Introduction
Dental implants have become an increasingly popular
choice of treatment for replacing missing teeth (1). Peri-implantitis is the development of
serious inflammatory lesions following dental implant therapy. The
surrounding hard and soft tissues are affected in peri-implantitis,
which can lead to the loss of the implant (2). Previous studies of these therapeutic
strategies have not been able to identify a single treatment
regimen that is suitably effective against peri-implantitis
(3-5).
Host inflammatory cytokines and
osteo-immunoinflammatory mediators can lead to the loss of
supporting tissues around the implant (4). Matrix metalloproteinases (MMPs) are
important for bone repair, development and inflammation. As one of
the collagenases, matrix metalloproteinase 2 (MMP2) produced by
inflamed cells is involved in several inflammatory diseases
(6). In murine arthritis induced
by group B streptococci, MMP2 is involved in the production
of pro-inflammatory cytokines and the degradation of extracellular
matrix components (7). In human
astroglia, the ERKk1/2-NF-κB signaling pathway mediates
Toxoplasma gondii induced MMP2 production to cleave
fibronectin (8). In apical
periodontitis, the upregulation of MMP2 and MMP9 is modulated by
the TLR2MyD88 signaling pathway (9).
A previous study showed that MMP2 was upregulated
when stimulated by Porphyromonas gingivalis (P.
gingivalis) lipopolysaccharide (PG-LPS) in THP-1 macrophages
(10). However, the regulatory
mechanism and signal transduction pathway by which MMP2 exerts its
effects in patients who have not received implants have not been
assessed previously, to the best of our knowledge. The aim of the
present study was to characterize the regulatory mechanism of MMP2
in peri-implantitis.
Materials and methods
Peri-implant crevicular fluid (PICF)
collection
In the present study, 10 cases of healthy implants
(age, 45±4.6 years; sex, five males and five females) and 10 cases
of peri-implantitis implants (age, 46±4.4 years; sex, five males
and five females) were studied. Each patient had ≥ two dental
implants, and both implants were investigated. The diagnostic
criteria, exclusion criteria and inclusion criteria have been
described previously used in our preliminary work (10). Sterile Periopapers (Oraflow) were
used to collect PICF samples, which were then placed on the
gingival sulcus and implant for 30 sec. For further testing, 50 mM
phosphate buffer containing 0.1 mM phenylmethylsulfonyl fluoroether
was used to elute the PICF contents.
This study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
The Hospital Affiliated to Qingdao University (approval no.
3/6/2018 #20180306; Qingdao, China). All patients provided written
informed consent (1).
Isolation of human macrophages
Human macrophages were obtained from the peripheral
blood of healthy volunteers after receiving approval from the
Ethics Committee of The Affiliated Hospital of Qingdao University.
The mononuclear fraction was isolated by density gradient
centrifugation at 400 x g for 30 min at room temperature using
Ficoll Paque Plus according to the manufacturer's protocol (TBD
Biotech). After washing with PBS at room temperature, cells were
centrifuged at 200 x g for 5 min at room temperature and suspended
in RPMI-1640 media (Hyclone; Cytiva) at a density of
1x106 cells/ml. Cells were confirmed to be macrophages
(macrophages >95%) by Diff-Quik (cat. no. G1541; Beijing
Solarbio Science & Technology Co., Ltd.) stain (Diff-Quik I for
10 sec and Diff-Quik II for 15 sec at room temperature).
Bacterial culture
P. gingivalis, provided by the Oral
Laboratory of the Hospital affiliated to Qingdao University, were
grown overnight in an incubator at 37˚C under anaerobic conditions
(90% N and 5% CO2 and 25% H2) in gifu
anaerobic medium (cat. no. LA4500; Beijing Solarbio Science &
Technology Co., Ltd.) broth with an optical density of 650 nm of
1.0 (1x109 colony forming units/ml) (11). The P. gingivalis was added
to RPMI-1640 media to infect the human macrophages after washing
the cells twice by RPMI-1640. The multiplicity of infection (MOI)
used was 20.
MMP2 expression in human macrophages
infected with P. gingivalis
Western blotting and reverse
transcription-quantitative (RT-q)PCR were used to detect MMP2
expression in cells treated with live P. gingivalis bacteria
after 0, 1/4, 1/2, 1, 4 and 16 h, to measure the expression of MMP2
in human macrophages infected with P. gingivalis.
To reveal the role of dectin-1 and LOX-1 in the
production of MMP2 following infection with P. gingivalis
bacteria, dectin-1 (cat. no. AF1859) or LOX-1 (cat. no. MAB1798)
neutralizing antibodies (10 µg/ml; R&D Systems, Inc.) (12) and the LOX-1 inhibitor, polyinosinic
acid (Poly I; Sigma-Aldrich; Merck KGaA; 0.25 mg/ml) (13) or a dectin-1 inhibitor, laminarin
(Sigma-Aldrich; Merck KGaA; 0.3 mg/ml) (1) were used to pretreat human macrophages
for 2 h at room temperature, which were then treated with P.
gingivalis bacteria 2 h later at room temperature.
To investigate the effects of Erk1/2 and JNK on MMP2
production with P. gingivalis, human macrophages were
pretreated with the JNK inhibitor, SP600125 (Selleck Chemicals; 40
µM) and the Erk1/2 inhibitor, U0126 (Selleck Chemicals; 20 µM)
(1), 2 h at room temperature
before treatment with live P. gingivalis bacteria at room
temperature. The controls were DMSO, sterile water and goat IgG (10
µg/ml; cat. no. AB-108-C; R&D Systems, Inc.). Live P.
gingivalis were used to treat human macrophages for 0 and 16 h
at room temperature for Western blot analysis and for 0 and 4 h at
room temperature at a MOI of 20 for RT-qPCR.
