Introduction
Malignant mixed Müllerian tumors (MMMT), widely
known as carcinosarcomas, are extremely rare and highly malignant
neoplasms when diagnosed in the female genital tract (1). Field literature recognizes the uterus,
cervix and ovary as the most common primary sites of these
malignancies (2). While the
endometrium is the most frequent known site for carcinosarcomas,
their development in the fallopian tube is a rare condition, only
accounting for 0.1 to 0.5% among all gynecological malignancies
(3,4). Usually fallopian carcinosarcomas
develop in the fifth to sixth decade in postmenopausal women, and
the preoperative non-specific aspects and multiple similarities to
hydrosalpinx, ovarian malignancies or tuboovarian abscess lead in
most cases to a misdiagnosis. Symptomatology has no specific
elements; the presenting symptom being usually abdominal pain
mostly in the hypogastric area, followed by abnormal vaginal
bleeding or abdominal distension, and exceptionally with an acute
clinical picture (5,6). Due to all the mentioned elements, a
diagnosis of certitude is extremely difficult to confirm, often
being verified only by the final histology result, but in some
cases cervical cytology or endometrial curettage may guide the
specialist (7).
Regarding the histological features, MMMTs integrate
both stromal and epithelial, carcinomatous and sarcomatous
elements, typically high grade, with a significantly aggressive
progress and a poor patient prognosis. In addition, this type of
tumor usually metastasizes and disseminates rapidly among the
pelvic organs in approximately 60% of the cases, but also to the
peritoneum, paraaortic lymphatic nodes, even distant metastasis to
the lungs, liver or bones (8,9).
The present article presents one case of fallopian
MMMT with heterologous elements synchronous with an endometrial
serous carcinoma surgically operated on in the First Obstetrics and
Gynecology Clinic, ‘George Emil Palade’ University of Medicine,
Pharmacy, Science, and Technology, Târgu Mureș, Romania. In
addition, a meta-analysis of the medical literature was performed
in order to find correlations between the patient medical data and
prognosis.
Patients and methods
A synchronous fallopian MMMT together with an
early-stage endometrial serous carcinoma is further described.
Moreover, the present study incorporates all the data found in
field literature regarding MMMTs, statistically analyzed in order
to identify potential associations between specific characteristics
and the described management of each patient and the post-treatment
survival. The data available in English literature was found
through Medline search, using the following keywords: ‘fallopian
carcinosarcoma’, ‘tubal carcinosarcoma’ and ‘fallopian malignant
mixed tumor’.
During the Medline search, 94 patients were reported
between 1902 and 2019. Ten cases were excluded because the patients
were lost to follow-up, or because of the lack of reported
information. Finally, 84 cases presented in Table I (4,5,7,8,10-69),
together with the one case surgically operated on by our team were
included in the present analysis. The reported cases were divided
into 2 groups according to patient outcome at the end of the
follow-up period in each case: No Evidence of Disease (NED) group
including 33 patients reported to be without any residual disease
at the end of the follow-up period; death of disease (DOD) group
including 51 patients who died due to the progression of fallopian
carcinosarcoma or its complications. The collected data concerned
the patient age at diagnosis, signs and symptoms at presentation,
imaging findings, the accuracy of the first diagnosis, surgical,
histological and oncological aspects.
 | Table IPreviously reported cases of
fallopian MMMT. |
Table I
Previously reported cases of
fallopian MMMT.
| Patient no. | Author (Refs.) | Year of report | Age of patients
(years) | FIGO stage | Outcome |
|---|
| 1 | Motta (10) | 1926 | 14 | IV | DOD |
| 2 | Zacho (11) | 1933 | N/D | IIIC | DOD |
| 3 | Platz (12) | 1940 | 58 | IV | DOD |
| 4 | Bochner (13) | 1961 | 58 | N/D | DOD |
| 5 | Williams and
Woodruff (14) | 1963 | 35 | IV | DOD |
| 6 | Malnasy and Gaal
(15) | 1963 | 45 | IIB | DOD |
| 7 | McQueeney et
al (16) | 1964 | 69 | IIB | DOD |
| 8 | De Queiroz and Roth
(17) | 1970 | 64 | IIIC | DOD |
| 9 | Wu et al
(18) | 1973 | 57 | IA | NED |
| 10 | Acosta et al
(19) | 1974 | 46 | IIB | DOD |
| 11 | Acosta et al
(19) | 1974 | 62 | IV | DOD |
| 12 | Acosta et al
(19) | 1974 | 48 | IC | DOD |
| 13 | Aggarwal et
al (20) | 1976 | 50 | IIIC | DOD |
| 14 | Manes and Taylor
(21) | 1976 | 76 | IA | DOD |
| 15 | Manes and Taylor
(21) | 1976 | 74 | IA | DOD |
| 16 | Manes and Taylor
(21) | 1976 | 47 | IA | NED |
| 17 | Manes and Taylor
(21) | 1976 | 58 | IA | NED |
| 18 | Henderson et
al (22) | 1977 | 62 | IIB | DOD |
| 19 | Jain (23) | 1977 | 52 | IA | NED |
| 20 | Oka et al
(24) | 1978 | 57 | IA | NED |
| 21 | Hanjani et
al (25) | 1980 | 62 | IV | DOD |
| 22 | Viniker et
al (26) | 1980 | 63 | IA | NED |
| 23 | Holst and Erichsen
(27) | 1981 | 65 | IIIC | NED |
| 24 | O'Toole et
al (28) | 1982 | 71 | IV | DOD |
| 25 | Egorov (29) | 1982 | 53 | N/D | DOD |
| 26 | Kahanpää et
al (30) | 1983 | 65 | III | NED |
