase inhibitors (10). Based on the aforementioned
findings, the present study hypothesized that VEGF could be
involved in BP-induced ONJ. Therefore, the present study aimed to
investigate whether VEGF could affect the apoptosis and
differentiation of ZA-stimulated osteoblasts. The present results
may provide new insights into the development of novel approaches
for the treatment or prevention of BP-induced ONJ.
Materials and methods
Cell culture and treatment
The murine osteoblast cell line MC3T3-E1 was
obtained from the American Type Culture Collection. The cells were
grown in α-minimal essential medium (α-MEM) supplemented with
ribonucleosides, deoxyribonucleosides, 2 mM L-glutamine, 1 mM
sodium pyruvate (Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS
(Thermo Fisher Scientific, Inc.), without the addition of ascorbic
acid. MCT3T-E1 cells were stimulated with different doses (0.1, 1
or 5 µM) of ZA (Selleck Chemicals) which was dissolved in sterile
distilled water to evaluate cell apoptosis and differentiation.
Murine VEGF (R&D Systems, Inc.) at a concentration of 10 ng/ml
in sterile PBS was selected to treat ZA-stimulated MCT3T-E1 cells,
as previously described (11).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MC3T3-E1 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA purity was
quantified by determining the optical density (OD)260/OD280 ratio
using a spectrophotometer (Mettler-Toledo GmbH). RNA quality was
estimated by agarose electrophoresis. Subsequently, RNA was reverse
transcribed into complementary DNA (cDNA) with RevertAid First
Strand cDNA Synthesis kit according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc.) a
LightCycler® 480 system (Roche Diagnostics). The
amplification of the target cDNAs were performed using the
BeyoFast™ SYBR Green One-Step RT-qPCR kit (Beyotime Institute of
Biotechnology). The thermocycling conditions were as follows:
Initial denaturation at 95˚C for 5 min, followed by 40 cycles of
denaturation for 15 sec at 95˚C and annealing for 30 sec at 60˚C.
The primers were as follows: VEGF forward,
5'-CTGCCGTCCGATTGAGACC-3', reverse: 5'-CCCCTCCTTGTACCACTGTC-3';
GAPDH, forward: 5'-AGGTCGGTGTGAACGGATTTG-3', reverse:
5'-GGGGTCGTTGATGGCAACA-3'. Lastly, the relative expression levels
of the target genes were measured using the 2-ΔΔCq
method (12).
Western blot analysis
Proteins were extracted from MC3T3-E1 cells with
RIPA lysis buffer (Absin Biotechnology Co., Ltd.) and the protein
concentration was measured using a BCA kit (Beyotime Institute of
Biotechnology). The protein samples were then boiled at 100˚C for
3-5 min to achieve complete protein denaturation, followed by
separation by 12% SDS-PAGE (30 µg/lane; Beijing Solarbio Science
& Technology Co., Ltd.). Subsequently, the proteins were
transferred onto a PVDF membrane (Corning, Inc.). Following
blocking with 5% bovine serum albumin at room temperature (Absin
Biotechnology Co., Ltd.) for 2 h, the membranes were incubated with
primary antibodies [Bcl-2, cat. no. 3498, 1:1,000; Bax, cat. no.
2772, 1:1,000; runt-related transcription factor 2 (RUNX2), cat.
no. 12556, 1:1,000; NLRP3, cat. no. 15101, 1:1,000; caspase 1, cat.
no. 83383, 1:1,000; GSDMD, cat. no. 39754, 1:1,000; GAPDH, cat. no.
5174, 1:1,000, Cell Signaling Technology, Inc.; VEGF, cat. no.
ab214424, 1:1,000; osteocalcin, cat. no. ab133612, 1:1,000;
osteopontin, ab283656, 1:1,000; all Abcam] diluted in TBS-Tween-20
(0.1% Tween-20, TBST) on a shaking bed at 4˚C overnight. Membranes
were washed with TBST, then incubated with the corresponding
horseradish peroxidase-conjugated secondary antibody (Anti-rabbit
IgG; cat. no. 7074, 1:10,000, Cell Signaling Technology, Inc.) at
room temperature for 2 h. Finally, the protein bands were
visualized using an ECL kit (Beyotime Institute of Biotechnology).
GAPDH served as the loading control.
Cell Counting Kit-8 (CCK-8) assay
The viability of ZA-treated MC3T3-E1 cells
overexpressing VEGF or not was determined using a CCK-8 assay
(Beyotime Institute of Biotechnology). Briefly, a 100-µl cell
suspension was added into a 96-well plate at a density of
2x103 cells/well. Following treatment, 10 µl of CCK-8
solution were added to each well and cells were incubated for 1 h
at 37˚C. Cell viability was assessed by measuring the OD value in
each well at a wavelength of 450 nm using a spectrophotometer
(Mettler-Toledo GmbH).
TUNEL staining
Adherent MC3T3-E1 cells were fixed with 4%
paraformaldehyde at room temperature for 30 min (Shanghai Aladdin
Bio-Chem Technology Co., Ltd.), and were first incubated with 0.3%
Triton X-100 (Thermo Fisher Scientific, Inc.) at room temperature
for 5 min and then with 0.3% H2O2 (Merck
KGaA) in PBS (Biofount; Beijing Biote Pharmaceutical Co., Ltd.) for
an additional 20 min at room temperature. Prior to each step, cells
were washed thrice with Hanks' balanced salt solution (HBSS;
Sigma-Aldrich; Merck KGaA). Apoptotic cells were stained using
TUNEL Apoptosis Assay kit by according to the manufacturer's
guidance (Beyotime Institute of Biotechnology). The nuclei were
stained using DAPI (0.1 µg/ml) at room temperature for 5 min in the
dark. The cells were observed under a fluorescence microscope
(magnification, x200) and six random fields were chosen after
Antifade Mounting Medium (Beyotime Institute of Biotechnology) was
used to block sections.