Western blotting
Total proteins from human macrophages were lysed by
RIPA Lysis Buffer (Beijing Solarbio Science & Technology Co.,
Ltd.). The total protein contents were quantified using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Protein was loaded into each well (60 µg) of a 10% SDS-gel (Bio-Rad
Laboratories, Inc.), resolved by SDS-PAGE and transferred to a PVDF
membrane (EMD Millipore). The membranes were put in blocking buffer
(cat. no. P0023B; Beyotime Institute of Biotechnology) for 2 h. The
membranes were incubated overnight with an anti-β-actin (1:1,000
Cell Signaling Technologies, Inc.) or anti-MMP2 (1:500 ProteinTech
Group, Inc.) primary antibodies at 4˚C, followed by incubation with
the corresponding HRP-tagged secondary antibodies at room
temperature for 2 h (1:1,000 cat. nos. 7074 and 7076; Cell
Signaling Technologies, Inc.). Clarity Western ECL Substrate (cat.
no. 1705061; Bio-Rad Laboratories, Inc.) combined with the Fusion
Solo System (FUSION-FX7 advanced; Vilber Lourmat) was used to
visualize signals (ImageJ 1.48; National Institutes of Health).
Immunofluorescence staining
MMP2 immunofluorescence was used to stain small
tissue fragments of superficial gingiva in patients with
peri-implantitis. The immunofluorescence protocol was performed as
described previously (10).
Anti-MMP2 antibody (1:50; ProteinTech Group, Inc.) was used as the
primary antibody overnight at 4˚C, and an Alexa Fluor
488-conjugated goat anti-rabbit IgG (1:1,000; cat. no. 4412, Cell
Signaling Technology, Inc.) was used as the secondary antibody at
room temperature for 1 h, followed by DAPI staining at room
temperature for 10 min (20 µg/ml; cat. no. 4083; Cell Signaling
Technology, Inc.). Images were captured using a fluorescence
microscope (magnification, x400).
RT-qPCR
RT-qPCR was performed as described previously
(10). At a pre-set time, the cell
culture medium was carefully removed before 500 µl RNAiso Plus
(cat. no. 9109; Takara Bio, Inc.) was added into the cell culture
plate followed by incubation at room temperature for 5 min to
facilitate cell lysis. cDNA template for PCR was prepared from RNA
(2 µg) from human macrophages by reverse transcription (cat. no.
RR066A; PrimeScript™ RT-PCR Kit Perfect Real Time; Takara Bio,
Inc.). The reaction temperature of reverse transcription is 37˚C
for 15 min, 85˚C for 5 sec. DEPC-treated water was used to dilute
cDNA products 1:25. PCR was assayed in 20 µl reaction system
consisting of 2 µl of 1 µg diluted cDNA (1:25), 10 µl of TB
Green® Premix Ex Taq™ (cat. no. RR420A; Takara Bio,
Inc.), 1 µl 100 µM diluted primers (1:9) and 7 µl DEPC-treated
water) for qPCR. All reactions were performed with the following
cycling parameters: 95˚C for 30 sec, followed by 40 cycles of 95˚C
for 5 sec, 60˚C for 30 sec and a final stage of 95˚C for 15 sec,
60˚C for 30 sec and 95˚C for 15 sec. The primer pair sequences
were: β-actin forward, 5'-TGGCACCCAGCACAATGAA-3' and reverse,
5'-CTAAGTCATAGTCCGCCTAGAAGCA-3' and MMP2 forward,
5'-AGTTTCCATTCCGCTTCCAG-3' and reverse, 5'-CGGTCGTAGTCCTCAGTGGT-3'.
β-actin was used as the loading control. Data were quantified using
the 2-ΔΔCq method (14).
Statistical analysis
One-way ANOVA followed by a post-hoc Tukey's test
was used to compare the differences between groups with SPSS 19.0
(IBM Corp.), which was presented as the mean ± SD from three
experimental repeats. P<0.05 was considered to indicate a
statistically significant difference.