| 27 | Deppe et al
(31) | 1984 | 68 | IIIB | NED |
| 28 | Punnonen et
al (32) | 1985 | 68 | IIIC | DOD |
| 29 | Buchino and Buchino
(33) | 1987 | 61 | IIIC | DOD |
| 30 | Yabushita et
al (34) | 1987 | 53 | IIA | NED |
| 31 | Chen and Wolk
(35) | 1988 | 56 | IC | DOD |
| 32 | Muntz et al
(36) | 1989 | 57 | IIIC | DOD |
| 33 | Muntz et al
(36) | 1989 | 60 | IIIA | DOD |
| 34 | Muntz et al
(36) | 1989 | 61 | IV | DOD |
| 35 | Axelrod et
al (37) | 1989 | 62 | IIIC | NED |
| 36 | Kinoshita et
al (38) | 1989 | 79 | IC | NED |
| 37 | van Dijk et
al (39) | 1990 | 45 | IIA | DOD |
| 38 | van Dijk et
al (39) | 1990 | 67 | IIIB | DOD |
| 39 | Seraj et al
(40) | 1990 | 62 | IIIC | DOD |
| 40 | Seraj et al
(40) | 1990 | 53 | IIIC | DOD |
| 41 | Liang et al
(41) | 1990 | 63 | IIIC | DOD |
| 42 | Chang et al
(42) | 1991 | 66 | III | DOD |
| 43 | Chiou et al
(43) | 1991 | 63 | IIIC | DOD |
| 44 | Imachi et al
(5) | 1992 | 60 | IIIC | DOD |
| 45 | Imachi et al
(5) | 1992 | 67 | IV | DOD |
| 46 | Moore et al
(44) | 1992 | 66 | IIIC | DOD |
| 47 | Carlson et
al (45) | 1993 | 72 | IIIC | DOD |
| 48 | Carlson et
al (45) | 1993 | 56 | IIIC | NED |
| 49 | Carlson et
al (45) | 1993 | 60 | IB | NED |
| 50 | Carlson et
al (45) | 1993 | 44 | IA | NED |
| 51 | Carlson et
al (45) | 1993 | 59 | IIIB | NED |
| 52 | Weber et al
(46) | 1993 | 74 | IIA | NED |
| 53 | Zorlu et al
(47) | 1994 | 38 | III | DOD |
| 54 | Horn et al
(48) | 1996 | 62 | IIIB | DOD |
| 55 | Horn et al
(48) | 1996 | 64 | IIB | DOD |
| 56 | Horn et al
(48) | 1996 | 69 | IIIC | DOD |
| 57 | Horn et al
(48) | 1996 | 71 | IV | DOD |
| 58 | Ebert et al
(49) | 1998 | 70 | IA | NED |
| 59 | Maitra et al
(50) | 2004 | 29 | IIIA | DOD |
| 60 | Moustafa et
al (51) | 2004 | 75 | IIA | DOD |
| 61 | Humble and Carter
(52) | 2004 | 63 | IIIC | DOD |
| 62 | Lim et al
(53) | 2004 | 57 | IA | NED |
| 63 | Gagner and Mittal
(54) | 2005 | 77 | IV | DOD |
| 64 | Kuroda et al
(55) | 2005 | 65 | IIB | DOD |
| 65 | Das et al
(56) | 2005 | 49 | III | NED |
| 66 | Das et al
(56) | 2005 | 80 | IIB | DOD |
| 67 | Hudelist et
al (57) | 2006 | 57 | IIB | NED |
| 68 | Kuroda et al
(58) | 2007 | 77 | IIIC | DOD |
| 70 | Kawaguchi et
al (59) | 2008 | 69 | IC | NED |
| 71 | Kourea et al
(60) | 2008 | 72 | IIIC | NED |
| 72 | Piura et al
(61) | 2009 | 46 | IIIC | NED |
| 73 | Shen et al
(8) | 2010 | 58 | III | DOD |
| 74 | Malhotra et
al (62) | 2012 | 60 | IIIC | DOD |
| 75 | Watanabe et
al (7) | 2012 | 53 | IIIC | NED |
| 76 | Tsai et al
(63) | 2012 | 57 | IIIA | NED |
| 77 | Gupta and Jenison
(64) | 2011 | 74 | IIIC | DOD |
| 78 | Takemoto et
al (65) | 2015 | 56 | IIIC | DOD |
| 79 | Narin et al
(66) | 2015 | 68 | IIA | NED |
| 80 | Vale-Fernandes
et al (67) | 2015 | 57 | IIA | NED |
| 81 | Ji et al
(4) | 2015 | 60 | IIIC | NED |
| 82 | Monsalve et
al (68) | 2015 | 71 | III | NED |
| 83 | Zhang et al
(1) | 2018 | 70 | IIIB | NED |
| 84 | Bécsi et al
(69) | 2019 | 70 | IIIB | NED |
Statistical analysis
Data were gathered from the previously reported
cases in the literature and processed using Microsoft Excel. For
the statistical analysis, the GraphPad InStat software (GraphPad
Software, Inc.) was used, made available by ‘George Emil Palade’
University of Medicine, Pharmacy, Science, and Technology of Târgu
Mureș, Romania. Quantitative variables were revealed as mean and
median, qualitative and categorical variables being expressed as
integer and percentage values. For all variable groups the
normality of distribution was evaluated by applying
Kolmogonov-Smirnov test. Quantitative analysis was performed using
the Student's t-test for groups with Gaussian distribution of
values and Mann-Whitney test for groups with abnormal distribution.
Inferential statistics consisting in odds ratio (OR) calculations
for mentioned pre-treatment, surgical, histopathological and
oncological data was conducted with Fisher's exact test, this
offering a higher accuracy. The level of statistical significance
was established at a P-value of 0.05, with a 95% confidence
interval for all the investigated parameters.
Case report Clinical and paraclinical
findings
A female patient aged 65, primigravidae, primiparous
presented with a moderate lower abdomen discomfort and a light
atypical vaginal bleeding for 2 weeks. The patient was
postmenopausal from the age of 50, this being the first bleeding
episode. At the clinical gynecologic exam, no vaginal or cervical
macroscopic pathologies were detected, but abdominal palpation
revealed a moderate sensitivity in the hypogastric area,
accentuated in both iliac fossa. Transvaginal ultrasonography
uncovered images suggesting a bilateral hydrosalpinx of 92x33 mm on
the right side and 45x12 mm on the left side, also showing an
intracavitary image pleading for a large endometrial polyp of 19x23
mm. These ultrasonography findings did not raise any suspicions or
the necessity of substantial imagistic explorations, due to the
absence of criteria which could indicate a neoplastic disease.
After appropriate counseling and considering the patient age and
associated medical conditions, the patient was scheduled for an
operative hysteroscopy followed by laparoscopy and
histopathological exam.
Intraoperative appearance