Alizarin Red S (ARS) staining
The mineralization of MC3T3-E1 cells was evaluated
using the ARS Staining kit for Osteogenesis (Sigma-Aldrich; Merck
KGaA). Briefly, cells were seeded into a 24-well plate at a density
of 250 cells/well and grown in complete culture medium for 3 days
until 95% confluency. Subsequently, cell mineralization was induced
following supplementation with osteogenic α-MEM with 10% FBS, 20 mM
β-glycerophosphate and 100 mg/ml ascorbic acid, 7.5 mM glycerol
phosphate and 50 mg/ml ascorbic acid. After fixation at room
temperature in 95% ethanol for 10 min and washing with sterilized
water, ARS was utilized to stain cells at room temperature for 30
min. Finally, prior to an inverted light microscopy observation
(Olympus), stained cells were washed with distilled water.
Alkaline phosphatase (ALP) activity
assay
ALP activity was measured using Mouse ALP ELISA kit
(cat. no. 69-50082, MSK Biotechnology Co., Ltd.) to evaluate the
osteogenic ability of MC3T3-E1 cells. Briefly, 5 µl cells
(2.5x105 cells/well) were lysed using Cell Lysis Buffer
(cat. no. P0013J, Beyotime Institute of Biotechnology) and added
into a 96-well plate and mixed with the solutions prepared
according to the manufacturer's instructions. Following incubation
for 10 min at 37˚C, the absorbance in each well was measured at a
wavelength of 405 nm.
Statistical analysis
The differences among different groups were compared
by one-way ANOVA followed by Tukey's post hoc test. All data were
analyzed and plotted with GraphPad Prism 6 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference. All data are expressed as the mean ± SD.
All experiments were performed in triplicate.
Results
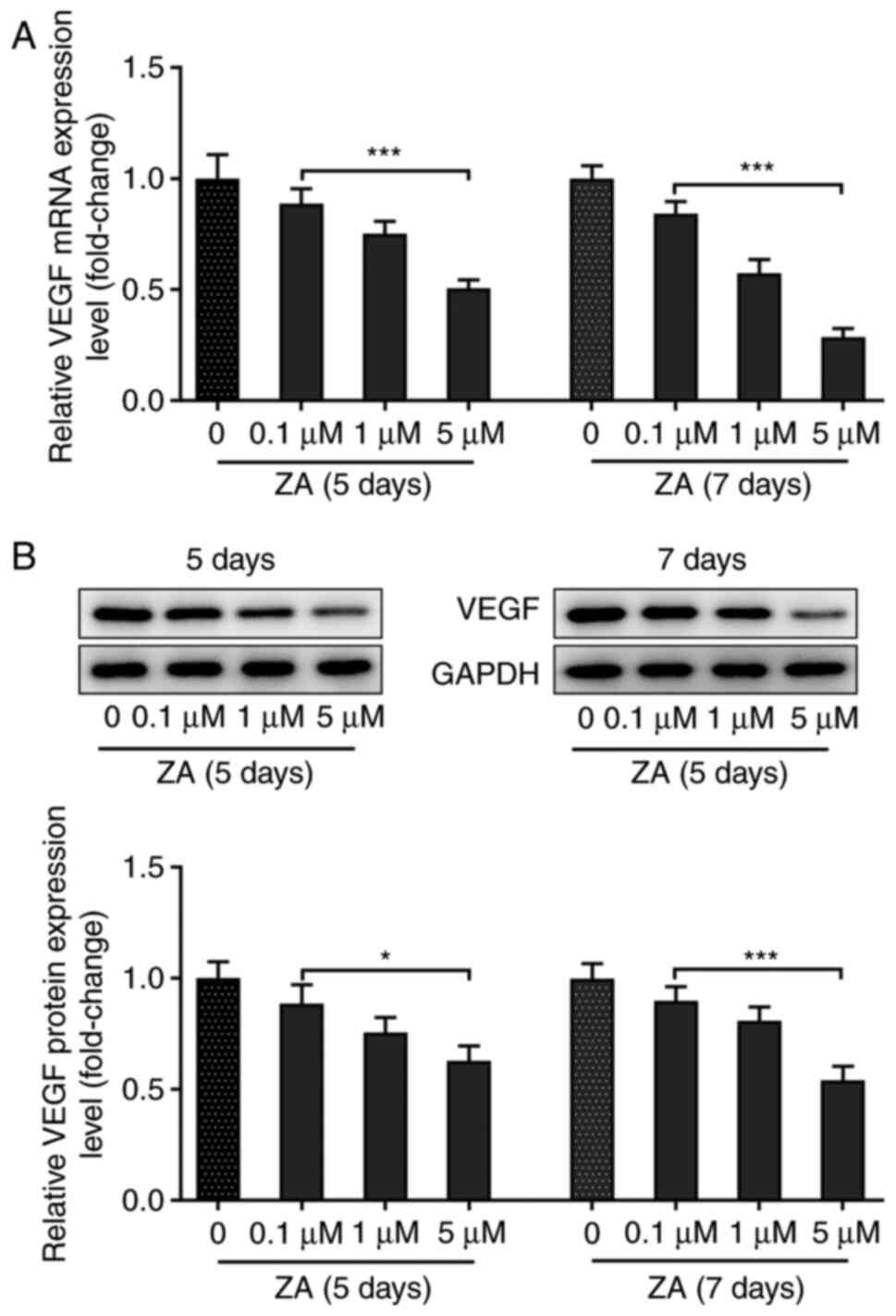
MC3T3-E1 cell stimulation with ZA downregulates
VEGF. MC3T3-E1 cells were stimulated with ZA for 5 and 7 days,
and the mRNA and protein expression levels of VEGF were assessed by
RT-qPCR and western blot analysis, respectively. The results
indicated that the expression level of VEGF in MC3T3-E1 cells was
decreased following cell treatment with ZA in a dose-dependent
manner at both the mRNA and protein levels (Fig. 1A and B). The expression of VEGF was not notably
changed in MCT3T3-E1 cells stimulated with ZA for 7 days compared
with those stimulated for 5 days (Fig.