Results
MMP2 expression is increased in
peri-implantitis
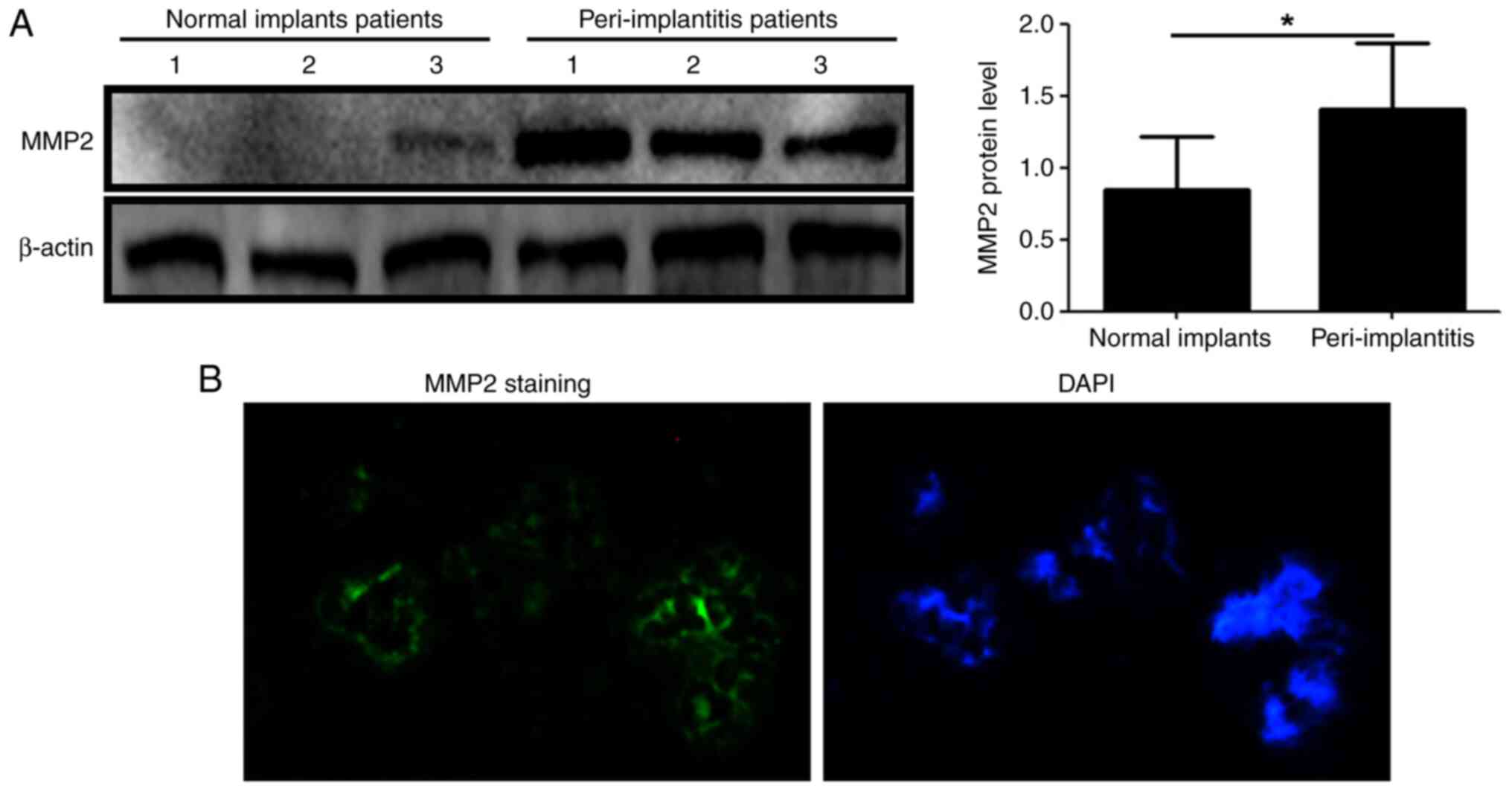
Compared to healthy implants, the MMP2 protein
expression levels were higher in the PICF of patients with
peri-implantitis (P<0.05; Fig.
1A). The superficial staining of peri-implantitis patients
around implants also showed expression of MMP2 (green) (Fig. 1B). The results demonstrated that
MMP2 may be involved in the pathogenesis of dental
peri-implantitis.
MMP2 expression is increased in P.
gingivalis infected human macrophages
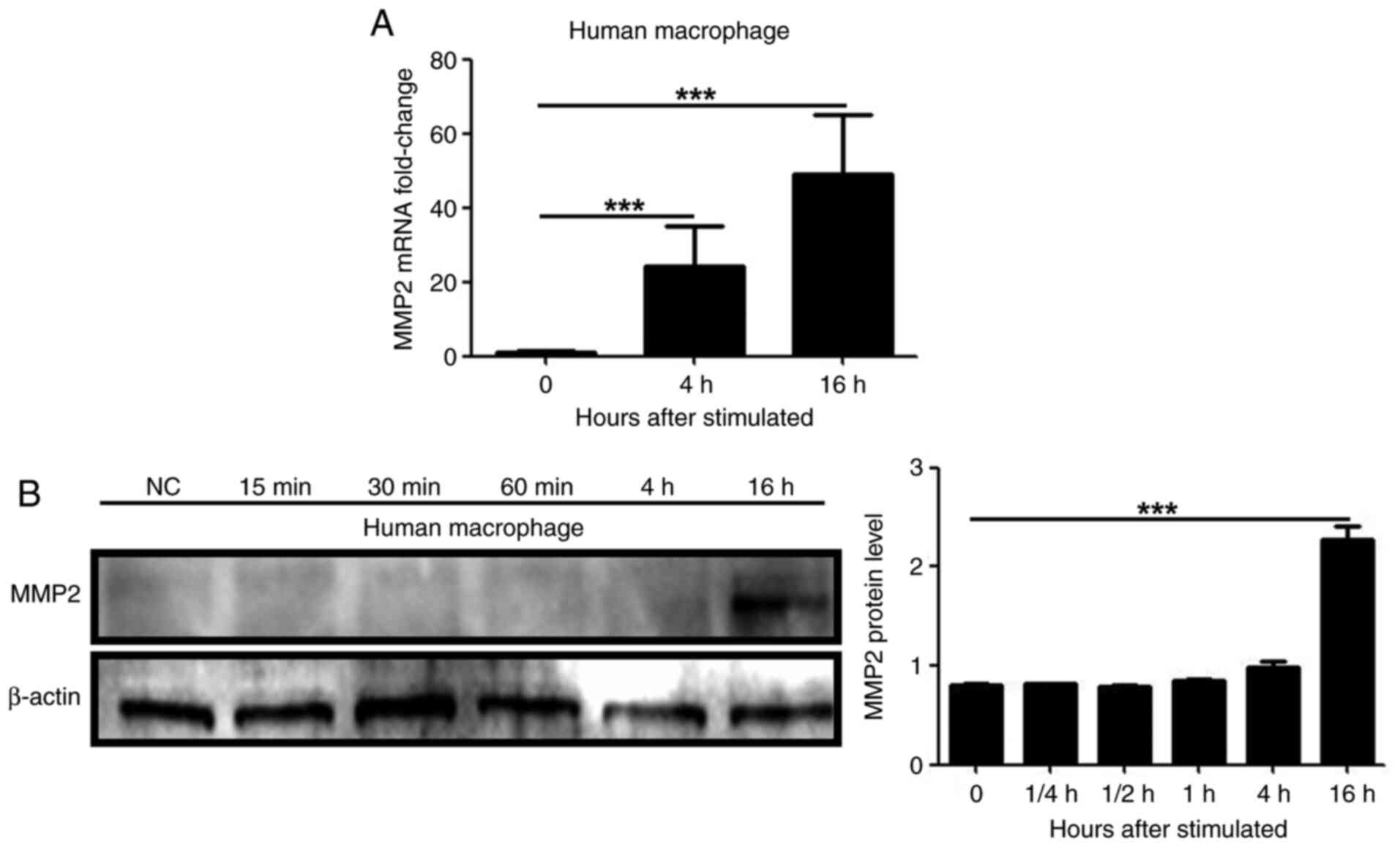
MMP2 mRNA expression was increased after 4 and 16 h
following infection of human macrophages with live P.