On October 2019, the patient was admitted to the
First Obstetrics and Gynecology Clinic, Targu Mures Emergency
Clinical County Hospital, Romania, for a combined hysteroscopic and
laparoscopic approach. Under general anesthesia, a diagnostic
hysteroscopy was performed, which revealed an atrophic endometrium
with permeable tubal ostia together with an endometrial tumor
suggesting a polyp. Thus, a hysteroscopic polypectomy was performed
and the specimen was sent for histopathological examination.
During the laparoscopic phase, extended perianexial
adhesions on the left side were found and an atrophic uterus and
ovaries. Both fallopian tubes were enlarged and tumoral, similar to
a hydrosalpinx with thick walls, sinuous, measuring 7x2x3 cm on the
left side and 8x7x4 cm on the right side, without noticeable
vegetation on the tubal surface but with mixed content, both fluid
and cerebroid, expelled through the pavilion. A bilateral
adnexectomy was performed and the specimen was carefully extracted
through a mini laparotomy in the left iliac fossa and sent for
frozen section, which confirmed malignancy. Subsequently, a
laparotomy approach was chosen and a total hysterectomy, pelvic and
paraaortic lymphadenectomy, appendectomy, total omentectomy were
performed, without intraoperative complications and with no
residual disease in the abdomen. Her postoperative recovery was
uneventful under antibiotic prophylaxis and anticoagulant
treatment. The patient was discharged on the 7th postoperative day.
After surgery and the final pathology result, the patient completed
6 cycles of systemic chemotherapy with carboplatin and paclitaxel
and has NED.
Histopathological examination
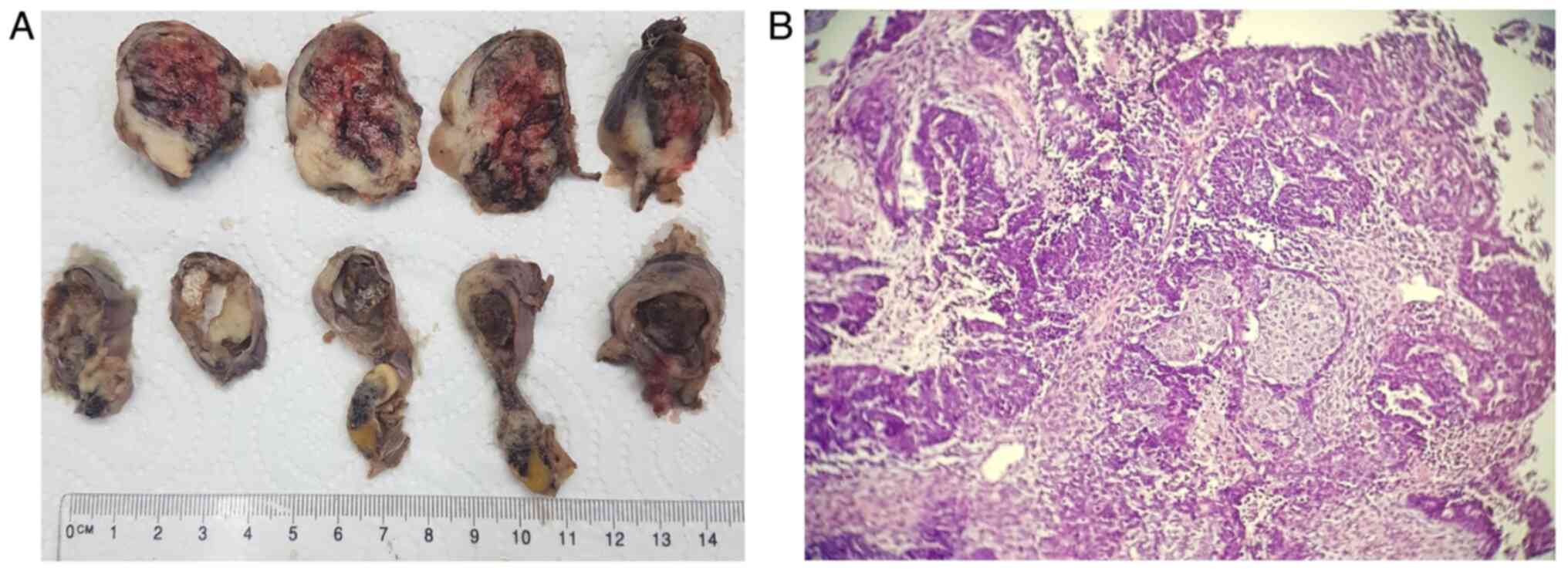
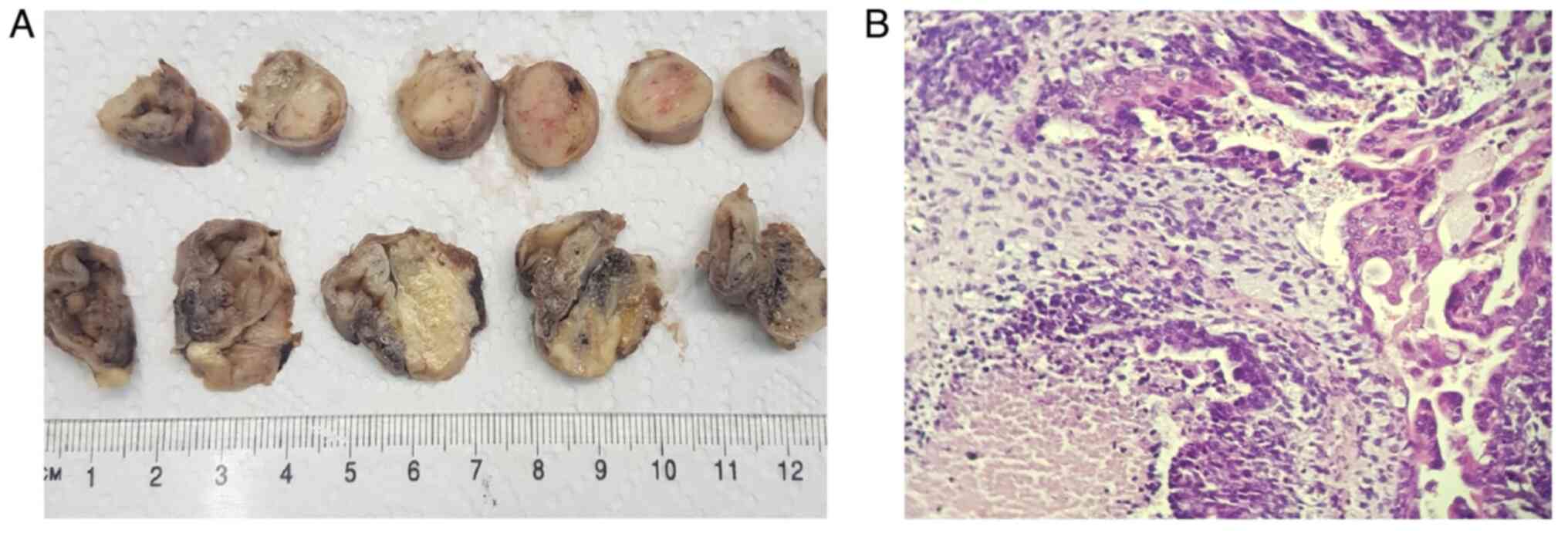
Macroscopic and microscopic features of the two
excised fallopian tubes are presented in Figs. 1 and 2. The right fallopian tube measured 110x45
mm, exhibited an increase in volume and dilated on the entire
length, presenting a ruptured serosa in several portions and a
friable white tumoral mass which filled and enlarged the lumen in
all performed sections, with many necrotic associated with
hemorrhagic areas. The left fallopian tube measured 50x15 mm, with
dilated portions and the examined sections unveiling a white
vegetant tumoral mass extended in the entire length of the
organ.
Microscopically, in both fallopian tubes, the same
type of infiltrative tumor was found, with mixed aspect: an
epithelial component of high-grade serous carcinoma associated with
heterologous elements, such as chondrosarcoma, liposarcoma and
undifferentiated sarcoma, with extended areas of necrosis and
hemorrhage. In the tubal epithelium, multiple serous
intraepithelial carcinoma zones with an increased mitotic index
were observed. In the right tube, the tumor was found to infiltrate
the entire wall, and tumoral cells were found on the serosa. In the
left tube, the tumor infiltrated only the muscular wall. The
microscopic examination revealed lymphovascular emboli but without
tumoral invasion in the ovaries and without metastases in all the
58 pelvic and paraaortic removed lymph nodes. The omentum and
appendix were tumor-free.
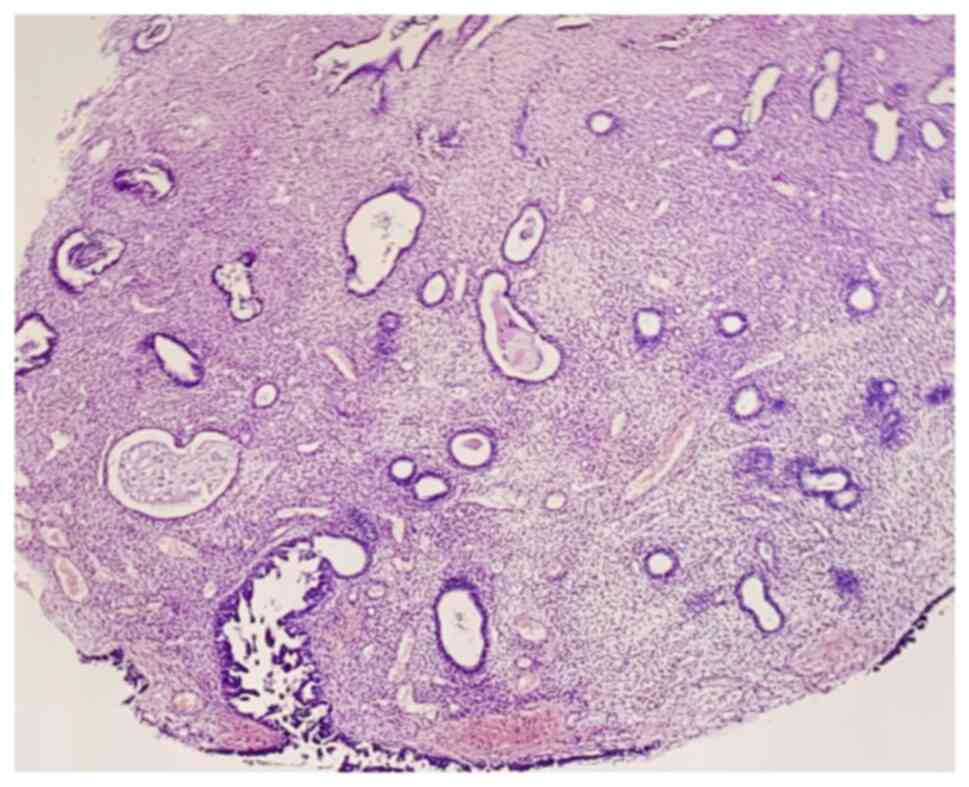
Regarding the uterus, the endometrial mass appeared
as a polypoid lesion with predominant atrophic glands, but with
serous endometrial intraepithelial carcinoma features on the
surface, with no invasion. The microscopic aspect is presented in
Fig. 3. The final histological
diagnosis was bilateral tubal carcinosarcoma (MMMT) and synchronous
serous endometrial intraepithelial carcinoma, FIGO stage IC2 and
pTNM stage pT1c2.
Results
The pre-treatment assessment of the cases previously
reported in the literature (4,5,7,8,10-69)
is presented in Table II. The mean
age was not significantly different in the study groups. Regarding
the patients' repartition by decades, the age interval 41-60 years
was a statistically significant protective factor towards death
(OR=0.3684, P=0.0419). Patients' age <40 years and 61-80 years
represented a higher risk for a negative outcome, although the
results were not statistically significant. Regarding the symptoms,
the abdominal distention reported in the died of disease (DOD)
group was confirmed to be the only one directly affecting prognosis
and could be considered a risk factor for death with an OR=3.955
(P=0.0226). In addition, ascites was found to influence the
outcome, being present in 17.6% of the patients included in the DOD
group, but the calculated P-value for its OR was above the level of
statistical significance. Tumor evidence on imaging could better
guide treatment, prolonging the life of patients (OR=0.2500,
P=0.0216). Cancer antigen (CA)125 level was not shown to be a
statistically significant prognostic factor, but the published data
when performed by routine in patients with suspicious tubal
malignancies were poor. The accuracy of the initial diagnosis was
low due to the multiple non-specific elements of the disease as
previously mentioned, more frequent patients being diagnosed with
ovarian or pelvic tumor, followed by hydrosalpinx in both the NED
and DOD groups. None of the initial misdiagnoses affected prognosis
from the statistical perspective.