1A and B). Collectively, these
results demonstrated that treatment of MC3T3-E1 cells with ZA
reduced the expression of VEGF in a dose-dependent manner.
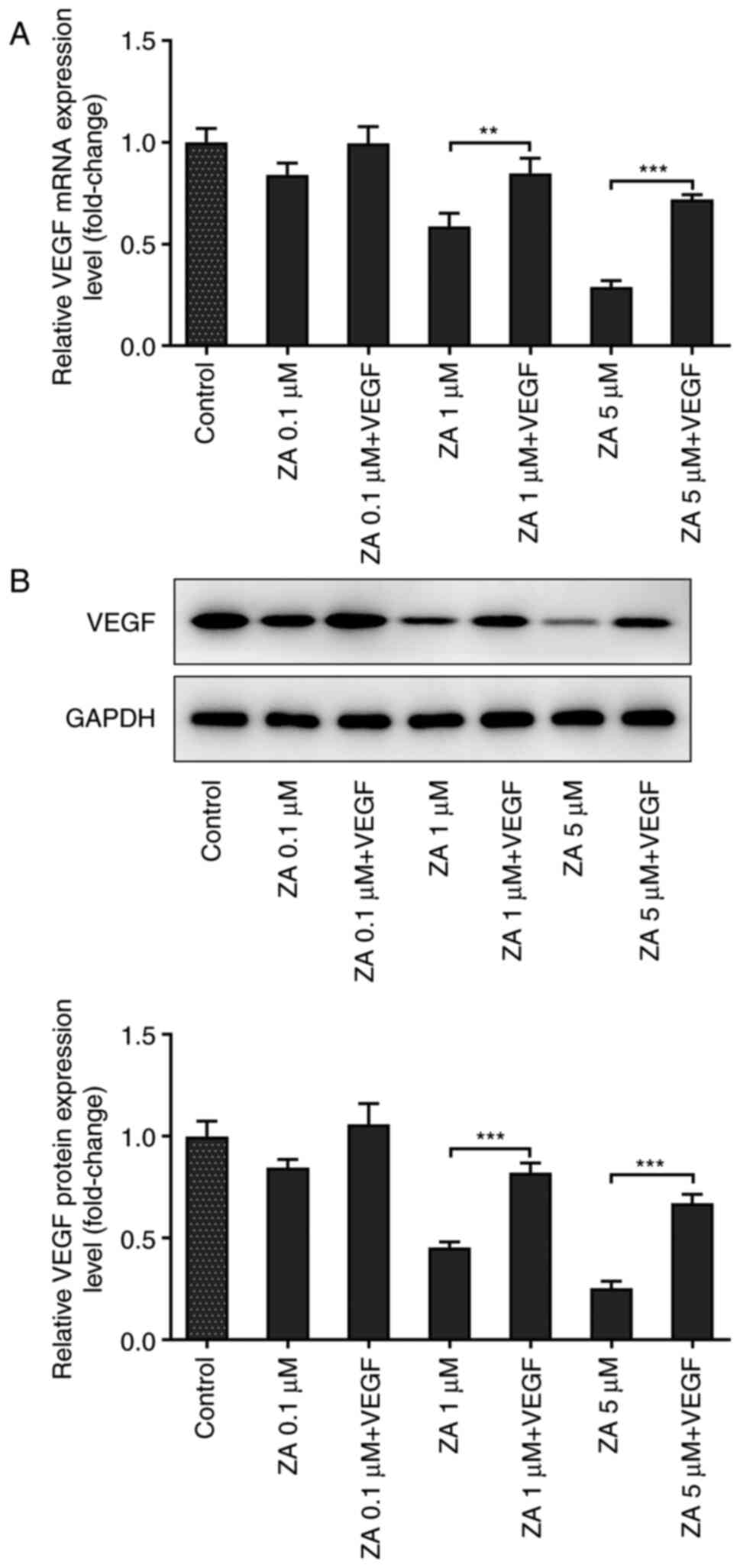
VEGF improves the viability of
ZA-stimulated MC3T3-E1 cells
To reveal whether VEGF could affect cell viability,
ZA-stimulated MC3T3 cells were treated with murine VEGF at a
concentration of 10 ng/ml. RT-qPCR and western blot analysis
confirmed that VEGF levels were increased in cells treated with
exogenous VEGF (Fig. 2A and
B). As presented in Fig. 3A, the OD values obtained by CCK-8
assays were increased in ZA-stimulated MC3T3 cells following VEGF
addition. The present finding indicated that VEGF addition could
enhance the viability of ZA-stimulated MC3T3-E1 cells.