gingivalis bacteria (P<0.01; Fig. 2A). MMP2 production also increased
at the protein level 16 h after infection with P. gingivalis
bacteria (P<0.01; Fig. 2B).
These results demonstrated that MMP2 production in human
macrophages could be induced by P. gingivalis.
MMP2 production in P. gingivalis
infected human macrophages is mediated by LOX-1
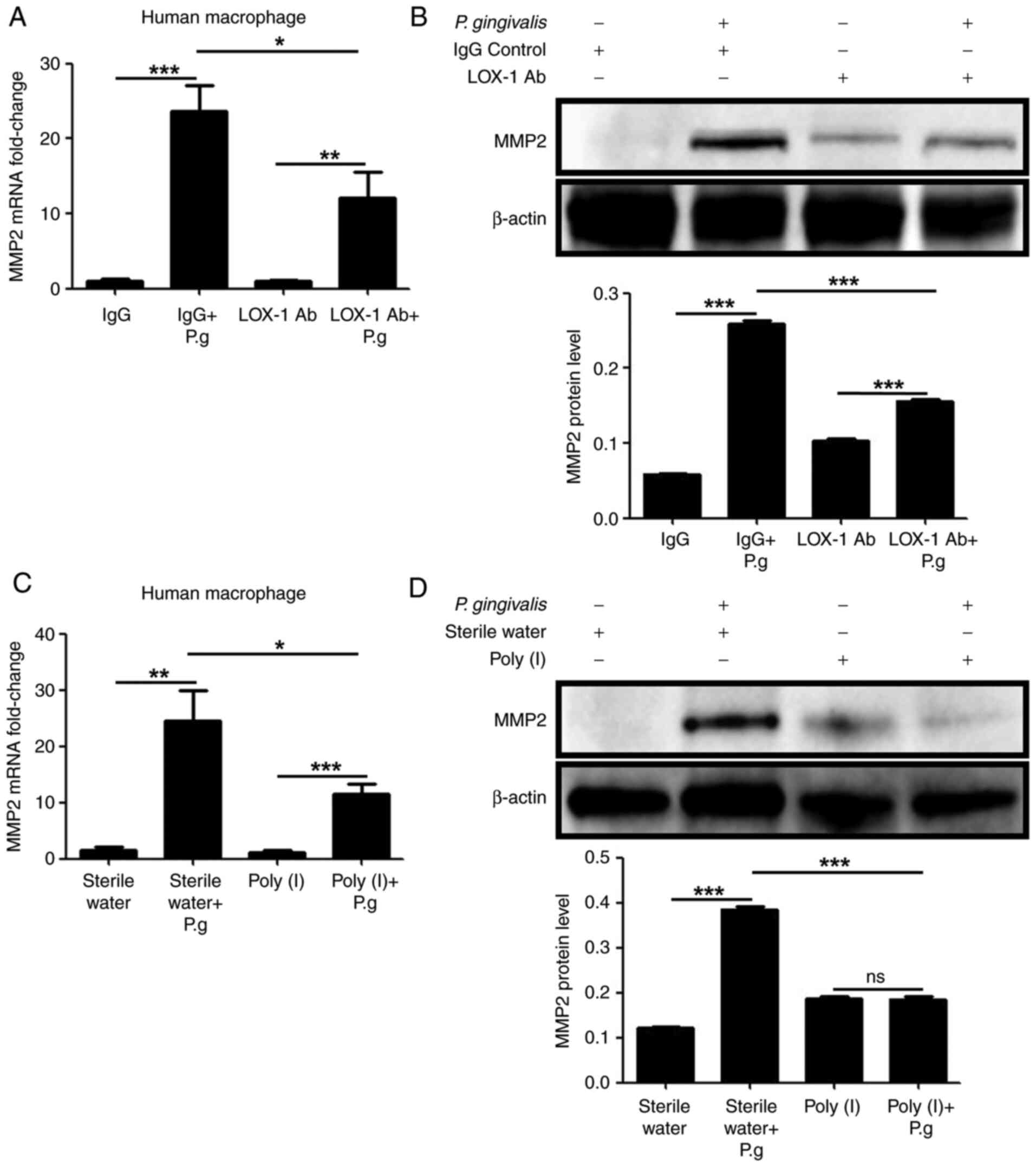
To study the function of LOX-1 in human macrophages
in the production of MMP2 in cells infected with P.
gingivalis, LOX-1 expression was decreased using neutralizing
antibodies or inhibitors. The increase in MMP2 expression in
infected macrophages at the mRNA (P<0.05; Fig. 3A and C) and protein (P<0.01; Fig. 3B and D) levels were decreased by the LOX-1
neutralizing antibody or inhibitor pre-treatments. These results
demonstrated that LOX-1 mediated MMP2 production induced by the
infection of human macrophages with live P. gingivalis
bacteria.