 | Table IIPre-treatment evaluation in the field
literature. |
Table II
Pre-treatment evaluation in the field
literature.
| Features | No evidence of
disease (NED) group (n=33), n (%) | Death of disease
(DOD) group (n=51), n (%) | Odds ratio
(OR) | P-value |
|---|
| Age of the patients
(years) | | | | |
|
<40 | 0 (0) | 3 (5.9) | 4.8350 | NS |
|
41-60 | 19 (57.6) | 17 (33.3) | 0.3684 | 0.0419 |
|
61-80 | 14 (42.4) | 31 (60.8) | 2.1040 | NS |
| Mean age | 60.27 | 61.25 | - | NS |
| Signs and
symptoms | | | | |
|
Atypical
vaginal bleeding | 16 (48.5) | 21 (41.2) | 0.7438 | NS |
|
Pelvic
mass | 10 (30.3) | 12 (23.5) | 0.7077 | NS |
|
Abdominal
pain | 13 (39.4) | 25 (49.0) | 1.4790 | NS |
|
Abdominal
distention | 4 (12.1) | 18 (35.3) | 3.9550 | 0.0226 |
|
Fever | 1 (3.0) | 2 (3.9) | 1.3060 | NS |
|
Ascites | 2 (6.0) | 9 (17.6) | 3.3210 | NS |
| Other pre-treatment
findings | | | | |
|
CT/RMN tumor
evidence | 10 (30.3) | 5 (9.8) | 0.2500 | 0.0216 |
| CA125 | | | | |
|
Normal | 3 (9.1) | 1 (2.0) | 0.2000 | NS |
|
Elevated
(>35 U/ml) | 4 (12.1) | 4 (7.8) | 0.6170 | NS |
|
No
evidence | 25 (75.8) | 48 (94.1) | - | - |
| Accuracy of first
diagnosis | | | | |
|
Accurate
diagnosis | 1 (3.0) | 3 (5.9) | 2.0000 | NS |
|
Ovarian
tumor | 8 (24.2) | 8 (15.7) | 0.5814 | NS |
|
Pelvic
tumor | 4 (12.1) | 6 (11.8) | 0.9667 | NS |
|
Hydrosalpinx | 3 (9.1) | 1 (2.0) | 0.2000 | NS |
|
Uterine
tumor | 1 (3.0) | 1 (2.0) | 0.6400 | NS |
In Table III, the
surgical management and pathology reports are shown. Concerning the
surgical treatment, a total hysterectomy and bilateral
salpingo-oophorectomy were performed for most of the patients.
Omentectomy proved to be a statistically significant protective
factor, increasing the survival (OR=0.3545, P=0.0269). Similar data
were found regarding pelvic lymphadenectomy, performed more
frequent in the NED group (42.4%), with an OR=0.3732 and P=0.05.
The need for bowel resection in fallopian MMMT patients could
highly predispose to a poor prognosis, but the calculated chance
rates (OR=7.925) were not statistically significant. Other surgical
procedures, such as appendectomy, paraaortic lymphadenectomy,
peritonectomy or metastases resection did not present statistical
importance in the patient evolution.
 | Table IIISurgical management of fallopian
MMMT. |
Table III
Surgical management of fallopian
MMMT.
| Feature | No evidence of
disease (NED) group (n=33), n (%) | Death of disease
(DOD) group (n=51), n (%) | Odds ratio
(OR) | P-value |
|---|
| Surgical
procedure | | | | |
|
Hysterectomy | 32 (96.9) | 42 (82.4) | 0.1458 | NS |
|
Bilat.
salpingo-oophorectomy | 32 (96.9) | 47 (92.2) | 0.3672 | NS |
|
Omentectomy | 20 (60.6) | 18 (35.3) | 0.3545 | 0.0269 |
|
Appendectomy | 7 (21.2) | 8 (15.7) | 0.6910 | NS |
|
Pelvic
lymphadenectomy | 14 (42.4) | 11 (21.6) | 0.3732 | 0.0500 |
|
Paraaortic
lymphadenectomy | 6 (18.2) | 7 (13.7) | 0.7159 | NS |
|
Peritonectomy | 1 (3.0) | 2 (3.9) | 1.3060 | NS |
|
Bowel
resection | 0 (0) | 5 (9.8) | 7.9250 | NS |
|
Metastases
resection | 5 (15.2) | 6 (11.8) | 0.7467 | NS |
| Presence of
extragenital metastases | | | | |
|
Omentum | 7 (21.2) | 18 (35.3) | 2.0260 | NS |
|
Appendix | 2 (6.1) | 2 (3.9) | 0.6327 | NS |
|
Lymph
nodes | 5 (15.2) | 18 (35.3) | 3.0550 | 0.0491 |
|
Peritoneum | 7 (21.2) | 17 (33.3) | 2.0000 | NS |
|
Bowel | 2 (6.1) | 11 (21.6) | 4.2630 | 0.0500 |
|
Distant | 0 (0) | 9 (17.6) | 14.9760 | 0.0103 |
| FIGO staging | | | | |
|
I (A-C) | 13 (39.4) | 4 (7.8) | 0.1309 | 0.0007 |
|
II
(A-B) | 6 (18.2) | 8 (16.7) | 0.8372 | NS |
|
III
(A-B) | 6 (18.2) | 7 (13.7) | 0.7159 | NS |
|
IIIC | 8 (24.2) | 23 (45.1) | 2.5670 | 0.0500 |
|
IV | 0 (0) | 9 (17.7) | 14.9760 | 0.0103 |
| Tumor
localization | | | | |
|
Intraluminal | 16 (48.5) | 14 (27.4) | 0.0636 | NS |
|
Fimbria | 2 (6.1) | 11 (21.6) | 4.2630 | NS |
|
No
evidence | 15 (45.4) | 26 (51.0) | - | - |
| Histological
type | | | | |
|
Homologous | 18 (54.5) | 15 (29.4) | 0.3472 | 0.0247 |
|
Heterologous | 15 (45.5) | 36 (70.6) | 2.8800 | 0.0247 |
|
Chondrosarcoma | 13 (39.4) | 26 (51.0) | 1.6000 | NS |
|
Rhabdomyosarcoma | 4 (12.1) | 12 (23.5) | 2.2231 | NS |
|
Osteosarcoma | 2 (6.1) | 1(2) | 0.3100 | NS |
|
Liposarcoma,
angiosarcoma | 4 (12.1) | 0 (0) | 0.0636 | 0.0212 |
The presence of extragenital metastases proved to be
risk factors for death, especially involving the lymph nodes
(OR=3.055, P=0.0491), bowel (OR=4.263, P=0.05) or distant organs
and tissues (OR=14.976, P=0.0103). Although omentectomy has been
proven to be a protective factor, the evidence of omentum
metastases was not highly reported in the reviewed articles, thus
the OR for this parameter (OR=2.026) was not statistically
significant. FIGO staging was another important aspect searched in
previous reports. FIGO stage I can be considered a positive
prognosis factor, the results fitting into protective factor
intervals towards death (OR=0.1309, P=0.0007). Patients with FIGO
staged IIIC (OR=2.567, P=0.05) and IV (OR=14.976, P=0.0103) were
more susceptible to negative post-treatment outcomes.
The histologic features were also analyzed,
depending on the existing evidence in the reviewed articles.
Despite the lack of existing data regarding the tumor localization
in different segments of the fallopian tube, an intraluminal
development of the tumor could be a protective factor in relation
to death (OR=0.0636), while fimbrial localization is more probable
to be a risk factor (OR=4.263), but none of the parameters
presented statistical significance. Analyzing the histological type
of the MMMT, homologous type was a protective factor for death
(OR=0.3472) while heterologous type could be considered a risk
factor (OR=2.880), both parameters being extremely significant
(P=0.0247). Due to insufficient evidence concerning the
heterologous-specific elements, the obtained results are not of
statistical importance.