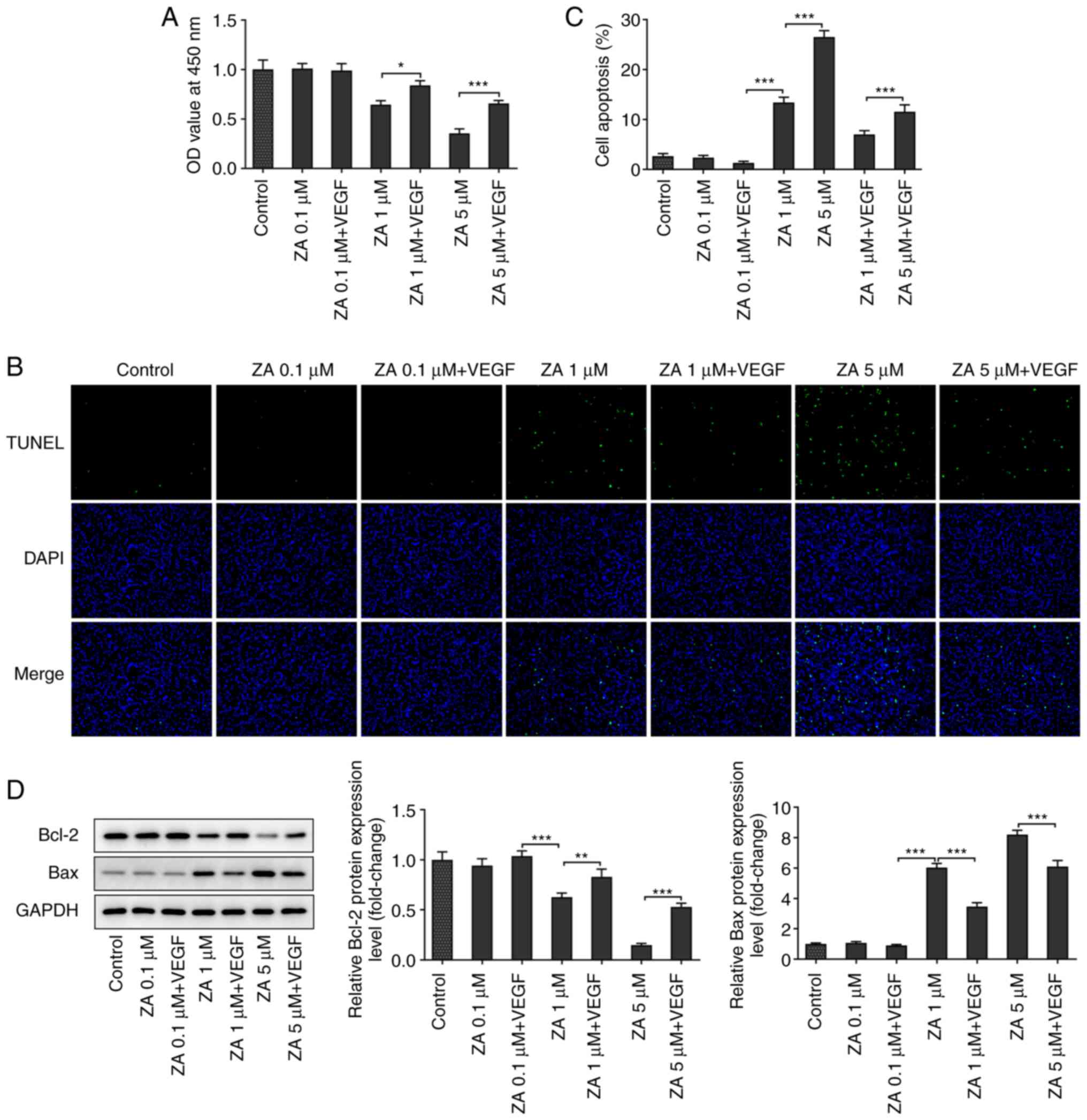
VEGF addition attenuates the apoptosis
of ZA-stimulated MC3T3-E1 cells
The effect of VEGF on cell apoptosis was evaluated
via TUNEL staining. The results indicated that cell treatment with
exogenous VEGF increased the number of viable ZA-stimulated
MC3T3-E1 cells (Fig. 3B and
C). In addition, western blot
analysis was carried out to determine the expression levels of the
apoptosis-related proteins Bcl2 and Bax. The analysis revealed that
the expression levels of the anti-apoptotic protein Bcl2 were
increased, while those of the pro-apoptotic protein Bax were
decreased in ZA-stimulated MC3T3-E1 cells after VEGF addition
(Fig. 3D). Taken together, the
present results suggested that VEGF attenuated the apoptosis of
ZA-stimulated MC3T3-E1 cells.