MMP2 production in P. gingivalis
infected human macrophages is independent of dectin-1
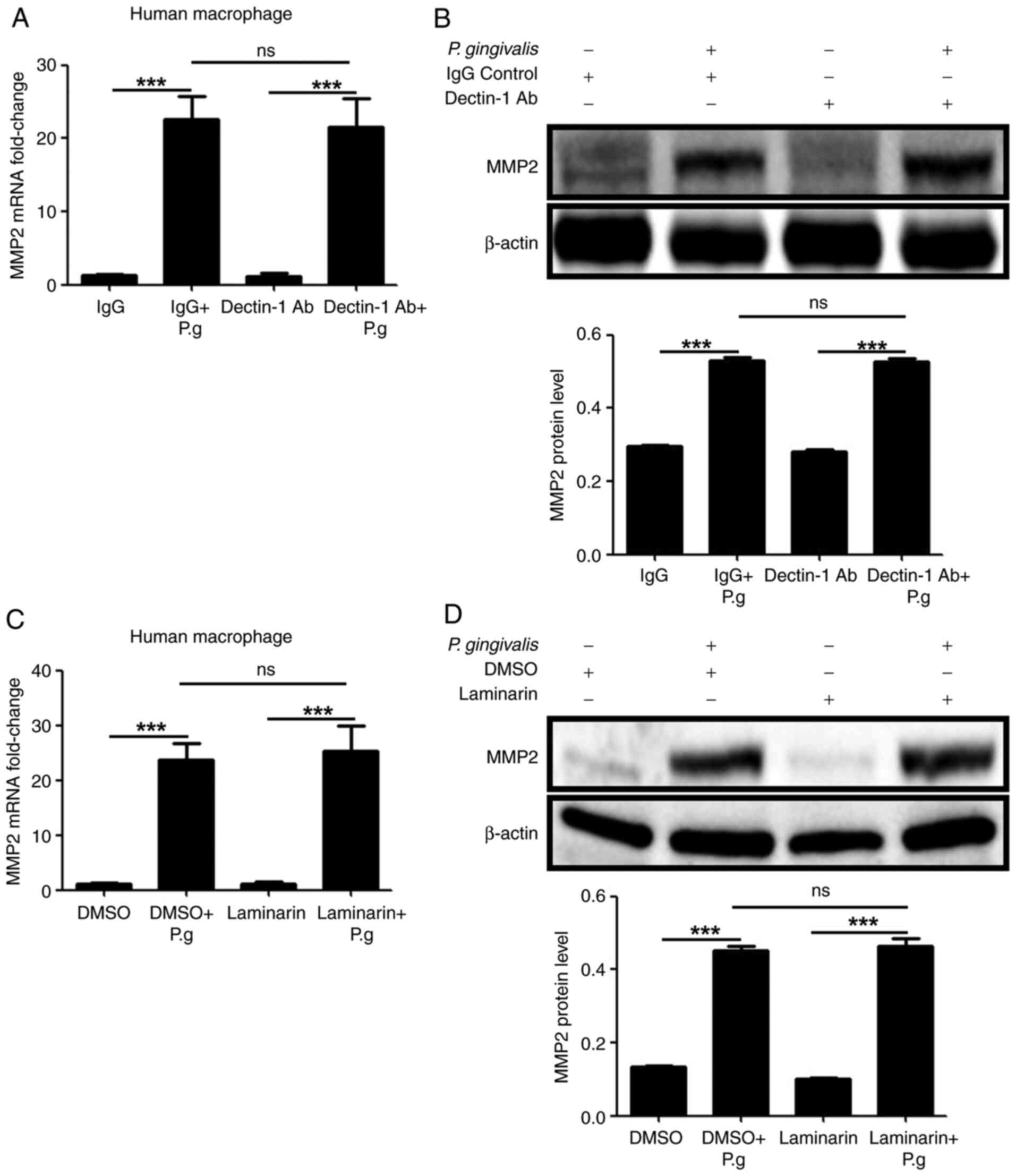
To determine the function of dectin-1 in the MMP2
production in human macrophages infected with live P.
gingivalis bacteria, neutralizing antibodies and inhibitors
were used to inhibit dectin-1 activity. The MMP2 mRNA (P>0.05;
Fig. 4A and C) and protein (P>0.05; Fig. 4B and D) levels did not differ significantly
between treatment with the dectin-1 neutralizing antibody or
inhibitor pre-treatment compared to the control untreated cells.
These results showed that MMP2 production was independent of
dectin-1 in the macrophages infected with live P. gingivalis
bacteria.