Oncological approach and patient follow-up data are
presented in Table IV. In the NED
group, the majority of patients (72.7%) received systemic
chemotherapy, the statistical analysis results confirming that
chemotherapy administration is a very significant protective factor
against death, with an OR=0.2679 and P=0.0070, while the absence of
chemotherapy in the treatment of fallopian MMMT is an uncontestable
risk factor (OR=3.733). Regarding the chemotherapy agents reported,
the only regimen with a statistical positive impact on the survival
of patients was carboplatin + paclitaxel (OR=0.2857, P=0.0293). The
necessity of using multiple therapeutic lines during the treatment
may suggest a negative outcome (OR=2.1330, OR=2.2140), but without
statistical significance. Concerning radiotherapy, the evidence
gathered from the literature did not suggest any significant
involvement in disease progression from a statistical
perspective.
 | Table IVOncological approach and
follow-up. |
Table IV
Oncological approach and
follow-up.
| Treatment
approach | No evidence of
disease (NED) group (n=33), n (%) | Death of disease
(DOD) group (n=51), n (%) | Odds ratio
(OR) | P-value |
|---|
| Chemotherapy | | | | |
|
Received | 24 (72.7) | 20 (39.2) | 0.2679 | 0.0070 |
|
Not
received | 9 (27.3) | 28 (54.9) | 3.7330 | 0.0070 |
|
No
evidence | 0 (0) | 3 (5.9) | - | - |
| First-line
chemotherapy agents | | | | |
|
Carboplatin+paclitaxel | 11 (45.8) | 6 (30.0) | 0.2857 | 0.0293 |
|
Cisplatin+doxorubicin+cyclophosphamide | 7 (29.2) | 8 (40.0) | 0.7429 | NS |
|
Cyclophosphamide+vincristine+doxorubicin | 2 (8.3) | 1 (5.0) | 0.3298 | NS |
|
Vincristine+actynomicin
D+cyclophosphamide | 1 (4.2) | 2 (10.0) | 2.1330 | NS |
|
Unknown
agents | 3 (12.5) | 3 (15.0) | - | - |
|
Multiple
therapeutic lines | 2 (8.3) | 6 (30.0) | 2.2140 | NS |
| Radiotherapy | | | | |
|
Received | 12 (36.4) | 16 (31.4) | 0.8750 | NS |
|
Not
received | 21 (63.6) | 32 (62.7) | 1.1430 | NS |
|
No
evidence | 0 (0) | 3 (5.9) | - | - |
| Follow-up
(months) | | | | |
|
Average | 33.40 | 13.19 | - |
<0.0001 |
|
Median | 29 | 8 | | |
| FIGO stage | | | | |
|
Stage I
(A-C) | 46.53 | 26 | - | NS |
|
Stage II
(A-B) | 29 | 11.33 | - | 0.0256 |
|
Stage III
(A-B) | 40.17 | 29.42 | - | NS |
|
Stage
IIIC | 31.75 | 12.13 | - | 0.0034 |
|
Stage
IV | - | 18.5 | - | - |
Regarding the follow-up period, after eliminating
the outlier values, the average was 33.40 months in the NED group
and 13.19 months in the DOD group, the differences being
statistically significant (P<0.0001). The median survival was 29
months in the NED and 8 months in the DOD group. The follow-up
period depending on FIGO stage also presented several differences
between the two groups. For stages I (A-C) and III (A-B), there
were no statistically significant differences regarding the average
follow-up period in the NED and DOD group. For FIGO stage II (A-B),
the average follow-up was 29 months in the NED and 11.33 months in
the DOD group, differences being statistically significant
(P=0.0256). For stage IIIC, the average follow-up was significantly
higher in the NED (31.75 months) than in the DOD group (12.13
months) (P=0.0034). It is also notable that were no FIGO stage IV
patients in the NED group.
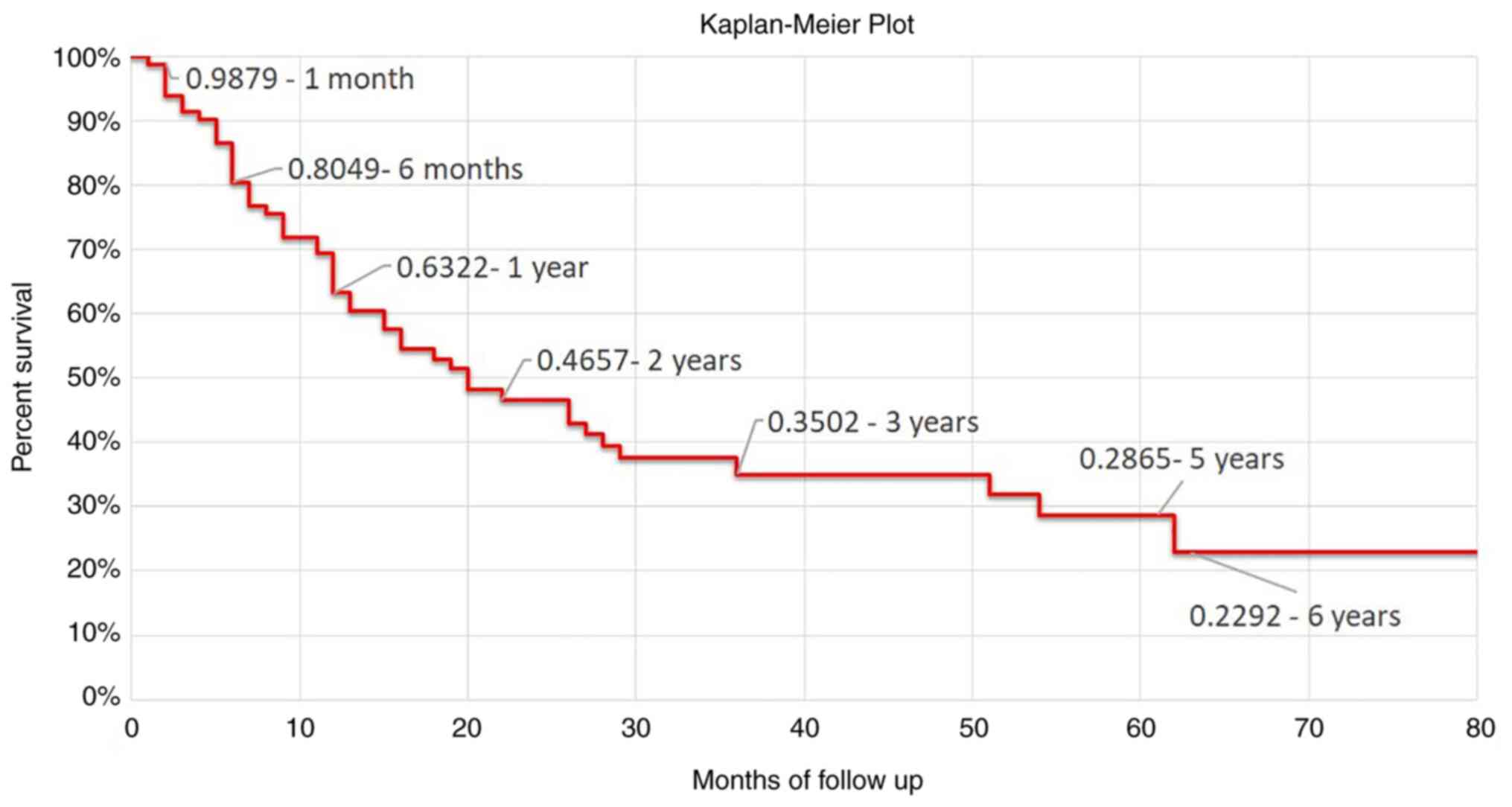
Fig. 4 presents the
Kaplan-Meier survival analysis and survival rates at different
checkpoints. The survival rate was 0.9879 at 1 month of follow-up,
0.8049 at 6 months, 0.4657 at 2 years, while at 5 years of
follow-up it was 0.2865.
MMMT accounts for about 2.4% of all fallopian tube
malignancies, and only 4% from this histologic type develop in the
fallopian tube as a primary tumor. The rarity of fallopian MMMTs
could be correlated with the reduced cyclical activity and lower
hormonal responsiveness of the tubal stroma, as compared with
endometrial stroma where these tumors occur almost 10 times more
frequently (16). These tumors are
associated with high invasiveness and poor patient prognosis,
especially if the diagnosis is delayed. Due to the extremely low
incidence of fallopian carcinosarcoma, clinical protocols for these
tumors are not clearly established (22,70).
Discussion
Previously published literature reviews (Table I) (4,5,7,8,10-69)
have revealed that the average age of the 85 patients was 59.7
years. Regarding the symptomatology at presentation, our results
are in accordance with previous reports by reporting atypical
vaginal bleeding and pelvic pain or discomfort, although there is
no relevant evidence for which these symptoms may be correlated
with prognosis. MMMTs can present, although rarely, as acute
abdomen, cases in which are associated with torsion or rupture,
leading to hemoperitoneum (64,66).