VEGF addition promotes the
differentiation of ZA-stimulated MC3T3-E1 cells
The mineralization of ZA-stimulated MC3T3-E1 cells
was evaluated by ARS staining. As presented in Fig. 4A, red staining was more prominent
in cells treated with exogenous VEGF compared with cells in the
control group. ALP activity was also determined to further evaluate
the mineralization of ZA-stimulated MC3T3-E1 cells. The results
demonstrated that ALP activity was attenuated in a dose-dependent
manner following cell treatment with ZA. This effect was restored
after treatment with exogenous VEGF (Fig. 4B). Additionally, the expression
levels of differentiation-related proteins were detected by western
blot analysis and the results indicated that osteopontin,
osteocalcin and RUNX2 were downregulated in ZA-stimulated MC3T3-E1
cells, and were then upregulated following VEGF addition (Fig. 4C and D). The aforementioned results suggested
that VEGF addition promoted the differentiation of ZA-stimulated
MC3T3-E1 cells.
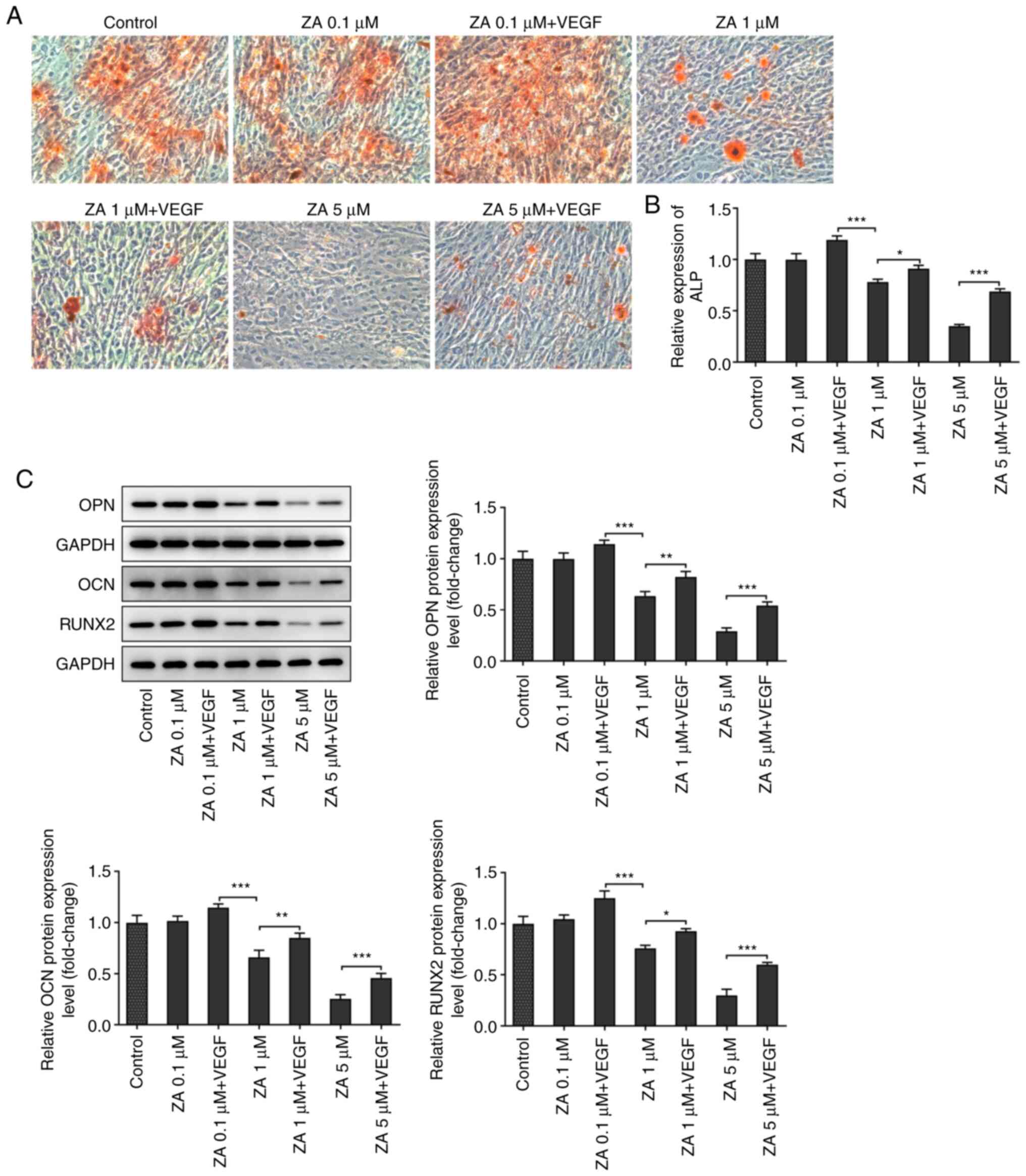 | Figure 4(A) Mineralization of ZA-stimulated
MC3T3-E1 cells in the absence or presence of VEGF, detected by
Alizarin red S staining. (B) ALP activity in ZA-stimulated MC3T3-E1
cells in the absence or presence of VEGF, detected by ALP assay
kit. (C) Expression levels of differentiation-related proteins OPN,
OCN and RUNX2 in ZA-stimulated MC3T3-E1 cells in the absence or
presence of VEGF, detected by western blot assay, then quantified
and analyzed. *P<0.05, **P<0.01,
***P<0.001. ZA, zoledronic acid; ALP, alkaline
phosphatase; OPN, osteopontin; OCN, osteocalcin; RUNX2,
runt-related transcription factor 2. |
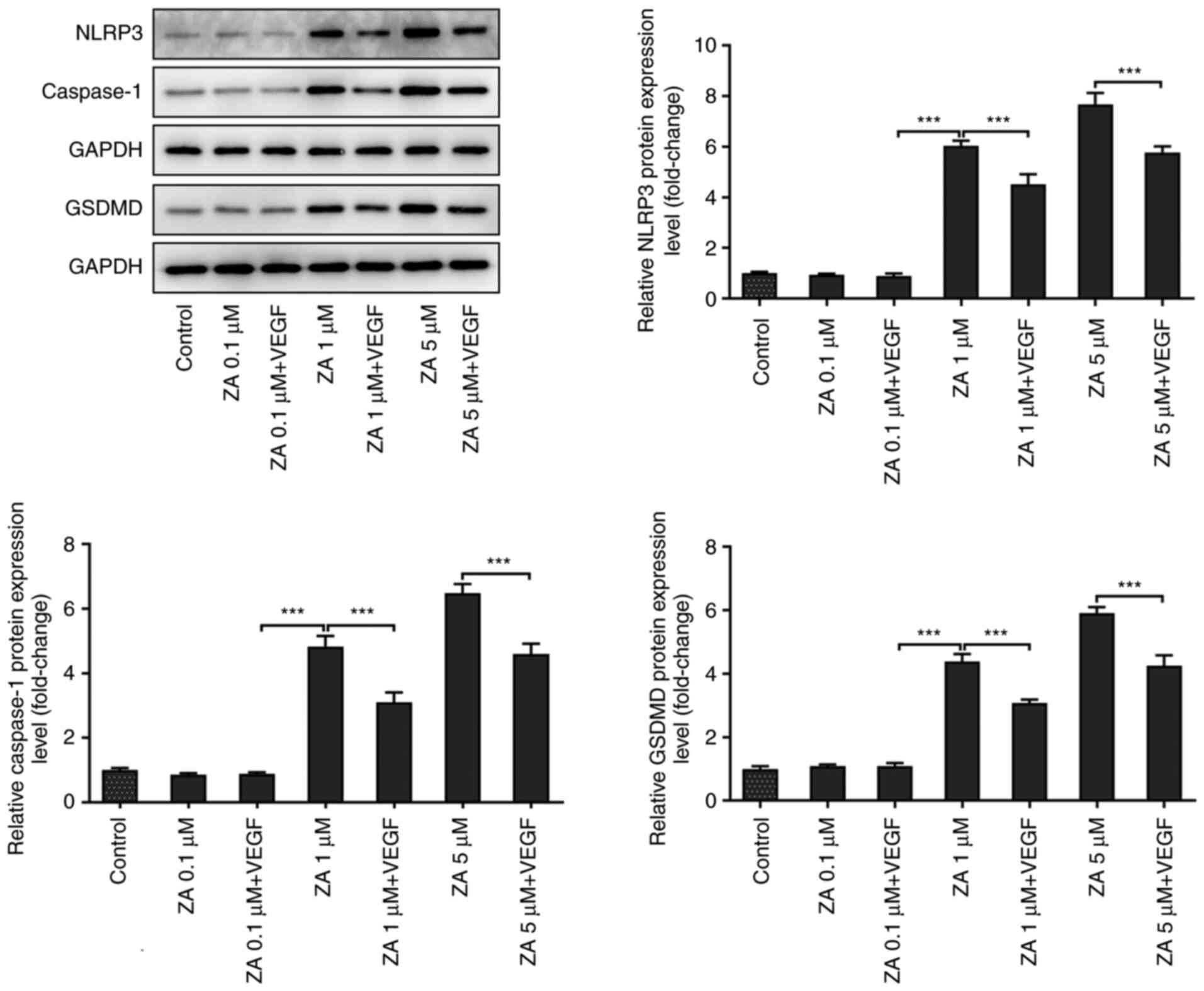
VEGF addition inhibits the expression