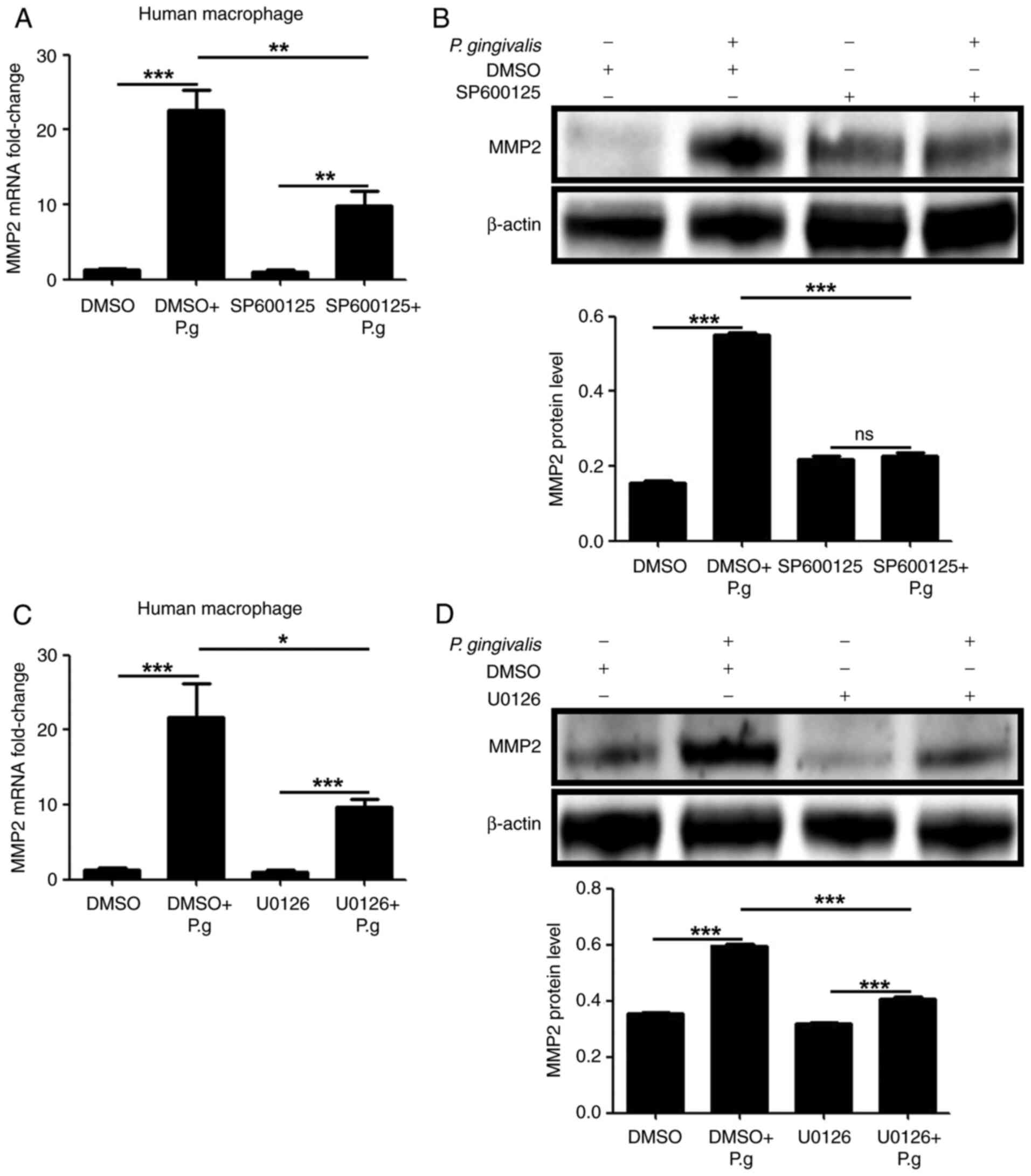
JNK and Erk1/2 are responsible for P.
gingivalis induced MMP2 production
JNK and Erk1/2 inhibitors were used to pretreat the
human macrophages to investigate their role in MMP2 production
following P. gingivalis infection . The results indicated
that the upregulated MMP2 mRNA (P<0.05; Fig. 5A and C) and protein (P<0.01; Fig. 5B and D) levels, when infected with live P.
gingivalis bacteria, were decreased by JNK or Erk1/2
inhibition. These results demonstrated that LOX-1 upregulated MMP2
with the infection of P. gingivalis bacteria was mediated by
Erk1/2.
Discussion
As one of the collagenases, MMP2 mediates multiple
functions associated with oral inflammation, bone development and
repair. Gonçalves et al (15) found that the levels of MMP2, MMP3,
MMP8, MMP9, MMP12 and MMP13 in gingival crevicular fluid were
significantly correlated with the severity of localized aggressive
periodontitis. da Costa et al (16) identified MMP2 and TNF-α as novel
biomarkers of inflammation in the alveolar bone and neighboring
tissues of patients with chronic periodontitis. Fu et al
(17) found that cyclosporin-A
prevented periodontal bone loss in patients with periodontitis by
suppressing the expression of MMP2 and MMP9. Araújo et al
(18) found that the inflammation
and bone loss in patients with periodontitis was reduced when
treated with olmesartan by decreasing MMP2, MMP9 and RANKL
expression, and increasing osteoprotegerin production. In the
present study, it was shown that the expression of MMP2 was
upregulated in the PICF of patients with peri-implantitis. This
observation is consistent with a report showing that active MMP2
complexes contributed to periodontal destruction and was found at
higher levels in the periodontal ligament and gingival crevicular
fluid of patients with periodontitis (19).
Osteoclastogenesis and severe macrophage derived
inflammatory responses can cause peri-implantitis (20). Thus, human macrophages were
selected for infection by P. gingivalis to assess the
regulatory mechanism of MMP2 in peri-implantitis. Kong et al
(21) revealed that the secretion
of MMP1 and MMP2 induced by P. gingivalis stimulation was
significantly inhibited by theaflavins in human gingival
fibroblasts. Mieszkowska et al (22) found that an increase of MMP2, IL-1β
and IL-8 following infection with P. gingivalis was
decreased by rhamnogalacturonan-Is treatment, which can reduce
inflammation and enhance implant integration. Consistent with these
studies, the results of the present study showed that when cells
were infected with P. gingivalis, MMP2 levels were increased
in the normal human macrophages.
LOX-1 and dectin-1, as pattern recognition receptors
of the C-type lectin superfamily, are associated with
osteoclastogenesis (23-25).
Bacteria, fungi, apoptotic cells and oxidized low-density
lipoproteins are the primary ligands of LOX-1 (26,27).