Due to the common symptoms of MMMTs, rarity and localization, an
initial definitive diagnosis is very difficult to achieve and
prove, in the majority of cases remaining uncertain until the
histological examination is performed; the most frequent
preoperative and intraoperative diagnosis is related to an ovarian
tumor, underlining its inaccuracy (67). Concerning imaging examination, MRI
is more sensitive compared to CT to distinguish various tumor
characteristics that may facilitate the preoperative diagnosis and
further treatment, although imaging reports of carcinosarcoma of
the fallopian tube are limited, as well as the role of
CA125(71).
Despite the lack of therapeutic protocols for
fallopian MMMTs and the small number of reported cases, the
strategy involves a primary surgical procedure aimed to resect all
visible tumors, followed by oncological treatment intensely debated
in the past decades (72). For
proper staging, ascites or peritoneal washings must be collected
for cytological examinations, followed by a thorough exploration of
all peritoneal surfaces; a total hysterectomy and bilateral
salpingo-oophorectomy must be performed, together with omentectomy,
lymphadenectomy and peritoneal biopsies, depending on the
intraoperative findings, achieving a maximal cytoreduction when
possible (48,73). As already demonstrated, omentectomy
could be an important positive prognostic factor, together with
pelvic lymphadenectomy, but the extent of surgery might be variable
and sometimes demanding because of pelvic modified anatomy,
requiring a retroperitoneal dissection (74). Regarding the possible metastatic
sites, the most frequent are the contralateral tube, ovaries,
uterus, but also the peritoneal surface, emphasizing that pelvic
and paraaortic lymphatic nodes are not often involved, while
distant metastasis is extremely rare (63), but our meta-analysis has confirmed
that omentum, lymph node and distant metastases in fallopian MMMTs
are often described. Sometimes, when the fallopian tumor invades
other pelvic organs, different types of exenterative procedure must
be performed, but these situations are rare (75).
A proper staging concerning fallopian
carcinosarcomas is essential to adopt a therapeutic strategy, the
survival rates being directly dependent on this parameter. As the
results of the meta-analysis demonstrated, FIGO stage I presented
the best survival outcomes, while FIGO stages IIIC and IV were
prone to death due to disease. The prognosis of a primary fallopian
tube malignancy is usually poor and depends rather on staging than
on histological criteria, such as tumor type or grade (76).
Previous histological macroscopic descriptions of
the tumor were similar with the present case report, revealing a
dilated lumen of the tube containing polypoid or infiltrative grey
or white colored mass, more frequent with necrosis and hemorrhage
areas (69). Microscopically two
mentioned components of the MMMT were often reported-a serous
carcinoma with high-grade malignancy associated with a neoplastic
proliferation of the conjunctive tissue, the presence of
chondrosarcoma detected in about 50% of cases (38,60).
The current meta-analysis has shown that a fimbrial localization of
the tumor could predispose to a more aggressive tumor evolution, an
issue explained by the fact that intraluminal fluid can be
discharged through the uterus in the case of fimbrial atresia. But
when the end of the fimbria remains open or the tumor develops at
this level, it is more likely for tumor cells to be implanted into
the abdominal cavity, situations in which the prognosis is poor
(77). The histological type is
also known to be a prognostic factor in many gynecological
malignancies. Current meta-analysis results reporting that the
heterologous type of fallopian carcinosarcoma could negatively
affect survival and, by contrary, homologous MMMTs are thought to
be associated with a better prognosis (78).
Systemic chemotherapy significantly improves
survival, especially associated with an optimal cytoreductive
surgery, as mentioned before. Over the past several decades,
multiple regimens have been tried, demonstrating that adjuvant
chemotherapy containing platinum agents is the most effective
treatment for fallopian carcinosarcomas (79). GOG Study analyzed the association
between ifosfamide and cisplatin, confirming no survival advantage,
with the cost of increased toxicity (80). Currently, the combination of
paclitaxel and carboplatin has been intensely studied and gained
popularity in a great variety of gynecological malignant diseases,
due to its important activity, acceptable toxicity and ease of
administration; the results of current meta-analysis have also
revealed that patients who received this drug combination exhibit
better survival outcomes (59).
Radiotherapy has no influence on prognosis and no benefit on
survival (25,54,57,65,81).
To date, the few reported fallopian MMMTs emphasize
its extremely low incidence and its high malignancy, fulminant
progression, and high incidence of local and distant metastases,
all associated with poor survival outcomes. As an early definite
diagnosis is extremely difficult to achieve even with high
performance imaging examinations, most of the cases are finally
diagnosed after histological evaluation. Fallopian MMMTs should be
considered as a differential diagnosis in all postmenopausal
patients who present with a pelvic mass, vaginal bleeding,
abdominal pain or distension and with no other significant
findings. Due to the non-specific presentation, symptomatology and
low incidence of this neoplasia, the success of conducting large
randomized trials in order to improve diagnosis accuracy, treatment
options and establish international therapeutic protocols is
limited. Reporting this rare pathology could be essential for
obtaining more precise information regarding the diagnostic
methods, targeted treatment and prognosis, in order to improve the
survival and quality of life in patients with MMMTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received to complete the present
literature research.
Availability of data and materials
Data was gathered from previously published reports
and collected into a database. The dataset used and analyzed during
the current study is available from the corresponding author on
reasonable request, all the results being included in this
article.
Authors' contributions
ALC conceived, directed the project and prepared the
manuscript. MEC was the leading surgeon for the surgical procedure
described in our case report. AAM and NB supervised the work and
revised the article. SM prepared and histologically analyzed the
specimens. ALC, MG, SLK, AF and MS are part of the surgical team
since 2016 and collected data from previously published articles.
MG, SLK and MS were involved in designing and drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable. Yet, informed consent was obtained
for publication of the patient's data.
Patient consent for publication
The patient provided written informed consent for
the scientific publication of any associated data and accompanying
images.
Competing interests
Authors declare no competing interests relevant to
this article.
References
|
1
|
Zhang Q, Liu A, Wu JJ, Niu M, Zhao Y, Tian
SF, Chen A and Zhong L: Primary malignant mixed Müllerian tumors of
the fallopian tube with cervix metastasis: A rare case report and
literature review. Medicine (Baltimore). 97(e11311)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lorusso D, Martinelli F, Mancini M, Sarno
I, Ditto A and Raspagliesi F: Carboplatin-paclitaxel versus
cisplatin-ifosfamide in the treatment of uterine carcinosarcoma: A
retrospective cohort study. Int J Gynecol Cancer. 24:1256–1261.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schink JC and Lurain JR: Rare gynecologic
malignancies. Curr Opin Obstet Gynecol. 3:78–90. 1991.PubMed/NCBI
|
|
4
|
Ji J, Zuo P, Li L and Wang Y: Primary
malignant mixed Müllerian tumor of the fallopian tube after
subtotal hysterectomy: A case report and literature review. Arch
Gynecol Obstet. 291:1187–1190. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Imachi M, Tsukamoto N, Shigematsu T,
Watanabe T, Uehira K, Amada S, Umezu T and Nakano H: Malignant
mixed Müllerian tumor of the fallopian tube: Report of two cases
and review of literature. Gynecol Oncol. 47:114–124.
1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xue Q, Wu QY and Wang J: Ultrasonographic
diagnosis of carcinosarcoma of the fallopian tube: A report of 1
case. Chin J Med Imaging. 21(942)2013.
|
|
7
|
Watanabe T, Sugino T, Furukawa S, Soeda S,
Nishiyama H and Fujimori K: Malignant mixed Müllerian tumor of the
fallopian tube: A case report. Eur J Gynaecol Oncol. 33:223–226.
2012.PubMed/NCBI
|
|
8
|
Shen YM, Xie YP, Xu L, Yang KX, Yu N, Yu Y
and Wang JH: Malignant mixed Müllerian tumor of the fallopian tube:
Report of two cases and review of literature. Arch Gynecol Obstet.
281:1023–1028. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hellström AC, Tegerstedt G, Silfverswärd C
and Pettersson F: Malignant mixed Müllerian tumors of the ovary:
Histopathologic and clinical review of 36 cases. Int J Gynecol
Cancer. 9:312–316. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Motta G: Contributto alla conoscenza dei
tumori misti rari dell' apperato genitale fimminile (carcinosarcoma
della salpinge). Ann Obstet Ginecol. 48:611–625. 1926.
|
|
11
|
Zacho A: Sur le carcinoma primaire dans la
trompe de fallope: Avec exposé D'un cas. Acta Obstet Gynecol Scand.