of pyroptosis-related proteins in ZA-stimulated MC3T3-E1 cells
The expression levels of the pyroptosis-related
proteins involved in the Nod-like receptor family pyrin
domain-containing 3 (NLRP3)/caspase 1/gasdermin D (GSDMD) signaling
pathway were detected in ZA-stimulated MC3T3-E1 cells to further
investigate the effect of VEGF on BP-induced ONJ. As presented in
Fig. 5, the expression of NLRP3
and caspase 1 was notably upregulated in ZA (1 µM)-stimulated
MC3T3-E1 cells when compared with Control group, and was restored
after treatment with exogenous VEGF. However, the changes in the
expression levels of GSDMD were not significantly different between
the VEGF-treated and untreated ZA-stimulated MC3T3-E1 cells.
Overall, the present findings suggested that VEGF addition could
exert an inhibitory effect on the NLRP3/caspase 1/GSDMD signaling
pathway in ZA-stimulated MC3T3-E1 cells.
Discussion
BPs, including alendronate, risedronate, ZA and
ibandronate, are a type of drug commonly used to treat patients
with osteoporosis, since they can improve mineral density and
reduce the risk of bone fractures (13). However, patients undergoing
treatment with BPs may often develop ONJ, accompanied by jaw pain,
tooth loss, oral infection or even osteomyelitis (14,15).
Osteoblasts and osteoclasts interact with each other to renew bone
tissues in order for the sequestrum to be resorbed and regenerated.
It has been suggested that BP-induced ONJ may be associated with
the accumulation of BP on the bone surface, thus attenuating the
bone resorption ability via inhibiting osteoclast viability.
Therefore, the reduced absorption capacity of the bones could be
maintained for a long time after the withdrawal of BP therapy
(16-18).