Nakayachi et al (23) found
that osteoclast differentiation could potentially be inhibited by
decreasing the cell-cell fusion of preosteoclasts via LOX-1.
Dectin-1, found predominantly on the cell membrane of the cells of
a myeloid lineage, can recognize β-glucans, some bacteria and other
unidentified molecules (28).
Yamasaki et al (24) found
that osteoclast differentiation and NFATc1 expression could be
negatively regulated by the dectin-1/syk signaling pathway. In the
present study, the data showed that MMP2 production was regulated
by LOX-1 in human macrophages with P. gingivalis. The
results of the present study are in agreement with previous studies
that have shown that LOX-1 neutralizing antibody treatment can
reduce MMP2 and MMP9 expression induced by gasoline and diesel
engine exhaust (29). The results
of the present study also revealed that MMP2 production was
independent of dectin-1 in infected human macrophages with P.
gingivalis.
JNK and Erk1/2, as members of the serine-threonine
protein kinases, play significant roles in cellular responses to
proinflammatory cytokines. Li et al (30) found that the expression of IL-1β
and TNF-α induced by sialidase of P. gingivalis could be
inhibited by JNK blocking epi4 cells. Ren et al (31) found that P. gingivalis
induced IFN-γ and IL-8 expression was mediated by Erk1/2 and p38
MAPK in HTR8 cells. JNK and Erk1/2 are also involved in MMP2
secretion in inflammatory diseases. Jiang et al (32) showed that neuropathic pain induced
by chronic constrictive injury was significantly attenuated by
tetramethylpyrazine via the JNK-MMP2/9 signaling pathway. Wang
et al (33) found that
Erk1/2/NF-κB pathway could upregulate MMP2 and MMP9 expression in
murine mast cells infected with Toxoplasma gondii. The
results of the present study suggested that JNK and Erk1/2 were
responsible for P. gingivalis induced MMP2 production. The
Erk1/2 inhibitor was considerably more potent than the JNK
inhibitor in suppressing MMP2 production.
In conclusion, the results of the present study,
obtained through evidence from patients and cell-culture studies,
showed that MMP2 was involved in peri-implantitis, and may have
been regulated by LOX-1, in a JNK and Erk1/2 signaling pathway
dependent manner. The LOX-1/MMP2 signaling pathway may thus be a
potential target for management of peri-implantitis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was sponsored by the Traditional
Chinese Medicine Research Project of Qingdao (grant no.
2020-zyy055) and the Science and Technology Project of Qingdao West
Coast New District (grant no. 2020-54).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ designed the study and wrote the majority of the
manuscript. TX and NB performed the majority of the experiments and
wrote part of the manuscript. FT and HZ performed some of the
experiments. JL designed the study and revised the manuscript. All
authors have read and approved the final manuscript. QZ and TX
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Qingdao University
(Qingdao, China) and performed in accordance with the Helsinki
Declaration. Each patient in this study provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Q, Xu H, Bai N, Tan F, Xu H and Liu
J: Matrix metalloproteinase 9 is regulated by LOX-1 and erk1/2
pathway in dental peri-implantitis. Curr Pharm Biotechnol.
21:862–871. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang Q, Liu J, Ma L, Bai N and Xu H:
LOX-1 is involved in TLR2 induced RANKL regulation in
peri-implantitis. Int Immunopharmacol. 77(105956)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roccuzzo A, Stähli A, Monje A, Sculean A
and Salvi GE: Peri-implantitis: A clinical update on prevalence and
surgical treatment outcomes. J Clin Med. 10(1107)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang Y, Zhang Y and Miron RJ: Health,
maintenance, and recovery of soft tissues around implants. Clin
Implant Dent Relat Res. 18:618–634. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barootchi S and Wang HL: Peri-implant
diseases: Current understanding and management. Int J Oral
Implantol (Berl). 14:263–282. 2021.PubMed/NCBI
|
|
6
|
Corry DB, Rishi K, Kanellis J, Kiss A,
Song LZ, Xu J, Feng L, Werb Z and Kheradmand F: Decreased allergic
lung inflammatory cell egression and increased susceptibility to
asphyxiation in MMP2-deficiency. Nat Immunol. 3:347–353.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Puliti M, Momi S, Falcinelli E, Gresele P,
Bistoni F and Tissi L: Contribution of matrix metalloproteinase 2
to joint destruction in group B Streptococcus-induced murine
arthritis. Arthritis Rheum. 64:1089–1097. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu CY and Lai SC: Induction of matrix
metalloproteinase-2 and -9 via Erk1/2-NF-κB pathway in human
astroglia infected with Toxoplasma gondii. Acta Trop. 127:14–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barreiros D, Filho NP, Paula-Silva FWG,
Oliveira KMH, Lucisano MP, Rossi AD, Silva LAB, Küchler EC and
Silva RAB: MMP2 and MMP9 are associated with apical periodontitis
progression and might be modulated by TLR2 and MyD88. Braz Dent J.
29:43–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Che C, Liu J, Ma L, Xu H, Bai N and Zhang
Q: LOX-1 is involved in IL-1β production and extracellular matrix
breakdown in dental peri-implantitis. Int Immunopharmacol.
52:127–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park E, Na HS, Kim SM, Wallet S, Cha S and
Chung J: Xylitol, an anticaries agent, exhibits potent inhibition
of inflammatory responses in human THP-1-derived macrophages
infected with Porphyromonas gingivalis. J Periodontol.