13:283–291. 1933.
|
|
12
|
Platz J: Über sechs weitere Fälle von
primärem Tubencarcinom. Arch Gynaekol. 170:604–615. 1940.
|
|
13
|
Bochner K: Primary uterine tube
malignancy. Obstet Gynecol. 18:767–769. 1961.PubMed/NCBI
|
|
14
|
Williams TJ and Woodruff JD: Malignant
mixed mesenchymal tumor of the uterine tube: Report of a case.
Obstet Gynecol. 21:618–621. 1963.PubMed/NCBI
|
|
15
|
Malnasy J and Gaal M: Primary
carcinosarcoma of the fallopian tube. Gynaecologia. 156:203–208.
1963.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McQueeney AJ, Carswell BL and Sheehan WJ:
Malignant mixed Müllerian tumor primary in uterine tube: Review of
the literature and report of an additional case. Obstet Gynecol.
23:338–343. 1964.PubMed/NCBI
|
|
17
|
De Queiroz AC and Roth LM: Malignant mixed
Müllerian tumor of the fallopian tube. Report of a case. Obstet
Gynecol. 36:554–557. 1970.PubMed/NCBI
|
|
18
|
Wu JP, Tanner WS and Fardal PM: Malignant
mixed Müllerian tumor of the uterine tube. Obstet Gynecol.
41:707–712. 1973.PubMed/NCBI
|
|
19
|
Acosta AA, Kaplan AL and Kaufmann RH:
Mixed Müllerian tumors of the oviduct. Obstet Gynaecol. 44:84–90.
1974.PubMed/NCBI
|
|
20
|
Aggarwal S, Devi PK and Aikat M: Malignant
mixed Müllerian tumour of the fallopian tube. J Obstet Gynaecol
India. 85:224–236. 1976.
|
|
21
|
Manes JL and Taylor HB: Carcinosarcoma and
mixed Müllerian tumors of the fallopian tube: Report of four cases.
Cancer. 38:1687–1693. 1976.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Henderson SR, Harper RC, Salazar OM and
Rudolph JH: Primary carcinoma of the fallopian tube: Difficulties
of diagnosis and treatment. Gynecol Oncol. 5:168–179.
1977.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jain U: Mixed mesodermal tumor of the
fallopian tube: Report of a case and review of literature. Md State
Med J. 26:43–46. 1977.PubMed/NCBI
|
|
24
|
Oka M, Bassett EP and Gross S: Malignant
mixed Müllerian tumors. N Y State J Med. 78:1431–1434.
1978.PubMed/NCBI
|
|
25
|
Hanjani P, Petersen RO and Bonnell SA:
Malignant mixed Müllerian tumor of the fallopian tube. Report of a
case and review of literature. Gynecol Oncol. 9:381–393.
1980.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Viniker DA, Mantell BS and Greenstein RJ:
Carcinosarcoma of the fallopian tube: A case report and review of
the literature. Br J Obstet Gynaecol. 87:530–534. 1980.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Holst N and Erichsen A: Mixed mesodermal
tumour of the fallopian tube. A case report. Ann Chir Gynaecol.
70:207–209. 1981.PubMed/NCBI
|
|
28
|
O'Toole RV, Tuttle SE and Shah NT:
Heterologous carcinosarcoma of the fallopian tube. A case report. J
Reprod Med. 27:749–752. 1982.PubMed/NCBI
|
|
29
|
Egorov VP: Heterotopic mesodermal tumor of
the fallopian tube. Arkh Patol. 44:54–56. 1982.PubMed/NCBI(In Russian).
|
|
30
|
Kahanpää KV, Laine R and Saksela E:
Malignant mixed Müllerian tumor of the fallopian tube: Report of a
case with 5-year survival. Gynecol Oncol. 16:144–149.
1983.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deppe G, Zbella E, Friberg J and Thomas W:
Combination chemotherapy for mixed Müllerian tumor of the fallopian
tube. Cancer. 54:1517–1520. 1984.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Punnonen R, Lauslahti K and Pystynen P:
Primary malignancies of the fallopian tube. Ann Chir Gynaecol
Suppl. 197:15–18. 1985.PubMed/NCBI
|
|
33
|
Buchino JJ and Buchino JJ: Malignant mixed
Müllerian tumor of the fallopian tube. Arch Pathol Lab Med.
111:386–387. 1987.PubMed/NCBI
|
|
34
|
Yabushita H, Ogawa A, Hoshina S, Okamoto
T, Nakanishi M and Ishihara M: Malignant mixed mesodermal tumour of
the fallopian tube. Case report. Br J Obstet Gynaecol. 94:179–183.
1987.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen KT and Wolk RW: Extragenital
malignant mixed Müllerian tumor. Gynecol Oncol. 30:422–426.
1988.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Muntz HG, Rutgers JL, Tarraza HM and
Fuller AF Jr: Carcinosarcomas and mixed Müllerian tumors of the
fallopian tube. Gynecol Oncol. 34:109–115. 1989.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Axelrod JH, Herbold DR and Freel JH:
Carcinosarcoma of the fallopian tube. Gynecol Oncol. 32:398–400.
1989.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kinoshita M, Asano S, Yamashita M and
Matsuda T: Mesodermal mixed tumor primary in the fallopian tube.
Gynecol Oncol. 32:331–335. 1989.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van Dijk CM, Kooijman CD and van Lindert
AC: Malignant mixed Müllerian tumour of the fallopian tube.
Histopathology. 16:300–302. 1990.PubMed/NCBI
|
|
40
|
Seraj IM, King A and Chase D: Malignant
mixed Müllerian tumor of the oviduct. Gynecol Oncol. 37:296–301.
1990.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liang WW, Lin YN and Lee YN: Malignant
mixed Müllerian tumor of fallopian tube. Report of a case and
review of literature. Zhonghua Yi Xue Za Zhi (Taipei). 45:272–275.
1990.PubMed/NCBI
|
|
42
|
Chang HC, Hsueh S and Soong YK: Malignant
mixed Müllerian tumor of the fallopian tube. Case report and review
of the literature. Changgeng Yi Xue Za Zhi. 14:259–263.
1991.PubMed/NCBI
|
|
43
|
Chiou YK, Su IJ, Chen CA and Hsieh CY:
Malignant mixed Müllerian tumor of the fallopian tube. J Formos Med
Assoc. 90:793–795. 1991.
|
|
44
|
Moore DT, Taslimi MM and Kosanovich M:
Malignant mixed Müllerian tumor of the fallopian tube of the
heterologous type. J Tenn Med Assoc. 85:513–514. 1992.PubMed/NCBI
|
|
45
|
Carlson JA Jr, Ackerman BL and Wheeler JE:
Malignant mixed Müllerian tumor of the fallopian tube. Cancer.
71:187–192. 1993.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weber AM, Hewett WF, Gajewski WH and Curry
SL: Malignant mixed Müllerian tumors of the fallopian tube. Gynecol
Oncol. 50:239–243. 1993.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zorlu CG, Cobanoglu O, Kușcu E and Aribas
D: Malignant mixed Müllerian tumor of the fallopian tube. Acta
Obstet Gynecol Scand. 73:352–354. 1994.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Horn LC, Werschnik C, Bilek K and Emmert
C: Diagnosis and clinical management in malignant Müllerian tumors
of the fallopian tube. A report of four cases and review of recent
literature. Arch Gynecol Obstet. 258:47–53. 1996.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ebert AD, Perez-Canto A, Schaller G,
Entezami M, Hopp HS and Weitzel HK: Stage I primary malignant mixed
Müllerian tumor of the fallopian tube. Report of a case with
five-year survival after minimal surgery without adjuvant
treatment. J Reprod Med. 43:598–600. 1998.PubMed/NCBI
|
|
50
|
Maitra RN, Lee J, McConnell DT, Kenwright
DN and Dady P: Malignant mixed Müllerian tumour of the fallopian
tube occurring in a patient with Peutz-Jegher's syndrome. Aust N Z
J Obstet Gynaecol. 44:77–79. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Moustafa M, Beynon DW and Elmahallawy M:
Primary malignant mixed Müllerian tumour of the fallopian tube. J
Obstet Gynaecol. 24:940–941. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Humble S and Carter E: Pathologic quiz
case: A right adnexal mass in a postmenopausal patient. Malignant
mixed Müllerian tumor with heterologous elements arising in the
fallopian tube. Arch Pathol Lab Med. 128:e161–162. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lim BJ, Kim JW, Yang WI and Cho NH:
Malignant mixed Müllerian tumor of fallopian tube with multiple
distinct heterologous components. Int J Gynecol Cancer. 14:690–693.