It has been previously reported that in vivo treatment of
osteocytes with ZA reduced their number and function (19,20).
Another study demonstrated that osteoblast treatment with 1-100 µM
of BP exerted a suppressive effect on their proliferation,
differentiation and mineralization (21). Consistent with the aforementioned
studies, the present study demonstrated that stimulation of
MC3T3-E1 cells with ≥1 µM ZA significantly inhibited cell viability
and differentiation, and promoted MC3T3-E1 cell apoptosis.
VEGF serves a key role in regulating blood vessel
growth and angiogenesis, but is also involved in osteogenesis
(22). A previous study indicated
that patients with BP-induced ONJ exhibited reduced VEGF serum
levels. Therefore, VEGF levels are currently used as a biomarker
for BP-induced ONJ (23). In the
present study, the expression of VEGF was gradually decreased in
MC3T3-E1 cells after treatment with increasing concentrations of
ZA. It has been also reported that VEGF could regulate osteoblast
survival (24). For example, a
study on osteoarthritis revealed that the expression of VEGF was
downregulated by ACY-1215, a histone deacetylase 6 inhibitor, to
promote the apoptosis of osteoblasts (25). In the current study, VEGF
revitalized ZA-stimulated MC3T3-E1 cells, as evidenced by the
reduced apoptotic cell percentage and expression of the
pro-apoptotic protein Bax, and the increased expression of the
anti-apoptotic protein Bcl2. Another study revealed that, during
the bone healing process, VEGF could improve osteoblast
differentiation and facilitate bone formation (26). The same osteogenic effects of VEGF
were observed in the mineralization and differentiation of
adipose-derived stem cells (27).
The results of the present study indicated that ZA-stimulation
impeded the mineralization of MC3T3-E1 cells and reduced ALP
activity. However, VEGF treatment partially reversed the
aforementioned effects. Furthermore, the reduced expression levels
of differentiation-related proteins in ZA-stimulated MC3T3-E1 cells
were also restored following cell treatment with exogenous VEGF.
These findings indicated that VEGF could alleviate ZA-induced
apoptosis and abrogate the inhibitory effect of ZA on the
differentiation of murine osteoblasts.
To further examine the effects of VEGF, the present
study also investigated whether VEGF could affect the NLRP3/caspase
1/GSDMD signaling pathway. Emerging evidence has reported that
NLRP3 and its downstream target, caspase 1, are closely associated
with disc degeneration (28). In
addition, another study demonstrated that BPs could activate
NLRP3/caspase 1 signaling to induce osteoporosis in diabetic mice
(28,29). Likewise, the results of the present
study indicated that the expression of both NLRP3 and caspase 1 was
upregulated in ZA-stimulated MC3T3-E1 cells, which was suppressed
following VEGF treatment. Furthermore, it has also been reported
that increased pyroptosis in the alveolar bone was associated with
reduced osteoblast differentiation capacity (30). However, the lack of evidence to
support the role of ZA in NLRP3-mediated pyroptosis by VEGF
requires more extensive studies in osteoblasts, and whether
pyroptosis is involved in the occurrence of ONJ remains unknown.
VEGF exerted a mitigatory effect on hepatocyte pyroptosis, thus
protecting the liver from renal allograft ischemia-reperfusion
injury (31); however, in the
current study, ZA stimulation or VEGF treatment had no significant
effects on the expression of the pyroptosis-related protein GSDMD
in MC3T3-E1 cells.
Taken together, the present results suggested that
VEGF addition could alleviate cell apoptosis, suppress the
activation of the NLRP3/caspase 1 axis and reverse ZA-mediated
inhibition of MC3T3-E1 cell differentiation. These findings could
provide novel insights into the potential role of VEGF as a
therapeutic target in the treatment of BP-induced ONJ.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81701008);
Key research and development program of Shandong Province (grant
no. 2019GSF108187); Guangdong Basic and Applied Basic Research
Foundation (grant no. 2020A1515010150); Students Research Fund of
Shandong University (grant no. 2019298).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD, HJL, XHD, ZLG, XYX and YL contributed to the
conception and design of the work and the acquisition, analysis and
interpretation of data. YD, HJL and YL drafted the manuscript and
revised it critically for important intellectual content. All
authors read and approved the final version of the manuscript. YD,
HJL and YL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuznik A, Pazdzierniok-Holewa A, Jewula P
and Kuznik N: Bisphosphonates-much more than only drugs for bone
diseases. Eur J Pharmacol. 866(172773)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kates SL and Ackert-Bicknell CL: How do
bisphosphonates affect fracture healing? Injury. 47 (Suppl
1):S65–68. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rosini S, Rosini S, Bertoldi I and
Frediani B: Understanding bisphosphonates and osteonecrosis of the
jaw: Uses and risks. Eur Rev Med Pharmacol Sci. 19:3309–3317.
2015.PubMed/NCBI
|
|
4
|
Maines E, Monti E, Doro F, Morandi G,
Cavarzere P and Antoniazzi F: Children and adolescents treated with
neridronate for osteogenesis imperfecta show no evidence of any
osteonecrosis of the jaw. J Bone Miner Metab. 30:434–438.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Himelstein AL, Foster JC, Khatcheressian
JL, Roberts JD, Seisler DK, Novotny PJ, Qin R, Go RS, Grubbs SS,
O'Connor T, et al: Effect of longer-interval vs standard dosing of
zoledronic acid on skeletal events in patients with bone
metastases: A randomized clinical trial. JAMA. 317:48–58.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL,
Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF,
et al: Bone formation during distraction osteogenesis is dependent
on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 23:596–609.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Behr B, Leucht P, Longaker MT and Quarto
N: Fgf-9 is required for angiogenesis and osteogenesis in long bone
repair. Proc Natl Acad Sci USA. 107:11853–11858. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Emad B, Sherif EM, Basma GM, Wong RW,
Bendeus M and Rabie AB: Vascular endothelial growth factor augments
the healing of demineralized bone matrix grafts. Int J Surg.