85:e212–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Che C, Liu J, Ma L, Xu H, Bai N and Zhang
Q: Osteopontin is essential for IL-1β production and apoptosis in
peri-implantitis. Clin Implant Dent Relat Res. 20:384–392.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao G, Hu M, Li C, Lee J, Yuan K, Zhu G
and Che C: Osteopontin contributes to effective neutrophil
recruitment, IL-1β production and apoptosis in Aspergillus
fumigatus keratitis. Immunol Cell Biol. 96:401–412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gonçalves PF, Huang H, McAninley S, Alfant
B, Harrison P, Aukhil I, Walker C and Shaddox LM: Periodontal
treatment reduces matrix metalloproteinase levels in localized
aggressive periodontitis. J Periodontol. 84:1801–1808.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
da Costa T.A, Silva MJ, Alves PM, Chica
JE, Barcelos EZ, Giani MA, Garlet GP, da Silva JS, Júnior VR,
Rodrigues DB and Cardoso CR: Inflammation biomarkers of advanced
disease in nongingival tissues of chronic periodontitis patients.
Mediators Inflamm. 2015(983782)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fu MM, Fu E, Kuo PJ, Tu HP, Chin YT,
Chiang CY and Chiu HC: Gelatinases and extracellular matrix
metalloproteinase inducer are associated with cyclosporin-A-induced
attenuation of periodontal degradation in rats. J Periodontol.
86:82–90. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Araújo AA, de Souza GL, Souza TO, de
Castro BritoGA, Aragão KS, de Medeiros CA, Lourenço Y, Alves MS and
de Araújo RM Jr: Olmesartan decreases IL-1β and TNF-α levels;
downregulates MMP-2, MMP-9, COX-2, and RANKL; and upregulates OPG
in experimental periodontitis. Naunyn Schmiedebergs Arch Pharmacol.
386:875–884. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bildt MM, Bloemen M, Kuijpers-Jagtman AM
and Hoff JW: Collagenolytic fragments and active gelatinase
complexes in periodontitis. J Periodontol. 79:1704–1711.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tang H, Mattheos N, Yao Y, Jia Y, Ma L and
Gong P: In vivo osteoprotegerin gene therapy preventing bone loss
induced by periodontitis. J Periodontal Res. 50:434–443.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kong L, Qi X, Huang S, Chen S, Wu Y and
Zhao L: Theaflavins inhibit pathogenic properties of P. gingivalis
and MMPs production in P. gingivalis-stimulated human gingival
fibroblasts. Arch Oral Biol. 60:12–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mieszkowska A, Folkert J, Gaber T, Miksch
K and Gurzawska K: Pectin nanocoating reduces proinflammatory
fibroblast response to bacteria. J Biomed Mater Res A.
105:3475–3481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nakayachi M, Ito J, Hayashida C, Ohyama Y,
Kakino A, Okayasu M, Sato T, Ogasawara T, Kaneda T, Suda N, et al:
Lectin-like oxidized low-density lipoprotein receptor-1 abrogation
causes resistance to inflammatory bone destruction in mice, despite
promoting osteoclastogenesis in the steady state. Bone. 75:170–182.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamasaki T, Ariyoshi W, Okinaga T, Adachi
Y, Hosokawa R, Mochizuki S, Sakurai K and Nishihara T: The dectin 1
agonist curdlan regulates osteoclastogenesis by inhibiting nuclear
factor of activated T cells cytoplasmic 1 (NFATc1) through syk
kinase. J Biol Chem. 289:19191–19203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang H, Wang Q, Han L, Yang X, Zhao W, Lyu
L, Wang L, Yan H and Che C: Nerolidol inhibits the LOX-1/IL-1β
signaling to protect against the Aspergillus fumigatus keratitis
inflammation damage to the cornea. Int Immunopharmacol.
80(106118)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheng M, Lin J, Li C, Zhao W, Yang H, Lv L
and Che C: Wedelolactone suppresses IL-1β maturation and neutrophil
infiltration in Aspergillus fumigatus keratitis. Int
Immunopharmacol. 73:17–22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Che C, Li C, Lin J, Zhang J, Jiang N, Yuan
K and Zhao G: Wnt5a contributes to dectin-1 and LOX-1 induced host
inflammatory response signature in Aspergillus fumigatus keratitis.
Cell Signal. 52:103–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Drummond RA and Brown GD: The role of
dectin-1 in the host defence against fungal infections. Curr Opin
Microbiol. 14:392–399. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu Z, Sawamura T, Kurdowska AK, Ji HL,
Idell S and Fu J: LOX-1 deletion improves neutrophil responses,
enhances bacterial clearance, and reduces lung injury in a murine
polymicrobial sepsis model. Infect Immun. 79:2865–2870.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C, Yang X, Pan Y, Yu N, Xu X, Tong T,
Tang X, Zhang D, Liu J and Lin L: A sialidase-deficient
porphyromonas gingivalis mutant strain induces less
interleukin-1β and tumor necrosis factor-α in Epi4 cells than W83
strain through regulation of c-Jun N-terminal kinase pathway. J
Periodontol. 88:e129–e139. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ren H, Li Y, Jiang H and Du M:
Porphyromonas gingivalis induces IL-8 and IFN-gamma
secretion and apoptosis in human extravillous trophoblast derived
HTR8/SVneo cells via activation of ERK1/2 and p38 signaling
pathways. Placenta. 45:8–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang L, Pan CL, Wang CY, Liu BQ, Han Y,
Hu L, Liu L, Yang Y, Qu JW and Liu WT: Selective suppression of the
JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates
neuropathic pain in rats. J Neuroinflammation.
14(174)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang MF, Lu CY and Lai SC: Up-regulation
of matrix metalloproteinases-2 and -9 via an Erk1/2/NF-κB pathway
in murine mast cells infected with Toxoplasma gondii. J Comp
Pathol. 149:146–155. 2013.PubMed/NCBI View Article : Google Scholar
|