2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gagner JP and Mittal K: Malignant mixed
Müllerian tumor of the fimbriated end of the fallopian tube: Origin
as an intraepithelial carcinoma. Gynecol Oncol. 97:219–222.
2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kuroda N, Moriki T, Oguri H, Maeda N, Toi
M, Miyazaki E, Hiroi M, Fukaya T and Enzan H: Malignant Müllerian
mixed tumor (carcinosarcoma) of the fallopian tube: An
immunohistochemical study of neoplastic cells. APMIS. 113:643–646.
2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Das TK, Raha K, Bandyopadhyay A, Dasgupta
A, Ghosh D and Mondal AK: Malignant mixed Müllerian tumour of the
fallopian tube of heterologous variety-a case report. Indian J
Pathol Microbiol. 48:354–356. 2005.PubMed/NCBI
|
|
57
|
Hudelist G, Unterrieder K, Kandolf O, Alpi
G, Pucher S, Pollak G, Czerwenka K and Keckstein J: Malignant mixed
Müllerian tumor with heterologous component arising in the
fallopian tube-a case report. Eur J Gynaecol Oncol. 27:509–512.
2006.PubMed/NCBI
|
|
58
|
Kuroda N, Inui Y, Ohara M, Hirouchi T,
Mizuno K, Kubo A, Hayashi Y, Enzan H and Lee GH: Hyaline
globule-like structures in undifferentiated sarcoma cells of
malignant Müllerian mixed tumor of the fallopian tube. Med Mol
Morphol. 40:46–49. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kawaguchi W, Itamochi H, Kigawa J,
Kanamori Y, Oishi T, Shimada M, Sato S, Sato S and Terakawa N:
Chemotherapy consisting of paclitaxel and carboplatin benefits a
patient with malignant mixed Müllerian tumor of the fallopian tube.
Int J Clin Oncol. 13:461–463. 2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kourea HP, Adonakis G, Androutsopoulos G,
Zyli P, Kourounis G and Decavalas G: Fallopian tube malignant mixed
Müllerian tumor (carcinosarcoma): A case report with
immunohistochemical profiling. Eur J Gynaecol Oncol. 29:538–542.
2008.PubMed/NCBI
|
|
61
|
Piura B, Rabinovich A, Apel-Sarid L and
Shaco-Levy R: Carcinosarcoma of the fallopian tube with metastasis
of its epithelial component to the ovary, appendix and omentum. J
Obstet Gynaecol. 29:566–567. 2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Malhotra V, Nanda S, Chauhan MB, Marwah N
and Sen R: Heterologous malignant mixed Müllerian tumor of the
uterus and fallopian tube: A case report. J Gynacol Surg.
28:296–298. 2012.PubMed/NCBI
|
|
63
|
Tsai CP, Ho ES, Ke YM, Hsu ST, Wang RC and
Lu CH: Stage III malignant mixed Müllerian tumor of the fallopian
tube: A case of 5-year survival after optimal debulking and
adjuvant chemotherapy with paclitaxel plus carboplatin. Taiwan J
Obstet Gynecol. 51:294–296. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gupta R and Jenison E: A rare case of
carcinosarcoma of the fallopian tube presenting with torsion,
rupture and hemoperitoneum. Gynecol Oncol Case Rep. 2:4–5.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Takemoto Y, Ota T, Aoki Y, Ogura K,
Ogishima D and Matsumoto T: Carcinosarcoma of the fallopian tube
with disappearance of carcinoma cells by neoadjuvant chemotherapy:
Case study. Eur J Gynaecol Oncol. 36:618–622. 2015.PubMed/NCBI
|
|
66
|
Narin MA, Basaran D, Karalok A, Turan T
and Tulunay G: Primary fallopian tube carcinosarcoma: Report of two
cases. Yeditepe Med J. 11:945–948. 2015.
|
|
67
|
Vale-Fernandez E, Rodrigues F, Serrano P
and Silva AI: Primary malignant mixed Müllerian tumour of the
fallopian tube: A rare and difficult but possible diagnosis. BMJ
Case Rep. 2015(bcr2014209268)2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Monsalve N, Santos M, Petrosino P, Arenas
A and Sánchez Z: Tumor Mülleriano mixto heterólogo primario de
trompa uterine. Rev Obstet Ginecol Venez. 75:212–216. 2015.
|
|
69
|
Bécsi J, Szabó B, Szabó T, Onuș M, Mocan S
and Căpîlna M: Malignant mixed Müllerian tumor of fallopian tube:
Report of a case and review of the literature. Eur J Gynaecol
Oncol. 11:143–147. 2019.
|
|
70
|
Mackay HJ, Buckanovich RJ, Hirte H, Correa
R, Hoskins P, Biagi J, Martin LP, Fleming GF, Morgan R, Wang L, et
al: A phase II study single agent of aflibercept (VEGF Trap) in
patients with recurrent or metastatic gynecologic carcinosarcomas
and uterine leiomyosarco. A trial of the Princess Margret Hospital,
Chicago and California cancer phase II consortia. Gynecol Oncol.
125:136–140. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Makhija S, Howden N, Edwards R, Kelley J,
Townsend DW and Meltzer CC: Positron emission tomography/computed
tomography imaging for the detection of recurrent ovarian and
fallopian tube carcinoma: A retrospective review. Gynecol Oncol.
85:53–58. 2002.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Matulonis UA, Krag KJ, Krasner CN,
Atkinson T, Horowitz NS, Lee H and Penson RT: Phase II prospective
study of paclitaxel and carboplatin in older patients with newly
diagnosed Müllerian tumors. Gynecol Oncol. 112:394–399.
2009.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chiva L, Zanagnolo V, Querleu D,
Martin-Calvo N, Arevalo-Serrano J, Căpîlna ME, Fagotti A,
Kucukmetin A, Mom C, Chakalova G, et al: SUCCOR study: An
international European cohort observational study comparing
minimally invasive surgery versus open abdominal radical
hysterectomy in patients with stage IB1 cervical cancer. Int J
Gynecol Cancer. 30:1269–1277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Căpîlna ME, Szabo B, Rusu SC, Becsi J,
Moldovan B, Neagoe RM and Muhlfay G: Anatomical variations of the
obturator veins and their surgical implications. Eur J Gynaecol
Oncol. 38:263–265. 2017.PubMed/NCBI
|
|
75
|
Căpîlna ME, Moldovan B and Szabo B: Pelvic
exenteration-our initial experience in 15 cases. Eur J Gynaecol
Oncol. 36:142–145. 2015.PubMed/NCBI
|
|
76
|
Skafida E, Grammatoglou X, Katsamagkou E,
Glava C, Firfiris N and Vasilakaki T: Primary malignant mixed
Müllerian tumour of the fallopian tube. Report of a case. Eur J
Gynaecol Oncol. 31:126–128. 2010.PubMed/NCBI
|
|
77
|
Ma Y and Duan W: Clinical and survival
analysis of 36 cases of primary fallopian tube carcinoma. World J
Surg Onc. 12(311)2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mok JE, Kim YM, Jung MH, Kim KR, Kim DY,
Kim JH, Kim YT and Nam JH: Malignant mixed Müllerian tumors of the
ovary: Experience with cytoreductive surgery and platinum-based
combination chemotherapy. Int J Gynecol Cancer. 16:101–105.
2006.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yokoyama Y, Yokota M, Futagami M and
Mizunuma H: Carcinosarcoma of the fallopian tube: Report of four
cases and review of literature. Asia Pac J Clin Oncol. 8:303–311.
2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sutton G, Brunetto VL, Kilgore L, Soper
JT, McGehee R, Olt G, Lentz SS, Sorosky J and Hsiu JG: A phase III
trial of ifosfamide with or without cisplatin in carcinosarcoma of
the uterus: A gynecologic oncology group study. Gynecol Oncol.
79:147–153. 2000.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Căpîlna ME, Rusu SC, Laczko C, Szabo B and
Marian C: Three synchronous primary pelvic cancers-a case report.
Eur J Gynaecol Oncol. 36:216–218. 2015.PubMed/NCBI
|