4:160–166. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Cann T, Loyson T, Verbiest A, Clement
PM, Bechter O, Willems L, Spriet I, Coropciuc R, Politis C,
Vandeweyer RO, et al: Incidence of medication-related osteonecrosis
of the jaw in patients treated with both bone resorption inhibitors
and vascular endothelial growth factor receptor tyrosine kinase
inhibitors. Support Care Cancer. 26:869–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tan YY, Yang YQ, Chai L, Wong RW and Rabie
AB: Effects of vascular endothelial growth factor (VEGF) on
MC3T3-E1. Orthod Craniofac Res. 13:223–228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ganesan K, Bansal P, Goyal A and Roane D:
Bisphosphonate. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2021.
|
|
14
|
Gupta M and Gupta N: Bisphosphonate
Related Jaw Osteonecrosis. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2020.
|
|
15
|
Dhillon S: Zoledronic acid (Reclas
®, Aclasta ®): A review in osteoporosis.
Drugs. 76:1683–1697. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu W, Xu R, Du J, Fu Y, Li S, Zhang P,
Liu L and Jiang H: Zoledronic acid promotes TLR-4-mediated M1
macrophage polarization in bisphosphonate-related osteonecrosis of
the jaw. FASEB J. 33:5208–5219. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Endo Y, Kumamoto H, Nakamura M, Sugawara
S, Takano-Yamamoto T, Sasaki K and Takahashi T: Underlying
mechanisms and therapeutic strategies for bisphosphonate-related
osteonecrosis of the jaw (BRONJ). Biol Pharm Bull. 40:739–750.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Limones A, Saez-Alcaide LM, Diaz-Parreño
SA, Helm A, Bornstein MM and Molinero-Mourelle P:
Medication-related osteonecrosis of the jaws (MRONJ) in cancer
patients treated with denosumab VS. zoledronic acid: A systematic
review and meta-analysis. Med Oral Patol Oral Cir Bucal.
25:e326–e336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huja SS, Fernandez SA, Phillips C and Li
Y: Zoledronic acid decreases bone formation without causing
osteocyte death in mice. Arch Oral Biol. 54:851–856.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pozzi S, Vallet S, Mukherjee S, Cirstea D,
Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, et al:
High-dose zoledronic acid impacts bone remodeling with effects on
osteoblastic lineage and bone mechanical properties. Clin Cancer
Res. 15:5829–5839. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang X, Huang S, Guo F, Xu F, Cheng P, Ye
Y, Dong Y, Xiang W and Chen A: Dose-dependent inhibitory effects of
zoledronic acid on osteoblast viability and function in
vitro. Mol Med Rep. 13:613–622. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu K and Olsen BR: The roles of vascular
endothelial growth factor in bone repair and regeneration. Bone.
91:30–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vincenzi B, Napolitano A, Zoccoli A,
Iuliani M, Pantano F, Papapietro N, Denaro V, Santini D and Tonini
G: Serum VEGF levels as predictive marker of bisphosphonate-related
osteonecrosis of the jaw. J Hematol Oncol. 5(56)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Street J and Lenehan B: Vascular
endothelial growth factor regulates osteoblast survival-evidence
for an autocrine feedback mechanism. J Orthop Surg Res.
4(19)2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li L, Liu F, Huang W, Wang J, Wan Y, Li M,
Pang Y and Yin Z: Ricolinostat (ACY-1215) inhibits VEGF expression
via PI3K/AKT pathway and promotes apoptosis in osteoarthritic
osteoblasts. Biomed Pharmacother. 118(109357)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Clark D, Wang X, Chang S,
Czajka-Jakubowska A, Clarkson BH and Liu J: VEGF promotes
osteogenic differentiation of ASCs on ordered fluorapatite
surfaces. J Biomed Mater Res A. 103:639–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Q, Yu W, Lee S, Xu Q, Naji A and Le
AD: Bisphosphonate induces osteonecrosis of the jaw in diabetic
mice via NLRP3/caspase-1-dependent IL-1β mechanism. J Bone Miner
Res. 30:2300–2312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen ZH, Jin SH, Wang MY, Jin XL, Lv C,
Deng YF and Wang JL: Enhanced NLRP3, caspase-1, and IL-1β levels in
degenerate human intervertebral disc and their association with the
grades of disc degeneration. Anat Rec (Hoboken). 298:720–726.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Yang L, Liu J, Shan Q, Geng G and Shao P:
High glucose inhibits proliferation and differentiation of
osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys
Res Commun. 522:471–478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao H, Huang H, Alam A, Chen Q, Suen KC,
Cui J, Sun Q, Ologunde R, Zhang W, Lian Q and Ma D: VEGF mitigates
histone-induced pyroptosis in the remote liver injury associated
with renal allograft ischemia-reperfusion injury in rats. Am J
Transplant. 18:1890–1903. 2018.PubMed/NCBI View Article : Google Scholar
|